Abstract
Rationale/Objectives
Antibiotic selection for in-hospital treatment of pulmonary exacerbations (PEx) in people with cystic fibrosis (CF) is typically guided by previous respiratory culture results or past PEx antibiotic treatment. In the absence of clinical improvement during PEx treatment, antibiotics are frequently changed in search of a regimen that better alleviates symptoms and restores lung function. The clinical benefits of changing antibiotics during PEx treatment are largely uncharacterized.
Methods
This was a retrospective cohort study using the Cystic Fibrosis Foundation Patient Registry Pediatric Health Information System. PEx were included if they occurred in children with CF from 6 to 21 years old who had been treated with intravenous antibiotics between January 1, 2006, and December 31, 2018. PEx with lengths of stay <5 or >21 days or for which treatment was delivered in an intensive care unit were excluded. An antibiotic change was defined as the addition or subtraction of any intravenous antibiotic between Hospital Day 6 and the day before hospital discharge. Inverse probability of treatment weighting was used to adjust for disease severity and indication bias, which might influence a decision to change antibiotics.
Results
In all, 4,099 children with CF contributed 18,745 PEx for analysis, of which 8,169 PEx (43.6%) included a change in intravenous antibiotics on or after Hospital Day 6. The mean change in pre- to post-treatment percent predicted forced expiratory volume in 1 second (ppFEV1) was 11.3 (standard error, 0.21) among events in which an intravenous antibiotic change occurred versus 12.2 (0.18) among PEx without an intravenous antibiotic change (P = 0.001). Similarly, the odds of return to ⩾90% of baseline ppFEV1 were less for PEx with antibiotic changes than for those without changes (odds ratio [OR], 0.89 [95% confidence interval (CI), 0.80–0.98]). The odds of returning to ⩾100% of baseline ppFEV1 did not differ between PEx with versus without antibiotic changes (OR, 0.94 [95% CI, 0.86–1.03]). In addition, PEx treated with intravenous antibiotic changes were associated with higher odds of future PEx (OR, 1.17 [95% CI, 1.12–1.22]).
Conclusions
In this retrospective study, changing intravenous antibiotics during PEx treatment in children with CF was common and not associated with improved clinical outcomes.
Keywords: CF, antibacterial agents, hospitalization
Pulmonary exacerbations (PEx) are associated with substantial morbidity in people with cystic fibrosis (CF). CF PEx are diagnosed on the basis of specific signs and symptoms, including increased cough and sputum production, weight loss, and lung function decline (1–4). Although the U.S. Cystic Fibrosis Foundation has published PEx treatment recommendations (5), most of these are consensus based rather than evidence based. Unsurprisingly, substantial variation is seen in antibiotic selection for PEx treatment, including the number of antibiotics used, route(s) of administration, and dosing (6–8). In clinical practice, PEx antibiotic selection is frequently guided by previous respiratory culture results or by past PEx antibiotic treatment. In the absence of clinical improvement during PEx treatment, antibiotic regimens are frequently (9, 10) changed by addition or substitution in an attempt to achieve greater symptom reduction and to restore lung function.
Two previous studies have formally examined the benefits of changing antibiotics during PEx treatment. A recent retrospective, single-center study of almost 400 PEx in adults and children with CF found that PEx treatments in which antibiotics were changed did not result in better outcomes, on average, than treatments without antibiotic changes (9). In addition, we have previously found no association between antibiotic changes during PEx treatment and time to next PEx treated with intravenous (IV) antibiotics among 6,000 pediatric PEx using the Pediatric Health Information System (PHIS) dataset (10). The former study was limited by a smaller sample size, affecting generalizability, and the latter was limited by the absence of important CF demographic and clinical covariates known to be associated with short- and long-term outcomes after PEx.
Using the Cystic Fibrosis Foundation Patient Registry (CFFPR) (11)-PHIS (12, 13) dataset, we describe the frequency and patterns of IV antibiotic regimen changes among children with CF hospitalized for PEx and compare average clinical outcomes for PEx treatments in which IV antibiotics were changed versus those in which they were not. We hypothesized that an IV antibiotic change during in-hospital PEx treatment would be associated with a larger pre- to post-PEx lung function treatment change, a higher likelihood of returning to lung function baseline, and lower odds of having future PEx requiring IV antibiotics when compared with PEx treatments lacking antibiotic changes.
Methods
Study Design
This was a retrospective cohort study of the CFFPR-PHIS dataset, a recently linked dataset that contains clinical and demographic data for >10,000 children with CF living in the United States. The dataset includes comprehensive in-hospital data on all medications prescribed (e.g., antibiotics, date of antibiotic administration) and relevant outpatient demographic and clinical information (e.g., lung function and microbiology results) needed to define the study exposure and outcomes. Exact or close match (defined as CFFPR and PHIS encounters occurring within 14 d of each other) and PHIS-only encounters were included for analysis (14). CFFPR-only encounters were not included for analysis because in-hospital antibiotic use could not be determined.
The primary study aims were 1) to describe the frequency of antibiotic changes among PEx included in the CFFPR-PHIS linked dataset and 2) to determine whether an IV antibiotic change (compared with PEx treatment without an antibiotic change) was associated with a larger absolute pre- to post-PEx change in percent predicted forced expiratory volume in 1 second (ppFEV1), a higher likelihood of returning to lung function baseline, and lower odds of having future PEx requiring IV antibiotics.
Study Definitions
Baseline ppFEV1 was defined as the mean of all recorded ppFEV1 measurements within 6 months before a PEx event (15). As done previously (16, 17), pre-PEx treatment lung function was defined as the lowest ppFEV1 recorded within 30 days before admission up to and including the day of admission. Post-PEx treatment lung function was defined as the last in-hospital ppFEV1 measurement on the day of discharge or the earliest ppFEV1 measurement recorded after the hospital stay up to Day 42, whichever came first. To evaluate the odds of returning to lung function baseline, the best ppFEV1 within 3 months after the study PEx was compared with the baseline ppFEV1. Specifically, we compared the proportion of events in which return to ⩾90% and return to ⩾100% of baseline ppFEV1 was achieved between the two treatment groups. We compared the proportion of PEx with a subsequent hospitalization for a PEx treated with IV antibiotics up to 12 months after the study PEx between PEx treated with versus without an IV antibiotic change. We evaluated the proportion with a subsequent PEx outcome as a binary outcome with a 12-month cutoff (calculated as odds ratio [OR] rather than time to next PEx calculated as a hazard ratio); this decision was made because we considered it unlikely that PEx management during a given PEx would be pertinently associated with a repeat PEx requiring IV antibiotics more than 1 year later. When no subsequent PEx occurred during the 12-month interval, data for those children were censored at the last CFFPR encounter date.
IV antibiotics prescribed on any hospital day up to and including Day 5 were considered the baseline PEx antibiotic treatment; we chose Hospital Day 5 rather than hospital admission to exclude any IV antibiotic regimen change that may have occurred because of drug allergy or intolerance (i.e., excluding antibiotic regimen changes more likely to be performed for a reason other than an attempt to improve clinical outcomes). An IV antibiotic regimen change was defined as an addition, subtraction, or substitution (i.e., change within an antibiotic class, such as ceftazidime to cefepime) of an IV antibiotic between Hospital Day 6 up to the day before hospital discharge. We excluded any antibiotic changes on the day of discharge because IV antibiotics are discontinued in most cases before a child leaves the hospital. Changes in oral and/or inhaled antibiotics were not included in the antibiotic regimen change definition.
Inclusion and Exclusion Criteria
Children with CF were included if hospitalized for a PEx between 2006 and 2018, were 6–21 years of age at the time of discharge, and had IV antibiotic use within 48 hours after admission. We included people with CF up to 21 years of age with the presumption that the vast majority would be hospitalized at a pediatric hospital (PHIS center) rather than an adult hospital that would not be captured in PHIS. To assess the return to baseline ppFEV1, eligible PEx required a minimum drop in ppFEV1 of ⩾3% from baseline to pre-PEx treatment lung function. Participants with a history of malignancy or solid organ transplant were excluded. Additional exclusion criteria included 1) culture positivity for nontuberculous mycobacteria, Achromobacter xylosoxidans, or Burkholderia cepacia complex species in the 12 months before a study PEx; 2) intensive care unit stay; 3) PEx requiring IV antibiotics within the preceding 3 months; or 4) length of hospital stay <5 or >21 days. We excluded PEx requiring IV antibiotics within the preceding 3 months to allow a participant to recover to lung function baseline before another PEx event.
Analytic Methods
Clinical characteristics and cohort demographics with medians and interquartile ranges (IQRs) for continuous variables and counts and percentages for categorical variables were described. The unit of analysis was the PEx; we constructed generalized estimating equations (GEEs) and allowed repeat PEx events. Change in pre- to post-PEx ppFEV1 was analyzed using a GEE model with a normal distribution. Return to ⩾90% and ⩾100% of baseline ppFEV1 was regressed on IV antibiotic switching (yes or no) using GEEs with a binomial distribution and logit link function accounting for clustering within hospitals. PHIS center was included as a random effect in all the models.
We accounted for the possibility that changes in IV antibiotics might occur more commonly in people with CF with the most severe disease by way of inverse probability of treatment weighting to reweight our sample and balance measured confounders between groups. Variables included in the propensity score model (the full variable list is included in Table 1) were selected on the basis of a priori assessment of the potential for confounding (i.e., variables we thought might influence the decision to switch IV antibiotics during PEx treatment). A directed acyclic graph is also available in the data supplement. All analytic assumptions were verified and all analyses were performed using SAS version 9.4 (SAS Institute). The Seattle Children’s Hospital Institutional Review Board deemed this research as not involving human subjects.
Table 1.
PEx–level characteristics, by antibiotic regimen change
| All PEx | PEx with IV Antibiotic Change | PEx without IV Antibiotic Change | |
|---|---|---|---|
| Demographics | |||
| No. | 18,745 | 8,169 | 10,576 |
| Age at PEx, yr, median (IQR) | 14 (11, 17) | 15 (11, 17) | 14 (11, 17) |
| Female sex, n (%) | 10,585 (56.5) | 4,665 (57.1) | 5,920 (56.0) |
| White race, n (%) | 16,325 (87.1) | 7,420 (90.8) | 8,905 (84.2) |
| Ethnicity, n (%) | |||
| Hispanic | 2,696 (14.4) | 996 (12.2) | 1,700 (16.1) |
| Non-Hispanic | 12,490 (66.6) | 5,743 (70.3) | 6,747 (63.8) |
| Unknown | 3,559 (19.0) | 1,430 (17.5) | 2,129 (20.1) |
| Insurance, n (%) | |||
| Private | 8,583 (45.8) | 3,691 (45.2) | 4,892 (46.3) |
| Public | 9,765 (52.1) | 4,313 (52.8) | 5,452 (51.6) |
| None/other | 397 (2.1) | 165 (2.0) | 232 (2.2) |
| Clinical | |||
| Baseline ppFEV1, median (IQR)* | 66.5 (49.6, 80.5) | 65.4 (48.1, 79.8) | 67.3 (50.9, 81.2) |
| 100+ , n (%) | 709 (3.8) | 293 (3.6) | 417 (3.9) |
| 70 to <100, n (%) | 7,557 (40.3) | 3,152 (38.6) | 4,405 (41.7) |
| 40 to <70, n (%) | 8,034 (42.9) | 3,529 (43.2) | 4,505 (42.6) |
| <40, n (%) | 2,445 (13.0) | 1,195 (14.6) | 1,250 (11.8) |
| ppFEV1 drop before PEx, median (IQR) | 14.5 (8.7, 22.4) | 14.4 (8.6, 22.0) | 14.6 (8.7, 22.6) |
| ABPA, n (%) | 1,969 (10.5) | 846 (10.4) | 1,123 (10.6) |
| CF-related diabetes, n (%) | 6,484 (34.6) | 3,053 (37.4) | 3,431 (32.4) |
| Prior-year IV-treated PEx, n (%) | |||
| 0 | 4,090 (21.8) | 1,626 (19.9) | 2,464 (23.3) |
| 1 | 4,257 (22.7) | 1,794 (22.0) | 2,463 (23.3) |
| 2+ | 10,398 (55.5) | 4,749 (58.1) | 5,649 (53.4) |
| Pancreatic enzyme use, n (%) | 17,772 (94.8) | 7,769 (95.1) | 10,003 (94.6) |
| Ursodiol use (liver disease), n (%) | 6,370 (34.0) | 3,867 (35.1) | 3,503 (33.1) |
| Microbiologic | |||
| P. aeruginosa isolated | 11,088 (59.2) | 5,129 (62.8) | 5,959 (56.3) |
| MRSA isolated | 8,918 (47.6) | 4,141 (50.7) | 4,777 (45.2) |
| MSSA isolated | 10,101 (53.9) | 4,232 (51.8) | 5,869 (55.5) |
| S. maltophilia isolated | 4,744 (25.3) | 2,169 (26.6) | 2,575 (24.4) |
| Medicinal | |||
| Past year CFTR modulator use, n (%) | 1,795 (9.6) | 842 (10.3) | 953 (9.0) |
| Past year AZM use, n (%) | 8,756 (46.7) | 4,055 (49.6) | 4,701 (44.5) |
| Past year chronic inhABX use, n (%) | 4,792 (26.1) | 1,881 (23.0) | 3,011 (28.5) |
| Past year dornase use, n (%) | 18,295 (97.6) | 7,994 (97.9) | 10,301 (97.4) |
| Past year hypertonic saline use, n (%) | 13,372 (71.3) | 6,014 (73.6) | 7,358 (69.6) |
| Oral ABX at admission, n (%) | 976 (5.2) | 458 (5.6) | 518 (4.9) |
| InhABX at admission, n (%) | 4,892 (26.1) | 1,881 (23.0) | 3,011 (28.5) |
| Oral steroids during PEx, n (%) | 4,954 (26.4) | 2,180 (26.7) | 2,774 (26.2) |
Definition of abbreviations: ABPA = allergic bronchopulmonary aspergillosis; AZM = azithromycin; CF = cystic fibrosis; CFTR = cystic fibrosis transmembrane conductance receptor; InhABX = inhaled antibiotics; IQR = interquartile range; IV = intravenous; MRSA = methicillin-resistant Staphylococcus aureus; MSSA = methicillin-sensitive Staphylococcus aureus; P. aeruginosa = Pseudomonas aeruginosa; PEx = pulmonary exacerbations; ppFEV1 = percentage predicted forced expiratory volume in 1 second; S. maltophilia = Stenotrophomonas maltophilia.
Defined as the average of ppFEV1 recorded within 6 months before a PEx.
Sensitivity Analysis
To ensure that children with CF who contributed multiple PEx did not bias our findings (i.e., a participant whose antibiotics were switched in a prior PEx might more likely have an antibiotic regimen change during a subsequent PEx), we performed a sensitivity analysis that included only the first PEx from each participant during the study period.
Results
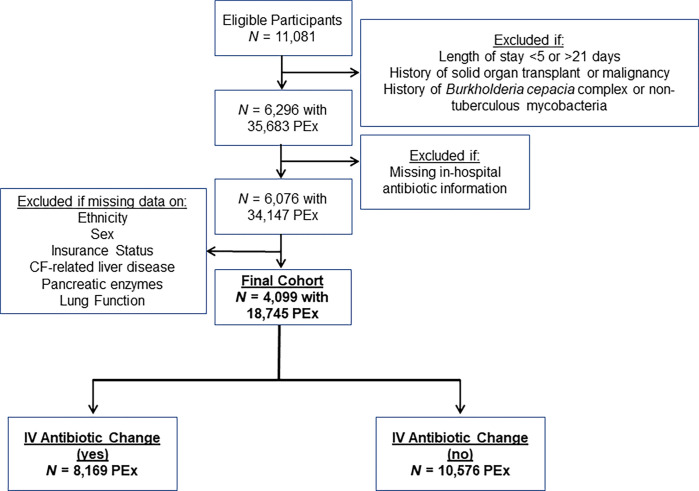
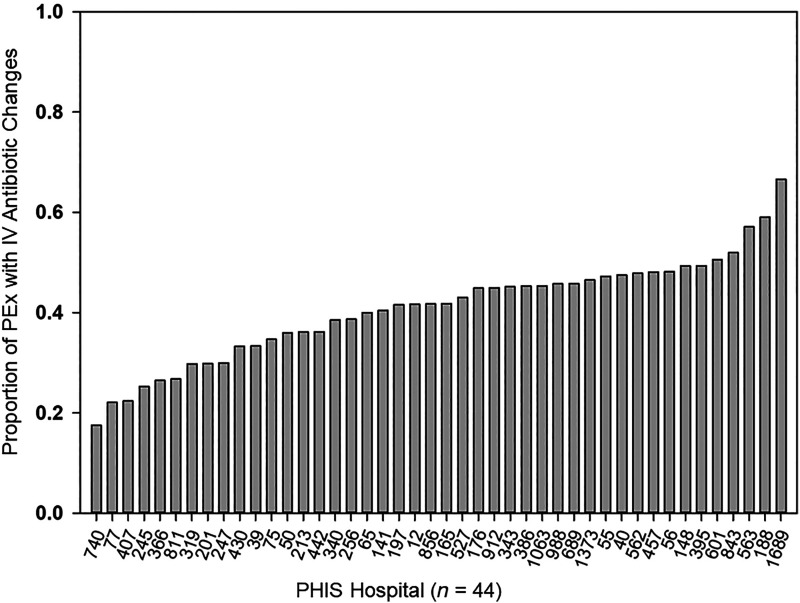
A total of 4,099 children with CF contributed 18,745 PEx for analysis (Figure 1), with 8,169 PEx treatments (43.6%) having an IV antibiotic change between Day 6 and the day before hospital discharge. The median length of stay for PEx without IV antibiotic changes was 13 days (IQR, 10–14 d) versus 11 days (8–14 d) for PEx with IV antibiotic changes. Substantial variability was seen in the proportion of PEx with IV antibiotic changes by PHIS hospital (range, 17–67%) (Figure 2).
Figure 1.
Flow diagram illustrating cohort selection. CF = cystic fibrosis; IV = intravenous; PEx = pulmonary exacerbations.
Figure 2.
Proportion of pulmonary exacerbations (PEx) with IV antibiotic changes, by Pediatric Health Information System (PHIS) hospital. The number below each PHIS hospital indicates the number of PEx included in the analysis from each PHIS hospital. IV = intravenous.
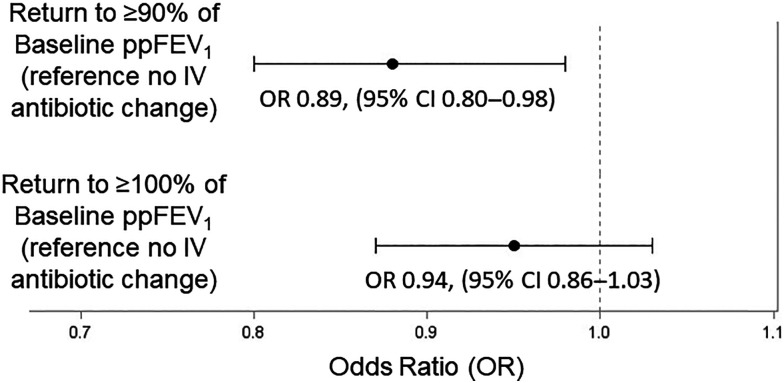
Median ppFEV1 drops from baseline at admission were 14.4 (IQR, 8.6–22.0) and 14.6 (IQR, 8.7–22.6) among PEx with and without an IV antibiotic change, respectively. Return to ⩾90% of baseline ppFEV1 was seen among 72% and 75% of PEx with versus without an IV antibiotic change, respectively, whereas return to ⩾100% of baseline ppFEV1 was seen among 34% and 36% of PEx with versus without an IV antibiotic change, respectively. PEx in which an IV antibiotic change occurred during treatment had a statistically significant lower improvement in pre- to post-PEX treatment ppFEV1 (mean, 11.3; standard error [SE], 0.21) among PEx with an IV antibiotic change versus 12.2 (0.18) among PEx without an IV antibiotic change (P = 0.001) In addition, PEx in which an IV antibiotic change occurred were less likely to return to ⩾90% of baseline ppFEV1 (OR, 0.89 [95% confidence interval (CI), 0.80–0.98]; P = 0.02) and were not more likely to return to ⩾100% of baseline ppFEV1 (OR, 0.94 [0.86–1.03]; P = 0.19) when compared with children with CF whose PEx treatment did not include an IV antibiotic change (Figure 3). In addition, when compared with PEx treatment without an IV antibiotic change, PEx treatment with an IV antibiotic change was associated with higher odds of subsequent PEx requiring IV antibiotics within 12 months (OR, 1.17 [95% CI, 1.12–1.22]; P < 0.0001).
Figure 3.
Comparison of return to ⩾90% and ⩾100% of baseline ppFEV1 between PEx with versus without an IV antibiotic change. CI = confidence interval; IV = intravenous; OR = odds ratio; PEx = pulmonary exacerbations; ppFEV1 = percentage predicted forced expiratory volume in 1 second.
When the cohort was restricted to one PEx per participant (n = 4,099), no statistically significant differences were seen in the pre- to post-PEx treatment ppFEV1 (12.3 [SE, 0.55] and 13.5 [SE, 0.37]) among PEx with versus without an IV antibiotic change, respectively (P = 0.15), the odds of returning to ⩾90% (OR, 0.91 [95% CI, 0.70–1.17]; P = 0.58), or ⩾100% (1.15 [95% CI, 0.93–1.41]; P = 0.20) of baseline ppFEV1 or in the odds of having future PEx requiring IV antibiotics (hazard ratio, 0.97 [95% CI, 0.87–1.09]; P = 0.64).
Discussion
Using the CFFPR-PHIS linked dataset, this study of more than 18,000 PEx from almost 4,100 children with CF found 1) substantial variability among CF centers in changing IV antibiotics during PEx treatment and 2) that changing an IV antibiotic during in-hospital PEx treatment was not associated, on average, with improved clinical outcomes when compared with PEx without antibiotic changes. More specifically, an IV antibiotic change was associated with a smaller pre- to post-PEx ppFEV1 treatment change, lower odds of returning to ⩾90% of baseline ppFEV1, and higher odds of having a future PEx requiring IV antibiotics than PEx without antibiotic changes. This observation of poorer average outcomes associated with PEx treatments in which IV antibiotics were changed is consistent with the hypothesis both that changes were driven largely by a search for better PEx outcomes by treating clinicians when initial treatment responses were poor and that changing regimens did not appear to improve those outcomes. Although we did adjust for many relevant covariates in our models, it is possible that unmeasured confounding might in part explain the negative associations between changing IV antibiotics and clinical outcomes after PEx treatment; however, we think it is very unlikely that adjusting for such potential unmeasured confounders would reverse this association (i.e., IV antibiotic changes would be associated with improved outcomes).
To our knowledge, only two studies have systematically examined the effects of antibiotic changes on clinical outcomes among people with CF undergoing IV antibiotic treatment. Investigators from Toronto performed a single-center, retrospective cohort study of 399 PEx from children and adults with CF over a 6-year period, of which 105 PEx included a change in antibiotics (defined as a change in IV or oral antibiotics). Using a multivariable regression model, those investigators found no significant differences in absolute or relative ppFEV1 response at the end of PEx treatment or at follow-up (9) among PEx that included versus lacked a change in antibiotics. In addition, we previously performed a study using the PHIS dataset alone (before CFFPR-PHIS linkage) examining the relationship between antibiotic switching (defined in that study as a change in IV, oral, and/or inhaled antibiotics) and time to next PEx requiring IV antibiotics. Among 6,451 IV-treated PEx from January 2011 through December 2016, antibiotic switching occurred in 54% of PEx and was not associated with a reduced hazard of having future PEx requiring IV antibiotics compared with PEx without an antibiotic change (10). The present study, which used the CFFPR-PHIS dataset, specifically examined changes in IV antibiotics, but not oral or inhaled antibiotics, which likely explains the discrepancy in antibiotic change frequency observed between the two studies (54% and 43.6% in the PHIS-only and CFFPR-PHIS studies, respectively). Taken together, the results of these three studies provide no evidence that changing IV antibiotics during in-hospital PEx treatment increases the likelihood or extent of clinical recovery after PEx.
Because of limitations in the CFFPR-PHIS dataset, in this study, we were unable to determine reasons for IV antibiotic changes during PEx treatments. The most common reasons for an antibiotic change in children with CF found in the Toronto study included a poor initial ppFEV1 response and culture positivity for another CF-related microorganism (9). Other possibilities for changing IV antibiotics among children with CF include lack of expected symptom improvement, discordance of antibiotic regimens with antimicrobial susceptibility testing results, unwanted antimicrobial side effects (e.g., acute kidney injury, liver toxicity), or local practice patterns (e.g., individual CF provider experience). The latter possibility could also explain in part the substantial variability seen in changing IV antibiotics among PHIS hospitals. Nevertheless, there is growing evidence that antimicrobial susceptibility testing does not predict clinical outcomes of CF antimicrobial treatment (18), indicating that switching for new such data is unsupportable. In addition, our results, when paired with the Toronto data, suggest that changing IV antibiotics only because of a lack of improvement in ppFEV1 or symptoms might not be warranted. More research is needed to better determine the optimal treatment strategies for children with CF in whom early ppFEV1 and symptom recovery are not achieved during PEx treatment.
Strengths of this study include its large sample size (>18,000 PEx, which is much larger than the two previously published studies), the ability to include children with CF from more than 40 (N = 44) CF care centers, and the use of a dataset found to be generalizable to children with CF living in the United States (13). In addition, we used a rigorous methodological approach (i.e., propensity scores) in an attempt to address indication bias. In contrast to the two prior published studies, this analysis included short-term (e.g., pre- to post-PEx treatment ppFEV1), medium-term (return to baseline ppFEV1), and long-term (time to next PEx treated with IV antibiotics) clinical outcomes. Last, PHIS medication data have been shown to be highly accurate when compared with aggregate electronic health record data (19), making it less likely that an IV antibiotic change was incorrectly coded or misclassified.
Limitations
This study does have several important limitations, the most important of which relates to possible confounding by indication. Although we used inverse probability of treatment weighting to address indication bias, unmeasured confounders (including the rationale for changing IV antibiotics) might have affected the study results. Our primary analysis included multiple PEx per child with CF, so it is possible that some children in the IV antibiotic switching group might have characteristics that could predispose them to poorer outcomes, which could influence clinical outcomes in the entire IV antibiotic switching group. We attempted to address this concern in our sensitivity analysis that only included one PEx per child with CF; this analysis (similar to our primary analysis) found no statistically significant improvements in any clinical outcomes between PEx with and without IV antibiotic switching, thus strengthening this observation. Our dataset was limited to the study years, and results from this study might not be generalizable to adults with CF, to children with CF cared for outside the United States, or in the context of new maintenance therapies, such as highly effective CFTR modulators. Finally, because of limitations of the dataset, we were unable to describe any antibiotic-associated adverse effects (e.g., antibiotic-related allergy or intolerance) that might have contributed to antibiotic selection or changing antibiotics or to describe whether a child with CF was discharged home with IV antibiotics.
Conclusions
In conclusion, the results of this study identified no evidence that changing IV antibiotics improves clinical outcomes after in-hospital PEx treatment. Additional studies are needed to better determine optimal PEx treatment strategies, particularly among children with CF who are not meeting early PEx treatment milestones.
Acknowledgments
Acknowledgment
The authors thank both the Cystic Fibrosis Foundation for the use of Cystic Fibrosis Foundation Patient Registry data and the Children’s Hospital Association team for providing Pediatric Health Information System data to conduct this study. In addition, the authors thank the people with CF, care providers, and clinical coordinators at CF centers throughout the United States for their contributions to the Cystic Fibrosis Foundation Patient Registry. Lastly, the authors thank Dr. Dutch VanDevanter for his comments on study design and results interpretation.
Footnotes
Supported by the Cystic Fibrosis Foundation (SANDER18Y7 [J.E.S.] and SINGH19R0 [L.R.H.]) and the National Institutes of Health Clinical Center (K24HL141669 [L.R.H.]).
Author Contributions: J.D.C. conceptualized and designed the study, drafted the initial manuscript, and reviewed and revised the manuscript. J.E.S. and A.V.F. conceptualized and designed the study, conducted the analyses, and critically reviewed and revised the manuscript. D.B.S., R.S., R.L.G., L.R.H., and C.L.R. participated in study design and critically reviewed and revised the manuscript.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Ferkol T, Rosenfeld M, Milla CE. Cystic fibrosis pulmonary exacerbations. J Pediatr . 2006;148:259–264. doi: 10.1016/j.jpeds.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 2. Goss CH, Burns JL. Exacerbations in cystic fibrosis. 1: epidemiology and pathogenesis. Thorax . 2007;62:360–367. doi: 10.1136/thx.2006.060889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Waters V, Stanojevic S, Atenafu EG, Lu A, Yau Y, Tullis E, et al. Effect of pulmonary exacerbations on long-term lung function decline in cystic fibrosis. Eur Respir J . 2012;40:61–66. doi: 10.1183/09031936.00159111. [DOI] [PubMed] [Google Scholar]
- 4. Britto MT, Kotagal UR, Hornung RW, Atherton HD, Tsevat J, Wilmott RW. Impact of recent pulmonary exacerbations on quality of life in patients with cystic fibrosis. Chest . 2002;121:64–72. doi: 10.1378/chest.121.1.64. [DOI] [PubMed] [Google Scholar]
- 5. Flume PA, Mogayzel PJ, Jr, Robinson KA, Goss CH, Rosenblatt RL, Kuhn RJ, et al. Clinical Practice Guidelines for Pulmonary Therapies Committee Cystic fibrosis pulmonary guidelines: treatment of pulmonary exacerbations. Am J Respir Crit Care Med . 2009;180:802–808. doi: 10.1164/rccm.200812-1845PP. [DOI] [PubMed] [Google Scholar]
- 6. Cogen JD, Oron AP, Gibson RL, Hoffman LR, Kronman MP, Ong T, et al. Characterization of inpatient cystic fibrosis pulmonary exacerbations. Pediatrics . 2017;139:e20162642. doi: 10.1542/peds.2016-2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wagener JS, Rasouliyan L, VanDevanter DR, Pasta DJ, Regelmann WE, Morgan WJ, et al. Investigators and Coordinators of the Epidemiologic Study of Cystic Fibrosis Oral, inhaled, and intravenous antibiotic choice for treating pulmonary exacerbations in cystic fibrosis. Pediatr Pulmonol . 2013;48:666–673. doi: 10.1002/ppul.22652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sanders DB, Solomon GM, Beckett VV, West NE, Daines CL, Heltshe SL, et al. STOP Study Group Standardized Treatment of Pulmonary Exacerbations (STOP) study: observations at the initiation of intravenous antibiotics for cystic fibrosis pulmonary exacerbations. J Cyst Fibros . 2017;16:592–599. doi: 10.1016/j.jcf.2017.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zikic A, Ratjen F, Shaw M, Tullis E, Waters V. The effect of antibiotic changes during treatment of cystic fibrosis pulmonary exacerbations. J Cyst Fibros . 2022;21:759–765. doi: 10.1016/j.jcf.2022.05.010. [DOI] [PubMed] [Google Scholar]
- 10. Cogen JD, Whitlock KB, Gibson RL, Hoffman LR, VanDevanter DR. The use of antimicrobial susceptibility testing in pediatric cystic fibrosis pulmonary exacerbations. J Cyst Fibros . 2019;18:851–856. doi: 10.1016/j.jcf.2019.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Knapp EA, Fink AK, Goss CH, Sewall A, Ostrenga J, Dowd C, et al. The Cystic Fibrosis Foundation Patient Registry. Design and methods of a national observational disease registry. Ann Am Thorac Soc . 2016;13:1173–1179. doi: 10.1513/AnnalsATS.201511-781OC. [DOI] [PubMed] [Google Scholar]
- 12.Children’s Hospital Association. https://www.childrenshospitals.org/Programs-and-Services/Data-Analytics-and-Research/Pediatric-Analytic-Solutions/Pediatric-Health-Information-System
- 13. Cogen JD, Hall M, Loeffler DR, Gove N, Onchiri F, Sawicki GS, et al. Linkage of the CF Foundation Patient Registry with the pediatric health information system database. Pediatr Pulmonol . 2019;54:721–728. doi: 10.1002/ppul.24272. [DOI] [PubMed] [Google Scholar]
- 14. Cogen JD, Faino AV, Onchiri F, Hall M, Fink AK. Evaluation of hospitalization data for the CFFPR-PHIS linked data set. Pediatr Pulmonol . 2020;55:30–32. doi: 10.1002/ppul.24527. [DOI] [PubMed] [Google Scholar]
- 15. Wagener JS, VanDevanter DR, Konstan MW, Pasta DJ, Millar SJ, Morgan WJ. Lung function changes before and after pulmonary exacerbation antimicrobial treatment in cystic fibrosis. Pediatr Pulmonol . 2020;55:828–834. doi: 10.1002/ppul.24577. [DOI] [PubMed] [Google Scholar]
- 16. Cogen JD, Faino AV, Onchiri F, Hoffman LR, Kronman MP, Nichols DP, et al. Association between number of intravenous antipseudomonal antibiotics and clinical outcomes of pediatric cystic fibrosis pulmonary exacerbations. Clin Infect Dis . 2021;73:1589–1596. doi: 10.1093/cid/ciab525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cogen JD, Faino AV, Onchiri F, Gibson RL, Hoffman LR, Nichols DP, et al. Clinical outcomes of antipseudomonal versus other antibiotics among children with cystic fibrosis without Pseudomonas aeruginosa. Ann Am Thorac Soc . 2022;19:1320–1327. doi: 10.1513/AnnalsATS.202111-1294OC. [DOI] [PubMed] [Google Scholar]
- 18. VanDevanter DR, Heltshe SL, Hilliard JB, Konstan MW. Pseudomonas aeruginosa antimicrobial susceptibility test (AST) results and pulmonary exacerbation treatment responses in cystic fibrosis. J Cyst Fibros . 2021;20:257–263. doi: 10.1016/j.jcf.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Courter JD, Parker SK, Thurm C, Kronman MP, Weissman SJ, Shah SS, et al. Accuracy of administrative data for antimicrobial administration in hospitalized children. J Pediatric Infect Dis Soc . 2018;7:261–263. doi: 10.1093/jpids/pix064. [DOI] [PubMed] [Google Scholar]