Abstract
Rationale
Loss of pharyngeal dilator muscle activity is a key determinant of respiratory events in obstructive sleep apnea (OSA). After the withdrawal of wakefulness stimuli to the genioglossus at sleep onset, mechanoreceptor negative pressure and chemoreceptor ventilatory drive feedback govern genioglossus activation during sleep, but the relative contributions of drive and pressure stimuli to genioglossus activity across progressive obstructive events remain unclear. We recently showed that drive typically falls during events, whereas negative pressures increase, providing a means to assess their individual contributions to the time course of genioglossus activity.
Objectives
For the first time, we critically test whether the loss of drive could explain the loss of genioglossus activity observed within events in OSA.
Methods
We examined the time course of genioglossus activity (EMGgg; intramuscular electromyography), ventilatory drive (intraesophageal diaphragm electromyography), and esophageal pressure during spontaneous respiratory events (using the ensemble-average method) in 42 patients with OSA (apnea–hypopnea index 5–91 events/h).
Results
Multivariable regression demonstrated that the falling-then-rising time course of EMGgg may be well explained by falling-then-rising drive and rising negative pressure stimuli (model R = 0.91 [0.88–0.98] [95% confidence interval]). Overall, EMGgg was 2.9-fold (0.47–∞) more closely associated with drive than pressure stimuli (ratio of standardized coefficients, βdrive:βpressure; ∞ denotes absent pressure contribution). However, individual patient results were heterogeneous: approximately one-half (n = 22 of 42) exhibited drive-dominant responses (i.e., βdrive:βpressure > 2:1), and one-quarter (n = 11 of 42) exhibited pressure-dominant EMGgg responses (i.e., βdrive:βpressure < 1:2). Patients exhibiting more drive-dominant EMGgg responses experienced greater event-related EMGgg declines (12.9 [4.8–21.0] %baseline/standard deviation of βdrive:βpressure; P = 0.004, adjusted analysis).
Conclusions
Loss of genioglossus activity precipitating events in patients with OSA is strongly associated with a contemporaneous loss of drive and is greatest in those whose activity tracks drive rather than pressure stimuli. These findings were upheld for events without prior arousal. Responding to falling drive rather than rising negative pressure during events may be deleterious; future therapeutic strategies whose aim is to sustain genioglossus activity by preferentially enhancing responses to rising pressure rather than falling drive are of interest.
Keywords: upper airway pathophysiology, dilator muscle, chemical drive, negative pressure
Obstructive sleep apnea (OSA) is a highly prevalent disorder associated with adverse long-term health outcomes (1) for which novel therapeutic approaches are the focus of considerable investigation (2–4). A defining feature of OSA is the loss of pharyngeal dilator muscle activity that precipitates respiratory events during sleep. It is now well established that sustaining pharyngeal muscle activity during sleep could ameliorate OSA. First, pharyngeal collapse occurs exclusively during sleep, meaning that the neural sources of activity present in wakefulness are always sufficient to maintain airway patency, even in highly predisposed individuals. Second, dampened responsiveness of the genioglossus, the largest phasic dilator muscle, during sleep is a key trait discriminating between patients with OSA and healthy control subjects with similar pharyngeal collapsibility (5–7). Third, increasing genioglossus activity pharmacologically via adrenergic stimulation (8–10), or via surgically implanted devices (3, 11), can prevent respiratory events in OSA (10, 12–14). Moreover, when genioglossus activity is augmented spontaneously within sleep (e.g., in deeper sleep and/or at greater respiratory drive), periods of stable breathing can occur, even in patients with otherwise severe OSA (15). Notably, the precise physiological mechanisms underlying the spontaneous loss of genioglossus muscle activity during sleep that precipitates respiratory events remain unclear.
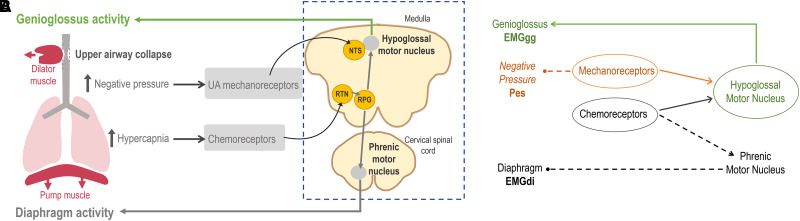
At sleep onset, a withdrawal of feed-forward wake-related facilitatory inputs to the hypoglossal motor nucleus results in a loss of genioglossus activity (16–21). Perplexingly, genioglossus activity continues to progressively decline within obstructive events in OSA during sleep (15, 22) despite the consequent rise in mechanical load, which in principle should stimulate at least one of two distinct feedback inputs (23–29): 1) negative pressure swings develop acutely with pharyngeal obstruction and are sensed largely by superficial mechanoreceptors in the upper airway and are relayed via the nucleus tractus solitarius to the hypoglossal motor nuclei (Figure 1), and 2) a load-induced reduction in ventilation and ensuing hypercapnia are subsequently sensed at peripheral (i.e., carotid body) and central chemoreceptors (e.g., retrotrapezoid nucleus), relayed in the form of increased ventilatory drive at the rostral ventrolateral medulla to both the phrenic and hypoglossal motor nuclei (Figure 1) (30). As intrathoracic negative pressure swings are generated by drive-induced contraction of inspiratory pump muscles, ventilatory drive was assumed to rise in synchrony with negative pressures during events. The ongoing and progressive loss of genioglossus activity (Figure 2) within events during sleep, despite increasing negative pressures, therefore appeared attributable to an ongoing loss of wake or arousal-related inputs.
Figure 1.
(A) Simplified conceptual diagram illustrating the separate major pathways for chemoreceptor “drive” and mechanoreceptor “pressure” inputs that determine genioglossus activity (EMGgg) during sleep. Laryngeal negative pressures are transduced by mechanoreceptors in the upper airway via the superior laryngeal nerve to the NTS to provide negative pressure reflex input to the hypoglossal motor nucleus (HGN). Hypercapnia is sensed by peripheral and central chemoreceptors and transduced to chemosensitive neurons in the medulla (e.g., at the RTN) that innervate the RPGs, which provide a common ventilatory drive signal to both the hypoglossal and phrenic motor nuclei. Additional pathways, including wakefulness or arousal inputs and (potential) pulmonary stretch receptor inputs to the HGN, are not shown. (B) Directed acyclic graph summarizing the statistical analysis approach. Multivariable regression was used to quantify separate mechanoreceptor and chemoreceptor inputs to the hypoglossal motor nucleus and the downstream EMGgg; Pes was used to represent mechanoreceptor input, and EMGdi was used to represent chemoreceptor input. EMGdi = diaphragm electromyography; NTS = nucleus tractus solitarius; Pes = esophageal pressure; RPG = respiratory pattern generator; RTN = retrotrapezoid nucleus.
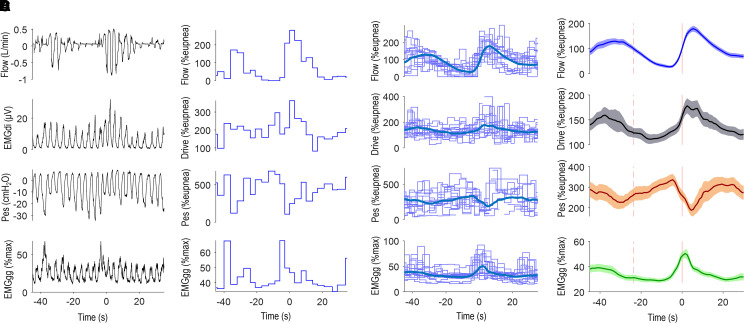
Figure 2.
Analysis of respiratory signals. (A) Example flow, diaphragm electromyography (EMGdi), esophageal pressure (Pes), and peak genioglossus activity (EMGgg) signals during a respiratory event in an example patient. Note that during the event, negative pressure swings increase progressively with obstruction, but EMGdi swings first fall and then rise later in the event. (B) For the same period, breath-by-breath values of ventilation (VE; presented as percentage eupnea %eupnea), drive (peak minus initial EMGdi swing, normalized by the ratio of EMGdi to VE during wakefulness breaths, presented as %eupnea), pressure (peak minus initial Pes swing, normalized by the ratio of Pes to VE during wakefulness breaths, presented as %eupnea), and EMGgg (peak EMGgg value, presented as %max). (C and D) For each signal, breath-by-breath data from all events were aligned at event termination (every fifth event is plotted for clarity) and ensemble averaged to provide a mean time course for each subject (C, thick blue line), also shown in D with shaded 95% confidence interval region. %max = percentage maximum.
Contrary to the prevailing view, we recently showed that ventilatory drive typically falls during respiratory events yet manifests rising negative pressure swings (15) indirectly via increased mechanical load in the presence of greater upper airway resistance. These findings not only illustrate that drive and pressure stimuli may often provide asynchronous and independent stimulation of the genioglossus during respiratory events but also raise the possibility that falling drive provides a sufficient explanation for the previously perplexing loss of genioglossus (despite increasing mechanical load) within OSA events.
Accordingly, in the present study, we aimed to investigate the separate, independent contributions of ventilatory drive and negative pressure to the time course of genioglossus activity during spontaneous events in patients with OSA using multivariable regression. Conceptually, if falling drive leads to a contemporaneous loss of genioglossus activity, an attendant increase in obstruction, and greater negative pressures, but genioglossus activity continues to fall, this ongoing decline in genioglossus activity would be attributable to the falling drive stimuli. Alternatively, if the obstruction-related negative pressure increase led to a contemporaneous increase in genioglossus activity, then the changes in genioglossus activity would be more attributable to the pressure stimuli. On this basis, we tested the hypothesis that falling ventilatory drive stimuli, independent of negative pressure stimuli, provide a quantitative explanation for the fall in genioglossus activity within events in patients with OSA. By revealing more about the potential mechanisms behind falling genioglossus activity during sleep, we sought to provide insight into underlying pathophysiology of OSA and a more precise target for future OSA therapies.
Methods
Participants, Procedure, and Setup
The present study is a secondary analysis of a larger physiology study designed to describe the typical physiological changes in ventilation, ventilatory drive, and dilator muscle activity during respiratory events in patients with OSA (15, 31, 32). Sixty-two participants with suspected or diagnosed OSA were enrolled; exclusion criteria included the use of respiratory stimulants or depressants (including opioids and benzodiazepines), heart failure or lung diseases, central sleep apnea, and pregnancy. Participants gave written informed consent, and study approval was granted by the Partners Institutional Review Board (2017P001255). Forty-two patients who exhibited at least mild OSA (apnea–hypopnea index > 5 events/h) and could tolerate the full equipment setup provided data for the present analyses.
Patients underwent overnight polysomnography with additional physiological measurements of ventilation (pneumotachography via sealed oronasal mask), ventilatory drive (via intraesophageal diaphragm electromyography EMG] catheter), and esophageal pressure (pressure-tipped catheter). For catheter positioning, nose–ear–xyphoid distances were measured in each individual; catheters were inserted through a lidocaine-anesthetized nostril to approximately nose–ear–xyphoid + 5 cm, then withdrawn slowly until optimal position was confirmed (see the data supplement) and taped at the nares. Genioglossus EMG was performed using two Teflon-coated (Cooner Wire Co.) stainless-steel fine-wire intramuscular electrodes (Cooner Wire Co.); 2 mm of Teflon was removed from the tip. After the application of topical anesthesia, electrodes were inserted using a 25-gauge needle ∼0.4 cm on either side of the frenulum to a depth of ∼1 cm (3). All data were acquired using Spike 2 software (Cambridge Electronic Design).
Sleep, arousals, and respiratory events were scored according to standard criteria (hypopnea was defined as a 30% reduction in airflow with ⩾3% desaturation or arousal [33]). Before any signal analysis for each individual, periods of time with sustained artifacts for any signal were manually listed for exclusion. Acute artifacts on esophageal pressures (e.g., large positive deflections with swallowing) were excluded via an automated algorithm. Diaphragm EMG and esophageal pressure signals were processed to remove electrocardiographic artifact and provide a moving time (root-mean-squared) signal for analysis. Breath-by-breath peak-minus-onset swings were tabulated and calibrated using wakefulness data to provide magnitudes as percentages of each patient’s estimated eupneic ventilation (Figures 2A and 2B; for more details, see the data supplement). Genioglossus EMG was rectified and moving-time averaged (100-ms time constant) (34). Breath-by-breath peak genioglossus muscle activity was calculated and presented as percentage maximum (measured during wakefulness via the maximal isometric tongue protrusion maneuver; see Figures 2A and 2B) or percentage baseline (mean pre-event peak genioglossus baseline from ensemble-average analysis; see below). Tonic genioglossus muscle levels (nadir within breath) were also examined in the supplementary analysis.
Ensemble-Average Respiratory Event Analysis
Relationships between genioglossus muscle activity and both drive and pressure were assessed using ensemble-average event profiles (1, 35, 36): within a patient, drive, pressure, and peak genioglossus activity from each event were aligned at event termination and overlaid to provide a mean time course for each signal (“individual patient” analysis; see Figures 2C and 2D). At least 10 events were required for analysis. The averaging of breath-by-breath data across events or disturbances has been used widely in physiological analyses (36, 37–41) and was used here to minimize the effects of measurement noise on the associations observed (see the data supplement).
To provide a single representative time course of drive, pressure, and genioglossus activity across events for all patients (“multipatient” analysis), all individual patient averages were again ensemble averaged (15), aligning start and end times to match the average event duration (stretching or compressing individual patient ensemble averages as needed).
Statistical Analyses
To address the primary hypothesis that falling genioglossus activity is associated with falling ventilatory drive during events independent of negative pressure, we assessed the association between peak genioglossus activity (the dependent variable) and drive and pressure swings (the independent variables) in each patient using multivariable linear regression analyses applied to ensemble-averaged data. Specifically, for each patient, we separately modeled genioglossus activity ∼ βdrive × drive + βpressure × pressure. We emphasize that regression analysis describes correlations and is not designed to establish causality; rather, the goal of the study was to determine the relative influence of two previously established contributing factors to genioglossus activation during spontaneous respiratory events. The multivariable analysis approach was designed to quantify the sensitivity of the separate pathways by which drive and pressure are detected (Figure 1): multivariable βdrive (Figure 1, gray pathway) was taken as an estimate of the direct effect of ventilatory drive on genioglossus activity, without the indirect effect of drive on genioglossus activity through generating negative pressure (captured by βpressure; Figure 1, orange pathway). Likewise, multivariable βpressure captures the sensitivity of the negative pressure sensing pathway that includes both upstream drive and resistance contributions to generating observed negative pressure swings. Analyses were limited to data within the bounds of each individual’s average respiratory event; that is, pre-event arousal periods were intentionally excluded to minimize the role of the withdrawal of wake or arousal-related stimuli, which was not the focus of the present study. In primary analyses, drive, pressure, and genioglossus signals were standardized by 1 standard deviation (SD) to facilitate the interpretation of how much variability in genioglossus activity is explained by the observed changes in pressure versus drive. An independent role for drive in the loss of genioglossus activity during events was accepted if the lower 95% confidence bound for multivariable βdrive exceeded zero (mixed-model analysis). P values <0.05 were considered to indicate statistical significance.
In exploratory analysis, we examined whether multivariable βdrive typically exceeded βpressure (mixed-model analysis).
In alternative (secondary) analysis, the above analyses were repeated using the multipatient ensemble-averaged data that describe a single typical time course of the three physiological signals.
In an additional exploratory analysis designed to further minimize the potential influence of postarousal inputs on drive, we repeated the primary analysis isolated to events without prior arousal. For events to be used, arousals could not be present within 15 seconds of any event start time.
On the basis that patients varied considerably in whether genioglossus activity predominantly tracked drive (βdrive) or pressure (βpressure) during events, we calculated the drive-versus-pressure contribution to genioglossus activity for each individual by calculating the ratio of βdrive to βpressure from the primary multivariable analysis. βdrive:βpressure = ∞ indicates that genioglossus activity is positively associated with drive but not with pressure (βpressure is ⩽0), βdrive:βpressure = 0 indicates that genioglossus activity is positively associated with pressure but not drive (βdrive is ⩽0), and βdrive:βpressure near 1 indicates that genioglossus activity is associated with both drive and pressure with similar magnitudes. We also assessed whether this drive-versus-pressure contribution may be a novel deleterious trait contributing to the magnitude of the loss of genioglossus activity during events (pre-event baseline to event nadir) using multivariable linear regression (adjusting for absolute changes in drive and pressure during events).
Results
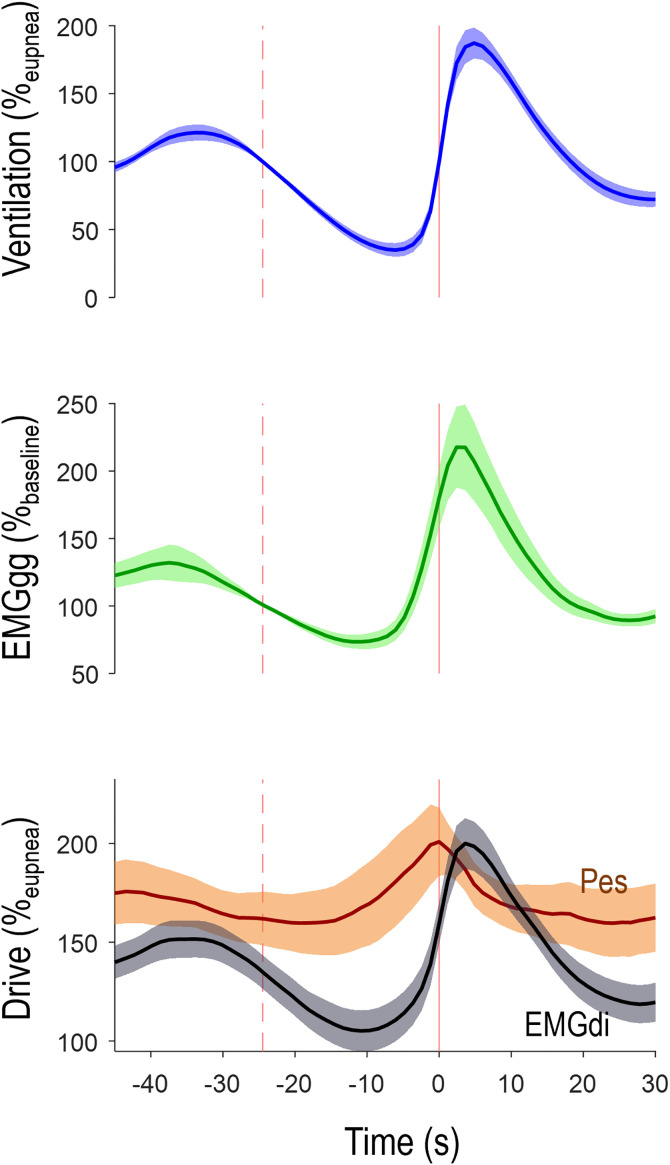
Baseline characteristics are detailed in Table 1. Patients exhibited a broad range of OSA severities (5–91 events/h) on the study night. Overall, genioglossus activity fell early during events (median reduction, 18 [interquartile range (IQR), −1 to 35] %baseline), accompanied by a reduction in drive (reduction, 19 [11–33] %baseline), but no systematic reduction in pressure swings was observed (increase, 7 [−4 to 22] %baseline) (see Figure 3 and Table 2).
Table 1.
Patient characteristics
| Characteristic | All Subjects (N = 42) |
|---|---|
| Demographics | |
| Age, yr | 57.4 ± 9.6 |
| Sex | |
| Male | 27 |
| Female | 15 |
| Race | |
| Black | 12 |
| White | 29 |
| Asian | 0 |
| Other | 1 |
| Body mass index, kg/m2 | 32.8 ± 6.4 |
| Neck circumference, cm | 41.7 ± 4.8 |
| Polysomnography* | |
| OSA severity | |
| Mild | 13 |
| Moderate | 8 |
| Severe | 21 |
| Apnea–hypopnea index, total (events/h) | 26 (13–44) |
| Fraction of hypopneas | 52 (34–87) |
| Fraction of central events | 0 (0–0) |
| Fraction of events ending in arousal | 89 (77–96) |
| Mean event duration, s | 22 ± 6 |
| Sleep time, spontaneous breathing off CPAP, min | 193 (88–283) |
| N1, % sleep off CPAP | 40 (23–63) |
| N2, % sleep off CPAP | 41 (32–58) |
| N3, % sleep off CPAP | 1 (0–11) |
| REM sleep, % sleep off CPAP | 4 (0–11) |
Definition of abbreviations: CPAP = continuous positive airway pressure; N = non–rapid eye movement sleep stage; OSA = obstructive sleep apnea; REM = rapid eye movement.
Values are mean ± SD, n, or median (interquartile range) as determined by normality.
Polysomnographic respiratory event data refer to the period off CPAP. OSA severity categories are as follows: mild (5–15 events/h), moderate (15–30 events/h), and severe (⩾30 events/h).
Figure 3.
Average time course of ventilation, peak genioglossus activity (EMGgg), and the ventilatory drive (EMGdi, gray) and intrathoracic pressure (Pes, orange) stimuli during events across all patients (N = 42; shading denotes 95% confidence interval). The vertical dashed line illustrates the average event start, and the solid vertical line illustrates event end. Note that the time course of EMGgg visibly matches the time course of drive but not that of pressure. EGMdi = diaphragm electromyography; Pes = esophageal pressure.
Table 2.
Drive-related event pathophysiology
| Characteristic | All Subjects |
|---|---|
| Reduction in EMGgg, %baseline | 18 (−1 to 35) |
| Increase in negative pressure swings, Pes, %baseline | 7 (−4 to 22) |
| Reduction in drive, EMGdi, %baseline | 19 (11 to 33) |
| Reduction in ventilation, %baseline | 65 (55 to 76) |
| Increase in obstruction, %baseline | 56 (35 to 75) |
Definition of abbreviations: EMGdi = diaphragm electromyography; EMGgg = genioglossus activity; Pes = esophageal pressure.
Continuous data are shown as median (interquartile range). Values are taken from ensemble-average event analysis for each patient and represent the change in each respiratory variable from pre-event baseline to the point of nadir ventilation. Note that pressure swings increase during events, in contrast to falling EMGgg, drive, and ventilation. Increase in obstruction is calculated from the percentage reduction in flow:drive ratio.
Time Course of Genioglossus Activity within Events
Individual Patient Analysis
Multivariable regression applied to individual subject data (a unique statistical model for each subject) showed that both ventilatory drive and pressure signals were associated with the time course of peak genioglossus activity during events, producing good model fits (median model R = 0.91 [IQR, 0.88–0.98]; Table 3). Notably, the magnitude of the multivariable association with genioglossus activity was greater for drive (median βdrive = 0.85; Table 3) compared with pressure (median βpressure = 0.31) (mixed-model estimated difference = +0.30 [0.02–0.59]; P = 0.035; median βdrive:βpressure = 2.9). The estimated absolute change in genioglossus activity per change in drive (per unstandardized model, βdrive = 9.4 [3.8–17.3] %baseline per 10%eupnea; 10%eupnea is equivalent to ∼0.9 cm H2O) was approximately threefold greater than the absolute changes in genioglossus activity per change in negative pressure (βpressure = 2.8 [−2.1 to 24.8] %baseline per 10%eupnea). The findings were unchanged when analysis was confined to non–rapid eye movement sleep only (see Table E1 in the data supplement). Replacing peak genioglossus activity with tonic genioglossus activity did not weaken the association with drive (see Table E2).
Table 3.
Bivariate and multivariable analysis of time course of genioglossus activity during events
| Peak Genioglossus |
||
|---|---|---|
| Independent Variable | Individual Patient Ensemble Average | Multipatient Ensemble Average |
| Median (IQR) | Estimate (95% CI) | |
| ΔEMGgg per reduction in drive (standardized) | 0.85 (0.43 to 0.96) | 0.75 (0.63 to 0.86) P = 1 × 10−10 |
| ΔEMGgg per reduction in pressure (standardized) | 0.31 (−0.17 to 0.88) | 0.50 (0.38 to 0.61) P = 5 × 10−8 |
| Drive-vs.-pressure contribution* | 2.90 (0.47 to ∞) | 1.50 (1.16 to 1.99) |
| ΔEMGgg per reduction in drive (unstandardized) | 9.4 (3.8 to 17.3) | 14.4 (12.2 to 16.5) P = 1 × 10−10 |
| ΔEMGgg per reduction in pressure (unstandardized) | 2.8 (−2.1 to 24.8) | 7.3 (5.6 to 8.9) P = 5 × 10−8 |
Definition of abbreviations: %max = percentage maximum; CI = confidence interval; EMGgg = genioglossus activity; IQR = interquartile range; Pes = esophageal pressure; SD = standard deviation.
For individual patient analysis, values are determined from β coefficients from individual regression models fit to each patient and presented as median (IQR) across subjects. For multipatient analysis, values are determined from a single regression model fit to the ensemble-averaged data (Figure 2). Data were first standardized by 1 SD so the relative contributions of drive and pressure could be compared (1 SD in drive = 9.5%eupnea, Pes = 12.4%eupnea, peak genioglossus activity = 18.3%baseline or 1.8%max). Percentage baseline refers to the pre-event baseline peak genioglossus value. Unstandardized multivariate ensemble-average results describe the reduction in EMGgg in percentage baseline per 10%eupnea reduction in drive or pressure; a 10%eupnea change in pressure is equivalent to 0.9 cm H2O average. Model fits were good (median [IQR] individual multivariate model R = 0.91 [0.88–0.98], multipatient multivariate model R = 0.97). EMGgg refers to breath-by-breath peak value of the moving time-averaged signal.
Drive-vs.-Pressure contribution reflects the ratio of the standardized drive and pressure β coefficients.
Multipatient Analysis
With all patient data pooled to provide a single aggregate time course for each signal (Figure 3), results were similar. Multivariable regression showed that typical drive and pressure signals adequately describe the typical time course of genioglossus activity (model R = 0.97; Figure 3) and that genioglossus activity again tracked drive more closely than pressure (βdrive = 0.75 [0.63–0.86], βpressure = 0.50 [0.38–0.61]; Table 3; difference = 0.25 [0.07–0.42]; P = 0.007; βdrive:βpressure = 1.50]).
Analysis of Events without Prior Arousal
Sensitivity analysis examining events without prior arousal yielded similar findings compared with all events in the same patients (n = 26, βdrive = 0.85 [0.60–1.05] without arousal compared with 0.87 [0.45–0.91] for all events; see Table E3). The absolute loss of genioglossus activity per decrement in drive was also similar without arousal (8.5 [3.5–14.3] %baseline per 10%eupnea) versus all events (9.9 [7.2–16.2] %baseline per 10%eupnea in this subset).
Heterogeneity across Subjects
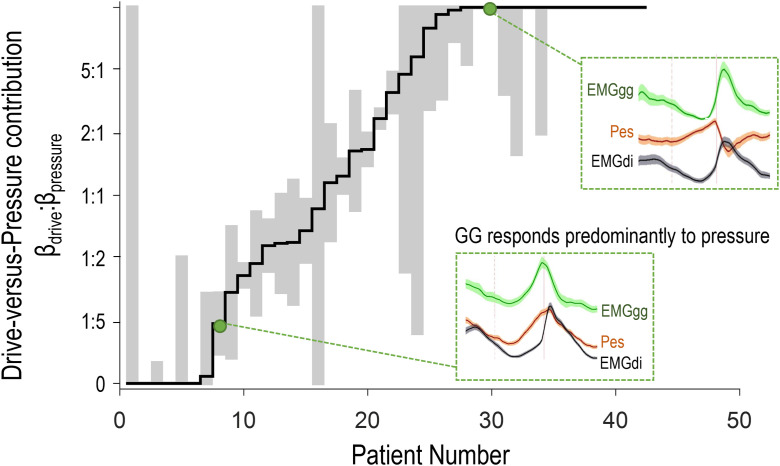
Individual patient analysis revealed wide variability across subjects in the relative contributions of drive and pressure stimuli (βdrive:βpressure) to the time course of genioglossus activity (Figure 4). Approximately one-half of patients (n = 22 of 42) lacked a meaningful association between genioglossus activity and pressure (the association of genioglossus activity with drive was more than twice that with pressure βdrive:βpressure > 2:1]; i.e., these patients were “drive dominant”). Conversely, one-quarter (n = 11 of 42) exhibited “pressure-dominant” genioglossus associations (βdrive:βpressure < 1:2), and the remainder (n = 9 of 42) exhibited balanced associations with both drive and pressure stimuli (1:2 ⩽ βdrive: βpressure ⩽ 2:1). Subgroup-level patient characteristics are documented in Table E4.
Figure 4.
Individual data demonstrating the prevalence of patients whose genioglossus activity (EMGgg) during events is predominantly explained by drive (high drive-vs.-pressure contribution, i.e., βdrive:βpressure > 2:1; n = 22 of 42), by pressure (low drive-vs.-pressure contribution, i.e., βdrive:βpressure < 1:2; n = 11 of 42), or by a more balanced combination of both drive and pressure stimuli (βdrive similar to βpressure; n = 9). Inset plots show the signal time course of EMGgg compared with esophageal pressure (Pes) and drive diaphragm electromyography (EMGdi) stimuli during events for two representative example patients (as per Figure 2). Note that EMGgg closely tracks EMGdi in patient number 30 (high drive-versus-pressure contribution) and closely tracks Pes in patient 8 (low drive-vs.-pressure contribution). Gray bars (indicating 95% confidence interval) demonstrate that for most individuals, separate drive-versus-pressure contributions to EMGgg could be differentiated using multivariable regression. βdrive describes the association between EMGgg and drive (standardized coefficient, adjusting for pressure); βpressure describes the association between EMGgg and pressure (adjusting for drive).
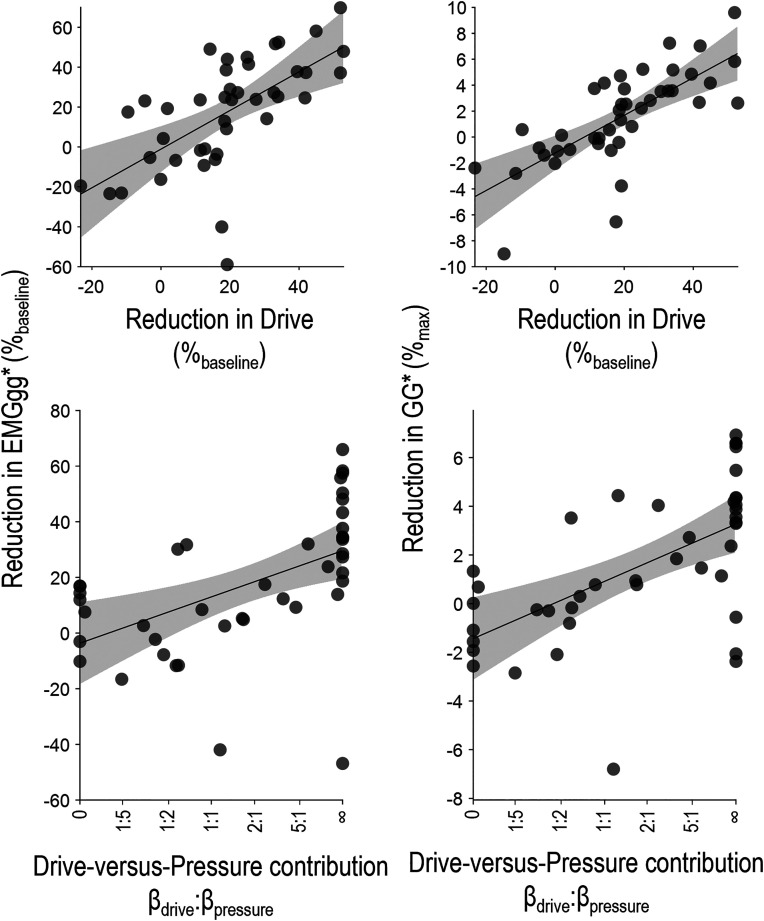
Magnitude of Genioglossus Reduction during Events
The magnitude of reduction in genioglossus activity during events varied considerably among patients (median, 18 [IQR, −1–35] %baseline; Table 2). A greater reduction in genioglossus activity was independently associated with a higher drive-versus-pressure contribution (βdrive:βpressure), independent of changes in drive and pressure (Table 4 and Figure 5). That is, patients with pressure-dominant responses exhibited smaller reductions in genioglossus activity during events (Tables 4 and E5). The reduction in genioglossus activity was also independently associated with the magnitude of change in drive, but not with the magnitude of change in pressure during events.
Table 4.
Multivariable analysis of reduction in genioglossus activity during events
| Independent Variable | Reduction in EMGgg |
|
|---|---|---|
| %baseline | %max | |
| Reduction in drive | 17.8 (8.7 to 26.8) P = 0.0003 |
2.7 (1.6 to 3.7) P = 8 × 10−6 |
| Reduction in pressure | −3.0 (−11.6 to 5.5) P = 0.5 |
−0.4 (−1.4 to 0.6) P = 0.4 |
| Drive-vs.-pressure contribution (βdrive:βpressure) | 12.9 (4.8 to 21.0) P = 0.003 |
1.8 (0.9 to 2.8) P = 0.0003 |
Definition of abbreviations: %max = percentage maximum; EMGgg = genioglossus activity; SD = standard deviation.
Reduction in EMGgg was regressed against the reduction in drive, the reduction in pressure, and the drive-versus-pressure contribution (βdrive:βpressure, transformed). Independent variables were 1-SD standardized and to facilitate comparisons. Reduction in EMGgg, drive, and pressure were all calculated at nadir ventilation. Model R2 = 0.32 (left) and 0.43 (right).
Figure 5.
The magnitude of the reduction in genioglossus activity (EMGgg) during events differed from patient to patient. A greater reduction in genioglossus activity was independently associated with a greater reduction in drive (top) and a tendency of the genioglossus activity to track drive rather than pressure (drive-vs.-pressure contribution, bottom). Findings were similar when examining the loss of EMGgg in units of percentage baseline (mean pre-event peak EMGgg, left) or in units of percentage maximum (right). Models are described in Table 4. *Top panels show the independent associations between reduction in EMGgg and drive after adjusting for the reduction in esophageal pressure (Pes) and the drive-versus-pressure contribution. Bottom panels show the independent associations between reduction in EMGgg and drive-versus-pressure contribution after adjusting for the reduction in drive and the reduction in Pes.
Discussion
The present study demonstrated for the first time how genioglossus activity changes during events in association with drive and pressure stimuli. Overall, genioglossus activity falls during events in OSA, with a time course that more closely tracks the fall in ventilatory drive, as opposed to changes in intrathoracic pressures. Specifically, using individual subject–level event time-course data, a 1-SD reduction in ventilatory drive was independently associated with a 0.85-SD fall in genioglossus activity, whereas a 1-SD increase in intrathoracic pressure swings was independently associated with a smaller 0.31-SD rise in genioglossus activity. Combined, both stimuli provide a good fit to the typical falling-then-rising time course of genioglossus activity during events (median R = 0.91). Overall, falling ventilatory drive stimuli provides a novel quantitative explanation for the previously puzzling observation that genioglossus activity continues to fall during events, even in the face of the rising negative pressure stimuli.
The present study also revealed considerable interpatient heterogeneity in response to drive versus pressure during events: although ∼50% of patients exhibited meaningfully greater associations between ventilatory drive and genioglossus activity (>2:1 drive vs. pressure), roughly one-quarter of patients exhibited stronger responses to pressure rather than drive (<1:2 drive vs. pressure). As intrathoracic pressures rise sooner than ventilatory drive in the face of obstruction, a tendency to respond to falling drive instead of rising pressures may be a novel deleterious trait contributing to the pathophysiology of OSA (in ∼50% of patients).
Physiological Insight
Our finding that genioglossus activity tracks drive more closely than negative pressures during events initially appears unexpected. Physiological studies have established negative intrathoracic pressures generated by respiratory effort (i.e., in the presence of obstruction) as a leading stimulus for genioglossus muscle activation (24, 42–44). Notably, awake healthy control subjects exhibit robust genioglossus responses to negative pressures generated under experimental conditions with absent ventilatory drive (42). Although these studies support a robust genioglossus response to negative pressures in individuals without OSA, evidence for dominant negative pressure responses in patients with OSA within respiratory events is inconclusive.
An unsolved mystery in sleep apnea pathophysiology has been why, for most patients, genioglossus activity continues to fall throughout obstructive respiratory events despite rising negative pressure stimuli. The present study extended our previous finding—that drive typically falls whereas pressures rise during events (15)—to show that the falling drive appears to provide a leading explanation for the transient loss of dilator muscle activity that precipitates obstruction within sleep in patients with OSA. Prior studies provide support for this conclusion: Jordan and colleagues (36) showed, in 10–12 patients, that genioglossus and tensor palatini activity initially fall and then later rise during events, whereas epiglottic pressure swings progressively rise (diaphragm EMG was not performed). Notably, Lo and colleagues showed, in 11 healthy control subjects, that the genioglossus response to CO2 (slope) was not affected by continuous positive airway pressure (CPAP)–related attenuation of negative pressure involvement (25); that is, chemical drive raises genioglossus activity directly (e.g., via parafacial respiratory group [45]), with minimal indirect action of drive mediated by negative pressure afferent pathways. In another study of control subjects (29), removal of hypercapnic/hypoxic stimuli did, however, attenuate genioglossus pressure responses during non–rapid eye movement sleep, suggesting that baseline chemoreceptor activation may in part regulate the response to negative pressure. Taken together, it is now clear that the progressive fall in genioglossus activity during events is adequately accounted for by its response to falling ventilatory drive, even in the face of increased obstructive load.
Typically, genioglossus responsiveness is measured experimentally during challenges that fully occlude or obstruct the airway and provide simultaneous increases in negative pressure and drive stimuli. Here, in the first study (to our knowledge) to tease apart the separate drive and pressure inputs to genioglossus activity in patients with OSA during sleep, we identified a potential new subendotype of pharyngeal dilator muscle deficiency: a tendency to respond to drive in preference to negative pressure. In the context of pharyngeal obstruction typically led by a reduction in drive (15), we consider the pitfalls of such a physiological defense strategy. Sudden obstruction leads immediately to an increase in negative pressure, but only after multiple breaths of compromised gas exchange does obstruction eventually manifest an increased chemical drive. Hence, it is possible that preferential responses to drive over pressure are a novel causal factor in OSA pathogenesis. The study also provides a potential explanation for why most patients, even those with strong experimentally induced genioglossus responses (to combined drive and pressure stimuli), remain vulnerable to obstructive events, as a reliance on drive stimuli will yield events whenever drive is reduced.
Clinical Implications
Preventing the loss of upper airway muscle activity that precipitates events is a major target for future sleep apnea intervention. On the basis of our findings, a future therapeutic strategy may be to preferentially augment responses to rising negative pressure stimuli to provide for sustained muscle activity in the face of falling ventilatory drive during events. Indeed, topical potassium channel inhibition in the upper airway to target the negative pressure reflex has been considered as an approach to improve pharyngeal function in OSA (46). Raising the responsiveness of the genioglossus to ventilatory drive stimuli per se (i.e., increasing the slope without changing baseline activity), such that genioglossus activity is highly contingent on the maintenance of ventilatory drive, may be a less effective strategy. These therapeutic strategies will require a greater understanding of the basic neurobiological mechanisms underlying responses to stimuli and the transmission pathways for respiratory drive from the rostral medulla through to the upper-airway motoneurons.
Limitations
The present study has several limitations.
-
1.
We did not directly stimulate the upper airway with isolated drive and pressure stimuli to determine mechanisms. Such experiments have already been performed (23–28); the aim here was instead to assess how these known distinct pathways may contribute to genioglossus activity variability within events in the context of OSA pathogenesis.
-
2.
Although our study is large for a complex physiological study, the sample is underpowered to provide insight into age, sex, or obesity contributions.
-
3.
We considered the possibility that a progressive decline in state-related (wakefulness or arousal) drive inputs to both genioglossus activity and phrenic output (drive) during events could be responsible for the main findings in our study (i.e., a loss of wakefulness stimuli as sleep deepens may explain the strong association between falling drive and genioglossus activity in events). We could not definitively discriminate between declining wakefulness sources and chemical sources of drive in our main analyses, though our analyses were confined to periods within events to minimize known arousal effects. Exploratory analysis showed that a strong drive–genioglossus association was present (see Table E3) even in the absence of a prior arousal. This additional finding leads us to interpret that drive changes within events are likely of “chemical drive” rather than “wakefulness drive” origin, but a role for more gradual changes in wakefulness inputs to drive (and in turn genioglossus activity) within events cannot be excluded. Notably, ongoing withdrawal of wake or arousal stimuli is now not the only potential explanation for the observed progressive decline in genioglossus activity throughout respiratory events in OSA. (We note that our work does not diminish the known importance of state-dependent inputs on pharyngeal dilator muscles at sleep onset, nor does it challenge the notion that sleep is an essential precondition for the described pathophysiology.)
-
4.
In our main analysis we did not consider the potential additional input of pulmonary stretch receptors to genioglossus activity, which would theoretically become disinhibited with the reduction in stretch in the presence of event-related obstruction and falling tidal volume (47). In human sleeping adults, unlike negative pressure stimulation, pulmonary stretch receptor disinhibition has not been demonstrated to contribute to genioglossus activity in the context of resistive load (44, 48). If this third input were present, we expect that our study would have overestimated the genioglossus response to pressure (as a reduction in tidal stretch accompanies rising negative pressure) and underestimated the genioglossus response to drive (as the disinhibition with reduced tidal stretch may have partially counteracted a larger underlying drive related loss of genioglossal activity).
-
5.
The present study was focused on peak genioglossus activity, which captures the sum of both phasic and tonic components. Exploratory analysis of tonic activity continued to show greater associations between genioglossus activity and drive versus pressure (see Table E2).
-
6.
We measured esophageal pressure rather than local pressure at the laryngeal mechanoreceptors directly. In an exploratory analysis, we examined a subset of patients (n = 15) in whom we collected simultaneous epiglottic pressure measurements. Notably, the time courses of esophageal and epiglottic pressure swings during events were strongly correlated (R = 0.95; see Figure E1). Replacement of esophageal pressure with epiglottic pressure in multivariate analysis did not yield an increased role of pressure (βpressure = 0.63 for both in multipatient analysis).
-
7.
Our modeling of the roles of drive and pressure assumes separate additive inputs, yet we also considered that lowered drive might dampen responses to negative pressure (i.e., a more than additive interaction), as seen in iron-lung experiments (29, 42). Exploratory analysis including a drive–pressure interaction term in the multipatient average analysis indeed identified a modest interaction effect (estimated βdrive × pressure = 0.22 [95% confidence interval, 0.18–0.27]) that slightly improved the model R (from 0.97 to 0.996) that indeed indicated a stronger genioglossus response to pressure in the presence of higher drive. Thus, falling drive may be additionally deleterious to upper airway muscle activity by indirectly attenuating the response to negative pressure. We emphasize that the main findings described above remained relevant with the interaction included (see the data supplement), and the interaction effect explained relatively little of the total variance (5%).
-
8.
One-quarter of patients exhibited pressure-dominant responses characterized by an increased correlation between genioglossus activity and negative pressure. We considered whether an increase in correlation with pressure may be an artificial byproduct of a less severe event-related increase in obstruction (and thus a smaller load-induced increase in pressure swings), such that pressure simply better tracks falling genioglossus activity and drive. If so, such an effect would imply an even greater role for drive than described. Several factors lead us to conclude that the pressure correlation was not overly inflated in these individuals. First, we reason that a smaller pressure range would be expected to yield a smaller measured correlation in the presence of random noise. Second, we did not see evidence of reduced multivariable model quality in this subgroup (see Figure E2) that would suggest that there was greater difficulty discriminating between pressure and drive contributions in this subgroup. Finally, we emphasize that genioglossus activity fell less in this group (vs. drive-dominant patients) despite a greater reduction in drive (see Table E5), supporting the view that this group authentically exhibited a degree of genioglossus activity that was better preserved by tracking pressure- versus drive-dominant patients.
-
9.
The genioglossus is an important phasic pharyngeal dilator muscle whose responsiveness is known to influence OSA (5, 23, 25, 27, 42, 44), but other dilator muscles may behave differently. We note that nongenioglossus dilator muscles are generally believed to track state-related input more closely (49), so studying their drive versus pressure contributions was not prioritized.
-
10.
We considered whether genioglossus electrode placement could sample more selectively from motor units that receive drive inputs in some patients and pressure inputs in others and thus affect patient subendotype. However, this possibility appears unlikely, as our EMG recordings pick up signals comprising multiple motor units between the two electrodes (each approximately 4 mm on either side of the lingual frenulum).
Conclusions
Overall, genioglossus activity tracks ventilatory drive more closely than intrathoracic pressure during events in patients with OSA. We now consider that the leading theory underlying the ongoing decline in genioglossus activity, despite rising negative pressures, is that genioglossus activity responds more closely to drive. Notably, the weak temporal association between genioglossus activity and rising negative pressure stimuli seen in many patients highlights an opportunity for novel therapeutic intervention.
Footnotes
Supported by National Heart, Lung, and Blood Institute grant R01HL146697; American Heart Association grants 15SDG25890059 (parent study data collection) and 19CDA34660137 (A.A.); American Heart Association Postdoctoral Fellowship Awards 907613 (L.K.G.) and 20POST35210530 (D.V.); American Academy of Sleep Medicine Foundation grants 228-SR-20 (S.A.S.) and 188-SR-17 (A.A.); and National Institutes of Health grant R01HL153874 (A.A.).
Author Contributions: Conception: L.K.G., A.W., and S.A.S. Study design: L.K.G. and S.A.S. Data collection and analysis: L.K.G., D.V., N.C., S.A.S., L.T.-M., and L.H. All authors interpreted data, edited the manuscript for important intellectual content, and approved the final draft. L.K.G. and S.A.S. act as guarantors for the paper and take responsibility for the integrity of the work as a whole.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Azarbarzin A, Sands SA, Stone KL, Taranto-Montemurro L, Messineo L, Terrill PI, et al. The hypoxic burden of sleep apnoea predicts cardiovascular disease-related mortality: the Osteoporotic Fractures in Men Study and the Sleep Heart Health Study. Eur Heart J . 2019;40:1149–1157. doi: 10.1093/eurheartj/ehy624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Taranto-Montemurro L, Messineo L, Wellman A. Targeting endotypic traits with medications for the pharmacological treatment of obstructive sleep apnea: a review of the current literature. J Clin Med . 2019;8:1846. doi: 10.3390/jcm8111846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Eastwood PR, Barnes M, Walsh JH, Maddison KJ, Hee G, Schwartz AR, et al. Treating obstructive sleep apnea with hypoglossal nerve stimulation. Sleep (Basel) . 2011;34:1479–1486. doi: 10.5665/sleep.1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Eckert DJ. Phenotypic approaches to obstructive sleep apnoea—new pathways for targeted therapy. Sleep Med Rev . 2018;37:45–59. doi: 10.1016/j.smrv.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 5. Sands SA, Eckert DJ, Jordan AS, Edwards BA, Owens RL, Butler JP, et al. Enhanced upper-airway muscle responsiveness is a distinct feature of overweight/obese individuals without sleep apnea. Am J Respir Crit Care Med . 2014;190:930–937. doi: 10.1164/rccm.201404-0783OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Eckert DJ, White DP, Jordan AS, Malhotra A, Wellman A. Defining phenotypic causes of obstructive sleep apnea: identification of novel therapeutic targets. Am J Respir Crit Care Med . 2013;188:996–1004. doi: 10.1164/rccm.201303-0448OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Patil SP, Schneider H, Marx JJ, Gladmon E, Schwartz AR, Smith PL. Neuromechanical control of upper airway patency during sleep. J Appl Physiol (1985) . 2007;102:547–556. doi: 10.1152/japplphysiol.00282.2006. [DOI] [PubMed] [Google Scholar]
- 8. Chan E, Steenland HW, Liu H, Horner RL. Endogenous excitatory drive modulating respiratory muscle activity across sleep-wake states. Am J Respir Crit Care Med . 2006;174:1264–1273. doi: 10.1164/rccm.200605-597OC. [DOI] [PubMed] [Google Scholar]
- 9. Taranto-Montemurro L, Edwards BA, Sands SA, Marques M, Eckert DJ, White DP, et al. Desipramine increases genioglossus activity and reduces upper airway collapsibility during non-REM sleep in healthy subjects. Am J Respir Crit Care Med . 2016;194:878–885. doi: 10.1164/rccm.201511-2172OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Taranto-Montemurro L, Messineo L, Sands SA, Azarbarzin A, Marques M, Edwards BA, et al. The combination of atomoxetine and oxybutynin greatly reduces obstructive sleep apnea severity: a randomized, placebo-controlled, double-blind crossover trial. Am J Respir Crit Care Med . 2019;199:1267–1276. doi: 10.1164/rccm.201808-1493OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Strollo PJ, Jr, Soose RJ, Maurer JT, de Vries N, Cornelius J, Froymovich O, et al. STAR Trial Group Upper-airway stimulation for obstructive sleep apnea. N Engl J Med . 2014;370:139–149. doi: 10.1056/NEJMoa1308659. [DOI] [PubMed] [Google Scholar]
- 12. Taranto-Montemurro L, Messineo L, Azarbarzin A, Vena D, Hess L, Calianese N, et al. Effects of the combination of atomoxetine and oxybutynin on obstructive sleep apnea endotypic traits. Chest . 2020;157:1626–1636. doi: 10.1016/j.chest.2020.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kezirian EJ, Goding GS, Jr, Malhotra A, O’Donoghue FJ, Zammit G, Wheatley JR, et al. Hypoglossal nerve stimulation improves obstructive sleep apnoea: 12-month outcomes. J Sleep Res . 2014;23:77–83. doi: 10.1111/jsr.12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Op de Beeck S, Wellman A, Dieltjens M, Strohl KP, Willemen M, Van de Heyning PH, et al. STAR Trial Investigators Endotypic mechanisms of successful hypoglossal nerve stimulation for obstructive sleep apnea. Am J Respir Crit Care Med . 2021;203:746–755. doi: 10.1164/rccm.202006-2176OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gell LK, Vena D, Alex RM, Azarbarzin A, Calianese N, Hess LB, et al. Neural ventilatory drive decline as a predominant mechanism of obstructive sleep apnoea events. Thorax . 2022;77:707–716. doi: 10.1136/thoraxjnl-2021-217756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Worsnop C, Kay A, Pierce R, Kim Y, Trinder J. Activity of respiratory pump and upper airway muscles during sleep onset. J Appl Physiol (1985) . 1998;85:908–920. doi: 10.1152/jappl.1998.85.3.908. [DOI] [PubMed] [Google Scholar]
- 17. Fogel RB, Trinder J, Malhotra A, Stanchina M, Edwards JK, Schory KE, et al. Within-breath control of genioglossal muscle activation in humans: effect of sleep-wake state. J Physiol . 2003;550:899–910. doi: 10.1113/jphysiol.2003.038810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fogel RB, Trinder J, White DP, Malhotra A, Raneri J, Schory K, et al. The effect of sleep onset on upper airway muscle activity in patients with sleep apnoea versus controls. J Physiol . 2005;564:549–562. doi: 10.1113/jphysiol.2005.083659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mezzanotte WS, Tangel DJ, White DP. Influence of sleep onset on upper-airway muscle activity in apnea patients versus normal controls. Am J Respir Crit Care Med . 1996;153:1880–1887. doi: 10.1164/ajrccm.153.6.8665050. [DOI] [PubMed] [Google Scholar]
- 20. Horner RL, Liu X, Gill H, Nolan P, Liu H, Sood S. Effects of sleep-wake state on the genioglossus vs.diaphragm muscle response to CO2 in rats. J Appl Physiol (1985) . 2002;92:878–887. doi: 10.1152/japplphysiol.00855.2001. [DOI] [PubMed] [Google Scholar]
- 21. Horner RL, Kozar LF, Kimoff RJ, Phillipson EA. Effects of sleep on the tonic drive to respiratory muscle and the threshold for rhythm generation in the dog. J Physiol . 1994;474:525–537. doi: 10.1113/jphysiol.1994.sp020042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Younes M. Role of respiratory control mechanisms in the pathogenesis of obstructive sleep disorders. J Appl Physiol (1985) . 2008;105:1389–1405. doi: 10.1152/japplphysiol.90408.2008. [DOI] [PubMed] [Google Scholar]
- 23. Horner RL, Innes JA, Holden HB, Guz A. Afferent pathway(s) for pharyngeal dilator reflex to negative pressure in man: a study using upper airway anaesthesia. J Physiol . 1991;436:31–44. doi: 10.1113/jphysiol.1991.sp018537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Akahoshi T, White DP, Edwards JK, Beauregard J, Shea SA. Phasic mechanoreceptor stimuli can induce phasic activation of upper airway muscles in humans. J Physiol . 2001;531:677–691. doi: 10.1111/j.1469-7793.2001.0677h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lo YL, Jordan AS, Malhotra A, Wellman A, Heinzer RC, Schory K, et al. Genioglossal muscle response to CO2 stimulation during NREM sleep. Sleep . 2006;29:470–477. doi: 10.1093/sleep/29.4.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Onal E, Lopata M, O’Connor TD. Diaphragmatic and genioglossal electromyogram responses to CO2 rebreathing in humans. J Appl Physiol . 1981;50:1052–1055. doi: 10.1152/jappl.1981.50.5.1052. [DOI] [PubMed] [Google Scholar]
- 27. Malhotra A, Pillar G, Fogel RB, Edwards JK, Ayas N, Akahoshi T, et al. Pharyngeal pressure and flow effects on genioglossus activation in normal subjects. Am J Respir Crit Care Med . 2002;165:71–77. doi: 10.1164/ajrccm.165.1.2011065. [DOI] [PubMed] [Google Scholar]
- 28. Horner RL, Innes JA, Guz A. Reflex pharyngeal dilator muscle activation by stimuli of negative airway pressure in awake man. Sleep . 1993;16:S85–S86. doi: 10.1093/sleep/16.suppl_8.s85. [DOI] [PubMed] [Google Scholar]
- 29. Stanchina ML, Malhotra A, Fogel RB, Ayas N, Edwards JK, Schory K, et al. Genioglossus muscle responsiveness to chemical and mechanical stimuli during non-rapid eye movement sleep. Am J Respir Crit Care Med . 2002;165:945–949. doi: 10.1164/ajrccm.165.7.2108076. [DOI] [PubMed] [Google Scholar]
- 30. Ramirez JM, Garcia AJ, III, Anderson TM, Koschnitzky JE, Peng YJ, Kumar GK, et al. Central and peripheral factors contributing to obstructive sleep apneas. Respir Physiol Neurobiol . 2013;189:344–353. doi: 10.1016/j.resp.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sands SA, Edwards BA, Terrill PI, Taranto-Montemurro L, Azarbarzin A, Marques M, et al. Phenotyping pharyngeal pathophysiology using polysomnography in patients with obstructive sleep apnea. Am J Respir Crit Care Med . 2018;197:1187–1197. doi: 10.1164/rccm.201707-1435OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Messineo L, Eckert DJ, Taranto-Montemurro L, Vena D, Azarbarzin A, Hess LB, et al. Ventilatory drive withdrawal rather than reduced genioglossus compensation as a mechanism of obstructive sleep apnea in REM. Am J Respir Crit Care Med . 2022;205:219–232. doi: 10.1164/rccm.202101-0237OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Berry RB, Budhiraja R, Gottlieb DJ, Gozal D, Iber C, Kapur VK, et al. American Academy of Sleep Medicine; Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine Rules for scoring respiratory events in sleep: update of the 2007 AASM manual for the scoring of sleep and associated events. J Clin Sleep Med . 2012;8:597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Taranto-Montemurro L, Sands SA, Grace KP, Azarbarzin A, Messineo L, Salant R, et al. Neural memory of the genioglossus muscle during sleep is stage-dependent in healthy subjects and obstructive sleep apnoea patients. J Physiol . 2018;596:5163–5173. doi: 10.1113/JP276618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vena D, Azarbarzin A, Marques M, Op de Beeck S, Vanderveken OM, Edwards BA, et al. Predicting sleep apnea responses to oral appliance therapy using polysomnographic airflow. Sleep (Basel) . 2020;43:zsaa004. doi: 10.1093/sleep/zsaa004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jordan AS, White DP, Lo YL, Wellman A, Eckert DJ, Yim-Yeh S, et al. Airway dilator muscle activity and lung volume during stable breathing in obstructive sleep apnea. Sleep . 2009;32:361–368. doi: 10.1093/sleep/32.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jordan AS, Eckert DJ, Wellman A, Trinder JA, Malhotra A, White DP. Termination of respiratory events with and without cortical arousal in obstructive sleep apnea. Am J Respir Crit Care Med . 2011;184:1183–1191. doi: 10.1164/rccm.201106-0975OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Xiao SC, He BT, Steier J, Moxham J, Polkey MI, Luo YM. Neural respiratory drive and arousal in patients with obstructive sleep apnea hypopnea. Sleep (Basel) . 2015;38:941–949. doi: 10.5665/sleep.4746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ratnavadivel R, Stadler D, Windler S, Bradley J, Paul D, McEvoy RD, et al. Upper airway function and arousability to ventilatory challenge in slow wave versus stage 2 sleep in obstructive sleep apnoea. Thorax . 2010;65:107–112. doi: 10.1136/thx.2008.112953. [DOI] [PubMed] [Google Scholar]
- 40. Carberry JC, Jordan AS, White DP, Wellman A, Eckert DJ. Upper airway collapsibility (Pcrit) and pharyngeal dilator muscle activity are sleep stage dependent. Sleep (Basel) . 2016;39:511–521. doi: 10.5665/sleep.5516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cori JM, Thornton T, O’Donoghue FJ, Rochford PD, White DP, Trinder J, et al. Arousal-induced hypocapnia does not reduce genioglossus activity in obstructive sleep apnea. Sleep (Basel) . 2017;40:zsx057. doi: 10.1093/sleep/zsx057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pillar G, Fogel RB, Malhotra A, Beauregard J, Edwards JK, Shea SA, et al. Genioglossal inspiratory activation: central respiratory vs mechanoreceptive influences. Respir Physiol . 2001;127:23–38. doi: 10.1016/s0034-5687(01)00230-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. White DP, Edwards JK, Shea SA. Local reflex mechanisms: influence on basal genioglossal muscle activation in normal subjects. Sleep . 1998;21:719–728. doi: 10.1093/sleep/21.7.719. [DOI] [PubMed] [Google Scholar]
- 44. Malhotra A, Fogel RB, Edwards JK, Shea SA, White DP. Local mechanisms drive genioglossus activation in obstructive sleep apnea. Am J Respir Crit Care Med . 2000;161:1746–1749. doi: 10.1164/ajrccm.161.5.9907109. [DOI] [PubMed] [Google Scholar]
- 45. Jordan AS, White DP. Pharyngeal motor control and the pathogenesis of obstructive sleep apnea. Respir Physiol Neurobiol . 2008;160:1–7. doi: 10.1016/j.resp.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Osman AM, Mukherjee S, Altree TJ, Delbeck M, Gehring D, Hahn M, et al. Topical potassium channel blockage improves pharyngeal collapsibility: a translational, placebo-controlled trial. Chest . 2023;163:953–965. doi: 10.1016/j.chest.2022.11.024. [DOI] [PubMed] [Google Scholar]
- 47. Gauda EB, Carroll TP, Schwartz AR, Smith PL, Fitzgerald RS. Mechano- and chemoreceptor modulation of respiratory muscles in response to upper airway negative pressure. J Appl Physiol (1985) . 1994;76:2656–2662. doi: 10.1152/jappl.1994.76.6.2656. [DOI] [PubMed] [Google Scholar]
- 48. Horner RL. Neural control of the upper airway: integrative physiological mechanisms and relevance for sleep disordered breathing. Compr Physiol . 2012;2:479–535. doi: 10.1002/cphy.c110023. [DOI] [PubMed] [Google Scholar]
- 49. Oliven R, Cohen G, Dotan Y, Somri M, Schwartz AR, Oliven A. Alteration in upper airway dilator muscle coactivation during sleep: comparison of patients with obstructive sleep apnea and healthy subjects. J Appl Physiol (1985) . 2018;124:421–429. doi: 10.1152/japplphysiol.01067.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]