Abstract
Adipose tissue (AT) is a complicated metabolic organ consisting of a heterogeneous population of cells that exert wide-ranging effects on the regulation of systemic metabolism and in maintaining metabolic homeostasis. Various obesity-related complications are associated with the development of dysfunctional AT. As an essential transmitter of intercellular information, extracellular vesicles (EVs) have recently been recognized as crucial in regulating multiple physiological functions. AT-derived extracellular vesicles (ADEVs) have been shown to facilitate cellular communication both inside and between ATs and other peripheral organs. Here, the role of EVs released from ATs in the homeostasis of metabolic and cardiovascular diseases, cancer, and neurological disorders by delivering lipids, proteins, and nucleic acids between different cells is summarized. Furthermore, the differences in the sources of ADEVs, such as adipocytes, AT macrophages, AT-derived stem cells, and AT-derived mesenchymal stem cells, are also discussed. This review may provide valuable information for the potential application of ADEVs in metabolic syndrome, cardiovascular diseases, cancer, and neurological disorders.
Keywords: Adipose tissues, Adipose tissue-derived extracellular vesicles, metabolic syndrome, Cardiovascular diseases, Cancers
1. Introduction
Obesity has rapidly become a widespread public health concern, the incidence of which has gradually increased over numerous decades. By 2025, the World Health Organization predicts that one in five adults will be obese globally (1). Obesity is a highly heterogeneous and complicated disorder caused by unbalanced energy metabolism. In addition, obesity is also closely associated with the pathogenesis of various metabolic diseases, such as type 2 diabetes mellitus (T2D), dyslipidemia, and cardiovascular diseases (CVDs), including hypertension and stroke, neurological disorders, musculoskeletal disease, and certain types of cancer (for example, breast, liver, ovarian, kidney, prostate and colon cancer) (2). Furthermore, several studies have found that obese patients with associated comorbidities are more susceptible to SARS-CoV-2, exhibiting a higher risk of death (3–5).
Adipose tissues (ATs) are complex tissues that primarily exhibit a regulatory function. In mammals, ATs are primarily classified into white AT (WATs) and brown AT (BATs). In addition to metabolizing fat into energy, ATs also serve as a critical endocrine organ that can regulate energy metabolism, immunological responses, and cardiovascular balance by secreting a variety of adipokines, peptide hormones, and cytokines (6). Over the years, ~100 adipokines have been identified (7). Adipokines have a wide range of physiological effects on tissues and organs in different systems, such as the nervous system, immune system, and vascular system (8). For example, ATs secrete adiponectin that can promote insulin sensitivity and the antiatherosclerotic properties of cells by binding to adiponectin receptor (AdipoR) 1 and AdipoR2 (9–11). An inverse association exists between circulating adiponectin and obesity-related cancer incidence (11). Furthermore, adiponectin acts as an essential metabolic reprogramming factor by promoting the interaction between adaptor protein, phosphotyrosine interacting with PH domain, and leucine zipper 1 (APPL1) and AMP-activated protein kinase (AMPK), promoting glucose uptake through glucose transporter 4 (GLUT4) (12). The release of leptin by adipocytes was correlated with alterations in cell metabolism, such as the switch from mitochondrial β-oxidation to aerobic glycolysis (13). Chronic inflammation is another well-established characteristic of obesity (14). In obesity, there is an increase in oxidative stress and inflammation, leading to an increased release of proinflammatory adipokines, which can contribute to insulin resistance in the liver, muscles, and ATs, resulting in metabolic abnormalities (15). Studies have shown a positive correlation between obesity, insulin levels, insulin resistance, and increased tumor necrosis factor (TNF)-α production in human adipocytes (16).
In addition to the classical polypeptide adipokines and cytokines, ATs can also produce and secrete extracellular vesicles (EVs) (17). The composition of all EVs is similar to that of the parent cells, packed with bioactive molecules such as lipids, proteins, and DNA delivered to cells within ATs or in distant organs, mediating intercellular and interorgan communication (14). In this context, AT-derived extracellular vesicles (ADEVs) have been identified as crucial players in the cellular communication of immune and metabolic responses, regulating cellular processes in local and distant tissues (18,19). This review will focus on the compositions and functions of ADEVs from different cellular sources in ATs and their contribution to AT homeostasis and the development of metabolic complications, such as metabolic diseases, CVDs, several types of cancer, and neurological disorders.
2. Introduction to EVs
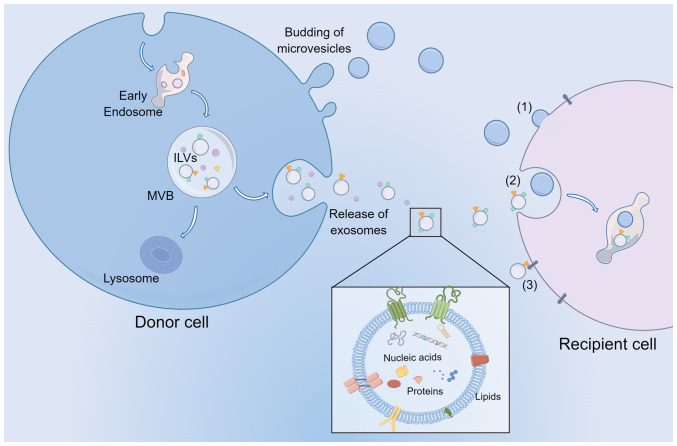
Initially, EVs were viewed as a quality control system to eliminate harmful or unnecessary molecules from the cell (20). EVs are now identified as a group of submicron-sized membrane-bound organelles secreted by almost all cells, carrying several biological cargoes, such as lipids, fatty acids (FAs), and nucleic acids, capable of targeting and transferring their contents to various receptor cells within the tissue or distal tissues (Fig. 1). EVs primarily consist of exosomes, microvesicles (MVs) and apoptotic bodies (21). Apoptotic bodies are known to be produced during apoptotic cell death and have a diameter >5 µm (22). MVs are small vesicles (100–1,000 nm size range) formed by plasma membrane fusion and budding. Although the exact process by which MVs are formed is not fully understood, cytoskeletal elements such as actin and microtubules, coat proteins, and fusion machinery, such as SNAREs, are hypothesized to be necessary. Specifically, coat proteins such as clathrin and cytoplasmic coat protein complex, are drawn to the membrane to reshape the flat membranes into rounded buds, cargo, and vesicle-SNAREs (v-SNAREs, primarily including VAMP) are integrated into the budding vesicle by attaching to coat subunits, for example, adaptor protein (AP) complexes (23). In addition, the molecular composition of MVs primarily consists of cytoplasmic and plasma membrane-associated proteins since MVs are formed by the outward budding of the membrane, and they may vary greatly depending on the cell type (24). In addition, MVs were first described as subcellular material originating from platelets and were demonstrated to play a role in blood coagulation (25,26). More recently, they have been reported to transfer cargo to target cells, thus playing an essential role in cell communication (27). Exosomes are vesicles 30–150 nm in diameter secreted by the endosome pathway. During the biogenesis of exosomes, endocytosis-mediated invagination of the plasma membrane (PM) forms early endosomes. Endosomal membranes bud inward into the lumen to create intraluminal vesicles (ILVs). These late endosomes contain ILVs called multivesicular bodies (MVBs). MVBs can fuse with lysosomes to be degraded or fuse with the PM to release ILVs as exosomes into the extracellular environment (28,29).
Figure 1.
EV biogenesis, release, and communication with recipient cells. Exosomes and MVs released from cells are primarily stratified based on size. In addition, MVs can bud from the plasma membrane, while exosomes are derived from ILVs within the lumen of MVBs. MVBs then fuse with the plasma membrane to release the exosomes. EVs transport nucleic acids, proteins, and lipids to target cells by (1) membrane fusion, (2) endocytosis, and (3) ligand binding mechanisms to exert their biological functions. EV, extracellular vesicle; MVs, microvesicles; ILVs, intraluminal vesicles; MVBs, multivesicular bodies.
The nature and abundance of EV cargoes are specific to the cell type. They are frequently affected by the state of donor cells and the molecular processes that result in their biogenesis (30,31). EVs are loaded with various biomolecular components, such as nucleic acids and proteins, contributing to their functional diversity, heterogeneity, and complexity. Proteins commonly found in EVs are those associated with biogenetic mechanisms, including those related to endosomal pathways. Several membrane proteins and transcription factors can also be found in EVs (32,33). EVs are rich in sphingomyelin, cholesterol, desaturated lipids, phosphatidylserine, and ceramide (34). In addition, a range of genetic material is found in EVs, such as DNA, mRNAs, microRNAs (miRNAs), and several noncoding RNAs (ncRNAs). As soon as EVs bind to target cells, they may remain in the PM or be ingested through endocytosis, direct membrane fusion, and ligand binding mechanisms (35–37).
Proteins of the tetraspanin family, such as CD81 and CD9, are enriched in EVs and considered unique markers of EVs, including exosomes and MVs (38). However, researchers have demonstrated that CD81, CD63, and CD9 are exosome markers in a recent study on the difference between exosomes and MVs (39). At the same time, they emphasized that Annexin A1 is present in MVs, not exosomes. Furthermore, MVs also contain several biological molecules, such as integrins, selectins, and CD40 ligands, which may facilitate the formation of MVs (40,41). Therefore, more research is required to distinguish them from each other. In the present review, ‘EVs’ is used to refer to exosomes and MVs only.
3. AT and ADEVs
AT
ATs are complex metabolic organs with profound effects on regulating systemic metabolism, energy storage, and homeostasis. The primary characteristic of BATs is the presence of multilocular lipid droplets and several mitochondria expressing high levels of uncoupling protein 1 (UCP1), responsible for nonshivering thermogenesis, leading to increased energy expenditure (42). WATs primarily consist of white adipocytes, which carry large lipid droplets and fewer mitochondria, making them the primary site for storing and releasing energy (42). In addition, studies have shown that WATs can undergo a process called ‘browning’, during which part of the white adipose tissue can be transformed into beige adipose tissue (BeATs), morphologically distinct from WATs and BATs (43,44). Browning occurs under certain circumstances, such as in the cold and as a result of exercise. Moreover, medicines, such as β-adrenergic receptor and peroxisome proliferator-activated receptor (PPAR)γ agonists, can also trigger browning by promoting the decomposition of triglyceride and glucose in ATs or by inducing the expression of thermogenesis-related genes, respectively, which ultimately encourages lipolysis and thermogenesis (45–47). Characteristically, beige adipocytes contain several small lipid droplets, are typically larger than brown adipocytes, have more mitochondria than white adipocytes, and express UCP1 (48). Additionally, these types of ATs differ in critical ways that include aspects of their gene expression profile and secretome. WAT, known as the active endocrine organ, can release cytokines and adipokines such as leptin and adiponectin (6). In contrast, BAT/BeAT have fewer secretory functions than WAT. In addition to UCP1, Cell death-inducing DFFA like effector a, Cytochrome c oxidase subunit 7A1, and ELOVL fatty acid elongase 3 are specifically expressed in BAT, while BeAT expresses T-box transcription factor 1, Solute carrier family 27 member 1, Cbp/p300-interacting transactivator with Glu/Asp-rich carboxy-terminal domain 1, CD40 and CD137 (49,50). Since the UCP1 expression of beige adipocytes is considerably lower than those of classical brown adipocytes, BeATs were once considered unimportant in whole-body energy expenditure (51). However, in adults with a minimal classic BAT reserve, BeATs are the primary energy source for nonshivering thermogenesis (52). Additionally, BeATs regulate whole-body energy metabolism and glucose homeostasis through an UCP1 independent mechanism (51). According to the report, activation of BeATs with β-adrenergic agonist CL316243 enhanced the selective uptake of fatty acids from triglyceride-rich lipoproteins by ATs, and reduced plasma TG and cholesterol levels, thereby alleviating hypercholesterolemia and atherosclerosis (53). Another study showed that prolonged maintenance of thermogenically active BeATs enhanced whole-body energy expenditure and protected mice from diet-induced obesity and insulin resistance (54). As the most abundant form of AT, WAT is distributed throughout the body and are active endocrine organs. They release free fatty acids and adipokines, such as leptin, adiponectin, TNF-α, and IL-6, which act on distal tissues, including the brain, liver, and muscle tissue, to regulate food intake, energy homeostasis, and insulin sensitivity (55). The ATs found in the hypodermis layer are called subcutaneous ATs (SCATs), and form a connective tissue in the dermis between the aponeurosis and the muscle fascia, insulating and storing energy. SCATs are primarily distributed in the abdominal and gluteofemoral regions of the human body, storing >80% of total body fat (56). Dermal WATs (DATs), located directly below the reticular dermis (primarily above the SCATs), have been reported to be involved in insulation, hair regeneration, wound healing, and the prevention of skin infections (57,58). In addition, WATs that accumulate around internal organs are visceral ATs (VATs), which are primarily found in the intrathoracic region and abdominal cavity, such as epicardial and pericardial fat and perigonadal, mesenteric, perirenal, and retroperitoneal fat, protecting the internal organs of rodents and humans and storing 5–20% of total body fat (59,60).
Cellular composition of ATs
ATs are connective tissues primarily made up of lipid-rich cells known as adipocytes. Adipocytes, the parenchymal cells of ATs, are critical regulators of energy metabolism and endocrine modulators engaged in numerous physiological or pathological processes, such as appetite regulation and immunological response (61,62). In addition to adipocytes, there are several nonadipocyte compartments termed the stromal vascular fraction (SVF), composed of AT-derived stem cells (ADSCs), preadipocytes, endothelial cells, and a broad spectrum of adaptive and innate immune cells (63–66). Preadipocytes can differentiate into mature adipocytes to maintain adipogenesis and homeostasis in adipose tissue (67). The ADSCs in ATs are mesenchymal stem cells (MSCs) of mesodermal origin, serving as progenitors responsible for adipocyte regeneration and replenishment. ADSCs also have potent self-renewal capacity and a high capacity for classical adipogenic, osteogenic, and chondrogenic differentiation. In addition to mesenchymal cells, ADSCs can differentiate into nonmesenchymal cell lineages, such as endothelial cells, myocytes, and neuronal lineages (68). Endothelial cells and pericytes provide vasculature to ATs by forming capillaries (69–71). The immune cell types and functions of ATs have been widely discussed, primarily in the context of obesity. Various immune cells form a dynamic immunological microenvironment with variable metabolic status, including macrophages, eosinophils, dendritic cells (DCs), invariant natural killer cells (iNKT cells), T cells, and B cells (72–74). For example, under conditions of obesity or chronic metabolic stress, increased infiltration and activation of proinflammatory immune cells can accelerate WAT inflammation, thus influencing the effect of insulin and other metabolic hormones on parenchymal cells, thus further damaging the glucose and lipid metabolism process of metabolic organs (75–77).
Working model and the source of ADEVs
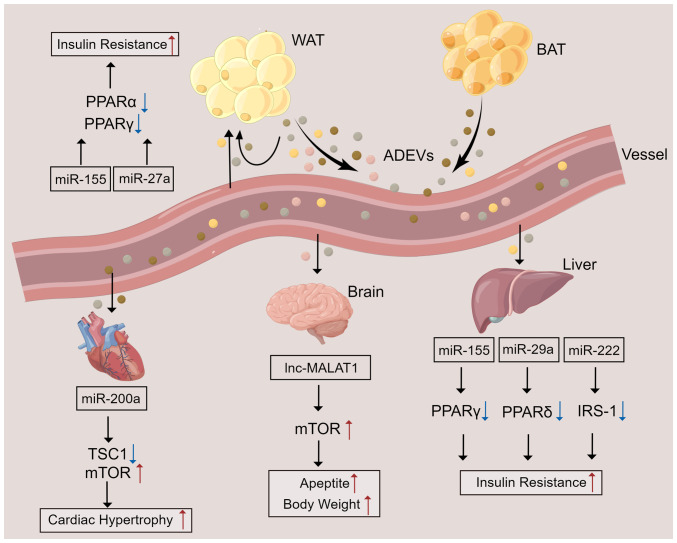
Although EVs are physiologically released from cells, pathophysiological stimuli can regulate their biogenesis and release. Furthermore, certain proteins and mRNAs can be selectively packaged into EVs during physiological changes or pathological injuries. Similar to normal EVs, ADEVs exert their biological functions by transporting bioactive cargos such as miRNAs, ncRNAs, proteins, and lipids to receptor cells. Studies have shown that ADEVs can not only modulate the immune responses of local ATs through cellular communication but can also regulate systemic insulin sensitivity and glycolipid metabolic processes through their remote effects on other metabolic organs (for example, the brain and liver) (78–80) (Fig. 2). Research has shown that ADEVs directly modulate glucose tolerance and insulin sensitivity in adipocytes, myocytes, and hepatocytes through modulation of PPARγ and perhaps fibroblast growth factor 21 (81,82). In the brain, ADEVs derived from adipocytes have been shown to carry the long noncoding RNA (lncRNA) metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) and to activate the mTOR signaling pathway through miR-181b and miR-144 in hypothalamic anorexigenic pro-opiomelanocortin (POMC) neurons, thereby leading to increased appetite and body weight (83). In addition, analysis of the protein profiles of EVs derived from adipocytes confirmed that EVs from obese mice are enriched in proteins and enzymes involved in the metabolism and transport of lipids, such as caveolin 1, lipoprotein lipase, and aquaporin 7, which may be associated with ectopic lipid accumulation and lead to mitochondrial energy metabolism disturbance, and systemic insulin resistance (84,85). In particular, quantitative proteomic analysis of EVs released by 3T3-L1 adipocytes showed that enzymes related to de novo lipogenesis, including glucose-6-phosphate dehydrogenase and fatty acid synthase, were selectively enriched in EVs from adipocytes, promoting lipid accumulation in recipient adipocytes and preadipocytes (85).
Figure 2.
ADEV-mediated crosstalk between ATs and other organs. ATs secrete EVs containing various components into the blood circulation to affect ATs and distant organs. In ATs and the liver, miRNAs contained in ADEVs result in insulin resistance by inhibiting the activation or expression of PPARα, PPARγ, PPARδ, or IRS-1. In the brain, ADEVs contain lncRNA MALAT1, which enhances mTOR signaling in POMC neurons, leading to increased appetite and a gain in body gain. miR-200a contained in ADEVs can be delivered to cardiomyocytes to inhibit TSC1 and activate the mTOR pathway, leading to cardiac hypertrophy. ATs, adipose tissues; ADEVs, AT-derived extracellular vesicles; WAT, white AT; BAT, brown AT; miR, microRNA; lnc, long non-coding RNA; TSC1, tuberous sclerosis complex 1; PPAR, peroxisome proliferator-activated receptor; IRS-1, insulin receptor substrate 1; MALAT1, metastasis-associated lung adenocarcinoma transcript 1.
Most of the current body of knowledge on ADEVs comes from studies using 3T3-L1 cells. These studies found that ADEVs are highly adipocyte-specific and can be identified from complicated heterogeneous origins, such as plasma (86,87). Currently, multiple types of cells in ATs, such as adipocytes, ADSCs, and macrophages, have been shown to release EVs, mediating intercellular and interorgan crosstalk and regulating ATs and systemic homeostasis (88,89). EVs derived from adipocytes have been reported to carry proteins or enzymes involved in fatty acid oxidation (FAO), which can induce metabolic reprogramming and stimulate the migration and invasion of melanoma cells when EVs are taken up by tumor cells, thus amplifying the deleterious dialog between cancer cells and adipocytes (78,90). Furthermore, EVs derived from AT macrophages (ATMs) can modulate mouse glucose tolerance and insulin sensitivity (81,91). ADSCs have a high capacity for differentiation into multiple cell types and play an essential role in immune regulation (92–94). EVs released from ADSCs may at least be partially responsible for some of these functions. ADSC-derived EVs (ADSC-EVs) obtained from patients with or without cancer show equivalent miRNA content, which suggests that ADSC-EVs have the same therapeutic paracrine effects regardless of the health status of the donor (95). Previous studies have found that delivering ADSC-EVs from lean mice to obese mice showed desirable effects on alleviating obesity and IR (96). Additionally, ADSC-EVs obtained from human WATs and BATs can induce ADSCs to differentiate into WATs and BATs, respectively, attenuating diet-induced obesity, glucose tolerance, and liver steatosis (97). These findings suggest that ADEVs can be used as cell-free therapeutics for AT regeneration and remodeling.
4. ADEVs in disease
ADEVs regulate metabolic disorders
AT dysfunction is accompanied by chronic low-grade inflammation, in which excessive adipokine synthesis and secretion are closely related to cardiovascular, chronic liver, and kidney diseases as well as other systemic metabolic disorders (98,99). The inflammation of WATs caused by obesity is characterized by the accumulation of macrophages, including monocyte-derived macrophages (moMacs). Moreover, obese WAT monocytes can differentiate locally into moMacs and contribute to ATM pools (100). During the development of obesity, macrophages transition from an anti-inflammatory phenotype to a proinflammatory phenotype, producing proinflammatory factors and exacerbating adipose inflammation (101). Initially, ADEVs were shown to be taken up by peripheral monocytes, which then differentiated into macrophages that increased TNF-α and IL-6 secretion, leading to insulin resistance and glucose intolerance through the Toll-like receptor 4/TIR domain-containing adaptor molecule 1 pathway (102). Notably, a recent study revealed a mechanism by which adipocytes regulate ATM polarization through EVs. ADEVs derived from mature adipocytes containing miR-34a inhibited macrophage M2 polarization by downregulating Krüppel-like factor 4, a crucial transcription factor for maintaining an adipose M2 macrophage phenotype, thereby leading to adipose inflammation (103). Several immunomodulatory proteins, such as macrophage migration inhibitory factor, retinol-binding protein 4, and soluble adiponectin, have been identified in EVs from cultured human adipocytes and human AT explants, which may induce monocytes to differentiate into M1-phenotype macrophages, exacerbating adipose inflammation and insulin resistance (86).
Insulin resistance (IR) is a disordered biological response in which the body cannot respond to high insulin levels or absorb and utilize glucose normally, causing the body to produce more insulin in response. It is the critical cause of several metabolic syndromes, such as T2D and obesity (104). As mentioned above, ADEVs from the VATs of obese mice were enriched in fatty acid binding protein 4 (FABP4) and induced the differentiation of macrophages to the M1 phenotype. In addition, those ADEVs were shown to be taken up by monocytes and to induce IR (102). Ying et al (91) found that injecting ATM-derived EVs from obese mice into lean mice inhibited the expression of PPARγ, which can promote whole-body lipid metabolism and insulin sensitivity, and GLUT4 (a PPARγ target gene), thereby reducing adipocyte sensitivity to insulin. Specifically, EVs containing miR-155 derived from ATMs target PPARγ in adipocytes, the liver, and the muscle, regulating insulin activity (91). In addition, adipocytes secrete EVs containing miR-27a, a negative regulator of PPARγ, leading to PPARγ-dependent obesity-induced IR by directly binding to the 3′UTR of PPARγ (105). Subsequently, ATM-derived EVs from obese mice were confirmed to possess miR-29a and to cause IR by binding to the 3′UTR of PPARδ (a potent modulator of insulin sensitivity) in adipocytes, myocytes, and hepatocytes (81). Another study showed that miR-222 from gonadal WAT-derived EVs promoted IR in the liver and skeletal muscle by suppressing IRS-1 expression via binding to the 3′UTR of IRS-1 (106). In contrast, ADSC-EVs facilitated metabolic homeostasis and improved insulin sensitivity in obese mice by alternatively activating M2 macrophage polarization and reducing inflammation by activating arginase-1 through STAT3 carried by EVs (96). In addition, miR-27a was enriched in adipocyte-derived EVs isolated from the VATs of obese individuals, and those EVs were shown to contribute to IR in the liver and skeletal muscle by inhibiting insulin-induced Akt phosphorylation and PPARα expression (107).
Given that obesity is a risk factor for the development of T2D, research has focused on the relationship between ATs and diabetes/diabetic complications. As no definitive marker of ADEVs has yet been identified, it is challenging to elucidate the detailed role of ADEVs in obesity and metabolic syndromes such as T2D. The production and specific cargo of ADEVs are altered under metabolic stresses (108). Perilipin A levels were higher in circulating adipocyte-derived EVs from obese mice and humans with metabolic diseases (109). AT-derived miRNAs are the main circulating EV miRNAs (18). A study showed that miR-20b-5p was abundant in serum EVs of T2D patients, and miR-20b-5p modulated insulin action in skeletal muscle by downregulating Akt interacting protein (AKTIP) and STAT3 expression (110). In addition, certain ADEVs exert beneficial effects on T2D. A previous study assessed the relationship between metabolic syndrome and adipose tissue-derived EV markers, and revealed that individuals with CD14-positive EVs had a 16% lower risk of developing T2D after 6.5 years of follow-up (111). EVs derived from activated beige adipocytes contain diabetes-preventing factors. When administered to primary white adipocytes, these EVs improved insulin sensitivity and insulin-stimulated glucose uptake (112). Together, based on these studies, adipose-derived EVs are viewed as a novel cellular communication tool within ATs and perhaps between ATs and distant organs to regulate T2D.
ADEVs and CVD
Dysfunctional ATs in obese individuals can lead to an increased risk of CVD, which remains one of the principal causes of death worldwide, despite advances in risk factor management (113–115). To date, efficient treatments for CVD are lacking. Recently, ADEVs have emerged as critical actors in the crosstalk between obesity and CVD progression.
Aside from polarization, macrophage foaming also plays a vital role in the progression of atherosclerotic lesions. ADEVs from VATs in obese mice facilitate macrophage foam cell generation by downregulating ATP binding cassette subfamily A member 1 (ABCA1) and ATP binding cassette subfamily G member 1 (ABCG1)-mediated cholesterol efflux and exacerbated atherosclerosis (116). ADEVs from adipocytes and their miRNA contents were confirmed to reduce macrophage cholesterol efflux by targeting ABCA1, thus promoting the development of atherosclerosis (117). In contrast, another study identified the beneficial effects of EVs derived from ADSCs in cardiac recovery, highlighting their potential in regenerative therapy (118). In addition, studies on 3T3-L1 models showed that adipocyte-derived EVs containing miR-802-5p promoted IR in cardiomyocytes by downregulating heat shock protein 60, which has been proven to prevent inflammation, mitochondrial dysfunction, and even insulin resistance (119).
Coronary artery disease and atherosclerosis are caused by endothelial dysfunction during the early stages of the disease (120). It is not well understood how EVs are exchanged between adipocytes and endothelial cells during obesity despite extensive evidence of proinflammatory crosstalk between ATMs and ADEVs. It was found that hypertrophic and dysfunctional adipocytes release EVs that impair vascular endothelial cell function, potentially contributing to obesity-related atherosclerosis (121). In addition, research has demonstrated that hypoxia and inflammation promote synergistic EV production from adipocytes. These ADEVs promote the expression of endothelial vascular cell adhesion molecule 1 (VCAM-1), which increases the subsequent attachment of leukocytes to endothelial cells and exacerbates vascular disease in obesity (122).
One of the causes of heart failure is cardiac hypertrophy (CH). Research has shown that miR-200a in ADEVs derived from adipocytes can be transferred into cardiomyocytes to inhibit TSC1 and activate the mTOR pathway, leading to CH. It was also shown that inhibition of miR-200a could abrogate CH (123). In addition, Gan et al (124) demonstrated that ADEVs derived from diabetic adipocytes were delivered to cardiomyocytes where they facilitated the pathogenic interaction between the heart and defective ATs, aggravating ischemic heart damage in obese/diabetic individuals. miR-130b-3p was found to be a vital agent mediating this proapoptotic effect of diabetic adipocyte-derived EVs and identified AMPK as a novel target of miR-130b-3p, in which miR-130b-3p was shown to impair the expression of AMPK, the latter of which is a crucial regulator of metabolic disorder-induced cellular malfunction and cell death (124). However, the precise role of ADEVs is still poorly understood in the context of CVD and requires further investigation.
ADEVs as major actors in cancer
An increasing body of data from animal and human studies indicates that obesity increases the risk of developing cancer, cancer-associated mortality, and cancer recurrence following treatment (125). Previously, studies on the communication between adipocytes and tumor cells have been limited to cytokines such as endorphin, leptin, or chemokines. Then, the discovery of cancer-associated adipocytes (CAAs) revealed a vicious cycle in which tumors activate CAAs, and CAAs can further contribute to tumor progression by secreting adipokines, inflammatory cytokines, and metabolites (126,127). More recently, the role of ADEVs in tumor–adipose tissue communication has also been confirmed, and obesity modifies ADEV secretion quantitatively and qualitatively, thus amplifying their effect on tumor aggressiveness.
The first line of evidence linking ADEVs with cancer showed that ADEVs from different adipocyte models enhanced melanoma cell migration, invasion, and lung metastases in the context of obesity (90). Subsequently, Wang et al (128) showed that EVs derived from 3T3-L1 adipocytes induced lung tumor metastasis by increasing MMP9 activity of 3LL lung cancer cells, in which MMP9 has been shown to promote tumor invasion and metastasis. Another study demonstrated that ADEVs from AT-derived mesenchymal stem cells (ADMSCs) could foster the invasion, migration, and proliferation of osteosarcoma cells by increasing galactosyl transferase 2 and MMP2/9 expression (129). Gangadaran et al (130) demonstrated that ADSC-EVs contain angiogenic proteins such as IL-8, CCL2, TIMP-1, TIMP-2, and VEGF-D. Following internalization of ADSC-EVs, endothelial cells undergo differentiation, develop a tube-like formation and promote angiogenesis in vitro and in vivo. Khanh et al (131) showed that in patients with T2D, ADEVs derived from ADMSCs can promote breast cancer metastasis by targeting the JAK/STAT3 pathway. In conditions of obesity, ADEVs from AT macrophages are rich in miR-155, which is not only involved in IR but also plays an oncogenic/antiapoptotic role through caspase-3 and Bcl-2 in breast cancer cells (132).
By contributing local FAs to the process of FAO within tumor cells, a novel beneficial metabolic route is activated that increases tumor aggressiveness and proliferation, and adipocytes also aid in the evolution of tumors through metabolic collaboration (90,133,134). The proteins, including the enzymes needed for FAO, can be transferred to cancer cells or across farther distances by ADEVs released by adipocytes. Lazar et al (90) demonstrated that proteins implicated in FAO were enriched in ADEVs, which were then taken up by melanoma cells, leading to increased lung metastases and an increase in FAO in tumor cells. Clement et al (78) revealed the role of ADEVs in the crosstalk between melanoma cells and adipocytes, which triggers metabolic remodeling and ultimately facilitates the FAO process and tumor aggressiveness. In addition to FAO enzyme transfer, ADEVs also deliver FAs to tumor cells to enhance the FAO process, reinforcing the effect of ADEVs on obesity. Together, this research revealed that ADEVs are involved in guiding the growth, invasion, metabolic reprogramming, and metastasis of cancer cells by modulating the acquisition and maintenance of cancer markers.
Effects of ADEVs on neurological disorders
Neurological disorders, including neurodegenerative diseases such as Alzheimer's disease (AD) and Parkinson's disease (PD), as well as ischemic stroke, are often characterized by neuroinflammation. Treating neurological disorders has long been challenging as many therapeutics do not cross the blood-brain barrier (BBB). To date, EVs carrying biological molecules are recognized as an excellent tool for treating central nervous system (CNS)-related ailments since they are prospective drug delivery systems that can cross the BBB (135).
The deposition of β-amyloid peptide (Aβ) in the brain and neurofibrillary tangles (NFTs) formed by hyperphosphorylated Tau protein plays a critical role in the pathogenesis of AD (136,137). According to Katsuda et al (138), ADSC-derived EVs carried enzymatically active neprilysin (NEP), the brain's most important Aβ-degrading enzyme. Furthermore, Aβ levels were decreased when ADSC-derived EVs were transfused into N2a neuroblastoma cells in vitro. Another study demonstrated that ADSC-derived EVs inhibited inflammatory polarization of activated human microglia, which has been shown to mediate several CNS inflammatory processes, such as AD (139). In another rat model of ischemic stroke, ADSC-derived EVs containing miR-126 were shown to prevent ischemic stroke, promote neurogenesis, and vasculogenesis following ischemic stroke, and inhibit ischemic stroke-induced microglial activation and inflammatory responses (140). Jiang et al (141) showed that EVs derived from ADSCs suppress autophagy by inhibiting the expression of Beclin-1 and Atg5 via miR-30d-5p, thereby promoting microglial M2 polarization and ultimately preventing acute ischemic stroke. These characteristics make ADSC-derived EVs promising candidates for therapeutic relevance in neurodegenerative diseases.
5. Conclusions and future directions
In addition to the classical polypeptide adipokines and cytokines, the critical role of ADEVs in communication between ATs and other organs has been gradually deciphered. More recently, growing evidence from both epidemiologic and preclinical studies further highlights the effects of ADEVs on mediating cell-to-cell communication within ATs, and between ATs and other peripheral organs. A comprehensive understanding of the interaction patterns between ATs and other tissues and the molecular changes in AT dysfunction during obesity, such as ADEVs and their cargoes, may provide novel avenues for developing new therapeutic interventions in obesity-related metabolic diseases.
EVs have long been recognized as crucial intercellular communication tools. In addition, EV-mediated cellular communication is implicated in various diseases and biological events, including certain immune responses such as inflammation. Therefore, EVs are also considered a therapeutic target for multiple diseases (142). In addition, due to their endogenous origin, EVs have been widely explored as next-generation nanoscale drug delivery systems, allowing them to circumvent certain drawbacks associated with existing therapies (143). The impact of obesity on the biological components of ADEVs from different cell origins has been described previously (144). Therefore, the critical function of ADEVs and the role of ADEVs and their cargoes in multiple diseases were emphasized here.
The present review summarizes the composition and function of ADEVs derived from different cell sources in ATs, such as adipocytes, preadipocytes, macrophages, and MSCs. In addition, ADEVs participate in developing pathologies associated with metabolic diseases, CVDs, and several types of cancer (Table I). Understanding the mechanisms behind the effects of ADEVs on obesity or metabolic disorders and CVDs may contribute to the development of novel therapeutic strategies. However, the vast majority of current research is currently in the early stages, and no definitive marker of ADEV has yet been identified, complicating the isolation of ADEVs from ATs with high purity. In current research models, ADEVs from different sources are frequently derived from in vitro cell cultures. Therefore, further investigation is required to reveal the detailed characteristics of ADEVs.
Table I.
Summary of ADEV cargos and their functions in recipient cells.
| A, ADEVs in metabolic disorders | |||
|---|---|---|---|
|
| |||
| Origin | Cargo | Functions | (Refs.) |
| Adipose tissue-EVs | N.D. | Promote M1 polarization of macrophages; Induce IR | (102) |
| Adipocyte-EVs | miR-34a | Inhibit M2 polarization of macrophages | (103) |
| Human adipocyte-EVs | MIF, M-CSF, TNF-α | Promote M1 polarization of macrophages | (86) |
| ATM-EVs | miR-155 | Reduce the insulin sensitivity in adipocytes, the liver, and the muscle | (91) |
| Adipocyte-EVs | miR-27a | Induce hepatic and skeletal muscle IR | (105,107) |
| ATM-EVs | miR-29a | Induce IR in adipocytes, myocytes, and hepatocytes | (81) |
| WAT-EVs | miR-222 | Promote IR in the liver and skeletal muscle | (106) |
| Human adipocyte-EVs | miR-20b-5p | Impair insulin action in skeletal muscle | (110) |
|
| |||
| B, ADEVs and CVDs | |||
|
| |||
| Origin | Cargo | Functions | (Refs.) |
|
| |||
| VAT-EVs | N.D. | Facilitate macrophage foam cell generation and exacerbate atherosclerosis | (116) |
| Adipocyte-EVs | miRNAs | Increase macrophage cholesterol efflux | (117) |
| 3T3-L1-EVs | miR-802-5p | Promote insulin resistance in cardiomyocytes | (119) |
| Adipocyte-EVs | N.D. | Impair the function of vascular endothelial cells | (121) |
| Adipocyte-EVs | N.D. | Promote the attachment of leukocytes to endothelial cells | (122) |
| Adipocyte-EVs | miR-200a | Impair the function of cardiomyocytes, and promote the process of cardiac hypertrophy | (123) |
| Adipocyte-EVs | miR-130b-3p | Exacerbate ischemic heart injury | (124) |
|
| |||
| C, ADEVs in cancer | |||
|
| |||
| Origin | Cargo | Functions | (Refs.) |
|
| |||
| Adipocyte-EVs | MMP3 | Promote lung tumor metastasis | (128) |
| ADMSC-EVs | N.D. | Promote the invasion, migration, and proliferation of osteosarcoma cells | (129) |
| ADSC-EVs | Angiogenic proteins | Promote the tube-like formation of endothelial cells | (130) |
| ADMSC-EVs | N.D. | Promote the metastasis of breast cancer cells | (131) |
| Adipocyte-EVs | FA substrates, proteins implicated in FAO | Promote lung tumor metastasis | (90) |
| Adipocyte-EVs | FAO enzyme, FA substrates | Trigger metabolic remodeling, facilitate FAO and tumor aggressiveness | (78) |
N.D., not detected; ADEVs, adipose tissue-derived extracellular vesicles; ATM, adipose tissue macrophage; WAT, white adipose tissue; VAT, visceral adipose tissue; ADSC, adipose tissue-derived stem cells; ADMSC, adipose tissue-derived mesenchymal stem cells; FAO, fatty acid oxidation; FA, fatty acids; miR/miRNA, microRNA; IR, insulin resistance; MIF, macrophage migration inhibitory factor; M-CSF, macrophage colony stimulating factor 1; TNF-α, tumor necrosis factor-α; MMP3, matrix metallopeptidase 3.
Acknowledgments
Not applicable.
Glossary
Abbreviations
- EVs
extracellular vesicles
- ADEVs
adipose tissue-derived extracellular vesicles
- T2D
type 2 diabetes
- CVD
cardiovascular disease
- AD
Alzheimer's disease
- CH
cardiac hypertrophy
- WATs
white adipose tissues
- BATs
brown adipose tissues
- BeATs
beige adipose tissues
- MVs
microvesicles
- ILVs
intraluminal vesicles
- MVBs
multivesicular bodies
- ncRNAs
noncoding RNAs
- miRNAs
microRNAs
- SCATs
subcutaneous ATs
- DATs
dermal WATs
- VAT
visceral ATs
- ADSCs
adipose tissue-derived stem cells
- ATMs
adipose tissue macrophages
- IR
insulin resistance
- GLUT4
glucose transporter 4
- PPAR
peroxisome proliferator-activated receptor
- FAs
fatty acids
- FAO
fatty acid oxidation
Funding Statement
This work was supported by the National Natural Science Foundation of China (grant no. 82000003), the Natural Science Foundation of Zhejiang Province, China (grant no. LY23HO60009), the Natural Science Foundation of Zhejiang Province, China (grant no. LGF20H040009), and the China Postdoctoral Science Foundation (grant no. 2020M671748).
Availability of data and materials
Not applicable.
Authors' contributions
XBY, JYH, and JL wrote the manuscript. XHK and XLL conceived the subject of review and edited the manuscript. XHK designed and created the schematic representations. Data authentication is not applicable. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Mohammed MS, Sendra S, Lloret J, Bosch I. Systems and WBANs for Controlling Obesity. J Healthc Eng. 2018;2018:1564748. doi: 10.1155/2018/1564748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kusminski CM, Bickel PE, Scherer PE. Targeting adipose tissue in the treatment of obesity-associated diabetes. Nat Rev Drug Discov. 2016;15:639–660. doi: 10.1038/nrd.2016.75. [DOI] [PubMed] [Google Scholar]
- 3.Abu-Farha M, Al-Mulla F, Thanaraj TA, Kavalakatt S, Ali H, Abdul Ghani M, Abubaker J. Impact of Diabetes in Patients Diagnosed With COVID-19. Front Immunol. 2020;11:576818. doi: 10.3389/fimmu.2020.576818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goodman KE, Magder LS, Baghdadi JD, Pineles L, Levine AR, Perencevich EN, Harris AD. Impact of sex and metabolic comorbidities on coronavirus disease 2019 (COVID-19) mortality risk across age groups: 66 646 inpatients across 613 U.S. Hospitals. Clin Infect Dis. 2021;73:e4113–e4123. doi: 10.1093/cid/ciaa1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Piroth L, Cottenet J, Mariet AS, Bonniaud P, Blot M, Tubert-Bitter P, Quantin C. Comparison of the characteristics, morbidity, and mortality of COVID-19 and seasonal influenza: A nationwide, population-based retrospective cohort study. Lancet Respir Med. 2021;9:251–259. doi: 10.1016/S2213-2600(20)30527-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ottaviani E, Malagoli D, Franceschi C. The evolution of the adipose tissue: A neglected enigma. Gen Comp Endocrinol. 2011;174:1–4. doi: 10.1016/j.ygcen.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 7.Unamuno X, Gomez-Ambrosi J, Rodriguez A, Becerril S, Fruhbeck G, Catalan V. Adipokine dysregulation and adipose tissue inflammation in human obesity. Eur J Clin Invest. 2018;48:e12997. doi: 10.1111/eci.12997. [DOI] [PubMed] [Google Scholar]
- 8.Burhans MS, Hagman DK, Kuzma JN, Schmidt KA, Kratz M. Contribution of adipose tissue inflammation to the development of type 2 diabetes Mellitus. Compr Physiol. 2018;9:1–58. doi: 10.1002/cphy.c170040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berg AH, Combs TP, Du X, Brownlee M, Scherer PE. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med. 2001;7:947–953. doi: 10.1038/90992. [DOI] [PubMed] [Google Scholar]
- 10.Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, Sugiyama T, Miyagishi M, Hara K, Tsunoda M, et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423:762–769. doi: 10.1038/nature01705. [DOI] [PubMed] [Google Scholar]
- 11.Hefetz-Sela S, Scherer PE. Adipocytes: Impact on tumor growth and potential sites for therapeutic intervention. Pharmacol Ther. 2013;138:197–210. doi: 10.1016/j.pharmthera.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Igata M, Motoshima H, Tsuruzoe K, Kojima K, Matsumura T, Kondo T, Taguchi T, Nakamaru K, Yano M, Kukidome D, et al. Adenosine monophosphate-activated protein kinase suppresses vascular smooth muscle cell proliferation through the inhibition of cell cycle progression. Circ Res. 2005;97:837–844. doi: 10.1161/01.RES.0000185823.73556.06. [DOI] [PubMed] [Google Scholar]
- 13.Douros JD, Baltzegar DA, Reading BJ, Seale AP, Lerner DT, Grau EG, Borski RJ. Leptin stimulates cellular glycolysis through a STAT3 dependent mechanism in Tilapia. Front Endocrinol (Lausanne) 2018;9:465. doi: 10.3389/fendo.2018.00465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang Z, Xu A. Adipose extracellular vesicles in intercellular and inter-organ crosstalk in metabolic health and diseases. Front Immunol. 2021;12:608680. doi: 10.3389/fimmu.2021.608680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Padilla J, Vieira-Potter VJ, Jia G, Sowers JR. Role of perivascular adipose tissue on vascular reactive oxygen species in type 2 diabetes: A give-and-take relationship. Diabetes. 2015;64:1904–1906. doi: 10.2337/db15-0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kern PA, Ranganathan S, Li C, Wood L, Ranganathan G. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am J Physiol Endocrinol Metab. 2001;280:E745–E751. doi: 10.1152/ajpendo.2001.280.5.E745. [DOI] [PubMed] [Google Scholar]
- 17.Keller S, Sanderson MP, Stoeck A, Altevogt P. Exosomes: From biogenesis and secretion to biological function. Immunol Lett. 2006;107:102–108. doi: 10.1016/j.imlet.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 18.Thomou T, Mori MA, Dreyfuss JM, Konishi M, Sakaguchi M, Wolfrum C, Rao TN, Winnay JN, Garcia-Martin R, Grinspoon SK, et al. Adipose-derived circulating miRNAs regulate gene expression in other tissues. Nature. 2017;542:450–455. doi: 10.1038/nature21365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rome S, Blandin A, Le Lay S. Adipocyte-Derived extracellular vesicles: State of the art. Int J Mol Sci. 2021;22:1788. doi: 10.3390/ijms22041788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vidal M. Exosomes: Revisiting their role as ‘garbage bags’. Traffic. 2019;20:815–828. doi: 10.1111/tra.12687. [DOI] [PubMed] [Google Scholar]
- 21.van der Pol E, Boing AN, Harrison P, Sturk A, Nieuwland R. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol Rev. 2012;64:676–705. doi: 10.1124/pr.112.005983. [DOI] [PubMed] [Google Scholar]
- 22.Tricarico C, Clancy J, D'Souza-Schorey C. Biology and biogenesis of shed microvesicles. Small GTPases. 2017;8:220–232. doi: 10.1080/21541248.2016.1215283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cai H, Reinisch K, Ferro-Novick S. Coats, tethers, Rabs, and SNAREs work together to mediate the intracellular destination of a transport vesicle. Dev Cell. 2007;12:671–682. doi: 10.1016/j.devcel.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 24.Escola JM, Kleijmeer MJ, Stoorvogel W, Griffith JM, Yoshie O, Geuze HJ. Selective enrichment of tetraspan proteins on the internal vesicles of multivesicular endosomes and on exosomes secreted by human B-lymphocytes. J Biol Chem. 1998;273:20121–20127. doi: 10.1074/jbc.273.32.20121. [DOI] [PubMed] [Google Scholar]
- 25.Giebel B, Helmbrecht C. Methods to Analyze EVs. Methods Mol Biol. 2017;1545:1–20. doi: 10.1007/978-1-4939-6728-5_1. [DOI] [PubMed] [Google Scholar]
- 26.Wolf P. The nature and significance of platelet products in human plasma. Br J Haematol. 1967;13:269–288. doi: 10.1111/j.1365-2141.1967.tb08741.x. [DOI] [PubMed] [Google Scholar]
- 27.Al-Nedawi K, Meehan B, Micallef J, Lhotak V, May L, Guha A, Rak J. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat Cell Biol. 2008;10:619–624. doi: 10.1038/ncb1725. [DOI] [PubMed] [Google Scholar]
- 28.Harding C, Heuser J, Stahl P. Endocytosis and intracellular processing of transferrin and colloidal gold-transferrin in rat reticulocytes: Demonstration of a pathway for receptor shedding. Eur J Cell Biol. 1984;35:256–263. [PubMed] [Google Scholar]
- 29.Pan BT, Teng K, Wu C, Adam M, Johnstone RM. Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes. J Cell Biol. 1985;101:942–948. doi: 10.1083/jcb.101.3.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kalra H, Drummen GP, Mathivanan S. Focus on extracellular vesicles: Introducing the next small big thing. Int J Mol Sci. 2016;17:170. doi: 10.3390/ijms17020170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Minciacchi VR, Freeman MR, Di Vizio D. Extracellular vesicles in cancer: Exosomes, microvesicles and the emerging role of large oncosomes. Semin Cell Dev Biol. 2015;40:41–51. doi: 10.1016/j.semcdb.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raposo G, Stoorvogel W. Extracellular vesicles: Exosomes, microvesicles, and friends. J Cell Biol. 2013;200:373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mulcahy LA, Pink RC, Carter DR. Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles. 2014;3 doi: 10.3402/jev.v3.24641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Llorente A, Skotland T, Sylvanne T, Kauhanen D, Rog T, Orlowski A, Vattulainen I, Ekroos K, Sandvig K. Molecular lipidomics of exosomes released by PC-3 prostate cancer cells. Biochim Biophys Acta. 2013;1831:1302–1309. doi: 10.1016/j.bbalip.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 35.Laulagnier K, Javalet C, Hemming FJ, Chivet M, Lachenal G, Blot B, Chatellard C, Sadoul R. Amyloid precursor protein products concentrate in a subset of exosomes specifically endocytosed by neurons. Cell Mol Life Sci. 2018;75:757–773. doi: 10.1007/s00018-017-2664-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vargas A, Zhou S, Ethier-Chiasson M, Flipo D, Lafond J, Gilbert C, Barbeau B. Syncytin proteins incorporated in placenta exosomes are important for cell uptake and show variation in abundance in serum exosomes from patients with preeclampsia. FASEB J. 2014;28:3703–3719. doi: 10.1096/fj.13-239053. [DOI] [PubMed] [Google Scholar]
- 37.Kamerkar S, LeBleu VS, Sugimoto H, Yang S, Ruivo CF, Melo SA, Lee JJ, Kalluri R. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature. 2017;546:498–503. doi: 10.1038/nature22341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Niel G, D'Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19:213–228. doi: 10.1038/nrm.2017.125. [DOI] [PubMed] [Google Scholar]
- 39.Jeppesen DK, Fenix AM, Franklin JL, Higginbotham JN, Zhang Q, Zimmerman LJ, Liebler DC, Ping J, Liu Q, Evans R, et al. Reassessment of exosome composition. Cell. 2019;177:428–445. e18. doi: 10.1016/j.cell.2019.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Corrado C, Raimondo S, Saieva L, Flugy AM, De Leo G, Alessandro R. Exosome-mediated crosstalk between chronic myelogenous leukemia cells and human bone marrow stromal cells triggers an interleukin 8-dependent survival of leukemia cells. Cancer Lett. 2014;348:71–76. doi: 10.1016/j.canlet.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 41.Ailawadi S, Wang X, Gu H, Fan GC. Pathologic function and therapeutic potential of exosomes in cardiovascular disease. Biochim Biophys Acta. 2015;1852:1–11. doi: 10.1016/j.bbadis.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Marken Lichtenbelt W. Brown adipose tissue and the regulation of nonshivering thermogenesis. Curr Opin Clin Nutr Metab Care. 2012;15:547–552. doi: 10.1097/MCO.0b013e3283599184. [DOI] [PubMed] [Google Scholar]
- 43.Lee YH, Kim SN, Kwon HJ, Granneman JG. Metabolic heterogeneity of activated beige/brite adipocytes in inguinal adipose tissue. Sci Rep. 2017;7:39794. doi: 10.1038/srep39794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Keipert S, Jastroch M. Brite/beige fat and UCP1 - is it thermogenesis? Biochim Biophys Acta. 2014;1837:1075–1082. doi: 10.1016/j.bbabio.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 45.Wang Z, Ning T, Song A, Rutter J, Wang QA, Jiang L. Chronic cold exposure enhances glucose oxidation in brown adipose tissue. EMBO Rep. 2020;21:e50085. doi: 10.15252/embr.202050085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shamsi BH, Ma C, Naqvi S, Xiao Y. Effects of pioglitazone mediated activation of PPAR-ү on CIDEC and obesity related changes in mice. PLoS One. 2014;9:e106992. doi: 10.1371/journal.pone.0106992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Giampietro L, Gallorini M, De Filippis B, Amoroso R, Cataldi A, di Giacomo V. PPAR-ү agonist GL516 reduces oxidative stress and apoptosis occurrence in a rat astrocyte cell line. Neurochem Int. 2019;126:239–245. doi: 10.1016/j.neuint.2019.03.021. [DOI] [PubMed] [Google Scholar]
- 48.Jung SM, Sanchez-Gurmaches J, Guertin DA. Brown adipose tissue development and metabolism. Handb Exp Pharmacol. 2019;251:3–36. doi: 10.1007/164_2018_168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lau P, Tuong ZK, Wang SC, Fitzsimmons RL, Goode JM, Thomas GP, Cowin GJ, Pearen MA, Mardon K, Stow JL, Muscat GE. Roralpha deficiency and decreased adiposity are associated with induction of thermogenic gene expression in subcutaneous white adipose and brown adipose tissue. Am J Physiol Endocrinol Metab. 2015;308:E159–E171. doi: 10.1152/ajpendo.00056.2014. [DOI] [PubMed] [Google Scholar]
- 50.Wu J, Bostrom P, Sparks LM, Ye L, Choi JH, Giang AH, Khandekar M, Virtanen KA, Nuutila P, Schaart G, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150:366–376. doi: 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ikeda K, Maretich P, Kajimura S. The common and distinct features of brown and beige adipocytes. Trends Endocrinol Metab. 2018;29:191–200. doi: 10.1016/j.tem.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pinckard KM, Stanford KI. The heartwarming effect of brown adipose tissue. Mol Pharmacol. 2022;102:460–471. doi: 10.1124/molpharm.121.000328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Berbee JF, Boon MR, Khedoe PP, Bartelt A, Schlein C, Worthmann A, Kooijman S, Hoeke G, Mol IM, John C, et al. Brown fat activation reduces hypercholesterolaemia and protects from atherosclerosis development. Nat Commun. 2015;6:6356. doi: 10.1038/ncomms7356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Altshuler-Keylin S, Shinoda K, Hasegawa Y, Ikeda K, Hong H, Kang Q, Yang Y, Perera RM, Debnath J, Kajimura S. Beige adipocyte maintenance is regulated by autophagy-induced mitochondrial clearance. Cell Metab. 2016;24:402–419. doi: 10.1016/j.cmet.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rosen ED, Spiegelman BM. Adipocytes as regulators of energy balance and glucose homeostasis. Nature. 2006;444:847–853. doi: 10.1038/nature05483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Arner P. Regional adipocity in man. J Endocrinol. 1997;155:191–192. doi: 10.1677/joe.0.1550191. [DOI] [PubMed] [Google Scholar]
- 57.Chen SX, Zhang LJ, Gallo RL. Dermal white adipose tissue: A newly recognized layer of skin innate defense. J Invest Dermatol. 2019;139:1002–1009. doi: 10.1016/j.jid.2018.12.031. [DOI] [PubMed] [Google Scholar]
- 58.Zhang LJ, Guerrero-Juarez CF, Hata T, Bapat SP, Ramos R, Plikus MV, Gallo RL. Innate immunity. Dermal adipocytes protect against invasive Staphylococcus aureus skin infection. Science. 2015;347:67–71. doi: 10.1126/science.1260972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fruhbeck G. Overview of adipose tissue and its role in obesity and metabolic disorders. Methods Mol Biol. 2008;456:1–22. doi: 10.1007/978-1-59745-245-8_1. [DOI] [PubMed] [Google Scholar]
- 60.Salvador J, Silva C, Pujante P, Fruhbeck G. Abdominal obesity: An indicator of cardiometabolic risk. Endocrinol Nutr. 2008;55:420–432. doi: 10.1016/S1575-0922(08)75079-4. (In English, Spanish) [DOI] [PubMed] [Google Scholar]
- 61.Scheja L, Heeren J. The endocrine function of adipose tissues in health and cardiometabolic disease. Nat Rev Endocrinol. 2019;15:507–524. doi: 10.1038/s41574-019-0230-6. [DOI] [PubMed] [Google Scholar]
- 62.Stern JH, Rutkowski JM, Scherer PE. Adiponectin, leptin, and fatty acids in the maintenance of metabolic homeostasis through adipose tissue crosstalk. Cell Metab. 2016;23:770–784. doi: 10.1016/j.cmet.2016.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Eto H, Suga H, Matsumoto D, Inoue K, Aoi N, Kato H, Araki J, Yoshimura K. Characterization of structure and cellular components of aspirated and excised adipose tissue. Plast Reconstr Surg. 2009;124:1087–1097. doi: 10.1097/PRS.0b013e3181b5a3f1. [DOI] [PubMed] [Google Scholar]
- 64.Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 65.Brown JC, Shang H, Li Y, Yang N, Patel N, Katz AJ. Isolation of adipose-derived stromal vascular fraction cells using a novel point-of-care device: Cell characterization and review of the literature. Tissue Eng Part C Methods. 2017;23:125–135. doi: 10.1089/ten.tec.2016.0377. [DOI] [PubMed] [Google Scholar]
- 66.Wu H, Ballantyne CM. Metabolic inflammation and insulin resistance in obesity. Circ Res. 2020;126:1549–1564. doi: 10.1161/CIRCRESAHA.119.315896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hollenberg CH, Vost A. Regulation of DNA synthesis in fat cells and stromal elements from rat adipose tissue. J Clin Invest. 1969;47:2485–2498. doi: 10.1172/JCI105930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Panina YA, Yakimov AS, Komleva YK, Morgun AV, Lopatina OL, Malinovskaya NA, Shuvaev AN, Salmin VV, Taranushenko TE, Salmina AB. Plasticity of adipose tissue-derived stem cells and regulation of angiogenesis. Front Physiol. 2018;9:1656. doi: 10.3389/fphys.2018.01656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cao Y. Adipose tissue angiogenesis as a therapeutic target for obesity and metabolic diseases. Nat Rev Drug Discov. 2010;9:107–115. doi: 10.1038/nrd3055. [DOI] [PubMed] [Google Scholar]
- 70.Mahlakoiv T, Flamar AL, Johnston LK, Moriyama S, Putzel GG, Bryce PJ, Artis D. Stromal cells maintain immune cell homeostasis in adipose tissue via production of interleukin-33. Sci Immunol. 2019;4:eaax0416. doi: 10.1126/sciimmunol.aax0416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sun C, Berry WL, Olson LE. PDGFRα controls the balance of stromal and adipogenic cells during adipose tissue organogenesis. Development. 2017;144:83–94. doi: 10.1242/dev.135962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mclaughlin T, Ackerman SE, Shen L, Engleman E. Role of innate and adaptive immunity in obesity-associated metabolic disease. J Clin Invest. 2017;127:5–13. doi: 10.1172/JCI88876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rochette L, Mazini L, Malka G, Zeller M, Cottin Y, Vergely C. The crosstalk of adipose-derived stem cells (ADSC), oxidative stress, and inflammation in protective and adaptive responses. Int J Mol Sci. 2020;21:9262. doi: 10.3390/ijms21239262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hui X, Zhang M, Gu P, Li K, Gao Y, Wu D, Wang Y, Xu A. Adipocyte SIRT1 controls systemic insulin sensitivity by modulating macrophages in adipose tissue. EMBO Rep. 2017;18:645–657. doi: 10.15252/embr.201643184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hotamisligil GS. Inflammation, metaflammation and immunometabolic disorders. Nature. 2017;542:177–185. doi: 10.1038/nature21363. [DOI] [PubMed] [Google Scholar]
- 76.Hotamisligil GS. Foundations of immunometabolism and implications for metabolic health and disease. Immunity. 2017;47:406–420. doi: 10.1016/j.immuni.2017.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Man K, Kutyavin VI, Chawla A. Tissue immunometabolism: Development, physiology, and pathobiology. Cell Metab. 2017;25:11–26. doi: 10.1016/j.cmet.2016.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Clement E, Lazar I, Attane C, Carrie L, Dauvillier S, Ducoux-Petit M, Esteve D, Menneteau T, Moutahir M, Le Gonidec S, et al. Adipocyte extracellular vesicles carry enzymes and fatty acids that stimulate mitochondrial metabolism and remodeling in tumor cells. EMBO J. 2020;39:e102525. doi: 10.15252/embj.2019102525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hartwig S, De Filippo E, Goddeke S, Knebel B, Kotzka J, Al-Hasani H, Roden M, Lehr S, Sell H. Exosomal proteins constitute an essential part of the human adipose tissue secretome. Biochim Biophys Acta Proteins Proteom. 2019;1867:140172. doi: 10.1016/j.bbapap.2018.11.009. [DOI] [PubMed] [Google Scholar]
- 80.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 81.Liu T, Sun YC, Cheng P, Shao HG. Adipose tissue macrophage-derived exosomal miR-29a regulates obesity-associated insulin resistance. Biochem Biophys Res Commun. 2019;515:352–358. doi: 10.1016/j.bbrc.2019.05.113. [DOI] [PubMed] [Google Scholar]
- 82.Geng L, Lam K, Xu A. The therapeutic potential of FGF21 in metabolic diseases: From bench to clinic. Nat Rev Endocrinol. 2020;16:654–667. doi: 10.1038/s41574-020-0386-0. [DOI] [PubMed] [Google Scholar]
- 83.Gao J, Li X, Wang Y, Cao Y, Yao D, Sun L, Qin L, Qiu H, Zhan X. Adipocyte-derived extracellular vesicles modulate appetite and weight through mTOR signalling in the hypothalamus. Acta Physiol (Oxf) 2020;228:e13339. doi: 10.1111/apha.13339. [DOI] [PubMed] [Google Scholar]
- 84.Lee JE, Moon PG, Lee IK, Baek MC. Proteomic Analysis of extracellular vesicles released by adipocytes of otsuka long-evans tokushima fatty (OLETF) Rats. Protein J. 2015;34:220–235. doi: 10.1007/s10930-015-9616-z. [DOI] [PubMed] [Google Scholar]
- 85.Sano S, Izumi Y, Yamaguchi T, Yamazaki T, Tanaka M, Shiota M, Osada-Oka M, Nakamura Y, Wei M, Wanibuchi H, et al. Lipid synthesis is promoted by hypoxic adipocyte-derived exosomes in 3T3-L1 cells. Biochem Biophys Res Commun. 2014;445:327–333. doi: 10.1016/j.bbrc.2014.01.183. [DOI] [PubMed] [Google Scholar]
- 86.Kranendonk ME, Visseren FL, van Balkom BW, Nolte-'t Hoen EN, van Herwaarden JA, de Jager W, Schipper HS, Brenkman AB, Verhaar MC, Wauben MH, Kalkhoven E. Human adipocyte extracellular vesicles in reciprocal signaling between adipocytes and macrophages. Obesity (Silver Spring) 2014;22:1296–1308. doi: 10.1002/oby.20679. [DOI] [PubMed] [Google Scholar]
- 87.Phoonsawat W, Aoki-Yoshida A, Tsuruta T, Sonoyama K. Adiponectin is partially associated with exosomes in mouse serum. Biochem Biophys Res Commun. 2014;448:261–266. doi: 10.1016/j.bbrc.2014.04.114. [DOI] [PubMed] [Google Scholar]
- 88.Crewe C, Scherer PE. Intercellular and interorgan crosstalk through adipocyte extracellular vesicles. Rev Endocr Metab Disord. 2022;23:61–69. doi: 10.1007/s11154-020-09625-x. [DOI] [PubMed] [Google Scholar]
- 89.Connolly KD, Wadey RM, Mathew D, Johnson E, Rees DA, James PE. Evidence for adipocyte-derived extracellular vesicles in the human circulation. Endocrinology. 2018;159:3259–3267. doi: 10.1210/en.2018-00266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lazar I, Clement E, Dauvillier S, Milhas D, Ducoux-Petit M, Legonidec S, Moro C, Soldan V, Dalle S, Balor S, et al. Adipocyte Exosomes Promote Melanoma Aggressiveness through Fatty Acid Oxidation: A Novel Mechanism Linking Obesity and Cancer. Cancer Res. 2016;76:4051–4057. doi: 10.1158/0008-5472.CAN-16-0651. [DOI] [PubMed] [Google Scholar]
- 91.Ying W, Riopel M, Bandyopadhyay G, Dong Y, Birmingham A, Seo JB, Ofrecio JM, Wollam J, Hernandez-Carretero A, Fu W, et al. Adipose tissue macrophage-derived exosomal miRNAs Can modulate in vivo and in vitro insulin sensitivity. Cell. 2017;171:372–384.e12. doi: 10.1016/j.cell.2017.08.035. [DOI] [PubMed] [Google Scholar]
- 92.Bassi EJ, Moraes-Vieira PM, Moreira-Sa CS, Almeida DC, Vieira LM, Cunha CS, Hiyane MI, Basso AS, Pacheco-Silva A, Camara NO. Immune regulatory properties of allogeneic adipose-derived mesenchymal stem cells in the treatment of experimental autoimmune diabetes. Diabetes. 2012;61:2534–2545. doi: 10.2337/db11-0844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mizuno H, Tobita M, Uysal AC. Concise review: Adipose-derived stem cells as a novel tool for future regenerative medicine. Stem Cells. 2012;30:804–810. doi: 10.1002/stem.1076. [DOI] [PubMed] [Google Scholar]
- 94.Gonzalez MA, Gonzalez-Rey E, Rico L, Buscher D, Delgado M. Adipose-derived mesenchymal stem cells alleviate experimental colitis by inhibiting inflammatory and autoimmune responses. Gastroenterology. 2009;136:978–989. doi: 10.1053/j.gastro.2008.11.041. [DOI] [PubMed] [Google Scholar]
- 95.Garcia-Contreras M, Vera-Donoso CD, Hernandez-Andreu JM, Garcia-Verdugo JM, Oltra E. Therapeutic potential of human adipose-derived stem cells (ADSCs) from cancer patients: A pilot study. PLoS One. 2014;9:e113288. doi: 10.1371/journal.pone.0113288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhao H, Shang Q, Pan Z, Bai Y, Li Z, Zhang H, Zhang Q, Guo C, Zhang L, Wang Q. Exosomes from adipose-derived stem cells attenuate adipose inflammation and obesity through polarizing M2 macrophages and beiging in white adipose tissue. Diabetes. 2018;67:235–247. doi: 10.2337/db17-0356. [DOI] [PubMed] [Google Scholar]
- 97.Jung YJ, Kim HK, Cho Y, Choi JS, Woo CH, Lee KS, Sul JH, Lee CM, Han J, Park JH, et al. Cell reprogramming using extracellular vesicles from differentiating stem cells into white/beige adipocytes. Sci Adv. 2020;6:eaay6721. doi: 10.1126/sciadv.aay6721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fuster JJ, Ouchi N, Gokce N, Walsh K. Obesity-Induced changes in adipose tissue microenvironment and their impact on cardiovascular disease. Circ Res. 2016;118:1786–1807. doi: 10.1161/CIRCRESAHA.115.306885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhao S, Kusminski CM, Scherer PE. Adiponectin, leptin and cardiovascular disorders. Circ Res. 2021;128:136–149. doi: 10.1161/CIRCRESAHA.120.314458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, Vaddi K, Charo I, Leibel RL, Ferrante AJ. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest. 2006;116:115–124. doi: 10.1172/JCI24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Deng ZB, Poliakov A, Hardy RW, Clements R, Liu C, Liu Y, Wang J, Xiang X, Zhang S, Zhuang X, et al. Adipose tissue exosome-like vesicles mediate activation of macrophage-induced insulin resistance. Diabetes. 2009;58:2498–2505. doi: 10.2337/db09-0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pan Y, Hui X, Hoo RLC, Ye D, Chan CYC, Feng T, Wang Y, Lam KSL, Xu A. Adipocyte-secreted exosomal microRNA-34a inhibits M2 macrophage polarization to promote obesity-induced adipose inflammation. J Clin Invest. 2019;129:834–849. doi: 10.1172/JCI123069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.James DE, Stockli J, Birnbaum MJ. The aetiology and molecular landscape of insulin resistance. Nat Rev Mol Cell Biol. 2021;22:751–771. doi: 10.1038/s41580-021-00390-6. [DOI] [PubMed] [Google Scholar]
- 105.Yu Y, Du H, Wei S, Feng L, Li J, Yao F, Zhang M, Hatch GM, Chen L. Adipocyte-Derived exosomal MiR-27a induces insulin resistance in skeletal muscle through repression of PPARү. Theranostics. 2018;8:2171–2188. doi: 10.7150/thno.22565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Li D, Song H, Shuo L, Wang L, Xie P, Li W, Liu J, Tong Y, Zhang CY, Jiang X, et al. Gonadal white adipose tissue-derived exosomal MiR-222 promotes obesity-associated insulin resistance. Aging (Albany NY) 2020;12:22719–22743. doi: 10.18632/aging.103891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kranendonk ME, Visseren FL, van Herwaarden JA, Nolte-'t Hoen EN, de Jager W, Wauben MH, Kalkhoven E. Effect of extracellular vesicles of human adipose tissue on insulin signaling in liver and muscle cells. Obesity (Silver Spring) 2014;22:2216–2223. doi: 10.1002/oby.20847. [DOI] [PubMed] [Google Scholar]
- 108.Gao X, Salomon C, Freeman DJ. Extracellular vesicles from adipose tissue-A potential role in obesity and type 2 diabetes? Front Endocrinol (Lausanne) 2017;8:202. doi: 10.3389/fendo.2017.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Eguchi A, Lazic M, Armando AM, Phillips SA, Katebian R, Maraka S, Quehenberger O, Sears DD, Feldstein AE. Circulating adipocyte-derived extracellular vesicles are novel markers of metabolic stress. J Mol Med (Berl) 2016;94:1241–1253. doi: 10.1007/s00109-016-1446-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Katayama M, Wiklander OPB, Fritz T, Caidahl K, El-Andaloussi S, Zierath JR, Krook A. Circulating exosomal miR-20b-5p is elevated in type 2 diabetes and could impair insulin action in human skeletal muscle. Diabetes. 2019;68:515–526. doi: 10.2337/db18-0470. [DOI] [PubMed] [Google Scholar]
- 111.Kranendonk ME, de Kleijn DP, Kalkhoven E, Kanhai DA, Uiterwaal CS, van der Graaf Y, Pasterkamp G, Visseren FL, SMART Study Group Extracellular vesicle markers in relation to obesity and metabolic complications in patients with manifest cardiovascular disease. Cardiovasc Diabetol. 2014;13:37. doi: 10.1186/1475-2840-13-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Su S, Guntur AR, Nguyen DC, Fakory SS, Doucette CC, Leech C, Lotana H, Kelley M, Kohli J, Martino J, et al. A renewable source of human beige adipocytes for development of therapies to treat metabolic Syndrome. Cell Rep. 2018;25:3215–3228,e9. doi: 10.1016/j.celrep.2018.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Connolly KD, Rees DA, James PE. Role of adipocyte-derived extracellular vesicles in vascular inflammation. Free Radic Biol Med. 2021;172:58–64. doi: 10.1016/j.freeradbiomed.2021.04.031. [DOI] [PubMed] [Google Scholar]
- 114.Dai W, Liu Z, Yang S, Kong J. Inflamed adipose tissue: Therapeutic Targets for obesity-related endothelial injury. Endocrinology. 2023;164:bqad094. doi: 10.1210/endocr/bqad094. [DOI] [PubMed] [Google Scholar]
- 115.Koenen M, Hill MA, Cohen P, Sowers JR. Obesity, adipose tissue and vascular dysfunction. Circ Res. 2021;128:951–968. doi: 10.1161/CIRCRESAHA.121.318093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Xie Z, Wang X, Liu X, Du H, Sun C, Shao X, Tian J, Gu X, Wang H, Tian J, Yu B. Adipose-Derived exosomes exert proatherogenic effects by regulating macrophage foam cell formation and polarization. J Am Heart Assoc. 2018;7:e007442. doi: 10.1161/JAHA.117.007442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Barberio MD, Kasselman LJ, Playford MP, Epstein SB, Renna HA, Goldberg M, Deleon J, Voloshyna I, Barlev A, Salama M, et al. Cholesterol efflux alterations in adolescent obesity: Role of adipose-derived extracellular vesical microRNAs. J Transl Med. 2019;17:232. doi: 10.1186/s12967-019-1980-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fleury A, Martinez MC, Le Lay S. Extracellular vesicles as therapeutic tools in cardiovascular diseases. Front Immunol. 2014;5:370. doi: 10.3389/fimmu.2014.00370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wen Z, Li J, Fu Y, Zheng Y, Ma M, Wang C. Hypertrophic adipocyte-derived exosomal miR-802-5p contributes to insulin resistance in cardiac myocytes through targeting hSP60. Obesity (Silver Spring) 2020;28:1932–1940. doi: 10.1002/oby.22932. [DOI] [PubMed] [Google Scholar]
- 120.Monteiro JP, Bennett M, Rodor J, Caudrillier A, Ulitsky I, Baker AH. Endothelial function and dysfunction in the cardiovascular system: The long non-coding road. Cardiovasc Res. 2019;115:1692–1704. doi: 10.1093/cvr/cvz154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Muller G. Microvesicles/exosomes as potential novel biomarkers of metabolic diseases. Diabetes Metab Syndr Obes. 2012;5:247–282. doi: 10.2147/DMSO.S32923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wadey RM, Connolly KD, Mathew D, Walters G, Rees DA, James PE. Inflammatory adipocyte-derived extracellular vesicles promote leukocyte attachment to vascular endothelial cells. Atherosclerosis. 2019;283:19–27. doi: 10.1016/j.atherosclerosis.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 123.Fang X, Stroud MJ, Ouyang K, Fang L, Zhang J, Dalton ND, Gu Y, Wu T, Peterson KL, Huang HD, et al. Adipocyte-specific loss of PPARү attenuates cardiac hypertrophy. JCI Insight. 2016;1:e89908. doi: 10.1172/jci.insight.89908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gan L, Xie D, Liu J, Bond LW, Christopher TA, Lopez B, Zhang L, Gao E, Koch W, Ma XL, Wang Y. Small extracellular microvesicles mediated pathological communications between dysfunctional adipocytes and cardiomyocytes as a novel mechanism exacerbating ischemia/reperfusion injury in diabetic mice. Circulation. 2020;141:968–983. doi: 10.1161/CIRCULATIONAHA.119.042640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Parekh N, Chandran U, Bandera EV. Obesity in cancer survival. Annu Rev Nutr. 2012;32:311–342. doi: 10.1146/annurev-nutr-071811-150713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dirat B, Bochet L, Dabek M, Daviaud D, Dauvillier S, Majed B, Wang YY, Meulle A, Salles B, Le Gonidec S, et al. Cancer-associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Cancer Res. 2011;71:2455–2465. doi: 10.1158/0008-5472.CAN-10-3323. [DOI] [PubMed] [Google Scholar]
- 127.Nieman KM, Kenny HA, Penicka CV, Ladanyi A, Buell-Gutbrod R, Zillhardt MR, Romero IL, Carey MS, Mills GB, Hotamisligil GS, et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat Med. 2011;17:1498–1503. doi: 10.1038/nm.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wang J, Wu Y, Guo J, Fei X, Yu L, Ma S. Adipocyte-derived exosomes promote lung cancer metastasis by increasing MMP9 activity via transferring MMP3 to lung cancer cells. Oncotarget. 2017;8:81880–81891. doi: 10.18632/oncotarget.18737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wang Y, Chu Y, Li K, Zhang G, Guo Z, Wu X, Qiu C, Li Y, Wan X, Sui J, et al. Exosomes secreted by adipose-derived mesenchymal stem cells foster metastasis and osteosarcoma proliferation by increasing COLGALT2 expression. Front Cell Dev Biol. 2020;8:353. doi: 10.3389/fcell.2020.00353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gangadaran P, Rajendran RL, Oh JM, Oh EJ, Hong CM, Chung HY, Lee J, Ahn BC. Identification of angiogenic cargo in extracellular vesicles secreted from human adipose tissue-derived stem cells and induction of angiogenesis in vitro and in vivo. Pharmaceutics. 2021;13:495. doi: 10.3390/pharmaceutics13040495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Khanh VC, Fukushige M, Moriguchi K, Yamashita T, Osaka M, Hiramatsu Y, Ohneda O. Type 2 diabetes mellitus induced paracrine effects on breast cancer metastasis through extracellular vesicles derived from human mesenchymal stem cells. Stem Cells Dev. 2020;29:1382–1394. doi: 10.1089/scd.2020.0126. [DOI] [PubMed] [Google Scholar]
- 132.Mattiske S, Suetani RJ, Neilsen PM, Callen DF. The oncogenic role of miR-155 in breast cancer. Cancer Epidemiol Biomarkers Prev. 2012;21:1236–1243. doi: 10.1158/1055-9965.EPI-12-0173. [DOI] [PubMed] [Google Scholar]
- 133.Balaban S, Shearer RF, Lee LS, van Geldermalsen M, Schreuder M, Shtein HC, Cairns R, Thomas KC, Fazakerley DJ, Grewal T, et al. Adipocyte lipolysis links obesity to breast cancer growth: Adipocyte-derived fatty acids drive breast cancer cell proliferation and migration. Cancer Metab. 2017;5:1. doi: 10.1186/s40170-016-0163-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kuo CY, Ann DK. When fats commit crimes: Fatty acid metabolism, cancer stemness and therapeutic resistance. Cancer Commun (Lond) 2018;38:47. doi: 10.1186/s40880-018-0317-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhuang X, Xiang X, Grizzle W, Sun D, Zhang S, Axtell RC, Ju S, Mu J, Zhang L, Steinman L, et al. Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol Ther. 2011;19:1769–1779. doi: 10.1038/mt.2011.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hardy J. Amyloid, the presenilins and Alzheimer's disease. Trends Neurosci. 1997;20:154–159. doi: 10.1016/S0166-2236(96)01030-2. [DOI] [PubMed] [Google Scholar]
- 137.Selkoe DJ. The cell biology of beta-amyloid precursor protein and presenilin in Alzheimer's disease. Trends Cell Biol. 1998;8:447–453. doi: 10.1016/S0962-8924(98)01363-4. [DOI] [PubMed] [Google Scholar]
- 138.Katsuda T, Tsuchiya R, Kosaka N, Yoshioka Y, Takagaki K, Oki K, Takeshita F, Sakai Y, Kuroda M, Ochiya T. Human adipose tissue-derived mesenchymal stem cells secrete functional neprilysin-bound exosomes. Sci Rep. 2013;3:1197. doi: 10.1038/srep01197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Garcia-Contreras M, Thakor AS. Human adipose tissue-derived mesenchymal stem cells and their extracellular vesicles modulate lipopolysaccharide activated human microglia. Cell Death Discov. 2021;7:98. doi: 10.1038/s41420-021-00471-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Geng W, Tang H, Luo S, Lv Y, Liang D, Kang X, Hong W. Exosomes from miRNA-126-modified ADSCs promotes functional recovery after stroke in rats by improving neurogenesis and suppressing microglia activation. Am J Transl Res. 2019;11:780–792. [PMC free article] [PubMed] [Google Scholar]
- 141.Jiang M, Wang H, Jin M, Yang X, Ji H, Jiang Y, Zhang H, Wu F, Wu G, Lai X, et al. Exosomes from MiR-30d-5p-ADSCs reverse acute ischemic stroke-induced, autophagy-mediated brain injury by promoting M2 microglial/macrophage polarization. Cell Physiol Biochem. 2018;47:864–878. doi: 10.1159/000490078. [DOI] [PubMed] [Google Scholar]
- 142.Takahashi Y, Takakura Y. Extracellular vesicle-based therapeutics: Extracellular vesicles as therapeutic targets and agents. Pharmacol Ther. 2023;242:108352. doi: 10.1016/j.pharmthera.2023.108352. [DOI] [PubMed] [Google Scholar]
- 143.Greening DW, Xu R, Ale A, Hagemeyer CE, Chen W. Extracellular vesicles as next generation immunotherapeutics. Semin Cancer Biol. 2023;90:73–100. doi: 10.1016/j.semcancer.2023.02.002. [DOI] [PubMed] [Google Scholar]
- 144.Kwan HY, Chen M, Xu K, Chen B. The impact of obesity on adipocyte-derived extracellular vesicles. Cell Mol Life Sci. 2021;78:7275–7288. doi: 10.1007/s00018-021-03973-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.