Abstract
Pulmonary microvascular endothelial cells contribute to the integrity of the lung gas exchange interface, and they are highly glycolytic. Although glucose and fructose represent discrete substrates available for glycolysis, pulmonary microvascular endothelial cells prefer glucose over fructose, and the mechanisms involved in this selection are unknown. 6-Phosphofructo-2-kinase/fructose-2, 6-bisphosphatase 3 (PFKFB3) is an important glycolytic enzyme that drives glycolytic flux against negative feedback and links glycolytic and fructolytic pathways. We hypothesized that PFKFB3 inhibits fructose metabolism in pulmonary microvascular endothelial cells. We found that PFKFB3 knockout cells survive better than wild-type cells in fructose-rich medium under hypoxia. Seahorse assays, lactate and glucose measurements, and stable isotope tracing showed that PFKFB3 inhibits fructose–hexokinase–mediated glycolysis and oxidative phosphorylation. Microarray analysis revealed that fructose upregulates PFKFB3, and PFKFB3 knockout cells increase fructose-specific GLUT5 (glucose transporter 5) expression. Using conditional endothelial-specific PFKFB3 knockout mice, we demonstrated that endothelial PFKFB3 knockout increases lung tissue lactate production after fructose gavage. Last, we showed that pneumonia increases fructose in BAL fluid in mechanically ventilated ICU patients. Thus, PFKFB3 knockout increases GLUT5 expression and the hexokinase-mediated fructose use in pulmonary microvascular endothelial cells that promotes their survival. Our findings indicate that PFKFB3 is a molecular switch that controls glucose versus fructose use in glycolysis and help better understand lung endothelial cell metabolism during respiratory failure.
Keywords: GLUT5 (SLC2A5), hexokinase, ketohexokinase, hypoxia, 2-deoxyglucose
Fructose metabolism has been an important evolutionary strategy used by animals to evade extinction (1, 2). Fructose increases glycolytic flux, which enables the rapid production of energy and carbon sources for cell proliferation while promoting the storage of fat, glycogen, and water, which can be used under challenging environmental conditions. Naked mole rats, which are mouse-sized rodents that live underground in a hypoxic environment (3), uniquely rewire their metabolic pathways to use fructose to fuel anaerobic glycolysis to tolerate extreme degrees of hypoxia that are generally lethal to laboratory mice (4, 5). It is unknown whether fructose metabolism can be used by other rodents that normally reside in a normoxic environment to better endure hypoxic stresses and, if not, whether it is because their fructose use is suppressed by a molecular regulator. Elucidating this question may help better understand metabolic shifts that occur during human hypoxic respiratory failure and other diseases that induce tissue-level microenvironmental hypoxia and potentially identify therapeutic targets.
Despite the significant link between fructose metabolism and hypoxia (6), little is known regarding whether and how the lungs use fructose to meet their metabolic demands. The lungs are highly glycolytic at baseline, with at least 60% of glucose being converted to lactate (6), and glucose is used by the lungs at a 20-fold higher rate than is fructose (7). In isolated and ventilated rat lungs, fructose is metabolized mainly to fructose 1-phosphate by ketohexokinase (KHK; fructokinase) (Figure 1A) and incorporated into fatty acids and phospholipids rather than being converted to lactate (8). Although the role of fructose in lung metabolism seems limited at baseline, some transformed lung cells use fructose as an alternative fuel for glycolysis to promote their growth via upregulation of the GLUT5 (glucose transporter 5) (SLC2A5 [solute carrier family 2 member 5]) fructose transporter (9). Thus, it is possible that lung cells use fructose metabolism under pathologic conditions. Furthermore, although shifts in blood glucose and insulin concentrations do not affect KHK activity, they sensitively alter the activity of hexokinase (HK), the first rate-limiting enzyme of glycolysis. These findings suggest that fructose may be a stable alternative energy source and signaling mediator of the lungs, especially under pathologic conditions that alter metabolic homeostasis, such as hypoxia.
Figure 1.
6-Phosphofructo-2-kinase/fructose-2, 6-bisphosphatase 3 (PFKFB3) decreased PMVEC survival in a fructose-rich environment. (A) Schematic representation of our hypothesis. Fructose can be converted either to F-1-P, catalyzed by KHK, or to fructose 6-phosphate (F-6-P), mediated by hexokinase (HK). PFKFB3 regulates the conversion from F-6-P to fructose 2,6-bisphosphate, which directly links the glycolysis and fructolysis pathways. We hypothesized that PFKFB3 suppresses fructose–HK–mediated glycolysis in PMVECs. (B) PFKFB3 knockout (KO) PMVECs were generated using the CRISPR–Cas9 technique. Western blot analysis showed nondetectable PFKFB3 protein in PFKFB3 KO cell lysate, indicating successful deletion of the target gene. (C–F) Survival assays were performed on wild-type and PFKFB3 KO PMVECs. (C and D) ATP concentrations (C) and BrdU incorporation (D) were lower in wild-type cells in fructose medium under normoxia and hypoxia, indicating decreased cell viability and proliferation, respectively. (E and F) Propidium iodide (E) and annexin V (F) staining intensities were higher in wild-type cells in fructose medium under hypoxia, indicating increased necrosis and apoptosis in these cells, respectively. (G) Representative images of propidium iodide and annexin V double-stained cells are shown. At least four independent experiments were performed. Three-way ANOVA and Šidák’s multiple-comparisons test were used to compare different groups. Scale bars, 170 µm. *Significant difference (P < 0.05). Data are expressed as mean ± SD. 2DG = 2-deoxyglucose; BrdU = bromodeoxyuridine; Cas9 = CRISPR-associated protein 9; DHAP = dihydroxyacetone phosphate; EX/EM = excitation/emission; F-1,6-P2 = fructose 1,6-bisphosphate; F-1-P = fructose 1-phosphate; F-2,6-P2 = fructose 2,6-bisphosphate; G3P = glycerol 3-phosphate; GA = glyceraldehyde; GA3P = glyceraldehyde 3-phosphate; Glucose 6-P = glucose 6-phosphate; hypox. = hypoxia; KHK = ketohexokinase; normox. = normoxia; ns = not significant; OXPHOS = oxidative phosphorylation; PFK = phosphofructokinase; PMVEC = pulmonary microvascular endothelial cell; TxRED = Texas red; WT = wild-type.
Pulmonary microvascular endothelial cells (PMVECs) are at the forefront of the gas exchange interface, and they depend primarily on aerobic glycolysis (10, 11). Under hypoxic conditions, endothelial cells upregulate the expression of a series of glycolytic enzymes, including 6-phosphofructo-2-kinase/fructose-2, 6-bisphosphatase 3 (PFKFB3) (12). Proteins of the PFKFB family possess two mutually exclusive catalytic domains, kinase and phosphatase domains (13), and the PFKFB3 isoform exhibits a higher kinase:phosphatase activity ratio than other PFKFB isoforms (12, 14). PFKFB3 facilitates the conversion of fructose 6-phosphate, the third major substrate in the glycolysis pathway, to fructose-2,6-bisphosphate (F-2,6-P2) (Figure 1A). F-2,6-P2 is a potent allosteric activator of 6-phosphofructo-1-kinase (PFK-1), the rate-limiting glycolytic enzyme that is negatively controlled by ATP (15). PFKFB3 kinase activity increases F-2,6-P2 production and enhances PFK-1 activity, overriding negative regulation of PFK-1 by excessive ATP; PFKFB3 exerts a powerful influence on glycolytic flux. These actions of PFKFB3 influence adaptive and maladaptive vascular remodeling. PFKFB3 promotes angiogenesis (12) and mediates pulmonary arterial vasculopathy (16) and cardiac remodeling (17) in pulmonary arterial hypertension.
Although PFKFB3 regulates fructose 6-phosphate, which is a direct link to the fructose pathway, limited studies have investigated the significance of this link, probably because HK affinity for glucose is significantly higher than it is for fructose (18, 19). Yet in an interesting zebrafish study, investigators reported that angiogenesis was impaired in PFKFB3 knockout fish, an effect that could be rescued by fructose but not by glucose (20). Altogether, these findings indicate that PFKFB3 may have a direct regulatory role on fructose metabolism in endothelial cells, especially upon exposure to hypoxia. Therefore, we hypothesized that PFKFB3 serves as a molecular switch in PMVECs, inhibiting fructose metabolism while promoting glucose metabolism.
Methods
PMVEC Isolation and Generation of PFKFB3-depleted Cells
All procedures were approved by the University of South Alabama Institutional Animal Care and Use Committee. PMVECs were isolated from a Sprague-Dawley rat (11). PFKFB3 knockout PMVECs were generated using the CRISPR–Cas9 (CRISPR-associated protein 9) technique (21, 22). PFKFB3 knockout was evaluated using western blot (21).
Survival Assays
PMVECs were grown to confluence, then washed, and medium containing glucose or fructose (with or without bromodeoxyuridine [BrdU]) was loaded. Cells were incubated in normoxia (21% O2) or hypoxia (1% O2) with 5% CO2 for 24 or 48 hours. ATP, BrdU incorporation, propidium iodide staining, annexin V staining, and Hoechst staining were performed.
Real-Time Metabolic Analysis
Extracellular acidification rate (ECAR) and oxygen consumption rate (OCR) were measured on PMVECs using a Seahorse (Agilent) extracellular flux analyzer.
Lactate and Glucose Measurement
Cells were grown to confluence, then medium was replaced with medium containing different concentrations of glucose and fructose with or without 2 deoxyglucose (2DG) and were incubated in normoxia or hypoxia for 24, 48, or 96 hours. Supernatant or lysate lactate and glucose concentrations were measured.
Ultrahigh-Pressure Liquid Chromatography–Mass Spectrometry–based Metabolomics Analyses
PMVECs were grown to confluence in unlabeled glucose medium. Cells were rinsed and treated with medium containing labeled D-glucose (U-13C) or D-fructose (U-13C) and incubated under normoxia or hypoxia for 18 hours. Cell supernatant and pellets were shipped to the University of Colorado School of Medicine Metabolomics Facility. Metabolites from cell pellets and supernatant were measured and analyzed as described previously (23, 24).
RNA Sequencing and Confirmatory Quantitative PCR
PMVECs were treated with glucose or fructose medium under normoxia or hypoxia. Twenty-four hours later, RNA was isolated. RNA samples were processed and analyzed by Novogene. Quantitative real-time PCR was performed by us (25).
Mouse Fructose Challenge
PFKFB3fl/fl;Cdh5(PAC)-CreERT2 mice were used. One week after the last dose of tamoxifen, animals were gavaged with fructose. Two hours later, lactate was measured in the blood and lung tissue lysate.
Human BAL Fluid
Patients were enrolled from the ICU at the University of Alabama at Birmingham Hospital. All studies were approved by the Institutional Review Boards of the University of Alabama at Birmingham Hospital. BAL fluid (BALF) fructose, lactate, and glucose were measured in mechanically ventilated patients anytime within 14 days of admission.
Statistics for Data Other Than RNA Sequencing Analysis
Student’s t tests, one-way ANOVA and Bonferroni post hoc tests, and two-way or three-way ANOVA and Šidák’s multiple-comparisons test were used as indicated. Significance was considered at P < 0.05.
See the data supplement for further details.
Results
PFKFB3 Decreased PMVEC Survival in a Hypoxic Fructose-Rich Environment
To study the role of PFKFB3 in fructose metabolism, we first generated PFKFB3 knockout PMVECs using the CRISPR–Cas9 technique. Successful deletion of the PFKFB3 gene was confirmed using western blot (Figure 1B). To identify whether PFKFB3 contributes to cellular ATP concentration, proliferation, and/or apoptosis, we incubated wild-type and PFKFB3 knockout cells in either glucose or fructose medium under either normoxic or hypoxic conditions (1% O2 with 5% CO2). Two days after glucose or fructose treatment, cells exhibited visible differences in their viability, concomitant with measurements of ATP, BrdU incorporation, propidium iodide staining, and annexin V staining. In glucose medium, there were no differences in ATP concentrations (Figure 1C), BrdU incorporation (Figure 1D), and propidium iodide (Figure 1E) and annexin V (Figure 1F) staining intensity between wild-type and PFKFB3 knockout cells under normoxia and hypoxia. In contrast, in fructose medium, ATP concentrations (Figure 1C) and BrdU incorporation (Figure 1D) were lower in wild-type cells compared with PFKFB3 knockout cells, indicating an inhibitory effect of fructose on wild-type cell viability and proliferation, respectively, under normoxia and hypoxia. There was a significant difference between wild-type and PFKFB3 knockout cells in ATP concentrations (Figure 1C) but not in BrdU incorporation (Figure 1D) in fructose medium. Fructose medium increased wild-type cell necrosis and apoptosis under hypoxia, but not under normoxia, as assessed by increased propidium iodide (Figure 1E and G) and annexin V (Figure 1F and G) staining, respectively, in wild-type cells under hypoxia. PFKFB3 knockout cells demonstrated an unchanged phenotype with preserved survival under hypoxia. These findings indicate that PFKFB3 inhibits PMVEC survival in a hypoxic fructose-rich environment.
Seahorse Assays and Lactate and Glucose Measurements Suggested That PFKFB3 Decreases Fructose–HK–mediated Glycolysis in PMVECs
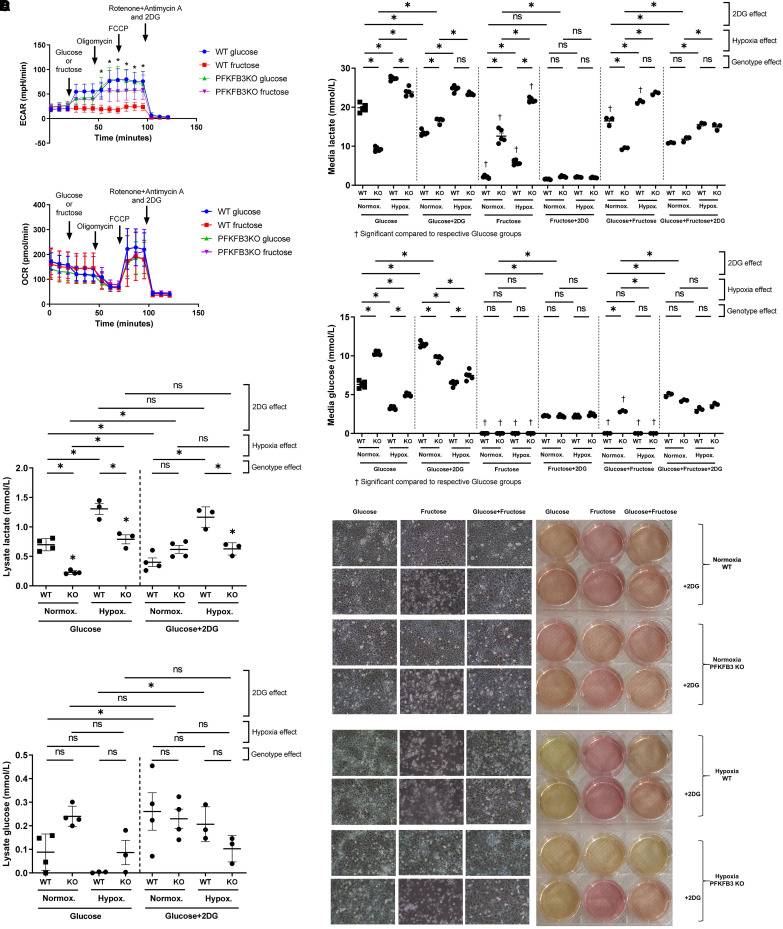
To further characterize the metabolic profile that promotes survival differences among wild-type and knockout cells in fructose medium, we exposed wild-type and PFKFB3 knockout cells to glucose or fructose medium, followed by sequential introduction of glycolytic and mitochondrial stressors, while monitoring real-time ECAR and OCR as surrogate markers for the degree of glycolysis and oxidative phosphorylation, respectively. Upon glucose challenge, both wild-type and PFKFB3 knockout cells had increased ECAR (Figure 2A). However, in response to fructose loading, wild-type cells showed no change in ECAR, whereas PFKFB3 knockout cells had increased ECAR, to the same degree as was seen with glucose exposure. Interestingly, both wild-type and PFKFB3 knockout cells showed dynamic OCR changes in response to mitochondrial stressors, as expected. These findings indicate that the inhibitory effect of PFKFB3 on fructose metabolism starts within 30 minutes of fructose exposure, and it affects primarily the glycolytic pathway rather than oxidative phosphorylation during this acute phase.
Figure 2.
Seahorse assays and lactate and glucose measurements suggested that PFKFB3 decreases fructose–hexokinase–mediated glycolysis in PMVECs. (A) A Seahorse extracellular flux analyzer was used to measure real-time fructose use by wild-type and PFKFB3 knockout PMVECs. Cells were sequentially challenged by glycolytic and mitochondrial stressors, including glucose (25 mM) or fructose (25 mM), oligomycin (1 μM), FCCP (1 μM), and a combination of rotenone (1 μM), antimycin A (1 μM), and 2DG (100 mM). Glucose-induced increases in extracellular acidification rates (ECARs), a surrogate for aerobic glycolysis, in both wild-type and PFKFB3 knockout cells. Fructose did not change ECAR in wild-type but increased ECAR in PFKFB3 knockout cells, suggesting impaired fructose-mediated glycolysis in wild-type cells. OCR, a surrogate for oxidative phosphorylation, was not affected by glucose or fructose loading and dynamically changed in response to mitochondrial stressors, as expected, in both wild-type and PFKFB3 knockout cells. Together, these findings indicate that wild-type cells do not use fructose for glycolysis but use it for oxidative phosphorylation. One-way ANOVA and Bonferroni post hoc tests were used to compare different groups. (B) Wild-type and PFKFB3 knockout PMVECs were incubated in glucose medium (25 mM), fructose medium (25 mM), or a combination of glucose (12.5 mM) and fructose (12.5 mM) media with and without 2DG (5 mM) final concentrations under normoxia or hypoxia for 3 days. Supernatant and cell lysate lactate and glucose concentrations were measured using the YSI 2500 (YSI Life Sciences) lactate and glucose analyzer. In glucose medium, wild-type PMVECs showed higher glycolysis activity compared with PFKFB3 knockout cells. Although 2DG treatment decreased glycolysis in wild-type cells, it paradoxically increased glycolysis in PFKFB3 knockout cells, indicating that glycolytic pathways were rewired in these PFKFB3 knockout cells. In fructose medium, PFKFB3 knockout cells showed dramatically higher glycolysis compared with wild-type cells, and 2DG profoundly suppressed glycolysis in all fructose medium conditions, indicating that PFKFB3 knockout cells actively use fructose for their glycolysis, and it is mediated by hexokinase rather than ketohexokinase. When glucose and fructose were mixed equally, the resulting metabolic flux represented an averaging of the effect of each substrate alone. Hypoxia generally enhanced lactate and decreased glucose in supernatant. Two-way ANOVA and Šidák’s multiple-comparisons test were used to compare different groups. (C) Cell pellet analysis was equivalent with supernatant lactate and glucose concentrations. Three-way ANOVA and Šidák’s multiple-comparisons test were used to compare different groups. (D) Representative images of cells and their pH-sensitive media color are shown. At least three independent experiments were performed. Scale bar, 100 µm. *Significant difference (P < 0.05). Data are expressed as mean ± SD. FCCP = carbonyl cyanide p-(trifluoromethoxy) phenylhydrazone; OCR = oxygen consumption rate.
We then extended the timeline and incubated wild-type and PFKFB3 knockout PMVECs in glucose medium, fructose medium, or a combination of glucose and fructose media under normoxic and hypoxic conditions for 3 days. Lactate and glucose concentrations were measured as an index of glycolytic flux. In glucose medium, wild-type cells had higher lactate and lower glucose concentrations in supernatant compared with PFKFB3 knockout cells, consistent with its allosteric regulation of PFK-1 (Figure 2B). 2DG is a competitive HK inhibitor. Interestingly, whereas 2DG treatment decreased supernatant lactate in wild-type cells, it increased supernatant lactate in PFKFB3 knockout cells. In fructose medium, wild-type cells exhibited lower supernatant lactate concentrations compared with PFKFB3 knockout cells, and 2DG profoundly suppressed supernatant lactate concentrations in all fructose medium conditions. When glucose and fructose media were equally mixed, lactate and glucose concentrations represented an average of the effect of either substrate alone. These data indicate that PFKFB3 promotes glucose metabolism to lactate while inhibiting fructose metabolism to lactate.
Hypoxia generally enhanced lactate and decreased glucose supernatant concentrations. As we found the paradoxical 2DG response in PFKFB3 knockout cells in glucose medium to be intriguing, we also analyzed cell pellet lactate and glucose concentrations in glucose-treated conditions. Lactate and glucose concentrations in the cell pellet matched those of the supernatant, indicating that the paradoxical response to 2DG in PFKFB3 knockout cells was not due to a transcellular shift in lactate but rather to an increase in glycolysis (Figure 2C). Representative images of the pH-sensitive medium color changes and the microscopic appearance of cells are shown in Figure 2D; these images match with the pattern of survival and glycolytic activity.
Together, these results may indicate that PFKFB3 promotes glucose-mediated glycolysis and inhibits fructose-mediated glycolysis, suggesting that it functions as a molecular switch in control of substrate metabolism through glycolysis. PFKFB3 seems to be required for 2DG to suppress glucose-mediated glycolysis, and the finding that 2DG suppresses fructose-mediated glycolysis suggests that HK, rather than KHK, catalyzes fructose-mediated glycolysis in PMVECs.
Metabolomic Analysis Reveals That PFKFB3 Inhibits Fructose-mediated Glycolysis and Oxidative Phosphorylation in PMVECs
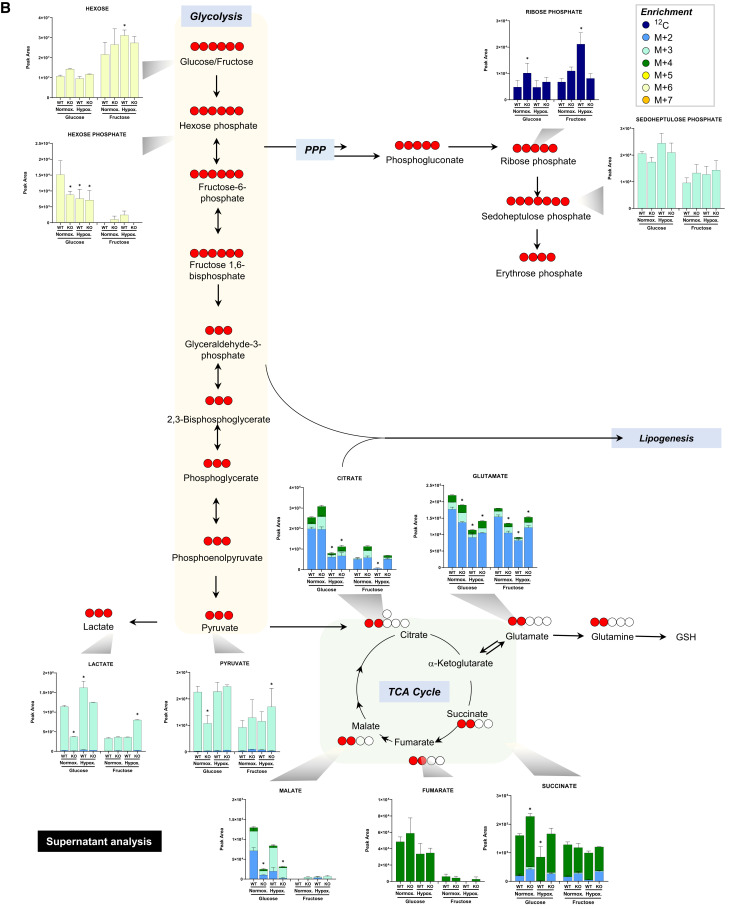
To validate our Seahorse, lactate and glucose data and expand our understanding of the global PMVEC metabolic program, we cultured wild-type and PFKFB3 knockout cells with stable isotope-labeled D-glucose (U-13C6) or D-fructose (U-13C6) for 18 hours under normoxic or hypoxic conditions. Metabolites from cells and supernatants were separated and measured using an ultrahigh-pressure liquid chromatography–mass spectrometry–based platform. In glucose medium, PFKFB3 did not seem to affect intracellular concentrations of glycolytic intermediates (Figure 3A); however, significantly higher supernatant lactate concentrations suggest that glycolysis and lactate export are both higher in wild-type cells (Figure 3B). Enrichment of most tricarboxylic acid (TCA) intermediates was lower in wild-type cells, indicating lower TCA flux in glucose medium (Figure 3A). These findings suggest that PFKFB3 promotes glycolysis but suppresses TCA flux in PMVECs under normoxic conditions. Hypoxia generally increased glycolysis and decreased TCA flux in glucose medium, consistent with canonical anaerobic responses of PMVECs.
Figure 3.
Metabolomic analysis reveals that PFKFB3 inhibits fructose-mediated glycolysis and oxidative phosphorylation in PMVECs. (A and B) Relative concentrations of metabolites in glycolysis, the pentose phosphate pathway (PPP), and the tricarboxylic acid (TCA) cycle were measured in wild-type and PFKFB3 knockout PMVEC (A) cell pellet and (B) supernatant cultured in the presence of U-13C glucose or U-13C fructose (25 mM) under normoxia or hypoxia for 18 hours. The data suggest that PFKFB3 promotes glucose-mediated glycolysis but suppresses TCA and PPP flux, while PFKFB3 inhibits fructose-mediated glycolysis and TCA but promotes PPP under normoxia. Hypoxia enhanced glycolysis and suppressed TCA flux. Three independent experiments were performed. Two-way ANOVA and Tukey’s multiple-comparisons test were used to compare different groups. *Significant difference (P < 0.05) from wild-type normoxia for each glucose or fructose condition. Data are expressed as mean ± SEM. Purple circles, 12C; dark blue circles, M+2; light blue circles, M+3; green circles, M+4; yellow circles, M+5; white circles, M+6; orange circles, M+7. GSH = glutathione.
In heavy fructose-supplemented medium, wild-type cells showed minimal metabolic flux, whereas PFKFB3 knockout cells exhibited dramatically increased fructose-mediated glycolysis (Figure 3A). TCA flux was also increased in PFKFB3 knockout cells in fructose medium (Figure 3A). Notably, PFKFB3 knockout cells tended to retain glycolytic and TCA intermediates intracellularly, resulting in disproportionally lower glycolytic and TCA intermediates in the medium (Figures 3A and 3B), which indicates that PFKFB3 may be involved in transcellular substrate transport. Hypoxia appeared to decrease fructose-mediated glycolytic and TCA flux, on the basis of the cell pellet analysis (Figure 3A). However, significantly higher medium lactate under hypoxia, compared with normoxia (Figure 3B), indicates equivalent or increased fructose-mediated glycolytic flux under hypoxic conditions. Hypoxia decreased TCA flux in fructose medium, similar to the observations in glucose medium (Figure 3A).
The ratio of phosphogluconate to hexose phosphate was determined to gain insight on hexose flux through the pentose phosphate pathway. In glucose medium, PFKFB3 knockout cells had increased pentose phosphate pathway flux (Figure 3A). In fructose medium, PFKFB3 had no significant effect on pentose phosphate pathway flux (Figure 3A). In addition, we quantified the ratio of M+6 (i.e., fully 13C uniformly labeled) fructose bisphosphate to hexose phosphate, which can serve as a readout of PFKFB enzymatic activity as a kinase versus bisphosphatase (Figure 3A). In our experimental settings, this ratio represents the activities of all PFKFB isoforms, including PFKFB1, PFKFB2, PFKFB3 (only for wild-type), and PFKFB4. In glucose medium, the ratio was not significantly different among groups (Figure 3A). In fructose medium, the ratio was higher in PFKFB3 knockout cells under normoxic conditions (Figure 3A), indicating higher kinase activities in these cells, while hypoxia resolved this difference. Although neither this ratio nor F-2,6-P2 was different between wild-type and PFKFB3 knockout cells at the time of sample collection, significantly higher glycolytic intermediates in wild-type cell medium (Figure 3B) may indicate a higher early phase of glycolytic flux that slowed down at a later phase because of negative feedback from accumulating metabolites and ATP. A complete list of the total metabolite plots is shown in Figure E1 in the data supplement.
Together, these findings indicate that PFKFB3 promotes glucose-mediated glycolysis while suppressing TCA and pentose phosphate pathway flux and, further, that PFKFB3 inhibits fructose-mediated glycolysis and TCA flux under normoxia. Hypoxia generally enhances glycolysis and suppresses TCA flux. Thus, metabolomic studies affirm the role of PFKFB3 as a molecular switch in the control of glycolytic substrate use.
RNA Sequencing Analysis Suggests That Fructose Increases PFKFB3 Expression and PFKFB3 Knockout Increases GLUT5 Expression, Which Subsequently Promotes Fructose Use in PMVECs
We next sought to resolve the gene expression matrices that affect how PFKFB3 inhibits fructose metabolism in PMVECs. To do this, we performed RNA sequencing on wild-type and PFKFB3 knockout cells after 24 hours of incubation in glucose or fructose medium in both normoxic and hypoxic conditions. The principal-component analysis (Figure 4A) and heat map (Figure 4B) demonstrated clear separation of gene expression patterns between wild-type and PFKFB3 knockout cells. Although wild-type cells dynamically changed gene expression patterns in response to fructose or hypoxia, PFKFB3 knockout cells seemed to be modestly affected by the same environmental stimuli.
Figure 4.
RNA sequencing analysis suggests that fructose increases PFKFB3 expression and PFKFB3 knockout increases GLUT5 (glucose transporter 5) expression, which subsequently promotes fructose use in PMVECs. Wild-type and PFKFB3 knockout PMVECs were incubated in medium containing glucose (25 mM) or fructose (25 mM) under normoxia or hypoxia for 24 hours, and then RNA sequencing was performed on cell lysates. (A and B) PCA (A) and heat map clustering (B) of genes showed clear separation among experimental groups. Wild-type cells showed more dramatic shifts in the PCA distributions and heat map clustering in response to fructose or hypoxia challenge, whereas PFKFB3 knockout cells tend to cluster for the same environmental changes. (C) Genes linked to canonical fructose metabolism were selected and confirmatory quantitative PCR was performed. Fructose exposure increased PFKFB3 expression in wild-type cells. PFKFB3 knockout cells expressed the GLUT5 fructose transporter at higher concentrations in glucose medium. Three-way ANOVA and Šidák’s multiple-comparisons test were used to compare different groups. (D) GO and KEGG pathway enrichment analysis showed significantly suppressed survival pathways, including those linked to DNA metabolism and the cell cycle in wild-type cells in fructose medium, which matches with our survival assay findings. PFKFB3 knockout cells increased lipid metabolism upon fructose exposure, most likely indicating upregulated lipogenesis as a subsequent destination of fructose metabolism substrates. Three independent experiments were performed. *Significant difference (P < 0.05). Data are expressed as mean ± SD. DEG = differentially expressed gene; fruc. = fructose; gluc. = glucose; GO = Gene Ontology; KEGG = Kyoto Encyclopedia of Genes and Genomes; LDHB = lactate dehydrogenase B; padj = adjusted P value; PC1 = principal component 1; PC2 = principal component 2; PCA = principal-component analysis; PPAR = peroxisome proliferator activated receptor; SLC2A5 = solute carrier family 2 member 5.
We then screened genes that are known to be linked to canonical fructose metabolic pathways and highlighted significant differences between any of the experimental conditions in the analysis of differentially expressed genes. We performed confirmatory quantitative PCR experiments. PFKFB1, PFKFB2, PFKFB3, PFKFB4, GLUT5, HK1, ALDOC (aldolase, fructose-bisphosphate C), LDHB (lactate dehydrogenase B), and LDHD (lactate dehydrogenase D) were selected for the initial screening. Among the selected genes, PFKFB3, GLUT5, and LDHB showed the most notable fold changes between experimental groups (Figure 4C). Interestingly, fructose exposure increased PFKFB3 gene expression in wild-type cells, and PFKFB3 deletion increased GLUT5 fructose transporter expression, regardless of whether cells were in a glucose- or fructose-rich environment. These findings indicate that wild-type cells increase PFKFB3, leading to the suppression of GLUT5 expression and fructose transport, which subsequently inhibits fructose metabolism. Profoundly suppressed LDHB expression in PFKFB3 knockout cells may suggest that LDHB contributes to glucose-mediated glycolysis but not fructose-mediate glycolysis in PMVECs.
Gene Ontology and Kyoto Encyclopedia of Genes and Genomes enrichment analysis was performed on all groupings. In fructose medium, pathways related to DNA metabolism and the cell cycle were significantly higher in PFKFB3 knockout cells, and similar pathways were downregulated by fructose treatment in wild-type cells, consistent with our findings of the detrimental effects of fructose on wild-type cell survival (Figure 4D). Unlike in wild-type cells, the fructose effect was relatively minor in PFKFB3 knockout pathways; the most significant changes were limited to lipid metabolism, which is a major subsequent pathway linked to fructolysis (26). Overall, these gene expression changes match with our survival and metabolic/metabolomic studies, and they support the idea that PFKFB3 knockout increases GLUT5 expression, fructose metabolism, and subsequently cell survival in PMVECs.
Endothelial PFKFB3 Decreases Lung Tissue Glycolysis after Fructose Gavage in Mice
We next examined the physiological relevance of our findings in vitro and in vivo. Although we used 25 mM glucose and fructose media for our in vitro studies, circulating blood glucose and fructose concentrations are lower in most physiological conditions in humans. Therefore, we repeated lactate and glucose assays in 6 mM glucose or 100 μM fructose medium, the concentrations closer to those of normal circulating blood. In 6 mM glucose medium, PFKFB3 knockout PMVECs showed lower supernatant lactate compared with wild-type cells in 24 hours, which is consistent with the finding shown in 25 mM glucose medium (Figure 5A). However, unlike in 25 mM glucose medium, hypoxia did not significantly increase supernatant lactate in wild-type cells. This is most likely due to relatively limited substrate availability, considering that a starting supernatant concentration of 6 mM glucose falls to an undetectable concentration by 24 hours in hypoxic conditions (data not shown). Next, we tested 100 μM fructose medium in the same conditions with an extension of the endpoint to 48 hours to better detect differences between experimental groups. Compared with wild-type cells, PFKFB3 knockout cells showed higher supernatant lactate under both normoxia and hypoxia (Figure 5B), which is consistent with the finding shown in 25 mM fructose medium. These results suggest that the regulatory role of PFKFB3 on fructose metabolism persists in micromolar concentrations of fructose, and our findings are relevant to in vivo and clinical settings.
Figure 5.
Endothelial PFKFB3 decreases lung tissue glycolysis in mice. Physiological relevance was examined in in vitro and in vivo settings. Medium concentrations of glucose and fructose were lowered to mimic circulating systemic human blood to investigate the role of PFKFB3 in PMVEC metabolism. (A) Wild-type and PFKFB3 knockout PMVECs were incubated in 6 mM glucose medium under normoxia and hypoxia for 24 hours. Supernatant lactate concentrations were measured using the YSI 2500 lactate and glucose analyzer. Wild-type PMVECs showed higher lactate compared with PFKFB3 knockout cells. Hypoxia increased lactate in both groups, but the degree of increase was limited by glucose availability in the media. Two-way ANOVA and Šidák’s multiple-comparisons test were used to compare different groups. (B) Wild-type and PFKFB3 knockout PMVECs were incubated in 100 μM fructose medium under normoxia and hypoxia for 2 days. Supernatant lactate concentrations were higher in PFKFB3 knockout PMVECs, and hypoxia increased lactate in all groups, similar to the findings shown in 25 mM fructose medium. Two-way ANOVA and Šidák’s multiple-comparisons test were used to compare different groups. (C) Tamoxifen-inducible endothelial-specific PFKFB3 knockout mice (PFKFB3fl/fl;Cdh5[PAC]-CreERT2) were used to investigate the role of PFKFB3 in fructose metabolism in vivo. Cre-positive PFKFB3 knockout mice (PFKFB3Cdh5) were compared with Cre-negative littermate control mice (PFKFB3fl/fl). All animals were treated with tamoxifen daily (72 mg/kg, intraperitoneal injection) for 5 days. One week after the last dose of tamoxifen, animals were gavaged with fructose (2 g/kg body weight). Two hours after fructose gavage, blood was sampled from the right ventricle, and lung tissue was collected. Lactate was measured on blood and lung tissue lysate. There was no difference in blood lactate, but lung tissue lactate was significantly higher in PFKFB3Cdh5 compared with PFKFB3fl/fl, suggesting an inhibitory role of pulmonary endothelial PFKFB3 on fructose-mediated glycolysis. Student’s t tests were used to compare different groups. (D) Wild-type and PFKFB3 knockout PMVECs and pulmonary arterial endothelial cells (PAECs) were incubated in 25 mM glucose or fructose medium under normoxia or hypoxia for 96 hours. Supernatant lactate concentrations were measured using the YSI 2500 lactate and glucose analyzer. In glucose medium, supernatant lactate was lower in PAECs compared with wild-type PMVECs. In fructose medium, there was no significant difference in supernatant lactate between wild-type PMVECs and PAECs. Three-way ANOVA and Šidák’s multiple comparisons tests were used to compare different groups. *Significant difference (P < 0.05). Data are expressed as mean ± SD. At least three independent experiments were performed.
We then investigated whether PFKFB3 plays any role in fructose metabolism in vivo. For this study, we used tamoxifen-inducible endothelial-specific PFKFB3 knockout mice (PFKFB3fl/fl;Cdh5[PAC]-CreERT2). Cre-positive PFKFB3 knockout mice (PFKFB3Cdh5) were compared with Cre-negative littermate control mice (PFKFB3fl/fl). All animals were treated with tamoxifen regardless of Cre positivity. To study the effect of fructose on pulmonary endothelial metabolism, animals were gavaged with fructose. Two hours after fructose gavage, lactate was measured in the blood and lung tissue lysate. There was no difference in blood lactate, but a significant increase in lung tissue lactate in PFKFB3Cdh5 mice compared with PFKFB3fl/fl was observed (Figure 5C). These findings suggest that PFKFB3 inhibits fructose use in pulmonary endothelial cells, which is consistent with our in vitro findings.
As PFKFB3 deletion in our PFKFB3Cdh5 mice is not limited to PMVECs, and pulmonary endothelial cells are heterogeneous in their metabolic characteristics (10), we studied how pulmonary arterial endothelial cells (PAECs) use fructose compared with PMVECs in vitro. Wild-type and PFKFB3 knockout PMVECs and PAECs were incubated in 25 mM glucose or fructose medium for 4 days, and lactate and glucose concentrations were measured in supernatant. In glucose medium, lactate was lower in PAECs (Figure 5D), as expected from our previous work (11). In fructose medium, there was no difference between wild-type PMVECs and PAECs in their lactate concentrations. Hypoxia uniformly increased lactate in all groups. These findings suggest that wild-type PMVECs and PAECs are similar in that they minimally use fructose for glycolysis. Whether PAEC PFKFB3 contributed to the increased lung tissue fructose use in PFKFB3Cdh5 mice is yet to be determined.
Pneumonia Increases Fructose in the BALF of ICU Patients
Having seen fructose-induced glycolytic activity changes in mouse lung tissue, we wondered whether fructose is detected in BALF and how pneumonia that generates hypoxic microenvironments affects BALF fructose concentrations. To answer this question, we measured fructose in BALF obtained from mechanically ventilated ICU patients anytime within 14 days of ICU admission. Patients were grouped on the basis of BALF bacterial culture results. Table 1 shows the baseline characteristics of these patients, illustrating that there were no significant differences in the measured variables, except for a higher rate of vasopressor use in control patients. BALF fructose was significantly higher in patients with pneumonia compared with control subjects (Figure 6A). We also measured lactate (Figure 6B) and glucose (Figure 6C) on the same BALF samples for comparison, which did not reveal any significant differences between groups. Our findings suggest that fructose is readily detectable in BALF and that fructose BALF concentrations increase during pneumonia.
Table 1.
Baseline Characteristics of University of Alabama at Birmingham Hospital Patients
| Characteristic | Control | Pneumonia | Total |
|---|---|---|---|
| (n = 9) | (n = 14) | (n = 23) | |
| Age, yr, median (range) | 53 (20–87) | 52.5 (20–74) | 53 (20–87) |
| Male sex, n/total (%) | 3/9 (33.3) | 9/14 (64.3) | 12/23 (52.2) |
| Race, n/total (%) | |||
| White | 6/9 (66.7) | 10/14 (71.4) | 16/23 (69.6) |
| Black | 2/9 (22.2) | 3/14 (21.4) | 5/23 (21.7) |
| Hispanic | 1/9 (11.1) | 1/14 (7.1) | 3/23 (8.7) |
| Primary diagnosis, n/total (%) | |||
| Motor vehicle collision/trauma | 5/9 (55.6) | 8/14 (57.1) | 13/23 (56.5) |
| Brain hemorrhage | 0/9 (0.0) | 3/14 (21.4) | 3/23 (13.0) |
| Burn | 1/9 (11.1) | 1/14 (7.1) | 2/23 (8.7) |
| Bowel perforation | 1/9 (11.1) | 0/14 (0.0) | 1/23 (4.93) |
| Myasthenic crisis | 1/9 (11.1) | 0/14 (0.0) | 1/23 (4.93) |
| Status epilepticus | 1/9 (11.1) | 0/14 (0.0) | 1/23 (4.93) |
| Tongue mass | 0/9 (0.0) | 1/14 (7.1) | 1/23 (4.93) |
| Mechanical ventilator, n/total (%) | 9/9 (100) | 14/14 (100) | 23/23 (100) |
| Vasopressor, n/total (%) | 8/9 (88.9)* | 5/14 (35.7)* | 13/23 (56.5) |
| Length of ICU stay, d, median (range) | 19 (8–31) | 15 (6–105) | 18 (6–105) |
| Length of hospital stay, d, median (range) | 32 (11–69) | 20.5 (10–105) | 21 (10–105) |
| Deceased, n/total (%) | 2/9 (22.2) | 4/14 (28.6) | 6/23 (26.1) |
| BAL culture, n/total (%) | |||
| S. aureus | — | 6/14 (42.9) | — |
| K. pneumoniae | — | 2/14 (14.3) | — |
| P. aeruginosa | — | 1/14 (7.1) | — |
| H. influenzae | — | 1/14 (7.1) | — |
| S. pyogenes | — | 1/14 (7.1) | — |
| S. aureus and P. aeruginosa | — | 1/14 (7.1) | — |
| H. influenzae and S. maltophilia | — | 1/14 (7.1) | — |
Definition of abbreviations: H. influenzae = Haemophilus influenzae; K. pneumoniae = Klebsiella pneumoniae; P. aeruginosa = Pseudomonas aeruginosa; S. aureus = Staphylococcus aureus; S. maltophilia = Stenotrophomonas maltophilia; S. pyogenes = Streptococcus pyogenes.
Chi-square and Wilcoxon rank sum tests were used for categorical and quantitative data, respectively.
Significant difference (P < 0.05), control versus pneumonia.
Figure 6.
Pneumonia increases fructose in the BAL fluid (BALF) of ICU patients. BALF was collected from mechanically ventilated patients anytime within 14 days of ICU admission. Patients were grouped into control versus pneumonia on the basis of BALF culture results. (A) BALF fructose was higher in patients with pneumonia compared with control subjects. (B and C) There was no difference in (B) BALF lactate or (C) glucose between groups. (D) A schematic representation of how PFKFB3 functions as a molecular switch for glucose versus fructose use in glycolysis is shown. In wild-type PMVECs, glucose-mediated glycolysis is increased, whereas fructose-mediated glycolysis is decreased because of decreased GLUT5 expression and fructose uptake. In PFKFB3 knockout PMVECs, glucose-mediated glycolysis is decreased, whereas fructose-mediated glycolysis is increased via increased GLUT5 expression and fructose uptake. Unlike the canonical fructolytic pathway, which is mediated by KHK, fructose-mediated glycolysis in PMVECs is mediated primarily by HK. Therefore, PFKFB3 serves as a molecular switch that determines glucose versus fructose use in glycolysis in PMVECs. Wilcoxon rank sum tests were used to compare different groups. *Significant difference (P < 0.05).
Discussion
In this study, we investigated the role that PFKFB3 plays in the regulation of fructose metabolism in PMVECs. We report six key findings: 1) PFKFB3 decreases PMVEC survival in a hypoxic, fructose-rich environment; 2) Seahorse assays and lactate and glucose measurements suggest that PFKFB3 decreases fructose–HK–mediated glycolysis in PMVECs; 3) metabolomic analysis reveals that PFKFB3 inhibits fructose-mediated glycolysis and oxidative phosphorylation in PMVECs; 4) RNA sequencing suggests that fructose increases PFKFB3 expression and PFKFB3 knockout increases GLUT5 expression, which subsequently promotes fructose use in PMVECs; 5) endothelial PFKFB3 decreases lung tissue glycolysis after fructose gavage in mice; and 6) pneumonia increases fructose in the BALF of ICU patients. These findings reveal that PFKFB3 functions as a molecular switch in control of substrate use for glycolysis (Figure 5). PFKFB3 could be a potential therapeutic target to enhance fructose use in PMVECs.
Detailed molecular interactions responsible for the PFKFB3-dependent inhibition of fructose metabolism in PMVECs remain incompletely understood. GLUT5 is upregulated in PFKFB3 knockout cells. It is therefore possible that transcellular fructose transport in wild-type cells is low simply because GLUT5 expression and/or activity is also low. However, Seahorse assays showed that although fructose does not fuel glycolysis in wild-type cells, it supports oxidative phosphorylation at a degree comparable with what is seen in the PFKFB3 knockout cells. This finding is inconsistent with the idea that impaired fructose-mediated glycolysis in wild-type cells is due solely to slower fructose transcellular transport. It still may be possible that the relationship between fructose transport and oxidative phosphorylation is a time-dependent phenomenon due to a slower cycling rate of oxidative phosphorylation, where suppressed oxidative phosphorylation in wild-type cells becomes measurable later in the time course when samples were collected for metabolomic analysis. In the future, studies addressing mitochondrial function over an extended time course will help resolve the link between fructose transport into the cell and oxidative phosphorylation. Hypoxia-induced acceleration of wild-type cell death in fructose medium seems to indicate that wild-type cells rely on oxidative phosphorylation for survival under normoxia, and when they are exposed to hypoxia, anaerobic suppression of oxidative phosphorylation leads to cell death, as wild-type cells have no glycolytic reserve in fructose medium. It is most likely that PFKFB3 inhibits GLUT5 expression and fructose transcellular transport, which results in decreased fructose-mediated glycolysis and, subsequently, oxidative phosphorylation. Further studying transcriptional regulation of GLUT5 and PFKFB3 and exploring potential involvement of noncoding RNAs in linking these mediators of metabolism may offer additional insights and therapeutic targets for yet to be uncovered clinical applications (27).
Fructose is the second most abundant blood glucose in humans (9), but peripheral plasma fructose concentrations are typically in the micromolar range (28). Plasma concentrations can reach low millimolar amounts after fructose ingestion, but the majority of fructose is extracted by the liver, decreasing circulating concentrations to the micromolar range within a couple of hours (29). Under physiological conditions, 70% of intestinally absorbed fructose is converted into glycogen and fat in the liver, and relatively small amounts are distributed to other organs (30). However, studies measuring fructose extraction rate by the liver did not specify whether blood samples were obtained from the right side or the left side of the heart (7). If blood was taken from the systemic circulation, it is possible that the fraction of fructose extracted by the lungs could have been included in “the fraction of fructose extracted by the liver.” Considering that fructose is not under tight endocrinological control, as glucose is, it is unclear how dynamically fructose concentrations fluctuate, particularly during pathologic conditions that involve hepatic dysfunction or other metabolic derangements. In our studies, both 100 μM and 25 mM fructose medium induced similar metabolic changes, and cell exposure to similar glucose and fructose concentrations induced a mixed metabolic phenotype, representing a balance of the metabolic activity of each substrate alone. Fructose assays are not part of routine laboratory tests in most hospital settings; fructose plasma and tissue-level monitoring, especially in the setting of global and tissue hypoxia, would offer valuable insight into the patient’s metabolic status.
Naked mole rats exhibit a fascinating use of fructose to support their metabolic demands under extreme hypoxia. Yet significant concerns exist regarding the impacts of fructose metabolism on human health (31). In chronic lung disease settings, concerns exist regarding the link between a high-fructose diet and increased inflammation in asthma, COPD, and cigarette smoking–related parenchymal lung diseases (32–34). However, these studies are oftentimes confounded by simultaneous high-fat diet and underlying obesity. In acute settings, intravenous fructose infusion is believed to be potentially dangerous, as KHK bypasses the negative glycolytic feedback by ATP, and unopposed fructolysis cycling can lead to depletion of phosphate and increased production of lactate and uric acid (35, 36). However, this phenomenon is relevant only if fructose is infused at high concentrations and metabolized solely via KHK. KHK is expressed primarily in the liver, kidney, and small intestine (37), and our RNA sequencing data showed that KHK concentrations are extremely low or undetectable in PMVECs. Similar findings of nonfructolytic cells using HK rather than KHK and increasing GLUT5 to support their proliferation have been reported (38). We demonstrated that 2DG treatment profoundly inhibits fructolysis, which also indicates that fructose-mediated glycolysis is mediated by HK rather than KHK in PMVECs. We found that 2DG toxicity is more powerful in fructose medium than it is in glucose medium, most likely because of the difference in the fructose and glucose Michaelis constants (Km) for HK. The fructose Km for HK is higher (19) than the glucose Km for HK; therefore, 2DG binding to HK is higher in fructose medium than in glucose medium. Furthermore, in a preliminary study (data not shown), we found that the KHK inhibitor (PF-06835919, also known as MDK1846) has no effect on fructose-mediated glycolysis in our experimental settings, which further supports the idea that fructose metabolism is mediated by HK rather than by KHK in PMVECs.
How 2DG causes cytotoxicity and how some cells develop resistance to 2DG are poorly understood (31). In our study, 2DG inhibited glucose-mediated glycolysis in wild-type cells, which is consistent with previous reports (39, 40). However, 2DG paradoxically increased glucose-mediated glycolysis in PFKFB3 knockout cells. This paradoxical effect of 2DG on PFKFB3 knockout cells was not seen in the Seahorse assays. This finding can be related to a dose-related difference, as a 20 times higher concentration of 2DG was used for Seahorse assays. Alternatively, these findings may indicate that the paradoxical 2DG effect is an indirect cellular response to 2DG. It is questionable whether there is any noncanonical glycolytic pathway suppressed by PFKFB3 that bypasses 2DG targets. The fact that the paradoxical effect of 2DG is not maintained under hypoxia could indicate that this potential noncanonical glycolytic pathway is not under hypoxic regulation. Together, our data suggest that PFKFB3 may rewire glucose-mediated glycolytic pathways, but further investigation is warranted to validate this idea.
Metabolomic and RNA sequencing data were consistent with findings from the survival and metabolic assays, and they offered additional insights. In comparing cell pellet and supernatant metabolomic analyses, we found that PFKFB3 knockout cells retain many glycolytic and TCA intermediates inside the cell, out of proportion with their extracellular concentrations. This was particularly intriguing because, during our preliminary studies, in which we extended the experimental duration for up to 6 days, we observed that PFKFB3 knockout cells slowly acidify the media. We believe that there are two important implications of this finding. First, decreased proton secretion for a given lactate production may serve as a strategy to control extracellular acidosis, while maintaining a high degree of glycolysis. This possibility is relevant to critically ill patients with sepsis, who often develop uncontrollable acidosis because of excessive metabolism. Second, increased intracellular fructose metabolite accumulation may facilitate lipogenesis, especially considering our RNA sequencing data showing strong upregulation of lipid metabolic pathways in these cells. Emerging evidence indicates that fatty acids contribute to the pathogenesis of pulmonary arterial hypertension (41), and considering the critical role of PFKFB3 in glycolysis, angiogenesis, and lung injury and repair (16, 17, 42, 43), further elucidating molecular mechanisms involved in the fructose–PFKFB3 axis will offer helpful insight into how lungs handle the second most abundant sugar in humans, fructose, under stressful environments.
We probed for potential clinical relevance of our findings with in vivo and clinical data. Increased lung tissue lactate production after fructose gavage in PFKFB3Cdh5 mice, independent of blood lactate changes, suggests PFKFB3-mediated inhibition of fructose use in the pulmonary circulation. However, further studies, such as isotope tracing of lung tissue or metabolic assays, on isolated pulmonary endothelial cells from PFKFB3Cdh5 mice, will be needed to validate our interpretation. Our BALF fructose data on mechanically ventilated patients with pneumonia is interesting, as this is the first study to assess fructose concentrations in BALF. However, our studies are limited by small sample numbers. Whether fructose is increased in pneumonia BALF because of increased uptake or decreased use by lung tissue, and whether it plays any role in disease progression, remains to be determined.
Conclusions
We report that PFKFB3 inhibits fructose metabolism and cell survival, particularly under hypoxic conditions, in PMVECs. Our work reveals that PFKFB3 functions as an essential molecular switch, coordinating glucose versus fructose use in glycolysis. Our findings suggest that inhibition of PFKFB3 could be a potential therapeutic strategy for critically ill patients with hypoxic respiratory failure, in an effort to increase fructose use and enhance pulmonary capillary integrity.
Footnotes
Supported by American Heart Association grant 23PRE1020594 (R.P.S.); U.S. Department of Defense grants W81XWH2110161 (M.F.A.) and W81XWH2110669 (M.F.A.); National Science Foundation grants RAPID 2030080 (G.M.B.), 2219900 (G.M.B.), 2223547 (G.M.B.), 2243532 (G.M.B.), and CNS-1726069 (G.M.B.); National Institutes of Health grants HL160988 (J.Y.L., T.S.), HL140182 (T.S.), HL148069 (J.Y.L., T.S.), HL66299 (T.S.), HL60024 (T.S.), OD010944 (M.F.A.), HL135872 (B.B.G.), HL165873 (R.P.S.), HL167997 (T.S.), and HL152961 (B.B.G., K.R.S.); National Heart, Lung, and Blood Institute grant 1S10OD025089 (M.F.A.); National Center for Advancing Translational Sciences grant UL1TR001417 (G.M.B.); and National Institute of General Medical Sciences grants GM127584 (B.M.W.) and GM127584-S1 (B.M.W.).
Author Contributions: J.Y.L., R.P.S., and T.S. conceived the study. J.Y.L., R.P.S., J.A.R., D.N., A.D’A., B.B.G., K.R.S., and T.S. designed the study. J.Y.L., V.V.P., V.M.P., N.K., M.F.A., J.A.R., D.N., A.D’A., A.K., M.S.G., J.T.R., G.M.B., B.M.W., and J.-F.P. contributed to the acquisition of data. All authors interpreted the data. J.Y.L., J.A.R., D.N., A.D’A., and T.S. wrote the manuscript. All authors reviewed and edited the manuscript.
Data Sharing: The RNA-seq data presented in this article have been submitted to the National Center for Biotechnology Information Gene Expression Omnibus repository (GEO) (http://www.ncbi.nlm.nih.gov/geo/). The GEO accession number is GSE165500.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2022-0443OC on May 18, 2023
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Johnson RJ, Stenvinkel P, Andrews P, Sánchez-Lozada LG, Nakagawa T, Gaucher E, et al. Fructose metabolism as a common evolutionary pathway of survival associated with climate change, food shortage and droughts. J Intern Med . 2020;287:252–262. doi: 10.1111/joim.12993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nakagawa T, Sanchez-Lozada LG, Andres-Hernando A, Kojima H, Kasahara M, Rodriguez-Iturbe B, et al. Endogenous fructose metabolism could explain the Warburg effect and the protection of SGLT2 inhibitors in chronic kidney disease. Front Immunol . 2021;12:694457. doi: 10.3389/fimmu.2021.694457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Arieli R, Ar A. Ventilation of a fossorial mammal (Spalax ehrenbergi) in hypoxic and hypercapnic conditions. J Appl Physiol . 1979;47:1011–1017. doi: 10.1152/jappl.1979.47.5.1011. [DOI] [PubMed] [Google Scholar]
- 4. Park TJ, Reznick J, Peterson BL, Blass G, Omerbašić D, Bennett NC, et al. Fructose-driven glycolysis supports anoxia resistance in the naked mole-rat. Science . 2017;356:307–311. doi: 10.1126/science.aab3896. [DOI] [PubMed] [Google Scholar]
- 5. Park TJ, Smith ESJ, Reznick J, Bennett NC, Applegate DT, Larson J, et al. African naked mole-rats demonstrate extreme tolerance to hypoxia and hypercapnia. Adv Exp Med Biol . 2021;1319:255–269. doi: 10.1007/978-3-030-65943-1_9. [DOI] [PubMed] [Google Scholar]
- 6. Pérez-Díaz J, Martín-Requero A, Ayuso-Parrila MS, Parrilla R. Metabolic features of isolated rat lung cells: I. Factors controlling glucose utilization. Am J Physiol . 1977;232:E394–E400. doi: 10.1152/ajpendo.1977.232.4.E394. [DOI] [PubMed] [Google Scholar]
- 7. Das DK, Neogi A, Steinberg H. Nutritional and hormonal control of glucose and fructose utilization by lung. Clin Physiol Biochem . 1985;3:240–248. [PubMed] [Google Scholar]
- 8. Das DK, Neogi A, Steinberg H. Fructose utilization by lung. J Appl Physiol . 1984;56:333–337. doi: 10.1152/jappl.1984.56.2.333. [DOI] [PubMed] [Google Scholar]
- 9. Chen WL, Jin X, Wang M, Liu D, Luo Q, Tian H, et al. GLUT5-mediated fructose utilization drives lung cancer growth by stimulating fatty acid synthesis and AMPK/mTORC1 signaling. JCI Insight . 2020;5:e131596. doi: 10.1172/jci.insight.131596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Parra-Bonilla G, Alvarez DF, Al-Mehdi AB, Alexeyev M, Stevens T. Critical role for lactate dehydrogenase A in aerobic glycolysis that sustains pulmonary microvascular endothelial cell proliferation. Am J Physiol Lung Cell Mol Physiol . 2010;299:L513–L522. doi: 10.1152/ajplung.00274.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee JY, McMurtry SA, Stevens T. Single cell cloning generates lung endothelial colonies with conserved growth, angiogenic, and bioenergetic characteristics. Pulm Circ . 2017;7:777–792. doi: 10.1177/2045893217731295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Xu Y, An X, Guo X, Habtetsion TG, Wang Y, Xu X, et al. Endothelial PFKFB3 plays a critical role in angiogenesis. Arterioscler Thromb Vasc Biol . 2014;34:1231–1239. doi: 10.1161/ATVBAHA.113.303041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kim S-G, Manes NP, El-Maghrabi MR, Lee Y-H. Crystal structure of the hypoxia-inducible form of 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase (PFKFB3): a possible new target for cancer therapy. J Biol Chem . 2006;281:2939–2944. doi: 10.1074/jbc.M511019200. [DOI] [PubMed] [Google Scholar]
- 14. Shi L, Pan H, Liu Z, Xie J, Han W. Roles of PFKFB3 in cancer. Signal Transduct Target Ther . 2017;2:17044. doi: 10.1038/sigtrans.2017.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Van Schaftingen E, Jett MF, Hue L, Hers HG. Control of liver 6-phosphofructokinase by fructose 2,6-bisphosphate and other effectors. Proc Natl Acad Sci U S A . 1981;78:3483–3486. doi: 10.1073/pnas.78.6.3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cao Y, Zhang X, Wang L, Yang Q, Ma Q, Xu J, et al. PFKFB3-mediated endothelial glycolysis promotes pulmonary hypertension. Proc Natl Acad Sci U S A . 2019;116:13394–13403. doi: 10.1073/pnas.1821401116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kassa B, Kumar R, Mickael C, Sanders L, Vohwinkel C, Lee MH, et al. Endothelial cell PHD2-HIF1α-PFKFB3 contributes to right ventricle vascular adaptation in pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol . 2021;321:L675–L685. doi: 10.1152/ajplung.00351.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Johnson RJ, Perez-Pozo SE, Sautin YY, Manitius J, Sanchez-Lozada LG, Feig DI, et al. Hypothesis: could excessive fructose intake and uric acid cause type 2 diabetes? Endocr Rev . 2009;30:96–116. doi: 10.1210/er.2008-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Van den Berghe G. Fructose: metabolism and short-term effects on carbohydrate and purine metabolic pathways. Prog Biochem Pharmacol . 1986;21:1–32. [PubMed] [Google Scholar]
- 20. Chun C, Chun H, Chen C, Mohandas R, Segal M. Abstract 12570: fructolysis is essential and sufficient in angiogenesis. Circulation . 2018;138:A12570–A12570. [Google Scholar]
- 21. Lee JY, Alexeyev M, Kozhukhar N, Pastukh V, White R, Stevens T. Carbonic anhydrase IX is a critical determinant of pulmonary microvascular endothelial cell pH regulation and angiogenesis during acidosis. Am J Physiol Lung Cell Mol Physiol . 2018;315:L41–L51. doi: 10.1152/ajplung.00446.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Balczon R, Morrow KA, Zhou C, Edmonds B, Alexeyev M, Pittet JF, et al. Pseudomonas aeruginosa infection liberates transmissible, cytotoxic prion amyloids. FASEB J . 2017;31:2785–2796. doi: 10.1096/fj.201601042RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nemkov T, Hansen KC, D’Alessandro A. A three-minute method for high-throughput quantitative metabolomics and quantitative tracing experiments of central carbon and nitrogen pathways. Rapid Commun Mass Spectrom . 2017;31:663–673. doi: 10.1002/rcm.7834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nemkov T, Reisz JA, Gehrke S, Hansen KC, D’Alessandro A. High-throughput metabolomics: isocratic and gradient mass spectrometry-based methods. Methods Mol Biol . 2019;1978:13–26. doi: 10.1007/978-1-4939-9236-2_2. [DOI] [PubMed] [Google Scholar]
- 25. Tan YB, Pastukh VM, Gorodnya OM, Mulekar MS, Simmons JD, Machuca TN, et al. Enhanced mitochondrial DNA repair resuscitates transplantable lungs donated after circulatory death. J Surg Res . 2020;245:273–280. doi: 10.1016/j.jss.2019.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shi Y-N, Liu Y-J, Xie Z, Zhang WJ. Fructose and metabolic diseases: too much to be good. Chin Med J (Engl) . 2021;134:1276–1285. doi: 10.1097/CM9.0000000000001545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Caruso P, Dunmore BJ, Schlosser K, Schoors S, Dos Santos C, Perez-Iratxeta C, et al. Identification of microrna-124 as a major regulator of enhanced endothelial cell glycolysis in pulmonary arterial hypertension via ptbp1 (polypyrimidine tract binding protein) and pyruvate kinase m2. Circulation . 2017;136:2451–2467. doi: 10.1161/CIRCULATIONAHA.117.028034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kawasaki T, Akanuma H, Yamanouchi T. Increased fructose concentrations in blood and urine in patients with diabetes. Diabetes Care . 2002;25:353–357. doi: 10.2337/diacare.25.2.353. [DOI] [PubMed] [Google Scholar]
- 29. Sugimoto K, Hosotani T, Kawasaki T, Nakagawa K, Hayashi S, Nakano Y, et al. Eucalyptus leaf extract suppresses the postprandial elevation of portal, cardiac and peripheral fructose concentrations after sucrose ingestion in rats. J Clin Biochem Nutr . 2010;46:205–211. doi: 10.3164/jcbn.09-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Weng Y, Zhu J, Chen Z, Fu J, Zhang F. Fructose fuels lung adenocarcinoma through GLUT5. Cell Death Dis . 2018;9:557. doi: 10.1038/s41419-018-0630-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gaby AR. Adverse effects of dietary fructose. Altern Med Rev . 2005;10:294–306. [PubMed] [Google Scholar]
- 32. DeChristopher LR, Tucker KL. Excess free fructose, high-fructose corn syrup and adult asthma: the Framingham Offspring Cohort. Br J Nutr . 2018;119:1157–1167. doi: 10.1017/S0007114518000417. [DOI] [PubMed] [Google Scholar]
- 33. Qian G, Adeyanju O, Sunil C, Huang SK, Chen S-Y, Tucker TA, et al. Dedicator of cytokinesis 2 (dock2) deficiency attenuates lung injury associated with chronic high-fat and high-fructose diet–induced obesity. Am J Pathol . 2022;192:226–238. doi: 10.1016/j.ajpath.2021.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Suehiro CL, Toledo-Arruda AC, Vieira RP, Almeida FM, Olivo CR, Martins MA, et al. A possible association between fructose consumption and pulmonary emphysema. Sci Rep . 2019;9:9344. doi: 10.1038/s41598-019-45594-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Woods HF, Alberti KGMM. Dangers of intravenous fructose. Lancet . 1972;2:1354–1357. doi: 10.1016/s0140-6736(72)92791-2. [DOI] [PubMed] [Google Scholar]
- 36. Tappy L, Lê K-A. Metabolic effects of fructose and the worldwide increase in obesity. Physiol Rev . 2010;90:23–46. doi: 10.1152/physrev.00019.2009. [DOI] [PubMed] [Google Scholar]
- 37. Miller CO, Yang X, Lu K, Cao J, Herath K, Rosahl TW, et al. Ketohexokinase knockout mice, a model for essential fructosuria, exhibit altered fructose metabolism and are protected from diet-induced metabolic defects. Am J Physiol Endocrinol Metab . 2018;315:E386–E393. doi: 10.1152/ajpendo.00027.2018. [DOI] [PubMed] [Google Scholar]
- 38. Liang RJ, Taylor S, Nahiyaan N, Song J, Murphy CJ, Dantas E, et al. GLUT5 (SLC2A5) enables fructose-mediated proliferation independent of ketohexokinase. Cancer Metab . 2021;9:12. doi: 10.1186/s40170-021-00246-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Verdegem D, Moens S, Stapor P, Carmeliet P. Endothelial cell metabolism: parallels and divergences with cancer cell metabolism. Cancer Metab . 2014;2:19. doi: 10.1186/2049-3002-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lee JY, Stevens RP, Kash M, Zhou C, Koloteva A, Renema P, et al. Kd025 shifts pulmonary endothelial cell bioenergetics and decreases baseline lung permeability. Am J Respir Cell Mol Biol . 2020;63:519–530. doi: 10.1165/rcmb.2019-0435OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lee MH, Sanders L, Kumar R, Hernandez-Saavedra D, Yun X, Ford JA, et al. Contribution of fatty acid oxidation to the pathogenesis of pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol . 2022;323:L355–L371. doi: 10.1152/ajplung.00039.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Stevens RP, Paudel SS, Johnson SC, Stevens T, Lee JY. Endothelial metabolism in pulmonary vascular homeostasis and acute respiratory distress syndrome. Am J Physiol Lung Cell Mol Physiol . 2021;321:L358–L376. doi: 10.1152/ajplung.00131.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Vohwinkel CU, Burns N, Coit E, Yuan X, Vladar EK, Sul C, et al. HIF1A-dependent induction of alveolar epithelial PFKFB3 dampens acute lung injury. JCI Insight . 2022;7:e157855. doi: 10.1172/jci.insight.157855. [DOI] [PMC free article] [PubMed] [Google Scholar]