Abstract
Protection of podocytes is one of the important means to delay the progression of diabetic nephropathy (DN), and glucagon-like peptide-1 (GLP-1) has been shown to have a protective effect on the kidney in DN models, but whether it has a protective effect on podocytes and the potential mechanisms of action remain largely unknown. In the present study, we established a type 2 diabetes mellitus (T2DM) mouse model by high-fat diet feeding combined with streptozotocin (STZ) induction and administered the intervention for 14 weeks. We found that liraglutide significantly ameliorated podocyte injury in DN mice. Mechanistically, we detected glucagon-like peptide-1 receptor (GLP-1R) protein expression levels in kidney tissues by immunohistochemical staining, immunofluorescence staining, and western blotting and found that podocytes could express GLP-1R and liraglutide treatment could restore GLP-1R expression in the kidney tissues of DN mice. Furthermore, we found that NLRP3-induced inflammation and pyroptosis were positively correlated with podocyte injury in DN mice, and liraglutide inhibited the expression of NLRP3-induced inflammation and pyroptosis-related proteins. Our results suggest that liraglutide protects DN mouse podocytes by regulating GLP-1R in renal tissues and by regulating NLRP3-induced inflammation and pyroptosis.
Keywords: liraglutide, diabetic nephropathy, podocytes, glucagon-like peptide-1 receptor, pyroptosis
Introduction
Diabetic nephropathy (DN) is one of the microvascular complications of diabetes, and according to the International Diabetes Federation, the number of people with diabetes is expected to exceed 780 million by 2045 (1). Diabetic nephropathy has become the leading cause of chronic kidney disease and end-stage renal disease (ESRD) worldwide in recent years, and with its high mortality rate, it is a major burden on health care worldwide (2). The classical albuminuric phenotype of diabetic nephropathy (DN) is mainly due to damage to glomerular podocytes (3). Podocytes, the highly specialized glomerular epithelial cells, form the glomerular basement membrane (GFB) together with the fenestrated endothelium and the glomerular basement membrane (3, 4, 5). Podocyte injury is an early event in the development of DN (6), and the loss and fusion of podocytes can be observed in the early stages of DN (7). Podocytes have a limited proliferative capacity, and the loss of more than 20% of podocytes represents an irreversible alteration in DN pathogenesis, leading to glomerular scar formation and ESRD progression (8, 9). In-depth investigation of the damage process of podocytes and protection of podocytes are important tools for the treatment of glomerular diseases and key targets for the prevention and treatment of glomerular diseases (10, 11).
Glucagon-like peptide-1 (GLP-1) is an endogenous entero-insulinotropic hormone whose main target of action is GLP-1 receptor (GLP-1R). GLP-1R is expressed in islet β-cells and several extrapancreatic tissues, including the small inlet arteries, proximal tubules, and collecting ducts of the kidney (12, 13). GLP-1R activity is significantly reduced in chronic kidney disease (14), and several studies have shown that GLP-1 has a protective effect on the kidney (15, 16, 17).
The mechanism of renal protection by GLP-1 analogs is not well understood. To further investigate the protective effect of liraglutide on podocytes in T2DM nephropathy in addition to its hypoglycemic effect, this study was conducted to establish a T2DM mouse model using a high-fat diet (HFD) combined with streptozotocin (STZ) induction and to investigate the protective effect of liraglutide on podocytes and its related molecular mechanism by detecting the differences in the expression levels of specific proteins and the pathological changes in the kidneys of these model mice. It is expected to provide a more theoretical basis for preventing the occurrence of albuminuria, delaying the progression of renal injury in T2DM patients, and providing a more theoretical basis for the rational clinical application of these drugs.
Materials and methods
Animals and group treatment
We purchased male-specific pathogen-free (SPF) C57BL/6J mice, aged 8 weeks, weighing 22–28 g from the Experimental Animal Center of Beijing Weitong Company and raised them in SPF conditions (Animal Quality Certificate No.: SCXK (Beijing) 2021-0006). These mice were alternately exposed to 45–55% humidity and light and darkness for 12 h in turn at a temperature of 18–22°C, with adequate food and water intake. All experiments performed were approved by the Ethical Committee of Experimental Animal Care of Yangtze University. The code is No.202301005.
After 1 week of adaptive feeding on a normal diet, 32 C57BL/6J male mice were divided into a normal diet group (14% fat) (NC, n = 8) and a HFD group (60% fat) (HFD, n = 24) using a simple random sampling method. After 4 weeks of different feedings, six mice in each group were randomly selected for glucose tolerance test. The mice were fasted but given adequate water for 12 h, glucose at a concentration of 20% (2 g/kg) was injected intraperitoneally, blood was taken from the tail vein, and blood glucose was measured by a glucometer at the corresponding time points of 0 min, 15 min, 30 min, 60 min, and 120 min. Then the HFD group was given a 1% STZ (S0130, Sigma) intraperitoneal injection (for 5 days of continuous administration, 50 mg/kg day dissolved in 0.1 M citrate buffer, pH 4.5). The NC group was injected with an equal amount of citrate buffer. After 7 days, the HFD group mice showed the symptoms of diabetes as polyuria, polydipsia, polyphagia, and weight loss, and the random blood glucose (GLU) levels were measured over 16.7 mmol/L by a blood glucose meter (Accu-Chek, Roche) for 2 consecutive days, which was considered T2DM mice (18). These T2DM mice were divided into the T2DM nephropathy model group (DN group, n = 8), liraglutide treatment group (DN + Lira group, n = 8) (Novo Nordisk, 400 μg/kg day, subcutaneous injection), and degludec insulin treatment group (DN + RI group, n = 8) (Novo Nordisk, 1–2 U/kg day, subcutaneous injection). The dosage of degludec insulin was adjusted according to the level of GLU. The NC and DN groups received daily subcutaneous injections of an equal volume of saline. All injections were completed at 18:00–19:00 daily.
24-h urinary protein determination and biochemical parameters
Tail-tip blood was collected regularly every week for GLU measurements in each group. At 8 weeks and 14 weeks after administration, each mouse was placed in a metabolic cage to collect 24-h urine for urinary protein quantification according to the method of the urinary protein test kit (C035-2-1, Nanjing Jiancheng, Nanjing, China). The mice were euthanized at the end of 14 weeks after administration, blood was collected from the heart, serum was centrifuged, and serum total cholesterol, creatinine, and urea nitrogen were examined by an automatic biochemical instrument (AU600, Olympus).
Pathomorphological observation of renal pathology
Mouse kidneys were fixed in 4% paraformaldehyde, dehydrated, paraffin-embedded, and made into sections (3 μm thick) for hematoxylin and eosin (H&E) (BA4025, Baso, Zhuhai, China) and periodic acid–Schiff (PAS) (BA4080A, Baso, China). Finally, the sections were sealed with glycerin gelatin (BA4348, Baso, China), and the results were observed under a microscope (DMI 8, Laica, Germany).
Ultrastructural changes under transmission electron microscope
Fresh intact renal cortex of 1 mm3 size was fixed in 2.5% glutaraldehyde, 4℃ for 48 h, dehydrated by acetone gradient, embedding, sectioning, lead citrate staining, uranyl acetate staining, sections were dried naturally and imaged and observed by transmission electron microscopy (HT7800).
Immunohistochemistry
Paraffin sections were dewaxed and hydrated, and thermal antigen repair was performed by antigen EDTA pH = 9.0 (G1203, Servicebio, China) according to the primary antibody instructions, hydrogen peroxide for 10 min to eliminate endogenous peroxidase, blocking, corresponding primary antibodies nephrin (1:1500, ab216341, Abcam), NPHS2 (1:2000, ab181143, Abcam), GLP-1R (1:250, ab218532, Abcam), overnight at 4°C, HRP-labeled goat anti-rabbit secondary antibody immunoglobulin G (IgG) (1:50, A0208, Beyotime, Shanghai, China) was incubated at room temperature for 1 h, DAB color (ZLI-9018, Zsbio, Beijing, China) development for 10 min, and hematoxylin for 10 s, followed by dehydration, transparency, blocking, and microscopic examination.
Immunofluorescence
Paraffin sections were dewaxed and hydrated, antigen repaired, exposed to hydrogen peroxide for 10 min, blocked, and incubated with corresponding primary antibodies against nephrin (1:500), GLP-1R (1:250), IL-1β (1:200), and NLRP3 (1:200) overnight at 4°C, FITC/CY3-labeled fluorescent secondary antibody (1:200) for 1 h. DAPI (MAO128, Meilunbio, China) stained nuclei for 5 min, the anti-fluorescence quencher was blocked, and the fluorescence microscopy images were captured.
Western blot
Mouse kidney tissues were lysed in the lysis solution containing RIPA and PMSF, ground in a tissue grinder, and then placed on ice for 30 min to obtain total protein. The supernatant was centrifuged and the protein concentration was determined by a BCA kit. The boiled 20–40 μg of total protein were separated by gel electrophoresis, transferred, and blocked, followed by the addition of corresponding primary antibodies nephrin (1:1000, ab216341, Abcam), NPHS2 (1:2500, ab181143, Abcam), IL-1β (1:1000, 12242, CST, USA), GSDMD (1:1000, 39754, CST, Danvers, MA, USA), cleaved GSDMD (1:1000, 10137, CST, USA), caspase-1 (1:1000, 24232, CST, USA), cleaved caspase-1 (1:1000, 89332, CST, USA), internal reference GAPDH (1:5000, 10494-1-AP, Proteintech, China), and β-actin (1:3000, 20536-1-AP, Proteintech, China) overnight at 4℃ in a shaking bed; 40 μg of total protein cooked at 70℃ were separated by gel electrophoresis, transferred to a membrane and blocked, followed by the addition of a primary antibody GLP-1R (1:1000, ab218532, Abcam) and an internal reference GAPDH (1:5000) overnight at 4℃ in a shaking bed. After washing, the secondary antibody (1:3000) was added and incubated in a shaker at room temperature, and after washing, ECL luminescence solution was added dropwise and exposed to a chemiluminescence imager for photography. The expression of the target proteins was analyzed by ImageJ software for comparison.
Statistical analysis
All data are presented as the mean ± s.e.m. Multiple group comparisons were assessed using one-way ANOVA Statistical analyses were performed using GraphPad Prism 8.0 software. P < 0.05 was considered statistically significant.
Results
High-fat diet induces insulin resistance
After 4 weeks of different feedings, compared with the NC group, the mice in HFD group showed significantly higher blood glucose levels at 0 min, 30 min, 60 min, 90 min, and 120 min after intraperitoneal injection of glucose (all P < 0.001) (Fig. 1A). The area under the glucose curve AUC in the HFD group was significantly increased compared with that in the NC group (P < 0.0001) (Fig. 1B). It indicated that the HFD induced insulin resistance successfully in the HFD group of mice.
Figure 1.
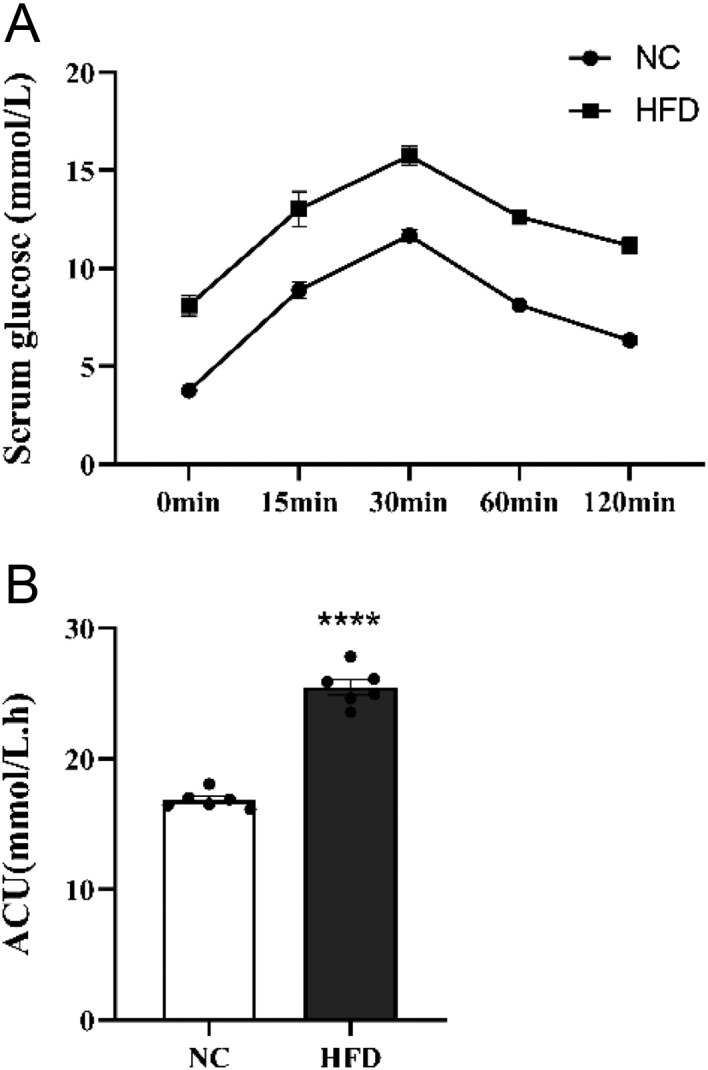
(A) IPGTT experiment result. (B) AUC. **** P < 0.0001 vs NC.
Liraglutide improves urinary protein in DN mice
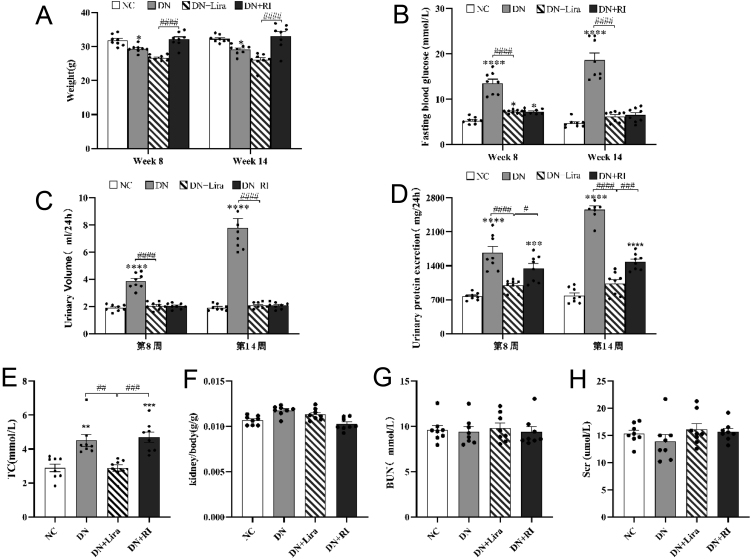
Compared with the NC group, the weight of mice in the DN group decreased at week 8 and week 14 after drug administration, which was consistent with the weight loss in type 2 diabetes models (T2DM) (P < 0.05). Compared with the DN + RI group, the body weight of the DN + Lira group decreased (P < 0.0001), indicating that liraglutide had a weight-reducing effect (Fig. 2A). Compared with the NC group, fasting blood glucose and 24-h urinary volume in the DN group were significantly increased at week 8 and week 14 after drug administration (both P < 0.0001), which was consistent with the characteristics of polyuria and hyperglycemia T2DM. But compared with the DN group, the above indexes were significantly decreased in the DN + Lira group (both P < 0.0001) (Fig. 2B and C). This suggests that both liraglutide and degludec insulin can improve hypermetabolic state and lower blood glucose. Compared with the NC group, the 24-h urinary protein in the DN and DN + RI groups was significantly increased at week 8 and week 14 after drug administration (both P < 0.0001). The presence of proteinuria suggests progression to DN, and the treatment with degludec fails to reduce urinary protein. Compared with the DN and DN + RI groups, the urinary protein in the DN + Lira group was decreased (both P < 0.05) (Fig. 2D). It suggests that liraglutide is more effective than degludec in reducing urinary protein in the progression of DN.3.3. Liraglutide lowers total cholesterol in DN mice.
Figure 2.
Physiological and biochemical index results of each group of mice: (A) body weight; (B) fasting blood glucose; (C) 24-h urine volume; (D) 24-h urinary protein; (E) total cholesterol; (F) kidney weight/body ratio; (G) serum urea nitrogen; and (H) serum creatinine. *P < 0.05, **P < 0.01, ***P <0.001, ****P <0.0001 vs NC; #P < 0.05, ##P < 0.01, ###P < 0.001, ####P < 0.0001 vs DN + Lira.
At week 14 after drug administration, total cholesterol (TC) was elevated in the DN and DN + RI groups compared with the NC group, consistent with the manifestation of hyperlipidemia in T2DM (both P < 0.01); total cholesterol (TC) was decreased in the DN + Lira group compared with the DN and DN + RI groups (both P < 0.01) (Fig. 2E). It shows that liraglutide has a hypolipidemic effect compared to degludec. The differences in serum creatinine (Scr), urea nitrogen (BUN), and the renal weight/body weight ratio were not statistically significant in each group of mice (Fig. 2F, G, and H).
Liraglutide attenuates pathological renal injury in DN mice
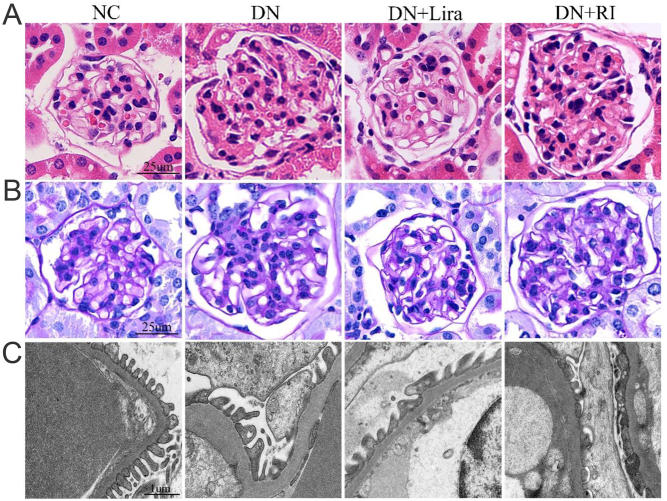
H&E staining and PAS staining showed that the glomerular thylakoid stroma was hyperplastic, and the glomerular volume was increased in the DN and DN + RI groups compared with the NC group; the above pathological changes were reduced in the DN + Lira group compared with the DN and DN + RI groups. The results of transmission electron microscopy showed that the mice in the DN and DN + RI groups showed a large fusion of podocyte peduncles, reduced number of peduncles, and thickening of glomerular basement membrane compared with the NC group; the mice in the DN + Lira group showed significantly improved fusion of podocyte peduncles, reduced number of peduncles, and thickening of glomerular basement membrane compared with the DN and DN + RI groups (Fig. 3A, B, and C). This suggests that treatment with liraglutide is more effective in delaying renal pathology compared to degludec insulin.
Figure 3.
Renal pathological injury in each group of mice: (A) H&E staining of mouse kidney tissues; (B) PAS staining of mouse kidney tissues; and (C) transmission microscopic observation of basement membrane thickness and pedicle width in mouse kidney cortex.
Liraglutide attenuates podocyte damage in DN mice
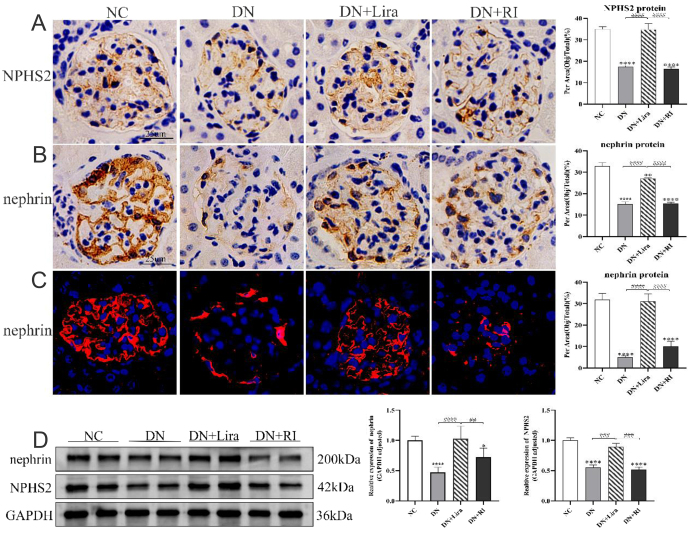
Immunohistochemical staining and semiquantitative analysis showed that the expression of podocyte marker proteins NPHS2 and nephrin decreased in the kidneys of DN and DN + RI mice compared with the NC group (both P < 0.0001); the expression of NPHS2 and nephrin increased in the DN + Lira group compared with the DN and DN + RI groups (both P < 0.0001) (Fig. 4A and B). Western blot results of NPHS2 and nephrin protein and immunofluorescence staining and semiquantitative analysis of nephrin protein showed the same trend (all P < 0.05) (Fig. 4C and D). NPHS2 and nephrin protein expression levels decreased in both the DN and DN + RI groups, indicating that the number and function of podocytes were decreased in T2DM and that insulin hypoglycemic intervention did not significantly improve podocyte damage. NPHS2 and nephrin protein increased in the DN + Lira group compared with both the DN and DN + RI groups, indicating that liraglutide treatment attenuated podocyte damage and had a protective effect on podocytes in T2DM. In contrast, no protection of podocytes was observed with degludec insulin treatment.
Figure 4.
Liraglutide attenuates podocyte injury in each group of mice. (A) Immunohistochemical observation and semi-quantitative analysis of mouse glomerular NPHS2 protein expression. (B) Immunohistochemical observation and semiquantitative analysis of mouse glomerular nephrin protein expression. (C) Immunofluorescence observation and semiquantitative analysis of mouse glomerular nephrin protein expression. (D) Western blot semiquantitative analysis of the protein expression levels of NPHS2 and nephrin in mouse kidneys. *P < 0.05, ****P <0.0001 vs NC; ##P < 0.01, ####P < 0.001, ####P < 0.0001 vs DN + Lira.
Liraglutide restores the reduced GLP-1R protein in the kidneys of DN mice
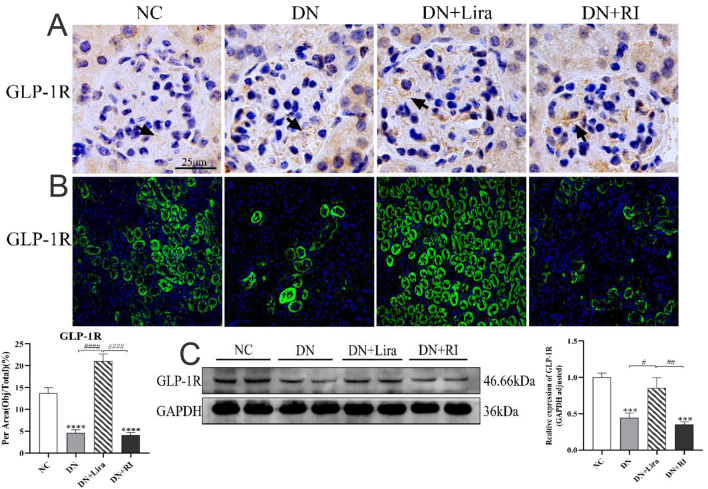
Immunohistochemical results showed that GLP-1R could be expressed in the glomerular and renal tubular epithelium (Fig. 5A). Immunofluorescence staining and semiquantitative analysis showed that the expression of GLP-1R protein in the kidney decreased in the DN and DN + RI groups compared with the NC group (both P <0.0001) and increased in the DN + Lira group compared with the DN and DN + RI groups (both P < 0.0001) (Fig. 5B). Western blot results also showed the same trend as immunofluorescence (all P < 0.05) (Fig. 5C).
Figure 5.
Changes in the expression site and amount of GLP-1R in the kidney of each group of mice. (A) Immunohistochemical method to observe the expression of GLP-1R protein in mouse glomeruli. (B) Immunofluorescence was used to observe and semi-quantitatively analyze the expression of GLP-1R protein in mouse kidney. (C) Western blot semiquantitative analyses of the expression level of GLP-1R protein in mouse kidneys. *** P < 0.001, ****P <0.0001 vs NC; #P < 0.05, ##P < 0.01, ####P < 0.0001 vs DN + Lira.
In the DN and DN + RI groups, we observed a decreasing trend in renal GLP-1R protein expression compared with the NC group, which verified that there was a reduction in renal GLP-1R protein expression in the T2DM mouse model. The hypoglycemic effect of Degu insulin does not reverse this change. In this experiment, we also found that liraglutide binds to GLP-1R expressed in renal tissues, and the level of GLP-1R protein expression in the kidneys of mice was significantly restored after its treatment.
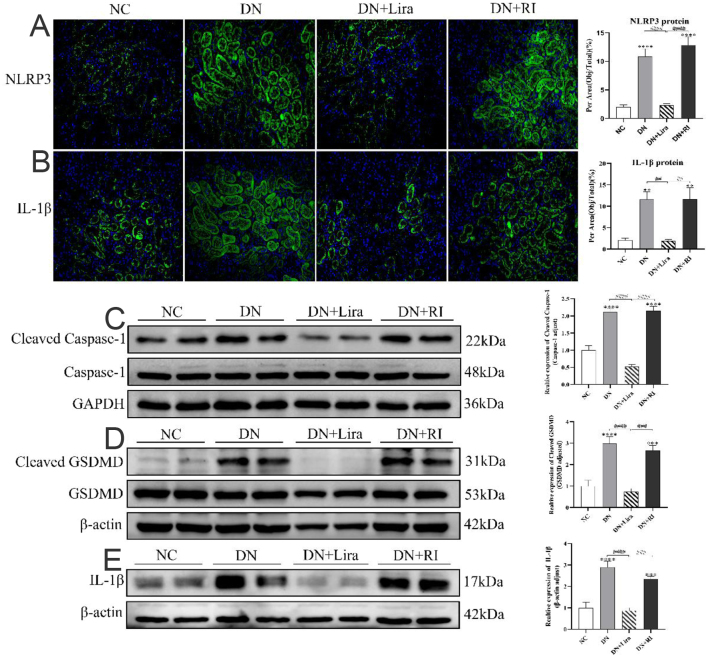
Liraglutide ameliorates renal injury through NLRP3-mediated inflammation and pyroptosis
Immunofluorescence staining and semiquantitative analysis showed that the expression of NLRP3 and IL-1β proteins in the kidneys of mice in the DN and DN + RI groups was significantly increased compared with that in the NC group (all P < 0.01). The expression of NLRP3 and IL-1β proteins in the kidneys of mice in the DN + Lira group was significantly decreased compared with that in the DN and DN + RI groups (both P < 0.01) (Fig. 6A and B). The western blot results of IL-1β, cleaved caspase-1, and cleaved GSDMD proteins showed the same trend (both P < 0.001) (Fig. 6C, D, and E).
Figure 6.
Expression of kidney inflammation and pyroptosis proteins in each group of mice. (A) Immunofluorescence observation and semiquantitative analysis of the expression level of mouse kidney NLRP3 protein. (B) Immunofluorescence observation and semiquantitative analysis of mouse kidney IL-1β protein expression. (C) Western blot semiquantitative analysis of mouse kidney caspase-1/cleaved caspase-1 protein expression. (D) Western blot semiquantitative analysis of mouse kidney GSDMD/cleaved GSDMD protein expression. (E) Western blot semiquantitative analysis of kidney IL-1β protein expression in mice. **P < 0.01, ***P < 0.001, ****P < 0.0001 vs NC; ##P < 0.01, ####P < 0.001, ####P < 0.0001 vs DN + Lira.
The expression levels of NLRP3, IL-1β, cleaved GSDMD, and cleaved caspase-1 proteins were increased in the DN and DN + RI groups, indicating that inflammation and pyroptosis were accompanied by the process of kidney injury in T2DM.The hypoglycemic effect of degludec insulin did not ameliorate these changes. The above indexes were decreased in the DN+Lira group compared with the DN and DN + RI groups, indicating that liraglutide reduced renal inflammation and pyroptosis in T2DM and had a protective effect on the kidney.
Discussion
In recent years, the treatment of diabetic nephropathy has focused on glycemic control and blood pressure management, but these measures have had little impact on the decrease in glomerular filtration rate and progression to ESRD (19). Liraglutide is a glucose-lowering agent approved for clinical use (20) and has received much attention in recent years for its protective effects in addition to its role in glycemic control (21). Several studies have shown that GLP-1RA attenuates proteinuria and delays the progression of renal injury in DN patients (22, 23). In the present study, by comparing the renal pathologic findings in mice from different administration treatment groups,we confirmed that liraglutide is effective in reducing kidney injury in the treatment of type 2 diabetic nephropathy compared to the pure hypoglycemic effect of insulin.
In the progression of DN, damage to podocytes is closely associated with proteinuria (24, 25). In the present experiment, we observed that liraglutide reduced proteinuria in DN mice, and combined with the transmission electron microscopy results of significantly improved pedicle fusion in DN+Lira group mice, we speculated that the protective effect of liraglutide on podocytes was one of the main mechanisms for its renal protective effect. In this study, we further detected the expression levels of kidney podocyte-specific proteins (NPHS2 and nephrin) in each group by immunohistochemistry, immunofluorescence and Western blotting, and the results showed that the expression levels of NPHS2 protein and nephrin protein in DN+Lira group mice were increased compared with those in the DN group and DN+RI group, which verified the protective effect of liraglutide on DN mouse podocytes This is consistent with the findings of previous study. This is consistent with the findings of previous studies (25, 26, 27). The same conclusion was reached in experiments related to mouse podocytes cultured in vitro (28). Because no improvement in renal injury was observed in the DN+RI group in this trial, we speculate that the renal protective effect of liraglutide is not related to its hypoglycemic effect or that there are additional multiple mechanisms of protection.
GLP-1R agonists exert protective effects on the kidney through various molecular mechanisms such as endothelial protection and antifibrosis (29). In an obesity-associated glomerular disease model, GLP-1 also exerts a protective effect on the kidney by promoting the translocation of Glut4 to the cell membrane and inhibiting cellular autophagy, thereby attenuating podocyte injury (30). While whether GLP-1R plays a role in the protection of renal podocytes by liraglutide has not been clearly verified (25, 29, 31). Given that the main acting receptor of liraglutide is GLP-1R and it can be expressed in the kidney, whether GLP-1R plays a role in the protection of renal podocytes by liraglutide is still a question worth exploring. In the present experiment, we observed the expression of GLP-1R protein in the kidney of each group of mice by immunohistochemistry and immunofluorescence staining and found that both glomerular podocytes and renal tubular epithelium of mice could express GLP-1R, which further improved the previous study (31, 32, 33). Therefore, we further speculated that the protective effect of liraglutide on podocytes might be related to the renal expression of GLP-1R. To further verify the above speculation, we analyzed the expression level of GLP-1R protein in mouse kidney tissues by immunofluorescence semiquantitative and western blot semiquantitative analysis. The experimental results all showed that the expression level of GLP-1R protein in renal tissues of mice in the DN group and DN + RI group was reduced compared with that of mice in the normal control group, while the expression level of GLP-1R protein in renal tissues was significantly restored after liraglutide treatment. Through this experiment, we found that the expression of GLP-1R protein in renal tissues was reduced by the DN environment, suggesting that the reduction of GLP-1R protein may be one of the mechanisms of action of renal injury in T2DM. And liraglutide can bind to GLP-1R expressed in renal tissues in DN and ameliorate this adverse effect of GLP-1R by the DN environment. Since we observed less GLP-1R protein expressed by podocytes in immunohistochemical staining results, we suggest that the protective effect of liraglutide on podocytes may be partly dependent on changes in GLP-1R expressed by podocytes, and there may also be a non-GLP-1R-dependent protective mechanism.
DN is considered an inflammatory disease, and several studies have shown that activation of inflammatory vesicles (NLRP3) and their mediated cellular scorching can promote the development of multiple complications of diabetes, including DN and diabetic neuropathy (34, 35, 36). NLRP3 induces cellular scorching, a specific form of inflammation-driven apoptosis (37). Activation of NLRP3 is observed in mouse models of diabetic nephropathy and in podocytes after angiotensin-II-aldosterone stimulation, and in these models, blockade of the pharmacological mechanism of NLRP3 ameliorates albuminuria and podocyte injury (38, 39, 40). Meanwhile, it has been demonstrated that podocyte injury also activates the NLRP3–ASC–caspase-1 axis, releasing mature IL-1β and IL-1 (41, 42, 43), and activates pro-caspase-1, which cleaves to activated caspase-1 (44) and cleaves GSDMD to GSDMD-C and GSDMD-N (i.e. cleaved-GSDMD in this paper) (45, 46), further causing cellular scorching (47) and accelerating the progression of DN. Therefore, we speculate that the protective effect of liraglutide on podocytes in the treatment of diabetic nephropathy may be related to the improvement of renal inflammation and cellular scorching. To verify this conjecture, this experiment further detected the expression of inflammation and cell scorching-related proteins in the kidney tissue of DN mice by Western blot and immunofluorescence assays, and the results showed that inflammation and cell scorching in the kidneys of mice were significantly improved after liraglutide treatment. On contrast, this phenomenon was not observed in the degludec insulin-treated group. This indicates that liraglutide can improve podocyte injury in diabetic nephropathy by regulating NLRP3-mediated cell scorching.
In this study, due to the lack of kidney biopsy samples from patients with diabetic nephropathy who only used liraglutide intervention, the conclusions of this experiment lack clinical data support, and clinical samples need to be collected at a later stage to further investigate the role of liraglutide in the damage of podocytes in diabetic nephropathy. In addition, whether the protective effect of liraglutide on podocytes is mediated through GLP-1R needs to be further verified by animal GLP-1R knockout models.
In conclusion, liraglutide can protect podocytes by regulating renal inflammation and cellular scorching, thereby reducing glomerular injury in diabetic nephropathy mice, decreasing proteinuria production, and delaying the progression of diabetic nephropathy to end-stage renal disease. This protective effect may be partly related to GLP-1R expressed by podocytes.
Declaration of interest
The authors declare no conflict of interest.
Funding
This work was supported by Jingzhou Science and Technology Project (2022HC46), Innovation Fund Project of Yangyze University of Medical Department (202206).
Author contribution statement
TM conceived the project; SS and XC designed the experiments; SS performed all experiments, data collection, and data analysis; WY and XK prepared the reagents and collected the samples; TM and XC supervised the study; SS and XC wrote the manuscript.
References
- 1.Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, Stein C, Basit A, Chan JCN, Mbanya JC, et al. IDF Diabetes Atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Research and Clinical Practice 2022183109119. ( 10.1016/j.diabres.2021.109119) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dong R Yu JL Yu FX Yang S Qian Q & Zha Y. IGF-1/IGF-1R blockade ameliorates diabetic kidney disease through normalizing Snail 1 expression in a mouse model. American Journal of Physiology-Endocrinology and Metabolism 2019317E686–E698. ( 10.1152/ajpendo.00071.2019) [DOI] [PubMed] [Google Scholar]
- 3.Barutta F Bellini S & Gruden G. Mechanisms of podocyte injury and implications for diabetic nephropathy. Clinical Science 2022136493–520. ( 10.1042/CS20210625) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scott RP & Quaggin SE. Review series: the cell biology of renal filtration. Journal of Cell Biology 2015209199–210. ( 10.1083/jcb.201410017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garg P. A review of podocyte biology. American Journal of Nephrology 201847(Supplement 1) 3–13. ( 10.1159/000481633) [DOI] [PubMed] [Google Scholar]
- 6.Mohandes S Doke T Hu H Mukhi D Dhillon P & Susztak K. Molecular pathways that drive diabetic kidney disease. Journal of Clinical Investigation 2023133. ( 10.1172/JCI165654) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meyer TW Bennett PH & Nelson RG. Podocyte number predicts long-term urinary albumin excretion in Pima Indians with Type II diabetes and microalbuminuria. Diabetologia 1999421341–1344. ( 10.1007/s001250051447) [DOI] [PubMed] [Google Scholar]
- 8.Li J, Zheng S, Ma C, Chen X, Li X, Li S, Wang P, Chen P, Wang Z, Li W, et al. Research progress on exosomes in podocyte injury associated with diabetic kidney disease. Frontiers in Endocrinology 2023141129884. ( 10.3389/fendo.2023.1129884) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mukhi D & Susztak K. The transcriptomic signature of the aging podocyte. Kidney International 2020981079–1081. ( 10.1016/j.kint.2020.08.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Du P, Fan B, Han H, Zhen J, Shang J, Wang X, Li X, Shi W, Tang W, Bao C, et al. NOD2 promotes renal injury by exacerbating inflammation and podocyte insulin resistance in diabetic nephropathy. Kidney International 201384265–276. ( 10.1038/ki.2013.113) [DOI] [PubMed] [Google Scholar]
- 11.Li J, Niu J, Min W, Ai J, Lin X, Miao J, Zhou S, Liang Y, Chen S, Ren Q, et al. B7-1 mediates podocyte injury and glomerulosclerosis through communication with Hsp90ab1-LRP5-β-catenin pathway. Cell Death and Differentiation 2022292399–2416. ( 10.1038/s41418-022-01026-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muskiet MHA Smits MM Morsink LM & Diamant M. The gut-renal axis: do incretin-based agents confer renoprotection in diabetes? Nature Reviews. Nephrology 20141088–103. ( 10.1038/nrneph.2013.272) [DOI] [PubMed] [Google Scholar]
- 13.Korner M Stockli M Waser B & Reubi JC. GLP-1 receptor expression in human tumors and human normal tissues: potential for in vivo targeting. Journal of Nuclear Medicine 200748736–743. ( 10.2967/jnumed.106.038679) [DOI] [PubMed] [Google Scholar]
- 14.Kim SJ Kwon SK Kim HY Kim SM Bae JW & Choi JK. DPP-4 inhibition enhanced renal tubular and myocardial GLP-1 receptor expression decreased in CKD with myocardial infarction. BMC Nephrology 20192075. ( 10.1186/s12882-019-1243-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kodera R, Shikata K, Kataoka HU, Takatsuka T, Miyamoto S, Sasaki M, Kajitani N, Nishishita S, Sarai K, Hirota D, et al. Glucagon-like peptide-1 receptor agonist ameliorates renal injury through its anti-inflammatory action without lowering blood glucose level in a rat model of type 1 diabetes. Diabetologia 201154965–978. ( 10.1007/s00125-010-2028-x) [DOI] [PubMed] [Google Scholar]
- 16.Yang Q, Lang Y, Yang W, Yang F, Yang J, Wu Y, Xiao X, Qin C, Zou Y, Zhao Y, et al. Efficacy and safety of drugs for people with type 2 diabetes mellitus and chronic kidney disease on kidney and cardiovascular outcomes: a systematic review and network meta-analysis of randomized controlled trials. Diabetes Research and Clinical Practice 2023198110592. ( 10.1016/j.diabres.2023.110592) [DOI] [PubMed] [Google Scholar]
- 17.Moellmann J, Klinkhammer BM, Onstein J, Stohr R, Jankowski V, Jankowski J, Lebherz C, Tacke F, Marx N, Boor P, et al. Glucagon-like peptide 1 and its cleavage products are renoprotective in murine diabetic nephropathy. Diabetes 2018672410–2419. ( 10.2337/db17-1212) [DOI] [PubMed] [Google Scholar]
- 18.Li D Jiang C Mei G Zhao Y Chen L Liu J Tang Y Gao C & Yao P. Quercetin Alleviates Ferroptosis of Pancreatic beta Cells in Type 2 Diabetes. Nutrients 202012. ( 10.3390/nu12102954) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Forst T Mathieu C Giorgino F Wheeler DC Papanas N Schmieder RE Halabi A Schnell O Streckbein M & Tuttle KR. New strategies to improve clinical outcomes for diabetic kidney disease. BMC Medicine 202220337. ( 10.1186/s12916-022-02539-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burcelin R & Gourdy P. Harnessing glucagon-like peptide-1 receptor agonists for the pharmacological treatment of overweight and obesity. Obesity Reviews 20171886–98. ( 10.1111/obr.12465) [DOI] [PubMed] [Google Scholar]
- 21.Bendotti G, Montefusco L, Lunati ME, Usuelli V, Pastore I, Lazzaroni E, Assi E, Seelam AJ, El Essawy B, Jang J, et al. The anti-inflammatory and immunological properties of GLP-1 Receptor agonists. Pharmacological Research 2022182106320. ( 10.1016/j.phrs.2022.106320) [DOI] [PubMed] [Google Scholar]
- 22.Gerstein HC, Sattar N, Rosenstock J, Ramasundarahettige C, Pratley R, Lopes RD, Lam CSP, Khurmi NS, Heenan L, Del Prato S, et al. Cardiovascular and renal outcomes with Efpeglenatide in Type 2 diabetes. New England Journal of Medicine 2021385896–907. ( 10.1056/NEJMoa2108269) [DOI] [PubMed] [Google Scholar]
- 23.Tuttle KR Lakshmanan MC Rayner B Busch RS Zimmermann AG Woodward DB & Botros FT. Dulaglutide versus insulin glargine in patients with type 2 diabetes and moderate-to-severe chronic kidney disease (AWARD-7): a multicentre, open-label, randomised trial. Lancet. Diabetes and Endocrinology 20186605–617. ( 10.1016/S2213-8587(1830104-9) [DOI] [PubMed] [Google Scholar]
- 24.Kravets I & Mallipattu SK. The role of podocytes and podocyte-associated biomarkers in diagnosis and treatment of diabetic kidney disease. Journal of the Endocrine Society 20204bvaa029. ( 10.1210/jendso/bvaa029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dalbøge L, Christensen M, Madsen M, Secher T, Endlich N, Drenic' V, Manresa-Arraut A, Hansen H, Rune I, Fink L, et al. Nephroprotective effects of semaglutide as mono- and combination treatment with Lisinopril in a mouse model of hypertension-accelerated diabetic kidney disease. Biomedicines 2022101661. ( 10.3390/biomedicines10071661) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang J Zhou Y Long D Wu Y & Liu F. GLP-1 receptor agonist, liraglutide, protects podocytes from apoptosis in diabetic nephropathy by promoting white fat browning. Biochemical and Biophysical Research Communications 2023664142–151. ( 10.1016/j.bbrc.2023.04.012) [DOI] [PubMed] [Google Scholar]
- 27.Ye Y Zhong X Li N & Pan T. Protective effects of liraglutide on glomerular podocytes in obese mice by inhibiting the inflammatory factor TNF-α-mediated NF-κB and MAPK pathway. Obesity Research and Clinical Practice 201913385–390. ( 10.1016/j.orcp.2019.03.003) [DOI] [PubMed] [Google Scholar]
- 28.Shi JX & Huang Q. Glucagonlike peptide1 protects mouse podocytes against high glucoseinduced apoptosis, and suppresses reactive oxygen species production and proinflammatory cytokine secretion, through sirtuin 1 activation in vitro. Molecular Medicine Reports 2018181789–1797. ( 10.3892/mmr.2018.9085) [DOI] [PubMed] [Google Scholar]
- 29.Yaribeygi H Atkin SL Montecucco F Jamialahmadi T & Sahebkar A. Renoprotective effects of incretin-based therapy in diabetes mellitus. BioMed Research International 202120218163153. ( 10.1155/2021/8163153) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo H Wang B Li H Ling L Niu J & Gu Y. Glucagon-like peptide-1 analog prevents obesity-related glomerulopathy by inhibiting excessive autophagy in podocytes. American Journal of Physiology. Renal Physiology 2018314F181–F189. ( 10.1152/ajprenal.00302.2017) [DOI] [PubMed] [Google Scholar]
- 31.Barrera-Chimal J & Jaisser F. Pathophysiologic mechanisms in diabetic kidney disease: a focus on current and future therapeutic targets. Diabetes, Obesity and Metabolism 202022(Supplement 1) 16–31. ( 10.1111/dom.13969) [DOI] [PubMed] [Google Scholar]
- 32.Choi JH Kim SJ Kwon SK Kim HY & Jeon H. Renal tubular glucagon-like Peptide-1 receptor expression is increased in early sepsis but reduced in chronic kidney disease and sepsis-induced kidney injury. International Journal of Molecular Sciences 201920. ( 10.3390/ijms20236024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nistala R, Habibi J, Aroor A, Sowers JR, Hayden MR, Meuth A, Knight W, Hancock T, Klein T, DeMarco VG, et al. DPP4 inhibition attenuates filtration barrier injury and oxidant stress in the Zucker obese rat. Obesity 2014222172–2179. ( 10.1002/oby.20833) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ding H Li J Li Y Yang M Nie S Zhou M Zhou Z Yang X Liu Y & Hou FF. MicroRNA-10 negatively regulates inflammation in diabetic kidney via targeting activation of the NLRP3 inflammasome. Molecular Therapy 2021292308–2320. ( 10.1016/j.ymthe.2021.03.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Al Mamun A Ara Mimi A Wu Y Zaeem M Abdul Aziz M Aktar SS Alyafeai E Munir F & Xiao J. Pyroptosis in diabetic nephropathy. Clinica Chimica Acta 2021523131–143. ( 10.1016/j.cca.2021.09.003) [DOI] [PubMed] [Google Scholar]
- 36.Zheng S, Zhang K, Zhang Y, He J, Ouyang Y, Lang R, Ao C, Jiang Y, Xiao H, Li Y, et al. Human umbilical cord mesenchymal stem cells inhibit pyroptosis of renal tubular epithelial cells through miR-342-3p/Caspase1 signaling pathway in diabetic nephropathy. Stem Cells International 202320235584894. ( 10.1155/2023/5584894) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu P Zhang ZD & Li Y. Relevance of the pyroptosis-related inflammasome pathway in the pathogenesis of diabetic kidney disease. Frontiers in Immunology 202112603416. ( 10.3389/fimmu.2021.603416) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao M Bai M Ding GX Zhang Y Huang SM Jia ZJ & Zhang AH. Angiotensin II stimulates the NLRP3 inflammasome to induce podocyte injury and mitochondrial dysfunction. Kidney Diseases 2018483–94. ( 10.1159/000488242) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiang MZ Zhao M Bai M Lei J Yuan YG Huang SM Zhang Y Ding GX Jia ZJ & Zhang AH. SIRT1 alleviates aldosterone-induced podocyte injury by suppressing mitochondrial dysfunction and NLRP3 inflammasome activation. Kidney Diseases 20217293–305. ( 10.1159/000513884) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shahzad K Fatima S Khawaja H Elwakiel A Gadi I Ambreen S Zimmermann S Mertens PR Biemann R & Isermann B. Podocyte-specific Nlrp3 inflammasome activation promotes diabetic kidney disease. Kidney International 2022102766–779. ( 10.1016/j.kint.2022.06.010) [DOI] [PubMed] [Google Scholar]
- 41.Komada T & Muruve DA. The role of inflammasomes in kidney disease. Nature Reviews. Nephrology 201915501–520. ( 10.1038/s41581-019-0158-z) [DOI] [PubMed] [Google Scholar]
- 42.Swanson KV Deng M & Ting JPY. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nature Reviews. Immunology 201919477–489. ( 10.1038/s41577-019-0165-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu J & Nunez G. The NLRP3 inflammasome: activation and regulation. Trends in Biochemical Sciences 202348331–344. ( 10.1016/j.tibs.2022.10.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen X He WT Hu LC Li JX Fang Y Wang X Xu XZ Wang Z Huang K & Han JH. Pyroptosis is driven by non-selective gasdermin-D pore and its morphology is different from MLKL channel-mediated necroptosis. Cell Research 2016261007–1020. ( 10.1038/cr.2016.100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fu JN & Wu H. Structural mechanisms of NLRP3 inflammasome assembly and activation. Annual Review of Immunology 202341301–316. ( 10.1146/annurev-immunol-081022-021207) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Burdette BE Esparza AN Zhu H & Wang S. Gasdermin D in pyroptosis. Acta Pharmaceutica Sinica. B 2021112768–2782. ( 10.1016/j.apsb.2021.02.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li W Sun J Zhou X Lu Y Cui W & Miao L. Mini-review: GSDME-mediated pyroptosis in diabetic nephropathy. Frontiers in Pharmacology 202112780790. ( 10.3389/fphar.2021.780790) [DOI] [PMC free article] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a