Abstract
Long COVID is characterized by persistent signs and symptoms that continue or develop for more than 4 weeks after acute COVID-19 infection. Patients with Long COVID experience a cardiovascular autonomic imbalance known as dysautonomia. However, the underlying autonomic pathophysiological mechanisms behind this remain unclear. Current hypotheses include neurotropism, cytokine storms, and inflammatory persistence. Certain immunological factors indicate autoimmune dysfunction, which can be used to identify patients at a higher risk of Long COVID. Heart rate variability can indicate autonomic imbalances in individuals suffering from Long COVID, and measurement is a non-invasive and low-cost method for assessing cardiovascular autonomic modulation. Additionally, biochemical inflammatory markers are used for diagnosing and monitoring Long COVID. These inflammatory markers can be used to improve the understanding of the mechanisms driving the inflammatory response and its effects on the sympathetic and parasympathetic pathways of the autonomic nervous system. Autonomic imbalances in patients with Long COVID may result in lower heart rate variability, impaired vagal activity, and substantial sympathovagal imbalance. New research on this subject must be encouraged to enhance the understanding of the long-term risks that cardiovascular autonomic imbalances can cause in individuals with Long COVID.
Keywords: Long COVID, autonomic nervous system, autonomic diseases, pathophysiology, inflammation, heart rate variability
1. Introduction
Long COVID Syndrome, know as Long COVID, can be described as the persistence signs and symptoms that continue or develop for more than 4 weeks after acute COVID-19 infection (1). During clinical evaluation, differential diagnosis and the identification of associated pathologies unrelated to SARS-CoV-2 infection are essential (2). Manifestations of Long COVID occur in approximately 50%–80% of previously symptomatic patients with COVID-19 who have recovered (3). Cardiovascular autonomic dysfunction or dysautonomia involves inadequate autonomic nervous system (ANS) function, resulting in various cardiovascular symptoms. Dysautonomia can be acute, chronic, progressive, irreversible, or variable and can accompany infectious or non-infectious diseases. Cardiovascular autonomic dysfunction can be assessed by measuring heart rate variability (HRV) using linear (time or frequency domain) and non-linear methods (4). HRV measures RR interval variation, autonomic function and neurocardiac (5) from simple bedside analysis to more sophisticated markers (6), in patients with cardiac or non-cardiac diseases (7) with analysis of vagal function (8). The cardiovascular autonomic pathophysiology of Long COVID is unclear, but neurotropism, hypoxia, or viral-mediated pathways may be associated with this condition. The symptoms of cardiovascular autonomic dysfunction include orthostatic intolerance, chest pain, palpitations, reduced exercise tolerance, and “brain fog” (9, 10).
According to Raveendran et al. (2021) (10), the symptoms of Long COVID occur due to chronic inflammatory persistence or immune responses related to antibody production. Individuals with altered immunity or those who are reinfected may develop viraemia (11). To better understand Long COVID, investigating whether elevated levels of inflammatory markers are present long-term is necessary, potentially improving the prognostic stratification of this disease (12–16).
Autonomic tests can be used to analyse the possible causal relationships of dysautonomia (17) in individuals with Long COVID. Associations between HRV and various inflammatory markers appear to alter cardiovascular autonomic modulation. HRV monitoring may also result in better disease stratification in patients with Long COVID. The objective of this review was to provide information on long-term cardiovascular autonomic dysfunction in patients with Long COVID and its impact on morbidity and mortality in this patient population.
2. Autonomic pathophysiology of long COVID
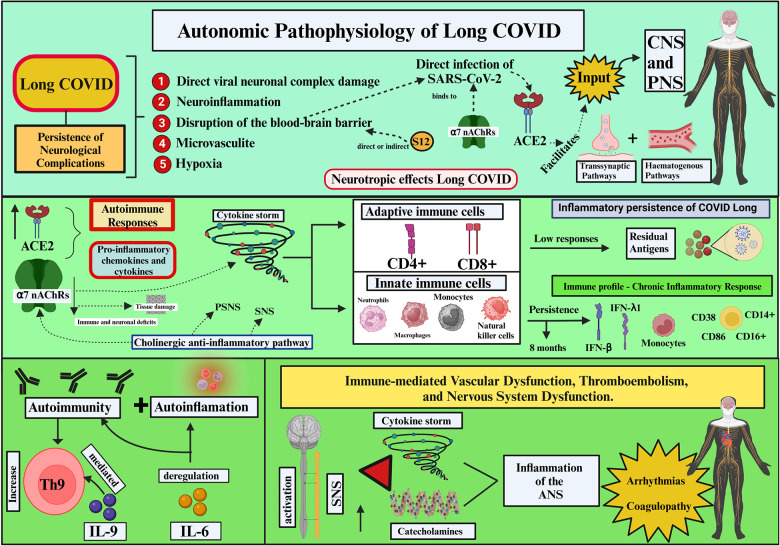
The persistent neurological complications observed in Long COVID likely result from damage to the central nervous system (CNS) and peripheral nervous system (PNS). This may involve a complex pathophysiology, including direct viral neuronal damage, neuroinflammation, disruption of the blood-brain barrier (BBB), microvasculitis, and hypoxia (18). Through the angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 (the causative agent of COVID-19) binds to nervous system (NS) cells in the brain, the choroid plexus, and the ventral posterior nucleus of the thalamus. SARS-CoV-2 also binds to α7 nicotinic acetylcholine receptors (α7 nAChRs) (19). Limited data are available on the SARS-CoV-2 neuroinvasive routes and NS infection (20); however, neuroinflammation with substantial immune infiltration has been observed (21).
After direct infection by SARS-CoV-2, neurotropic effects play a considerable role in the pathogenesis of Long COVID. SARS-CoV-2 may use ACE2 to enter CNS and PNS cells through haematogenous or transsynaptic pathways (22). Although the neurotropic pathways are unclear, the virus is likely to cross the BBB or directly or indirectly damage it. The SARS-CoV-2 spike protein (S12) can also damage the integrity of the BBB, either independently or in conjunction with other cell mediators (23). Indirect damage occurs through endothelial cells and pericytes or activation of the autoimmune response (24).
ACE2 may enhance the cytokine storm immune response through chemokine and pro-inflammatory cytokine expressions (25). These may activate adaptive immune cells (CD4 + and CD8 + T cells) and recruit innate immune cells (neutrophils, monocytes, macrophages, and natural killer cells) (26).
The long-term inflammatory persistence of Long COVID may be explained by the presence of residual SARS-CoV-2 antigens, resulting from low adaptive immune responses. Persistent activation of SARS-CoV-2-specific T cells has been observed at the beginning of infection and 6 months post-infection. Patients with Long COVID have a specific increase in CD4 + T and CD8 + T cells at the end of their recovery (27). According to Galán et al. (2022) (28), individuals with Long COVID have lasting cytotoxic persistence as indicated by high levels of CD8 + T and CD4 + T cells and PD-1 exhaustion markers on CD3 + . This may explain why this population develops a potent memory response against SARS-CoV-2.
The innate immune response is highly activated in patients with Long COVID, and this activation persists for 8 months after initial infection by SARS-CoV-2. This results in the increased expression of type I interferon (IFN-β) and type III interferon (IFN-β, -λ1) cytokines, T lymphocyte types CD38, CD86, CD14+, and CD16+, as well as monocytes. Immunological profiles of the patients revealed a chronic inflammatory response (29). Patients with Long COVID are likely to be in a state of autoimmunity conferred by a significant increase in interleukin (IL)-9-mediated Th9 cells. Autoinflammation and autoimmunity may also be maintained through IL-6 dysregulation (30).
Neuronal nicotinic acetylcholine receptors (nAChRs) are primarily found in the CNS and PNS. α7 nAChRs are found in the immune system of patients with COVID-19 and may persist long-term in various immune cells, such as macrophages, B cells, T cells, and dendritic cells. Activation of α7 nAChRs in the cholinergic anti-inflammatory pathway inhibits the production of pro-inflammatory cytokines (19). α7 nAChRs are present in neuronal and non-neuronal cells; a decrease in their levels would cause tissue damage and overproduction of cytokines. The Y674-R685 region of protein S can bind to α7 nAChRs and regulate ACE2. α7 nAChR expression ligands may affect SARS-CoV-2 infectivity and COVID-19 progression (31).
α7 nAChR deficiency causes immune and neuronal deficits. The SARS-CoV-2 spike protein (S12) facilitates viral cell entry. At a cellular level, S12 can suppress α7 nAChR. The S12 immunoreaction suggests a contribution to the onset of cardiovascular diseases independent of viral infection (23). In the ANS autoimmune imbalance, disparities autoantibodies at the G protein-coupled receptors contribute to the development of clinical and autonomic symptoms in patients with Long COVID (32, 33).
The α7 nAChR subtype is overexpressed in the hippocampus and is the most important mediator of the anti-inflammatory properties of the cholinergic system; dysregulation in this system could potentially cause the uncontrolled inflammatory response observed in COVID-19 (34). At the end of the inflammatory response, α7-nAChR is activated by acetylcholine, a neurotransmitter used by the parasympathetic nervous system (PSNS) (35).
After an inflammatory response, afferent signals are conducted through the vagus nerve (the primary nerve of the PSNS) to the nucleus tractus solitarius. Posteriorly, the vagus nerve is responsible for a reflex action called the cholinergic anti-inflammatory pathway(CAP) (36). This inflammatory reflex is dynamic, and immune responses can be influenced by the sympathetic nervous system (SNS). The SNS is connected to different central circuits and works as a protective neural system, performing tissue repair and global recovery through interactions between the immune system and the brain. The brain receives immune signals through cytokines and modulates immune system reactivity through the SNS and possibly the sympatho-adrenal and hypothalamic-pituitary-adrenal systems. During inflammation, cytokines are involved in nociceptor sensitisation in SNS fibres (37).
The ANS controls inflammation through the CAP (32). ANS and the immune system interact with invading pathogens. Acetylcholine and noradrenaline regulate cytokine release, and cholinergic signals from the efferent vagus nerve and α7 nAChR have anti-inflammatory functions. Intracellular signals interrupt NF-κB and activation of JAK2/STAT3 cascades, and inflammasome-mediated cholinergic signals using α7 nAChR stop the production of TNF, IL-1β, and other pro-inflammatory cytokines. The resolution of inflammation occurs through the synchronized action of protective mechanisms, lipoxins and eguasins, and increased activity of neutrophils and macrophages. The vagus nerve acts as an integrator during anti-inflammatory control (38).
Other mechanisms leading to Long COVID may include an association between immune-mediated vascular dysfunction, thromboembolism, and NS dysfunction. A relationship between the activation of the SNS and an increase in catecholamines accompanied by a cytokine storm and impairment mediated by ANS inflammation is likely to be established (39). Autonomic dysfunction in Long COVID can be caused by the direct viral action of SARS-CoV-2 or by the immune response impacting the ANS (40).
Additionally, prolonged inflammatory persistence and cellular damage from fibrotic changes can reduce cell adhesion and lead to arrhythmias (palpitations) such as coagulopathies and postural orthostatic tachycardia syndrome (POTS) (41). According to Glynne et al. (2022) (42), patients with Long COVID may report improvements in symptoms, but symptoms of dysautonomia may persist (Figure 1).
Figure 1.
Autonomic Pathophysiology of Long COVID (Created with BioRender.com). SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; ACE2, angiotensin-converting enzyme 2; S12, SARS-CoV-2 spike protein; α7 nAChRs, α7 nicotinic acetylcholine receptors; CNS, central nervous system; PNS, peripheral nervous system; PSNS, parasympathetic nervous system; SNS, sympathetic nervous system; CD4+, T lymphocyte CD4+; CD8+, T lymphocyte CD8+; type III interferon (IFN-β, -γ1), T lymphocyte types: CD38, CD86, CD14α, CD16α; IL-9, interleukin 9; IL-6, interleukin 6; ANS, autonomic nervous system.
3. HRV and inflammatory markers
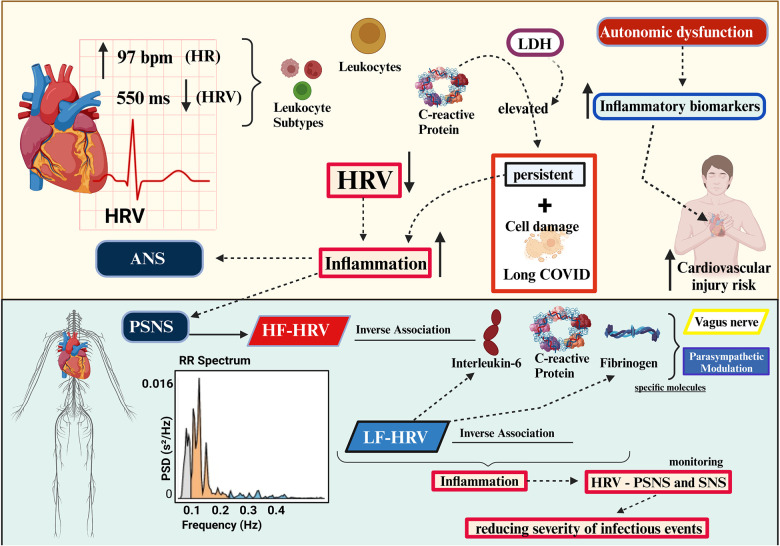
In the meta-analysis performed by Williams et al. (2019), the link between HRV and inflammation was clarified. Higher HRV (all indices), particularly vagal HRV, was associated with lower levels of inflammation. Over time, the SDNN index proved to be the most consistent, while the high-frequency HRV (HF-HRV) was the strongest when examining frequency; these data support the involvement of the CAP. Examining the vagal index, both HF-HRV and RMSSD were related to inflammation. The inflammatory markers C-reactive protein (c-RP) and white blood cell (WBC) count exhibited negative associations with different levels of HRV (36).
According to Sotak (2022), inflammatory biochemical markers can be used to support and monitor infection diagnosis and treatment efficacy (43). Studies evaluating inflammatory markers and HRV in Long COVID are scarce, although Aeschbacher et al. (2017) (44) reported that the inflammatory parameters c-RP, leukocytes, and leukocyte subtypes may be associated with increased heart rate (HR) and decreased HRV (SDNN). These results suggest an association between inflammation and the ANS as well as increased cardiovascular morbidity and mortality. Autonomic dysfunction may put individuals with elevated inflammatory biomarkers at increased cardiovascular injury risk.
Elevated c-RP levels are associated with a chronic inflammatory state that functions as a peripheral marker in clinical research and may be associated with disease complications (45, 46). According to Pasini et al. (2021) (47), patients with Long COVID have high levels of c-RP and lactate dehydrogenase, resulting in cell damage and persistent inflammation.
HRV has the potential to aid in early inflammatory response detection and tracking. However, further HRV studies are required to study chronic inflammation states (48). The PSNS participates in inflammatory processes through HF-HRV and is inversely associated with IL-6, c-RP, and fibrinogen levels. This suggests that parasympathetic modulation of inflammation by the vagus nerve may act on specific inflammatory molecules. Similar inverse associations were observed between low frequency HRV (LF-HRV) and IL6 and c-RP (49).
The inverse association between HRV and the inflammatory markers IL-6 and c-RP in healthy individuals and those with cardiovascular disease (CVD) was reported by Haensel et al. (2008) (50). This indicated that HRV is associated with inflammation. The CAP driven by the vagus nerve is well described, and sympathetic and parasympathetic modulation substantially contribute to the modulation of cytokine production (51).
As in the study by Wegeberg et al. (2020) (52) pointing out the inflammation associated with HRV involving the SNS and PSNS. A cohort study (1,255 participants) conducted by Cooper et al. (2015) (53) found that LF-HRV was inversely associated with IL-6, c-RP, and fibrinogen levels, whereas HF-HRV was inversely associated with c-RP and fibrinogen. Vagal activity regulates systemic inflammation and prevents damage from excessive inflammatory responses (Figure 2). Implementing HRV monitoring associated with the monitoring of inflammatory markers could substantially contribute to reducing the severity of infectious events through early detection and reductions in mortality (54) (Figure 2).
Figure 2.
Association of heart rate variability and inflammatory markers (created with BioRender.com). HRV, heart rate variability; LDH, lactate dehydrogenase; ANS, autonomic nervous system; PNS, peripheral nervous system; PSNS, parasympathetic nervous system; SNS, sympathetic nervous system; HF-HRV, high-frequency HRV; LF-HRV, low-frequency HRV.
4. Cardiovascular autonomic dysautonomia in long COVID
The negative and positive feedback mechanisms of the heart and vessels are regulated through the balance of inhibition and activation of sympathetic and parasympathetic afferent and efferent neurons of the Intrinsic Cardiac Nervous System. Cardiac neural regulation interacts reflexively to modulate heart rate and blood pressure. Stimulation of cardiac sympathetic afferents reflexively increase sympathetic efferents and inhibits efferent vagal cardiac fibers, generating an increase in HR and blood pressure (BP). While the parasympathetic cardiomotor function helps to reduce HR, blood pressure. Baroreceptor controls also reflect HR and BP fluctuations, wich convey responses for sympathetic and parasympathetic modulation (55, 56).
Long COVID may involve cardiovascular dysautonomia. POTS is the most prevalent type of CVD, defined as a persistent HR increase of at least 30 beats per minute when standing (upright). It may be associated with palpitations, chest pain, and exercise or orthostatic intolerance. POTS is more prevalent in women (80%) and can be precipitated by viral illnesses or serious infections (57, 58).
Eldokla et al. (2022) reported autonomic dysfunction in 332 individuals with Long COVID (59). The authors found a high prevalence of dysautonomia symptoms (76.7%) when using the Composite Autonomic Symptom Score 31 questionnaire (COMPASS-31). The high score (>16.4) found suggested that autonomic dysfunction was initially associated with a longer duration of Long COVID symptoms.
A high prevalence of dysautonomia (66%) was also reported by Larsen et al. (2022) (60) when using the COMPASS-31 questionnaire to evaluate 2,314 individuals with Long COVID; they observed moderate-to-severe autonomic scores. However, according to Hovaguimian (2023) (61), whether Long COVID is directly involved in autonomic impairment, causes POTS symptoms, or orthostatic intolerance through previously identified chronic disease mechanisms remains unclear.
Heart rate recovery provides a surrogate measure of autonomic health in patients with Long COVID. Long COVID POTS patients may benefit from conservative treatments to recovery HR and dysautonomia symptoms (62, 63), treatment may involve graded exercise, use of compression stockings, control of high fluid and sodium intake, and cognitive-behavioral therapy (57).
Dysautonomia was also reported by a cohort in a study by Zanin et al. (2023) performing a battery of autonomic tests on patients with Long COVID. Patients in this study had disabling symptoms and severe COVID-19. The authors showed more evident parasympathetic dysfunctions than sympathetic dysfunctions with fluctuating and polymorphic symptoms. Among the battery of tests, sympathetic and parasympathetic autonomic tests, neuropathy scores (Kale Score), and autonomic severity (evaluated using the Composite Autonomic Severity Score) were used. They found that 37.5% of patients had at least one abnormal test result, which was associated with mild autonomic failure in 83% and moderate autonomic failure in 16%. The cardiovascular system and sudomotor function were the most affected (64).
Dysautonomia can persist for almost a year due to symptom heterogeneity, and monitoring of cardiovascular autonomic dysfunction in patients with Long COVID is challenging. The impairment of these functions decreases the quality of life of patients, affecting their daily activities and hampering their ability to return to work (65).
The physical inactivity and at rest result in cardiovascular deconditioning and impair cardiovascular neural control (55, 66). HRV feedback training aims to increase vagal tone by metronomic breathing (5). Post-COVID-19 rehabilitation with an aerobic and resistance training program can provide important cardiorespiratory, cardiovascular, functional and autonomic responses (67).
5. Association of ANS and HRV
HRV measurement assesses the ANS with its interconnected sympathetic and parasympathetic branches (68). Heart rate and rhythm are considerably influenced by the ANS; parasympathetic activity decreases HR through the vagus nerve and sympathetic resolution by activating β-adrenergic receptors. HRV can be measured using either linear or nonlinear methods. High HRV or one that is reduced are linked to sympathetic (“escape”) and parasympathetic activation (“recovery”), respectively (69).
In general, autonomic imbalances refer to the inappropriate ability of the autonomic system to respond physiologically to stimuli, either by increased or decreased modulation of one of the two branches. Inappropriate increased or decreased of vagal or sympathetic modulation can be detrimental (7, 70, 71), and generate clinical implications (72). The dysautonomia can be found due to prolonged bed rest, so early initiation of rehabilitation can combat its symptoms (5). Physically inactive people (mild to moderate infection) have greater autonomic dysfunctions (increased sympathetic activity—LF) compared to physically active controls (increased parasympathetic activity—HF) (73).
With HRV reduction, neuroautonomic disconnections or a decrease in baroreflex sensitivity can be observed. HRV reduction can be estimated by reductions in time-related factors (DNN, RMSSD, and pNN50) or frequency (LF/HF ratio reductions or LF increases). Infectious diseases can reduce HRV, and ANS imbalances can reduce the vagal tone and compromise physiology by uncoupling distinct mechanisms (74).
A high HRV indicates a sympathovagal balance and good ANS adaptability. A reduced HRV may indicate abnormal ANS control (75). Activation of the inflammatory reflex has been proposed to interfere with vagal modulation of the HR as inflammation in the acute and chronic phases modifies cardiac function through changes in HRV (76).
The vagus nerve contributes to inflammation control through its afferent and efferent pathways, playing a dual anti-inflammatory role. Low vagal tone, visualised by HRV, is a marker of sympathovagal balance (77). A wide range of symptoms are observed in the population with Long COVID. Therefore, autonomic testing, including checking blood pressure, heart rate, and HRV by long-term Holter monitoring (24 h) or short-term heart rate monitoring (5–15 min) is imperative. These tests can be adopted in clinical practice (65, 78, 79) (Figure 3).
Figure 3.
Autonomic nervous system and heart rate variability in Long COVID (created with BioRender.com). SNS, sympathetic nervous system; PSNS, parasympathetic nervous system; HRV, heart rate variability; HR, heart rate.
6. Heart rate variability in long COVID
HRV is considered an indirect biomarker of cardiac autonomic control. A reduced HRV can be observed in cardiac and non-cardiac patients and is related to a worse prognosis (80). During viral infections, changes in long-term autonomic control and individual responses to HRV can be observed (81).
HRV can indicate cardiovascular dysautonomia in individuals suffering from Long COVID. These patients may have reduced HRV compared with healthy individuals. Data predicting autonomic dysfunction in patients that have recovered from SARS-CoV-2 infection are limited (82, 83). Dysautonomia was also reported by Liviero et al. (2023) in paucisymptomatic patients that had recovered from acute COVID-19. With analysis of HRV frequency and time, persistent sympathetic activity (increase in LF and LF/HF) and decreases in vagal activity due to decreases in HF were observed, in addition to reductions in SDNN and RMSSD. Patients in the acute phase of COVID-19 with autonomic dysregulation can progress to Long COVID; HRV works as a marker of worsening clinical condition in the long term (84).
To verify dysautonomia, Acanfora et al. (2022) examined HRV in 30 individuals with Long COVID and found impaired vagal activity and sympathovagal imbalances. The authors found lower SDNN, SDANN, and SDNNi values in patients with Long COVID. Examining frequency, patients presented lower total power for VLF and HF components. When stratifying patients with Long COVID and controls, the SDNN parameter was lower in Long COVID, whereas the LF/HF ratio, D-dimer, NT-Pro-BNP, and IL-6 were significantly higher (85).
Vagal dysfunction and sympathovagal alterations identified through HRV can be visualised by changes in HF components and LF/HF ratio. Persistent parasympathetic activity has been reported in individuals with Long COVID by Menezes Júnior et al. (2022). Patients with Long COVID showed lower HF values than healthy individuals but had LF increases. The authors discussed that increases in parasympathetic activity (for example, in RMSSD) might be related to symptoms of Long COVID and inflammatory marker increases (brain natriuretic peptide, D-dimer, and c-RP) (86).
An increase in parasympathetic tone initially overcome by an increase in sympathetic tone was visualised in 60 patients with Long COVID (>12 weeks post-COVID) in a study by Asarcikli et al. (2022). In comparison to healthy controls, the Long COVID group showed a significant increase in SDNN, RMSSD, and HF when examined using by the VFC in the domain of time and frequency. Prolonged parasympathetic activity may be responsible for the varied symptoms seen in Long COVID (87). However, it is noteworthy that in this study the authors excluded asymptomatic patients or with severe COVID-19 infection, as well as patients with depression, renal failure, morbid obesity diabetes, obstructive sleep apnoea and overt cardiovascular diseases (all known to have a reduced vagal tone), such exclusion criteria were not considered, which may generate selection bias since their result can only be applied to a small category of Long COVID patients, but ultimately not to all patients with Long COVID.
Shah et al. (2022) analysed cardiovascular dysautonomia in 92 patients that had recovered from COVID-19 by examining HRV over time. They found that patients had significantly reduced HRV and higher levels of inflammatory markers than controls. Reduced HRV negatively correlated with RMSSD in relation to the c-RP and IL-6 markers. The HRV time domain (RMSSD) was the most relevant measure for short-term ANS analysis (83).
Lampsas et al. (2022) evaluated patients with Long COVID who had autonomic dysfunction and were hospitalised (88). These patients experienced decreases in overall SDNN-HRV when evaluated with 24-hour ambulatory Holter analysis. The SDNN improved within 6 months of hospitalisation, corroborating the association between autonomic dysfunction and Long COVID. In contrast, Kurtoğlu et al. (2022) examined 50 patients with Long COVID with cardiovascular autonomic dysfunction who were not hospitalised (89). They found that cardiovascular autonomic dysfunction was associated with a decrease in RMSSD parameters, pNN50, HF, and LF, as well as a decrease in signal complexity seen by approximate and sample entropies.
Many authors have reported a loss of autonomic function due to SARS-CoV-2 infection; Freire et al. (2023) (90) reported that seven immunised (full immunisation) young adult patients with Long COVID experienced restoration of their autonomic function over approximately 5 months after mild to moderate infection. The improvement in autonomic function should be further investigated to follow the progression of SARS-CoV-2 infection and enhance the understanding of its long-term effects on the ANS.
7. Importance of HRV for long COVID
For better long-term management, physicians should be aware that patients with mild to severe symptoms of COVID-19, as well as asymptomatic people, can develop symptoms of Long COVID regardless of their health status (91). Long COVID symptoms can last for weeks, months, or even years after the acute phase of the illness. Melatonin is a neuroprotective drug that has shown promise in aiding Long COVID recovery as it helps combat cytokine reactions and advancing symptom severity, helps prevent neurological disorders, and controls cognitive deterioration (“brain fog”) (92).
HRV is influenced by the circadian rhythm; it can decrease throughout the day with higher morning values, with RMSSD and SDNN being higher during the day. The cardiovagal response to stress generates higher HRV at rest, and cardiovagal control is essential to reduce chronic disease symptoms (93). Patients with Long COVID often have insomnia and circadian rhythm problems (94), and treatment with melatonin can substantially combat these deficits in addition to decreasing dysautonomia and increasing HRV (95).
Different HRV responses are observed in patients with Long COVID. Karakayali et al. (2023) examined patients with Long COVID (mild to moderate COVID-19) that were symptomatic or asymptomatic; symptomatic patients had higher parasympathetic tone (RMSSD, SDNN, SD1, and SD2). The parasympathetic system increases HRV, whereas vagal dysfunction reduces it (96).
HRV monitoring results in better stratification in patients with Long COVID. HRV can be used to monitor Long COVID (symptomatic), post-COVID-19 (asymptomatic), and uncertain Long COVID (unclear) individuals (97). Long COVID can range in severity from mild to severely debilitating (98). HRV monitoring in these groups should identify early diagnosis of autonomic changes, predict the severity of Long COVID, limit disease progression, improve clinical outcomes, and lead to new therapeutic strategies.
Analyses of complex and simple HRV markers over time and frequency have been performed to detect early changes in autonomic involvement. Patients with low HRV are at risk of arrhythmia and sudden death (6); furthermore, reduced HRV can be considered predictive of malignant arrhythmias (99).
Different clinical groups of patients with Long COVID were analysed by Marques et al. (2022) using HRV measurement with linear and non-linear methods. They found increased sympathetic activity (LF, LF/HF) at rest and reduced parasympathetic tone (RMSSD, SDNN, SD1, and HF). These alterations can lead to increases in heart rate and blood pressure, predisposing patients to cardiovascular complications, chronic diseases, and sudden death (78).
Owing to its affordability, HRV monitoring can be adopted into evaluation protocols to monitor therapies and the improvement of patients with Long COVID who have autonomic dysfunction (100). Severely ill patients who do not show improvements in long-term HRV require a longer follow-up period to eliminate the virus. HRV markers can be associated with clinical disease severity, acting as non-invasive monitoring resources and predictors of clinical outcomes (101).
HRV is the most appropriate tool for diagnosing patients with Long COVID who have cardiovascular autonomic dysfunction as it generates a quantitative score independent of cognitive function. In addition, HRV can be used as a predictor of inflammatory and autonomous states using diagnostic and predictive methods for cardiovascular function (102). This provides further information on the vagal anti-inflammatory role (53), indicators of cardiovascular health, and the mortality prognosis for the Long COVID population (103, 104).
Neurocognitive “brain fog” symptoms can remain elevated up to 2 years after SARS-CoV-2 infection, as described in a cohort of a retrospective study of nearly 1.3 million patients (105). Autonomic dysfunction can also occur with the presence of persistent dyspnoea, and 60% of patients with Long COVID progress without improvement (106). Monitoring HRV from the initial clinical diagnosis to full recovery may be fundamental for understanding the autonomic balance of patients with Long COVID.
8. Future research
Future studies are needed to clarify the changes in cardiovascular autonomic modulation seen in the Long COVID population. Although proposals for pathophysiological mechanisms have been discussed, the data in the literature remain unclear. It is necessary to identify whether there is a relationship between inflammatory markers and an autonomic origin related to the cardiovascular system.
9. Conclusions
Dysautonomia in patients with Long COVID is multifactorial, and many of the autonomic symptoms can be understood by vagal dysfunction; the parasympathetic and sympathetic interactions involved still need to be extensively studied. Understanding cardiovascular autonomic dysfunction and the importance of association with inflammatory biomarkers in patients with Long COVID can contribute to more accurate diagnoses, better knowledge of the clinical presentation of the ANS pathway when reacting to an infectious disease, and better prognoses. Future studies are needed to understand how the recovery of the autonomic pathway occurs in patients with Long COVID.
Acknowledgments
The authors would like to thank the support of the Federal University of Pará for this publication through the PROPESP/UFPA (PAPQ—Qualified Publication Support Program).
Funding Statement
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
This research was funded by the Amazon Foundation for Research Support (FAPESPA), grant number #006/2020, the Secretary of Science, Technology, and Higher, Professional, and Technological Education (SECTET), grant number #09/2021, the Higher Education Personnel Improvement Coordination—Brazil (CAPES), grant number “Finance code 001, notice no 13/2020”; and the National Council for Scientific and Technological Development—Brazil, grant number “INCT: 406360/2022-7”.
Author contributions
KM: Conceptualization, Investigation, Writing – original draft. JQ: Conceptualization, Supervision, Validation, Writing – review & editing, Visualization. LF: Conceptualization, Supervision, Validation, Visualization, Writing – review & editing.
Conflict of interest
JASQ is a member of the editorial board of Frontiers in Cardiovascular Medicine. This had no impact on the peer review process and the final decision.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1.Szabo S, Zayachkivska O, Hussain A, Muller V. What is really ‘long COVID'? Inflammopharmacology. (2023) 31:551–7. 10.1007/s10787-023-01194-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sisó-Almirall A, Brito-Zerón P, Conangla Ferrín L, Kostov B, Moragas Moreno A, Mestres J, et al. Long COVID-19: proposed primary care clinical guidelines for diagnosis and disease management. Int J Environ Res Public Health. (2021) 18:4350. 10.3390/ijerph18084350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dotan A, David P, Arnheim D, Shoenfeld Y. The autonomic aspects of the post-COVID19 syndrome. Autoimmun Rev. (2022) 21:103071. 10.1016/j.autrev.2022.103071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barizien N, Le Guen M, Russel S, Touche P, Huang F, Vallée A. Clinical characterization of dysautonomia in long COVID-19 patients. Sci Rep. (2021) 11:14042. 10.1038/s41598-021-93546-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schnekenberg L, Sedghi A, Schoene D, Pallesen LP, Barlinn J, Woitek F, et al. Assessment and therapeutic modulation of heart rate variability: potential implications in patients with COVID-19. J Cardiovasc Dev Dis. (2023) 10:297. 10.3390/jcdd10070297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cygankiewicz I, Zareba W. Heart rate variability. Handb Clin Neurol. (2013) 117:379–93. 10.1016/B978-0-444-53491-0.00031-6 [DOI] [PubMed] [Google Scholar]
- 7.Malik M. Heart rate variability. Ann Noninvasive Electrocardiol. (1996) 1:151–81. 10.1111/j.1542-474x.1996.tb00275.x [DOI] [Google Scholar]
- 8.Arakaki X, Arechavala RJ, Choy EH, Bautista J, Bliss B, Molloy C, et al. The connection between heart rate variability (HRV), neurological health, and cognition: a literature review. Front Neurosci. (2023) 17:1055445. 10.3389/fnins.2023.105544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bisaccia G, Ricci F, Recce V, Serio A, Iannetti G, Chahal AA, et al. Post-acute sequelae of COVID-19 and cardiovascular autonomic dysfunction: what do we know? J Cardiovasc Dev Dis. (2021) 8:156. 10.3390/jcdd8110156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raveendran AV, Jayadevan R, Sashidharan S. Long COVID: an overview. Diabetes Metab Syndr. (2021) 15:869–75. 10.1016/j.dsx.2021.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bosco J, Titano R. Severe post-COVID-19 dysautonomia: a case report. BMC Infect Dis. (2022) 22:214. 10.1186/s12879-022-07181-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gorog DA, Storey RF, Gurbel PA, Tantry US, Berger JS, Chan MY, et al. Current and novel biomarkers of thrombotic risk in COVID-19: a consensus statement from the international COVID-19 thrombosis biomarkers colloquium. Nat Rev Cardiol. (2022) 19:475–95. 10.1038/s41569-021-00665-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lionte C, Sorodoc V, Haliga RE, Bologa C, Ceasovschih A, Petris OR, et al. Inflammatory and cardiac biomarkers in relation with post-acute COVID-19 and mortality: what we know after successive pandemic waves. Diagnostics. (2022) 12:1373. 10.3390/diagnostics12061373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chakraborty A, Johnson JN, Spagnoli J, Amin N, Mccoy M, Swaminathan N, et al. Long-term cardiovascular outcomes of multisystem inflammatory syndrome in children associated with COVID-19 using an institution based algorithm. Pediatr Cardiol. (2023) 44:367–80. 10.1007/s00246-022-03020-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sabanoglu C, Inanc IH, Polat E, Peker SA. Long-term predictive value of cardiac biomarkers in patients with COVID-19 infection. Eur Rev Med Pharmacol Sci. (2022) 26:6396–403. 10.26355/eurrev_202209_29667 [DOI] [PubMed] [Google Scholar]
- 16.Zhu Z, Wang M, Lin W, Cai Q, Zhang L, Chen D, et al. Cardiac biomarkers, cardiac injury, and comorbidities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. Immun Inflamm Dis. (2021) 9:1071–100. 10.1002/iid3.471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shouman K, Vanichkachorn G, Cheshire WP, Suarez MD, Shelly S, Lamotte GJ, et al. Autonomic dysfunction following COVID-19 infection: an early experience. Clin Auton Res. (2021) 31:385–94. 10.1007/s10286-021-00803-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hugon J, Msika E-F, Queneau M, Farid K, Paquet C. Long COVID: cognitive complaints (brain fog) and dysfunction of the cingulate cortex. J Neurol. (2022) 269:44–6. 10.1007/s00415-021-10655-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O'Brien B-V, Weber L, Hueffer K, Weltzin MM. SARS-CoV-2 spike ectodomain targets α7 nicotinic acetylcholine receptors. J Biol Chem. (2023) 299:104707. 10.1016/j.jbc.2023.104707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwabenland M, Salié H, Tanevski J, Killmer S, Lago MS, Schlaak AE, et al. Deep spatial profiling of human COVID-19 brains reveals neuroinflammation with distinct microanatomical microglia-T-cell interactions. Immunity. (2021) 54:1594–1610.e11. 10.1016/j.immuni.2021.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vanderheiden A, Klein RS. Neuroinflammation and COVID-19. Curr Opin Neurobiol. (2022) 76:102608. 10.1016/j.conb.2022.102608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stefanou M-I, Palaiodimou L, Bakola E, Smyrnis N, Papadopoulou M, Paraskevas GP, et al. Neurological manifestations of long-COVID syndrome: a narrative review. Ther Adv Chronic Dis. (2022) 13:20406223221076890. 10.1177/20406223221076890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tillman TS, Chen Q, Bondarenko V, Coleman JA, Xu Y, Tang P. SARS-CoV-2 spike protein downregulates cell surface α7nAChR through a helical motif in the spike neck. ACS Chem Neurosci. (2023) 14:689–98. 10.1021/acschemneuro.2c00610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Theoharides TC. Could SARS-CoV-2 spike protein be responsible for long-COVID syndrome? Mol Neurobiol. (2022) 59:1850–61. 10.1007/s12035-021-02696-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amruta N, Chastain WH, Paz M, Solch RJ, Murray-Brown IC, Befeler JB, et al. SARS-CoV-2 mediated neuroinflammation and the impact of COVID-19 in neurological disorders. Cytokine Growth Factor Rev. (2021) 58:1–15. 10.1016/j.cytogfr.2021.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jing H, Wu X, Xiang M, Liu L, Novakovic VA, Shi J. Pathophysiological mechanisms of thrombosis in acute and long COVID-19. Front Immunol. (2022) 13:992384. 10.3389/fimmu.2022.992384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Opsteen S, Files JK, Fram T, Erdmann N. The role of immune activation and antigen persistence in acute and long COVID. J Investig Med. (2023) 71:545–62. 10.1177/10815589231158041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Galán M, Vigón L, Fuertes D, Murciano-Antón MA, Casado-Fernández G, Domínguez-Mateos S, et al. Persistent overactive cytotoxic immune response in a spanish cohort of individuals with long-COVID: identification of diagnostic biomarkers. Front Immunol. (2022) 13:848886. 10.3389/fimmu.2022.848886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Phetsouphanh C, Darley DR, Wilson DB, Howe A, Munier CML, Patel SK, et al. Immunological dysfunction persists for 8 months following initial mild-to-moderate SARS-CoV-2 infection. Nat Immunol. (2022) 23:210–6. 10.1038/s41590-021-01113-x [DOI] [PubMed] [Google Scholar]
- 30.Acosta-Ampudia Y, Monsalve DM, Rojas M, Rodríguez Y, Zapata E, Ramírez-Santana C, et al. Persistent autoimmune activation and proinflammatory state in post-coronavirus disease 2019 syndrome. J Infect Dis. (2022) 225:2155–62. 10.1093/infdis/jiac017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chrestia JF, Oliveira AS, Mulholland AJ, Gallagher T, Bermúdez I, Bouzat C. A functional interaction between Y674-R685 region of the SARS-CoV-2 spike protein and the human α7 nicotinic receptor. Mol Neurobiol. (2022) 59:6076–90. 10.1007/s12035-022-02947-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hajiasgharzadeh K, Jafarlou M, Mansoori B, Dastmalchi N, Baradaran B, Khabbazi A. Inflammatory reflex disruption in COVID-19. Clin Exp Neuroimmunol. (2022) 29:10.1111/cen3.12703. 10.1111/cen3.12703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Malkova AM, Shoenfeld Y. Autoimmune autonomic nervous system imbalance and conditions: chronic fatigue syndrome, fibromyalgia, silicone breast implants, COVID and post-COVID syndrome, sick building syndrome, post-orthostatic tachycardia syndrome, autoimmune diseases and autoimmune/inflammatory syndrome induced by adjuvants. Autoimmun Rev. (2023) 22:103230. 10.1016/j.autrev.2022.103230 [DOI] [PubMed] [Google Scholar]
- 34.Lagoumintzis G, Chasapis CT, Alexandris N, Kouretas D, Tzartos S, Eliopoulos E, et al. Nicotinic cholinergic system and COVID-19: in silico identification of interactions between α7 nicotinic acetylcholine receptor and the cryptic epitopes of SARS-Co-V and SARS-CoV-2 spike glycoproteins. Food Chem Toxicol. (2021) 149:112009. 10.1016/j.fct.2021.112009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tanmay S, Labrou D, Farsalinos K, Poulas K. Is SARS-CoV-2 spike glycoprotein impairing macrophage function via α7-nicotinic acetylcholine receptors? Food Chem Toxicol. (2021) 152:112184. 10.1016/j.fct.2021.112184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Williams DP, Koenig J, Carnevali L, Sgoifo A, Jarczok MN, Sternberg EM, et al. Heart rate variability and inflammation: a meta-analysis of human studies. Brain Behav Immun. (2019) 80:219–26. 10.1016/j.bbi.2019.03.009 [DOI] [PubMed] [Google Scholar]
- 37.Jänig W. Sympathetic nervous system and inflammation: a conceptual view. Auton Neurosci. (2014) 182:4–14. 10.1016/j.autneu.2014.01.004 [DOI] [PubMed] [Google Scholar]
- 38.Pavlov VA, Chavan SS, Tracey KJ. Molecular and functional neuroscience in immunity. Annu Rev Immunol. (2018) 36:783–812. 10.1146/annurev-immunol-042617-053158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maltezou HC, Pavli A, Tsakris A. Post-COVID syndrome: an insight on its pathogenesis. Vaccines. (2021) 9:497. 10.3390/vaccines9050497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dani M, Dirksen A, Taraborrelli P, Torocastro M, Panagopoulos D, Sutton R, et al. Autonomic dysfunction in “long COVID”: rationale, physiology and management strategies. Clin Med. (2021) 21:e63–7. 10.7861/clinmed.2020-0896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koc HC, Xiao J, Liu W, Li Y, Chen G. Long COVID and its management. Int J Biol Sci. (2022) 18:4768–80. 10.7150/ijbs.75056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Glynne P, Tahmasebi N, Gant V, Gupta R. Long COVID following mild SARS-CoV-2 infection: characteristic T cell alterations and response to antihistamines. J Investig Med. (2022) 70:61–7. 10.1136/jim-2021-002051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sotak Š. Inflammatory markers in clinical practice. Vnitr Lek. (2022) 68:11–6. 10.36290/vnl.2022.100 [DOI] [PubMed] [Google Scholar]
- 44.Aeschbacher S, Schoen T, Dörig L, Kreuzmann R, Neuhauser C, Schmidt-Trucksäss A, et al. Heart rate, heart rate variability and inflammatory biomarkers among young and healthy adults. Ann Med. (2017) 49:32–41. 10.1080/07853890.2016.1226512 [DOI] [PubMed] [Google Scholar]
- 45.Aguglia A, Natale A, Fusar-Poli L, Gnecco GB, Lechiara A, Marino M, et al. C-Reactive protein as a potential peripheral biomarker for high-lethality suicide attempts. Life. (2022) 12:1557. 10.3390/life12101557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bouayed MZ, Laaribi I, Chatar CEM, Benaini I, Bouazzaoui MA, Oujidi Y, et al. C-Reactive protein (CRP): a poor prognostic biomarker in COVID-19. Front Immunol. (2022) 13:1040024. 10.3389/fimmu.2022.1040024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pasini E, Corsetti G, Romano C, Scarabelli TM, Chen-Scarabelli C, Saravolatz L, et al. Serum metabolic profile in patients with long-COVID (PASC) syndrome: clinical implications. Front Med. (2021) 8:714426. 10.3389/fmed.2021.714426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Frasch MG. Heart rate as a non-invasive biomarker of inflammation: implications for digital health. Front Immunol. (2022) 13:930445. 10.3389/fimmu.2022.930445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alen N V, Parenteau AM, Sloan RP, Hostinar CE. Heart rate variability and circulating inflammatory markers in midlife. Brain Behav Immun Health. (2021) 15:100273. 10.1016/j.bbih.2021.100273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haensel A, Mills PJ, Nelesen RA, Ziegler MG, Dimsdale JE. The relationship between heart rate variability and inflammatory markers in cardiovascular diseases. Psychoneuroendocrinology. (2008) 33:1305–12. 10.1016/j.psyneuen.2008.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alen NV. The cholinergic anti-inflammatory pathway in humans: state-of-the-art review and future directions. Neurosci Biobehav Rev. (2022) 136:104622. 10.1016/j.neubiorev.2022.104622 [DOI] [PubMed] [Google Scholar]
- 52.Wegeberg A-ML, Okdahl T, Fløyel T, Brock C, Ejskjaer N, Riahi S, et al. Circulating inflammatory markers are inversely associated with heart rate variability measures in type 1 diabetes. Mediators Inflamm. (2020) 2020:3590389. 10.1155/2020/3590389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cooper TM, McKinley PS, Seeman TE, Choo T-H, Lee S, Sloan RP. Heart rate variability predicts levels of inflammatory markers: evidence for the vagal anti-inflammatory pathway. Brain Behav Immun. (2015) 49:94–100. 10.1016/j.bbi.2014.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kurul Ş, van Ackeren N, Goos TG, Ramakers CRB, Been JV, Kornelisse RF, et al. Introducing heart rate variability monitoring combined with biomarker screening into a level IV NICU: a prospective implementation study. Eur J Pediatr. (2022) 181:3331–8. 10.1007/s00431-022-04534-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Legramante JM, Raimondi G, Massaro M, Lellamo F. Positive and negative feedback mechanisms in the neural regulation of cardiovascular function in healthy and spinal cord–injured humans. Circulation. (2001) 103:1250–5. 10.1161/01.CIR.103.9.1250 [DOI] [PubMed] [Google Scholar]
- 56.Goldberger JJ, Arora R, Buckley U, Shivkumar K. Autonomic nervous system dysfunction: JACC focus seminar. J Am Coll Cardiol. (2019) 73:1189–206. 10.1016/j.jacc.2018.12.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chadda KR, Blakey EE, Huang CL-H, Jeevaratnam K. Long COVID-19 and postural orthostatic tachycardia syndrome- is dysautonomia to be blamed? Front Cardiovasc Med. (2022) 9:860198. 10.3389/fcvm.2022.860198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Johansson M, Ståhlberg M, Runold M, Nygren-Bonnier M, Nilsson J, Olshansky B, et al. Long-haul post-COVID-19 symptoms presenting as a variant of postural orthostatic tachycardia syndrome: the Swedish experience. JACC Case Rep. (2021) 3:573–80. 10.1016/j.jaccas.2021.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Eldokla AM, Mohamed-Hussein AA, Fouad AM, Abdelnaser MG, Ali ST, Makhlouf NA, et al. Prevalence and patterns of symptoms of dysautonomia in patients with long-COVID syndrome: a cross-sectional study. Ann Clin Transl Neurol. (2022) 9:778–85. 10.1002/acn3.51557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Larsen NW, Stiles LE, Shaik R, Schneider L, Muppidi S, Tsui CT, et al. Characterization of autonomic symptom burden in long COVID: a global survey of 2,314 adults. Front Neurol. (2022) 13:1012668. 10.3389/fneur.2022.1012668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hovaguimian A. Dysautonomia: diagnosis and management. Neurol Clin. (2023) 41:193–213. 10.1016/j.ncl.2022.08.002 [DOI] [PubMed] [Google Scholar]
- 62.Raman B, Bluemke DA, Lüscher TF, Neubauer S. Long COVID: post-acute sequelae of COVID-19 with a cardiovascular focus. Eur Heart J. (2022) 43:1157–72. 10.1093/eurheartj/ehac031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Raj SR, Arnold AC, Barboi A, Claydon VE, Limberg JK, Lucci VM, et al. Long-COVID postural tachycardia syndrome: an American autonomic society statement. Clin Auton Res. (2021) 31:365–8. 10.1007/s10286-021-00798-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zanin A, Amah G, Chakroun S, Testard P, Faucher A, Le TYV, et al. Parasympathetic autonomic dysfunction is more often evidenced than sympathetic autonomic dysfunction in fluctuating and polymorphic symptoms of “long-COVID” patients. Sci Rep. (2023) 13:8251. 10.1038/s41598-023-35086-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Becker RC. Autonomic dysfunction in SARS-COV-2 infection acute and long-term implications COVID-19 editor's Page series. J Thromb Thrombolysis. (2021) 52:692–707. 10.1007/s11239-021-02549-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stute NL, Stickford JL, Province VM, Augenreich MA, Ratchford SM, Stickford ASL. COVID-19 is getting on our nerves: sympathetic neural activity and haemodynamics in young adults recovering from SARS-CoV-2. J Physiol. (2021) 599:4269–85. 10.1113/JP281888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Delevatti RS, Danielevicz A, Sirydakis ME, de Melo PUG, de la Rocha Freitas C, Rech CR, et al. Effects of physical training on functional, clinical, morphological, behavioural and psychosocial outcomes in post-COVID-19 infection: COVID-19 and REhabilitation study (CORE-study)-a study protocol for a randomised controlled clinical trial. Trials. (2023) 24:39. 10.1186/s13063-022-07055-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schneider M, Schwerdtfeger A. Autonomic dysfunction in posttraumatic stress disorder indexed by heart rate variability: a meta-analysis. Psychol Med. (2020) 50:1937–48. 10.1017/S003329172000207X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Corrado J, Halpin S, Preston N, Whiteside D, Tarrant R, Davison J, et al. HEART Rate variability biofeedback for long COVID symptoms (HEARTLOC): protocol for a feasibility study. BMJ Open. (2022) 12:e066044. 10.1136/bmjopen-2022-066044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Thayer JF, Yamamoto SS, Brosschot JF. The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Int J Cardiol. (2010) 141:122–31. 10.1016/j.ijcard.2009.09.543 [DOI] [PubMed] [Google Scholar]
- 71.La Rovere MT, Pinna GD, Maestri R, Mortara A, Capomolla S, Febo O, et al. Short-term heart rate variability strongly predicts sudden cardiac death in chronic heart failure patients. Circulation. (2003) 107:565–70. 10.1161/01.cir.0000047275.25795 [DOI] [PubMed] [Google Scholar]
- 72.Jung I, Lee DY, Lee MY, Kwon H, Rhee E-J, Park C-Y, et al. Autonomic imbalance increases the risk for non-alcoholic fatty liver disease. Front Endocrinol. (2021) 12:752944. 10.3389/fendo.2021.752944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Freire APCF, Lira FS, Morano AEVA, Pereira T, Coelho-E-Silva MJ, Caseiro A, et al. Role of body mass and physical activity in autonomic function modulation on post-COVID-19 condition: an observational subanalysis of fit-COVID study. Int J Environ Res Public Health. (2022) 19:2457. 10.3390/ijerph19042457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Papaioannou VE, Verkerk AO, Amin AS, de Bakker JMT. Intracardiac origin of heart rate variability, pacemaker funny current and their possible association with critical illness. Curr Cardiol Rev. (2013) 9:82–96. 10.2174/157340313805076359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yang Z, Liu H, Meng F, Guan Y, Zhao M, Qu W, et al. The analysis of circadian rhythm of heart rate variability in patients with drug-resistant epilepsy. Epilepsy Res. (2018) 146:151–9. 10.1016/j.eplepsyres.2018.08.002 [DOI] [PubMed] [Google Scholar]
- 76.Garis G, Haupts M, Duning T, Hildebrandt H. Heart rate variability and fatigue in MS: two parallel pathways representing disseminated inflammatory processes? Neurol Sci. (2023) 44:83–98. 10.1007/s10072-022-06385-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bonaz B, Sinniger V, Pellissier S. Vagal tone: effects on sensitivity, motility, and inflammation. Neurogastroenterol Motil. (2016) 28:455–62. 10.1111/nmo.12817 [DOI] [PubMed] [Google Scholar]
- 78.Marques KC, Silva CC, da Trindade SS, de Santos MCS, Rocha RSB, da Vasconcelos PFC, et al. Reduction of cardiac autonomic modulation and increased sympathetic activity by heart rate variability in patients with long COVID. Front Cardiovasc Med. (2022) 9:862001. 10.3389/fcvm.2022.862001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stella A B, Furlanis G, Frezza NA, Valentinotti R, Ajcevic M, Manganotti P. Autonomic dysfunction in post-COVID patients with and witfhout neurological symptoms: a prospective multidomain observational study. J Neurol. (2022) 269:587–96. 10.1007/s00415-021-10735-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.De Maria B, Dalla Vecchia LA, Porta A, La Rovere MT. Autonomic dysfunction and heart rate variability with holter monitoring: a diagnostic look at autonomic regulation. Herzschrittmacherther Elektrophysiol. (2021) 32:315–9. 10.1007/s00399-021-00780-5 [DOI] [PubMed] [Google Scholar]
- 81.Hottenrott L, Gronwald T, Hottenrott K, Wiewelhove T, Ferrauti A. Utilizing heart rate variability for coaching athletes during and after viral infection: a case report in an elite endurance athlete. Front Sports Act Living. (2021) 3:612782. 10.3389/fspor.2021.612782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hasty F, García G, Dávila H, Wittels SH, Hendricks S, Chong S. Heart rate variability as a possible predictive marker for acute inflammatory response in COVID-19 patients. Mil Med. (2021) 186:e34–8. 10.1093/milmed/usaa405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shah B, Kunal S, Bansal A, Jain J, Poundrik S, Shetty MK, et al. Heart rate variability as a marker of cardiovascular dysautonomia in post-COVID-19 syndrome using artificial intelligence. Indian Pacing Electrophysiol J. (2022) 22:70–6. 10.1016/j.ipej.2022.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liviero F, Scapellato ML, Folino F, Moretto A, Mason P, Pavanello S. Persistent increase of sympathetic activity in post-acute COVID-19 of paucisymptomatic healthcare workers. Int J Environ Res Public Health. (2023) 20:830. 10.3390/ijerph20010830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Acanfora D, Nolano M, Acanfora C, Colella C, Provitera V, Caporaso G, et al. Impaired vagal activity in long-COVID-19 patients. Viruses. (2022) 14:1035. 10.3390/v14051035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.da Menezes Junior AS, Schröder AA, Botelho SM, Resende AL. Cardiac autonomic function in long COVID-19 using heart rate variability: an observational cross-sectional study. J Clin Med. (2022) 12:100. 10.3390/jcm12010100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Asarcikli LD, Hayiroglu Mİ, Osken A, Keskin K, Kolak Z, Aksu T. Heart rate variability and cardiac autonomic functions in post-COVID period. J Interv Card Electrophysiol. (2022) 63:715–21. 10.1007/s10840-022-01138-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lampsas S, Oikonomou E, Souvaliotis N, Goliopoulou A, Papamikroulis GA, Anastasiou A, et al. Impaired heart rate variability one and six months post acute COVID-19. Eur Heart J. (2022) 43:ehac544.402. 10.1093/eurheartj/ehac544.402 [DOI] [Google Scholar]
- 89.Kurtoğlu E, Afsin A, Aktaş İ, Aktürk E, Kutlusoy E, Çağaşar Ö. Altered cardiac autonomic function after recovery from COVID-19. Ann Noninvasive Electrocardiol. (2022) 27:e12916. 10.1111/anec.12916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Freire APCF, Amin S, Lira FS, von Morano AEA, Pereira T, Coelho-E-Silva M-J, et al. Autonomic function recovery and physical activity levels in post-COVID-19 young adults after immunization: an observational follow-up case-control study. Int J Environ Res Public Health. (2023) 20:2251. 10.3390/ijerph20032251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zadeh FH, Wilson DR, Agrawal DK. Long COVID: complications, underlying mechanisms, and treatment strategies. Arch Microbiol Immunol. (2023) 7:36–61. 10.26502/ami.936500103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Akanchise T, Angelova A. Potential of nano-antioxidants and nanomedicine for recovery from neurological disorders linked to long COVID syndrome. Antioxidants. (2023) 6:393. 10.3390/antiox12020393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vondrasek JD, Alkahtani SA, Al-Hudaib AA, Habib SS, Al-Masri AA, Grosicki GJ, et al. Heart rate variability and chronotype in young adult men. Healthcare. (2022) 10:2465. 10.3390/healthcare10122465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bonilla H, Peluso MJ, Rodgers K, Aberg JA, Patterson TF, Tamburro R, et al. Therapeutic trials for long COVID-19: a call to action from the interventions taskforce of the RECOVER initiative. Front Immunol. (2023) 14:1129459. 10.3389/fimmu.2023.1129459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cardinali DP, Brown GM, Pandi-Perumal SR. Possible application of melatonin in long COVID. Biomolecules. (2022) 12:1646. 10.3390/biom12111646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Karakayalı M, Artac I, Ilis D, Omar T, Rencuzogullari I, Karabag Y, et al. Evaluation of outpatients in the post-COVID-19 period in terms of autonomic dysfunction and silent ischemia. Cureus. (2023) 15:e40256. 10.7759/cureus.40256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Suh HW, Kwon CY, Lee B. Long-term impact of COVID-19 on heart rate variability: a systematic review of observational studies. Healthcare. (2023) 11:1095. 10.3390/healthcare11081095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Calabrese C, Kirchner E, Calabrese LH. Long COVID and rheumatology: clinical, diagnostic, and therapeutic implications. Best Pract Res Clin Rheumatol. (2022) 36:101794. 10.1016/j.berh.2022.101794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Krantz MJ, Mehler PS. Heart rate variability in women. Arch Intern Med. (2006) 166:247. 10.1001/archinte.166.2.247-a [DOI] [PubMed] [Google Scholar]
- 100.Jammoul M, Naddour J, Madi A, Reslan MA, Hatoum F, Zeineddine J, et al. Investigating the possible mechanisms of autonomic dysfunction post-COVID-19. Auton Neurosci. (2023) 245:103071. 10.1016/j.autneu.2022.103071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pan Y, Yu Z, Yuan Y, Han J, Wang Z, Chen H, et al. Alteration of autonomic nervous system is associated with severity and outcomes in patients with COVID-19. Front Physiol. (2021) 12:630038. 10.3389/fphys.2021.630038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Allendes FJ, Díaz HS, Ortiz FC, Marcus NJ, Quintanilla R, Inestrosa NC, et al. Cardiovascular and autonomic dysfunction in long-COVID syndrome and the potential role of non-invasive therapeutic strategies on cardiovascular outcomes. Front Med. (2023) 9:1095249. 10.3389/fmed.2022.1095249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Faust O, Hong W, Loh HW, Xu S, Tan R-S, Chakraborty S, et al. Heart rate variability for medical decision support systems: a review. Comput Biol Med. (2022) 145:105407. 10.1016/j.compbiomed.2022.105407 [DOI] [PubMed] [Google Scholar]
- 104.Shanks J, Abukar Y, Lever NA, Pachen M, LeGrice IJ, Crossman DJ, et al. Reverse re-modelling chronic heart failure by reinstating heart rate variability. Basic Res Cardiol. (2022) 117:4. 10.1007/s00395-022-00911-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Taquet M, Sillett R, Zhu L, Mendel J, Camplisson I, Dercon Q, et al. Neurological and psychiatric risk trajectories after SARS-CoV-2 infection: an analysis of 2-year retrospective cohort studies including 1 284 437 patients. Lancet Psychiatry. (2022) 9(10):815–27. 10.1016/S2215-0366(22)00260-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Perumal R, Shunmugam L, Naidoo K, Abdool Karim SS, Wilkins D, Garzino-Demo A, et al. Long COVID: a review and proposed visualization of the complexity of long COVID. Front Immunol. (2023) 14:1117464. 10.3389/fimmu.2023.1117464 [DOI] [PMC free article] [PubMed] [Google Scholar]