Abstract
Glycoside hydrolase (GH) family members act as virulence factors and regulate plant immune responses during pathogen infection. Here, we characterized the GH28 family member endopolygalacturonase VdEPG1 in Verticillium dahliae. VdEPG1 acts as a virulence factor during V. dahliae infection. The expression level of VdEPG1 was greatly increased in V. dahliae inoculated on cotton roots. VdEPG1 suppressed VdNLP1‐mediated cell death by modulating pathogenesis‐related genes in Nicotiana benthamiana. Knocking out VdEPG1 led to a significant decrease in the pathogenicity of V. dahliae in cotton. The deletion strains were more susceptible to osmotic stress and the ability of V. dahliae to utilize carbon sources was deficient. In addition, the deletion strains lost the ability to penetrate cellophane membrane, with mycelia showing a disordered arrangement on the membrane, and spore development was affected. A jasmonic acid (JA) pathway‐related gene, GhOPR9, was identified as interacting with VdEPG1 in the yeast two‐hybrid system. The interaction was further confirmed by bimolecular fluorescence complementation and luciferase complementation imaging assays in N. benthamiana leaves. GhOPR9 plays a positive role in the resistance of cotton to V. dahliae by regulating JA biosynthesis. These results indicate that VdEPG1 may be able to regulate host immune responses as a virulence factor through modulating the GhOPR9‐mediated JA biosynthesis.
Keywords: cell death, cotton, glycoside hydrolase, jasmonic acid, knock out, Verticillium dahliae
Verticillium dahliae secreted a virulence factor, VdEPG1, that interacted with GhOPR9, which is a jasmonic acid (JA) signal pathway gene in the plant cell membrane. GhOPR9 protected the plants against V. dahliae by regulating JA biosynthesis.

1. INTRODUCTION
The plant cell wall provides the first physical barrier against pathogen infection. To invade the host cell and supply themselves with nutrients, plant pathogens secrete cell wall‐degrading enzymes (CWDEs) to depolymerize plant cell walls (Kikot et al., 2009; Klockner et al., 2018). Some CWDEs act as virulence factors, such as the endo‐β‐1,4‐xylanase gene xyn11A of Botrytis cinerea (Brito et al., 2006), the endoxylanase gene xynB of Xanthomonas oryzae pv. oryzae (Rajeshwari et al., 2005), the sucrose nonfermenting 1 gene (VdSNF1) of Verticillium dahliae (Tzima et al., 2011), a pectate lyase gene VdPEL1 of V. dahliae (Yang et al., 2018), a Magnaporthe grisea cutinase gene CUT2 (Skamnioti & Gurr, 2007), the pectate lyase gene CcpelA of Colletotrichum coccodes (Ben‐Daniel et al., 2012), and the pectate lyase gene pelB of Colletotrichum gloeosporioides (Yakoby et al., 2001). Some CWDEs act as the inducers of immune responses by themselves or through degrading the plant cell wall into fragments (Hematy et al., 2009; Misas‐Villamil & van der Hoorn, 2008). For instance, polygalacturonase can degrade pectin to generate oligogalacturonides (OGs), and OGs are damage‐associated molecular patterns (DAMPs) that trigger plant defences (De Lorenzo et al., 2011; D'Ovidio et al., 2004; Prade et al., 1999). The xyloglucan‐specific endoglucanase PsXEG1 from Phytophthora sojae and the secreted xyloglucanase BcXYG1 from B. cinerea are pathogen‐associated molecular patterns (PAMPs) that activate plant immune responses (Ma et al., 2015; Zhang et al., 2014; Zhu et al., 2017). However, the mechanisms of hydrolytic enzymes of phytopathogens that can be recognized by host and trigger defence responses remain mostly unknown.
Glycoside hydrolases (GHs) are capable of hydrolytically cleaving glycosidic bonds in oligo‐ or polysaccharides, substrates that contain cellulose, hemicellulose, and pectin. In addition, these enzymes can cleave the linkage to side chains to remove modifications (such as methyl esters and acetylation) or to split linkages to lignin (Kubicek et al., 2014). Recently, it was reported that the GH12 protein PsXEG1 is required for virulence. Furthermore, soybean glucanase inhibitor protein 1 (GmGIP1) can interact with PsXEG1 to inhibit its enzyme activity and reduce P. sojae virulence. GmGIP1‐overexpressing soybean showed increased resistance to P. sojae (Ma et al., 2017). Six GH12 proteins have been characterized in V. dahliae V991, and two of these six proteins, VdEG1 and VdEG3, trigger cell death and PAMP‐triggered immunity (PTI) in Nicotiana benthamiana. VdEG1‐ and VdEG3‐triggered immunity needs the host protein BAK1, and VdEG1‐triggered immunity also needs the host protein SOBIR1 (Gui et al., 2017). Endopolygalacturonases, an important group of CWDEs, belong to the GH28 family with pectin‐degrading ability (Henrissat, 1991; Reignault et al., 2008). Many GH28 family members have been identified and characterized in various fungi. For example, BcPG1 and BcPG2 are important virulence factors for B. cinerea (Kars et al., 2005; Poinssot et al., 2003), an endoPG mutant of Alternaria citri causes light black rot symptoms on citrus (Isshiki et al., 2001), the endoPG homologue gene MGG_08938 of Magnaporthe oryzae is not associated with pathogenicity but is associated with conidial germination (Mori et al., 2008), and Lasiodiplodia theobromae endopolygalacturonase 1 (LtEPG1) plays important roles in pathogenesis, pectin degradation, and immune response regulation during successful infection (Thilini Chethana et al., 2020). However, the molecular of function of GH28 family members in V. dahliae is still unclear.
Jasmonic acid (JA) is a phytohormone that controls plant responses to various stresses, and regulates plant growth and development (Wasternack & Hause, 2013). The 12‐oxo‐phytodienoic acid reductases (OPRs) play a key role in JA biosynthesis (Liechti & Farmer, 2006; Schaller, 2001). In Arabidopsis thaliana, AtOPR3 modulates JA production and affects male gametophyte development (Stintzi & Browse, 2000). Overexpression of TaOPR1 in wheat enhances tolerance to salinity (Dong et al., 2013) and overexpression of AtOPR3 in wheat improves its freezing tolerance (Pigolev et al., 2018). Knockdown of SlOPR3 in Solanum lycopersicum increases susceptibility to B. cinerea (Scalschi et al., 2015). In upland cotton (Gossypium hirsutum), GhOPR3 regulates the resistance of cotton to V. dahliae by modulating JA biosynthesis (Hu et al., 2018).
V. dahliae is a plant vascular wilt fungal pathogen that causes a destructive disease on a large number of plant hosts, including high‐value crops such as cotton, tobacco, potato, and tomato (Fradin & Thomma, 2006; Klosterman et al., 2009). V. dahliae penetrates its host root and colonizes the plant xylem vessels. In recent years, many genes involved in pathogen penetration, stress response, and carbon source utilization have been characterized, including VdGAL4 (Wen et al., 2023), VdM35‐1, and VdASPF2 (Lv et al., 2022). In our previous study, we found that GhOPR9 can positively modulate the resistance of cotton to V. dahliae by regulating the JA pathway genes (Liu et al., 2020). To understand the GH28 members’ roles in V. dahliae, we identified GH28 members in V. dahliae, characterized a member, VdEPG1, involved in virulence, and confirmed the interaction between VdEPG1 and GhOPR9 in this study.
2. RESULTS
2.1. Identification and expression patterns of the GH28 family in the genome of V. dahliae
The Hidden Markov Model (HMM) of Glyco_hydro_28 (PF00295) was downloaded from the Pfam database to identify the GH28 family members using HMMER v. 3.1b2. We identified 11 GH28 family members in the V. dahliae strain VdLs.17 genome. These coding sequences of GH28s ranged from 1113 to 1410 nucleotides, with protein length ranging from 370 to 471 amino acids, isoelectric point pI from 4.86 to 8.68, and molecular weight from 37.9 to 52.6 kDa. The genes were distributed on seven chromosomes of V. dahliae. They all had a signal peptide, except VDAG_05974 (Table S1).
To investigate the relationships among GH28s in fungi, we used the 11 GH28 family members from V. dahliae, 11 GH28s from Fusarium oxysporum, 20 GH28s from C. gloeosporioides, 18 GH28s from B. cinerea, 19 GH28s from Phytophthora infestans, and two from M. oryzae (Table S2) to construct a phylogenetic tree (Figure S1a). The GH28s were divided into three groups. Group I contained nine VdGH28s, VDAG_09633 was classified into Group II, and VDAG_04977 was classified into Group III and showed a close relationship with MGG_08938 (Mori et al., 2008). All the GH28 family members in P. infestans were collected in Group III.
To further understand whether the VdGH28 gene expression levels were induced by cotton roots, we performed reverse transcription‐quantitative PCR (RT‐qPCR) to study the expression profiles of VdGH28 genes using a suspension of V991 spores that were treated with a cotton root extract or inoculated onto cotton roots (Figure S1b). The results showed that 10 of the VdGH28 genes were up‐regulated at 12 h postinfection (hpi) in the treated spores of V991, and VDAG_05974 was up‐regulated at 6 hpi; all VdGH28 gene expression levels increased to 24 hpi. In the inoculated root samples, nine VdGH28 genes were highly up‐regulated at 24 hpi and VDAG_04977 had increased by more than 48 times by 24 hpi; VDAG_05974 was not expressed in the roots. These results indicate that VdGH28 genes may play an important role in the virulence of V. dahliae.
2.2. The endopolygalacturonase VdEPG1 suppresses VdNLP1 protein‐induced cell death in N. benthamiana
To determine whether GH28 family genes in V. dahliae can induce cell death, an Agrobacterium‐mediated transient expression assay was used in N. benthamiana. The full‐length coding sequence of each GH28 family member was separately inserted into the PVX vector pGR107 and transformed into Agrobacterium tumefaciens GV3101. Transient expression of these genes in 6‐week‐old N. benthamiana leaves demonstrated that none of the VdGH28 family members induced cell death (Figure 1a). Semiquantitative RT‐PCR analysis of the total RNA extract from the agroinfiltrated leaf area confirmed the transcription of all VdGH28 genes in N. benthamiana (Figure 1b). We then co‐expressed VdNLP1 (NLP1) (Zhou et al., 2012) 24 h after infiltrating with the VdGH28 genes in 6‐week‐old N. benthamiana leaves. Cell death induced by NLP1 was only suppressed by VDAG_04977 (Figures 1c and S2). VDAG_04977 was named VdEPG1 (Table S1). VdEPG1 encodes an endopolygalacturuonase of 370 amino acids with a signal peptide from 1 to 18 amino acids (Figure S3). The yeast signal trap system assay showed that VdEPG1 is probably secreted into the extracellular space during infection (Figure 1d). Liquid chromatography mass spectrometry (LC–MS) was performed to test the endopolygalacturonase activity of purified VdEPG1 using pectin as a substrate. The results showed that VdEPG1 hydrolysed pectin to produce galacturonic acid (Figure S4).
FIGURE 1.

VdEPG1 suppresses the cell death induced by NLP1 in Verticillium dahliae. (a) Cell death assays for 11 V. dahliae genes in 6‐week‐old Nicotiana benthamiana leaves were performed by Agrobacterium‐mediated transient expression. Leaves were imaged 6 days after infiltration with Agrobacterium carrying the VdGH28 family genes. NLP1 and green fluorescent protein (GFP) were used as the positive and negative controls, respectively. Scale bar = 1 cm. (b) Semiquantitative reverse transcription‐PCR analysis of transiently expressed VdGH28 genes in N. benthamiana leaves 48 h after infiltration. NbActin was used as the control. (c) Detection of the cell death‐suppressing activity of VdEPG1. NLP1 was transiently expressed 24 h after infiltration with VdEPG1 in 6‐week‐old N. benthamiana leaves. Western blot analysis showed VdEPG1 was expressed in N. benthamiana leaves. 1, sample infiltrated with NLP1 only; 2, sample infiltrated with VdEPG1 only; 3, sample that was infiltrated with NLP1 24 h after infiltration with VdEPG1; 4, sample infiltrated with GFP only. Commassie brilliant blue (CBB) was used as the loading control. Scale bar = 1 cm. (d) Confirmation of the function of the signal peptide of VdEPG1 by yeast signal trap assay. Growth on YPRAA medium indicates the functionality of the signal peptide of VdEPG1. The signal peptide of Avr1b was used as the positive control. (e) and (f) Reverse transcription‐quantitative PCR analysis of the PAMP‐triggered immunity marker genes and pathogenesis‐related genes. N, E, and G represent NLP1, VdEPG1, and GFP, respectively. The blue, red, green, and black columns represent the samples which were infiltrated at 0, 12, 24 and 48 h, respectively. NbActin was used as a control. Values represent the mean ± standard deviation of three replicates. *p < 0.05, **p < 0.01, ***p < 0.001.
N. benthamiana leaves infiltrated with NLP1, VdEPG1 and NLP1, VdEPG1, or green fluorescent protein (GFP) were collected at 0, 12, 24 and 48 h postinfiltration (hpi) for reverse transcription‐quantitative PCR (RT‐qPCR) analysis. NbAcre31 (Kiba et al., 2018) and NbCYP71D20 (Heese et al., 2007) as PTI marker genes, and NbLOX, NbPR4 (Asai & Yoshioka, 2009), and NbWRKY7 (Ishihama et al., 2011) as pathogenesis‐related genes were selected. They were all significantly activated by NLP1 treatment (Figure 1e,f). NbAcre31, NbLOX, NbPR4, and NbWRKY7 were suppressed by co‐expressing NLP1 with VdEPG1 at 48 hpi, and NbAcre31, NbLOX, NbPR4, and NbWRKY7 expression levels in infiltrated VdEPG1 leaves were higher than those co‐infiltrated with NLP1 and VdEPG1 (Figure 1e,f). These results suggest that VdEPG1 can suppress the immune responses induced by NLP1.
2.3. VdEPG1 mutation causes sensitivity to osmotic stress
To investigate the function of VdEPG1 in the development and pathogenicity of V. dahliae, the knockout mutant ∆VdEPG1 was constructed using the homologous recombination method (Figure S5). Two independent deletion mutants, ∆VdEPG1‐8 and ∆VdEPG1‐11, were obtained and verified by PCR analysis (Figure S6a). In addition, the wild‐type VdEPG1 was reintroduced into the deletion mutants, yielding the complemented strains C‐∆VdEPG1‐8 and C‐∆VdEPG1‐11 (Figure S6b).
Compared with the wild‐type strain V991 (WT), the ∆VdEPG1 deletion strains were slightly reduced in mycelial growth on potato dextrose agar (PDA) (Figure 2a,b). In the presence of KCl, NaCl or sorbitol, the ∆VdEPG1‐8 and ∆VdEPG1‐11 strains were significantly reduced in radial growth. Under KCl stress especially, the growth of ∆VdEPG1‐8 and ∆VdEPG1‐11 strains was reduced almost six‐fold relative to the WT. In the presence of sodium dodecyl sulphate (SDS) or Congo red, the deletion strains showed no obvious sensitivity. Interestingly, the deletion strains grew slightly more in the presence of SDS compared with those on PDA. In addition, the deletion strains produced significantly fewer conidia than WT and complemented strains (Figure 2c). These results suggested that VdEPG1 participated in regulating the response to osmotic stress.
FIGURE 2.

VdEPG1 plays an important role in osmotic stress resistance and utilizing carbon sources. (a) Phenotype analysis of the wild‐type (WT), deletion mutant strains ∆VdEPG1‐8 and ∆VdEPG1‐11, and complemented strains C‐∆VdEPG1‐8 and C‐∆VdEPG1‐11 grown on potato dextrose agar (PDA) or PDA supplemented with 1 M KCl, 1 M NaCl, 1 M sorbitol, 0.002% sodium dodecyl sulphate (SDS), and 0.02% Congo red (CR) for 14 days. Scale bar = 1 cm. (b) The mycelial relative growth of the WT, ∆VdEPG1‐8, ∆VdEPG1‐11, C‐∆VdEPG1‐8, and C‐∆VdEPG1‐11 strains on PDA or supplemented PDA per day. (c) The spore concentration of the WT, ∆VdEPG1‐8, ∆VdEPG1‐11, C‐∆VdEPG1‐8, and C‐∆VdEPG1‐11 strains grown in liquid Czapek Dox medium for 3 days. Values represent the mean ± standard deviation of three replicates. (d) Phenotype analysis of the WT, ∆VdEPG1‐8, ∆VdEPG1‐11, C‐∆VdEPG1‐8, and C‐∆VdEPG1‐11 strains grown on Czapek Dox medium with sucrose, galactose, raffinose, pectin, or starch as the sole carbon source, or with no carbon source (W–S), for 14 days. Scale bar = 1 cm. (e) The mycelial relative growth of the WT, ∆VdEPG1‐8, ∆VdEPG1‐11, C‐∆VdEPG1‐8, and C‐∆VdEPG1‐11 strains on Czapek Dox medium per day. Values represent the mean ± standard deviation of three replicates. The asterisks represent significant differences performed by a t test (*p < 0.05, **p < 0.01) in comparison with the WT strain.
2.4. VdEPG1 is required to use different carbon sources
To analyse the ability of mutant strains to use different carbon sources, the mycelial radial growth of WT, deletion and complemented strains was performed using Czapek Dox solid medium with sucrose, galactose, raffinose, pectin, or starch as the sole carbon source. The results showed that the mycelial growth of all strains was significantly decreased on Czapek Dox medium without a carbon source (W‐S; Figure 2d,e). The radial growth of deletion strains was significantly reduced on Czapek Dox medium containing sucrose, pectin, raffinose, and starch compared to the WT. In the presence of galactose, the colony diameter was not significantly different among the different strains. These results suggest that VdEPG1 participates in carbon utilization.
2.5. VdEPG1 is required for full virulence on cotton
To examine the role of VdEPG1 in virulence, G. hirsutum ‘Jimian No. 11’ was used to perform the pathogenicity tests with the WT, ∆VdEPG1‐8 and ∆VdEPG1‐11 strains, and complemented strains C‐∆VdEPG1‐8 and C‐∆VdEPG1‐11. After inoculation by conidial suspensions of WT and complemented strains, the seedlings showed severe wilting and chlorosis of the leaves, and even defoliation, whereas the cotton plants inoculated with the deletion strains showed only slight wilting symptoms (Figure 3a). Fungal recovery assays from the stem sections demonstrated that the deletion strains still colonized the cotton plants but, compared to the plants infected by the WT and complemented strains, there was reduced recovery of fungal colonies from the stem sections on PDA (Figure 3b). Vascular discolouration, a typical symptom of Verticillium wilt (Klosterman et al., 2009), was observed. As expected, the plants inoculated with the ∆VdEPG1‐8 and ∆VdEPG1‐11 strains showed slight discolouration, while the plants infected with the WT and complemented strains displayed dark discolouration (Figure 3c). The disease index (DI) of plants infected with the deletion strains was significantly lower than that of plants infected with the WT and complemented strains (Figure 3d). In addition, fungal biomass analysis of the inoculated cotton stems was performed by quantitative PCR (qPCR) and the results showed that the biomass of the deletion strains was significantly lower than that of the WT and complemented strains (Figure 3e). These results show that VdEPG1 plays an important role in the virulence of V. dahliae.
FIGURE 3.
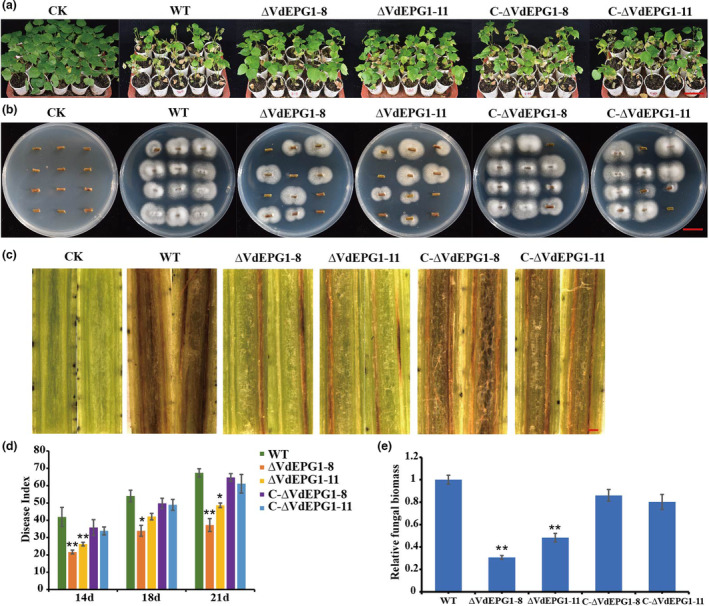
VdEPG1 plays a positive role in the virulence of Verticillium dahliae. (a) Disease symptoms of cotton after inoculation with wild‐type (WT), deletion mutants ∆VdEPG1‐8 or ∆VdEPG1‐11, and complemented strains C‐∆VdEPG1‐8, C‐∆VdEPG1‐11, or water (CK). Photographs were taken at 21 days after inoculation. Scale bar = 10 cm. (b) Reisolation of V. dahliae strains from the stem of cotton plants on potato dextrose agar inoculated at 25°C for 5 days. Scale bar = 1 cm. (c) Disease symptoms in stems of cotton 21 days after inoculation with WT, ∆VdEPG1‐8, ∆VdEPG1‐11, C‐∆VdEPG1‐8, and C‐∆VdEPG1‐11 strains. Scale bar = 0.1 cm. (d) Disease index of cotton plants at 14, 18, and 21 days after inoculation with WT, ∆VdEPG1‐8, ∆VdEPG1‐11, C‐∆VdEPG1‐8, and C‐∆VdEPG1‐11 strains. (e) Relative fungal biomass in stems of cotton 21 days after inoculation with WT, ∆VdEPG1‐8, ∆VdEPG1‐11, C‐∆VdEPG1‐8, and C‐∆VdEPG1‐11 strains. Values represent the mean ± standard deviation of three replicates. The asterisks represent significant differences by a t test (*p < 0.05, **p < 0.01) in comparison with the WT strain.
2.6. VdEPG1 is required for penetration of cellophane membrane
To investigate the role of VdEPG1 in the process of initial colonization by V. dahliae, the penetration ability of hyphae through a cellophane membrane laid on PDA was examined. The deletion strains showed similar colony morphology to that earlier displayed in Figure 2a on complete medium (CM) (Figure 4a). The colony diameter of the deletion strains was smaller than that of the WT and complemented strains at 3 days postinoculation (dpi). In addition, the hyphae of the deletion strains failed to penetrate the cellophane membrane and were not observed on the medium when the cellophane membrane was removed at 5 days postinoculation (dpi). However, the WT and complemented strain hyphae penetrated the cellophane membrane, and the hyphae were observed after removing the cellophane membrane at 5 dpi (Figure 4a). The hyphae on the cellophane membrane were observed under scanning electron microscopy. The mycelia of the WT and complemented strains showed normal and uniform arrangements, but the mycelia of deletion strains displayed a disordered arrangement (Figure 4b) and, the spores of mutant strains were altered (long spherical with rugged surface; Figure 4b). These results indicate that VdEPG1 can regulate the penetration abilities of hyphae during V. dahliae infection.
FIGURE 4.

The cellophane membrane penetration assay. (a) Penetration symptoms of the wild‐type (WT), deletion strains ∆VdEPG1‐8 and ∆VdEPG1‐11, and complemented strains C‐∆VdEPG1‐8, and C‐∆VdEPG1‐11 grown on potato dextrose agar (PDA) overlaid with a cellophane membrane (above) for 3 days and then after membrane removal for 5 days (below). P, the positive side of the PDA; N, the negative side of the PDA. Scale bar = 1 cm. (b) Scanning electron microscopy observation of mycelia and conidia on cellophane membrane. Scale bar = 100 μm.
2.7. VdEPG1 interacts with GhOPR9 in vitro and in vivo
To gain insights into the regulatory network of VdEPG1, the coding sequence VdEPG1 SP was cloned into the pGBKT7 vector to be used as a bait in a yeast two‐hybrid (Y2H) assay to screen a V. dahliae‐inoculated cotton root cDNA library. GhOPR9 (GH_D05G1193), a 12‐oxo‐phytodienoic acid reductase (OPR), was identified as an interacting protein of VdEPG1 (Figure 5a). As found in a previous study, GhOPR9 participates in regulating the resistance of cotton to V. dahliae through mediating the jasmonic acid (JA) pathway genes (Liu et al., 2020). In N. benthamiana, VdEPG1‐GFP was located in the plasma membrane and GhOPR9‐GFP was located in both the plasma membrane and the cytoplasm (Figure S7a). In onion epidermis, VdEPG1 was located in the plasma membrane, GhOPR9 was located in both the plasma membrane and cytoplasm, in accordance with that in N. benthamiana leaves (Figure S7b). The subcellular localization of GhOPR9‐GFP and VdEPG1‐RFP showed that VdEPG1 and GhOPR9 co‐located in the plasma membrane (Figure S7c).
FIGURE 5.
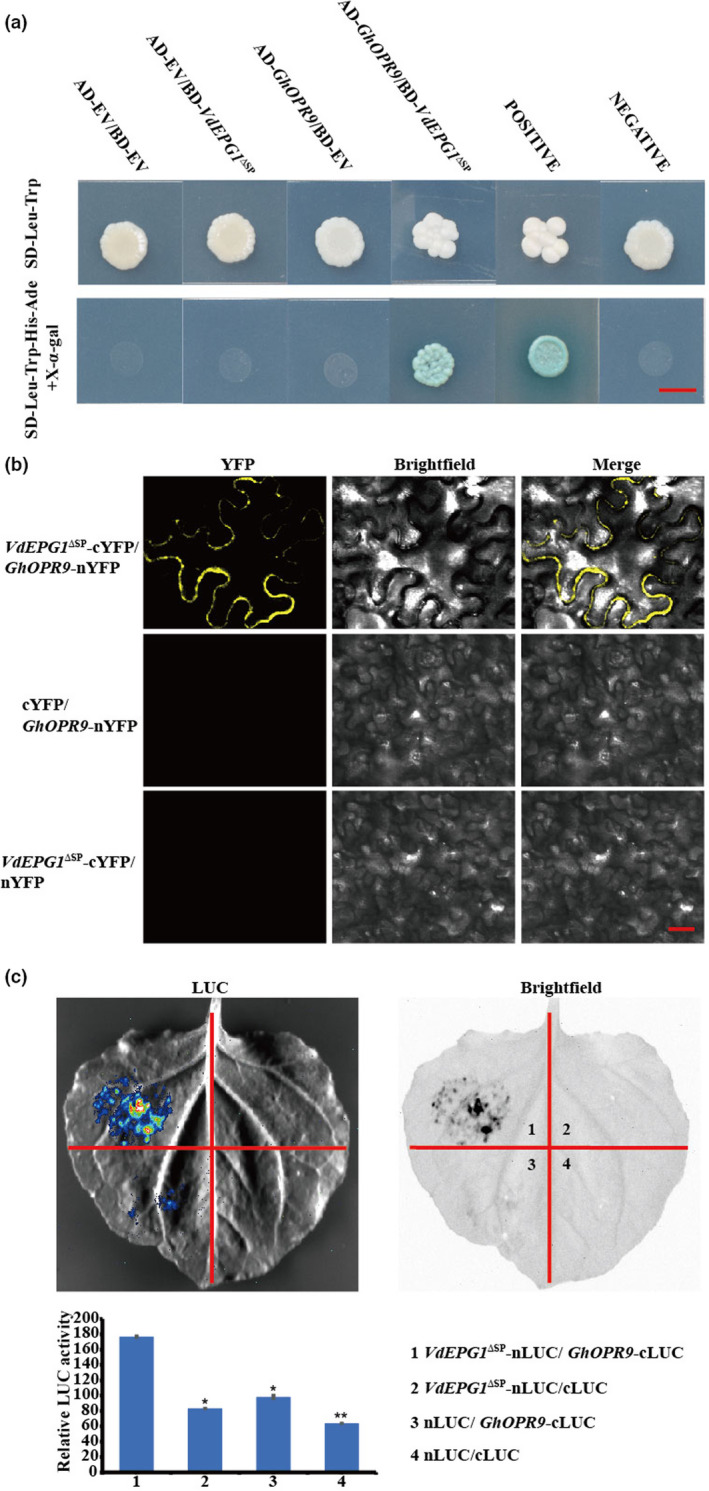
VdEPG1 interacts with GhOPR9. (a) The interaction between VdEPG1 and GhOPR9 was confirmed by yeast‐two‐hybrid assays. Scale bar = 0.5 cm. (b) Bimolecular fluorescence complementation assay showing that the interaction between GhOPR9‐nYFP and VdEPG1‐cYFP forms a functional yellow fluorescent protein (YFP) in the plasma membrane. Scale bar = 100 μm. (c) The interaction between VdEPG1 and GhOPR9 was confirmed by luciferase (LUC) assays in Nicotiana benthamiana leaves. The LUC signal was recorded in darkfield and brightfield. The experiment was repeated three times with similar results. Statistical analyses were performed using Student's t test: *p < 0.05, **p < 0.01.
Bimolecular fluorescence complementation (BiFC) assays were performed to test the interaction between VdEPG1 and GhOPR9 in N. benthamiana plant cells. VdEPG1‐SP‐cYFP and GhOPR9‐nYFP were co‐expressed in N. benthamiana leaves. Yellow fluorescent protein (YFP) was observed at the plasma membrane under laser confocal scanning microscopy at 488 nm, and The co‐infiltrated of cYFP with GhOPR9‐nYFP, or of VdEPG1‐SP‐cYFP with nYFP did not produce fluorescence (Figure 5b). In addition, a luciferase assay was used to verify the interaction between VdEPG1 and GhOPR9 in planta (Figure 5c). These results show that VdEPG1 interacts with GhOPR9 both in vitro and in vivo.
2.8. GhOPR9 plays a positive role in resistance to V. dahliae
To study the role of GhOPR9 in resistance against V. dahliae, cotton plants were infiltrated with the virus‐induced gene silencing (VIGS) constructs TRV:GhOPR9‐CDS, TRV:GhOPR9‐5′UTR, TRV:00, and TRV:GhPDS. When systemically infected leaves of the TRV:GhPDS lines displayed a photobleaching phenotype (Figure 6a), the gene‐silencing efficiency of TRV:GhOPR9‐CDS, TRV:GhOPR9‐5′UTR, and TRV:00 plants was measured by RT‐qPCR. The results showed that GhOPR9 was successfully silenced (Figure 6b). The silenced plants were inoculated with V. dahliae V991. The TRV:GhOPR9‐CDS and TRV:GhOPR9‐5′UTR lines displayed more severe symptoms than TRV:00 plants (Figure 6c,d). DI analysis, fungal biomass assays, and callose deposition assays showed that resistance to V. dahliae was more impaired in TRV:GhOPR9‐CDS lines than in TRV:GhOPR9‐5′UTR plants (Figure 6e–g). The transcription levels of genes of involved in JA synthesis increased during the early infection stages in GhOPR9‐silenced lines (Figure S8). The JA content accumulation decreased in silenced cotton and that in the TRV:GhOPR9‐CDS lines was lower than in TRV:GhOPR9‐5′UTR plants (Figure 6h).
FIGURE 6.
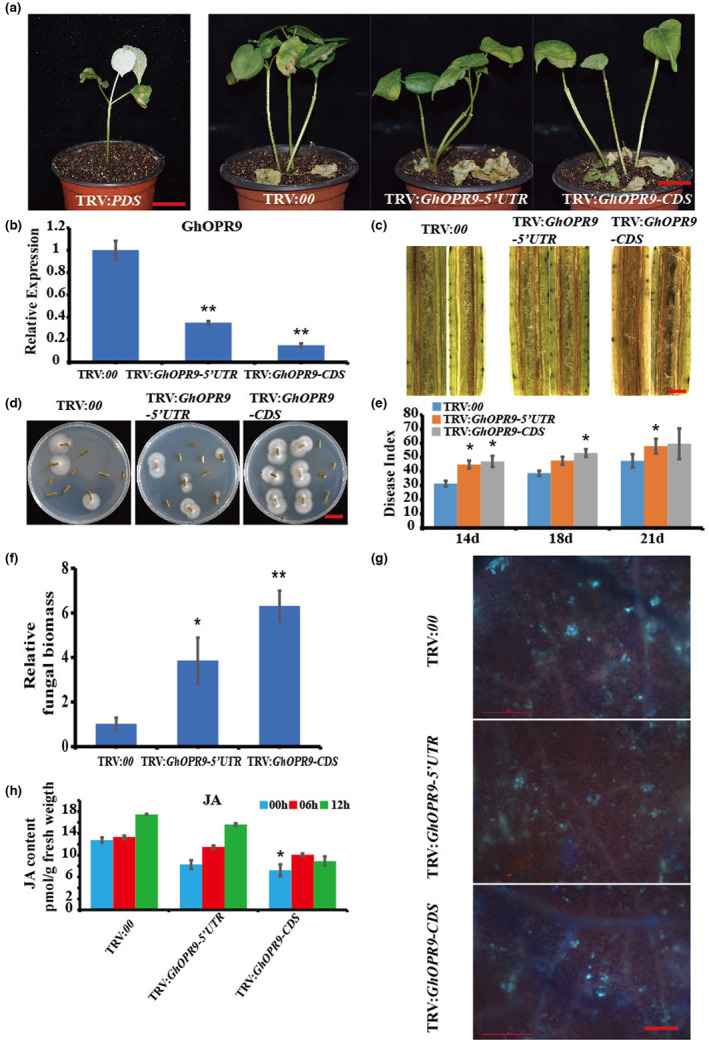
GhOPR9 positively regulates cotton resistance against Verticillium dahliae. (a) Disease symptoms of the cotton plants after V. dahliae V991 infection. Photographs were taken at 21 days after inoculation. TRV:PDS was used as the control to evaluate virus‐induced gene silencing. Scale bar = 2 cm. (b) The expression level of GhOPR9 in the treated plants. Total RNA was isolated from roots at 10 days postagroinfiltration. GhUB7 was used as the reference. Each experiment was performed using three independent replicates. (c) Disease symptoms in the stems of the treated plants at 21 days after V991 inoculation. Scale bar = 0.1 cm. (d) Reisolation of V. dahliae from the stem of cotton plants on potato dextrose agar inoculated at 25°C for 5 days. Scale bar = 1 cm. (e) Disease index of the treated plants at 14, 18, and 21 days after inoculation with V991. Each experiment was performed using three replicates. (f) Quantitative PCR analysis of the relative fungal biomass in stems of the treated plants at 21 days after V991 inoculation. Each experiment was performed using three replicates. (g) Callose deposition in leaves of the treated plants at 21 days after V991 inoculation. Leaves were stained with aniline blue and imaged using fluorescence microscopy. Scale bar = 200 μm. (h) GhOPR9 manipulates jasmonic acid (JA) biosynthesis in cotton. JA contents in roots from TRV:00, TRV:GhOPR9‐5′UTR, and TRV:GhOPR9‐CDS plants at 0, 6, and 12 h after V991 infection. Values are the mean ± standard deviation, n = 6. Statistical analyses were performed using Student's t test: *p < 0.05, **p < 0.01.
We also examined the effect of overexpression of GhOPR9 in Arabidopsis plants (Figure 7a,b). GhOPR9 was detected in the overexpression line by western blotting (Figure S9). After inoculation with V. dahliae V991, the Arabidopsis overexpressing GhOPR9 showed more resistance to V. dahliae (Figure 7b,c) and less fungal biomass was detected (Figure 7d). These results indicate that GhOPR9 plays a positive role in resistance to V. dahliae by modulating JA biosynthesis.
FIGURE 7.

Overexpression of GhOPR9 enhances Arabidopsis thaliana resistance to Verticillium dahliae. (a) Disease symptoms of A. thaliana plants after V. dahliae V991 infection. Photograph was taken at 14 days after inoculation. Scale bar = 1 cm. (b) The percentage of total number of A. thaliana leaves showing necrosis at 14 days after V991 infection. (c) Quantitative PCR analysis of the relative fungal biomass in leaves of the treatment plants at 14 days after V991 inoculation. Each experiment was performed using three replicates. Differences between groups were compared using the t test (*p < 0.05). OE, overexpression.
3. DISCUSSION
Compared to vertebrates, plants lack specialized immune cells to protect themselves from pathogens. Instead, plants have evolved sophisticated defence systems to make sure every plant cell has an effective immune response (Spoel & Dong, 2012). When phytopathogens attack the host, they release PAMPs or microbe‐associated molecular patterns (MAMPs), such as bacterial flagellin, fungal chitin, lipopolysaccharides, and peptidoglycans (Spoel & Dong, 2012; Zipfel, 2014). The first defence occurs at the plant cell wall, which is composed of complicated components, including pectin, cutin, cellulose, wax, and callose (Zipfel, 2014). The pattern‐recognition receptors (PRRs) that are localized at the plant cell surface perceive PAMPs or MAMPs and trigger downstream immunity responses, including cell death, reactive oxygen species (ROS) bursts, callose deposition, calcium ion (Ca2+) accumulation, and induction of defence‐related genes (Boller & Felix, 2009; Monaghan & Zipfel, 2012; Zipfel, 2009). This is called PTI. Furthermore, plant pathogens secrete effector proteins into plant cells to circumvent PTI (Dangl & Jones, 2001; Zipfel, 2014). Some effectors counter the PTI as virulence factors, inducing effector‐triggered susceptibility (ETS), and some effectors that are recognized by host surveillance systems lead to effector‐triggered immunity (ETI) (Dangl et al., 2013; Dodds & Rathjen, 2010). Nevertheless, the plant immune system during plant–pathogen interactions remains largely unknown.
In this study, we identified 11 glycoside hydrolase family 28 (GH28) members in the V. dahliae genome. When the cotton root was infected by V. dahliae V991, the expression of all GH28 genes was up‐regulated in the spore suspension, which indicates that the genes make a contribution to the virulence of V. dahliae. The expression level of VDAG_04977 was highest in the infected roots. This result suggests that VDAG_04977 plays an important role during infection. Many GH family members can induce cell death and trigger plant defence responses, such as XEG1 from P. sojae (Ma et al., 2015), EG1 and EG3 from V. dahliae (Gui et al., 2017), and EPG1 from L. theobromae (Thilini Chethana et al., 2020). In this study, an Agrobacterium‐mediated transient expression assay showed that the GH28 genes in V. dahliae did not induce cell death in N. benthamiana leaves, but VdEPG1 (VDAG_04977) suppressed cell death induced by NLP1. Furthermore, the pathogenesis‐related genes NbAcre31, NbCYP71D20, NbLOX, NbPR4, and NbWRKY7 activated by NLP1 were suppressed when VdEPG1 was co‐expressed with NLP1 in N. benthamiana leaves. In addition, VdEPG1 suppressed cell death induced by INF1 (Figure S10). These results suggest that VdEPG1 may function as virulence factor to suppress some plant immunity pathways during infection.
Many polygalacturonases have been confirmed to be virulence factors, for example Pg1 in F. oxysporum (Di Pietro & Roncero, 1998), BcPG1 and BcPG2 from B. cinerea (Kars et al., 2005; Poinssot et al., 2003), and EPG1 of L. theobromae (Thilini Chethana et al., 2020). Like these, VdEPG1 was also demonstrated to be a virulence factor. The pathogenicity of WT strains, ∆VdEPG1‐8 and ∆VdEPG1‐11 strains, complemented strains C‐∆VdEPG1‐8, and C‐∆VdEPG1‐11 showed that VdEPG1 may play a positive role in the virulence of V. dahliae. MGG_08938 (MDG1) is dispensable in the virulence of M. oryzae (Mori et al., 2008). A previous study showed that LtEPG1 has no influence on fungal morphology and colony characteristics (Thilini Chethana et al., 2020). Compared to LtEPG1, VdEPG1 influenced radial growth. These results suggest that the function of EPG1 in fungi appeared to be species‐specific. V. dahliae can live in soil for many years. In recent years, some genes have been demonstrated to be involved in response to various stresses. The VdGAL4 deletion mutants are more sensitive to SDS and sorbitol, and exhibit reduced pathogenicity of V. dahliae (Wen et al., 2023). VdPbs2 and VdSsk1 have also been shown to be involved in the response to high osmolarity stress (Tian et al., 2016; Zheng et al., 2019). VdPbs2 and VdSsk1 deletion mutants exhibit reduced virulence on plants. In our research, the stress treatment assays confirmed that deletion of VdEPG1 causes sensitivity to osmotic stresses, such as KCl, NaCl, and sorbitol, with significantly decreased mycelial growth on PDA under osmotic stresses. These results suggest that VdEPG1 may play an important role against osmotic stresses in soil during infection.
Many studies have shown that some genes participate in regulating the development of V. dahliae and affect the pathogenicity of V. dahliae in plants (Lv et al., 2022; Zhang et al., 2022). To counter plant cell wall protection, pathogenic fungi should degrade host cell walls. It is important to study the effect and utilization of cell wall components, including pectin, cellulose, lignin, and xylan (Vorwerk et al., 2004). VdOGDH deletion mutants grow slowly in medium with sucrose, pectin, xylan, starch, and galactose as the sole carbon source, indicating that VdOGDH is important for vegetative growth and carbon utilization of V. dahliae (Li et al., 2020). VdGAL4 deletion mutants exhibit reduced utilization of different carbon sources, such as raffinose and sucrose (Wen et al., 2023), but ΔVdHP1 strains were significantly increased on a medium with starch, cellulose, and skim milk, suggesting that VdHP1 might negatively regulate the utilization of skimmed milk, cellulose, and starch in V. dahliae. VdEPG1 encodes an endopolygalacturonase and can hydrolyse pectin to produce galacturonic acid (Gal‐A). ΔVdEPG1 strains showed significantly decreased growth on medium containing sucrose, pectin, raffinose, and starch. Furthermore, the penetration ability of hyphae plays a key role in the initial plant colonization by V. dahliae. Strains with deletions of VdNoxB, VdPls1, and VdSte11 cannot penetrate cellophane membranes, produce defective hyphopodia, and display significantly decrease virulence (Yu et al., 2019; Zhao et al., 2016). In this study, ∆VdEPG1 strains could not penetrate a cellophane membrane, and had a disordered mycelium arrangement, affected spore development, and showed reduced pathogenicity. These results indicate that VdEPG1 makes a contribution to the pathogenicity of V. dahliae as a virulence factor.
The yeast signal sequence trap system assay result suggested that VdEPG1 could be secreted by V. dahliae during infection. Furthermore, GhOPR9 was shown to interact with VdEPG1 by yeast two‐hybrid assay. As in a previous study, the GhOPR9 expression level increased more than 100‐fold at 24 h after V. dahliae inoculation of cotton root (Liu et al., 2020). In this study, VdEPG1 was also induced by cotton roots and the GhOPR9 expression level in cotton roots after infection with ∆VdEPG1 strains was down‐regulated (Figure S11a). These results indicate that VdEPG1 may induce GhOPR9 expression during infection. In a recent study, GhOPR3 interacted with GhCPK33 in the peroxisomes, and GhOPR3‐GFP and GhCPK33‐GFP were colocalized in peroxisomes in N. benthamiana leaves (Hu et al., 2018). In this research, GhOPR9 was localized in the plasma membrane and cytoplasm, VdEPG1 was localized in the plasma membrane in N. benthamiana leaves, and the interaction between VdEPG1 and GhOPR9 occurred in the plasma membrane in N. benthamiana leaves. These results indicate that OPR members that participate in cotton resistance to V. dahliae may have different targets in different spaces.
We silenced the 5′ untranslated region (UTR) sequences of GhOPR9 in this research. The TRV:GhOPR9‐5′ UTR lines displayed more resistance to V. dahliae than the TRV:GhOPR9‐CDS lines. This result suggests that these tandem duplication genes may have functional redundancy in cotton resistance to V. dahliae. Silencing of SlOPR3 in tomato can interrupt JA biosynthesis, lead to the expression of other JA synthesis reduction genes, and show more susceptibility to B. cinerea (Scalschi et al., 2015). In cotton, reduced GhOPR3 protein stability leads to a decrease in JA content and hence more susceptibility to V. dahliae (Hu et al., 2018). Silencing of GhOPR9 can affect the JA pathway genes and regulate the resistance of cotton to V. dahliae (Liu et al., 2020). In this study, the JA content was also reduced in GhOPR9‐silenced lines, which showed more susceptibility to V. dahliae. The transcriptional levels of other JA synthesis genes were increased in GhOPR9‐silenced lines and the JA content was reduced in GhOPR9‐silenced lines. OPR3‐mediated JA biosynthesis is the main pathway to regulate rapid and dramatic JA accumulation following pathogen challenge (Ruan et al., 2019). Our target gene GhOPR9 is a homologue of AtOPR2 (Liu et al., 2020). OPR2‐dependent JA biosynthesis is the subsidiary pathway (Chini et al., 2018). So when GhOPR9 was silenced in cotton, other genes of the JA biosynthesis pathway were increased, but the JA biosynthesis pathway was still broken and JA content was decreased. We also examined GhCPK33 expression level in plants by inoculation with WT and VdEPG1 mutant strains. GhCPK33 was reduced after infection and it was expressed more highly in the plants with VdEPG1 mutant strain infection (Figure S11b). This is consistent with a previous study that showed that GhCPK33 negatively regulates defence against V. dahliae (Hu et al., 2018). When GhOPR9 was reduced in plants by deletion mutant strain infection, GhOPR3 increased. To counter the JA accumulation caused by GhOPR3, GhCPK33 was up‐regulated. As shown in a previous study, LtEPG1 interacts with KINβ1 and manipulates the host immune response by interfering with the KINβ1‐mediated signalling pathway (Thilini Chethana et al., 2020). In this research, VdEPG1 was confirmed to interact with GhOPR9. Interestingly, the expression level of GhOPR9 was decreased with ∆VdEPG1 strain inoculation, and the JA content accumulation in cotton plants that were infected by the ∆VdEPG1 strain was higher than plants that were infected by WT and C‐∆VdEPG1 strains (Figure S11c). This result is consistent with Figure 6h. We suggest that when the GhOPR9 expression level in cotton was decreased, the main JA biosynthesis pathway genes were increased to regulate the JA content accumulation against V. dahliae infection. These results indicate that VdEPG1 may modulate the GhOPR9‐mediated JA biosynthesis pathway to regulate the resistance of cotton to V. dahliae. However, the mechanism by which VdEPG1 interferes with the GhOPR9‐mediated JA biosynthesis pathway needs to be further studied.
In summary, our results indicate that VdEPG1, a GH28 family member of V. dahliae, is a virulence factor. VdEPG1 can suppress the host immune responses caused by VdNLP1. VdEPG1 can help V. dahliae to infect successfully. VdEPG1 may regulate the ability of V. dahliae to respond to osmotic stresses in soil and affect the mycelial arrangement in the host root surface during infection. VdEPG1 interacts with the JA biosynthesis pathway protein GhOPR9, which plays a positive role in resistance to V. dahliae. These results suggest that VdEPG1 acts as a virulence factor and interferes with GhOPR9‐mediated JA biosynthesis to regulate host resistance to V. dahliae (Figure 8).
FIGURE 8.
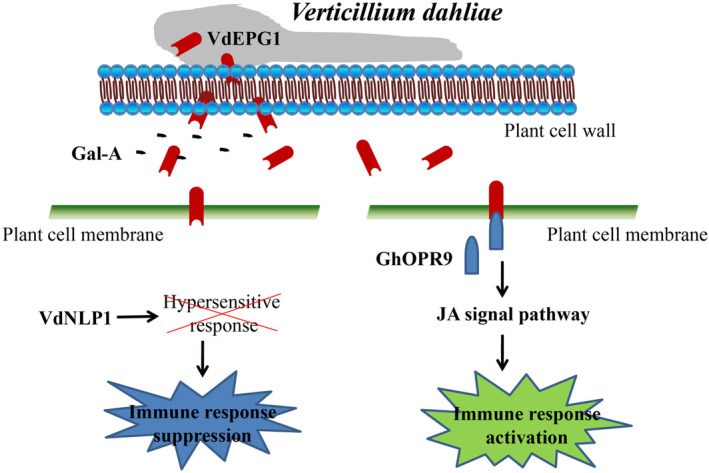
Schematic model of the interaction between VdEPG1 and GhOPR9. Verticillium dahliae secretes a virulence factor VdEPG1. VdEPG1 can locate the plant cell membrane, hydrolyse pectin to produce galacturonic acid (Gal‐A), and suppress the immune responses induced by NLP1. It is a positive regulator of V. dahliae virulence. GhOPR9, which is a component of the jasmonic acid (JA) signal pathway, interacts with VdEPG1 in the plant cell membrane and protects the plant against V. dahliae.
4. EXPERIMENTAL PROCEDURES
4.1. Fungal strain, plant material, and culture conditions
Verticillium dahliae V991 was cultured as described previously (Zhu et al., 2013). The fungus was cultured in liquid Czapek Dox medium or on PDA for 7 days at 25°C. Cotton plants (G. hirsutum ‘Jimian No. 11’) were grown in a greenhouse at 28°C with a 16 h/8 h day/night cycle. A. thaliana seedlings were grown in pots in a growth chamber at 24°C with a 16 h/8 h day/night cycle. N. benthamiana plants were grown at 25°C for 6 weeks for transient expression in greenhouse under a 14 h/10 h day/night regime.
4.2. PVX vector construction and transient expression assay
The full‐length coding sequences of the VdGH25 genes were amplified from cDNA of V. dahliae using specific primers. All sequences were integrated into the PVX vector pGR107 at the ClaI‐XmaI sites using the In‐Fusion HD Cloning Kit (Clontech). These PVX plasmids were transformed into Agrobacterium tumefaciens GV3101 and they were transiently expressed on N. benthamiana leaves using VdNLP1 (NLP1; Zhou et al., 2012) and GFP as positive and negative controls, respectively. To test the ability of VdEPG1 in suppression of cell death, A. tumefaciens cells carrying NLP1 were infiltrated 24 h after infiltration with VdEPG1. The leaves were collected at four time points (0, 12, 24, and 48 h) after Agrobacterium infiltration for RNA extraction. Symptoms were imaged at 6 dpi. Each assay was performed on three leaves from three individual plants and repeated three times. The primers used are listed in Table S3.
4.3. Yeast signal sequence trap system
The function of the predicted signal peptide VdEPG1 was verified as described previously (Jacobs et al., 1997). The predicted signal peptide of VdEPG1 was inserted into the vector pSUC2. The signal peptide of Avr1b was cloned as the positive control into pSUC2. Constructs pSUC2‐EPG1SP and pSUC2‐Avr1bSP were transformed into the yeast strain YTK12 and screened on CMD−W medium. The transformants were incubated on YPRAA medium (2% raffinose). The empty pSUC2 vector was used as the negative control. The primers used are listed in Table S3.
4.4. Fungal transformations
Homologous recombination was used to generate the ∆VdEPG1 mutants and complemented strains (Su et al., 2017). Flanking sequences of VdEPG1 (about 1.1 kb upstream and downstream) from genomic DNA of V991 and the hygromycin resistance cassette (HPH) were amplified, and these sequences were integrated into the knockout vector B303 via overlapping sequences used the In‐Fusion HD Cloning Kit (Clontech). The complementary plasmid construction was performed as described previously (Su et al., 2017). The 1.5‐kb promoter of VdEPG1 and complementary DNA of VdEPG1 were inserted into the pCAMBIA1302‐Neo vector with the geneticin resistance cassette. The knockout construct B303‐HPH‐VdEPG1 and complementation construct pCAMBIA1302‐Neo‐VdEPG1 were used to transform V. dahliae protoplasts as described previously (Rehman et al., 2016). Transformants were selected based on vector antibiotic resistance and confirmed by RT‐PCR. The primers used are listed in Table S3.
4.5. Growth of mutants on stress treatments
To examine mutants' sensitivity to osmotic stress, 1 M KCl, 1 M NaCl, or 1 M sorbitol were added to the PDA and the cultures were incubated for 14 days. For the cell wall stress assays, all strains were cultured on PDA containing 0.002% SDS or 0.02% Congo red for 14 days, as described previously (Zheng et al., 2019). The experiment was repeated six times.
4.6. Carbon source utilization assays
To analyse carbon source utilization by deletion strains, complementation strains, and WT, sucrose (30 g/L), galactose (10 g/L), pectin (10 g/L), raffinose (10 g/L), or starch (17 g/L) were mixed in Czapek Dox medium without sucrose for 14 days as described previously. The experiment was repeated six times.
4.7. Pathogenicity and penetration assays
All the strains, including deletion strains, complementation strains, and WT strain V991, were cultured as described previously (Zhu et al., 2013). The conidial suspensions were diluted to 107 spores/mL. Susceptible Jimian No. 11 cotton plants were used to assess the virulence of all strains. Seedlings' roots of plants at the two‐true‐leaf stage were dipped into conidial suspensions for 10 min as described previously (Li et al., 2019). The roots and leaves were collected at four time points (0, 12, 24, and 48 h) after inoculation for further study. The DI was calculated as described previously (Wei et al., 2019). The vascular wilt symptom of cotton was microscopically observed using seedling stems that were cut from each line at the same position at 21 dpi. Fungal recovery was performed using the stem sections of cotton at 21 dpi as described previously (Xu et al., 2014) and the fungal biomass assay was also performed as described previously (Hu et al., 2018). Total DNA of seedling stems at 3 weeks after inoculation was extracted for qPCR.
To observe the ability of all strains to penetrate the cellophane membrane, equal amounts of conidia of each strain were cultured on the cellophane membrane overlaid on PDA for 3 days. The hyphae on the cellophane membrane were imaged using scanning electron microscopy, and the cellophane membrane was removed and cultured for a further 5 days. All experiments were repeated three times. The primers used are listed in Table S3.
4.8. VdEPG1 expression and purification
VdEPG1 expression and purification were performed as described previously (Zhou et al., 2021). The sequences of VdEPG1 without the signal peptide coding region were inserted into EcoRI/HindIII restriction sites of pET28a. The construct was transformed into E. coli BL21, then VdEPG1 was purified using the AKTA Explorer system (Amersham Biosciencies) under optimum conditions. The concentration of purified protein was tested by microspectrophotometry (Thermo Fisher Scientific) and a BCA protein assay kit (Boster). The primers used are listed in Table S3.
4.9. Hydrolytic activity assays
LC–MS was used to analyse the pectin hydrolysate produced by the VdEPG1. Pectin from Sigma was used as the substrate. The experiment was repeated three times.
4.10. Y2H assays
The cDNA library of cotton roots after V. dahliae inoculation was constructed using the Matchmaker Gold Yeast Two‐Hybrid System (Clontech) for Y2H screening. The VdEPG1 ‐SSP gene was fused into pGBKT7 as a bait to screen for interacting proteins from the cDNA library. To verify the interaction between VdEPG1 and the identified protein GhOPR9, the pGBKT7 plasmid fused with VdEPG1 ‐SSP was transformed into the Y2H yeast strain and the pGADT7 plasmid fused with GhOPR9 was transformed into the Y187 yeast strain using the Transformation System (Clontech). The mating strains were plated on synthetic dropout (SD)−Leu−Trp and SD−Leu−Trp−His−Ade (with X‐α‐gal) media for selection as described by the manual (Clontech). The primers used are listed in Table S3.
4.11. BiFC analysis and subcellular localization
To detect the interaction between VdEPG1 and GhOPR9 in vivo, BiFC analysis was performed as described previously (Yu et al., 2008). The sequence of VdEPG1 ‐SSP was inserted into the vector pXY104cYFP and the full‐length coding sequence of GhOPR9 was cloned into the vector pXY106nYFP. The constructs were transformed into A. tumefaciens GV3101. The cell cultures possessing VdEPG1‐SP‐cYFP and GhOPR9‐nYFP were co‐infiltrated into N. benthamiana leaves. The sequences of VdEPG1 and GhOPR9 were fused into the vector pCAMBIA2300‐GFP to determine the subcellular localization, and the VdEPG1‐GFP and GhOPR9‐GFP constructs were transformed into A. tumefaciens GV3101. The cell cultures of VdEPG1‐GFP and GhOPR9‐GFP were infiltrated into N. benthamiana leaves. The GFP and YFP fluorescence signals were observed using a confocal microscope at 488 nm. The sequence of VdEPG1 was fused into the vector pBI121‐RFP to determine the subcellular localization with the GhOPR9‐GFP construct in N. benthamiana leaves. RFP (red fluorescence protein) fluorescence signals were observed using a confocal microscope at 594 nm. The primers used are listed in Table S3.
4.12. Firefly luciferase complementation imaging assay
The luciferase complementation imaging assay was performed as described previously (Chen et al., 2008). The constructs CLuc‐GhOPR9 and VdEPG1‐SP‐NLuc were transformed into A. tumefaciens GV3101 and cell cultures were simply mixed. Then the mixture was infiltrated into N. benthamiana leaves. The infiltrated leaves were sprayed with 1 mM beetle luciferin (Promega) and images were captured using a Photek camera (5200Multi). The primers used are listed in Table S3.
4.13. VIGS
The VIGS method was used to study the functions of GhOPR9. The fragments about 200 bp of the 5′ UTR and the coding sequence of GhOPR9 were amplified from Jimian No. 11 and cloned into the tobacco rattle virus (TRV) vector pYL156 to obtain the constructs TRV:GhOPR9‐5′UTR and TRV:GhOPR9‐CDS using a In‐Fusion HD Cloning Kit. The TRV:PDS construct was used as the positive marker for evaluating VIGS efficiency. All TRV constructs were infected into cotyledons of cotton seedlings as described previously (Tang et al., 2019). When the TRV:PDS lines displayed a photobleaching phenotype in their new true leaves, the silencing efficiency of GhOPR9 was examined in the new true leaves in the TRV:GhOPR9‐5′UTR and TRV:GhOPR9‐CDS lines. The successfully silenced lines were inoculated with V991 as described above. The roots and leaves were collected at four time points (0, 12, 24, and 48 h) after inoculation for further study. The DI, vascular wilt symptom, fungal recovery, and fungal biomass assay were performed as described above. Three weeks after inoculation with V991, callose depositions were visualized by using aniline blue staining as described previously (Liu et al., 2020). The stained leaves were imaged by fluorescence microscopy. The primers used are listed in Table S3.
4.14. Arabidopsis transformation
The coding sequence of GhOPR9 was amplified and inserted into the plant binary vector pCAMBIA2300 under control of the CaMV 35S promoter. The construct was transformed into A. tumefaciens GV3101 then used for Arabidopsis transformation via the floral dip method (Clough & Bent, 1998). The seeds were screened on Murashige and Skoog medium containing 50 mg/L kanamycin. The T3 transgenic lines were examined by PCR and used for the pathogenicity assay. The primers used are listed in Table S3.
4.15. Protein extraction and western blotting
Protein was extracted from N. benthamiana and A. thaliana leaves after treatment as described previously (Liu et al., 2021). Leaves were ground in liquid nitrogen after treatment and mixed with an equal volume of protein isolation buffer. The mixture was centrifuged for 10 min at 4°C at 13,000 g, then boiled in protein sample buffer for 5 min. Proteins were analysed by SDS‐PAGE and electroblotted onto polyvinylidene difluoride (PVDF) membranes.
4.16. Measurement of JA
Cotton root samples inoculated with WT, deletion strains, and complementation strains were collected. The endogenous concentration of JA was measured as described previously (Hu et al., 2018).
4.17. Nucleic acid extraction and expression analysis
Total RNA was extracted using the RNAprep Pure Plant Plus Kit (Polysaccharides & Polyphenolics‐rich) (TransGen Biotech) according to the manufacturer's instructions. Total DNA was extracted using the Fungal DNA Kit (Omega Bio‐tek) according to the manufacturer's instructions. First‐strand cDNA was synthesized using the All‐in‐One First‐Strand cDNA Synthesis Super Mix for qPCR Kit (TransGen Biotech). RT‐PCR and qPCR were performed according to a procedure described previously (Liu et al., 2020). The primers used are listed in Table S3.
4.18. Bioinformatics analysis
The hidden Markov model (HMM) of Glyco_hydro_28 (PF00295) was downloaded from the Pfam database to identify the GH28 family members (E < 1) by HMMER v. 3.1b2 software against fungal proteomes (El‐Gebali et al., 2019; Finn et al., 2011). GH28 members were used to construct a maximum‐likelihood phylogenetic tree via MEGA X software after multiple sequence alignment using MUSCLE (Edgar, 2004; Kumar et al., 2018). The fungal genome databases were downloaded from JGI (http://genome.jgi.doe.gov/).
CONFLICT OF INTEREST STATEMENT
Authors declare that they have no competing interests.
Supporting information
FIGURE S1 The reverse transcription‐quantitative PCR analysis of VdGH28s‐induced expression patterns. (a) The phylogenetic tree of the glycoside hydrolases 28 (GH28) family. The maximum‐likelihood tree was construct by the full‐length amino acid sequences of GH28 genes from Verticillium dahliae (VDAG), Magnaporthe oryzae (MGG), Phytophthora infestans (PITG), Fusarium oxysporum (FOXG), Botrytis cinerea (Bcin), and Colletotrichum gloeosporioides (CGGC), using MEGA X, with 1000 replicates. They were divided into three groups. Group I, Group II, and Group III are represented by green, blue, and orange, respectively. (b) The blue columns represent the expression patterns of VdGH28s of V991 spores that were treated with cotton root extract. The red columns represent the expression patterns of VdGH28s in cotton roots that were inoculated with a suspension of V991 spores. GhUB7 was used as a control. Values represent the mean ± standard deviation of three replicates. Asterisks showed the gene expressed significantly higher or lower at 6, 12 and 24 h than in 0 h by t test (*p < 0.05, **p < 0.01, ***p < 0.001)
FIGURE S2 VdEPG1 (VDAG_04977) suppresses the cell death induced by NLP1 in Verticillium dahliae. (a) Detection of the cell death‐suppressing activity of VdGH28s. NLP1 was transiently expressed at 24 h after infiltration with VdGH28s in 6‐week‐old Nicotiana benthamiana leaves. Scale bar = 1 cm. (b) Semiquantitative reverse transcription‐PCR analysis of transiently expressed VdGH28 genes in N. benthamiana leaves 48 h after infiltration. NbActin was used as the control
FIGURE S3 The protein sequence of VdEPG1. (a) The amino acid sequence of VdEPG1. VdEPG1 encodes 370 amino acids. (b) The prediction of VdEPG1 signal peptides in SignalP v. 5.0. The amino acids from 1 to 18 were predicted as the signal peptide of VdEPG1
FIGURE S4 Mass spectrogram of galacturonic acid (hydrolysates of pectin) by liquid chromatography mass spectrometry. (a) Mass spectrogram of biological reference standard d‐galacturonic acid. (b) Mass spectrogram of galacturonic acid, which was produced from the pectin treated by VdEPG1
FIGURE S5 The homologous recombination of VdEPG1 in Verticillium dahliae. HPH is the sequence of the hygromycin resistance gene cassette
FIGURE S6 Identification of VdEPG1 mutant transformations by PCR. (a) Identification of VdEPG1 gene knockout transformations by PCR. (b) Identification of VdEPG1 gene complementary transformations by PCR. VdEPG1 represents the sequence of VdEPG1 and HPH represents the sequence of the hygromycin resistance cassette. The red numbers represent the different mutant strains. The red columns represent the positive strains detected by PCR
FIGURE S7 Subcellular localization of VdEPG1 and GhOPR9 in Nicotiana benthamiana leaves. (a) VdEPG1‐GFP and GhOPR9‐GFP were expressed in N. benthamiana leaves. The results show that VdEPG1 was located in the plasma membrane and GhOPR9 was located in the plasma membrane and cytoplasm. Green fluorescent protein (GFP) was used as the control. (b) Subcellular localization of GhOPR9‐GFP and VdEPG1‐RFP on onion epidermis. (c) Subcellular co‐localization of GhOPR9‐GFP and VdEPG1‐RFP in N. benthamiana leaves. The results show that VdEPG1 and GhOPR9 are located in the plasma membrane. Scale bar = 100 μm
FIGURE S8 Reverse transcription‐quantitative PCR analysis of the expression of jasmonic acid (JA) biosynthesis pathway genes at 0, 1, 6 and 12 h after V991 inoculation in the treatment plants. (a) The expression levels of JA biosynthesis pathway genes after V991 inoculation in leaves. (b) The expression levels of JA biosynthesis pathway genes after V991 inoculation in roots. Values represent the mean ± standard deviation of three replicates. The TRV:00 plants at 0 h were used as the control. The asterisks showed the gene expressed significantly higher or lower at other time points than 0 h on t test (*p < 0.05, **p < 0.01, ***p < 0.001)
FIGURE S9 Western blot analysis of VdEPG1 expression in Arabidopsis thaliana plants
FIGURE S10 Detection of the cell death‐suppressing activity of VdEPG1. INF1 was transiently expressed at 24 h after infiltration with VdEPG1 in 6‐week‐old Nicotiana benthamiana leaves. Western blot analysis showed the VdEPG1 was expressed in N. benthamiana leaves. 1, sample infiltrated with INF1 only; 2, sample infiltrated with VdEPG1 only; 3, sample infiltrated with INF1 at 24 h after infiltration with VdEPG1; 4, sample infiltrated with green fluorescent protein (GFP) only. Commassie brilliant blue (CBB) was used as the loading control. Scale bar = 1 cm
FIGURE S11 VdEPG1 affects jasmonic acid (JA) biosynthesis in cotton by regulating the expression of GhOPR9. (a) GhOPR9 expression in cotton roots after inoculation by wild‐type (WT), ΔVdEPG1‐8, ΔVdEPG1‐11, C‐ΔVdEPG1‐8, and C‐ΔVdEPG1‐11 strains at 0, 1, 6, and 12 h. (b) GhCPK33 expression in cotton roots after inoculation by WT, ΔVdEPG1‐8, ΔVdEPG1‐11, C‐ΔVdEPG1‐8, and C‐ΔVdEPG1‐11 strains at 0, 1, 6, and 12 h. (c) JA contents in roots from plants that were infected by WT, ΔVdEPG1‐8, ΔVdEPG1‐11, C‐ΔVdEPG1‐8, and C‐ΔVdEPG1‐11 strains. Lines (CK) without Verticillium dahliae inoculation were used as the control. Values are the mean ± standard deviation, n = 6. Statistical analyses were performed using Student’s t test: *p < 0.05, **p < 0.01.
TABLE S1 Details of the GH28 of Verticillium dahliae protein physiochemical properties and gene annotation
TABLE S2 The members of GH28 in Verticillium dahliae, Magnaporthe oryzae, Phytophthora infestans, Fusarium oxysporum, Botrytis cinerea, and Colletotrichum gloeosporioides
TABLE S3 Primers used in this study
ACKNOWLEDGEMENTS
We thank Dr Jiahe Wu (Institute of Microbiology, Chinese Academy of Sciences) for kindly providing us with pXY104‐cYFP and pXY106‐nYFP vectors for BiFC, Dr Jianmin Zhou (Institute of Genetics and Developmental Biology, Chinese Academy of Sciences) for kindly providing us with nLUC and cLUC vectors for luciferase complementation imaging, and Dr Wenxiang Yang (College of Plant Protection, Hebei Agricultural University) for kindly providing us with pGR107‐PVX vector. This work was sponsored by the Natural Science Foundation of the Xinjiang Uygur Autonomous Region (2022D01E101), the National Natural Science Foundation of China (32172081), the Natural Science Foundation of Xinjiang Province (2022017) and the Agricultural Science and Technology Innovation Program of the Chinese Academy of Agricultural Sciences.
Liu, S. , Liu, R. , Lv, J. , Feng, Z. , Wei, F. , Zhao, L. et al. (2023) The glycoside hydrolase 28 member VdEPG1 is a virulence factor of Verticillium dahliae and interacts with the jasmonic acid pathway‐related gene GhOPR9 . Molecular Plant Pathology, 24, 1238–1255. Available from: 10.1111/mpp.13366
Shichao Liu, Ruibing Liu, and Junyuan Lv contributed equally to this work.
Contributor Information
Heqin Zhu, Email: heqinanyang@163.com.
Hongjie Feng, Email: fenghongjie@caas.cn.
DATA AVAILABILITY STATEMENT
The data that support the findings of this article are available from the corresponding author upon request.
REFERENCES
- Asai, S. & Yoshioka, H. (2009) Nitric oxide as a partner of reactive oxygen species participates in disease resistance to necrotrophic pathogen Botrytis cinerea in Nicotiana benthamiana . Molecular Plant‐Microbe Interactions, 22, 619–629. [DOI] [PubMed] [Google Scholar]
- Ben‐Daniel, B.H. , Bar‐Zvi, D. & Tsror, L. (2012) Pectate lyase affects pathogenicity in natural isolates of Colletotrichum coccodes and in pelA gene‐disrupted and gene‐overexpressing mutant lines. Molecular Plant Pathology, 13, 187–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boller, T. & Felix, G. (2009) A renaissance of elicitors: perception of microbe‐associated molecular patterns and danger signals by pattern‐recognition receptors. Annual Review of Plant Biology, 60, 379–406. [DOI] [PubMed] [Google Scholar]
- Brito, N. , Espino, J.J. & Gonzalez, C. (2006) The endo‐β‐1,4‐xylanase xyn11A is required for virulence in Botrytis cinerea . Molecular Plant‐Microbe Interactions, 19, 25–32. [DOI] [PubMed] [Google Scholar]
- Chen, H.M. , Zou, Y. , Shang, Y.L. , Lin, H.Q. , Wang, Y.J. , Cai, R. et al. (2008) Firefly luciferase complementation imaging assay for protein–protein interactions in plants. Plant Physiology, 146, 368–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chini, A. , Monte, I. , Zamarreno, A.M. , Hamberg, M. , Lassueur, S. , Reymond, P. et al. (2018) An OPR3‐independent pathway uses 4,5‐didehydrojasmonate for jasmonate synthesis. Nature Chemical Biology, 14, 171–178. [DOI] [PubMed] [Google Scholar]
- Clough, S.J. & Bent, A.F. (1998) Floral dip: a simplified method for Agrobacterium‐mediated transformation of Arabidopsis thaliana . The Plant Journal, 16, 735–743. [DOI] [PubMed] [Google Scholar]
- Dangl, J.L. , Horvath, D.M. & Staskawicz, B.J. (2013) Pivoting the plant immune system from dissection to deployment. Science, 341, 746–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dangl, J.L. & Jones, J.D.G. (2001) Plant pathogens and integrated defence responses to infection. Nature, 411, 826–833. [DOI] [PubMed] [Google Scholar]
- De Lorenzo, G. , Brutus, A. , Savatin, D.V. , Sicilia, F. & Cervone, F. (2011) Engineering plant resistance by constructing chimeric receptors that recognize damage‐associated molecular patterns (DAMPs). FEBS Letters, 585, 1521–1528. [DOI] [PubMed] [Google Scholar]
- Di Pietro, A. & Roncero, M.I.G. (1998) Cloning, expression, and role in pathogenicity of Pg1 encoding the major extracellular endopolygalacturonase of the vascular wilt pathogen Fusarium oxysporum . Molecular Plant‐Microbe Interactions, 11, 91–98. [DOI] [PubMed] [Google Scholar]
- Dodds, P.N. & Rathjen, J.P. (2010) Plant immunity: towards an integrated view of plant–pathogen interactions. Nature Reviews Genetics, 11, 539–548. [DOI] [PubMed] [Google Scholar]
- Dong, W. , Wang, M. , Xu, F. , Quan, T. , Peng, K. , Xiao, L. et al. (2013) Wheat oxophytodienoate reductase gene TaOPR1 confers salinity tolerance via enhancement of abscisic acid signaling and reactive oxygen species scavenging. Plant Physiology, 161, 1217–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Ovidio, R. , Mattei, B. , Roberti, S. & Bellincampi, D. (2004) Polygalacturonases, polygalacturonase‐inhibiting proteins and pectic oligomers in plant–pathogen interactions. Biochimica et Biophysica Acta, 1696, 237–244. [DOI] [PubMed] [Google Scholar]
- Edgar, R.C. (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research, 32, 1792–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El‐Gebali, S. , Mistry, J. , Bateman, A. , Eddy, S.R. , Luciani, A. , Potter, S.C. et al. (2019) The Pfam protein families database in 2019. Nucleic Acids Research, 47, D427–D432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn, R.D. , Clements, J. & Eddy, S.R. (2011) HMMER web server: interactive sequence similarity searching. Nucleic Acids Research, 39, W29–W37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fradin, E.F. & Thomma, B.P.H.J. (2006) Physiology and molecular aspects of verticillium wilt diseases caused by V. dahliae and V. albo‐atrum . Molecular Plant Pathology, 7, 71–86. [DOI] [PubMed] [Google Scholar]
- Gui, Y.J. , Chen, J.Y. , Zhang, D.D. , Li, N.Y. , Li, T.G. , Zhang, W.Q. et al. (2017) Verticillium dahliae manipulates plant immunity by glycoside hydrolase 12 proteins in conjunction with carbohydrate‐binding module 1. Environmental Microbiology, 19, 1914–1932. [DOI] [PubMed] [Google Scholar]
- Heese, A. , Hann, D.R. , Gimenez‐Ibanez, S. , Jones, A.M.E. , He, K. , Li, J. et al. (2007) The receptor‐like kinase SERK3/BAK1 is a central regulator of innate immunity in plants. Proceedings of the National Academy of Sciences of the United States of America, 104, 12217–12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hematy, K. , Cherk, C. & Somerville, S. (2009) Host–pathogen warfare at the plant cell wall. Current Opinion in Plant Biology, 12, 406–413. [DOI] [PubMed] [Google Scholar]
- Henrissat, B. (1991) A classification of glycosyl hydrolases based on amino acid sequence similarities. Biochemistry Journal, 280, 309–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, Q. , Zhu, L. , Zhang, X. , Guan, Q. , Xiao, S. , Min, L. et al. (2018) GhCPK33 negatively regulates defense against Verticillium dahliae by phosphorylating GhOPR3. Plant Physiology, 178, 876–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihama, N. , Yamada, R. , Yoshioka, M. , Katou, S. & Yoshioka, H. (2011) Phosphorylation of the Nicotiana benthamiana WRKY8 transcription factor by MAPK functions in the defense response. The Plant Cell, 23, 1153–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isshiki, A. , Akimitsu, K. , Yamamoto, M. & Yamamoto, H. (2001) Endopolygalacturonase is essential for citrus black rot caused by Alternaria citri but not brown spot caused by Alternaria alternata . Molecular Plant‐Microbe Interactions, 14, 749–757. [DOI] [PubMed] [Google Scholar]
- Jacobs, K.A. , Collins‐Racie, L.A. , Colbert, M. , Duckett, M. , Golden‐Fleet, M. , Kelleher, K. et al. (1997) A genetic selection for isolating cDNAs encoding secreted proteins. Gene, 198, 289–296. [DOI] [PubMed] [Google Scholar]
- Kars, I. , Krooshof, G.H. , Wagemakers, L. , Joosten, R. , Benen, J.A.E. & Van Kan, J.A.L. (2005) Necrotizing activity of five Botrytis cinerea endopolygalacturonases produced in Pichia pastoris . The Plant Journal, 43, 213–225. [DOI] [PubMed] [Google Scholar]
- Kiba, A. , Nakano, M. , Ohnishi, K. & Hikichi, Y. (2018) The SEC14 phospholipid transfer protein regulates pathogen‐associated molecular pattern‐triggered immunity in Nicotiana benthamiana . Plant Physiology and Biochemistry, 125, 212–218. [DOI] [PubMed] [Google Scholar]
- Kikot, G.E. , Hours, R.A. & Alconada, T.M. (2009) Contribution of cell wall degrading enzymes to pathogenesis of Fusarium graminearum: a review. Journal of Basic Microbiology, 49, 231–241. [DOI] [PubMed] [Google Scholar]
- Klockner, A. , Buhl, H. , Viollier, P. & Henrichfreise, B. (2018) Deconstructing the chlamydial cell wall. Biology of Chlamydia, 412, 1–33. [DOI] [PubMed] [Google Scholar]
- Klosterman, S.J. , Atallah, Z.K. , Vallad, G.E. & Subbarao, K.V. (2009) Diversity, pathogenicity, and management of Verticillium species. Annual Review of Phytopathology, 47, 39–62. [DOI] [PubMed] [Google Scholar]
- Kubicek, C.P. , Starr, T.L. & Glass, N.L. (2014) Plant cell wall‐degrading enzymes and their secretion in plant‐pathogenic fungi. Annual Review of Phytopathology, 52, 427–451. [DOI] [PubMed] [Google Scholar]
- Kumar, S. , Stecher, G. , Li, M. , Knyaz, C. & Tamura, K. (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Molecular Biology and Evolution, 35, 1547–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, T.G. , Wang, B.L. , Yin, C.M. , Zhang, D.D. , Wang, D. , Song, J. et al. (2019) The Gossypium hirsutum TIR‐NBS‐LRR gene GhDSC1 mediates resistance against verticillium wilt. Molecular Plant Pathology, 20, 857–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, X. , Su, X. , Lu, G. , Sun, G. , Zhang, Z. , Guo, H. et al. (2020) VdOGDH is involved in energy metabolism and required for virulence of Verticillium dahliae . Current Genetics, 66, 345–359. [DOI] [PubMed] [Google Scholar]
- Liechti, R. & Farmer, E.E. (2006) Jasmonate biochemical pathway. Science's STKE, 2006, cm3. [DOI] [PubMed] [Google Scholar]
- Liu, L. , Wang, Z. , Li, J. , Wang, Y. , Yuan, J. , Zhan, J. et al. (2021) Verticillium dahliae secreted protein Vd424Y is required for full virulence, targets the nucleus of plant cells, and induces cell death. Molecular Plant Pathology, 22, 1109–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, S.C. , Sun, R.B. , Zhang, X.J. , Feng, Z.L. , Wei, F. , Zhao, L.H. et al. (2020) Genome‐wide analysis of OPR family genes in cotton identified a role for GhOPR9 in Verticillium dahliae resistance. Genes, 11, 1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv, J. , Zhou, J. , Chang, B. , Zhang, Y. , Feng, Z. , Wei, F. et al. (2022) Two metalloproteases VdM35‐1 and VdASPF2 from Verticillium dahliae are required for fungal pathogenicity, stress adaptation, and activating immune response of host. Microbiology Spectrum, 10, e0247722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, Z.C. , Song, T.Q. , Zhu, L. , Ye, W.W. , Wang, Y. , Shao, Y.Y. et al. (2015) A Phytophthora sojae glycoside hydrolase 12 protein is a major virulence factor during soybean infection and is recognized as a PAMP. The Plant Cell, 27, 2057–2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, Z.C. , Zhu, L. , Song, T.Q. , Wang, Y. , Zhang, Q. , Xia, Y.Q. et al. (2017) A paralogous decoy protects Phytophthora sojae apoplastic effector PsXEG1 from a host inhibitor. Science, 355, 710–714. [DOI] [PubMed] [Google Scholar]
- Misas‐Villamil, J.C. & van der Hoorn, R.A.L. (2008) Enzyme‐inhibitor interactions at the plant–pathogen interface. Current Opinion in Plant Biology, 11, 380–388. [DOI] [PubMed] [Google Scholar]
- Monaghan, J. & Zipfel, C. (2012) Plant pattern recognition receptor complexes at the plasma membrane. Current Opinion in Plant Biology, 15, 349–357. [DOI] [PubMed] [Google Scholar]
- Mori, T. , Jung, H.Y. , Maejima, K. , Hirata, H. , Himeno, M. , Hamamoto, H. et al. (2008) Magnaporthe oryzae endopolygalacturonase homolog correlates with density‐dependent conidial germination. FEMS Microbiology Letters, 280, 182–188. [DOI] [PubMed] [Google Scholar]
- Pigolev, A.V. , Miroshnichenko, D.N. , Pushin, A.S. , Terentyev, V.V. , Boutanayev, A.M. , Dolgov, S.V. et al. (2018) Overexpression of Arabidopsis OPR3 in hexaploid wheat (Triticum aestivum L.) alters plant development and freezing tolerance. International Journal of Molecular Sciences, 19, 3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poinssot, B. , Vandelle, E. , Bentejac, M. , Adrian, M. , Levis, C. , Brygoo, Y. et al. (2003) The endopolygalacturonase 1 from Botrytis cinerea activates grapevine defense reactions unrelated to its enzymatic activity. Molecular Plant‐Microbe Interactions, 16, 553–564. [DOI] [PubMed] [Google Scholar]
- Prade, R.A. , Zhan, D.F. , Ayoubi, P. & Mort, A.J. (1999) Pectins, pectinases and plant–microbe interactions. Biotechnology and Genetic Engineering Reviews, 16, 361–391. [DOI] [PubMed] [Google Scholar]
- Rajeshwari, R. , Jha, G. & Sonti, R.V. (2005) Role of an in planta‐expressed xylanase of Xanthomonas oryzae pv. oryzae in promoting virulence on rice. Molecular Plant‐Microbe Interactions, 18, 830–837. [DOI] [PubMed] [Google Scholar]
- Rehman, L. , Su, X.F. , Guo, H.M. , Qi, X.L. & Cheng, H.M. (2016) Protoplast transformation as a potential platform for exploring gene function in Verticillium dahliae . BMC Biotechnology, 16, 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reignault, P. , Valette‐Collet, O. & Boccara, M. (2008) The importance of fungal pectinolytic enzymes in plant invasion, host adaptability and symptom type. European Journal of Plant Pathology, 120, 1–11. [Google Scholar]
- Ruan, J.J. , Zhou, Y.X. , Zhou, M.L. , Yan, J. , Khurshid, M. , Weng, W.F. et al. (2019) Jasmonic acid signaling pathway in plants. International Journal of Molecular Sciences, 20, 2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scalschi, L. , Sanmartin, M. , Camanes, G. , Troncho, P. , Sanchez‐Serrano, J.J. , Garcia‐Agustin, P. et al. (2015) Silencing of OPR3 in tomato reveals the role of OPDA in callose deposition during the activation of defense responses against Botrytis cinerea . The Plant Journal, 81, 304–315. [DOI] [PubMed] [Google Scholar]
- Schaller, F. (2001) Enzymes of the biosynthesis of octadecanoid‐derived signalling molecules. Journal of Experimental Botany, 52, 11–23. [PubMed] [Google Scholar]
- Skamnioti, P. & Gurr, S.J. (2007) Magnaporthe grisea cutinase2 mediates appressorium differentiation and host penetration and is required for full virulence. The Plant Cell, 19, 2674–2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoel, S.H. & Dong, X. (2012) How do plants achieve immunity? Defence without specialized immune cells. Nature Reviews Immunology, 12, 89–100. [DOI] [PubMed] [Google Scholar]
- Stintzi, A. & Browse, J. (2000) The Arabidopsis male‐sterile mutant, opr3, lacks the 12‐oxophytodienoic acid reductase required for jasmonate synthesis. Proceedings of the National Academy of Sciences of the United States of America, 97, 10625–10630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su, X.F. , Rehman, L. , Guo, H.M. , Li, X.K. , Zhang, R. & Cheng, H.M. (2017) AAC as a potential target gene to control Verticillium dahliae . Genes, 8, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, Y. , Zhang, Z.N. , Lei, Y. , Hu, G. , Liu, J.F. , Hao, M.Y. et al. (2019) Cotton WATs modulate SA biosynthesis and local lignin deposition participating in plant resistance against Verticillium dahliae . Frontiers in Plant Science, 10, 526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thilini Chethana, K.W. , Peng, J. , Li, X. , Xing, Q. , Liu, M. , Zhang, W. et al. (2020) LtEPG1, a secretory endopolygalacturonase protein, regulates the virulence of Lasiodiplodia theobromae in Vitis vinifera and is recognized as a microbe‐associated molecular patterns. Phytopathology, 110, 1727–1736. [DOI] [PubMed] [Google Scholar]
- Tian, L. , Wang, Y. , Yu, J. , Xiong, D. , Zhao, H. & Tian, C. (2016) The mitogen‐activated protein kinase kinase VdPbs2 of Verticillium dahliae regulates microsclerotia formation, stress response, and plant infection. Frontiers in Microbiology, 7, 1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzima, A.K. , Paplomatas, E.J. , Rauyaree, P. , Ospina‐Giraldo, M.D. & Kang, S. (2011) VdSNF1, the sucrose nonfermenting protein kinase gene of Verticillium dahliae, is required for virulence and expression of genes involved in cell‐wall degradation. Molecular Plant‐Microbe Interactions, 24, 129–142. [DOI] [PubMed] [Google Scholar]
- Vorwerk, S. , Somerville, S. & Somerville, C. (2004) The role of plant cell wall polysaccharide composition in disease resistance. Trends in Plant Science, 9, 203–209. [DOI] [PubMed] [Google Scholar]
- Wasternack, C. & Hause, B. (2013) Jasmonates: biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Annals of Botany, 111, 1021–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei, F. , Zhao, L.H. , Xu, X.M. , Feng, H.J. , Shi, Y.Q. , Deakin, G. et al. (2019) Cultivar‐dependent variation of the cotton rhizosphere and endosphere microbiome under field conditions. Frontiers in Plant Science, 10, 1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen, Y. , Zhou, J. , Feng, H. , Sun, W. , Zhang, Y. , Zhao, L. et al. (2023) VdGAL4 modulates microsclerotium formation, conidial morphology, and germination to promote virulence in Verticillium dahliae . Microbiology Spectrum, 11, e0351522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, L. , Zhang, W.W. , He, X. , Liu, M. , Zhang, K. , Shaban, M. et al. (2014) Functional characterization of cotton genes responsive to Verticillium dahliae through bioinformatics and reverse genetics strategies. Journal of Experimental Botany, 65, 6679–6692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakoby, N. , Beno‐Moualem, D. , Keen, N.T. , Dinoor, A. , Pines, O. & Prusky, D. (2001) Colletotrichum gloeosporioides pelB is an important virulence factor in avocado fruit–fungus interaction. Molecular Plant‐Microbe Interactions, 14, 988–995. [DOI] [PubMed] [Google Scholar]
- Yang, Y.K. , Zhang, Y. , Li, B.B. , Yang, X.F. , Dong, Y.J. & Qiu, D.W. (2018) A Verticillium dahliae pectate lyase induces plant immune responses and contributes to virulence. Frontiers in Plant Science, 9, 1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, J. , Li, T.Y. , Tian, L.Y. , Tang, C. , Klosterman, S.J. , Tian, C.M. et al. (2019) Two Verticillium dahliae MAPKKKs, VdSsk2 and VdSte11, have distinct roles in pathogenicity, microsclerotial formation, and stress adaptation. mSphere, 4, e00426‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, X.F. , Li, L. , Li, L. , Guo, M. , Chory, J. & Yin, Y.H. (2008) Modulation of brassinosteroid‐regulated gene expression by jumonji domain‐containing proteins ELF6 and REF6 in Arabidopsis . Proceedings of the National Academy of Sciences of the United States of America, 105, 7618–7623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, L.S. , Kars, I. , Essenstam, B. , Liebrand, T.W.H. , Wagemakers, L. , Elberse, J. et al. (2014) Fungal endopolygalacturonases are recognized as microbe‐associated molecular patterns by the Arabidopsis receptor‐like protein RESPONSIVENESS TO BOTRYTIS POLYGALACTURONASES1. Plant Physiology, 164, 352–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, X. , Zhao, L. , Liu, S. , Zhou, J. , Wu, Y. , Feng, Z. et al. (2022) Identification and functional analysis of a novel hydrophobic protein VdHP1 from Verticillium dahliae . Microbiology Spectrum, 10, e0247821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, Y.L. , Zhou, T.T. & Guo, H.S. (2016) Hyphopodium‐specific VdNoxB/VdPls1‐dependent ROS‐Ca2+ signaling is required for plant infection by Verticillium dahliae . PLoS Pathogens, 12, e1005793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, J. , Tang, C. , Deng, C. & Wang, Y. (2019) Involvement of a response regulator VdSsk1 in stress response, melanin biosynthesis and full virulence in Verticillium dahliae . Frontiers in Microbiology, 10, 606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, B.J. , Jia, P.S. , Gao, F. & Guo, H.S. (2012) Molecular characterization and functional analysis of a necrosis‐ and ethylene‐inducing, protein‐encoding gene family from Verticillium dahliae . Molecular Plant‐Microbe Interactions, 25, 964–975. [DOI] [PubMed] [Google Scholar]
- Zhou, J. , Feng, Z. , Liu, S. , Wei, F. , Shi, Y. , Zhao, L. et al. (2021) CGTase, a novel antimicrobial protein from Bacillus cereus YUPP‐10, suppresses Verticillium dahliae and mediates plant defence responses. Molecular Plant Pathology, 22, 130–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, H.Q. , Feng, Z.L. , Li, Z.F. , Shi, Y.Q. , Zhao, L.H. & Yang, J.R. (2013) Characterization of two fungal isolates from cotton and evaluation of their potential for biocontrol of verticillium wilt of cotton. Journal of Phytopathology, 161, 70–77. [Google Scholar]
- Zhu, W.J. , Ronen, M. , Gur, Y. , Minz‐Dub, A. , Masrati, G. , Ben‐Tal, N. et al. (2017) BcXYG1, a secreted xyloglucanase from Botrytis cinerea, triggers both cell death and plant immune responses. Plant Physiology, 175, 438–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipfel, C. (2009) Early molecular events in PAMP‐triggered immunity. Current Opinion in Plant Biology, 12, 414–420. [DOI] [PubMed] [Google Scholar]
- Zipfel, C. (2014) Plant pattern‐recognition receptors. Trends in Immunology, 35, 345–351. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FIGURE S1 The reverse transcription‐quantitative PCR analysis of VdGH28s‐induced expression patterns. (a) The phylogenetic tree of the glycoside hydrolases 28 (GH28) family. The maximum‐likelihood tree was construct by the full‐length amino acid sequences of GH28 genes from Verticillium dahliae (VDAG), Magnaporthe oryzae (MGG), Phytophthora infestans (PITG), Fusarium oxysporum (FOXG), Botrytis cinerea (Bcin), and Colletotrichum gloeosporioides (CGGC), using MEGA X, with 1000 replicates. They were divided into three groups. Group I, Group II, and Group III are represented by green, blue, and orange, respectively. (b) The blue columns represent the expression patterns of VdGH28s of V991 spores that were treated with cotton root extract. The red columns represent the expression patterns of VdGH28s in cotton roots that were inoculated with a suspension of V991 spores. GhUB7 was used as a control. Values represent the mean ± standard deviation of three replicates. Asterisks showed the gene expressed significantly higher or lower at 6, 12 and 24 h than in 0 h by t test (*p < 0.05, **p < 0.01, ***p < 0.001)
FIGURE S2 VdEPG1 (VDAG_04977) suppresses the cell death induced by NLP1 in Verticillium dahliae. (a) Detection of the cell death‐suppressing activity of VdGH28s. NLP1 was transiently expressed at 24 h after infiltration with VdGH28s in 6‐week‐old Nicotiana benthamiana leaves. Scale bar = 1 cm. (b) Semiquantitative reverse transcription‐PCR analysis of transiently expressed VdGH28 genes in N. benthamiana leaves 48 h after infiltration. NbActin was used as the control
FIGURE S3 The protein sequence of VdEPG1. (a) The amino acid sequence of VdEPG1. VdEPG1 encodes 370 amino acids. (b) The prediction of VdEPG1 signal peptides in SignalP v. 5.0. The amino acids from 1 to 18 were predicted as the signal peptide of VdEPG1
FIGURE S4 Mass spectrogram of galacturonic acid (hydrolysates of pectin) by liquid chromatography mass spectrometry. (a) Mass spectrogram of biological reference standard d‐galacturonic acid. (b) Mass spectrogram of galacturonic acid, which was produced from the pectin treated by VdEPG1
FIGURE S5 The homologous recombination of VdEPG1 in Verticillium dahliae. HPH is the sequence of the hygromycin resistance gene cassette
FIGURE S6 Identification of VdEPG1 mutant transformations by PCR. (a) Identification of VdEPG1 gene knockout transformations by PCR. (b) Identification of VdEPG1 gene complementary transformations by PCR. VdEPG1 represents the sequence of VdEPG1 and HPH represents the sequence of the hygromycin resistance cassette. The red numbers represent the different mutant strains. The red columns represent the positive strains detected by PCR
FIGURE S7 Subcellular localization of VdEPG1 and GhOPR9 in Nicotiana benthamiana leaves. (a) VdEPG1‐GFP and GhOPR9‐GFP were expressed in N. benthamiana leaves. The results show that VdEPG1 was located in the plasma membrane and GhOPR9 was located in the plasma membrane and cytoplasm. Green fluorescent protein (GFP) was used as the control. (b) Subcellular localization of GhOPR9‐GFP and VdEPG1‐RFP on onion epidermis. (c) Subcellular co‐localization of GhOPR9‐GFP and VdEPG1‐RFP in N. benthamiana leaves. The results show that VdEPG1 and GhOPR9 are located in the plasma membrane. Scale bar = 100 μm
FIGURE S8 Reverse transcription‐quantitative PCR analysis of the expression of jasmonic acid (JA) biosynthesis pathway genes at 0, 1, 6 and 12 h after V991 inoculation in the treatment plants. (a) The expression levels of JA biosynthesis pathway genes after V991 inoculation in leaves. (b) The expression levels of JA biosynthesis pathway genes after V991 inoculation in roots. Values represent the mean ± standard deviation of three replicates. The TRV:00 plants at 0 h were used as the control. The asterisks showed the gene expressed significantly higher or lower at other time points than 0 h on t test (*p < 0.05, **p < 0.01, ***p < 0.001)
FIGURE S9 Western blot analysis of VdEPG1 expression in Arabidopsis thaliana plants
FIGURE S10 Detection of the cell death‐suppressing activity of VdEPG1. INF1 was transiently expressed at 24 h after infiltration with VdEPG1 in 6‐week‐old Nicotiana benthamiana leaves. Western blot analysis showed the VdEPG1 was expressed in N. benthamiana leaves. 1, sample infiltrated with INF1 only; 2, sample infiltrated with VdEPG1 only; 3, sample infiltrated with INF1 at 24 h after infiltration with VdEPG1; 4, sample infiltrated with green fluorescent protein (GFP) only. Commassie brilliant blue (CBB) was used as the loading control. Scale bar = 1 cm
FIGURE S11 VdEPG1 affects jasmonic acid (JA) biosynthesis in cotton by regulating the expression of GhOPR9. (a) GhOPR9 expression in cotton roots after inoculation by wild‐type (WT), ΔVdEPG1‐8, ΔVdEPG1‐11, C‐ΔVdEPG1‐8, and C‐ΔVdEPG1‐11 strains at 0, 1, 6, and 12 h. (b) GhCPK33 expression in cotton roots after inoculation by WT, ΔVdEPG1‐8, ΔVdEPG1‐11, C‐ΔVdEPG1‐8, and C‐ΔVdEPG1‐11 strains at 0, 1, 6, and 12 h. (c) JA contents in roots from plants that were infected by WT, ΔVdEPG1‐8, ΔVdEPG1‐11, C‐ΔVdEPG1‐8, and C‐ΔVdEPG1‐11 strains. Lines (CK) without Verticillium dahliae inoculation were used as the control. Values are the mean ± standard deviation, n = 6. Statistical analyses were performed using Student’s t test: *p < 0.05, **p < 0.01.
TABLE S1 Details of the GH28 of Verticillium dahliae protein physiochemical properties and gene annotation
TABLE S2 The members of GH28 in Verticillium dahliae, Magnaporthe oryzae, Phytophthora infestans, Fusarium oxysporum, Botrytis cinerea, and Colletotrichum gloeosporioides
TABLE S3 Primers used in this study
Data Availability Statement
The data that support the findings of this article are available from the corresponding author upon request.


