Abstract
Birth defect surveillance in Eswatini in 2020–2021 identified 0.80% defects (197/24 599 live and stillborn infants). Neural tube defect (NTD) prevalence was 0.08%, 0.08%, and 0.15% for 4902 women on dolutegravir preconception, 17 285 HIV-negative women, and 1320 women on efavirenz preconception, respectively, more definitively refuting the dolutegravir preconception NTD safety signal.
Keywords: HIV, birth defect, dolutegravir, Eswatini, surveillance
An unexpected neural tube defect (NTD) safety signal with preconception dolutegravir (DTG) exposure was identified in a preliminary analysis from the Botswana Tsepamo birth surveillance study in May 2018 [1]. This brought into sharp focus the critical need for reliable data and improved pharmacovigilance to evaluate new antiretroviral (ARV) safety in pregnancy and women of reproductive potential. Evaluating the potential association of a drug with birth defects, which can be uncommon to rare, requires large numbers of exposures to adequately determine the existence and magnitude of risk; for example, a minimum of 2000 preconception exposures are necessary to rule out a 3-fold increase in NTD risk, given a 0.1% background NTD prevalence [2]. A substantial decline in NTD risk was observed in the Tsepamo study as additional preconception DTG exposures accumulated [3]. There is also marked geographic variation in both background NTD prevalence and national NTD prevention policies such as food folic acid fortification [4]. NTD prevalence appears highest in countries in Africa and Asia without existing NTD prevention programs, though many lack robust birth defect surveillance systems.
In Eswatini, HIV prevalence in pregnant women is 36% [5]. Transition to DTG-based therapy began in April 2019, with scale-up in February 2021 [6]. There is no national food folic acid fortification and no national population-based estimate of birth defects. We initiated sentinel surveillance for birth defects in live and stillborn infants using a design similar to the Tsepamo study, covering ∼77% of all births nationally (based on countrywide 2020 estimates), to provide a national estimate of birth defects and assess birth defect prevalence by HIV status, and among women with HIV, by antiretroviral therapy (ART) status and timing.
METHODS
This was a cross-sectional, observational design with data collection at time of hospital admission for delivery. Surveillance was conducted in the 5 highest-volume government hospitals in all 4 regions of Eswatini. Data are presented for ∼1 year, September 2021–September 2022, of a planned 2-year surveillance study. Routine data on HIV/ART status, pregnancy history, and birth outcomes for all women presenting to study sites for labor and delivery were abstracted from clinic records and extracted from national electronic databases. Per standard of care, clinic midwives, who received study-specific and defect identification training, performed surface examinations (including inside the mouth) of live and stillborn infants. Infants with detected defects were also examined by clinic physicians who advised on defect diagnoses. If defects were identified, mothers consented for interviews to capture more detailed medical and medication history and defect descriptions and birth defect photographs. Women undergoing medically induced abortions due to identification of a defect via diagnostic imagining were also enrolled, though ultrasounds were rarely used at study hospitals. Women could consent to the interview, photographs, or both study components.
Study data were captured by research assistants based at each study site. Photographs and interview data (blinded to HIV/ART status) were sent to an external medical geneticist for confirmatory diagnosis, consisting of the organ group and preferred term based on the Organ System Classification introduced by the Antiretroviral Pregnancy Registry. Defects were classified as major or minor based on definitions used by the Metropolitan Atlanta Congenital Defects Program [7]. In cases without photographs and/or interviews, typically because of study refusals or missed enrollments (due to a small proportion of missed deliveries occurring after normal working hours), clinic records or study surveillance was used to define defects if available. Defects without photographs or with photographs where the defect was not clearly captured were classified as confirmed without photos. If photographs clearly showed the area in question but the clinical diagnosis could not be confirmed, the defect was excluded from analysis.
Live/stillbirth was defined as either ≥28 weeks gestation or <28 weeks and (1) a live birth or (2) recorded as stillbirth by the hospital with a weight of ≥1000 g. Examinations were not routinely conducted for miscarriages and are excluded from this analysis. ART at conception was determined using date of drug initiation and last menstrual period (LMP) and defined as maternal ART received up to 8 weeks after the LMP date (≤6 weeks after estimated conception date). We described birth defect rates by HIV status and ART regimen at conception. Infants with ≥1 major defect with or without minor defects were classified as major; infants with ≥1 minor defect only were classified as minor. Major defects were categorized as NTD or non-NTD. Defect prevalence was calculated as number of events divided by total number of live and stillbirths; the numerator included an abortion with confirmed defects. Chromosomal trisomies identifiable by physical appearance were excluded from analysis. Confidence intervals were constructed using Wilson confidence limits for the binomial proportion.
Patient Consent
All participants provided written informed consent for interviews and/or photograph-taking. This protocol, including a waiver of consent for routine data collection, was approved by the Eswatini Health and Human Research Review Board (EHHRRB028/2021) and Advarra Institutional Review Board (Pro00055975) in the United States.
RESULTS
In total, 24 827 women had deliveries, with live births (n = 24 239), stillbirths (n = 566), or multiple live/stillbirths (n = 22); there was 1 medical abortion. Among women (median age [IQR], 26 [21–32] years) with deliveries, 17 285 (69.6%) were HIV-negative, 7536 (30.4%) had HIV, and 6 were missing HIV status. Of women with HIV, 4902 (65.0%) were on DTG at conception, 1320 (17.5%) were on efavirenz (EFV) at conception, 83 (1.1%) were on other ART at conception, and 1092 (14.5%) were not on ART at conception as they were newly diagnosed with HIV during pregnancy; nearly all (99.2%) initiated DTG during pregnancy. One hundred thirty-nine (1.8%) were missing ART regimen and/or timing of initiation.
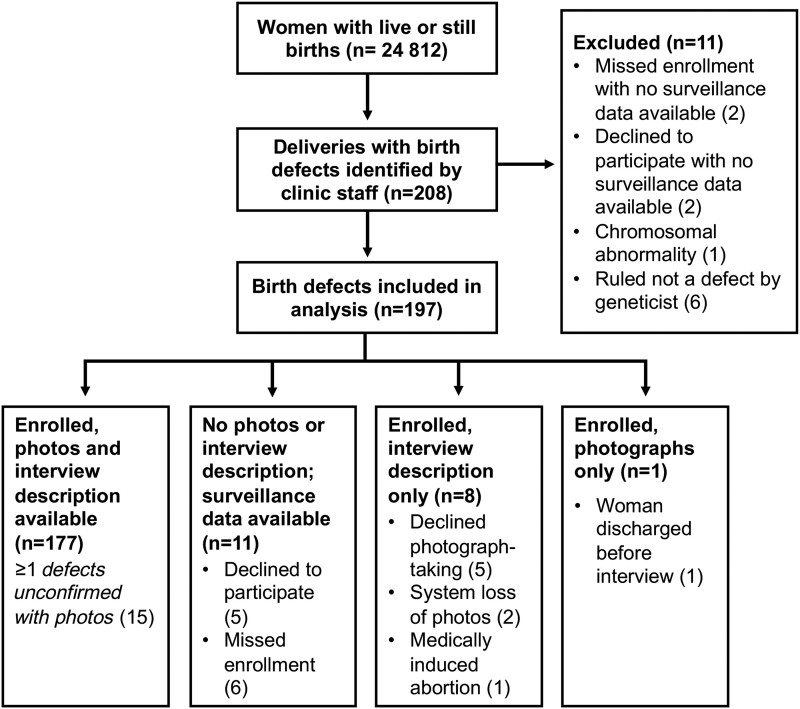
Of 208 deliveries with birth defects identified, eleven were excluded from analysis: 6 were determined not to be defects by the geneticist, 1 infant with Trisomy 21 and 4 infants without any defect information. See Figure 1 for details on participant refusals and missed enrollments. Of 24 599 births to HIV-negative women and women with HIV on DTG, EFV, or no ART at conception, 197 live or stillborn infants were diagnosed with confirmed external malformations and included in the analysis (0.80%; 95% CI, 0.70%–0.92%) (Table 1). No birth defects were identified in the infants of the remaining 228 women with unknown HIV status or ART regimen/timing, nor those receiving non-DTG/EFV-ART at conception, who were therefore excluded from the denominator (and are not shown in the table). All women of infants identified with birth defects who newly initiated ART during pregnancy were started on DTG. In 34 (17.3%) infants, ≥1 defect was diagnosed based on description only, including 2 NTDs (1 each in the HIV-negative and DTG preconception groups). Twelve infants were stillborn, and 18 infants died shortly after birth.
Figure 1.
Screening and enrollment of women with infants with identified birth defects.
Table 1.
Number and Rate of Birth Defects by HIV and ART Status
| No. (%, 95% CI) | |||||
|---|---|---|---|---|---|
| Women's HIV Status and Conception Regimen | Livebirths and Stillbirths | NTD | Major Non-NTD Birth Defects | Minor Birth Defects | Total Birth Defectsa |
| Total from subgroups with birth defectsb | 24 599 | 19 (0.08, 0.05–0.12) | 95 (0.39, 0.32–0.47) | 91 (0.37, 0.30–0.45) | 197 (0.80, 0.70–0.92) |
| HIV-negative | 17 285 | 13 (0.08, 0.04–0.13) | 65 (0.38, 0.30–0.48) | 60 (0.35, 0.27–0.45) | 133 (0.77, 0.65–0.91) |
| All women with HIVc | 7308 | 6 (0.08, 0.04–0.18) | 30 (0.41, 0.29–0.59) | 31 (0.42, 0.30–0.60) | 64 (0.88, 0.69–1.12) |
| DTG at conception | 4902 | 4 (0.08, 0.03–0.21) | 19 (0.39, 0.25–0.60) | 22 (0.45, 0.30–0.68) | 43 (0.88, 0.65–1.18) |
| EFV at conception | 1320 | 2 (0.15, 0.04–0.55) | 8 (0.61, 0.31–1.19) | 7 (0.53, 0.26–1.09) | 16 (1.21, 0.75–1.96) |
| No ART at conception (newly initiated on ART during pregnancy) | 1092 | 0 | 3 (0.27, 0.09–0.80) | 2 (0.18, 0.05–0.67) | 5 (0.46, 0.20–1.07) |
Abbreviations: ART, antiretroviral therapy; DTG, dolutegravir; EFV, efavirenz; NTD, neural tube defect.
One hundred ninety-seven infants were diagnosed with defects, but 8 had both NTD and other non-NTD major defects. Because NTD and major non-NTD estimates were calculated separately, the subgroups do not add up to the total number.
Total excludes infants of women with unknown HIV status (n = 6), women with HIV who were missing ART regimen/timing data (n = 139), and women with HIV receiving non-DTG/EFV ART at conception (n = 83).
As above, total excludes infants of women with HIV missing ART regimen/timing data and receiving non-DTG/EFV-ART at conception.
There were 19 NTDs (9.6% of defects). NTD prevalence was 0.08% (95% CI, 0.03%–0.21%), 0.08% (95% CI, 0.04%–0.13%), and 0.15% (95% CI, 0.04%–0.55%) for women on DTG at conception, HIV-negative women, and women on EFV at conception, respectively. Nine of the NTDs (47.4%) were myelomeningocele/meningocele, 2 associated with hydrocephalus. Eight infants had NTDs and other non-NTD major defects. Of 95 non-NTD major defects, the most common was varus foot malformation (26.3%, 25). Twenty-nine infants were diagnosed with multiple non-NTD major defects with or without minor defects. Among 91 deliveries with minor defects, most (n = 86, 94.5%) were polydactyly (postaxial hand or unspecified). Non-NTD major and minor defects were similar in women on DTG at conception and HIV-negative women (major 0.39% and 0.38%; minor 0.45% and 0.35%, respectively). Among women on EFV at conception, the rate was 0.61% and 0.53% for non-NTD major and minor defects. See Supplementary Table 1 for specific defects by organ group.
DISCUSSION
Most pregnant women with HIV in Eswatini are currently receiving DTG, with the majority receiving DTG at conception. There were similar rates of NTD and non-NTD defects between women on DTG at conception and HIV-negative women, which does not support an association of NTD with DTG at conception, providing evidence from another Sub-Saharan African country without national folic acid food supplementation that supports the updated Botswana Tsepamo results.
The NTD prevalence of 0.08% among women on DTG at conception in Eswatini was slightly lower than the 0.11% (95% CI, 0.06%–0.19%) prevalence reported by the Tsepamo study in July 2022 in women on DTG at conception [8]. Combining Tsepamo and our study data, there were >14 000 births among women on DTG at conception and 14 NTDs identified, giving an inverse variance weighted NTD prevalence of 0.10% (95% CI, 0.05%–0.15%). The NTD rate was the same for women with HIV overall and HIV-negative women in our study. The NTD rate among women on EFV at conception was higher than that of other groups, but there were fewer EFV-exposed births. Other ongoing surveillance in Kenya and South Africa similarly did not find association between major birth defects and HIV status or DTG use at conception [9].
The main study limitation is reliance on routine data for surveillance given inherent weaknesses with quality and missingness. For various reasons, we could not confirm 17% of birth defects with photos. However, due to data verification and quality checks, missingness for HIV/ART status and timing was <1%; birth defect diagnosis based on description only is similar to the approach used in other surveillance studies [3].
Despite its limitations, this study provides a population-based estimate of birth defects in a country with high HIV burden and nearly 5000 deliveries surveilled among women on DTG preconception. Following initial Tsepamo study results, there was concern regarding DTG use among women of reproductive potential. However, despite a clear decrease in the NTD signal in the Tsepamo study as further data accumulated [8] and updated 2019 WHO guidelines recommending DTG as the preferred ART regimen [10], transition to DTG ART in women continues to lag significantly behind men in many Sub-Saharan African countries [11, 12]. Together with updated Tsepamo results, our findings more definitively refute the NTD safety signal. They strengthen the case for DTG use among women of reproductive potential, hopefully helping to further address concerns and clear the path for use of this highly efficacious HIV treatment for women. The study also demonstrates the importance of active safety surveillance in pregnancy, which is critical to maintain, particularly as new ARVs are introduced, notably long-acting formulations, to help inform national and global HIV prevention and treatment policies.
Supplementary Material
Acknowledgments
The authors would also like to thank the research participants, research assistants, and clinic staff at the study sites. We would also like to acknowledge the ongoing support from the Eswatini Ministry of Health. As the study is still ongoing, the data are not publicly available.
Contributor Information
Michelle M Gill, Elizabeth Glaser Pediatric AIDS Foundation, Washington, DC, USA.
Philisiwe Khumalo, Elizabeth Glaser Pediatric AIDS Foundation, Mbabane, Eswatini.
Caspian Chouraya, Elizabeth Glaser Pediatric AIDS Foundation, Mbabane, Eswatini.
Mthokozisi Kunene, Elizabeth Glaser Pediatric AIDS Foundation, Mbabane, Eswatini.
Futhi Dlamini, Elizabeth Glaser Pediatric AIDS Foundation, Mbabane, Eswatini.
Heather J Hoffman, Department of Biostatistics and Bioinformatics, Milken School of Public Health, George Washington University, Washington, DC, USA.
Angela E Scheuerle, Southwestern Medical Center, University of Texas, Dallas, Texas, USA.
Bonisile Nhlabatsi, Eswatini Ministry of Health, Mbabane, Eswatini.
Wiseman Mngometulu, Eswatini Ministry of Health, Mbabane, Eswatini.
Ntombikayise Dlamini-Madlopha, Eswatini Ministry of Health, Mbabane, Eswatini.
Nompumelelo Mthunzi, Eswatini Ministry of Health, Mbabane, Eswatini.
Lynne Mofenson, Elizabeth Glaser Pediatric AIDS Foundation, Washington, DC, USA.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Author contributions. L.M. conceived the original idea. M.M.G., P.K., C.C., B.N., W.M., N.D.M., N.M., and L.M. designed the study. P.K., F.D., and M.K. were responsible for data collection and data management with study oversight from M.M.G., C.C., and B.N. P.K. and H.J.H. analyzed the data. A.S. provided the final outcome data used for the results. M.M.G. wrote the manuscript with significant contributions from L.M. All authors reviewed and approved the manuscript.
Financial support. This work was supported by ViiV Healthcare.
References
- 1. Zash R, Makhema J, Shapiro RL. Neural-tube defects with dolutegravir treatment from the time of conception. N Engl J Med 2018; 379:979–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Watts DH. Teratogenicity risk of antiretroviral therapy in pregnancy. Curr HIV/AIDS Rep 2007; 4:135–40. [DOI] [PubMed] [Google Scholar]
- 3. Zash R, Holmes L, Diseko M, et al. Neural-tube defects and antiretroviral treatment regimens in Botswana. N Engl J Med 2019; 381:827–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kancherla V. Neural tube defects: a review of global prevalence, causes, and primary prevention. Childs Nerv Syst 2023; 39:1703–10. [DOI] [PubMed] [Google Scholar]
- 5. Kingdom of Eswatini Ministry of Health, Monitoring and Evaluation Unit . Sexual and Reproductive Health 2021 Report. Kingdom of Eswatini Ministry of Health;2021. [Google Scholar]
- 6. Kingdom of Eswatini Ministry of Health . Swaziland Integrated HIV Management Guidelines. Kingdom of Eswatini Ministry of Health; 2018. [Google Scholar]
- 7. Centers for Disease Control and Prevention . Metropolitan Atlanta Congenital Defects Program (MACDP). Available at: https://www.cdc.gov/ncbddd/birthdefects/macdp.html#SnippetTab. Accessed May 24, 2023.
- 8. Zash R, Holmes LB, Diseko M, et al. Update on neural tube defects with antiretroviral exposure in the Tsepamo Study, Botswana. Paper presented at: 24th International AIDS Conference; 2022;. Montreal, Quebec, Canada, July 29–August 2, 2022.
- 9. Patel R, Mehta U, Humphrey J, et al. Dolutegravir exposure and congenital anomalies in Sub-Saharan Africa. Paper presented at: Conference on Retroviruses and Opportunistic Infections; 2023; Seattle, Washington, USA, February 19–23, 2023.
- 10. World Health Organization . Update of Recommendations on First- and Second-Line Antiretroviral Regimens. World Health Organization; 2019. [Google Scholar]
- 11. Shah N, Esber A, Cavanaugh JS, et al. Transitioning women to first-line preferred TLD regimen is lagging in Sub-Saharan Africa. Clin Infect Dis 2023; 76:e766–72. [DOI] [PubMed] [Google Scholar]
- 12. Dorward J, Sookrajh Y, Khubone T, et al. Implementation and outcomes of dolutegravir-based first-line antiretroviral therapy for people with HIV in South Africa: a retrospective cohort study. Lancet HIV 2023; 10:e284–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.