Abstract
Gastric cancer serves a major role in the global cancer burden, being the fourth most frequent cause of mortality among all types of cancer. Gastric squamous cell carcinoma (GSCC) is a rare histological variant accounting for 0.04-0.5% of all gastric cancer cases. Diagnostic work-up of GSCC is essential and involves multiple criteria: i) Tumour not located in the cardia, ii) no oesophageal extension of the tumour, and iii) no evidence of SCC in any other part of the body. Little is known about this rare variant in terms of pathogenesis, risk factors or evolution. Consequently, neither the European Society of Medical Oncology nor the National Comprehensive Cancer Network societies have published recommendations for GSCC. The aim of the present review is to provide an in-depth analysis of the current literature on this pathology, from pathophysiological hypothesis and clinical presentation to diagnostic work-up and treatment trends, in order to establish a possible management algorithm.
Keywords: GSCC, endoscopy
1. Introduction
Gastric cancer is the fifth most common cancer worldwide in terms of incidence, with >1 million new cases in 2020, and the fourth most frequently implicated in cancer-related deaths, with 768,793 deaths occurring per year (1). Incidence varies according to sex (15.7 cases/100,000 for men and 7.0 cases/100,000 for women) and age, with the highest incidence amongst people aged 55-80 years old (2). In addition, 50% of cases occur in Eastern Asia (3). Gastric adenocarcinoma (GC) represents 90% of all gastric cancer cases (4), whereas gastric squamous cell carcinoma (GSCC) accounts for 0.04-0.5% of cases (5,6). Other rare histological variants include adenosquamous carcinoma, carcinosarcoma, and oncocytic and malignant rhabdoid tumours (7). Rolleston and Trevor (8) described the first primary GSCC case in 1905 in a 39-year-old female patient. Akce et al (6) collected retrospective data on 61,215 gastric cancer cases diagnosed between 2004 and 2013 using the National Cancer Database. In this cohort, GSCC represented 1.4% of cases (n=836) with a mean age at diagnosis of 65.9 years (range, 23-90 years); in addition, a large proportion of the patients were male, 72.5% of all cases. Furthermore, in this cohort, 14.4% of patients with GC and 24.3% of patients with GSCC were African American (6). Using the Surveillance, Epidemiology, and End Results (SEER) database, Dong et al (9) revealed that 50% of patients with GSCC are diagnosed with stage IV disease. Mixed histology is also found in GSCC (10).
2. Pathogenesis and aetiology
The aetiology of GSCC is currently unknown. Pathophysiological hypotheses include differentiation of totipotent stem cells, the transformation of existing ectopic squamous tissue, squamous metaplasia resulting from chronic inflammation, a squamous transformation of a pre-existing adenocarcinoma, and infection with Epstein-Barr virus (EBV) or human papillomavirus (HPV) (5,11). First mentioned by Boswell and Helwig in 1965(12), squamous metaplasia is thought to be associated with certain factors, such as peptic ulcers, corrosive burns, congenital syphilis, chemoradiotherapy and long-term treatment with cyclophosphamide. These stressors create an inflammatory setting within the gastric mucosa, leading to structural changes and squamous metaplasia (13). In GC, the pathophysiological sequence is widely accepted to be a progression from chronic gastritis to atrophic gastritis and metaplasia, dysplasia, and, eventually, carcinoma (14).
GSCC and GC may share certain risk factors. Helicobacter pylori infection is a well-known risk factor for GC and gastritis, accounting for 90% of non-cardia cases; however, its association with GSCC has yet to be proven (15,16). Out of four case reports, only one has reported on the synchronous occurrence of H. pylori and GSCC (17-20). Smoking is also a known risk factor for GC (15), and three studies have highlighted smoking as a potential risk factor for GSCC (21-23). The hypothesis that EBV could be a risk factor comes from the fact that EBV is detected in 10% of gastric cancer cases (24) and that it was detected in two case reports of GSCC (17,25,26). Furthermore, HPV is known to cause dysplasia in squamous epithelia, and it is strongly associated with head/neck, cervix and anus squamous cell carcinoma (27); however, its association with GSCC is still unproven. Of the three case reports of GSCC using immunohistochemistry and/or in situ hybridisation for HPV detection, none were positive (17,19,25). Other established risk factors for GC, such as heavy alcohol consumption, foods preserved by salting, gastroesophageal reflux and obesity, may also be implicated in the pathogenesis of GSCC, but data are still insufficient (15). Ma et al (28) performed molecular analyses on six cases of GSCC and demonstrated an increase in the Sonic hedgehog signalling pathway-related molecules compared with in GC.
3. Prognosis
GC has a 5-year overall survival rate of 20%, whereas the 5-year overall survival rate for GSCC ranges between 13 and 51.9% (6,9,29,30). Notably, ~50% of GSCC cases are diagnosed as locally advanced or metastatic disease, which may explain the lower survival rate compared with GC (9). The median overall survival range is between 7 and 8.9 months (6,9,31). In a retrospective study, Akce et al (6) showed that, stage by stage, median overall survival for GSCC was lower compared with GC survival at any stage: Stage I, 16.1 vs. 32.2 months; stage II, 19.3 vs. 23.7 months; stage III, 14.2 vs. 16.4 months (6). As for GC, staging is the most important prognostic factor for GSCC (6,30). Furthermore, not receiving chemotherapy or surgery is associated with worse survival rates (6).
4. Clinical presentation
The clinical presentation of GSCC is similar to GC since symptoms are often non-specific. Common complaints include abdominal pain, weight loss, melena, as well as nausea and vomiting (9,30).
5. Diagnosis
As for GC, GSSC diagnosis requires histological proof obtained during a gastroscopy. The European Society of Gastrointestinal Endoscopy recommends that the gastroscopy last ≥7 min for an accurate diagnosis (32). Notably, GSCC cannot be differentiated from GC based on visual appearance during endoscopic procedures and thus histopathological analysis is required (9). Both GC and GSCC lesions usually present as a mass or a non-healing ulcer (33).
GSCC is referenced by the World Health Organization classification under the number: ICD 8070/3(7). The Japanese Gastric Cancer Association has set specific histological criteria for GSCC including: i) Tumour cells are exclusively SCC cells, with no adenocarcinomatous cell contingent, and ii) evidence that the GSCC arose from the gastric mucosa directly (34). Boswell and Helwig (12) were the first to set histological morphological criteria for GSCC. The lesion should include at least one of the following criteria: i) Keratinized cell masses forming keratin pearls, ii) mosaic cell arrangement, iii) presence of intercellular bridges, and iv) high concentration of sulfhydryl and/or disulfide groups, indicating the presence of keratin or prekeratin (12). Large biopsies are mandatory to exclude another associated component (adenocarcinoma in particular).
The morphological aspect is the cornerstone of the differential diagnosis between GSCC and GC. However, on biopsies, in case of diagnostic doubt between a poorly differentiated adenocarcinoma and a poorly differentiated SCC, complementary techniques such as staining with Alcian blue-PAS (in search of mucus in GC) or immunohistochemical markers (such as p40, which is positive in GSCC) can be used. Parks (35) first proposed diagnostic criteria to differentiate GSCC from SCC of the oesophagus or metastasis from another primary site: i) Tumour not located in the cardia, ii) no oesophageal extension of the tumour, and iii) no evidence of SCC in any other part of the body (35). To the best of our knowledge, molecular analyses have been performed in only two case reports (36,37), with one reporting a mismatch repair deficient lesion (36).
6. Staging
There is no specific recommendation for GSCC regarding staging. We recommend following the European Society for Medical Oncology (ESMO) GC staging recommendations for GSCC, including endoscopic ultrasound, with or without fine needle aspiration for the assessment of tumour depth and regional lymph node involvement, computed tomography (CT) of the thorax and abdomen and/or pelvis for detecting distant metastasis, and laparoscopy for peritoneum assessment (33). Schizas et al (30) reported the use of positron emission tomography (PET)-CT in preoperative staging in 18.3% of cases. This could prove to be a useful additional tool in the detection of distant metastasis, although it is not recommended routinely by the ESMO (4). To the best of our knowledge, there are currently no data on magnification endoscopy for GSCC; this tool has however proven to be useful in oesophageal SCC, enabling better identification of the margins, as well as predicting the depth of infiltration to optimize endoscopic treatment (38).
GSCC staging is also performed using the American Joint Committee on Cancer TNM classification for GC, which depends on the depth of invasion, lymph node invasion, and the presence or absence of distant metastases (39). The most common metastasis sites for GSCC are the liver, peritoneum, lung and bones (40). Another classification system by the Japanese Gastric Cancer Association is based on refined anatomic location, particularly on the lymph node stations (41). To the best of our knowledge, these staging systems have not been formally compared.
7. Treatment options
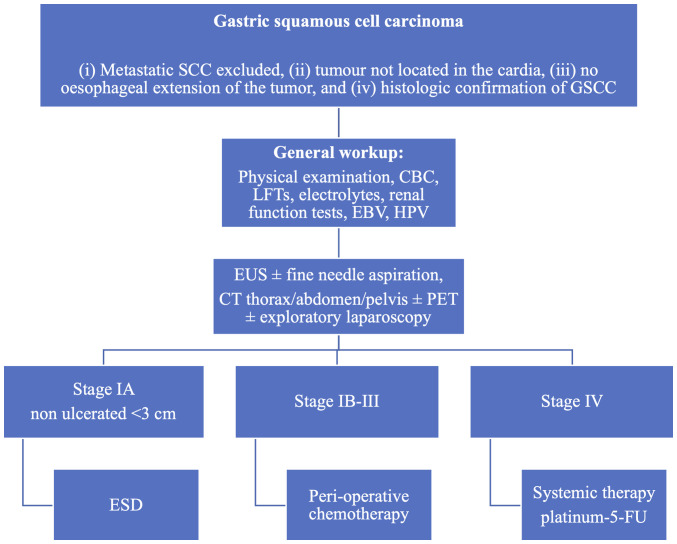
Neither the ESMO nor the National Comprehensive Cancer Network (NCCN) has recommendations for how to treat GSSC. Treatment trends were reported by Akce et al (6) in their study of 836 cases of GSCC. Surgical treatment was used in 26% of cases, compared with 32 and 95% reported in other studies (6,9,18) (Table I). They also reported that patients not receiving surgery were associated with worse survival rates (hazard ratio, 2.79) (6). In another study, Dong et al (9) reported that the 5-year overall survival rates were 17.4 and 59.2% in the surgery and non-surgery subgroups of patients with GSCC. Furthermore, Dong et al (9) reported radiation therapy in 23.3% of patients, compared with in 48.1% of patients reported by Akce et al (6). Akce et al (6) also observed that patients not treated with chemotherapy were associated with worse survival (hazard ratio, 1.71). A previous literature review conducted by Schizas et al (30) showed a 5-year overall survival rate of 51.9% and 3-year disease-free survival rate of 30.8% for patients undergoing surgical treatment for GSCC. In this review, only one patient received neoadjuvant chemotherapy with carboplatin/paclitaxel, 20 patients (60%) received adjuvant chemotherapy (mostly cisplatin or 5-fluorouracil based), and three patients (10%) benefited from radiation therapy (45 Gy in 25 fractions or 50.4 Gy in 28 fractions) (30). These survival results are inferior to the reported 70% 5-year survival rate for localized GC (42). To the best of our knowledge, no difference in resection margins has been reported for GSCC compared with GC. We propose the use of a management algorithm, as shown in Fig. 1.
Table I.
Gastric squamous cell carcinoma case series.
| First author, year | Number of included cases | Use of surgery, % | Use of radiation therapy, % | Use of systemic therapy, % | 5-year overall survival rate, % | (Refs.) |
|---|---|---|---|---|---|---|
| Akce, 2019 | 836 | 26.0 | 48.1 | 59.8 | 17.4 | (6) |
| Dong, 2016 | 163 | 31.9 | 23.3 | Not reported | 32.7 | (9) |
| Wakabayashi, 2014 | 56 | 94.6 | Not reported | 20 | Not reported | (18) |
Figure 1.
Proposed treatment algorithm for GSCC. GSCC, gastric squamous cell carcinoma; CBC, complete blood count; LFTs, liver function tests; EBV, Epstein-Barr virus; HPV, human papillomavirus; EUS, endoscopic ultrasound; CT, computed tomography; PET, positron emission tomography; ESD, endoscopic submucosal dissection; 5-FU, 5-fluorouracil.
Non-metastatic disease
A literature review performed by Schizas et al (30) suggested that radical resection is an important factor in improving patient survival. Although no data on the topic exists, we hypothesize that for early GSCC lesions (≤cT1 cN0) endoscopic submucosal dissection could be proposed for non-ulcerated lesions with a maximum diameter of 3 cm. This procedure could also serve as a ‘staging macro biopsy’ in case of histological features conferring a higher risk for nodal or systemic spread. For localised but more advanced disease (>cT1), Schizas et al (30) showed that most patients benefited from adjuvant therapy following resection, and despite the lack of strong evidence, we recommend a peri-operative chemotherapy approach with primary tumour resection and D2 lymphadenectomy, due to the poor prognosis of GSCC. Although without formal evidence we recommend the use of FLOT (5-fluorouracil, oxaliplatin and docetaxel) as a peri-operative regimen based on the fact that these drugs seem to have some level of efficacy in metastatic GSCC (4).
Metastatic disease
Very little is known about the treatment for metastatic GSCC. To the best of our knowledge, only nine cases have been published (Table II), with clinicians using platinum-fluoropyrimidine or platinum-taxane doublet (18,23,34,43-48). Chemoradiotherapy was used in one case with capecitabine and oxaliplatin and reported survival of 27 months (18). Due to the lack of reporting, it is not possible to generate strong evidence. In line with the published cases, we recommend using platinum-fluoropyrimidine or platinum-taxane doublet as the first line of treatment. Although molecular knowledge in GSCC is poor and response to targeted therapies has not been described in this pathology we are recommending testing metastatic GSCC, similar to GC, at least for HER-2 amplification and microsatellite instability. Notably, those molecular alterations and their associated therapies (namely, trastuzumab or anti-PD-1 immunotherapy) have shown efficacy in all but a few tumour types, independently of the tumour histology and localisation (4). Psychological and dietary support is also recommended with attention to vitamin and mineral deficiencies (4).
Table II.
Metastatic gastric squamous cell carcinoma case reports.
| First author, year | Sex | Age, years | Metastasis site | Treatment | Survival after diagnosis | (Refs.) |
|---|---|---|---|---|---|---|
| Amado Villanueva, 2022 | Female | 32 | Liver | Folinic acid, 5-FU, oxaliplatin | 8 months | (43) |
| Beattie, 2019 | Male | 53 | Liver | Carboplatin, paclitaxel | Not reported (>6 months) | (44) |
| Sabbah, 2021 | Female | 66 | Ovary | Symptomatic | Few months | (34) |
| Vailas, 2019 | Male | 66 | Lungs | Surgery, paclitaxel, carboplatin | Not reported (>12 months) | (45) |
| Von Waagner, 2015 | Male | 70 | Peritoneum, liver | Surgery, radiation, capecitabine, oxaliplatin | 27 months | (23) |
| Wakabayashi, 2014 | Male | 69 | Liver | Surgery, 5-FU, cisplatin, docetaxel | 36 months | (18) |
| Wu, 2016 | Male | 59 | Liver, spleen | Surgery, docetaxel, cisplatin | 16 months | (46) |
| Yamagata, 2019 | Male | 60 | Liver | Surgery, S-1 (novel 5-FU derivative) | 17 months | (48) |
| Yang, 2020 | Female | 51 | Peritoneum | Docetaxel, cisplatin | Not reported | (47) |
5-FU, 5-fluorouracil.
8. Treatment evaluation and follow-up
Due to the aggressiveness of the disease, we recommend a close follow-up of patients with GSSC. Due to the current lack of data, we recommend referring to the ESMO or NCCN guidelines described for GC for treatment assessment and follow-up of GSSC (4).
9. Conclusion
GSSC is a rare entity regarding which little is currently known. Similarly to other rare histological subtypes, a precise definition is needed to ensure an adequate diagnosis (35,49). The present review summarized the few reports regarding GSSC showing that patients diagnosed with GSSC seem to respond to molecules used to treat GC; therefore, our recommendation is currently to treat GSSC accordingly.
The present literature review has several limitations: First, a reporting bias could exist due to the rarity of the pathology of which only a handful of cases have been reported. Second, a selection bias may exist, in that not all cases used the diagnostic criteria set by Parks (35), Boswell and Helwig (12), or the Japanese Gastric Cancer Association (34). It is likely that database analyses, such as the SEER database analysed by Dong et al (9) or the National Cancer Database analysed by Akce et al (6), are unable to assess the correctness of the diagnosis based on the three criteria listed by Parks (35) and will have most likely misclassified some cases. Third, data to support endoscopic submucosal dissection in GSCC are lacking and would benefit from future research; the prognosis of GSCC is inferior to GC, and for this reason, endoscopic submucosal dissection should only be considered for stage IA non-ulcerated lesions <3 cm with no additional risk factors. Finally, the retrospective nature of the present review is prone to bias that can only be addressed by prospectively collecting and analysing GSSC data.
To improve our knowledge, we recommend systematically testing for H. pylori, EBV or HPV to determine their potential role in the pathogenesis of GSCC. Extensive molecular profiling could help to determine if GSSC is molecularly related to oesophageal SCC. This element is of utmost importance as GSSC has been classically treated as GC with chemotherapy; however, the chemoradiotherapy regimens used for oesophageal SCC could prove to be a valid option. Finally, we are pledging the creation of an international database of rare gastric cancer cases to improve the care of these patients.
Acknowledgements
The authors would like to thank Professor Mary Flannery (Department of English, University of Bern) for her help in the writing of this article.
Funding Statement
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
TK designed the study. GDL collected the data and conducted the literature review. GDL, TK, EC and AB interpreted the data and contributed to the writing of the draft manuscript. Data authentication is not applicable. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
TK discloses consulting or advisory roles for Merck Sharp & Dohme (MSD), Bristol-Myers Squibb (BMS), Lilly, Roche, Boehringer Ingelheim and Servier. All other authors declare that they have no competing interests.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Thrift AP, El-Serag HB. Burden of gastric cancer. Clin Gastroenterol Hepatol. 2020;18:534–542. doi: 10.1016/j.cgh.2019.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rahman R, Asombang AW, Ibdah JA. Characteristics of gastric cancer in Asia. World J Gastroenterol. 2014;20:4483–4490. doi: 10.3748/wjg.v20.i16.4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lordick F, Carneiro F, Cascinu S, Fleitas T, Haustermans K, Piessen G, Vogel A, Smyth EC. Gastric cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022;33:1005–1020. doi: 10.1016/j.annonc.2022.07.004. ESMO Guidelines Committee. Electronic address: Clinicalguidelines@esmo.org. [DOI] [PubMed] [Google Scholar]
- 5.Guzman Rojas P, Parikh J, Vishnubhotla P, Oharriz JJ. Primary gastric squamous cell carcinoma. Cureus. 2018;10(e2389) doi: 10.7759/cureus.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akce M, Jiang R, Alese OB, Shaib WL, Wu C, Behera M, El-Rayes BF. Gastric squamous cell carcinoma and gastric adenosquamous carcinoma, clinical features and outcomes of rare clinical entities: A National Cancer Database (NCDB) analysis. J Gastrointest Oncol. 2019;10:85–94. doi: 10.21037/jgo.2018.10.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182–188. doi: 10.1111/his.13975. WHO Classification of Tumours Editorial Board. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rolleston HD, Trevor RS. A case of columnar-celled carcinoma of the stomach showing squamous-celled metaplasia. J Pathol Bacteriol. 1905;10:418–422. [Google Scholar]
- 9.Dong C, Jiang M, Tan Y, Kong Y, Yang Z, Zhong C, Li D, Yuan Y. The clinicopathological features and prognostic factors of gastric squamous cell carcinoma. Medicine (Baltimore) 2016;95(e4720) doi: 10.1097/MD.0000000000004720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faria GR, Eloy C, Preto JR, Costa EL, Almeida T, Barbosa J, Paiva ME, Sousa-Rodrigues J, Pimenta A. Primary gastric adenosquamous carcinoma in a Caucasian woman: A case report. J Med Case Reports. 2010;4(351) doi: 10.1186/1752-1947-4-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Straus R, Heschel S, Fortmann DJ. Primary adenosquamous carcinoma of the stomach. A case report and review. Cancer. 1969;24:985–995. doi: 10.1002/1097-0142(196911)24:5<985::aid-cncr2820240518>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 12.Boswell JT, Helwig EB. Squamous cell carcinoma and adenoacanthoma of the stomach. A clinicopathologic study. Cancer. 1965;18:181–192. doi: 10.1002/1097-0142(196502)18:2<181::aid-cncr2820180209>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 13.Chang YS, Kim MS, Kim DH, Park S, You JY, Han JK, Kim SH, Lee HJ. Primary squamous cell carcinoma of the remnant stomach after subtotal gastrectomy. J Gastric Cancer. 2016;16:120–124. doi: 10.5230/jgc.2016.16.2.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mukkamalla SKR, Recio-Boiles A, Babiker HM. Gastric Cancer. In: StatPearls. StatPearls Publishing, Treasure Island (FL), 2023. [PubMed] [Google Scholar]
- 15.Morgan E, Arnold M, Camargo MC, Gini A, Kunzmann AT, Matsuda T, Meheus F, Verhoeven RHA, Vignat J, Laversanne M, et al. The current and future incidence and mortality of gastric cancer in 185 countries, 2020-40: A population-based modelling study. EClinicalMedicine. 2022;47(101404) doi: 10.1016/j.eclinm.2022.101404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee YC, Chiang T-H, Chou CK, Tu YK, Liao WC, Wu MS, Graham DY. Association between helicobacter pylori eradication and gastric cancer incidence: A systematic review and Meta-Analysis. Gastroenterology. 2016;150:1113–1124.e5. doi: 10.1053/j.gastro.2016.01.028. [DOI] [PubMed] [Google Scholar]
- 17.Callacondo D, Ganoza-Salas A, Anicama-Lima W, Quispe-Mauricio A, Longacre TA. Primary squamous cell carcinoma of the stomach with paraneoplastic leukocytosis: A case report and review of literature. Hum Pathol. 2009;40:1494–1498. doi: 10.1016/j.humpath.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 18.Wakabayashi H, Matsutani T, Fujita I, Kanazawa Y, Nomura T, Hagiwara N, Hosone M, Katayama H, Uchida E. A rare case of primary squamous cell carcinoma of the stomach and a review of the 56 cases reported in Japan. J Gastric Cancer. 2014;14:58–62. doi: 10.5230/jgc.2014.14.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamada R, Horiguchi SI, Shimizuguchi R, Nakano N, Motoi T, Monma K, Hishima T. A first case of primary gastric verrucous carcinoma with isolated squamous epithelium in the stomach. Virchows Arch. 2019;475:115–119. doi: 10.1007/s00428-019-02542-8. [DOI] [PubMed] [Google Scholar]
- 20.Zhou K, Faraz A, Magotra M, Tahir M. Exophytic primary gastric squamous cell carcinoma and H. pylori gastritis. BMJ Case Rep. 2019;12(e230310) doi: 10.1136/bcr-2019-230310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.González-Sánchez JA, Vitón R, Collantes E, Rodríguez-Montes JA. Primary squamous cell carcinoma of the stomach. Clin Med Insights Oncol. 2017;11(1179554916686076) doi: 10.1177/1179554916686076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Y, Zhu H, Xu F, Cao Y, Gu X, Wan Y, Gou H. Clinicopathological characteristics, treatment, and prognosis of 21 patients with primary gastric squamous cell carcinoma. Gastroenterol Res Pract. 2016;2016(e3062547) doi: 10.1155/2016/3062547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.von Waagner W, Wang Z, Picon AI. A Rare Case of a primary squamous cell carcinoma of the stomach presenting as a submucosal mass. Case Rep Surg. 2015;2015(482342) doi: 10.1155/2015/482342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takada K. Epstein-Barr virus and gastric carcinoma. Mol Pathol. 2000;53:255–261. doi: 10.1136/mp.53.5.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Katsura Y, Okabayashi T, Ozaki K, Shibuya Y, Iwata J. A case of Epstein Barr virus-associated primary squamous cell carcinoma of stomach. Surg Case Rep. 2021;7(240) doi: 10.1186/s40792-021-01301-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takita J, Kato H, Miyazaki T, Nakajima M, Fukai Y, Masuda N, Manda R, Fukuchi M, Kuwano H. Primary squamous cell carcinoma of the stomach: A case report with immunohistochemical and molecular biologic studies. Hepatogastroenterology. 2005;52:969–974. [PubMed] [Google Scholar]
- 27.Szymonowicz KA, Chen J. Biological and clinical aspects of HPV-related cancers. Cancer Biol Med. 2020;17:864–878. doi: 10.20892/j.issn.2095-3941.2020.0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma XL, Sun HJ, Wang YS, Huang SH, Xie JW, Zhang HW. Study of Sonic hedgehog signaling pathway related molecules in gastric carcinoma. World J Gastroenterol. 2006;12:3965–3969. doi: 10.3748/wjg.v12.i25.3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thomas RM, Sobin LH. Gastrointestinal cancer. Cancer. 1995;75:154–170. doi: 10.1002/1097-0142(19950101)75:1+<154::aid-cncr2820751305>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 30.Schizas D, Papaconstantinou D, Syllaios A, Ntomi V, Kykalos S, Tsourouflis G, Nastos C, Misiakos E, Pikoulis E. Oncologic outcomes of patients with resectable primary gastric squamous cell carcinoma: A systematic review. Ann Gastroenterol. 2022;35:376–382. doi: 10.20524/aog.2022.0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meng Y, Zhang J, Wang H, Zhang Y, Sun R, Zhang Z, Gao F, Huang C, Zhang S. Poorer prognosis in patients with advanced gastric squamous cell carcinoma compared with adenocarcinoma of the stomach: Case report. Medicine (Baltimore) 2017;96(e9224) doi: 10.1097/MD.0000000000009224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bisschops R, Areia M, Coron E, Dobru D, Kaskas B, Kuvaev R, Pech O, Ragunath K, Weusten B, Familiari P, et al. Performance measures for upper gastrointestinal endoscopy: A European Society of Gastrointestinal Endoscopy (ESGE) Quality Improvement Initiative. Endoscopy. 2016;48:843–864. doi: 10.1055/s-0042-113128. [DOI] [PubMed] [Google Scholar]
- 33.Evans JA, Chandrasekhara V, Chathadi KV, Decker GA, Early DS, Fisher DA, Foley K, Hwang JH, Jue TL, et al. The role of endoscopy in the management of premalignant and malignant conditions of the stomach. Gastrointest Endosc. 2015;82:1–8. doi: 10.1016/j.gie.2015.03.1967. ASGE Standards of Practice Committee. [DOI] [PubMed] [Google Scholar]
- 34.Sabbah M, Gharbi G, Bellil N, Helal I, Chamakhi C, Gargouri D. Primary gastric squamous cell carcinoma with a bilio-gastric fistula and Krukenberg syndrome. Clin Case Rep. 2021;9(e04325) doi: 10.1002/ccr3.4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parks RE. Squamous neoplasms of the stomach. Am J Roentgenol. 1967;101:447–449. doi: 10.2214/ajr.101.2.447. [DOI] [PubMed] [Google Scholar]
- 36.Jakubik J, Majos A, Jesionek-Kupnicka D, Wrona E, Kaufman-Szymczyk A, Lubecka-Gajewska K, Jakubik J. An unusual non-metastatic, mismatch repair-deficient primary gastric squamous cell carcinoma presenting as a large, exophytic, bleeding tumor: A case report. Oncol Lett. 2023;25(82) doi: 10.3892/ol.2023.13668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schonhart L, Kaiser R, Schmidt R, Sailer A, Steinestel K, Schneider-Kappus W, Beltzer C. Primary gastric squamous cell carcinoma-a case report of diagnosis, treatment, histological findings and follow-up. Z Gastroenterol. 2022;61:178–182. doi: 10.1055/a-1801-0370. [DOI] [PubMed] [Google Scholar]
- 38.Dekker E, Houwen BBSL, Puig I, Bustamante-Balén M, Coron E, Dobru DE, Kuvaev R, Neumann H, Johnson G, Pimentel-Nunes P, et al. Curriculum for optical diagnosis training in Europe: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement. Endoscopy. 2020;52:899–923. doi: 10.1055/a-1264-2634. [DOI] [PubMed] [Google Scholar]
- 39.Amin MB, Edge SB, Greene FL, et al. AJCC Cancer Staging Manual. Springer International Publishing, 2018. [Google Scholar]
- 40.Riihimäki M, Hemminki A, Sundquist K, Sundquist J, Hemminki K. Metastatic spread in patients with gastric cancer. Oncotarget. 2016;7:52307–52316. doi: 10.18632/oncotarget.10740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosa F, Costamagna G, Doglietto GB, Alfieri S. Classification of nodal stations in gastric cancer. Transl Gastroenterol Hepatol. 2017;2(2) doi: 10.21037/tgh.2016.12.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 43.Amado Villanueva PP, López González J, Lázaro Sáez M, Cuello Entrena E, Delgado Maroto A. An unusual presentation of advanced primary gastric squamous cell carcinoma in a young woman. Gastroenterol Hepatol. 2022;45 (Suppl 1):S39–S40. doi: 10.1016/j.gastrohep.2021.01.014. [DOI] [PubMed] [Google Scholar]
- 44.Beattie M, Mansour R, Thigpin D, Haus C. Metastatic primary gastric squamous cell carcinoma: An uncommon presentation of a rare malignancy. Case Rep Gastrointest Med. 2019;2019(5305023) doi: 10.1155/2019/5305023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vailas MG, Syllaios A, Hasemaki N, Sotiropoulou M, Mpaili E, Sarlanis H, Felekouras E, Papalampros A. A type of neoplasia deadlier than gastric adenocarcinoma? Report of a case of primary gastric squamous cell carcinoma. World J Surg Oncol. 2019;17(113) doi: 10.1186/s12957-019-1657-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu XD, Zhou Y, Fan RG, Zhou B, Shi Q, Jia J. Primary squamous cell carcinoma of the stomach presenting as a huge retroperitoneal tumor: A case report. Rev Esp Enferm Dig. 2016;108:283–284. doi: 10.17235/reed.2015.3795/2015. [DOI] [PubMed] [Google Scholar]
- 47.Yang F, Lan Z, He Y. Gastric squamous cell carcinoma presenting in ascites: Negative to P63 and P40 after one course of chemotherapy. Diagn Cytopathol. 2020;48:787–789. doi: 10.1002/dc.24457. [DOI] [PubMed] [Google Scholar]
- 48.Yamagata Y, Saito K, Ban S, Fujii A, Oya M. The origin of p40-negative and CDX2-positive primary squamous cell carcinoma of the stomach: Case report. World J Surg Oncol. 2019;17(53) doi: 10.1186/s12957-019-1594-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Astaras C, Bornand A, Koessler T. Squamous rectal carcinoma: A rare malignancy, literature review and management recommendations. ESMO Open. 2021;6(100180) doi: 10.1016/j.esmoop.2021.100180. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.