Abstract
The hippocampus is known to support processing of precise spatial information in recently learned environments. It is less clear, but crucial for theories of systems consolidation, to know whether it also supports processing of precise spatial information in familiar environments learned long ago and whether such precision extends to objects and numbers. In this fMRI study, we asked participants to make progressively more refined spatial distance judgments among well-known Toronto landmarks (whether landmark A is closer to landmark B or C) to examine hippocampal involvement. We also tested whether the hippocampus was similarly engaged in estimating magnitude regarding sizes of familiar animals and numbers. We found that the hippocampus was only engaged in spatial judgment. Activation was greater and lasted longer in the posterior than anterior hippocampus, which instead showed greater modulation as discrimination between spatial distances became more fine grained. These findings suggest that the anterior and posterior hippocampus have different functions which are influenced differently by estimation of differential distance. Similarly, parahippocampal-place-area and retrosplenial cortex were involved only in the spatial condition. By contrast, activation of the intraparietal sulcus was modulated by precision in all conditions. Therefore, our study supports the idea that the hippocampus and related structures are implicated in retrieving and operating even on remote spatial memories whenever precision is required, as posted by some theories of systems consolidation.
Keywords: parahippocampus, hippocampus longitudinal axis, spatial distance, object size, familiar environments
Introduction
It is well known that the hippocampus is implicated in navigation during the acquisition and short-term retention of precise allocentric spatial information, and operates on such information in rodents (Routtenberg et al. 1980; Spiers et al. 2018) and humans (Burgess et al. 2002; Miller et al. 2013; Howard et al. 2014; Brown et al. 2016; Epstein et al. 2017; Kunz et al. 2021; Sakon and Kahana 2022). The hippocampus has also been implicated in the perception (Aly et al. 2013), acquisition, and retention of precise spatial and nonspatial information, such as about objects and colors (Yassa and Stark 2011; Koen et al. 2017; Ekstrom and Yonelinas 2020). Less certain is whether the hippocampus is implicated in representing and operating on precise information about the layouts of familiar environments that were learned long ago and whether the hippocampus is similarly implicated in representing precise information about well-learned, nonspatial material such as objects (Yassa and Stark 2011). Thus, the main goal of this study was to investigate to what extent the hippocampus, as well as related scene network regions, play a role in supporting spatial processing in familiar environments. A subsidiary goal was to determine whether the hippocampus was similarly implicated in processing familiar objects.
There is evidence that people with damage to the hippocampus can navigate successfully in highly familiar environments, yet their knowledge of these environments is often deficient in detail and precision (Teng and Squire 1999; Rosenbaum et al. 2000, 2005; Maguire et al. 2006; Hirshhorn et al. 2012; Herdman et al. 2015). Likewise, functional neuroimaging studies suggest that although the hippocampus is clearly implicated in navigating in recently learned environments, there is conflicting evidence on the extent to which it is implicated in navigating and representing highly familiar environments (Maguire et al. 1997; Rosenbaum et al. 2004, 2007). For example, information about distances between locations is a feature of a map-like representation that is crucial for navigation. Some studies report that, over time, the hippocampus comes to share this role with extra-hippocampal structures, such as the retrosplenial cortex (Patai et al. 2019), while other studies report that the hippocampus continues to represent information about distances even in familiar environments (Barense et al. 2010; Hirshhorn et al. 2012; Peer et al. 2019). In addition, there are no comparable studies examining hippocampal involvement in representing precise information about very familiar, nonspatial material, such as objects, though a number of theories posit that the hippocampus would be similarly engaged (Yassa and Stark 2011; Ekstrom and Yonelinas 2020). Therefore, the first goal of this study is to determine how precisely the hippocampus and related extra-hippocampal structures represent information about highly familiar environments and objects.
In both humans and rodents, functional longitudinal gradients are observed along the long axis of the hippocampus, from anterior to posterior hippocampus in humans (Poppenk et al. 2013; Brunec et al. 2018; Ayhan et al. 2021; Bouffard et al. 2023; Liu et al. 2023) or from ventral to dorsal in rodents (Strange et al. 2014; Vogel et al. 2020). Thus, we were interested to know whether anterior–posterior gradients would be observed that varied with representational precision even for spatial information acquired long ago (Peer et al. 2019). Such gradients were evident on fMRI along the long axis of the hippocampus (Poppenk et al. 2013; Evensmoen et al. 2015) and in neocortical structures (Peer et al. 2019), with the anterior and posterior regions favored in making coarse and fine discriminations, or operating on large-scale or small-scale environments, respectively (Brunec et al. 2019). In addition to differing with regard to precision of spatial representations, the anterior and posterior hippocampus differ on other dimensions that likely are related to representational precision (Poppenk et al. 2013). Evidence has pointed to the posterior hippocampus having a more important role in navigation (Maguire et al. 2000), whereas the anterior hippocampus has a more important role in scene construction (Zeidman et al. 2015; Zeidman and Maguire 2016). We conjectured, therefore, that there would also be temporal differences along the long axis, with the anterior hippocampus showing an initial, early peak of activation associated with the construction of a scene, followed by a relatively rapid decline, whereas the posterior hippocampus would have more sustained activity as it operated on information afforded by the scene and coded the distance (Howard et al. 2014; Patai et al., 2019). Therefore, the second aim of this study was to determine whether there is a functional gradient or differentiation along the long axis of the hippocampus for processing precise spatial information about highly familiar places acquired long ago and whether similar gradients would be found for highly familiar objects. To achieve these research goals, we scanned participants while they made comparative judgments (1) about spatial distances between locations in a familiar city, and (2) about the size of well-known animals, with differences between distance and sizes being varied parametrically. We then examined the involvement of the anterior versus posterior hippocampus separately.
Since making comparative judgments of distance and size requires magnitude estimation, our third goal was to explore whether the precise size/distance judgment process also activates the left intraparietal sulcus, which is known to be engaged in symbolic/numeric processing (Piazza et al. 2004; Ansari 2008; Nieder and Dehaene 2009; Henik et al. 2012). Thus, we included a numerical magnitude judgment task, which would provide a basis of comparison with the other two conditions. Because processes such as pattern separation and completion, maintenance, and manipulation of information in working memory, and magnitude estimation are common to all three tasks, evidence of differences in hippocampal engagement among these three tasks would suggest that the determining factor in differential hippocampal engagement on these tasks is their underlying material specific representation.
Methods
Participants
Data from 24 healthy, right-handed volunteers with ages of 18–33 years (M = 24.79 years, SD = 3.90 years, 14 males) and years of education of 12–21 (M = 15.67 years, SD = 2.44 years) were analyzed. Twenty-eight people participated in the study, though four participants were excluded: One participant’s data were not usable due to excessive movement in the MRI scanner. A second person did not have enough knowledge of Toronto landmarks and did not follow instructions. A third responded that they are right-handed when asked verbally but on the handedness questionnaire it was clear that they were predominantly left-handed, and the fourth used marijuana frequently (daily) and long term. All participants had normal or corrected to normal vision and were free from neurological and psychiatric disease based on self-report. Informed consent was obtained from all participants as per the ethical guidelines of the University of Toronto and the Rotman Research Institute at Baycrest Centre for Geriatric Care. All participants were compensated with $50 and received either reimbursement for public transit fare or a free parking pass.
All participants had good knowledge of landmarks in downtown Toronto. They lived in Toronto for at least 2 years (mean = 16.48, SD = 8.96). The Toronto Public Places Test (TPPT; Rosenbaum et al. 2004), a spatial memory survey, was also used to determine participants’ familiarity with 54 landmarks in downtown Toronto on a scale of 1–5 (no familiarity to high familiarity). All participants had at least 23 landmarks, which they rated 4 or 5. The average number of landmarks given a rating of 4 or 5 was 35.54 (SD = 7.16). Participants reported their confidence in navigating on a rating from 1 to 10, scoring a mean rating of 9.38 (SD = 0.77) for the places that they had visited and 7.46 (SD = 1.61) for the places they knew of but had not visited.
Prior to the fMRI experiment, participants also completed a series of neuropsychological tests to confirm their eligibility for the current study. Specifically, participants all performed within the normal range on the Montreal Cognitive Assessment Test (Nasreddine et al. 2005) (M = 28.54, SD = 1.77). The Shipley vocabulary test (Shipley et al. 2009) indicated that all participants had a high level of English knowledge, indicating that communication ability was not an issue in the differential processing tasks used in the current study. Participants were highly right-handed (M = 1.21, SD = 0.27; Verdino and Dingman 1998). They had high normal phonemic (M = 47.79, SD = 11.08) and category fluency (M = 32.08, SD = 8.57; Tombaugh et al. 1999). A table top spatial test sensitive to hippocampal integrity (Smith and Milner 1984; Hirshhorn et al. 2011) was modified to a computer version in our laboratory for assessing hippocampal involvement. This indicated successful learning of locations and no impaired spatial processing. These neuropsychological tests, taken together with the knowledge of Toronto, their ability to learn the table top test, and their ratings of confidence with navigation, suggest that the participants had normal navigational skills.
fMRI tasks and procedure
Participants were first given practice trials of the fMRI task outside the scanner. Then, they performed 90 minutes of the experimental task during a functional scan (with 10 runs) followed by 15 minutes of structural MRI scans. The total scanning time for each participant was 1 hour and 45 minutes. There were three conditions in this fMRI experiment. The conditions of interest were a landmark spatial processing task (i.e. landmark task), the animal size processing task (i.e. animal task), and the number value processing task (i.e. number task).
Landmark task: differential distance comparison
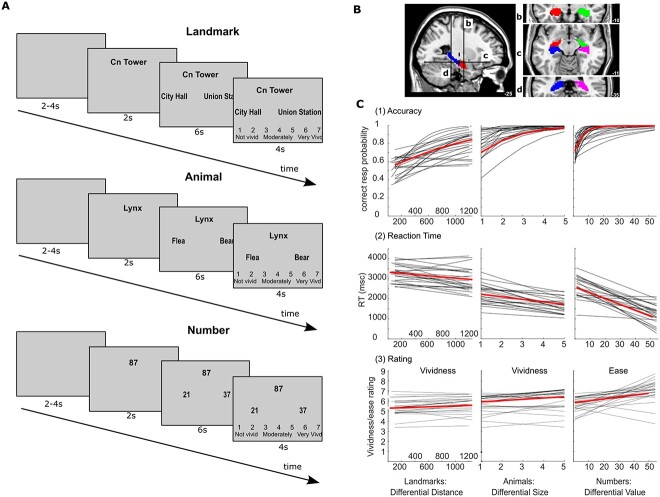
First, an individualized set of landmarks was generated for each participant. Specifically, we first chose the 20 most familiar landmarks (with rating ≥4 on the TPPT) for each participant. Then, we calculated the distance between any two landmarks using walking distance, a close approximation of Euclidean distance and readily available from Google Maps (Boscoe et al. 2012). Next, we chose 100 sets of 3 landmarks (one designated as starting location and the other two as targets) such that the differential distances, i.e. the distance between the starting location and Target 1 minus the distance between the starting location and Target 2, ranged from 50 to 1500 m. For example, with the walking distance from the starting location CN Tower to City Hall (Target 1) being 1300 m and CN Tower to Union Station (Target 2) being 750 m, the differential value was calculated as 1300 m − 750 m = 650 m. The angle from the two targets to the starting location was similarly distributed for trials with different level/magnitude of differential distances. Thus, differential distance between the two targets to the starting location was not correlated with the angle between them.
During each trial of the landmark task (Fig. 1A), the participants were first shown a cue landmark name, which was the starting location, for 2 seconds. Then, two different target landmark names were presented, and participants were asked to determine which of the two landmarks is geographically closer to the starting landmark. All three landmark names remained on the screen for 6 seconds. After the response, participants were asked to provide a vividness rating from 1 to 7 in a 4 second response window, in which a higher rating indicates stronger self-rated vividness of their mental images of the landmarks and neighborhood while making the differential distance comparison. Subjects utilized the 1 and 2 keys to move a box around a selected number in the 1 to 7 scale. The starting position was always 4. We presented half of the closer targets (i.e. the correct response) on the left side of the screen. The 100 trials in this condition were divided into 10 runs.
Fig. 1.
A) Typical trial for (a) landmark, (b) animal, and (c) number conditions. B) Anterior and Posterior hippocampal ROI: (a) left sagittal view of the anterior (red) and posterior (blue) hippocampus. Division (- - - -) is at the uncal apex. Coronal section view (b and d) and axial section view (c) are also presented. Masks were manually prepared and divided at the uncal apex for each participant. C) Behavioral performance at different differential values for each participant and each stimuli type: (1) accuracy (logistic regression), (2) response time (linear regression), (3) ratings (linear regression; vividness of judgment for landmark and animal and ease of judgment for the number task). Black lines represent the regression lines for each participant and the median regression lines are presented in red to reflect the relationship at the group level.
Animal task: differential size comparison
Twenty familiar animals were selected and their size was ranked (from 1 to 10) following Moyer (1973), with a larger number indicating a larger size. Then, similar to the landmark condition, 100 sets of 3 animals were selected such that the differential size ranged from 1 to 5. Next, we calculated the differential size using animal rankings; the minimum size (ranking) differential is 1 and the maximum is 5. For example, if the starting animal had a size ranking of 7 (e.g. Lynx), and Target 1 had a ranking of 1 (e.g. Flea), and Target 2 had a ranking of 8 (e.g. Bear), then the differential ranking would be ||1−7|−|8−7|| = 5 (| | denoting absolute value). Although all participants received the same animal sets, they were presented in individualized random order. All subjects reviewed the animal names prior to the experiment to be certain that they were familiar with each animal.
During each trial of the animal task, the name of the reference or starting animal was presented first, similarly to the landmark condition, followed by two different target animal names. Participants were asked to judge which of the two animals was closer in size to the starting item (Fig. 1A). After the response, participants were asked to provide a vividness rating from 1 to 7, where a higher rating indicates stronger vividness of their mental images of the animals during the size comparison. The 100 trials in this condition were divided into 10 runs. The left and right correct responses were also balanced within runs.
Number task: differential numerical value comparison
In this task, random numbers between 20 and 99 were used to create 100 sets of 3 numbers such that the differential differences are distributed between 1 and 54. For example, with 37 as the starting number, and 21 and 87 as the two targets, the differential value was calculated as follows: ||21−37|−|87−37|| = 34. All participants received the same trial sets presented in individualized random order.
For each trial of the number task, similar to the landmark task, participants first viewed a starting number, followed by two target numbers. Participants judged which of the two presented numbers was closer in value to the starting number (Fig. 1A). For the number differentials, however, participants rated the ease, instead of the vividness, with which they made the value comparison from 1 to 7. The 100 trials in this condition were divided into 10 runs. The left and right correct responses were also balanced within runs.
Thus, each of the 10 fMRI runs had 10 trials from the landmark, animal, and number tasks. These trials were distributed across a range from low to high differential landmark distance, animal size ranking, and numerical value. For each trial, there was a 2–4 second fixation cross presented at the center of the screen, which served as a jitter. In addition, we added two more trials in which participants were presented with names of landmarks in capital format and asked to count the vowels in the target landmark names and determine which of the two targets contained the least number of vowels (i.e. vowelcount trials). This condition was not analyzed in this study due to the low trial count. During the scan, participants were instructed to emphasize accuracy over speed when answering each question and to guess when they did not know the correct response. The response accuracy, response time, and vividness and ease scores were collected for each participant. Each run was 7 minutes 20 seconds long. Short breaks were allowed between each run.
Image acquisition
Anatomical and functional images were acquired at Baycrest Centre for Geriatric Care with a 3 T Siemens Magnetom Trio scanner (using Syngo MR B17 software) with a 12-channel matrix head coil. Functional MRI was assessed using the blood oxygenation level dependent signal and was obtained using axial, interleaved, multi-slice echo planar imaging (EPI, 30 slices, 5.0 mm thick, FOV = 20 by 20 cm, 64 by 64 acquisition matrix, 3.1 by 3.1 by 5.0 mm voxels, bandwidth = 2694 Hz/pixel, TE/TR = 30/2,000, flip angle = 70 degrees). Visual stimuli were presented on a back-projection screen using E-prime software, viewed with a mirror mounted on the head coil. Responses were collected with an fMRI-compatible response box. The task started 8 seconds after the MRI scanner was activated to allow magnetization to reach a steady state and to allow subjects to become accustomed to the sound of the scanner.
After the functional runs, we acquired a T1-weighted volumetric anatomical MRI for each participant (MPRAGE, 160 slices, 1.0 mm thick, FOV = 256 mm by 192 mm, 256 by 256 by 256 acquisition matrix, 1.0 by 1.0 by 1.0 mm voxels, bandwidth = 200 Hz/pixel, TI/TE/TR = 1100/2.63/2,000, flip angle = 9 degrees, averages = 1, scan time = 6:26).
Analysis
fMRI preprocessing
Functional image preprocessing was performed using SPM8 (Statistical Parametric Mapping v5236) software package with Matlab R2013a. First, the functional data were checked and no obvious artifact was found. Then the fMRI were slice-timing corrected and realigned using a six-parameter linear transformation. Next, the functional and anatomical images were co-registered and the anatomical images were then segmented into white matter, gray matter, and cerebrospinal fluid using SPM8 default tissue probability maps. These segmented images were then used to calculate the transformation parameters mapping from the individuals’ native space to the MNI template space. Next, the resulting transformation parameters were used to transform all functional and structural images to the MNI template. The functional images were re-sampled at 2 × 2 × 2 mm resolution and smoothed using a Gaussian kernel with the FWHM (full width at half maximum) of 6 mm.
fMRI task analysis
General Linear Model (GLM) design: SPM8 was used to conduct the first-level (i.e. individual) and second-level (i.e. group) region of interest (ROI) analyses. In the first-level event-related analyses, we first convolved trial onset from each condition, and in each run, with the canonical hemodynamic function as separate regressors. Each trial was defined from the onset of the starting stimuli to the end of the response collection (8 seconds in total). The two vowelcount trials were also convolved with the hemodynamic response function, but the regressor was treated as of no interest and not analyzed hereafter. To investigate whether differential values modulate brain activity in each condition, we adopted a parametric modulation analysis using the differential values (z-score transformed) calculated for each trial as additional regressors. These parametric modulators were entered for each condition and each run separately. No parametric modulator was entered for the vowelcount trials. Therefore, there were six regressors of interest in each run, with three condition mean activation regressors and three parametric modulation regressors. We also included six motion parameters in each run obtained from the image alignment processing as regressors of no interest. A default high-pass filter with a cut-off of 128 seconds was applied. In addition, a first-order autoregressive model AR(1) was used. To obtain the estimates for these regressors of interest across all 10 runs, we averaged the estimates of the 10 runs.
ROI analysis
ROI masks
Because the hippocampus is our major ROI, we conducted ROI analyses on the hippocampal activation in these conditions. To obtain the hippocampus ROI masks, we used Freesurfer recon-all, version 6.0 (Fischl 2012), to segment each participant’s anatomical image. The ROI masks were then normalized using the same parameters as used in the normalization of the functional data. The quality of the resulting hippocampus masks was confirmed by co-registering them with the participant’s anatomical image. To examine how the anterior and posterior hippocampus may play different roles in differential difference processing, we segmented the hippocampus into anterior and posterior subregions using the uncal apex as the boundary landmark, following Poppenk and Moscovitch (2011) and Weiss et al. (2005). This resulted in four ROIs: the left and right anterior and posterior hippocampus (Fig. 1B).
Hippocampal activation
Hippocampal activation in each condition was obtained using MarsBaR in SPM8 and the design matrix mentioned earlier. This step was implemented separately for the anterior and posterior hippocampus in the left and right hemisphere (i.e. the 4 ROIs). To ensure the results are robust, we treated individual ROI estimate values >2 SD as outliers, and used a winsorization method (i.e. suppressing extreme values that were beyond 2 SD to be equal to 2 SD), which affected less than 4% of data, to reduce the effect of the outliers. Repeated measure ANOVAs, with anterior versus posterior, left versus right hemisphere, and different conditions as factors, were then used to compare across conditions and ROIs at the second-level (i.e. group) analyses.
To confirm the analysis using the canonical hemodynamic function was accurate, we examined the time course of hippocampal activation during each differential processing condition using a Finite Impulse Response (FIR) model. Specifically, we used MarsBaR in SPM8 and the hippocampal masks to estimate brain responses within a 24 second (i.e. 12 TRs) window at each TR after the stimulus was presented. In this analysis, the conditions in the design were identical to the analysis described previously. The only difference was that instead of using the canonical hemodynamic function, we used 12 FIR to construct the regressors. We then baseline corrected the estimated response at each time point using the response value estimated at the first TR of the trial.
Differential value modulations
To test how differential value processing per se modulated the activation in different regions of the hippocampus, we similarly used MarsBaR in SPM8 and the design matrix mentioned earlier (with the canonical hemodynamic function) to obtain the ROI contrast estimate for the parametric modulation regressors in the landmark, animal, and number condition. To examine how these ROIs played a specific role in the landmark processing, we compared these ROI estimates in the landmark condition with those in the animal and number condition. Repeated ANOVA with the anterior versus posterior, left and right hemisphere, and task conditions as within-subject factors were used at these second-level analyses.
In repeated measures ANOVA with violations of sphericity, we used Greenhouse–Geisser (GG) correction for ε <0.75 and Huynh–Feldt (HF) correction of ε >0.75. The adjusted df was given and the results were indicated by GG or HF.
Whole-brain (voxel-wise) analyses
All major research questions of this study regarding the hippocampus were tested using ROI analyses. However, to explore other regions that may also participate in differential processing, such as the parahippocampus, we analyzed the task effects and linear modulation effects at the whole-brain single voxel level using SPM8 for each of the three conditions, landmark, animal, and number. Specifically, these voxel-wise contrast images obtained from the first-level analyses were used at the second-level analyses. One-sample t-test was used to test whether each contrast was statistically significant. To balance type I and II error and facilitate potential future meta-analyses (Lieberman and Cunningham 2009), we used a relatively lenient statistical threshold: P = 0.005, with 10 voxel extension. We note that the results of whole-brain activation for the landmark condition were also used to obtain the center coordinates for the parahippocampal place area (PPA) and retrosplenial cortex (RSC) ROIs (because no localization task was implemented in this study). Involvement of the PPA and RSC in these 3 conditions was then confirmed using trial-wise parametric ROI analyses (for details, see the Results section).
Finally, conjunction analyses were conducted to investigate what brain regions were commonly activated (i.e. condition mean effect) and commonly modulated by the trial-wise differential values (i.e. parametric modulation effect) in these three tasks which all involved magnitude processing. To this end, we calculated the intersection of the second-level whole-brain result images for the three conditions (mentioned in the previous analysis step), i.e. landmark ∩ animal ∩ number, each thresholded at P = 0.01 with 10 voxel extension. We conducted this analysis for both the condition activation and parametric modulation effects.
Results
Behavioral results
Accuracy
Mean accuracy of participants’ differential distance response was 68.25% (SD = 9.08), significantly above chance of 50%, t(23) = 9.85, P < 0.0001. To examine whether accuracy was related to the trial-by-trial differential distance, we performed a logistic regression analysis using trial-wise differential distance to predict the correctness (1: correct; 0: incorrect) of each trial Accuracy for all 100 trials for each individual participant, then tested the results at the group level. Results showed that larger differential distance predicted higher likelihood of correct response for landmarks, logistic regression coefficient β = 0.0014, t(23) = 6.041, P < 0.0001, see Fig. 1C(1).
Similar to the landmark condition, accuracy for animal and number condition was also significantly higher than chance (M = 93.92%, SD = 6.23, t(23) = 34.55, P < 0.0001, and M = 86.75%, SD = 5.885, t(23) = 30.59, P < 0.0001, for animal and number, respectively). Logistic regression showed that larger differential animal size or number value predicted higher accuracy for the animal condition, β = 9.527, t(23) = 2.36, P = 0.027, and number condition, β = 0.234, t(23) = 6.90, P < 0.0001, Fig. 1C(1).
Reaction time
Mean reaction time (RT) for the differential distance response in the landmark condition was 3117 ms (SD = 469.4 ms). To examine whether RT was related to the trial-by-trial differential distance processing, we performed a linear regression analysis using the trial-wise differential distance to predict the RT of each trial. Results for landmarks showed that higher differential distance predicted faster (lower) reaction times, regression coefficient β = −0.3010, t (23) = −3.88, P < 0.0001. The least square fit lines for each subject are presented in Fig. 1C(2) landmarks.
Similar to the landmark condition, we examined the RT in the animal and number conditions (M = 1951 ms, SD = 384.7 ms, and M = 2373 ms, SD = 447.5 ms, respectively). Regression analysis showed that, for the animal and number conditions, larger differential processing values led to faster responses (β = −146.3, t(23) = −7.611, P < 0.0001, β = −25.54, t(23) = −9.99, P < 0.0001; see Fig. 1C(2) animals and numbers).
Vividness and ease
Mean rating for vividness for the differential distance response in the landmark condition was 5.49 (SD = 0.8197). To examine whether the vividness rating was related to the trial-by-trial differential distance processing, we performed a linear regression analysis using the trial-wise differential distance to predict the rating of each trial. Results for landmarks showed that higher differential distance predicted higher vividness ratings, regression coefficient β = 0.0002, t(23) = 2.65, P < 0.0143. The least square fit lines for each subject are presented in Fig. 1C(3) landmarks.
Similar to the landmark condition, we examined the vividness rating in the animal and ease rating in the number condition (M = 5.88, SD = 0.94). Regression analysis showed, for the animal conditions, larger differential processing values were associated with higher vividness ratings (β = 0.0952, t(23) = 3.08, P < 0.005. See Fig. 1C(3) animals). Similarly, regression analysis showed that, for the number conditions (M = 5.90 ms, SD = 0.625 ms), larger differential processing values were associated with higher ease ratings (β = 0.0261, t(23) = 4.956, P < 0.0001; see Fig. 1C(3) numbers).
Participants rated all conditions similarly indicating that they may have responded with an ease judgment instead of a vividness judgment. The design itself made it difficult to differentiate between vividness versus ease judgments and therefore due to a question in the validity of this rating, we are not analyzing this further.
fMRI results: differential processing in different conditions: activation
Hippocampus ROI results
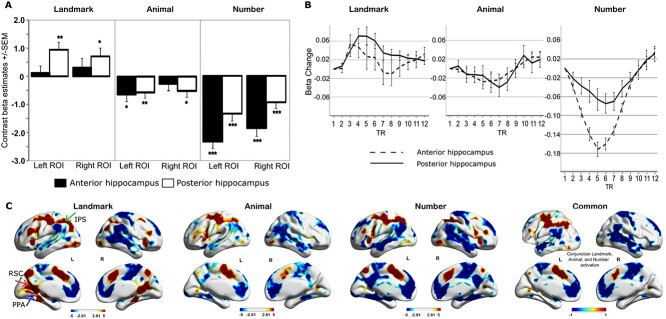
First, to test whether landmark processing engaged the hippocampus, and how different subregions along the longitudinal axis (i.e. anterior and posterior) of the hippocampus played a role in this type of differential distance processing, we conducted a 2 − 2 repeated measures ANOVA on landmark contrast values obtained from the first-level analysis, with longitudinal axis (anterior and posterior) and hemisphere (left and right) as two within-subject factors. The results showed that the landmark differential distance processing activation differentiated along the longitudinal axis such that the posterior hippocampus was more activated than its anterior counterpart (F(1,23) = 6.91, P = 0.015, η2 = 0.231; Fig. 2A). No differentiation was found between the hemispheres.
Fig. 2.
Differential processing effects in the landmark, animal, and number conditions. A) Differential processing effects in the bilateral anterior and posterior hippocampal ROIs. Condition effects: landmark > animal > number activation. Posterior–anterior differences: posterior ROI activation > anterior for landmark and number conditions. No longitudinal axis bias for animal condition. Laterality effects: right > left hemisphere bias for animal and number conditions. No hemisphere bias for landmarks condition. Significant changes in activity from baseline: *P < 0.05, **P < 0.01, ***P < 0.001. B) Time course of the differential processing effects (beta changes in %) in anterior and posterior hippocampal ROIs for landmark, animal, and number condition at each TR (means ± SEM). C) Voxel-wise whole-brain regions engaged by differential processing in the landmark (red arrow to RSC, blue arrow to PPA, green arrow to IPS), animal, and number tasks. Regions in warm color showed increased activation, and regions in cold color showed decreased activation. All peaks survived the threshold of P <0.005 with 10 voxel extension and no corrections. Common regions of positive (red) and negative (blue) activation across tasks of landmark, animal, and number differential value processing are also presented, i.e. the intersection of the effect from each condition (landmark ∩ animal ∩ number), P = 0.000001. The image presents common regions as a value of ±1.
Second, to test whether the animal task, which did not involve spatial differential processing, also recruited different hippocampal regions, we conducted a similar ANOVA separately on animal contrast values. The ANOVA only revealed a main effect of hemisphere (F(1, 23) = 5.30, P = 0.031, η2 = 0.187) with the right activity higher than the left (Fig. 2A). No other significant main and interaction effects were found for this condition. We also examined the number condition, which only engages general magnitude differentiation, and found two main effects: one of longitudinal axis (F(1, 23) = 34.42, P = 0.000, η2 = 0.599), where the posterior hippocampus had greater activity than the anterior hippocampus, and an effect of hemisphere (F(1, 23) = 12.59, η2 = 0.354, P = 0.002), with the right being greater than left. No other significant main and interaction effects were found (Fig. 2A). We note, however, that unlike for landmarks, activity in the two nonspatial conditions was below baseline.
Third, to test statistically whether the pattern of hippocampal involvement in the landmark condition was different from that in the animal and number condition, we conducted a 3-way repeated measures ANOVA, adding stimulus condition as another factor, i.e. a 3 (landmark, animal, number) × 2 (anterior and posterior) × 2 (left hemisphere and right hemisphere) ANOVA. The hippocampal activations, i.e. the landmark, animal, and number contrast values, were the dependent variable. The results revealed a main effect of stimulus condition, F(2, 46) = 50.52, P < 0.0001, η2 = 0.687, indicating that the overall hippocampal activation was different for these conditions. Post hoc tests using Bonferroni correction revealed landmark > animal > number activation (Ps < 0.05). A second significant main effect of longitudinal axis, F(1, 23) = 9.064, P = 0.006, η2 = 0.283, indicated an overall posterior > anterior activation. No significant main effect was found for hemisphere (F(1, 23) = 3.242, P = 0.094, η2 = 0.124). A significant interaction between the stimulus condition and longitudinal axis, F(1, 23) = 17.91, P < 0.0001, η2 = 0.438, was further analyzed with pairwise comparisons (with Bonferroni corrections) showing that, after collapsing across hemisphere, in the landmark (P = 0.012) and number (P < 0.0001) conditions, the posterior hippocampus exhibited higher activity than the anterior hippocampus, which was not the case for the animal condition.
As shown in Fig. 2A, although magnitude estimation and comparisons are implicated in all three tasks, it is only the landmark task that leads to above baseline activation of the hippocampus, more so in the posterior than anterior region. By comparison, hippocampal activity is below baseline when making comparative size judgments on objects and even more deactivated when making comparative value judgments on numbers.
Time course of the anterior and posterior hippocampus activation during landmark differential distance processing
The results mentioned previously were obtained from the analyses in which canonical hemodynamic function was used to model the fMRI signal. To confirm that the canonical hemodynamic function can model the hippocampal activity appropriately, we used a Finite Impulse Response model to obtain the time course of the anterior and posterior hippocampal activation in the three conditions. As shown in Fig. 2B, the landmark differential distance processing preferentially activates the posterior hippocampus over the anterior hippocampus, which is consistent with the results obtained using canonical hemodynamic functions in Fig. 2A. To further confirm this conclusion statistically, we conducted a 2 × 2 × 12 repeated measures ANOVA with hemisphere (i.e. left and right), longitudinal axis (i.e. anterior and posterior), and time point (i.e. 1–12 TRs) as factors. We found a significant interaction effect of longitudinal axis by time point (F(3.52, 80.93) = 3.64, P = 0.012, η2 = 0.137, GG). Post hoc testing with Bonferroni correction showed that, at TR 4, 5, 7, and 8, the posterior hippocampus showed stronger activation, significantly or at a trend level, than the anterior hippocampus (P = 0.085, 0.049, 0.083, 0.035), suggesting that the anterior hippocampus was engaged in the task for a shorter period of time, but the posterior hippocampal activation lasted longer. Moreover, Fig. 2B shows that the posterior hippocampal activation takes longer to reach its peak (occurring around TR 6, before responses were made), further indicating a stronger association between posterior hippocampal activation and spatial judgment when compared to the anterior hippocampus. Keeping in mind that activation was generally below baseline, for the animal condition, as shown in Fig. 2B, and the anterior and posterior hippocampus showed similar activation in all TRs, which was supported by nonsignificant main effects of the hippocampal longitudinal axis from a 2 × 12 (anterior/posterior × TRs) ANOVA (F(1,23) = 0.336, P = 0.568, η2 = 0.014). For the number condition, as can be seen from Fig. 2B, both the anterior and posterior hippocampus were deactivated, i.e. the activation level was lower than the baseline (i.e. at TR = 0). Moreover, the anterior hippocampus showed greater deactivation compared to the posterior hippocampus, which is supported by a significant main effect of the hippocampal longitudinal axis from a 2 × 12 (anterior/posterior × TRs) ANOVA (F(1,23) = 21.18, P < 0.001, η2 = 0.479, GG).
Therefore, the time-course results are consistent with the GLM results reported earlier using the canonical hemodynamic function.
Whole-brain results for differential processing
We also conducted a whole-brain voxel-wise analysis to reveal other brain regions that may be engaged by the differential distance/size/value comparison in the three conditions. The results are listed in Supplementary Table 1 and graphically illustrated in Fig. 2C (P < 0.005, with 10 voxel extension and no corrections). For the landmark condition, as shown in Fig. 2C and Supplementary Table 1a, the parahippocampal region, which includes the parahippocampal place area (PPA) (Epstein 2008; Vann et al. 2009) and the retrosplenial cortex (RSC), were involved in the differential distance judgment, but not in the animal and number condition. To confirm the differential involvement of PPA and RSC in the different tasks, we also made 8-mm spherical ROIs for the regions (using the maximum activation locations found in this analysis as the centers of the spherical ROIs) and tested whether PPA and RSC activation was modulated by trial-wise distance/size/magnitude values. Details are presented in the next section—trial-wise parametric modulation in differential processing. For completeness, the regions that were activated (positive) or deactivated (negative) in the animal and number condition are also presented in Fig. 2C and Supplementary Table 1b and c.
Common regions
As all the tasks involve magnitude estimation, we wanted to determine formally whether there were common regions of activation and deactivation, particularly in the left intraparietal sulcus (IPS), a region known to be implicated in magnitude estimation across modalities and tasks. To do so, we examined the whole-brain analysis activation contrasts during the three conditions/tasks and performed a conjunction analysis. We used a threshold of P = 0.01 with 10 voxel extension (uncorrected) to obtain the positive and negative activation in each condition, then found the overlapping regions, i.e. the intersection of the effect from all conditions (landmark ∩ animal ∩ number, P = 0.000001). Figure 2C-common represents the regions that engage similar general magnitude differentiation. We confirmed that IPS, especially on the left side, was activated in all three conditions.
fMRI results: trial-wise parametric modulation in differential processing
Hippocampus
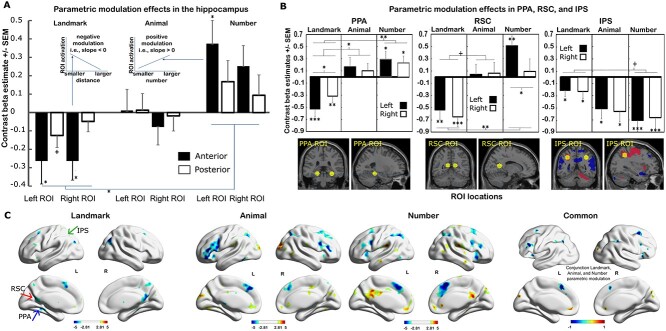
Next, we investigated whether the strength of the hippocampal activation is modulated by trial-wise differential distance in the landmark condition, and, if so, whether the anterior and posterior hippocampus are affected differently. To this end, we conducted second-level (i.e. group) t-tests in SPM on the parametric (trial-wise) modulation contrast values, obtained from the first-level (i.e. individual) analysis, in the different hippocampal ROIs (Fig. 3A). These parametric modulation analyses showed that smaller differential distance predicted stronger activation of the left and right anterior hippocampal ROIs (left: t = −2.11, P = 0.046, d = 0.436, right: t = −2.53, P = 0.019, d = 0.526, respectively), and the left posterior hippocampus at a trend level (t = −1.82, P = 0.081, d = 0.40). There was no effect in the right posterior hippocampal ROI (t = −0.961, P = 0.346, d = 0.180). As the estimate of this modulation is negative, the hippocampal modulation effect in Fig. 3A (landmark) is below 0, indicating that increasing differential distance, or decreasing spatial specificity, was associated with lower hippocampal activation, and vice versa. To test statistically whether the differential distance modulated the anterior and posterior hippocampus differentially, we included the bilateral anterior and posterior hippocampus ROIs to conduct a 2 × 2 repeated measures ANOVA on the landmark parametric effects obtained from SPM first-level analysis. The results showed a significant longitudinal axis effect such that the anterior hippocampus was modulated more strongly by the trial-wise differential distance value than the posterior hippocampus (F(1, 23) = 4.92, P = 0.037, η2 = 0.176) (Fig. 3A). No differentiation was found between the hemispheres (F(1, 23) = 0.645, P = 0.430, η2 = 0.027) and there were no interactions (F(1, 23) = 1.01, P = 0.326, η2 = 0.042).
Fig. 3.
Parametric modulation effects by trial-wise differential processing values in the landmark, animal, and number condition. A) Parametric modulation by trial-wise differential processing value in different hippocampal ROIs. B) Parametric modulation by trial-wise differential processing values in the parahippocampal place area (PPA), retrosplenial cortex region (RSC), and the intraparietal sulcus region (IPS). Locations of ROIs are also illustrated on brain section images. For (A) and (B), significant modulation effects and conditions differences are indicated by +P < 0.1, *P < 0.05, **P < 0.01, ***P < 0.001. The embedded schematic figures in (A) illustrate the meaning of the positive and negative modulation, i.e. how ROI activation changes when the trial-wise differential spatial distance or numerical magnitude changes. C) Voxel-wise whole-brain regions parametrically modulated by trial-wise differential processing. Larger differential values (distance, size, and magnitude) resulting in higher activation in warm colors, smaller differential values resulting in higher activation in cold colors. In the landmark condition results, RSC is indicated by a red arrow, PPA is indicated by a blue arrow, and IPS is indicated by a green arrow. All peaks survived the threshold of P <0.005 with 10 voxel extension and no corrections. Common regions of positive (red) and negative (blue) parametric modulation during the tasks of landmark, animal, and number differential value processing are also presented, i.e. the intersection of the effect from each condition (landmark ∩ animal ∩ number), P = 0.000001. The image presents common regions as a value of ±1.
The same parametric modulation analysis was conducted for the animal and number conditions. The results showed no significant modulation effect for the animal condition, i.e. the trial-wise animal differential size did not predict hippocampal activation (anterior left: t = 1.60, P = 0.874, d = 0.021; anterior right: t = −0.446, P = 0.660, d = 0.169; posterior left: t = 0.213, P = 0.833, d = 0.029; posterior right: t = −0.246, P = 0.810, d = 0.046; Fig. 3A). We found that differential difference in numbers showed significant modulation effects in the anterior hippocampal ROIs (left: t = 2.77, P = 0.011, d = 0.612; right: t = 1.96, P = 0.062, d = 0.469) but not for posterior ROIs (left: t = 1.44, P = 0.164, d = 0.300; right: t = 0.870, P = 0.394, d = 0.174). However, this modulation differed from the landmark condition, such that the smaller the differential number, the weaker was the activity in the anterior hippocampus (Fig. 3A, number condition). A similar 2 × 2 ANOVA confirmed the trial-wise modulation difference along the longitudinal axis in the number condition (F(1, 23) = 10.43, P = 0.004, η2 = 0.312).
Next, to confirm that the hippocampus ROIs were preferentially modulated in this parametric fashion (i.e. smaller, or more refined, differential value predicting stronger activation) by the landmark stimuli, we conducted another 3 × 2 × 2 repeated measures ANOVA with stimulus condition, hemisphere, and longitudinal axis as factors and the linear modulation contrast values as dependent variables. A main effect of stimulus condition (F(2, 46) = 4.40, η2 = 0.161, P = 0.018), followed by pairwise tests, supported that the modulation by the landmark differential distance is negative and significantly different from the positive modulation by the number difference (P = 0.016, with Bonferroni correction). The animal task does not demonstrate a trial-wise size modulation of the hippocampus, although the effect was not differentiated from the modulation of landmarks or numbers in the post hoc test. A significant interaction between stimulus condition and longitudinal axis, F(2,46) = 6.45, P = 0.003, η2 = 0.219, also confirmed that the modulation pattern by differential difference values along the hippocampal longitudinal axis was different among conditions. The anterior hippocampus showed more trial-wise parametric modulation (low differential distance, high activation) than the posterior hippocampus for landmarks (P = 0.037 with Bonferroni correction) while in the number condition, although the anterior hippocampus also showed stronger modulation effect than the posterior hippocampus (P = 0.004), the effect was in the opposite direction to the modulation effect in the landmark condition, i.e. when the differential number value was larger, the hippocampus showed smaller deactivation. Only the anterior hippocampus showed significantly different modulation between the landmark and number conditions (P = 0.002 with Bonferroni correction).
In summary, the modulation result, combined with the finding that the posterior hippocampus is activated more during the landmark task, showed that while the posterior hippocampus is required for all levels of differential distance with slight trial-wise modulation, the anterior hippocampus is particularly required for the smaller differential distance judgments and is less activated when the differential distance is larger.
Parahippocampal place area and retrosplenial cortex
Because the parahippocampal cortex (including the PPA and RSC) plays a key role in space/scene processing and navigation and was engaged by the landmark differential distance processing (Fig. 2C—landmark condition), we decided to test further whether these two localized regions also were modulated in a trial-wise (parametric) manner by the differential distance task. To this end, we first took as an ROI the region in the parahippocampal cortex that was engaged in the landmark task, using a sphere with r = 8 mm at the peak activation location [left, −27, −36, −16; right, 30, −36, −16] (Fig. 3B). This region coincides with the PPA (Epstein 2008; Baumann and Mattingley 2016). We then extracted the mean parametric trial-wise modulation value from each participant using this ROI. Finally, we ran a one-sample t-test at the group level. Results showed that bilaterally, this region was also parametrically modulated by the differential distances judgment task, t = −4.40, P = 0.0002, d = 0.897, and t = −3.22, P = 0.004, d = 0.656, for left and right PPA, respectively, in that PPA showed stronger activation when smaller differential distances were processed. In addition, the left PPA was modulated more strongly than the right PPA, t = −2.62, P < 0.01, d = −0.535, Fig. 3B. In the animal condition, the PPA, like the hippocampus, was not parametrically modulated by the trial-wise differential sizes, left: t = 1.0903, P = 0.289, d = 0.222; right: t = 0.865, P = 0.396, d = 0.176, with no hemisphere difference, t = 0.738, P = 0.468, d = 0.151. For the number condition, an opposite modulation pattern was found compared to the landmark condition. Higher PPA activation was found in trials with larger differential values, left: t = 2.254, P = 0.034, d = 0.468, right: t = 2.08, P = 0.049, d = 0.425, with no hemisphere difference, t = 0.684, P = 0.501, d = 0.139.
To further examine condition differences on the effects of differential processing in the PPA, we performed a 3 × 2 repeated measures ANOVA with stimulus condition, and hemisphere as factors, and the modulation effect in the PPA ROIs as dependent variables. Figure 3B illustrates the significant main effect of stimulus condition (F(2, 46) = 9.145, η2 = 0.284, P < 0.001), with landmark modulation in the PPA more negative than the animal (P < 0.02) and the number modulations (P < 0.001). The number and animal modulation values in the PPA were not significantly different from one another (P = 1.0). These results illustrate that in the PPA, the differential processing task elicits a similar modulation pattern as in the hippocampus and that the landmark differential processing elicits stronger activity for more refined spatial processing. There was also a condition by hemisphere modulation interaction (F(2, 46) = 3.564, η2 = 0.134, P < 0.036), indicating that only in the landmark condition the left and right PPA showed a different degree of modulation effect (P < 0.015).
We repeated this method on the left and right retrosplenial cortex (RSC; Fig. 3B). The sphere ROI selected for the RSC is located around the peak activation (left, −12, −52, 14, right, 14, −54, 14) in Fig. 2C (landmark condition). Results showed that bilaterally, the RSC was also parametrically modulated in a trial-wise manner by the differential distances in the spatial judgment task, t = −3.33, P = 0.0029, d = 0.680, and t = −4.27, P = 0.0003, d = 0.871, for left and right RSC, respectively. RSC showed stronger activation when differential distances were smaller, as we found in the hippocampus and PPA. There was no significant hemisphere difference, t = 1.149, P = 0.262, d = 0.235 (Fig. 3B). In the animal condition, as in the hippocampus, the RSC was not parametrically modulated by the differential sizes, left: t = 0.504, P = 0.619, d = 0.103; right: t = 0.584, P = 0.565, d = 0.119, with no hemisphere difference, t = −0.241, P = 0.812, d = −0.049. In the number condition, however, results showed parametric modulation only in the left RSC, again, with larger activation corresponding to trials with larger differential values, left: t = 3.18, P = 0.004, d = 0.651; right: t = 0.451, P = 0.656, d = 0.092, and there is a hemisphere difference with the left modulation of the RSC greater than the right hemisphere, t = 3.928, P = 0.0007, d = 0.801.
We repeated the 3 × 2 (stimulus condition by hemisphere) ANOVA with the modulation values from the RSC ROIs as dependent variables. As shown in Fig. 3B, there was a main effect of stimulus condition (F(2, 46) = 7.974, η2 = 0.257, P = 0.001) and a main effect of hemisphere (F(2, 46) = 14.103, η2 = 0.380, P = 0.001). Landmark modulation was more negative than the number modulation (P < 0.001) in the RSC and marginally different from the animal condition (P = 0.088). Number modulation was not significantly different from the animal modulation (P = 0.773). The condition differences on modulation effect in RSC are thus also similar to the hippocampal results. A significant interaction of condition × hemisphere (F(1.41, 32.32) = 5.542, η2 = 0.195, P = 0.016, GG) was also found, showing significant hemispheric difference in the number condition (P < 0.001).
In summary, the modulation results, combined with the finding that the posterior hippocampus is activated more during the landmark task, suggest that while the posterior hippocampus is required for all levels of differential distance decision with slight modulation, the anterior hippocampus is particularly required for the smaller differential distance judgments and is less activated when the differential distance is larger. For the animal task, the hippocampus is not modulated by the differential size judgment. While the activation is negative (i.e. below the baseline) for the number task (Fig. 2A), there is modulation of the hippocampus that is opposite to that of the landmark task, with the anterior hippocampus being more deactivated with smaller differential numeric judgments. The PPA and the RSC showed effects similar to that seen in the anterior hippocampus, i.e. more activity for smaller differential distances.
Intraparietal cortex
Because the intraparietal sulcus plays a key role in magnitude estimation, and all three conditions engaged this region (Fig. 2C), we tested whether this region was also modulated in a trial-wise (parametric) manner by the differential distance, the differential size, and the differential value tasks. To this end, we first took as an ROI the region in the left intraparietal sulcus that was engaged in the intersection region found above in the whole-brain analysis, using a sphere with r = 8 mm at the activation location [−33, −48, 43] (Fig. 3B). This region coincides with the meta-analyses indicating the link between magnitude processing and the IPS (Yeo et al. 2017; Hawes et al. 2019). As seen in Fig. 2C (common activation surface view) or 3B (section view), the right IPS was not engaged in all three tasks, specifically not in the animal condition; we used a mirror of the region selected on the left in the right IPS [33, −48, 43] to see whether the right IPS can still be modulated by trial-wise magnitude. We then extracted the mean parametric trial-wise modulation value from each participant using this ROI for each condition. Finally, we ran a one-sample t-test at the group level. Results showed that bilaterally, this IPS region was also parametrically modulated by the differential distances judgment (landmark) task, t = −4.395, P = 0.0002, d = −0.897, and t = −3.22, P = 0.004, d = −0.656, for left and right IPS, respectively. The left and right IPS modulation did not differ, t = −0.283, P = 0.426, d = −0.058, Fig. 3B. In the animal condition, different from the results reported so far, the IPS was parametrically modulated by the trial-wise differential sizes, left: t = −3.17, P = 0.004, d = −0.648, right: t = −3.57, P = 0.002, d = −0.728, with no hemisphere difference, t = 0.48, P = 0.635, d = 0.0981. Therefore, although the right IPS did not show significant activation during the whole animal size processing, different trial-wise animal size comparison did modulate the right IPS, i.e. this IPS region showed stronger activation when smaller animal differential sizes were processed. For the number condition, a similar modulation pattern was found, compared to the landmark and animal conditions. Higher IPS activation was found in trials with smaller differential values, left: t = −3.98, P = 0.00006, d = −0.812, right: t = −3.78, P = 0.001, d = −0.772, with no hemisphere difference, t = −0.48, P = 0.635, d = −0.0980. This ROI in the intraparietal sulcus showed increasing activation with more refined differential value decisions for all three differential processing judgments.
To further examine condition differences of differential processing in the IPS, we performed a 3 × 2 repeated measures ANOVA with stimulus condition, and hemisphere as factors and modulation values in the IPS ROIs as dependent variables. As shown in Fig. 3B (IPS), there was a marginally significant main effect of condition (F(1.42, 32.73) = 3.039, η2 = 0.116, P = 0.077, GG), indicating that although the IPS activation was similarly modulated by the differential processing of different stimuli, it was slightly less sensitive to (i.e. modulated to a lesser degree by) the processing of landmark distances, compared to other stimulus conditions (landmark > number P = 0.043 landmark > animal P = 0.113).
Whole-brain analysis: modulation by differential processing in the landmark condition
For completeness, we also conducted the whole-brain analysis for the three conditions to reveal other regions that could be parametrically modulated by the trial-wise differential value processing. The results are presented in Fig. 3C (P < 0.005, with 10 voxel extension and no corrections) and Supplementary Table 2a.
Common regions
To locate the common regions of the modulation by trial-wise quantitative processing of the three conditions, we calculated the intersection of the modulation effect from three differential processing tasks, landmark ∩ animal ∩ number. Similarly, we used a threshold of uncorrected P = 0.01 with voxel 10 extension for each condition and then obtained the overlapped regions (Fig. 3C—common regions). Regions such as the intraparietal sulcus, angular gyrus (AG), supramarginal gyrus (SMG) cortex, and precuneus showed increasing activation with more refined differential value decisions (P = 0.000001).
Discussion
Consistent with previous reports (Moyer and Landauer 1967; Moyer 1973), we found that for all differential judgment tasks—landmark, animal, and number—the smaller the differential magnitude, the longer the RTs and the lower the accuracy. Differences in magnitude estimation among the tasks, however, emerged at the neural level, particularly in the hippocampus and related structures. The hippocampus was recruited when making comparative distance judgments among highly familiar landmarks for which knowledge was acquired years ago, and thus presumably well-consolidated. Activation was greater, and lasted longer, in the posterior than anterior hippocampus. The anterior hippocampus, however, showed stronger modulation by distance than the posterior hippocampus, with increasing activation as differential distance declined. By contrast, hippocampal activation was undetectable, falling below baseline, for comparative judgment of the size of highly familiar objects and of the value of numbers. Other regions important for supporting scene/navigation processing, particularly the PPA and RSC, were also engaged in judging landmark distances and, like the hippocampus, were similarly modulated by the differential distance among them; no such involvement was evident in judging animal size and numerical value. Unlike the hippocampus and related structures, we found that the IPS was engaged in all three conditions, consistent with its well-documented role in magnitude estimation (Yeo et al. 2017; Hawes et al. 2019). Taken together, the findings suggest that the hippocampus is implicated in making precise and accurate judgments about distances in environments acquired long ago (Peer et al. 2019), but not about the size of comparably familiar objects and value of numbers.
Previous research had not determined to what extent, if any, the hippocampus is also implicated in representing in memory highly familiar environments that were encoded long ago and that presumably have undergone systems consolidation and transformation. Since the seminal finding of place cells in the hippocampus (O’Keefe and Dostrovsky 1971), numerous studies have found that the hippocampus is important for rodent and human navigation, cognitive map formation and usage, spatial coding, and path finding/integration in recently experienced environments (Maguire et al. 2000; Clark et al. 2007; Sheynikhovich et al. 2009; Morgan et al. 2010; Viard et al. 2011; Nadel et al. 2013; Chrastil et al. 2015; Lisman et al. 2017; Epstein et al. 2017; Zhao 2018; Peer et al. 2019; Liu et al. 2023). There are early reports that patients with extensive hippocampal damage can continue to navigate in familiar environments (Beatty et al. 1987; Habib and Sirigu 1987; Teng and Squire 1999; Rosenbaum et al. 2000; Corkin 2013), as can rodents (Winocur et al. 2005), but their knowledge of environments is impoverished, lacking precision and flexibility (for reviews, see Sekeres et al. 2018; Farzanfar et al. 2022). Functional neuroimaging studies in humans suggest that the hippocampus is recruited when precise direction/distance judgments are needed (Hirshhorn et al. 2012), or when planning routes (Spiers and Maguire 2006), though the hippocampus may relinquish its role in tracking distance to goal during navigation to RSC (Patai et al. 2019). Extending these lines of research, the current study found that the hippocampus was also involved in precise distance processing even when the landmarks are highly familiar and known for a long time.
As is the case with spatial memory, studies on mnemonic discrimination/pattern separation and precision (Koen et al. 2017; Ekstrom and Yonelinas 2020) also typically focus on recent memory and encoding of familiar objects (Yassa and Stark 2011) and their features, but not on retrieval of information about objects from remote memory. Unlike the observation that the hippocampus is engaged during the mnemonic discrimination task, particularly the dentate gyrus and CA2/3 subfields, which are more strongly represented in the posterior region, we found little hippocampal engagement in our object size discrimination task. Admittedly, our memory task involves magnitude estimation, whereas research using the pattern separation paradigm (Yassa and Stark 2011) and precision (Koen et al. 2017; Ekstrom and Yonelinas 2020) was concerned in discrimination of sensory features, such as shape, shading, and color. Future studies are needed to determine whether the discrepancy between our study and studies of object pattern separation and precision occurred because of the type of discrimination that was involved, or because retrieval in those cases was of recently acquired memories, whereas in ours it was of remote memory.
The pattern of hippocampal activation for judging distance between numerical values was different, yet again. Here, the hippocampus showed the reverse pattern to that seen with respect to spatial distance, i.e. when numerical distances decreased, hippocampal activation dropped even more below baseline. We have no ready explanation for this result. One possibility is that solving these numerical problems is a conceptual task that inhibits or interferes with hippocampal processing, and that the more difficult the task is, the greater the inhibition or interference becomes. This interference or inhibition may account for the poor memory exhibited by participants when they divide their attention during encoding, as the interfering task is often a concurrent mathematical task (Fernandes and Moscovitch 2000; Moscovitch et al. 2001; Fernandes et al. 2005).
Considering the extensive body of literature demonstrating the significance of the hippocampus in facilitating various forms of relational memory (e.g. Staresina & Davachi 2006; Kumaran et al. 2012; Tavares et al. 2015; Heusser et al. 2016; Kumaran et al. 2016; Ekstrom and Ranganath 2018; Park et al. 2020; Cohn-Sheehy et al. 2021), it is possible that spatial relations represent just one facet of the broader spectrum of relational memory that the hippocampus supports. However, we found the hippocampus did not play a role in the animal and number conditions, which also involves relational processing (i.e. comparing relationships between different items). Therefore, we can conclude that not all forms of relational processing necessitate the involvement of the hippocampus. It is likely that only relational processing associated with navigation and episodic memory requires support from the hippocampus.
To return to spatial memory and the pattern of hippocampal activation, the current study found that the posterior portion showed stronger engagement, compared to the anterior portion, in the landmark condition, which also is consistent with both the animal and human literature on the different roles that the dorsal–ventral (in rodents) or anterior–posterior (in humans) hippocampus may play in navigation or spatial processing. For example, in rodents, the place cells near the dorsal pole of the hippocampus have smaller, more stable, and more spatially selective firing fields, while cells in the ventral hippocampus have larger, less stable, and less spatially selective firing fields (Kjelstrup et al. 2008; Lyttle et al. 2013), reflecting a shift from processing fine-grained spatial information in the dorsal hippocampus to processing coarse-grained spatial or contextual (possibly nonspatial) inputs in the ventral hippocampus. Christensen et al. (2010) also found that dorsal hippocampus activity in rats plays a role in spatial learning and memory while ventral hippocampus activity is involved in anxiety-related behavior. Human studies also found that the anterior and posterior hippocampus have different connectivity patterns with other brain regions and are involved differently in navigation or spatial memory tasks (Woollett and Maguire 2011; Hirshhorn et al. 2012; Poppenk et al. 2013; Duarte et al. 2014; Brunec et al. 2019; Dalton et al. 2019, 2022; Barnett et al. 2021; Bouffard et al. 2022). For example, Evensmoen et al. (2013, 2015) found that activation of the posterior hippocampus is related to use of fine-grained, local route representations as compared to coarse, global route representations in the anterior hippocampus. Likewise, the posterior hippocampus was preferentially engaged when precision was required (Koen et al. 2017; Ekstrom and Yonelinas 2020). Moreover, in our study, when examining the time course of the involvement of the two hippocampal regions, we found a shift from processing information in both the anterior and posterior hippocampus initially upon participants seeing the stimuli, to a decrease in anterior hippocampus and a steady increase in posterior processing. The timing of the posterior and anterior onset is critically important in interpreting their involvement in the landmark condition. As we conjectured, this temporal difference in activation between the anterior and posterior hippocampus may be due to initial processing of landmark information to create a scene when the anterior hippocampus is being engaged (Hodgetts et al. 2016; Zeidman and Maguire 2016; Robin and Moscovitch 2017; Audrain et al. 2022). Once the scene is created, activation of the posterior hippocampus continues to rise to enable accurate judgments of differential distance.
To determine how these regions were specifically involved in these processes, we examined whether within-task, different magnitudes of the differential processing in each domain modified their activation. We found that the hippocampus, especially its anterior portion, showed stronger activation in trials where more fine-grained analysis of spatial distance was required. Thus, the hippocampal activity parametrically tracks spatial distance between memorized landmarks, which is in line with previous findings that the medial temporal lobe and related network play a key role in tracking travel distance to home or target location, integrating path, and navigation (Viard et al. 2011; Howard et al. 2014; Chrastil et al. 2015; Balaguer et al. 2016). Importantly, the current findings also suggest that the hippocampus can be still engaged in these processes even when the memory for a spatial environment was acquired long ago and, presumably, has already been consolidated (Patai et al. 2019). On the other hand, our results also showed that the hippocampus was engaged less when operating on more coarse-grained information about distances between landmarks. This result is also in line with previous findings that patients with extensive hippocampal damage can continue to navigate in familiar environments (Teng and Squire 1999; Rosenbaum et al. 2000), but their knowledge of environments is impoverished or lacks precision (for reviews see Sekeres et al. 2018; Farzanfar et al. 2022).
Contrary to our expectation, we found that activity in the anterior hippocampus varied more closely with differential distance between landmarks, compared to that in the posterior hippocampus. Although trials with smaller differential distances could be more challenging, we believe that the involvement of the anterior hippocampus is not driven by differences in task difficulty. This is because even though smaller size differences in the animal condition are more difficult to judge than larger size differences (see RT data), they did not activate this brain structure differentially. In addition, as depicted in Fig. 2B, the anterior hippocampus is engaged during the early stages of landmark trials, rather than the later stages where task difficulty becomes relevant, that is, when judgments need to be made. Instead, following Maguire and her colleagues, we think it is possible that the anterior hippocampus is implicated in scene construction (Maguire et al. 2016; Zeidman and Maguire 2016; Monk et al. 2021), whereas the posterior hippocampus is implicated in computing distances between landmarks located in the constructed scenes (Howard et al. 2014; Patai et al. 2019; Liu et al. 2023). In the current study, as differential distance decreases, more detailed scenes need to be constructed to afford accurate distance estimation. We speculate that once scene construction is complete, it is the posterior hippocampus that determines what the shortest navigational distance is, reflecting the temporal shift in activation of these two regions. Although future studies are needed to confirm the hypothesis, our finding that the anterior hippocampus was modulated more by differential distance than the posterior hippocampus is consistent with recent observations that changes in voxel-based temporal autocorrelations along the long axis were more evident in the anterior than posterior hippocampus in response both to different navigation conditions (Bouffard et al. 2022) and to transcranial magnetic stimulation applied to the angular gyrus (Coughlan et al. 2023). These findings suggest that the computations performed by the anterior and posterior hippocampus are different from one another, rather than simply varying along a continuum, consistent with their differing connectivity and function (Poppenk et al. 2013; Maguire et al. 2016; Zeidman and Maguire 2016; Peer et al. 2019; Monk et al. 2021). We note that the predicted gradient along the long axis is evident when participants conjure real-world spaces that vary in orders of magnitude from rooms to continents (Peer et al. 2019). However, it would be interesting to know whether results similar to ours would be obtained if participants made fine- versus coarse-grained distance judgments within each of these scales. For example, Rosenbaum et al. (2000) observed loss of precision in a patient with bilateral hippocampal lesions both at the neighborhood (city) level and at the country level.
In addition to the hippocampus, we also found that PPA and RSC showed activation in the landmark task, which was also modulated by trial-wise differential distances. Consistent with research using scene stimuli (Zeidman and Maguire 2016; Barry et al. 2021) and navigation (Patai et al. 2019), PPA and RSC, together with the hippocampus, play key roles in scene construction during navigation. PPA is thought to be involved in representing the local visual scene and the RSC involved in situating the scene within the broader spatial environment (Epstein 2008) and coding for distance to goal (Patai et al. 2019). The differential judgment task in the current study requires participants to imagine the particular landmark, the local visual scene, and then place it within the broader environment in order to accomplish the distance decision. Our results are consistent with Maguire and Mullally’s (2013) view that the anterior hippocampus constructs scenes likely by drawing on information from related structures such as PPA and RSC.
Despite similar behavioral results in the three tasks, judgments about the size of familiar objects (animal) or of numerical distance between numbers did not recruit the hippocampus. Lack of hippocampal modulation observed for nonspatial tasks suggests that it does not merely track general task performance (measured by reaction time and accuracy), and instead reflects the more specific role of hippocampus in making spatial judgments. Our findings are consistent with the observation that unilateral temporal lobectomy in either hemisphere does not affect patients’ ability to perform size judgments on objects including animals (Wilkins and Moscovitch 1978; Smith and Milner 1981). Studies with monkeys also found hippocampal lesions to be more likely to cause memory deficits in spatial memory (Hampton et al. 2004) rather than object memory (Basile et al. 2020). Our finding that processing number value did not result in activation above baseline levels in HPC, PPA, and RSC is consistent with a meta-analysis examining brain areas associated with numbers and calculations, which also did not show activation of any of these brain regions (Yeo et al. 2017; Arsalidou et al. 2018). As we do not see modulation in the hippocampus by the differential size of animals or by differential numerical distance, it is possible that regions other than the hippocampus and related spatial structures are being modulated. Consistent with that reasoning, we do, indeed, see such modulation in other regions.
Since all three tasks involve magnitude estimation, we, like others, observed that the IPS was modulated by magnitude in all three conditions, consistent with the robust findings that the IPS is engaged in symbolic/numeric processing (Ansari 2008; Henik et al. 2012, 2017; Arsalidou et al. 2018) and in representing numbers, space, and time (Bueti and Walsh 2009; Gabay et al. 2016). In addition, the angular gyrus, supramarginal gyrus, and precuneus showed increasing activation with more refined differential value decisions, consistent with the literature on magnitude estimation (Fabbri et al. 2012; Parkinson et al. 2014) and models (Verguts and Van Opstal 2005) that posit that numbers, space, and time are processed by a common analog magnitude system.
In conclusion, we found that when performing a comparative distance task in a highly familiar downtown Toronto neighborhood, both the anterior and posterior hippocampus are engaged in different ways. The posterior part, in general, was engaged more preferentially and over longer periods than the anterior part across all differential distances. By contrast, only activity in the anterior part was modulated by differential distances, such that it is more engaged when smaller distances need to be processed. These findings are consistent with our view that both anterior and posterior hippocampus are implicated in spatial navigation even when activating remote memories, and especially when making fine discriminations about landmarks in a familiar neighborhood and the distances between them (Rosenbaum et al. 2000; Maguire et al. 2006; Hirshhorn et al. 2012; Herdman et al. 2015). Our overall findings are in line with the idea that what determines the engagement of the hippocampus and related structures is not the memory’s age but the nature of its representation (Nadel and Moscovitch 1997; Winocur and Moscovitch 2011; Sekeres et al. 2018; Gilboa and Moscovitch 2021). Our results, however, do not support the hypothesis that the size of the differential distance is represented as a gradient that increases with differential size from anterior to posterior hippocampus. Instead, our results are more consistent with models that posit different functions for the anterior and posterior regions: scene and schema construction for the anterior hippocampus, and distance representation and estimation for the posterior hippocampus. How those functions are influenced by judgments of differential distance is more complicated than the simple gradient model suggests. Exactly how these different functions of the anterior and posterior hippocampus are related to the underlying gradient along the long axis remains to be determined (but see Peer et al. 2019; Peer and Epstein 2021; Farzanfar et al. 2022). Our results, nevertheless, suggest that as differential distance is reduced, more detailed scene construction is needed to support the precision of distance judgments. Similar to the hippocampus, the RSC and PPA were engaged incrementally as spatial processing became more refined contributing to scene construction (see above), and showed a similar lack of response as the hippocampus for objects and numerical processing. By comparison, the left IPS was sensitive to magnitude estimation across space, object, and number, consistent with the literature on magnitude estimation.
Supplementary Material
Acknowledgments
We thank Nick Hoang and Mariya Churina for their help with this research project.
Contributor Information
Marilyne G Ziegler, Psychology Department, University of Toronto, Toronto M5S 1A1, Canada.
Zhong-Xu Liu, Department of Behavioral Sciences, University of Michigan–Dearborn, 4901 Evergreen RD, Dearborn, United States.
Jessica Arsenault, Rotman Research Institute at Baycrest Health Sciences, Toronto M6A 2E1, Canada.
Christa Dang, Psychology Department, University of Toronto, Toronto M5S 1A1, Canada.
Cheryl Grady, Psychology Department, University of Toronto, Toronto M5S 1A1, Canada; Rotman Research Institute at Baycrest Health Sciences, Toronto M6A 2E1, Canada; Department of Psychiatry, University of Toronto, Toronto M5T 1R8, Canada.
R Shayna Rosenbaum, Rotman Research Institute at Baycrest Health Sciences, Toronto M6A 2E1, Canada; Department of Psychology and Centre for Vision Research, York University, Toronto M3J 1P3, Canada.
Morris Moscovitch, Psychology Department, University of Toronto, Toronto M5S 1A1, Canada; Rotman Research Institute at Baycrest Health Sciences, Toronto M6A 2E1, Canada.
Authors’ contributions
Marilyne G. Ziegler (Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing—original draft, Writing—review & editing), Zhong-Xu Liu (Conceptualization, Formal analysis, Investigation, Methodology, Visualization, Writing—original draft, Writing—review & editing), Jessica Arsenault (Conceptualization, Data curation, Investigation, Methodology), Christa Dang (Conceptualization, Data curation, Investigation, Methodology, Writing—review & editing), Cheryl Grady (Conceptualization, Investigation, Methodology, Writing—review & editing), R. Shayna Rosenbaum (Conceptualization, Investigation, Methodology, Writing—review & editing), and Morris Moscovitch (Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing—original draft, Writing—review & editing)
Supplementary material
Supplementary material is available at Cerebral Cortex online.
Funding
The study was financially supported by the Canadian Institutes of Health Research (CIHR) MOP125958 to MM.
Conflict of interest statement: None declared.
Data availability
The processed data can be shared on reasonable request to the corresponding author.
References
- Aly M, Ranganath C, Yonelinas AP. Detecting changes in scenes: the hippocampus is critical for strength-based perception. Neuron. 2013:78(6):1127–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari D. Effects of development and enculturation on number representation in the brain. Nat Rev Neurosci. 2008:9(4):278–291. [DOI] [PubMed] [Google Scholar]
- Arsalidou M, Pawliw-Levac M, Sadeghi M, Pascual-Leone J. Brain areas associated with numbers and calculations in children: meta-analyses of fMRI studies. Dev Cogn Neurosci. 2018:30:239–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audrain S, Gilmore AW, Wilson JM, Schacter DL, Martin A. A role for the anterior hippocampus in autobiographical memory construction regardless of temporal distance. J Neurosci. 2022:42(33):6445–6452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayhan F, Kulkarni A, Berto S, Sivaprakasam K, Douglas C, Lega BC, Konopka G. Resolving cellular and molecular diversity along the hippocampal anterior-to-posterior axis in humans. Neuron. 2021:109(13):2091–2105.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaguer J, Spiers H, Hassabis D, Summerfield C. Neural mechanisms of hierarchical planning in a virtual subway network. Neuron. 2016:90(4):893–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barense MD, Henson RNA, Lee ACH, Graham KS. Medial temporal lobe activity during complex discrimination of faces, objects, and scenes: effects of viewpoint. Hippocampus. 2010:20(3):389–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett AJ, Reilly W, Dimsdale-Zucker HR, Mizrak E, Reagh Z, Ranganath C. Intrinsic connectivity reveals functionally distinct cortico-hippocampal networks in the human brain. PLoS Biol. 2021:19(6):e3001275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry DN, Clark IA, Maguire EA. The relationship between hippocampal subfield volumes and autobiographical memory persistence. Hippocampus. 2021:31(4):362–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basile BM, Templer VL, Gazes RP, Hampton RR. Preserved visual memory and relational cognition performance in monkeys with selective hippocampal lesions. Sci Adv. 2020:6(29):eaaz0484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann O, Mattingley JB. Functional organization of the parahippocampal cortex: dissociable roles for context representations and the perception of visual scenes. J Neurosci. 2016:36(8):2536–2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty WW, Salmon DP, Bernstein N, Butters N. Remote memory in a patient with amnesia due to hypoxia. Psychol Med. 1987:17(3):657–665. [DOI] [PubMed] [Google Scholar]
- Boscoe FP, Henry KA, Zdeb MS. A nationwide comparison of driving distance versus straight-line distance to hospitals. Prof Geogr. 2012:64(2):188–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouffard NR, Golestani A, Brunec IK, Bellana B, Park JY, Barense MD, Moscovitch M. Single voxel autocorrelation uncovers gradients of temporal dynamics in the hippocampus and entorhinal cortex during rest and navigation. Cereb Cortex. 2023:33:3265–3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TI, Carr VA, LaRocque KF, Favila SE, Gordon AM, Bowles B, Bailenson JN, Wagner AD. Prospective representation of navigational goals in the human hippocampus. Science. 2016:352(6291):1323–1326. [DOI] [PubMed] [Google Scholar]
- Brunec IK, Bellana B, Ozubko JD, Man V, Robin J, Liu Z-X, Grady C, Rosenbaum RS, Winocur G, Barense MD, et al. Multiple scales of representation along the hippocampal anteroposterior axis in humans. Curr Biol. 2018:28(13):2129–2135.e6. [DOI] [PubMed] [Google Scholar]
- Brunec IK, Robin J, Patai EZ, Ozubko JD, Javadi A, Barense MD, Spiers HJ, Moscovitch M. Cognitive mapping style relates to posterior–anterior hippocampal volume ratio. Hippocampus. 2019:29(8):748–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bueti D, Walsh V. The parietal cortex and the representation of time, space, number and other magnitudes. Philos Trans R Soc B. 2009:364(1525):1831–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess N, Maguire EA, O’Keefe J. The human hippocampus and spatial and episodic memory. Neuron. 2002:35(4):625–641. [DOI] [PubMed] [Google Scholar]
- Chrastil ER, Sherrill KR, Hasselmo ME, Stern CE. There and back again: hippocampus and retrosplenial cortex track homing distance during human path integration. J Neurosci. 2015:35(46):15442–15452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen T, Bisgaard CF, Nielsen HB, Wiborg O. Transcriptome differentiation along the dorso–ventral axis in laser-captured microdissected rat hippocampal granular cell layer. Neuroscience. 2010:170(3):731–741. [DOI] [PubMed] [Google Scholar]
- Clark RE, Broadbent NJ, Squire LR. The hippocampus and spatial memory: findings with a novel modification of the water maze. J Neurosci. 2007:27(25):6647–6654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlan G, Bouffard NR, Golestani A, Thakral PP, Schacter DL, Grady C, Moscovitch M. Transcranial magnetic stimulation to the angular gyrus modulates the temporal dynamics of the hippocampus and entorhinal cortex. Cerebral Cortex. 2023:33:3255–3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn-Sheehy BI, Delarazan AI, Reagh ZM, Crivelli-Decker JE, Kim K, Barnett AJ, Zacks JM, Ranganath C. The hippocampus constructs narrative memories across distant events. Curr Biol. 2021:31(22):4935–4945.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corkin S. Permanent present tense: the unforgettable life of the amnesic patient, H. M. 1st. edition. ed. New York: Basic Books; 2013 [Google Scholar]
- Dalton MA, McCormick C, Maguire EA. Differences in functional connectivity along the anterior-posterior axis of human hippocampal subfields. NeuroImage. 2019:192:38–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton MA, D’Souza A, Lv J, Calamante F. New insights into anatomical connectivity along the anterior–posterior axis of the human hippocampus using in vivo quantitative fibre tracking. elife. 2022:11:e76143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte IC, Ferreira C, Marques J, Castelo-Branco M. Anterior/posterior competitive deactivation/activation dichotomy in the human hippocampus as revealed by a 3D navigation task. PLoS One. 2014:9(1):e86213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrom AD, Ranganath C. Space, time, and episodic memory: the hippocampus is all over the cognitive map. Hippocampus. 2018:28(9):680–687. [DOI] [PubMed] [Google Scholar]
- Ekstrom AD, Yonelinas AP. Precision, binding, and the hippocampus: precisely what are we talking about? Neuropsychologia. 2020:138:107341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein RA. Parahippocampal and retrosplenial contributions to human spatial navigation. Trends Cogn Sci. 2008:12(10):388–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein RA, Patai EZ, Julian JB, Spiers HJ. The cognitive map in humans: spatial navigation and beyond. Nat Neurosci. 2017:20(11):1504–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evensmoen HR, Lehn H, Xu J, Witter MP, Nadel L, Håberg AK. The anterior hippocampus supports a coarse, global environmental representation and the posterior hippocampus supports fine-grained, local environmental representations. J Cogn Neurosci. 2013:25(11):1908–1925. [DOI] [PubMed] [Google Scholar]
- Evensmoen HR, Ladstein J, Hansen TI, Møller JA, Witter MP, Nadel L, Håberg AK. From details to large scale: the representation of environmental positions follows a granularity gradient along the human hippocampal and entorhinal anterior–posterior axis. Hippocampus. 2015:25(1):119–135. [DOI] [PubMed] [Google Scholar]
- Fabbri M, Cancellieri J, Natale V. The A Theory Of Magnitude (ATOM) model in temporal perception and reproduction tasks. Acta Psychol. 2012:139(1):111–123. [DOI] [PubMed] [Google Scholar]
- Farzanfar D, Spiers HJ, Moscovitch M, Rosenbaum RS. From cognitive maps to spatial schemas. Nat Rev Neurosci. 2022:24(2):1–17. [DOI] [PubMed] [Google Scholar]
- Fernandes MA, Moscovitch M. Divided attention and memory: evidence of substantial interference effects at retrieval and encoding. J Exp Psychol Gen. 2000:129(2):155–176. [DOI] [PubMed] [Google Scholar]
- Fernandes MA, Moscovitch M, Ziegler M, Grady C. Brain regions associated with successful and unsuccessful retrieval of verbal episodic memory as revealed by divided attention. Neuropsychologia. 2005:43(8):1115–1127. [DOI] [PubMed] [Google Scholar]
- Fischl B. FreeSurfer. NeuroImage. 2012:62(2):774–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabay S, Kalanthroff E, Henik A, Gronau N. Conceptual size representation in ventral visual cortex. Neuropsychologia. 2016:81:198–206. [DOI] [PubMed] [Google Scholar]
- Gilboa A, Moscovitch M. No consolidation without representation: correspondence between neural and psychological representations in recent and remote memory. Neuron. 2021:109(14):2239–2255. [DOI] [PubMed] [Google Scholar]
- Habib M, Sirigu A. Pure topographical disorientation: a definition and anatomical basis. Cortex. 1987:23(1):73–85. [DOI] [PubMed] [Google Scholar]
- Hampton RR, Hampstead BM, Murray EA. Selective hippocampal damage in rhesus monkeys impairs spatial memory in an open-field test. Hippocampus. 2004:14(7):808–818. [DOI] [PubMed] [Google Scholar]
- Hawes Z, Sokolowski HM, Ononye CB, Ansari D. Neural underpinnings of numerical and spatial cognition: an fMRI meta-analysis of brain regions associated with symbolic number, arithmetic, and mental rotation. Neurosci Biobehav Rev. 2019:103:316–336. [DOI] [PubMed] [Google Scholar]
- Henik A, Leibovich T, Naparstek S, Diesendruck L, Rubinsten O. Quantities, amounts, and the numerical core system. Front Hum Neurosci. 2012:186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henik A, Gliksman Y, Kallai A, Leibovich T. Size perception and the foundation of numerical processing. Curr Dir Psychol Sci. 2017:26(1):45–51. [Google Scholar]
- Herdman KA, Calarco N, Moscovitch M, Hirshhorn M, Rosenbaum RS. Impoverished descriptions of familiar routes in three cases of hippocampal/medial temporal lobe amnesia. Cortex. 2015:71:248–263. [DOI] [PubMed] [Google Scholar]
- Heusser AC, Poeppel D, Ezzyat Y, Davachi L. Episodic sequence memory is supported by a theta-gamma phase code. Nat Neurosci. 2016:19(10):1374–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirshhorn M, Newman L, Moscovitch M. Detailed descriptions of routes traveled, but not map-like knowledge, correlates with tests of hippocampal function in older adults. Hippocampus. 2011:21(11):1147–1151. [DOI] [PubMed] [Google Scholar]
- Hirshhorn M, Grady C, Rosenbaum RS, Winocur G, Moscovitch M. Brain regions involved in the retrieval of spatial and episodic details associated with a familiar environment: An fMRI study. Neuropsychologia. 2012:50(13):3094–3106. [DOI] [PubMed] [Google Scholar]
- Hodgetts CJ, Shine JP, Lawrence AD, Downing PE, Graham KS. Evidencing a place for the hippocampus within the core scene processing network. Hum Brain Mapp. 2016:37(11):3779–3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard LR, Javadi AH, Yu Y, Mill RD, Morrison LC, Knight R, Loftus MM, Staskute L, Spiers HJ. The hippocampus and entorhinal cortex encode the path and Euclidean distances to goals during navigation. Curr Biol. 2014:24(12):1331–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjelstrup KB, Solstad T, Brun VH, Hafting T, Leutgeb S, Witter MP, Moser EI, Moser M-B. Finite scale of spatial representation in the hippocampus. Science. 2008:321(5885):140–143. [DOI] [PubMed] [Google Scholar]
- Koen JD, Borders AA, Petzold MT, Yonelinas AP. Visual short-term memory for high resolution associations is impaired in patients with medial temporal lobe damage. Hippocampus. 2017:27(2):184–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaran D, Melo HL, Duzel E. The emergence and representation of knowledge about social and nonsocial hierarchies. Neuron. 2012:76(3):653–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaran D, Banino A, Blundell C, Hassabis D, Dayan P. Computations underlying social hierarchy learning: distinct neural mechanisms for updating and representing self-relevant information. Neuron. 2016:92(5):1135–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz L, Brandt A, Reinacher PC, Staresina BP, Reifenstein ET, Weidemann CT, Herweg NA, Patel A, Tsitsiklis M, Kempter R, et al. A neural code for egocentric spatial maps in the human medial temporal lobe. Neuron. 2021:109(17):2781–2796.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman MD, Cunningham WA. Type I and type II error concerns in fMRI research: re-balancing the scale. Soc Cogn Affect Neurosci. 2009:4(4):423–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J, Buzsáki G, Eichenbaum H, Nadel L, Ranganath C, Redish AD. Viewpoints: How the hippocampus contributes to memory, navigation and cognition. Nat Neurosci. 2017:20(11):1434–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Chen D, Xiao X, Zhang H, Zhou W, Liang S, Kunz L, Schulze-Bonhage A, Axmacher N, Wang L. Multi-scale goal distance representations in human hippocampus during virtual spatial navigation. Curr Biol. 2023:33(10):2024–2033.e3. [DOI] [PubMed] [Google Scholar]
- Lyttle D, Gereke B, Lin KK, Fellous J-M. Spatial scale and place field stability in a grid-to-place cell model of the dorsoventral axis of the hippocampus. Hippocampus. 2013:23(8):729–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Mullally SL. The hippocampus: A manifesto for change. J Exp Psychol Gen. 2013:142(4):1180–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Frackowiak RS, Frith CD. Recalling routes around London: activation of the right hippocampus in taxi drivers. J Neurosci. 1997:17(18):7103–7110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Gadian DG, Johnsrude IS, Good CD, Ashburner J, Frackowiak RSJ, Frith CD. Navigation-related structural change in the hippocampi of taxi drivers. Proc Natl Acad Sci U S A. 2000:97(8):4398–4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Nannery R, Spiers HJ. Navigation around London by a taxi driver with bilateral hippocampal lesions. Brain. 2006:129(11):2894–2907. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Intraub H, Mullally SL. Scenes, spaces, and memory traces: what does the hippocampus do? Neuroscientist. 2016:22(5):432–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JF, Neufang M, Solway A, Brandt A, Trippel M, Mader I, Hefft S, Merkow M, Polyn SM, Jacobs J, et al. Neural activity in human hippocampal formation reveals the spatial context of retrieved memories. Science. 2013:342(6162):1111–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk AM, Dalton MA, Barnes GR, Maguire EA. The role of hippocampal–ventromedial prefrontal cortex neural dynamics in building mental representations. J Cogn Neurosci. 2021:33(1):89–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan L, MacEvoy S, Aguirre G, Epstein R. Adaptation for landmark identity and landmark location on a familiar college campus. J Vis. 2010:10(7):1236. [Google Scholar]
- Moscovitch M, Fernandes MA, Troyer A. Working-with-memory and cognitive resources: a component-process account of divided attention and memory. In: Naveh-Benjamin M, Moscovitch M, Roediger HL III, editors. Perspectives on human memory and cognitive aging: essays in honor of Fergus Craik. New York: Psychology Press; 2001. [Google Scholar]
- Moyer RS. Comparing objects in memory: evidence suggesting an internal psychophysics. Percept Psychophys. 1973:13(2):180–184. [Google Scholar]
- Moyer RS, Landauer TK. Time required for judgements of numerical inequality. Nature. 1967:215(5109):1519–1520. [DOI] [PubMed] [Google Scholar]
- Nadel L, Moscovitch M. Memory consolidation, retrograde amnesia and the hippocampal complex. Curr Opin Neurobiol. 1997:7(2):217–227. [DOI] [PubMed] [Google Scholar]
- Nadel L, Hoscheidt S, Ryan LR. Spatial cognition and the hippocampus: the anterior–posterior axis. J Cogn Neurosci. 2013:25(1):22–28. [DOI] [PubMed] [Google Scholar]
- Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005:53(4):695–699. [DOI] [PubMed] [Google Scholar]
- Nieder A, Dehaene S. Representation of number in the brain. Annu Rev Neurosci. 2009:32(1):185–208. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, Dostrovsky J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 1971:34(1):171–175. [DOI] [PubMed] [Google Scholar]
- Park SA, Miller DS, Nili H, Ranganath C, Boorman ED. Map making: constructing, combining, and inferring on abstract cognitive maps. Neuron. 2020:107(6):1226–1238.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson C, Liu S, Wheatley T. A common cortical metric for spatial, temporal, and social distance. J Neurosci. 2014:34(5):1979–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patai EZ, Javadi A-H, Ozubko JD, O’Callaghan A, Ji S, Robin J, Grady C, Winocur G, Rosenbaum RS, Moscovitch M, et al. Hippocampal and retrosplenial goal distance coding after long-term consolidation of a real-world environment. Cereb Cortex. 2019:29(6):2748–2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peer M, Ron Y, Monsa R, Arzy S. Processing of different spatial scales in the human brain. elife. 2019:8:e47492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peer M, Epstein RA. The human brain uses spatial schemas to represent segmented environments. Currv Biol. 2021:31:4677–4688.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza M, Izard V, Pinel P, Le Bihan D, Dehaene S. Tuning curves for approximate numerosity in the human intraparietal sulcus. Neuron. 2004:44(3):547–555. [DOI] [PubMed] [Google Scholar]
- Poppenk J, Moscovitch M. A hippocampal marker of recollection memory ability among healthy young adults: contributions of posterior and anterior segments. Neuron. 2011:72(6):931–937. [DOI] [PubMed] [Google Scholar]
- Poppenk J, Evensmoen HR, Moscovitch M, Nadel L. Long-axis specialization of the human hippocampus. Trends Cogn Sci. 2013:17(5):230–240. [DOI] [PubMed] [Google Scholar]
- Robin J, Moscovitch M. Details, gist and schema: hippocampal–neocortical interactions underlying recent and remote episodic and spatial memory. Current Opinion in Behavioral Sciences, Memory in time and space. 2017:17:114–123. [Google Scholar]
- Rosenbaum RS, Priselac S, Köhler S, Black SE, Gao F, Nadel L, Moscovitch M. Remote spatial memory in an amnesic person with extensive bilateral hippocampal lesions. Nat Neurosci. 2000:3(10):1044–1048. [DOI] [PubMed] [Google Scholar]
- Rosenbaum RS, Ziegler M, Winocur G, Grady CL, Moscovitch M. “I have often walked down this street before?”: fMRI Studies on the hippocampus and other structures during mental navigation of an old environment. Hippocampus. 2004:14(7):826–835. [DOI] [PubMed] [Google Scholar]
- Rosenbaum RS, Gao F, Richards B, Black SE, Moscovitch M. “Where to?” remote memory for spatial relations and landmark identity in former taxi drivers with Alzheimer’s disease and encephalitis. J Cogn Neurosci. 2005:17(3):446–462. [DOI] [PubMed] [Google Scholar]
- Rosenbaum RS, Winocur G, Grady CL, Ziegler M, Moscovitch M. Memory for familiar environments learned in the remote past: fMRI studies of healthy people and an amnesic person with extensive bilateral hippocampal lesions. Hippocampus. 2007:17(12):1241–1251. [DOI] [PubMed] [Google Scholar]
- Routtenberg A, O’Keefe J, Nadel L. The hippocampus as a cognitive map. Am J Psychol. 1980:93(1):177. [Google Scholar]
- Sakon JJ, Kahana MJ. Hippocampal ripples signal contextually mediated episodic recall. Proc Natl Acad Sci. 2022:119(40):e2201657119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekeres MJ, Winocur G, Moscovitch M. The hippocampus and related neocortical structures in memory transformation. Neuroscience Letters, New Perspectives on the Hippocampus and Memory. 2018:680:39–53. [DOI] [PubMed] [Google Scholar]
- Sheynikhovich D, Chavarriaga R, Strösslin T, Arleo A, Gerstner W. Is there a geometric module for spatial orientation? Insights from a rodent navigation model. Psychol Rev. 2009:116(3):540–566. [DOI] [PubMed] [Google Scholar]
- Shipley WC, Gruber CP, Martin TA, Klein AM. Shipley-2 manual. Los Angeles, CA: Western Psychological Services; 2009 [Google Scholar]
- Smith ML, Milner B. The role of the right hippocampus in the recall of spatial location. Neuropsychologia. 1981:19(6):781–793. [DOI] [PubMed] [Google Scholar]
- Smith ML, Milner B. Differential effects of frontal-lobe lesions on cognitive estimation and spatial memory. Neuropsychologia. 1984:22(6):697–705. [DOI] [PubMed] [Google Scholar]
- Spiers HJ, Maguire EA. Thoughts, behaviour, and brain dynamics during navigation in the real world. NeuroImage. 2006:31(4):1826–1840. [DOI] [PubMed] [Google Scholar]
- Spiers HJ, Olafsdottir HF, Lever C. Hippocampal CA1 activity correlated with the distance to the goal and navigation performance. Hippocampus. 2018:28(9):644–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staresina BP, Davachi L. Differential encoding mechanisms for subsequent associative recognition and free recall. J Neurosci. 2006:26(36):9162–9172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange BA, Witter MP, Lein ES, Moser EI. Functional organization of the hippocampal longitudinal axis. Nat Rev Neurosci. 2014:15(10):655–669. [DOI] [PubMed] [Google Scholar]
- Tavares RM, Mendelsohn A, Grossman Y, Williams CH, Shapiro M, Trope Y, Schiller D. A map for social navigation in the human brain. Neuron. 2015:87(1):231–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng E, Squire LR. Memory for places learned long ago is intact after hippocampal damage. Nature. 1999:400(6745):675–677. [DOI] [PubMed] [Google Scholar]
- Tombaugh TN, Kozak J, Rees L. Normative data stratified by age and education for two measures of verbal fluency: FAS and animal naming. Arch Clin Neuropsychol. 1999:14(2):167–177. [PubMed] [Google Scholar]
- Vann SD, Aggleton JP, Maguire EA. What does the retrosplenial cortex do? Nat Rev Neurosci. 2009:10(11):792–802. [DOI] [PubMed] [Google Scholar]
- Verdino M, Dingman S. Two measures of laterality in handedness: the Edinburgh Handedness Inventory and the Purdue Pegboard Test of manual dexterity. Percept Mot Skills. 1998:86(2):476–478. [DOI] [PubMed] [Google Scholar]
- Verguts T, Van Opstal F. Dissociation of the distance effect and size effect in one-digit numbers. Psychon Bull Rev. 2005:12(5):925–930. [DOI] [PubMed] [Google Scholar]
- Viard A, Doeller CF, Hartley T, Bird CM, Burgess N. Anterior hippocampus and goal-directed spatial decision making. J Neurosci. 2011:31(12):4613–4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel JW, La Joie R, Grothe MJ, Diaz-Papkovich A, Doyle A, Vachon-Presseau E, Lepage C, Vos de Wael R, Thomas RA, Iturria-Medina Y, et al. A molecular gradient along the longitudinal axis of the human hippocampus informs large-scale behavioral systems. Nat Commun. 2020:11(1):960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss AP, DeWitt I, Goff D, Ditman T, Heckers S. Anterior and posterior hippocampal volumes in schizophrenia. Schizophr Res. 2005:73(1):103–112. [DOI] [PubMed] [Google Scholar]
- Wilkins A, Moscovitch M. Selective impairment of semantic memory after temporal lobectomy. Neuropsychologia. 1978:16(1):73–79. [DOI] [PubMed] [Google Scholar]
- Winocur G, Moscovitch M. Memory transformation and systems consolidation. J Int Neuropsychol Soc. 2011:17(05):766–780. [DOI] [PubMed] [Google Scholar]
- Winocur G, Moscovitch M, Fogel S, Rosenbaum RS, Sekeres M. Preserved spatial memory after hippocampal lesions: effects of extensive experience in a complex environment. Nat Neurosci. 2005:8(3):273–275. [DOI] [PubMed] [Google Scholar]
- Woollett K, Maguire EA. Acquiring “the Knowledge” of London’s layout drives structural brain changes. Curr Biol. 2011:21(24):2109–2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Stark CEL. Pattern separation in the hippocampus. Trends Neurosci. 2011:34(10):515–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo DJ, Wilkey ED, Price GR. The search for the number form area: a functional neuroimaging meta-analysis. Neurosci Biobehav Rev. 2017:78:145–160. [DOI] [PubMed] [Google Scholar]
- Zeidman P, Maguire EA. Anterior hippocampus: the anatomy of perception, imagination and episodic memory. Nat Rev Neurosci. 2016:17(3):173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidman P, Mullally SL, Maguire EA. Constructing, perceiving, and maintaining scenes: hippocampal activity and connectivity. Cereb Cortex. 2015:25(10):3836–3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M. Human spatial representation: what we cannot learn from the studies of rodent navigation. J Neurophysiol. 2018:120(5):2453–2465. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The processed data can be shared on reasonable request to the corresponding author.