Abstract
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease. The complex relationships between race and ethnicity and social determinants of health (SDOH) in influencing SLE and its course are increasingly appreciated. Multiple SDOH have been strongly associated with lupus incidence and outcomes and contribute to health disparities in lupus. Measures of socioeconomic status, including economic instability, poverty, unemployment, and food insecurity, as well as features of the neighborhood and built environment, including lack of safe and affordable housing, crime, stress, racial segregation, and discrimination, are associated with race and ethnicity in the US and are risk factors for poor outcomes in lupus. In this scientific statement, we aimed to summarize current evidence on the role of SDOH in relation to racial and ethnic disparities in SLE and SLE outcomes, primarily as experienced in the U.S. Lupus Foundation of America's Health Disparities Advisory Panel, comprising 10 health disparity experts, including academic researchers and patients, who met 12 times over the course of 18 months in assembling and reviewing the data for this study. Sources included articles published from 2011 to 2023 in PubMed, Centers for Disease Control and Prevention data, and bibliographies and recommendations. Search terms included lupus, race, ethnicity, and SDOH domains. Data were extracted and synthesized into this scientific statement. Poorer neighborhoods correlate with increased damage, reduced care, and stress‐induced lupus flares. Large disparities in health care affordability, accessibility, and acceptability exist in the US, varying by region, insurance status, and racial and minority groups. Preliminary interventions targeted social support, depression, and shared‐decision‐making, but more research and intervention implementation and evaluation are needed. Disparities in lupus across racial and ethnic groups in the US are driven by SDOH, some of which are more easily remediable than others. A multidimensional and multidisciplinary approach involving various stakeholder groups is needed to address these complex challenges, address these diminish disparities, and improve outcomes.
Introduction
Racial and ethnic disparities in the burden of lupus in the US
Systemic lupus erythematosus (SLE) is an autoimmune disease with heterogenous presentation and manifestations ranging from mild to life threatening. Despite advances in the understanding and treatment of lupus over the past five decades, large racial and ethnic disparities persist. Because of its heterogeneity and often difficult or delayed diagnosis, capturing all cases of SLE has been a challenge for population‐based epidemiology studies. The Centers for Disease Control and Prevention (CDC)–funded registries in counties throughout California, Georgia, Michigan, and New York, along with Indian Health Service regions, provide estimates of SLE prevalence based on the 1997 American College of Rheumatology classification criteria (which have imperfect sensitivity and specificity) (1, 2, 3, 4). In the California Lupus Surveillance Project study, the age‐standardized physician‐reported prevalence of SLE per 100,000 between 2007 and 2009 was highest among Black and Hispanic women (5). The Manhattan Lupus Surveillance Program collected data during the same time period and found SLE prevalence to be approximately three times higher among Black people in comparison with White people (211 vs. 64 per 100,000 person‐years), as well as 2.2 higher for Hispanic populations and 1.4 times higher for Asian populations (1). Similarly, the Georgia Lupus Registry data demonstrated the incidence of SLE was almost threefold higher in Black women versus White women from 30 to 59 years of age (2). Summarizing the CDC's five national lupus registries, a 2018 meta‐analysis estimated overall SLE prevalence per 100,000 persons to be 271 for American Indian/Alaska Native women (acquired from the Indian Health Service registry only), 231 for Black women, 121 for Hispanic women, and 85 for White women (3). This evidence points to a strikingly high lupus prevalence among Black, Native American, and Hispanic people in the US, which is a disturbing health disparity (6, 7).
Additionally, a large body of literature documents that Asian, African American/Black, and Hispanic Americans have had poorer outcomes than non‐Hispanic White patients in the US with SLE‐related mortality highest among patients who are Black (8, 9). Large disparities in lupus nephritis incidence and outcomes have been found repeatedly across racial and ethnic groups in the US (10, 11). Among US Medicaid recipients, lupus nephritis was four times more common among Black than White people with SLE (7). Hispanic patients in the California Lupus Registry had over 3.5‐fold higher odds of kidney involvement compared with White patients (12). The risk of developing end‐stage renal disease (ESRD) necessitating dialysis or renal transplantation because of lupus nephritis has been much higher among Black, American Indian/Alaska Native, and Hispanic patients compared with Whites and Asian populations (2, 10, 13). In the Georgia Lupus Registry, newly diagnosed Black patients had a higher incidence of developing ESRD than White patients (14). Cardiovascular disease, the leading cause of death in SLE, is also characterized by large racial and ethnic disparities among people with SLE. Black race has been associated with elevated risk of myocardial infarction and stroke among patients with SLE (11, 15, 16, 17, 18). For example, a large cohort study found that Black patients with SLE had a 14% higher risk of cardiovascular disease compared with White patients (15). Additionally, in a study of inpatient data, both the likelihood of developing venous thromboembolism and hospitalization for it were two to three times higher among Black patients compared with White patients with SLE (18). Lastly, SLE‐related mortality rates are also much higher among non‐White racial and ethnic groups in the US. In nationwide data from 1968 to 2013 death certificates, the mortality rate from SLE was approximately four times higher for Black versus White people (19). Death certificate data from 2000 to 2015 showed that SLE was the fifth/sixth overall cause of death among Black and Hispanic females ages 15‐44 (8). Similarly, in the Georgia Lupus Registry, Black women in Fulton and Dekalb counties in Georgia died an average of 13 years earlier than did White women (20).
Thus, large disparities in SLE across racial and ethnic groups in the US are well established by past studies, and non‐White groups, in particular Black/African Americans, have much higher incidence, severity and complication rates, and mortality than Whites. Native American populations also appear to suffer a high incidence and severity of SLE, although population‐based data are not as plentiful (21). Empirical evidence from a county health rankings study examining the impact of modifiable health factors on health outcomes demonstrated that clinical care only accounted for 16% of overall health outcomes, whereas socioeconomic factors accounted for 46% (22). Low socioeconomic status (SES) is a risk factor for poorer outcomes in lupus. Low SES intersects and is interconnected to established domains of the social determinants of health (SDOH), including poverty, lack of access to a high‐quality education or employment, low access to adequate, quality care, neighborhood violence, toxic built environments, and unfavorable social and community conditions (23). Exposure to social disadvantage and low social positioning can have detrimental biological effects over the lifespan that consequentially lead to poorer health outcomes (24, 25). The objective of this scientific statement is to address the relationship between SDOH and racial and ethnic disparities and potential interventions to reduce these health disparities in lupus (Figure 1). Studies included highlight current knowledge about the contribution of SDOH to these racial and ethnic disparities in SLE in the US. This scientific statement is intended to be used by clinicians, researchers, public health practitioners, industry leaders, and policy makers to swing the pendulum toward health equity for historically marginalized individuals living with SLE.
Figure 1.
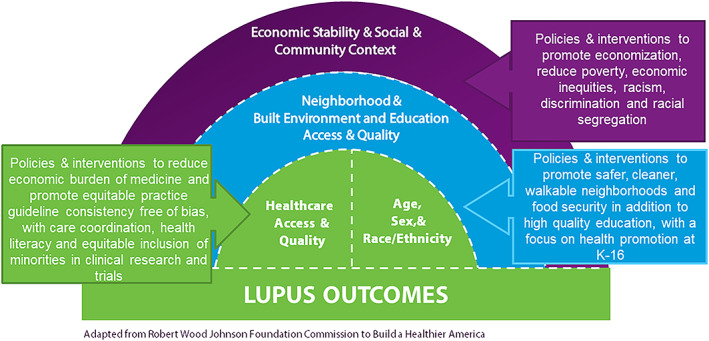
This figure presents an adapted illustration based on the Robert Wood Johnson Foundation's Commission to Build a Health America model. It encompasses various domains that mirror the SDOH, as delineated in the scientific statement. These SDOH correlate with adverse lupus outcomes in racially and ethnically marginalized communities, specifically among African American/Black and Hispanic communities. Our depicted model offers a framework that informs our scientific statement. It emphasizes the influence of SDOH on disproportionate outcomes and underscores suggested or strategies under investigation that are intended to mitigate racial and ethnic disparities in lupus. SDOH, social determinants of health.
Methods
In October 2020, the Lupus Foundation of America convened a panel of lupus and health disparity experts to address barriers and facilitators for reducing health disparities in people with lupus. Together the panel provided expert opinion about structural factors that result in unequal distribution of resources. This unequal distribution of resources drives the development of social inequities and barriers to access to care that facilitate the development of health disparities.
The Lupus Foundation of America's Health Disparities Advisory Panel is composed of individuals from various academic institutions, nonprofit organizations, and patients. All 10 members of the panel have expertise in health disparities research, lupus, and the abundantly nuanced challenges related to achieving health equity. The panel provided input and guidance to the structure and topics of this scientific statement/review. Studies were identified by searching PubMed for studies published from 2011 to 2023, other sources, including CDC data, study bibliographies, and recommendations, were also included. We identified both primary publications and review articles as well as referrals from members of the advisory panel. Database search terms included lupus, race, ethnicity, and domains of SDOH. Data related to the primary objective were extracted and results synthesized into this scientific statement. The advisory panel helped with identification of search terms and outlining the results of the scientific statement. The studies summarized in this scientific statement outline major epidemiological findings and novel findings related to social determinants that prove detrimental in SLE.
Results
SDOH and disparate lupus outcomes
As in many chronic diseases, low SES is a strong risk factor for poor outcomes in SLE, and racial and ethnic disparities in US patients with lupus are observed in groups with social, economic, or environmental disadvantages. Poverty and lower educational level are strong predictors of lower health‐related quality of life in SLE (26). Lower SES is also strongly associated with poor outcomes in SLE, including renal and cardiovascular disease (7, 17, 27). Historical and current anti‐Black racism, discrimination, and social exclusion also play roles in differences in health outcomes among people living with lupus as outlined in Figure 1 (28). Disentangling exactly how each of these connected social factors contributes to health disparities in lupus is challenging. Still, it is important to understand that SDOH impacts health outcomes outside clinical walls so that evidence‐based solutions, including policies and evidence‐based interventions addressing SDOH, can be developed to reduce disparities in lupus.
Economic instability
Economic stability is important for the ability to self‐manage lupus and includes adequate employment, food security, and affordable and safe housing (30). Studies demonstrate that living in poverty or having lower SES is associated with reduced SLE‐related physical functioning, increased incidence of depression, and increased risk of mortality (7, 30, 31). For example, data from the Georgians Organized Against Lupus study comprised patients with lupus from minority communities demonstrated that a lower poverty:income ratio (lower ratio indicates higher poverty) was associated with worse self‐reported physical functioning, potentially contributing to disparities in Black people with lupus (32). In the same cohort, Black people with lupus were twice as likely to experience job loss following diagnosis than Whites (33). Although standardized unemployment ratios comparing the risk of unemployment in the general population to those for people with lupus were similar across race and SES, they were greatest for people with lupus with severe disease activity and organ damage (33). A recent systematic review to synthesize data regarding the economic burden of SLE identified that SLE has significant indirect and direct cost implications for people with lupus (34). These studies suggest that people suffering from the greatest impact of lupus have a greater risk of having reduced work productivity, subsequently leading to transition into poverty or low SES.
Both low SES and poverty have been associated with increased mortality in lupus, irrespective of race. In a 2009 analysis of 807 subjects with lupus, after adjusting for age, poverty was associated with a 2.14 times greater risk of mortality (35). However, after adjusting for the extent of lupus‐related organ damage and age, poverty was no longer associated with an increased risk of mortality, suggesting that one potential mechanism by which poverty leads to higher SLE‐related mortality is through damage accumulation (35). Still, US Census Data analyses have shown that average annual mortality rates were highest among Black people with lupus living in average‐income, Southern and/or urban areas, and lowest among White, Hispanic, and Asian persons living in average‐income areas, suggesting that race as a social construct transcends SES alone as a risk factor for SLE‐related mortality risks (27). In the Hopkins Lupus Cohort, a graded relationship between income and having cardiovascular disease risk factors was observed (smoking, obesity, hypertension, hyperlipidemia, diabetes mellitus, myocardial infarction, and cerebrovascular accident) among White patients, but only current smoking status and diabetes were associated with lower income in Black people with lupus (17). In the same study, Black race was associated with obesity and hypertension, regardless of income. Although higher SES was associated with lower risk of myocardial infarction among White race, higher SES was not protective against myocardial infarction in Black people with lupus (17). These studies suggest that economic instability plays a role in creating disparate outcomes in lupus, but that increased income itself may not provide sufficient health benefits for all racial and ethnic minority groups. Additional studies are needed to understand the compounding effects of SES by various definitions and the social experience of race in the US, on overall lupus outcomes.
Neighborhood and built environment
Accessibility to safe and walkable neighborhoods and environments are conducive to optimal health and well‐being for all, including people with lupus. The Lupus Outcomes Study revealed that poor people with lupus living in residential areas of concentrated poverty had more damage accrual than impoverished people living in nonpoverty areas (36). In this same study, people who exited poverty permanently had similar accrual rates of lupus‐related organ damage over time as those who were never in poverty, but significantly less than those who remained in poverty (36). Depression, stress, and cognitive impairment may be related to increased damage accumulation for people living in poor or unsafe neighborhoods (36). In the Lupus Outcomes Study cohort, challenges in managing food, medical care, and housing insecurity, along with exposure to crime, were sources of continual stress for people living in impoverished neighborhoods. In the Black Women's Experiences Living with Lupus study, women living in highly segregated census tracts were at greatest risk of depression because of increased exposure to neighborhood disorder (37). According to data extracted from electronic health records at an urban US academic center, residence in a disadvantaged neighborhood was the strongest factor predicting poor retention in care for patients with lupus (38). In the same study, Black patients were 10 times more likely to live in the most disadvantaged neighborhood quartile (measured by validated area deprivation index) compared with White patients; race was not independently predictive of retention in care (38). These data suggest that solutions targeted at overcoming neighborhood context, including segregation, safety, and housing and food insecurity among others, might be beneficial in addressing lupus disparities.
Social and community context
Racism, discrimination, stress, abuse, and adverse childhood experiences (ACEs) also contribute to poor lupus outcomes. Racism, and not race, has been identified as a major driver of Black mortality in the US, and racism is arguably the most potent risk to the health of Black patients with lupus in the US (39). An analysis of racial discrimination in a subset of patients from the BeWell cohort indicated that SLE activity and organ damage were significantly associated with racial discrimination (40). In BeWell, anticipatory racism stress, defined as psychological and/or physiological arousal related to anticipation of experiencing racism in the near future, was also associated with significantly greater lupus disease activity, contributing to racial disparities in outcomes (41). The negative impacts of racism are seen not only from personal and anticipatory racism, but also from distress associated with witnessing racism, which was associated with greater lupus disease activity in the BeWell cohort (42, 43).
Childhood and adult stress‐related disorders have also been implicated as drivers of disease activity in lupus. In both the Nurses’ Health Study II and the Black Women's Cohort Study, exposure to childhood physical and emotional abuse were associated with almost threefold elevated risk of developing lupus among women (44, 45). In the California Lupus Epidemiology Study, reporting ACEs was more common among older people, Hispanic and Black people, and people without college degrees. People who reported ACEs were more likely to also have greater self‐reported SLE activity, depression, and worse health status (46).
In the LUMINA cohort study, Black participants had less social support than White participants, and less social support was a predictor of greater SLE disease activity over time (47). Similarly, the GOAL study qualitative data analysis demonstrated that social support needs were related to the magnitude of lupus severity among Black women (48). The GOAL study also found that social support protected against depression (48). A randomized trial of a theory‐based intervention centered on improving self‐efficacy and social support for patients with SLE demonstrated improved outcomes, including improved couple communication, self‐efficacy for SLE management, and mental health status, as well as decreased fatigue at 12 months (49). Further research on social and community context influencing lupus outcomes, including racism, discrimination, adverse experiences of childhood, and interpersonal violence, as well as interventions to improve social support and self‐efficacy, are needed.
Education access and quality
High‐quality education is associated with health beliefs that align with optimal health outcomes and greater knowledge to engage in self‐advocacy within the health care system. Lupus significantly limits opportunities to engage in postsecondary education in minority communities; in the Lupus Outcomes Study, Black versus White, adult patients with childhood‐onset SLE from diverse backgrounds were 37% less likely to go to college (50). Lower educational attainment and academic achievement intersect with decreased self‐efficacy, inappropriate medication‐taking behaviors, and missed medical appointments, particularly among people with lupus from racial and ethnic minority groups. When compounded, these can lead to worse lupus outcomes (51, 52, 53).
Multiple studies of people with lupus have associated lower education status with higher prevalence of depression or anxiety (54, 55). For example, in an analysis comparing individuals with childhood SLE and adult‐onset SLE, lower educational attainment, independent of poverty status, was most strongly associated with major depression risk in both groups, and the observed effect was greater for individuals with childhood versus adult‐onset lupus (55). Similarly, people with lupus with less than or high school level education reported higher need on the SLE Needs Questionnaire than did people with lupus with college or advanced degrees (54).
Health care access and quality
Health care access and quality are evaluated based on affordability, availability, accessibility, accommodation, and acceptability. To acquire access to high‐quality care, these factors must intersect with a patient's fortitude and motivation to engage in health‐seeking behaviors. Past studies have examined challenges patients with lupus experience related to health care affordability, accessibility, and acceptability.
Health care affordability reflects a person's capacity to afford direct, indirect, and opportunity costs associated with health care services. SLE is associated with significant direct and indirect costs limiting access to care. One study demonstrated that even in a single‐payer, publicly funded health care setting, low SES at SLE diagnosis was associated with significantly greater direct medical costs (56). Some direct cost estimates include $33,223 for all cost components for the general SLE population, $71,334 for people with LN, and $13,494 to $55,344 for severe or active SLE (57). Furthermore, the difference in predicted mean‐per‐person‐year incremental direct costs of SLE indicated that the difference in total cost between people with low SES and high SES is $1922 (56). People with SLE also experienced higher indirect costs ranging from $1252 to $20,046 for the general SLE population and up to $18,034 for people with LN, because of the decreased ability to provide childcare or conduct household work and unemployment leading to reduced access to employer benefits (57). An extensive analysis of claims data revealed a considerable increase in the annual cost of health care related to lupus. Specifically, the findings indicated that the incremental expenditure was approximately $10,984 higher when compared with the health care costs incurred by individuals without lupus. The analysis further delineated a notable disparity in health care costs among patients with lupus. Those individuals who experienced severe flares were found to incur health care costs that were approximately double those of patients with moderate to mild flares (58). Others have shown that maintaining a low lupus activity state more than half the time reduced annual direct medical costs by 25.9% (59). Additionally, research has highlighted significant differences in health care costs based on insurance types. A particular study showed that total unadjusted health care costs were notably higher for Medicaid‐insured individuals compared with their commercially insured counterparts with lupus (60). Cost‐related prescription adherence was evaluated in the Michigan Lupus Epidemiology and Surveillance study. This research revealed that patients with lupus were twice as likely to report cost‐related prescription nonadherence as a strategy to economize, which included behaviors such as skipping dosages, reducing medication intake, and delaying prescription refills, in comparison to agnostic patients with lupus (61).
Health care access can be more challenging for uninsured or under‐insured people with lupus or those with public insurance, possibly leading to worse outcomes. Studies demonstrate that those with lupus nephritis, especially from racial or ethnic minority groups (eg, Black people with lupus, who were most likely to be uninsured or without private insurance), are more likely to receive inadequate ESRD care, potentially worsening disease outcomes (10, 62, 63, 64). Another large cross‐sectional study found that uninsured patients with lupus received lower‐quality preventive care compared with those with health insurance, as measured by performance on 13 quality measures, such as sun avoidance counseling and traditional CV risk factor assessment (65). Furthermore, a study demonstrated linkages between being uninsured and experiencing depression among predominately Black women with chronic cutaneous suggesting that limited insurance coverage and subsequent limited access to care may increase the risk mental health challenges (66).
The substantial economic burden associated with SLE has the potential to impede patient access to adequate health care, which can trigger a cascade of consequences from increased flare severity, heightened, sustained disease activity, and ultimately amplification of existing disparities. Research conducted by Yazdany and colleagues highlighted that 16.5% of patients with SLE were readmitted to the hospital within 30 days of discharge. These patients were more likely to be from a marginalized racial or ethnic group or on either Medicare or Medicaid (67). The study further demonstrated that organ damage, particularly renal involvement, led to increased annual direct costs (67).
Research indicates that racial and ethnic minorities with lupus often experience significant challenges accessing high‐quality care. Health illiteracy, a key determinant in individual health care accessibility, may be a limitation. For example, a study of 323 patients from the California Lupus Epidemiology Study demonstrated that 38% of the people with lupus had limited health literacy, correlating with significantly worse scores in all patient‐reported outcomes (eg, physical functioning and SLE disease activity measures) except disease damage (68). Another observational study indicated that Black and Hispanic people with lupus received fewer rheumatologist referrals and were less likely to receive pre‐ESRD nephrology care than their White counterparts (63). The COVID‐19 pandemic further amplified these disparities by limiting access to care (69). Increased travel burden was associated with more missed appointments and medication nonadherence, particularly among Black patients with lupus, due to including reliance on caregivers, financial constraints, and physical limitations (70, 71). In an integrated care management program, 66% of patients reported concerns about medication access, and 61% about unmet transportation needs (61%) (72). Specialist diagnosis in American Indian/Alaskan Native people with lupus increased the likelihood of quality care indicators, such as having SLE classification criteria documented, being tested for biomarkers of disease activity, and receiving treatment with hydroxychloroquine (73). Medication quality and evaluation in minority groups have also proven challenging. People with lupus across racial and ethnic minority groups are less likely to enroll in lupus clinical trials that evaluate the effectiveness of medication on reducing disease activity and flares. Falasinnu et al found that racial and ethnic minorities only accounted for 49% of 193 clinical trial participants, while representing 70% of SLE cases in the US (27). This can exacerbate existing disparities in lupus as researchers cannot evaluate whether experimental interventions are safe and effective across different groups. Excessive consent forms, childcare, work, unpredicted personal and health‐related issues, misunderstanding of the scope and benefits of the intervention, mistrust related to racism, concerns about assignment to placebo groups, strict study exclusion criteria, and transportation issues contributed to decreased access (74, 75, 76). Researchers have proposed that these barriers could disproportionately affect people with lupus from racial and ethnic minority groups and hypothesized that interventions aimed at reducing these barriers could facilitate access to clinical trial participation and renal transplantation, for example (10, 64, 74).
Geography affects access to treatment, for example, people with lupus in the Northeast were more likely to receive immunosuppressive therapy compared with those living in the South (67). A review of Medicaid data showed that patients with lupus nephritis residing in the Northeast were more likely to receive immunosuppressive therapy compared with those living in the South and the Midwest, because of inadequate access to ambulatory care (77). In a cohort in the Bronx of patients with lupus mainly covered by public insurance, some were forced to live in shelters, putting them at high risk of immunosuppression‐related infections (78).
Lower‐quality, inconsistent care is often sought by racial and ethnic minority groups, even when affordability is not a barrier (79); causes are multifactorial (79). Groups who have been marginalized may perceive that health care environments are not welcoming or affordable and may be socially averse to environments that offer the highest quality care options (80). Conversely, the same provider may administer lower‐quality care to minority patients in comparison with their nonminority counterparts because of implicit bias, perceived difference in need/benefit, or culturally insensitive system designs that fail to meet the needs of minority groups (81). For example, Black and Hispanic people with lupus received fewer rheumatologist referrals and were less likely to receive pre‐ESRD nephrology care than their White counterparts in an observational study (63). Accordingly, significant disparities in the quality of care for low income, low education, and/or publicly insured people with lupus persist (82, 83). In an electronic health record study of people with lupus, a majority of whom were Black, study authors found that race, public insurance, and care fragmentation were independently associated with SLE‐derived damage. Moreover, Black and publicly insured people with lupus were more likely to experience care fragmentation compared with White or privately insured people with lupus (84). Another study reported that Black women with SLE who self‐reported unfair medical treatment were more likely to report greater damage compared with those who did not (85). Similarly, Black and Hispanic patients with lupus nephritis were less likely to receive pre‐ESRD care and less likely to be placed on the kidney transplant waiting list within the first year of ESRD diagnosis, compared with White patients (64). Further, Medicaid beneficiaries or uninsured individuals were less likely than those with private insurance to be placed on the waitlist and it has been hypothesized that this could be due to a lack of health care provider‐led patient education on ESRD treatment options (63, 64). Among Medicaid patients with SLE, those who were Black or American Indian/Alaskan Native patients prescribed hydroxychloroquine were less likely than White patients to receive recommended baseline retinal examinations (86). Although there have been technological advancements and improved maternal‐fetal outcomes for women with lupus, pregnancy can complicate active lupus and exacerbate compromised renal function (87). Thus, contraception is important to prevent pregnancy in women with lupus who are at increased risk for health complications or medication teratogenicity with unintended pregnancy. Compared with White women with SLE, Black women had 11% lower odds of any contraception dispensing and 29% lower odds of “highly effective contraception use” (use of long‐acting reversible contraception methods, intrauterine devices and implants, or sterilization) (88). Quality of care affects SLE‐related damage accrual rates and disease activity, particularly among Black and Hispanic patients and those publicly insured (89, 90, 91, 92).
Black patients with lupus and those with public insurance also have greater risk of hospitalization readmittance compared with White and/or privately insured people with lupus. Black patients with lupus have been reported to have significantly greater odds of having more lupus‐related hospitalization charges, discharges to rehabilitation or nursing facilities, and longer duration hospital stays compared with White patients (93, 94). Readmission has also been shown to occur more frequently among Black and Hispanic people with lupus compared with White people with lupus and those with public (Medicaid or Medicare) versus private insurance (62, 67). Patients with lupus from racial and ethnic minority groups have also been found to be more likely to be hospitalized due to a preventable cause. A study of Medicaid patients with lupus found that Black patients had a 22% greater risk of hospitalization due to vaccine‐preventable illness compared with White patients (95). Risk factors, including insurance status and race and ethnicity, have been shown to increase the risk of lupus hospitalization. An analysis of hospital claims data showed that specific racial and ethnic minority people with lupus had the highest odds for a preventable lupus hospitalization including those who were at least 40 years old, or on Medicare or Medicaid (94). In a different study, the cumulative mortality rate was highest among Medicaid beneficiaries with LN and highest among Black patients (96). Collectively, these studies suggest that social impacts of race and ethnicity may drive hospitalizations that were otherwise preventable with the right resources and tools.
High‐quality patient–physician relationships impact treatment decision‐making and lupus outcomes and must be acceptable to enhance a patient's perception of the various aspects of services and appropriateness of care. In a population‐based cohort of mostly Black people with lupus, disease activity and depression were independently associated with suboptimal quality of overall provider's interpersonal style; depression was independently associated with suboptimal quality of overall physician–patient communication (97). Authors postulated that shared‐decision‐making improvements were warranted given that this approach to care was only implemented “sometimes” (97). Patients’ trust in physicians can impact cooperativeness in shared decision‐making. A cross‐sectional study revealed Black patients with lupus agreed to immunosuppressive treatment with cyclophosphamide less often than White people with lupus if their lupus were to worsen (98). In that study, Black race was inversely associated with willingness to receive treatment, whereas patient–physician trust and perception of treatment effectiveness were associated with willingness to receive cyclophosphamide (98). Thus, improvements in patient–physician trust have the potential to impact decision‐making among people with lupus. Patient–physician relationships also influence lupus outcomes; a report showed that people living with lupus across racial and ethnic groups had lower odds of depression if they had visited a health care provider in the last year and if they reported better patient–physician interactions (66). A less‐developed body of evidence suggests that health literacy affects patient‐reported outcomes but not disease activity (68).
Proposed or studied interventions
In addition to the randomized trial of patients with lupus and their support persons, which proved the efficacy of an intervention to improve self‐efficacy and social support, the feasibility and effectiveness of the Peer Approaches to Lupus Self‐Management program, a peer mentoring tool to improve health behaviors, beliefs, and outcomes in Black women with SLE, has been reported (99, 100). Additional self‐management programs have been evaluated in pilot and feasibility studies (101, 102, 103). Proposed or ongoing interventions also include mobile health/technology‐advancing interventions (104, 105), mindfulness‐based cognitive interventions (106), and cardiovascular/resistance training exercise‐focused interventions (107). The majority of these aimed to improve disease self‐management and quality of life. A randomized pilot study of 30 Black patients with SLE, the Balancing Lupus Experiences with Stress Strategies study, focused on facilitating intervention workshops to reduce incidence of depression and stress and reported that the use of stress reduction techniques significantly affected depression, social capacity, health distress, fatigue, pain, and lupus self‐efficacy (103). However, evidence of similar interventions having an impact on SLE disease activity is still limited. Policy interventions being tested and implemented include care coordination interventions, as well as feasibility and accessibility studies of the use of novel quality indicators to improve care in rheumatology clinics (108, 109).
Discussion
Numerous SDOH could potentially underpin the racial and ethnic health disparities observed in SLE within the US. Financial instability, as indicated by employment status and income, is more prevalent among individuals with lupus from historically underrepresented racial and ethnic minority groups. This precarious economic condition may lead to an elevated disease burden and diminished access to health care. Similarly, lower educational attainment, predominantly observed among Black and Hispanic patients with lupus, correlates with poorer disease outcomes. Figure 1 encapsulates the SDOH and proposes potential policies and interventions that could alleviate some of the challenges delineated herein. Nonetheless, it is imperative to recognize that the mitigation of racism (as highlighted in Figure 1), which is postulated to be a primary driver of these disparities within the US, may not be achievable in the immediate future.
People with lupus from racial and ethnic minority groups, when compared with White people with lupus, are also more likely to have limited access to high‐quality lupus care and less likely to have medical insurance, both of which influence lupus disease outcomes. Quality of care may be linked to patient–physician mistrust, suggesting a need for solutions aiming to improve patient–physician dynamic, especially among people with lupus from racial and ethnic minority groups.
This scientific statement also captures the implications of reduced access to clinical trial opportunities and neighborhood and social factors (eg, neighborhood‐level poverty) on inequitable outcomes, including depression and anxiety, quality of life, and lupus disease activity. Still, we have identified gaps and/or areas for further study related to disparities and SDOH in lupus. First, limited evidence on SDOH influencing outcomes, such as cutaneous lupus, cardiovascular disease, and hospitalization outcomes, exists. Second, knowledge gaps related to racial and ethnic disparities associated with many core neighborhood and built environment factors classically included in social determinant of health definitions, including transportation accessibility, internet access, and exposure to air pollution, remain. Moreover, research on the role of community context in lupus outcomes beyond the neighborhood and built environment is missing from the lupus literature and was not immediately captured using our search strategy. Third, the role of social and community context related to attitudes and health‐seeking behaviors among patients with lupus from diverse backgrounds is still not well understood. Lastly, past interventions have not taken multidimensional approaches to address disparities in lupus, highlighting an unmet need in this area. In a study of Black patients with SLE, suboptimal interactions with physicians were shown to be impacted by a patient's symptoms, suggesting that patients with severe disease activity and depression could benefit from interventions focused on improving physician communication and interpersonal skills (97).
This scientific statement addresses the implications of various social factors and reduced access to clinical trials on inequitable outcomes in patients with lupus. However, there are several limitations to this statement that need to be acknowledged. First, our search strategy may have been incomplete, as most but not all published literature relevant to the topic has been reviewed. This may have resulted in some gaps in our understanding of the subject matter. Second, research on the role of community context in lupus outcomes beyond the neighborhood and built environment is missing from the lupus literature and was not immediately captured using our search strategy. This suggests that further exploration of these factors is necessary to better understand their impact on lupus outcomes. Third, studies on the impacts of SDOH on American Indian/Alaska Native populations, as it pertains to lupus, is largely missing from the extant literature. This highlights the need for additional research on this population to better understand the unique challenges they face in terms of lupus outcomes. Lastly, we did not examine the role of genetic or epigenetic factors in disparities among racial and ethnic minority groups, which may have been driven by bias and knowledge introduced by the advisory panel. Considering the potential influence of these factors on lupus outcomes, future research should address this limitation by incorporating genetic and epigenetic analyses. By acknowledging and addressing these limitations, future research can contribute to a more comprehensive understanding of the disparities and SDOH in lupus and help to improve outcomes for patients across different communities.
Known health disparities in SLE were brought into stark relief during the SARS‐CoV‐2 pandemic. Moving onward, it is critical to continue to study and implement mitigating solutions to improve disparities in SLE outcomes, especially those related to SDOH. These developments provide hope for a brighter future for individuals living with lupus as the Lupus Foundation of America, disparities researchers, and stakeholders continue to work together to brainstorm and implement solutions to address SDOH and disparities among individuals living with lupus.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published.
ROLE OF THE STUDY SPONSOR
AstraZeneca had no role in the study design or in the collection, analysis, or interpretation of the data, the writing of the manuscript, or the decision to submit the manuscript for publication. Publication of this article was not contingent upon approval by AstraZeneca. This boilerplate language aligns with AZs role in the project. They were solely a funder.
Supporting information
Disclosure Form
ACKNOWLEDGMENTS
Dr. Joy Buie expresses sincere gratitude to Jennifer Johnson for her vital role and guidance in coordinating the team of experts who contributed to the conceptualization of this manuscript. Dr. Buie's gratitude also extends to Dr. Melicent Miller and Melissa French for their astute and critical reading of their mansucript. Additionally, special thanks are extended to Kristen Backor and Greta Olesen for their indispensable manuscript development support.
This study was launched in partnership with Charles River Associates and was supported by Pharmaceutical Research and Manufacturers of America and AstraZeneca.
Author disclosures are available at https://onlinelibrary.wiley.com/doi/10.1002/acr2.11590.
REFERENCES
- 1. Izmirly PM, Wan I, Sahl S, et al. The incidence and prevalence of systemic lupus erythematosus in New York County (Manhattan), New York: the Manhattan Lupus Surveillance Program. Arthritis Rheumatol 2017;69:2006–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lim SS, Bayakly AR, Helmick CG, et al. The incidence and prevalence of systemic lupus erythematosus, 2002‐2004: the Georgia lupus registry. Arthritis Rheumatol 2014;66:357–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Izmirly PM, Parton H, Wang L, et al. Prevalence of systemic lupus erythematosus in the United States: estimates from a meta‐analysis of the Centers for Disease Control and Prevention National Lupus Registries. Arthritis Rheumatol 2021;73:991–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997;40:1725. [DOI] [PubMed] [Google Scholar]
- 5. Dall'Era M, Cisternas MG, Snipes K, et al. The incidence and prevalence of systemic lupus erythematosus in San Francisco County, California: the California Lupus Surveillance Project. Arthritis Rheumatol 2017;69:1996–2005. [DOI] [PubMed] [Google Scholar]
- 6. Li S, Gong T, Peng Y, et al. Prevalence and incidence of systemic lupus erythematosus and associated outcomes in the 2009–2016 US Medicare population. Lupus 2020;29:15–26. [DOI] [PubMed] [Google Scholar]
- 7. Feldman CH, Hiraki LT, Liu J, et al. Epidemiology and sociodemographics of systemic lupus erythematosus and lupus nephritis among US adults with Medicaid coverage, 2000‐2004. Arthritis Rheum 2013;65:753–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yen EY, Singh RR. Brief report: Lupus—an unrecognized leading cause of death in young females: a population‐based study using nationwide death certificates, 2000–2015. Arthritis Rheumatol 2018;70:1251–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Falasinnu T, Chaichian Y, Bass MB, et al. The representation of gender and race/ethnic groups in randomized clinical trials of individuals with systemic lupus erythematosus. Curr Rheumatol Rep 2018;20:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Costenbader KH, Desai A, Alarcón GS, et al. Trends in the incidence, demographics, and outcomes of end‐stage renal disease due to lupus nephritis in the US from 1995 to 2006. Arthritis Rheum 2011;63:1681–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Barbhaiya M, Feldman CH, Guan H, et al. Race/ethnicity and cardiovascular events among patients with systemic lupus erythematosus. Arthritis Rheumatol 2017;69:1823–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sandhu VK, Teh P, Zakhary B, et al. The Southern California Lupus Registry. I. Baseline characteristics of lupus patients in uncharted territory. Lupus 2020;29:1277–81. [DOI] [PubMed] [Google Scholar]
- 13. Hoover PJ, Costenbader KH. Insights into the epidemiology and management of lupus nephritis from the US rheumatologist's perspective. Kidney Int 2016;90:487–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Plantinga L, Lim SS, Patzer R, et al. Incidence of end‐stage renal disease among newly diagnosed systemic lupus erythematosus patients: the Georgia Lupus Registry. Arthritis Care Res (Hoboken) 2016;68:357–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Barbhaiya M, Feldman CH, Guan H, et al. Racial/ethnic variation in stroke rates and risks among patients with systemic lupus erythematosus. Semin Arthritis Rheum 2019;48:840–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pryor KP, Barbhaiya M, Costenbader KH, et al. Disparities in lupus and lupus nephritis care and outcomes among US Medicaid beneficiaries. Rheum Dis Clin North Am 2021;47:41–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Maynard JW, Fang H, Petri M. Low socioeconomic status is associated with cardiovascular risk factors and outcomes in systemic lupus erythematosus. J Rheumatol 2012;39:777–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kishore S, Jatwani S, Malhotra B, et al. Systemic lupus erythematosus is associated with a high risk of venous thromboembolism in hospitalized patients leading to poor outcomes and a higher cost: results from Nationwide Inpatient Sample Database 2003‐2011. ACR Open Rheumatol 2019;1:194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yen EY, Shaheen M, Woo JM, et al. 46‐year trends in systemic lupus erythematosus mortality in the United States, 1968 to 2013: a nationwide population‐based study. Ann Intern Med 2017;167:777–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lim SS, Helmick CG, Bao G, et al. Racial disparities in mortality associated with systemic lupus erythematosus — Fulton and DeKalb Counties, Georgia, 2002–2016. MMWR Morb Mortal Wkly Rep 2019;68:419–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ferucci ED, Johnston JM, Gaddy JR, et al. Prevalence and incidence of systemic lupus erythematosus in a population‐based registry of American Indian and Alaska Native people, 2007‐2009. Arthritis Rheumatol 2014;66:2494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hood CM, Gennuso KP, Swain GR, et al. County health rankings: relationships between determinant factors and health outcomes. Am J Prev Med 2016;50:129–35. [DOI] [PubMed] [Google Scholar]
- 23. Braveman P, Egerter S, Williams DR. The social determinants of health: coming of age. Annu Rev Public Health 2011;32:381–98. [DOI] [PubMed] [Google Scholar]
- 24. Shonkoff JP, Boyce WT, McEwen BS. Neuroscience, molecular biology, and the childhood roots of health disparities: building a new framework for health promotion and disease prevention. JAMA 2009;301:2252–9. [DOI] [PubMed] [Google Scholar]
- 25. Blane D. Social determinants of health: socioeconomic status, social class, and ethnicity. Am J Public Health 1995;85:903–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Elera‐Fitzcarrald C, Fuentes A, González LA, et al. Factors affecting quality of life in patients with systemic lupus erythematosus: important considerations and potential interventions. Expert Rev Clin Immunol 2018;14:915–31. [DOI] [PubMed] [Google Scholar]
- 27. Falasinnu T, Chaichian Y, Palaniappan L, et al. Unraveling race, socioeconomic factors, and geographical context in the heterogeneity of lupus mortality in the United States. ACR Open Rheumatol 2019;1:164–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Williams JN, Drenkard C, Lim SS. The impact of social determinants of health on the presentation, management and outcomes of systemic lupus erythematosus. Rheumatology (Oxford) 2023;62 Suppl 1:i10–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yelin E, Trupin L, Bunde J, et al. Poverty, neighborhoods, persistent stress, and systemic lupus erythematosus outcomes: a qualitative study of the patients' perspective. Arthritis Care Res (Hoboken) 2019;71:398–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Saez E, Zucman G. Wealth inequality in the United States since 1913: evidence from capitalized income tax data. Q J Econ 2016;131:519–78. [Google Scholar]
- 31. Semega J, Kollar M, Shrider E, et al. Income and poverty in the United States: 2019, Washington (DC): US Government Publishing Office; 2020. [Google Scholar]
- 32. Hoge C, Bowling CB, Lim SS, et al. Association of poverty income ratio with physical functioning in a cohort of patients with systemic lupus erythematosus. J Rheumatol 2020;47:983–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Drenkard C, Bao G, Dennis G, et al. Burden of systemic lupus erythematosus on employment and work productivity: data from a large cohort in the southeastern United States. Arthritis Care Res (Hoboken) 2014;66:878–87. [DOI] [PubMed] [Google Scholar]
- 34. Turchetti G, Yazdany J, Palla I, et al. Systemic lupus erythematosus and the economic perspective: a systematic literature review and points to consider. Clin Exp Rheumatol 2012;30 Suppl 73:S116–22. [PMC free article] [PubMed] [Google Scholar]
- 35. Yelin E, Yazdany J, Trupin L. Relationship between poverty and mortality in systemic lupus erythematosus. Arthritis Care Res (Hoboken) 2018;70:1101–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yelin E, Trupin L, Yazdany J. A prospective study of the impact of current poverty, history of poverty, and exiting poverty on accumulation of disease damage in systemic lupus erythematosus. Arthritis Rheumatol 2017;69:1612–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Martz CD, Hunter EA, Kramer MR, et al. Pathways linking census tract typologies with subjective neighborhood disorder and depressive symptoms in the Black Women's Experiences Living with Lupus (BeWELL) study. Health Place 2021;70:102587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bartels CM, Rosenthal A, Wang X, et al. Investigating lupus retention in care to inform interventions for disparities reduction: an observational cohort study. Arthritis Res Ther 2020;22:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Paradies Y, Ben J, Denson N, et al. Racism as a determinant of health: a systematic review and meta‐analysis. PLoS One 2015;10:e0138511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chae DH, Martz CD, Fuller‐Rowell TE, et al. Racial discrimination, disease activity, and organ damage: the Black Women's Experiences Living with Lupus (BeWELL) study. Am J Epidemiol 2019;188:1434–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Spears EC, Allen AM, Chung KW, et al. Anticipatory racism stress, smoking and disease activity: the Black women's experiences living with lupus (BeWELL) study. J Behav Med 2021;44:760–71. [DOI] [PubMed] [Google Scholar]
- 42. Martz CD, Allen AM, Fuller‐Rowell TE, et al. Vicarious racism stress and disease activity: the Black Women's Experiences Living with Lupus (BeWELL) study. J Racial Ethn Health Disparities 2019;6:1044–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lim SS, Drenkard C. Understanding lupus disparities through a social determinants of health framework: the Georgians organized against lupus research cohort. Rheum Dis Clin North Am 2020;46:613–21. [DOI] [PubMed] [Google Scholar]
- 44. Cozier YC, Barbhaiya M, Castro‐Webb N, et al. Association of child abuse and systemic lupus erythematosus in Black women during adulthood. Arthritis Care Res (Hoboken) 2021;73:833–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Feldman CH, Malspeis S, Leatherwood C, et al. Association of childhood abuse with incident systemic lupus erythematosus in adulthood in a longitudinal cohort of women. J Rheumatol 2019;46:1589–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. DeQuattro K, Trupin L, Li J, et al. Relationships between adverse childhood experiences and health status in systemic lupus erythematosus. Arthritis Care Res (Hoboken) 2020;72:525–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Alarcón GS, Calvo‐Alen J, McGwin G Jr, et al. Systemic lupus erythematosus in a multiethnic cohort: LUMINA XXXV. Predictive factors of high disease activity over time. Ann Rheum Dis 2006;65:1168–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jordan J, Thompson NJ, Dunlop‐Thomas C, et al. Relationships among organ damage, social support, and depression in African American women with systemic lupus erythematosus. Lupus 2019;28:253–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Karlson EW, Liang MH, Eaton H, et al. A randomized clinical trial of a psychoeducational intervention to improve outcomes in systemic lupus erythematosus. Arthritis Rheum 2004;50:1832–41. [DOI] [PubMed] [Google Scholar]
- 50. Lawson EF, Hersh AO, Trupin L, et al. Educational and vocational outcomes of adults with childhood‐ and adult‐onset systemic lupus erythematosus: nine years of followup. Arthritis Care Res (Hoboken) 2014;66:717–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Feldman CH, Yazdany J, Guan H, et al. Medication nonadherence is associated with increased subsequent acute care utilization among Medicaid beneficiaries with systemic lupus erythematosus. Arthritis Care Res (Hoboken) 2015;67:1712–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mehat P, Atiquzzaman M, Esdaile JM, et al. Medication nonadherence in systemic lupus erythematosus: a systematic review. Arthritis Care Res (Hoboken) 2017;69:1706–13. [DOI] [PubMed] [Google Scholar]
- 53. Son MB, Sergeyenko Y, Guan H, et al. Disease activity and transition outcomes in a childhood‐onset systemic lupus erythematosus cohort. Lupus 2016;25:1431–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Auerbach C, Beckerman NL. What social workers in health care should know about lupus: a Structural equation model. Health Soc Work 2011;36:269–78. [DOI] [PubMed] [Google Scholar]
- 55. Knight AM, Trupin L, Katz P, et al. Depression risk in young adults with juvenile‐ and adult‐onset lupus: twelve years of followup. Arthritis Care Res (Hoboken) 2018;70:475–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. McCormick N, Marra CA, Sadatsafavi M, et al. Socioeconomic status at diagnosis influences the incremental direct medical costs of systemic lupus erythematosus: a longitudinal population‐based study. Semin Arthritis Rheum 2020;50:77–83. [DOI] [PubMed] [Google Scholar]
- 57. Carter EE, Barr SG, Clarke AE. The global burden of SLE: prevalence, health disparities and socioeconomic impact. Nat Rev Rheumatol 2016;12:605–20. [DOI] [PubMed] [Google Scholar]
- 58. Kan HJ, Song X, Johnson BH, et al. Healthcare utilization and costs of systemic lupus erythematosus in Medicaid. BioMed Res Int 2013;2013:808391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yeo AL, Koelmeyer R, Kandane‐Rathnayake R, et al. Lupus low disease activity state and reduced direct health care costs in patients with systemic lupus erythematosus. Arthritis Care Res (Hoboken) 2020;72:1289–95. [DOI] [PubMed] [Google Scholar]
- 60. Clarke AE, Yazdany J, Kabadi SM, et al. The economic burden of systemic lupus erythematosus in commercially‐ and medicaid‐insured populations in the United States. Semin Arthritis Rheum 2020;50:759–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Minhas D, Marder W, Harlow S, et al. Access and cost‐related nonadherence to prescription medications among lupus patients and controls: the Michigan Lupus Epidemiology and Surveillance Program. Arthritis Care Res (Hoboken) 2021;73:1561–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bartels CM, Chodara A, Chen Y, et al. One quarter of medicare hospitalizations in patients with systemic lupus erythematosus readmitted within thirty days. Semin Arthritis Rheum 2021;51:477–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Plantinga LC, Drenkard C, Patzer RE, et al. Sociodemographic and geographic predictors of quality of care in United States patients with end‐stage renal disease due to lupus nephritis. Arthritis Rheumatol 2015;67:761–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Devlin A, Waikar SS, Solomon DH, et al. Variation in initial kidney replacement therapy for end‐stage renal disease due to lupus nephritis in the United States. Arthritis Care Res (Hoboken) 2011;63:1642–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Yazdany J, Trupin L, Tonner C, et al. Quality of care in systemic lupus erythematosus: application of quality measures to understand gaps in care. J Gen Intern Med 2012;27:1326–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hong J, Aspey L, Bao G, et al. Chronic cutaneous lupus erythematosus: depression burden and associated factors. Am J Clin Dermatol 2019;20:465–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Yazdany J, Marafino BJ, Dean ML, et al. Thirty‐day hospital readmissions in systemic lupus erythematosus: predictors and hospital‐ and state‐level variation. Arthritis Rheumatol 2014;66:2828–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Katz P, Dall'Era M, Trupin L, et al. Impact of limited health literacy on patient‐reported outcomes in systemic lupus erythematosus. Arthritis Care Res (Hoboken) 2021;73:110–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Fernandez‐Ruiz R, Paredes JL, Niewold TB. COVID‐19 in patients with systemic lupus erythematosus: lessons learned from the inflammatory disease. Transl Res 2021;232:13–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Williams EM, Ortiz K, Flournoy‐Floyd M, et al. Systemic lupus erythematosus observations of travel burden: a qualitative inquiry. Int J Rheum Dis 2015;18:751–60. [DOI] [PubMed] [Google Scholar]
- 71. Williams EM, Ortiz K, Zhang J, et al. The systemic lupus erythematosus travel burden survey: baseline data among a South Carolina cohort. BMC Res Notes 2016;9:246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Taber KA, Williams JN, Huang W, et al. Use of an integrated care management program to uncover and address social determinants of health for individuals with lupus. ACR Open Rheumatol 2021;3:305–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. McDougall JA, Helmick CG, Lim SS, et al. Differences in the diagnosis and management of systemic lupus erythematosus by primary care and specialist providers in the American Indian/Alaska Native population. Lupus 2018;27:1169–76. [DOI] [PubMed] [Google Scholar]
- 74. Arriens C, Aberle T, Carthen F, et al. Lupus patient decisions about clinical trial participation: a qualitative evaluation of perceptions, facilitators and barriers. Lupus Sci Med 2020;7:e000360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Drenkard C, Easley K, Bao G, et al. Overcoming barriers to recruitment and retention of African‐American women with SLE in behavioural interventions: lessons learnt from the WELL study. Lupus Sci Med 2020;7:e000391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Sneed RS, Mason M, Williams JN, et al. Using critical race theory to understand trial participation among black individuals with systemic lupus erythematosus: a qualitative study of patients and caregivers. Arthritis Care Res (Hoboken) 2021;73:1387–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Yazdany J, Feldman CH, Liu J, et al. Quality of care for incident lupus nephritis among medicaid beneficiaries in the United States. Arthritis Care Res (Hoboken) 2014;66:617–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Blanco I. SLE: serving the underserved in an academic medical center. Soc Work Health Care 2012;51:587–96. [DOI] [PubMed] [Google Scholar]
- 79. Agency for Healthcare Research and Quality . 2015 national healthcare quality and disparities report and 5th anniversary update on the national quality strategy. Rockville (MD): Agency for Healthcare Research and Quality; 2016. [Google Scholar]
- 80. Zestcott CA, Blair IV, Stone J. Examining the presence, consequences, and reduction of implicit bias in health care: a narrative review. Group Process Intergroup Relat 2016;19:528–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Hall WJ, Chapman MV, Lee KM, et al. Implicit racial/ethnic bias among health care professionals and its influence on health care outcomes: a systematic review. Am J Public Health 2015;105:e60–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Yelin E, Yazdany J, Tonner C, et al. Interactions between patients, providers, and health systems and technical quality of care. Arthritis Care Res (Hoboken) 2015;67:417–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Arora S, Yazdany J. Use of quality measures to identify disparities in health care for systemic lupus erythematosus. Rheum Dis Clin North Am 2020;46:623–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Walunas TL, Jackson KL, Chung AH, et al. Disease outcomes and care fragmentation among patients with systemic lupus erythematosus. Arthritis Care Res (Hoboken) 2017;69:1369–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Chae DH, Drenkard CM, Lewis TT, et al. Discrimination and cumulative disease damage among African American women with systemic lupus erythematosus. Am J Public Health 2015;105:2099–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Lin TC, Marmor MF, Barbhaiya M, et al. Baseline retinal examinations in patients with systemic lupus erythematosus newly initiating hydroxychloroquine treatment in a US Medicaid systemic lupus erythematosus population, 2000–2010. Arthritis Care Res (Hoboken) 2018;70:1700–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Essouma M, Nkeck JR, Motolouze K, et al. Outcomes of pregnancy and associated factors in sub‐Saharan African women with systemic lupus erythematosus: a scoping review. Lupus Sci Med 2020;7:e000400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Williams JN, Xu C, Costenbader KH, et al. Racial differences in contraception encounters and dispensing among female medicaid beneficiaries with systemic lupus erythematosus. Arthritis Care Res (Hoboken) 2021;73:1396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Verma SM, Okawa J, Propert KJ, et al. The impact of skin damage due to cutaneous lupus on quality of life. Br J Dermatol 2014;170:315–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Pons‐Estel GJ, Saurit V, Alarcón GS, et al. The impact of rural residency on the expression and outcome of systemic lupus erythematosus: data from a multiethnic Latin American cohort. Lupus 2012;21:1397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Bruce IN, O'Keeffe AG, Farewell V, et al. Factors associated with damage accrual in patients with systemic lupus erythematosus: results from the Systemic Lupus International Collaborating Clinics (SLICC) inception cohort. Ann Rheum Dis 2015;74:1706–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Ugarte‐Gil MF, Pimentel‐Quiroz VR, Vilá LM, et al. Factors associated with disease expression patterns in systemic lupus erythematosus patients: results from LUMINA (LXXVII), a multiethnic US cohort. Lupus 2017;26:650–5. [DOI] [PubMed] [Google Scholar]
- 93. Singh JA, Cleveland JD. Hospitalized infections in lupus: a nationwide study of types of infections, time trends, health care utilization, and in‐hospital mortality. Arthritis Rheumatol 2021;73:617–30. [DOI] [PubMed] [Google Scholar]
- 94. Brown EA, Gebregziabher M, Kamen DL, et al. Examining racial differences in access to primary care for people living with lupus: use of ambulatory care sensitive conditions to measure access. Ethn Dis 2020;30:611–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Feldman CH, Xu C, Costenbader KH. Avoidable acute care use for vaccine‐preventable illnesses among medicaid beneficiaries with lupus. Arthritis Care Res (Hoboken) 2021;73:1236–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Feldman CH, Broder A, Guan H, et al. Sex differences in health care utilization, end‐stage renal disease, and mortality among Medicaid beneficiaries with incident lupus nephritis. Arthritis Rheumatol 2018;70:417–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Drenkard C, Bao G, Lewis TT, et al. Physician–patient interactions in African American patients with systemic lupus erythematosus: demographic characteristics and relationship with disease activity and depression. Semin Arthritis Rheum 2019;48:669–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Vina ER, Masi CM, Green SL, et al. A study of racial/ethnic differences in treatment preferences among lupus patients. Rheumatology (Oxford) 2012;51:1697–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Williams EM, Egede L, Oates JC, et al. Peer approaches to self‐management (PALS): comparing a peer mentoring approach for disease self‐management in African American women with lupus with a social support control: study protocol for a randomized controlled trial. Trials 2019;20:529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Williams EM, Dismuke CL, Faith TD, et al. Cost‐effectiveness of a peer mentoring intervention to improve disease self‐management practices and self‐efficacy among African American women with systemic lupus erythematosus: analysis of the Peer Approaches to Lupus Self‐management (PALS) pilot study. Lupus 2019;28:937–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Drenkard C, Dunlop‐Thomas C, Easley K, et al. Benefits of a self‐management program in low‐income African‐American women with systemic lupus erythematosus: results of a pilot test. Lupus 2012;21:1586–93. [DOI] [PubMed] [Google Scholar]
- 102. Williams EM, Lorig K, Glover S, et al. Intervention to Improve Quality of life for African‐AmericaN lupus patients (IQAN): study protocol for a randomized controlled trial of a unique a la carte intervention approach to self‐management of lupus in African Americans. BMC Health Serv Res 2016;16:339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Williams EM, Penfield M, Kamen D, et al. an intervention to reduce psychosocial and biological indicators of stress in African American lupus patients: the Balancing Lupus Experiences with Stress Strategies study. Open J Prev Med 2014;4:22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Khan F, Granville N, Malkani R, et al. Health‐related quality of life improvements in systemic lupus erythematosus derived from a digital therapeutic plus tele‐health coaching intervention: randomized controlled pilot trial. J Med Internet Res 2020;22:e23868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Dantas LO, Weber S, Osani MC, et al. Mobile health technologies for the management of systemic lupus erythematosus: a systematic review. Lupus 2020;29:144–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Solati K, Mousavi M, Kheiri S, et al. The effectiveness of mindfulness‐based cognitive therapy on psychological symptoms and quality of life in systemic lupus erythematosus patients: a randomized controlled trial. Oman Med J 2017;32:378–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Abrahao MI, Gomiero AB, Peccin MS, et al. Cardiovascular training vs. resistance training for improving quality of life and physical function in patients with systemic lupus erythematosus: a randomized controlled trial. Scand J Rheumatol 2016;45:197–201. [DOI] [PubMed] [Google Scholar]
- 108. Anandarajah A. Designing an intervention to improve management of high‐risk lupus patients through care coordination. Rheum Dis Clin North Am 2020;46:723–34. [DOI] [PubMed] [Google Scholar]
- 109. Quinzanos I, Davis L, Keniston A, et al. Application and feasibility of systemic lupus erythematosus reproductive health care quality indicators at a public urban rheumatology clinic. Lupus 2015;24:203–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disclosure Form


