Abstract
By using different staining techniques, 479 stool specimens from 212 diarrheic patients with AIDS were examined for microsporidian spores. Calcofluor fluorescence staining of 119 specimens revealed fluorescent ovoid structures of microsporidian size. Staining of these samples according to the method of Weber et al. (R. Weber, R. T. Bryan, R. L. Owen, C. M. Wilcox, L. Gorelkin, and G. S. Visvesvara, N. Engl. J. Med. 326:161–166, 1992) with trichrome produced six specimens with pinkish spores containing the characteristic microsporidian belt-like structure. The 6 specimens were processed for transmission electron microscopy, as were another 21 specimens which did not present the belt-like structure after trichrome staining but which looked highly suspicious after fluorescence staining. In these 21 samples, only fungal spores and, particularly, bacterial Clostridium spores were demonstrated, whereas in the 6 samples diagnosed positive after trichrome staining, the existence of microsporidia could be verified by electron microscopy. Based on our observations, we propose that the belt-like structure seen with the Weber stains in microsporidian spores corresponds to structures existing in priming-stage spores. The results suggest that routine microscopical fecal diagnosis for microsporidian infection should include a screening by fluorescence staining and, subsequently, a confirmatory viewing of fluorescence-positive samples after trichrome staining.
Members of the phylum Microsporidia Balbiani 1882 are increasingly recognized as opportunistic parasites in patients with AIDS (24), as well as pathogens in immunocompetent persons (20, 22). Myositis (3), keratoconjunctivitis (1), and disseminated infection (14, 25) are reported in microsporidian infection, but the most common manifestation is chronic diarrhea caused by Enterocytozoon bieneusi or Encephalitozoon species (17, 24). Diagnosis is routinely based on the direct demonstration of microsporidian spores. However, microsporidian spores are difficult to detect because of their small size and their staining characteristics, which are similar to those of enteric bacteria and fungi. So, diagnosis of microsporidian infection by light microscopy examination is still a challenge, and the sensitivity and specificity of diagnosis strongly depend on the experience of the investigator.
The most common diagnostic method is to stain samples by using fluorescence brighteners, such as Calcofluor White M2R (21), Uvitex 2B (19), or Fungifluor (5), and by using Weber’s modified trichrome staining (23) or a modification of it (6, 12, 15, 16).
Fluorescence staining is very quick and easy to perform. Its specificity for microsporidian spores is high, if one accepts that other fluorescing spores (especially fungal spores) are of different sizes. Weber’s modified trichrome staining and its modifications also possess high specificity, because microsporidian spores can be identified by a diagonal or equatorial belt-like structure. However, the electron microscopical equivalent of this structure is unknown.
In this study, we evaluated whether spore size and the existence of the belt-like structure are sufficient criteria for a sensitive and specific diagnosis of microsporidian infection by fluorescence staining, Weber’s modified trichrome staining, and transmission electron microscopy.
MATERIALS AND METHODS
Stool specimens.
A total of 479 stool specimens from 212 AIDS patients with chronic diarrhea sent to our institute were investigated. Slides of stool specimens were prepared by stirring 10 μl of native stool in aqua destillata covering an area of about 2 cm2. The smears were air dried and fixed with methanol.
Calcofluor staining method.
The 479 stool samples were screened by fluorescence microscopy after Calcofluor White M2R (Sigma-Aldrich Chemicals F-6259, Deisenhofen, Germany) staining. The Calcofluor was prepared as a 0.1% solution in NaOH and stored in the dark at room temperature. Prior to use, the Calcofluor stain was filtered with filter paper (Schleicher & Schuell, Dassel, Germany) to remove precipitates. After the stool smears had been incubated with 1 or 2 drops of Calcofluor for 1.5 min, slides were rinsed with aqua destillata and counterstained with 0.5% Evans Blue (Sigma E-2129) in phosphate-buffered saline (pH 7.2) for 20 s. The slides were investigated under a Zeiss fluorescence microscope at a wavelength of 390 to 420 nm with a 450-nm-wavelength fluorescence filter.
Modified trichrome staining method.
A total of 119 specimens presenting fluorescing ovoid spores in Calcofluor staining were evaluated by Weber’s modified trichrome staining. The staining procedure was performed as described by Weber and colleagues (23) with chromotrope 2R (Sigma; Fluka 27140) and fast green FCF (Sigma; Fluka 44715).
Transmission electron microscopy.
Twenty-seven fecal samples of the 119 samples showing fluorescence-positive spores by Calcofluor staining were processed for electron microscopy: 21 revealing just ovoid fluorescing spores of microsporidian size, as well as 6 samples additionally presenting the belt-like structure in Weber’s modified trichrome staining. Samples were fixed for 6 days at 4°C in 2% buffered glutaraldehyde and postfixed for 30 min in 1% OsO4 after repeated rinsing with Soerensen buffer (pH 7.2). The material was dehydrated in graded ethanol and embedded in Spurr-Low medium. Ultrathin sections were prepared and stained for 5 min with saturated uranyl acetate in 50% ethanol and for 3 min in 0.03% lead citrate. They were examined with a Zeiss 900 electron microscope and documented on Agfa Scientia films.
RESULTS
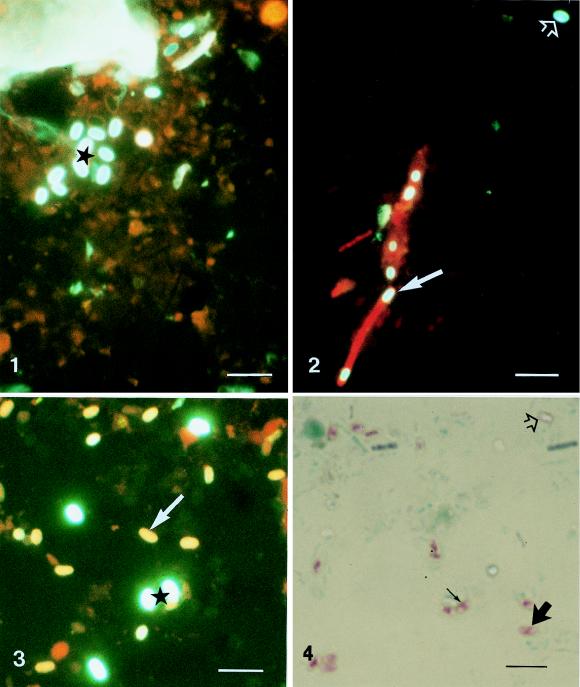
Smears of 479 stool samples from 212 patients were screened for microsporidian spores by Calcofluor staining. In 119 stool samples, ovoid spores with bright greenish-white fluorescence were detected (Fig. 1). In some stool samples, very similar looking spores inside red-stained bacteria were identified (Fig. 2). Six of the 119 specimens revealed ovoid but smaller spores with yellow-orange fluorescence (Fig. 3) in addition to the bright greenish-white spores. With application of Weber’s modified trichrome staining, in only these six samples ovoid pinkish-red spores (Fig. 4) were observed. Most of them were characterized by an internal belt-like structure oriented diagonally or equatorially.
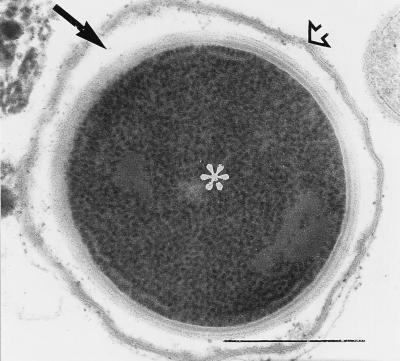
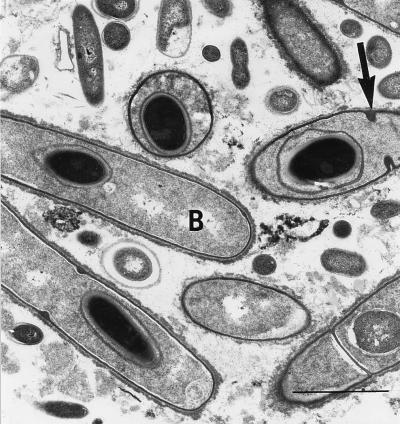
By electron microscopy of all 27 stool samples analyzed, bacterial spores morphologically and ultrastructurally identified as belonging to the genus Clostridium were demonstrated. Spores were 1 to 3 μm in diameter (depending on the plane in which the spores were cut) and displayed an electron-dense cytoplasm as well as a wall consisting of an inner electron-lucent layer and an outer electron-dense layer (Fig. 5). The cytoplasm contained densely packed ribosomes. In three stool samples, such spores were identified inside bacteria (Fig. 6).
FIG. 5.
Electron microscopy cross section of a spore appearing greenish-white after Calcofluor staining as shown in Fig. 1. The spore wall consists of two distinct layers: an electron-dense layer (➩) and an electron-lucent one (➞). The spore cytoplasm contains randomly and densely packed ribosomes (asterisk). Bar, 0.5 μm. Magnification, ×60,000.
FIG. 6.
Electron micrograph from a stool sample as shown by fluorescence microscopy in Fig. 2. The spores with a bilayered wall and electron-dense cytoplasm are situated in the interior of the bacteria (B). The characteristic binary fission of the bacteria can be seen (➞). Bar, 3 μm. Magnification, ×7,300.
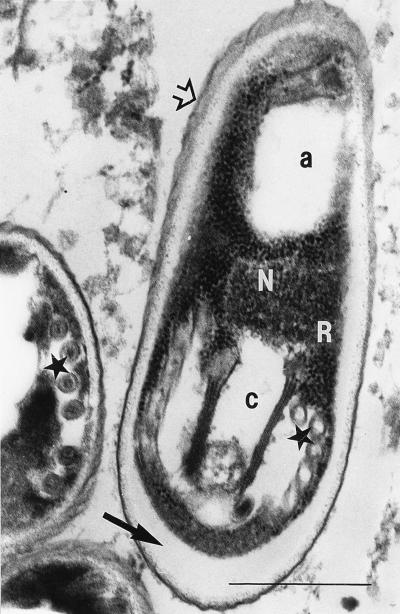
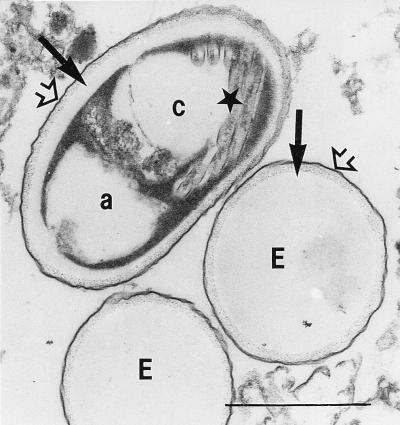
The spores revealing the belt-like structure after staining with Weber’s modified trichrome stain in six stool samples could be identified as microsporidian spores by transmission electron microscopy (Fig. 7 and 8). Typically for the genus Encephalitozoon, spores showed about six cross sections of the coiled part of the polar tube lying in one row. The spores were surrounded by a wall with an inner electron-lucent layer (commonly called endospore) and an outer electron-dense layer (commonly called exospore). Most of them showed an internal compartmentation: an anterior electron-lucent zone at the polaroplast level, an electron-lucent zone posterior to the nucleus, and a central electron-dense zone with modified cytoplasm and nucleus.
FIG. 7.
Electron micrograph of microsporidian spores from human stool samples as shown by light microscopy in Fig. 3 and 4. The characteristic polar tube can be seen in cross section (★). The bilayered spore wall consists of an outer electron-dense layer (exospore) (➩) and an inner electron-lucent layer (endospore) (➞). The spore cut in an axial plane shows two distinct electron-lucent compartments: an apical one (a) and caudal one (c) separated by a border of electron-dense cytoplasm containing polyribosomes (R) and part of the nuclear region (N). Bar, 0.5 μm. Magnification, ×60,000.
FIG. 8.
Microsporidian spores from human stool samples as shown by light microscopy in Fig. 3 and 4. Two spores are empty looking (E). One spore shows a distinct compartmentation built up of two electron-lucent areas (a and c, with cross sections of the polar tube [★] shown in area c) separated by an electron-dense area. All three spores reveal the bilayered spore wall consisting of an outer electron-dense layer (exospore) (➩) and an inner electron-lucent layer (endospore) (➞). Bar, 1 μm. Magnification, ×30,000.
DISCUSSION
After ingestion of microsporidian spores by the host, the spore can extrude its polar tube and inject the sporoplasm through the polar tube into a host cell. Following a proliferative vegetative phase, infective spores are formed which can infect new host cells or hosts by extruding their polar tube. During the extrusion process, spores undergo morphologic changes. Four different stages of the spore (the resting, priming, germinating, and discharged stages) can be distinguished (4). In this study, mature spores in the resting stage or immature stages like sporoblasts could not be observed by electron microscopy. However, spores in the discharged stage and in the priming stage were detected. Empty-looking spores are spores in the discharged stage, whereas the priming stage is characterized by an electron-lucent zone posterior to the nucleus, an electron-lucent anterior zone in the region of the polaroplast, and an electron-dense zone in the middle of the spore consisting of modified cytoplasm and nucleus. In contrast to Garcia and colleagues (8) and Ignatius and colleagues (9), who proposed that the belt-like structure in Weber’s modified trichrome staining is related to the microsporidian polar tube, this study demonstrated by electron microscopy that spores showing the belt-like structure are activated spores in the priming stage. The belt-like structure therefore is the result of the liquefaction of the polaroplast region and the region posterior to the nucleus.
Applying Uvitex 2B, van Gool and colleagues (19) found many spores with bright white fluorescence (“mature spores”) and few reddish-brown ones (“immature stages”) in the supernatant of cell culture. In contrast, in stool specimens, they saw many reddish-brown stages, but few spores with bright white fluorescence. We suggest that the reddish-brown stages observed by van Gool and colleagues, as well as the yellow-orange structures observed in this study with Calcofluor staining, are not immature stages, which would lack a well-developed inner wall layer, but are activated spores in the priming stage.
The regular associative occurrence of Clostridium spores and ovoid bright white fluorescing spores in Calcofluor staining leads to the conclusion that Clostridium spores also take up the fluorescent dye and therefore should be taken into consideration as a false-positive artifact by this staining method. The relevance of this detection is high, since infections with Clostridium spp. are increasingly being recognized in AIDS patients (10, 13).
With fluorescence staining, microsporidian spores are mixed up not only with bacterial spores but also with fungal spores. While distinguishing large budding fungal spores from microsporidian spores causes no problems, it is difficult to differentiate between microsporidia and small separate fungal spores. Considering studies which implicate that microsporidia are closely related to or even are fungi (7, 11), this should not be a surprise.
Regarding the size of ovoid fluorescing structures in stool specimens, the fact that spores of invertebrate microsporidia are likewise larger than spores found in stool specimens so far (E. bieneusi or Encephalitozoon spp.) also has to be taken into account. Most microsporidian species have been described in invertebrates, but as a matter of fact, their role as a source of human microsporidiosis has not been adequately evaluated yet. For example, Trammer and colleagues (18) demonstrated the progressive development of Nosema algerae, a microsporidian parasite of mosquitoes, in athymic mice, whereas Cali and colleagues (2) found a Nosema-like microsporidium in AIDS-associated myositis.
As a general practice for diagnosing microsporidian infection in stool specimens, we recommend that experienced persons use fluorescence staining as a cheap and practical tool for rapid screening of fecal smears. Successively, confirmation of fluorescence-positive smears should be done by light microscopy after Weber’s trichrome staining or a modification of it. (According to our own results, fluorescence staining and trichrome staining can be performed with the same smear without losing information.) However, one must be aware of the fact that discharged (empty) spores would pass that diagnostic filter unrecognized.
FIG. 1-4.
Light micrograph of ovoid greenish-white spores (★) in a Calcofluor-stained smear of a human stool sample. Bar, 6 μm. Magnification, ×1,780.
Fig. 2 Light micrograph of greenish-white spores outside (➩) and inside (➞) red-stained bacteria in a Calcofluor-stained smear of a human stool sample. Bar, 6 μm. Magnification, ×1,780.
Fig. 3 Light micrograph of ovoid, greenish-white spores (★) and smaller yellow-orange spores (➞) in a Calcofluor-stained smear of a human stool sample. Bar, 6 μm. Magnification, ×1,780.
Fig. 4 Smear of a human stool sample stained by Weber’s modified trichrome staining containing unstained, empty-looking spores (➩), as well as pink spores showing a belt-like structure with different internal localizations: equatorially (→) and diagonally (➞). Bar, 6 μm. Magnification, ×1,780.
REFERENCES
- 1.Cali A, Meisler D M, Lowder C Y, Lembach R, Ayers L, Takvorian P M, Rutherford I, Longworth D L, McMahon J, Bryan R T. Corneal microsporidiosis: characterization and identification. J Protozool. 1991;38:215S–217S. [PubMed] [Google Scholar]
- 2.Cali A, Takvorian P M, Lewin S, Rendel M, Sian C, Wittner M, Weiss L M. Identification of a new Nosema-like microsporidian associated with myositis in an AIDS patient. J Eukaryot Microbiol. 1996;43:108S. doi: 10.1111/j.1550-7408.1996.tb05028.x. [DOI] [PubMed] [Google Scholar]
- 3.Cali A, Takvorian P M, Keohane E, Weiss L M. Opportunistic microsporidian infections associated with myositis. J Eukaryot Microbiol. 1997;44:86S. doi: 10.1111/j.1550-7408.1997.tb05799.x. [DOI] [PubMed] [Google Scholar]
- 4.Chioralia G, Trammer T, Maier W A, Seitz H M. Morphologic changes in Nosema algerae (Microspora) during extrusion. Parasitol Res. 1998;84:123–131. doi: 10.1007/s004360050368. [DOI] [PubMed] [Google Scholar]
- 5.Conteas C, Donovan J, Berlin O G W, Sowerby T M, La Riviere M. Comparison of fluorescence and standard light microscopy for diagnosis of microsporidia in stools of patients with AIDS and chronic diarrhea. AIDS. 1997;11:386–387. [PubMed] [Google Scholar]
- 6.Didier E S, Orenstein J M, Aldras A, Bertucci D, Rogers L B, Janney F A. Comparison of three staining methods for detecting microsporidia in fluids. J Clin Microbiol. 1995;33:3138–3145. doi: 10.1128/jcm.33.12.3138-3145.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flegel T W, Pasharawipas T. A proposal for typical eukaryotic meiosis in microsporidians. Can J Microbiol. 1995;41:1–11. [Google Scholar]
- 8.Garcia L S, Shimizu R Y, Bruckner D A. Detection of microsporidial spores in fecal specimens from patients diagnosed with cryptosporidiosis. J Clin Microbiol. 1994;32:1739–1741. doi: 10.1128/jcm.32.7.1739-1741.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ignatius R, Henschel S, Liesenfeld O, Mansmann U, Schmidt W, Köppe S, Schneider T, Heise W, Futh U, Riecken E O, Hahn H, Ullrich R. Comparative evaluation of modified trichrome and Uvitex 2B stains for detection of low numbers of microsporidial spores in stool specimens. J Clin Microbiol. 1997;35:2266–2269. doi: 10.1128/jcm.35.9.2266-2269.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karlström O, Fryklund B, Tullus K, Burman L G the Swedish C. difficile Study Group. A prospective nationwide study of Clostridium difficile-associated diarrhea in Sweden. Clin Infect Dis. 1998;26:141–145. doi: 10.1086/516277. [DOI] [PubMed] [Google Scholar]
- 11.Keeling P J, Doolittle W F. Alpha-tubulin from early-diverging eukaryotic lineages and the evolution of the tubulin family. Mol Biol Evol. 1996;13:1297–1305. doi: 10.1093/oxfordjournals.molbev.a025576. [DOI] [PubMed] [Google Scholar]
- 12.Kokoskin E, Gyorkos T W, Camus A, Cedilotte L, Purtill T, Ward B. Modified technique for efficient detection of microsporidia. J Clin Microbiol. 1994;32:1074–1075. doi: 10.1128/jcm.32.4.1074-1075.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mastroianni, A., O. Coronado, A. Nanetti, R. Valentini, R. Manfredi, and F. Chiodo. 1997. Nosocomial Clostridium difficile-associated diarrhea in patients with AIDS: a three-year survey and review. Clin. Infect. Dis. 25(Suppl.):204S–205S. [DOI] [PubMed]
- 14.Mertens R B, Didier E S, Fishbein M C, Bertucci D C, Rogers L B, Orenstein J M. Encephalitozoon cuniculi microsporidiosis: infection of the brain, heart, kidneys, trachea, adrenal glands, and urinary bladder in a patient with AIDS. Mod Pathol. 1997;10:68–77. [PubMed] [Google Scholar]
- 15.Moura H, Schwartz D A, Bornay-Llinares F, Sodré F C, Wallace S, Visvesvara G S. A new and improved “quick-hot gram-chromotrope” technique that differentially stains microsporidian spores in clinical samples, including paraffin-embedded tissue sections. Arch Pathol Lab Med. 1997;121:888–893. [PubMed] [Google Scholar]
- 16.Ryan N J, Sutherland G, Coughlan K, Globan M, Doultree J, Marshall J, Baird R W, Pedersen J, Dwyer B. A new trichrome-blue stain for detection of microsporidial species in urine, stool, and nasopharyngeal specimens. J Clin Microbiol. 1993;31:3264–3269. doi: 10.1128/jcm.31.12.3264-3269.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwartz D A, Sobottka I, Leitch G J, Cali A, Visvesvara G S. Pathology of microsporidiosis. Emerging parasitic infections in patients with acquired immunodeficiency syndrome. Arch Pathol Lab Med. 1996;120:173–188. [PubMed] [Google Scholar]
- 18.Trammer T, Dombrowski F, Doehring M, Maier W A, Seitz H M. Opportunistic properties of Nosema algerae (Microspora), a mosquito parasite, in immunocompromised mice. J Eukaryot Microbiol. 1997;44:258–262. doi: 10.1111/j.1550-7408.1997.tb05709.x. [DOI] [PubMed] [Google Scholar]
- 19.van Gool T, Snjiders F, Reiss P, Eeftinck-Schattenkerk J K M, van den Bergh Weerman M A, Bartelsman J F W M, Bruins J J M, Canning E U, Dankert J. Diagnosis of intestinal and disseminated microsporidial infections in patients with HIV by a new rapid fluorescence technique. J Clin Pathol. 1993;46:694–699. doi: 10.1136/jcp.46.8.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Gool T, Vetter J C M, Weinmayr B, van Dam A, Dankert J. High seroprevalence of Encephalitozoon species in immunocompetent subjects. J Infect Dis. 1997;175:1020–1024. doi: 10.1086/513963. [DOI] [PubMed] [Google Scholar]
- 21.Vávra J, Dahbiová R, Hollister W S, Canning E U. Staining of microsporidian spores by optical brighteners with remarks on the use of brighteners for the diagnosis of AIDS associated human microsporidioses. Folia Parasitol. 1993;40:267–272. [PubMed] [Google Scholar]
- 22.Wanke C A, DeGirolami P, Federman M. Enterocytozoon bieneusi infection and diarrheal disease in patients who were not infected with human immunodeficiency virus: case report and review. Clin Infect Dis. 1996;23:816–818. doi: 10.1093/clinids/23.4.816. [DOI] [PubMed] [Google Scholar]
- 23.Weber R, Bryan R T, Owen R L, Wilcox C M, Gorelkin L, Visvesvara G S. Improved light-microscopical detection of microsporidia spores in stool and duodenal aspirates. N Engl J Med. 1992;326:161–166. doi: 10.1056/NEJM199201163260304. [DOI] [PubMed] [Google Scholar]
- 24.Weber R, Bryan R T, Schwartz D A, Owen R L. Human microsporidial infections. Clin Microbiol Rev. 1994;7:426–461. doi: 10.1128/cmr.7.4.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yachnis A T, Berg J, Martinez-Salazar A, Bender B S, Diaz L, Rojiani A M, Eskin T A, Orenstein J M. Disseminated microsporidiosis especially infecting the brain, heart, and kidneys. Report of a newly recognized pansporoblastic species in two symptomatic AIDS patients. Am J Clin Pathol. 1996;106:535–543. doi: 10.1093/ajcp/106.4.535. [DOI] [PubMed] [Google Scholar]