Abstract
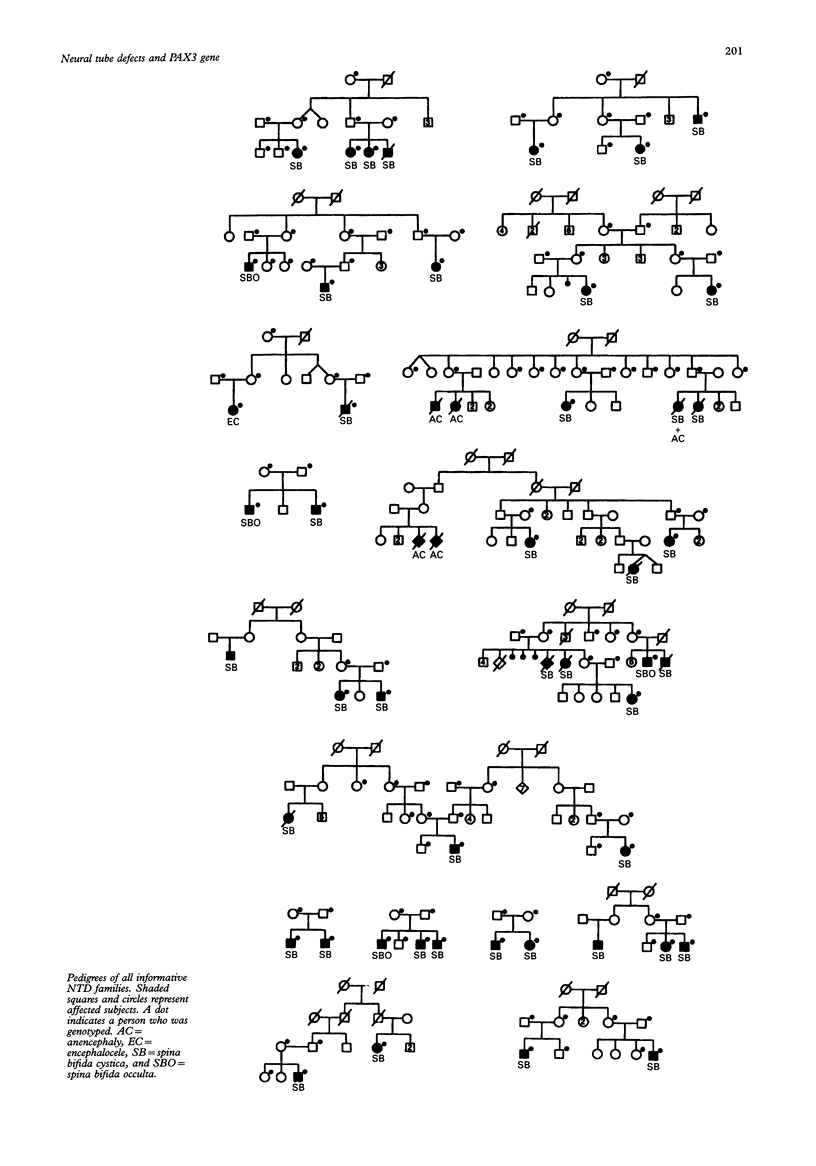
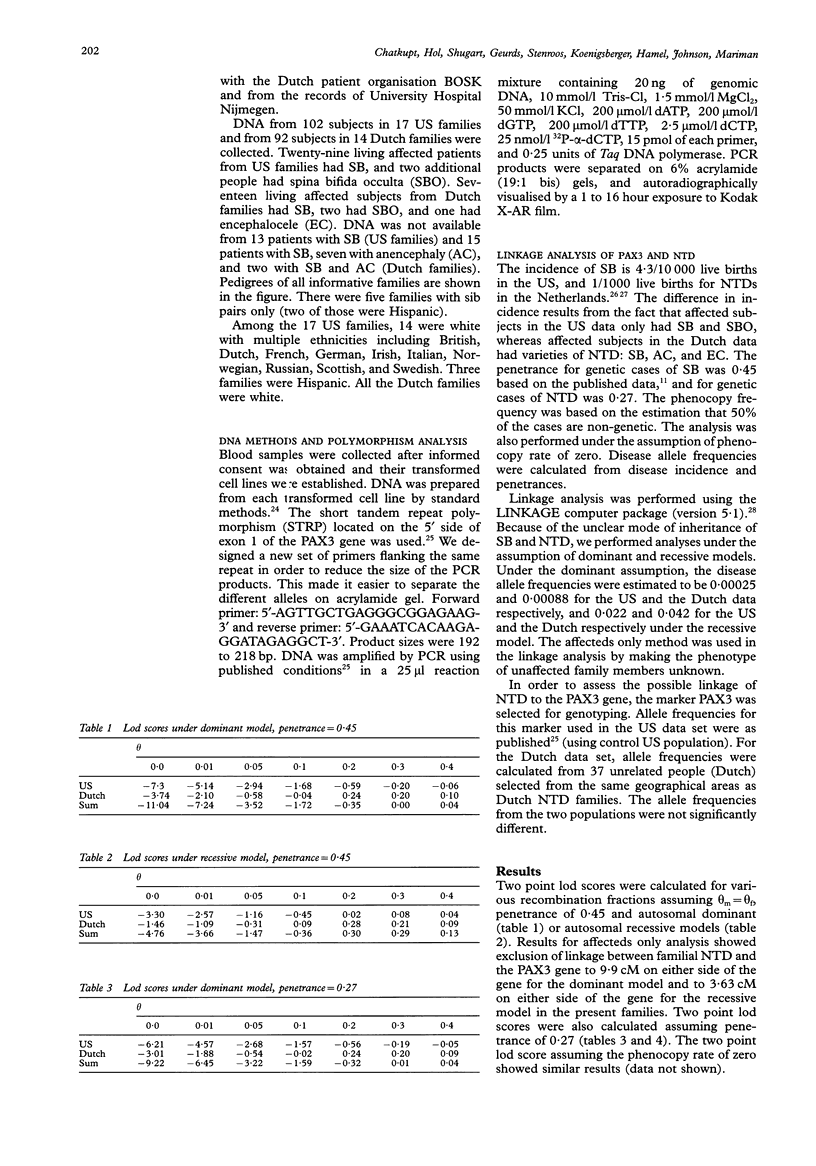
Neural tube defects (NTD) are among the most common and disabling birth defects. The aetiology of NTD is unknown and their genetics are complex. The majority of NTD cases are sporadic, isolated, nonsyndromic, and generally considered to be multifactorial in origin. Recently, PAX3 (formerly HuP2, the human homologue of mouse Pax-3), on chromosome 2q35-37, was suggested as a candidate gene for NTD because mutations of Pax-3 cause the mouse mutant Splotch (Sp), an animal model for human NTD. Mutations in PAX3 were also identified in patients with Waardenburg syndrome type 1 (WS1). At least eight patients with both WS1 and NTD have been described suggesting pleiotropy or a contiguous gene syndrome. Seventeen US families and 14 Dutch families with more than one affected person with NTD were collected and 194 people (50 affected) from both data sets were genotyped using the PAX3 polymorphic marker. The data were analysed using affecteds only linkage analysis. The lod scores were -7.30 (US), -3.74 (Dutch), and -11.04 (combined) at theta = 0.0, under the assumption of the autosomal dominant model. For the recessive model, the lod scores were -3.30 (US), -1.46 (Dutch), and -4.76 (combined) at theta = 0.0. Linkage between PAX3 and familial NTD was excluded to 9.9 cM on either side of the gene for the dominant model and to 3.63 cM on either side of the gene for the recessive model in the families studied. No evidence of heterogeneity was detected using the HOMOG program. Our data indicate that PAX3 is not a major gene for NTD.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin C. T., Hoth C. F., Amos J. A., da-Silva E. O., Milunsky A. An exonic mutation in the HuP2 paired domain gene causes Waardenburg's syndrome. Nature. 1992 Feb 13;355(6361):637–638. doi: 10.1038/355637a0. [DOI] [PubMed] [Google Scholar]
- Baraitser M., Burn J. Brief clinical report: neural tube defects as an X-linked condition. Am J Med Genet. 1984 Jan;17(1):383–385. doi: 10.1002/ajmg.1320170132. [DOI] [PubMed] [Google Scholar]
- Campbell L. R., Dayton D. H., Sohal G. S. Neural tube defects: a review of human and animal studies on the etiology of neural tube defects. Teratology. 1986 Oct;34(2):171–187. doi: 10.1002/tera.1420340206. [DOI] [PubMed] [Google Scholar]
- Carezani-Gavin M., Clarren S. K., Steege T. Waardenburg syndrome associated with meningomyelocele. Am J Med Genet. 1992 Jan 1;42(1):135–136. doi: 10.1002/ajmg.1320420127. [DOI] [PubMed] [Google Scholar]
- Carter C. O., David P. A., Laurence K. M. A family study of major central nervous system malformations in South Wales. J Med Genet. 1968 Jun;5(2):81–106. doi: 10.1136/jmg.5.2.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter C. O., Evans K. A., Till K. Spinal dysraphism: genetic relation to neural tube malformations. J Med Genet. 1976 Oct;13(5):343–350. doi: 10.1136/jmg.13.5.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalepakis G., Stoykova A., Wijnholds J., Tremblay P., Gruss P. Pax: gene regulators in the developing nervous system. J Neurobiol. 1993 Oct;24(10):1367–1384. doi: 10.1002/neu.480241009. [DOI] [PubMed] [Google Scholar]
- Chatkupt S., Chatkupt S., Johnson W. G. Waardenburg syndrome and myelomeningocele in a family. J Med Genet. 1993 Jan;30(1):83–84. doi: 10.1136/jmg.30.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatkupt S., Lucek P. R., Koenigsberger M. R., Johnson W. G. Parental sex effect in spina bifida: a role for genomic imprinting? Am J Med Genet. 1992 Nov 1;44(4):508–512. doi: 10.1002/ajmg.1320440426. [DOI] [PubMed] [Google Scholar]
- Chatkupt S., Skurnick J. H., Jaggi M., Mitruka K., Koenigsberger M. R., Johnson W. G. Study of genetics, epidemiology, and vitamin usage in familial spina bifida in the United States in the 1990s. Neurology. 1994 Jan;44(1):65–70. doi: 10.1212/wnl.44.1.65. [DOI] [PubMed] [Google Scholar]
- Clerget-Darpoux F., Bonaïti-Pellié C., Hochez J. Effects of misspecifying genetic parameters in lod score analysis. Biometrics. 1986 Jun;42(2):393–399. [PubMed] [Google Scholar]
- Demenais F., Le Merrer M., Briard M. L., Elston R. C. Neural tube defects in France: segregation analysis. Am J Med Genet. 1982 Mar;11(3):287–298. doi: 10.1002/ajmg.1320110305. [DOI] [PubMed] [Google Scholar]
- Edmonds L. D., James L. M. Temporal trends in the prevalence of congenital malformations at birth based on the birth defects monitoring program, United States, 1979-1987. MMWR CDC Surveill Summ. 1990 Dec;39(4):19–23. [PubMed] [Google Scholar]
- Farag T. I., Teebi A. S., Al-Awadi S. A. Nonsyndromal anencephaly: possible autosomal recessive variant. Am J Med Genet. 1986 Jul;24(3):461–464. doi: 10.1002/ajmg.1320240308. [DOI] [PubMed] [Google Scholar]
- Fellous M., Boué J., Malbrunot C., Wollman E., Sasportes M., Van Cong N., Marcelli A., Rebourcet R., Hubert C., Demenais F. A five-generation family with sacral agenesis and spina bifida: possible similarities with the mouse T-locus. Am J Med Genet. 1982 Aug;12(4):465–487. doi: 10.1002/ajmg.1320120410. [DOI] [PubMed] [Google Scholar]
- Fineman R. M., Jorde L. B., Martin R. A., Hasstedt S. J., Wing S. D., Walker M. L. Spinal dysraphia as an autosomal dominant defect in four families. Am J Med Genet. 1982 Aug;12(4):457–464. doi: 10.1002/ajmg.1320120409. [DOI] [PubMed] [Google Scholar]
- Goulding M. D., Chalepakis G., Deutsch U., Erselius J. R., Gruss P. Pax-3, a novel murine DNA binding protein expressed during early neurogenesis. EMBO J. 1991 May;10(5):1135–1147. doi: 10.1002/j.1460-2075.1991.tb08054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensson O., Arnason A., Gunnarsdottir H., Petursdottir I., Fossdal R., Hreidarsson S. A family showing apparent X linked inheritance of both anencephaly and spina bifida. J Med Genet. 1988 Apr;25(4):227–229. doi: 10.1136/jmg.25.4.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury M. J., Erickson J. D., James L. M. Etiologic heterogeneity of neural tube defects: clues from epidemiology. Am J Epidemiol. 1982 Apr;115(4):538–548. doi: 10.1093/oxfordjournals.aje.a113335. [DOI] [PubMed] [Google Scholar]
- LORBER J. THE FAMILY HISTORY OF SPINA BIFIDA CYSTICA. Pediatrics. 1965 Apr;35:589–595. [PubMed] [Google Scholar]
- Lathrop G. M., Lalouel J. M., Julier C., Ott J. Strategies for multilocus linkage analysis in humans. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3443–3446. doi: 10.1073/pnas.81.11.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariman E. C., Hamel B. C. Sex ratios of affected and transmitting members of multiple case families with neural tube defects. J Med Genet. 1992 Oct;29(10):695–698. doi: 10.1136/jmg.29.10.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moase C. E., Trasler D. G. Splotch locus mouse mutants: models for neural tube defects and Waardenburg syndrome type I in humans. J Med Genet. 1992 Mar;29(3):145–151. doi: 10.1136/jmg.29.3.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moline M. L., Sandlin C. Waardenburg syndrome and meningomyelocele. Am J Med Genet. 1993 Aug 1;47(1):126–126. doi: 10.1002/ajmg.1320470130. [DOI] [PubMed] [Google Scholar]
- Morell R., Friedman T. B., Moeljopawiro S., Hartono, Soewito, Asher J. H., Jr A frameshift mutation in the HuP2 paired domain of the probable human homolog of murine Pax-3 is responsible for Waardenburg syndrome type 1 in an Indonesian family. Hum Mol Genet. 1992 Jul;1(4):243–247. doi: 10.1093/hmg/1.4.243. [DOI] [PubMed] [Google Scholar]
- Nevin N. C., Johnston W. P. A family study of spina bifida and anencephalus in Belfast, Northern Ireland (1964 to 1968). J Med Genet. 1980 Jun;17(3):203–211. doi: 10.1136/jmg.17.3.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott J. Cutting a Gordian knot in the linkage analysis of complex human traits. Am J Hum Genet. 1990 Feb;46(2):219–221. [PMC free article] [PubMed] [Google Scholar]
- Risch N. Genetic linkage and complex diseases, with special reference to psychiatric disorders. Genet Epidemiol. 1990;7(1):3–45. doi: 10.1002/gepi.1370070103. [DOI] [PubMed] [Google Scholar]
- Seller M. J. Neural tube defects: are neurulation and canalization forms causally distinct? Am J Med Genet. 1990 Mar;35(3):394–396. doi: 10.1002/ajmg.1320350316. [DOI] [PubMed] [Google Scholar]
- Sever L. E. Spinal anomalies and neural tube defects. Am J Med Genet. 1983 Jun;15(2):343–345. doi: 10.1002/ajmg.1320150221. [DOI] [PubMed] [Google Scholar]
- Shaffer L. G., Marazita M. L., Bodurtha J., Newlin A., Nance W. E. Evidence for a major gene in familial anencephaly. Am J Med Genet. 1990 May;36(1):97–101. doi: 10.1002/ajmg.1320360119. [DOI] [PubMed] [Google Scholar]
- Stapleton P., Weith A., Urbánek P., Kozmik Z., Busslinger M. Chromosomal localization of seven PAX genes and cloning of a novel family member, PAX-9. Nat Genet. 1993 Apr;3(4):292–298. doi: 10.1038/ng0493-292. [DOI] [PubMed] [Google Scholar]
- Tassabehji M., Read A. P., Newton V. E., Harris R., Balling R., Gruss P., Strachan T. Waardenburg's syndrome patients have mutations in the human homologue of the Pax-3 paired box gene. Nature. 1992 Feb 13;355(6361):635–636. doi: 10.1038/355635a0. [DOI] [PubMed] [Google Scholar]
- Toriello H. V., Higgins J. V. Possible causal heterogeneity in spina bifida cystica. Am J Med Genet. 1985 May;21(1):13–20. doi: 10.1002/ajmg.1320210103. [DOI] [PubMed] [Google Scholar]
- Toriello H. V., Higgins J. V. X-linked midline defects. Am J Med Genet. 1985 May;21(1):143–146. doi: 10.1002/ajmg.1320210121. [DOI] [PubMed] [Google Scholar]
- Toriello H. V. Report of a third kindred with X-linked anencephaly/spina bifida. Am J Med Genet. 1984 Oct;19(2):411–412. doi: 10.1002/ajmg.1320190228. [DOI] [PubMed] [Google Scholar]
- Verheij J. B., Edens M., Cornel M. C., Groothoff J. W., ten Kate L. P. Incidentie en prevalentie van genetisch bepaalde aandoeningen in Nederland; een literatuuronderoek. Ned Tijdschr Geneeskd. 1994 Jan 8;138(2):71–77. [PubMed] [Google Scholar]
- Wilcox E. R., Rivolta M. N., Ploplis B., Potterf S. B., Fex J. The PAX3 gene is mapped to human chromosome 2 together with a highly informative CA dinucleotide repeat. Hum Mol Genet. 1992 Jun;1(3):215–215. doi: 10.1093/hmg/1.3.215-a. [DOI] [PubMed] [Google Scholar]


