Abstract
Targeted delivery of transgenes to tissue-resident stem cells and related niches offers avenues for interrogating pathways and editing endogenous alleles for therapeutic interventions. Here, we survey multiple adeno-associated virus (AAV) serotypes, administered via intranasal and retroorbital routes in mice, to target lung alveolar stem cell niches. We found that AAV5, AAV4, and AAV8 efficiently and preferentially transduce alveolar type-2 stem cells (AT2s), endothelial cells, and PDGFRA+ fibroblasts, respectively. Notably, some AAVs show different cell tropisms depending on the route of administration. Proof-of-concept experiments reveal the versatility of AAV5-mediated transgenesis for AT2-lineage labeling, clonal cell tracing after cell ablation, and conditional gene inactivation in both postnatal and adult mouse lungs in vivo. AAV6, but not AAV5, efficiently transduces both mouse and human AT2s in alveolar organoid cultures. Furthermore, AAV5 and AAV6 can be used to deliver guide RNAs and transgene cassettes for homologous recombination in vivo and ex vivo, respectively. Using this system coupled with clonal derivation of AT2 organoids, we demonstrate efficient and simultaneous editing of multiple loci, including targeted insertion of a payload cassette in AT2s. Taken together, our studies highlight the powerful utility of AAVs for interrogating alveolar stem cells and other specific cell types both in vivo and ex vivo.
Keywords: AAV, viral delivery, organoids, alveolar stem cells, alveolar stem cell niche
Tissue-resident stem cells and their surrounding niche cells coordinate the maintenance and repair of lost cells after damage. The reciprocal interactions between stem cells and niche cells make them a prime target for modulation of genetic pathways for regenerative medicine applications. Indeed, over the years, numerous studies have explored this approach in multiple organ systems, including muscle and hematopoietic tissues (1–6). However, a major challenge is targeting the delivery of transgenes specifically to the desired cells (7), especially in native tissues. This goal is particularly urgent now that CRISPR-based genome editing tools with unprecedented efficiency and specificity are becoming increasingly available for both therapeutic and research applications (1, 8–13).
In the lung, multiple region-specific stem and progenitor cells participate in tissue renewal and regeneration after injury. In particular, alveolar type-2 cells (AT2s) that reside in the millions of gas exchanging alveolar sacs can self-renew and differentiate into alveolar type-1 cells (AT1s) and thereby serve as stem cells of the alveoli (14–17). Clinical studies have revealed that mutations in multiple genes specific to AT2s—for example, ABCA3, SFTPB, and SFTPC—are associated with pediatric and adult interstitial lung diseases (18). Additionally, mutations in genes specific to other components of the alveolar niche have been identified to cause lung diseases (19). Therefore, targeted delivery of transgenes into AT2s and specific niche cells for transient or stable expression is of long-standing interest in pulmonary medicine. Significantly, this approach will also provide valuable tools to study the mechanisms that control the dynamic reciprocal interactions between these cell populations during regeneration.
Over the years, different delivery methods, including viral, chemical, peptide, and nanoparticle-based cargoes, have been developed to target airway and alveolar resident stem cells and their niches (20–22). Among these, adeno-associated viruses (AAVs) have been extensively explored for delivering transgenes into multiple cell populations (7, 23–31). AAVs are helper-dependent, single-stranded DNA viruses that naturally infect many species. Their ease of production in large quantities, serotype-specific cell tropism, known viral entry pathways, and propagation mechanisms, as well as their ability to exist as episomes without integration into host cells, all make AAV an ideal vehicle for delivering transgene cargo in both clinical and investigative research applications (32, 33). More important, several naturally occurring AAV variants and serotypes have been isolated from multiple species and tissues, and a series of hybrid and recombinant AAVs have been developed (33–35). These naturally occurring and recombinant libraries offer avenues for finding target cell–specific AAVs. Indeed, recent studies have identified tropism of the AAV6.2FF variant toward lung epithelium, including AT2s and airway club and ciliated cells (3, 26, 27). Similarly, the AAV5 serotype has been shown to infect airway ciliated and club cells in murine lungs (36). Pulmonary tropism of AAV4 in mice and the potential for transducing different cellular subsets in pig airways have also been demonstrated (37, 38). Furthermore, although comparative biology of different AAVs in ferret, pig, and human airway epithelia has also been assessed (39), we currently lack a clear understanding of which AAVs can target specific components of the alveolar stem cell niches (i.e., AT2s, endothelial cells, and fibroblasts). Similarly, we also lack AAVs that can efficiently transduce adult lung-derived AT2s in ex vivo three-dimensional organoid cultures.
Here, we survey the tropism of pseudotyped AAVs with capsids derived from AAV2/4, 2/5, 2/6, 2/8, and 2/9 within the alveolar stem cell niche delivered via either intranasal or systemic routes in mice. We identified AAV serotypes that can infect the alveolar stem cells, endothelial cells, and PDGFRA+ fibroblasts of alveolar tissues. Furthermore, we surveyed the tropism of pseudotyped AAVs in human and mouse alveolar stem cell–derived organoids. To demonstrate the versatility of potential research applications, we used AAV5 to deliver different transgenes into AT2s for stem cell biology applications, including cre-mediated lineage labeling, clonal tracing after cell ablation, and genetic interrogation in postnatal and adult alveoli. We then used AAV-mediated gene delivery for gene editing in alveolar stem cells both ex vivo and in vivo.
Methods
A detailed description of the Materials and Methods is available in the data supplement.
Results
Intranasal Administration of AAVs Reveal Alveolar Stem Cell–Tropic Serotypes In Vivo
To identify AAV serotypes that show tropism toward AT2s or their niche, we tested five AAV capsid serotypes: AAV4, -5, -6, -8, and -9. All serotypes carry a self-complementary vector expressing GFP driven by a truncated chicken β-actin minute virus of mice intron hybrid promoter. We administered AAV-GFP particles to mice at a dose of 2.5 × 1010 viral genomes per mouse intranasally, followed by tissue harvest on Day 6 for histological analysis (Figure 1A). All five AAV serotypes showed variable infection efficiency and cell-type specificity in the airway and alveolar tissues (Figures 1B and 1C). Co-immunostaining for GFP and cell type–specific markers revealed efficient GFP expression in AT2s (SFTPC+) in AAV4- and AAV5-infected lungs but only modest to low GFP expression in AAV9-infected lungs. In contrast, AAV6-, AAV8-, and AAV9-infected lungs showed GFP expression only in CD45+ immune cells in alveolar tissues (Figures 1B and 1C; also see Figure E1 in the data supplement). Notably, none of the serotypes infected AGER+ AT1s, despite their large surface area (Figure 1). In addition to cells in alveoli, AAV4- and AAV6-infected mice showed rare (AAV6) to moderate (AAV4) numbers of GFP-expressing club cells (SCGB1A1+) and small numbers of GFP-expressing ciliated cells (acetylated-TUBULIN) in airways (Figures 1C and E1C). We found no infection in tuft (DCLK1+) or neuroendocrine (CGRP+) cells (Figures E1C and E1D). Of note, none of the other serotypes showed GFP expression in airways (Figure 1C). Quantitative analyses revealed that AAV4 and AAV5 efficiently infect AT2s, whereas AAV6 and AAV8 target CD45+ immune cells (Figures 1D and 1E). Furthermore, in accordance with previous reports, we found that AAV9 infects AT2s. However, our data revealed that ∼50% of AAV9-GFP–infected cells are CD45+ immune cells. Taken together, our data show that intranasally delivered AAV4 and AAV5 have efficient AT2 cell tropism (Figure 1).
Figure 1.
Intranasal delivery of adeno-associated virus (AAV) serotypes transduce murine lung epithelial cells in vivo. (A) Schematic representation of experimental workflow for intranasal AAV delivery followed by tissue collection and analysis. (B) Immunostaining for GFP (green), SFTPC (red), and AGER (gray) on lung sections collected after intranasal delivery of AAV4, AAV5, AAV6, AAV8, or AAV9 serotype. Upper panel: low-magnification images. Middle panel: high-magnification images. Bottom panel: enlarged individual color channels depicted by a white boxed inset. Blue indicates DAPI staining of nuclei. Scale bars: upper panels, 50 μm; middle panels, 20 μm. (C) Immunostaining for GFP (green) and SCGB1A1 (red) on AAV-transduced lungs. White boxed insets are enlarged in the images below. Blue indicates DAPI staining of nuclei. Scale bars, 50 μm. (D) Quantification of GFP+ cells per field of view in lungs transduced with distinct AAVs. Data are presented as mean ± SEM. (n = 3 mice per condition). (E) Quantification of SFTPC+GFP+ and CD45+GFP+ cells out of total GFP+ cells. Data are presented as mean ± SEM. (n = 3 mice per condition). (F) Table summarizing cell tropism in the alveolar region of the lung after intranasal AAV delivery. d = day; FOV = field of view. Plus symbols indicate different levels of virus infection efficiency with four being the highest and one the lowest. Minus symbol indicates no infection.
AAV Cell Tropism Is Dependent on the Route of Administration
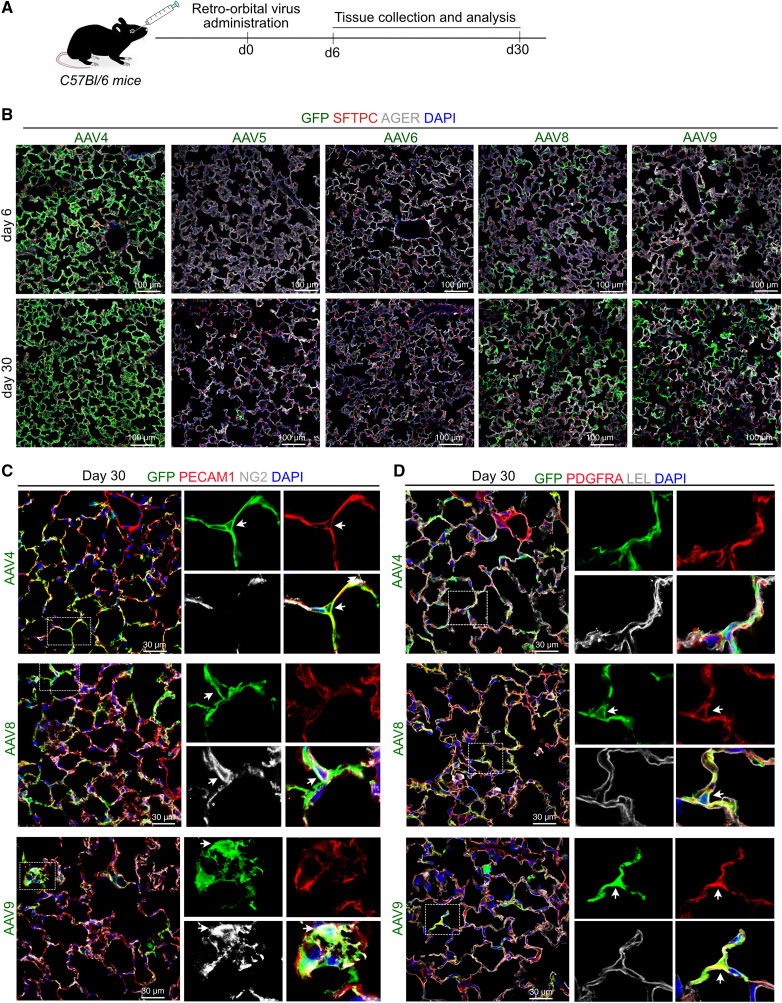
We then tested whether systemic (intravascular) administration would yield a similar pattern of cell type–specific infection to intranasal administration of the same virus. To do this, we administered all the aforementioned AAVs systemically via the retroorbital route, followed by tissue collection and analysis on Days 6 and 30 postinfection (Figure 2A; see Figure E2A). Immunostaining analysis for GFP revealed that retroorbital viral administration led to a dramatically different infection efficiency and tropism compared with intranasal administration (Figures 1, 2B, and E2B). Specifically, AAV4-, AAV8-, and AAV9-infected lungs showed a high number of GFP+ cells, whereas AAV5 and AAV6 showed rare GFP+ cells in alveolar tissues (Figure 2B). Further characterization using cell type–specific markers revealed that retroorbital administration of AAV4 showed high infection in PECAM+ endothelial cells but not in the alveolar epithelium or mesenchymal cells (PDGFRA+ fibroblasts and NG2+ pericytes) (Figures 2C, 2D, E2C, and E2D). In contrast, AAV8 and AAV9 showed GFP enrichment in PDGFRA+ fibroblasts and rarely in NG2+ pericytes but never in endothelial cells (Figures 2C, 2D, E2C, and E2D). Additionally, none of the serotypes transduced smooth muscle cells and immune cells, as shown by immunostaining for ACTA2 and CD45, respectively (Figure E2B). Of note, both AAV8 and AAV9 capsid serotypes only rarely infected Lycopersicon esculentum lectin–positive AT1 cells (Figure E2E). Together, these data reveal that AAV capsid variants display different cell tropisms depending on the route of administration in lung tissues.
Figure 2.
Cell tropism of AAV serotypes in murine lung after retro-orbital virus delivery. (A) Schematic representation of experimental design for retro-orbital AAV administration followed by tissue collection and analysis. (B) Immunostaining for GFP (green), SFTPC (red), and AGER (gray) on lung sections collected on Day 6 and Day 30 post–retro-orbital administration of AAV4, AAV6, AAV5, AAV8, or AAV9 serotype. Blue indicates DAPI staining of nuclei. Scale bars, 100 μm. (C) Immunostaining for GFP (green), endothelial cell marker PECAM1 (red), and pericyte cell marker NG2 (gray) on lung sections collected after retro-orbital delivery of AAV4 (top), AAV8 (middle), or AAV9 (bottom) and tissue collection on Day 30. White boxed insets are enlarged single channels shown on right. Blue indicates DAPI staining of nuclei. Scale bars, 30 μm. (D) Immunostaining for fibroblasts (PDGFRA, in red), alveolar type-1 stem cells (AT1s) (LEL, in gray), and GFP (in green) on lung sections harvested on Day 30 postinfection of mice with AAV4, AAV8, or AAV9. White boxed insets are enlarged single channels on right. Blue indicates DAPI staining of nuclei. Scale bars, 30 μm. LEL = Lycopersicon esculentum lectin. White arrows indicate co-localization of GFP and cell type specific marker.
Efficient AAV5-mediated Transgenesis in Postnatal and Adult Alveolar Stem Cells
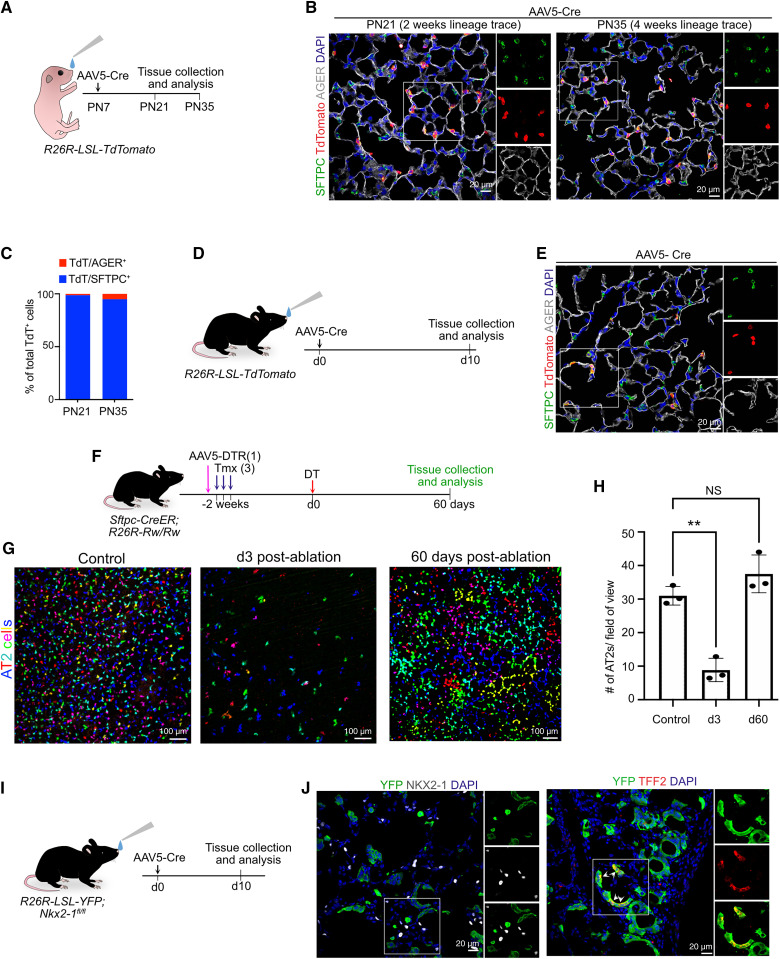
Having established that AAV5 efficiently infects AT2s, we then tested its utility for transgenesis in AT2s during postnatal development and in adult murine lungs in four series of experiments. First, to test whether infection is efficient postnatally, we intranasally administered AAV5 carrying EF1a promoter–driven cre recombinase to Rosa26-LSL-tdTomato (hereinafter termed R26R-tdT) pups on Postnatal Day 7 (Figure 3A). In this model, expression of cre leads to the excision of the stop cassette preceding tdTomato so that AT2s are permanently labeled with tdTomato, and their contribution to other cell types can be tracked over time. Lungs were collected 2 or 4 weeks postinfection for histological analysis. Immunostaining for tdTomato, SFTPC, and AGER revealed efficient tdTomato expression specifically in AT2s but not in AT1s, indicating that AAV5 can be used for targeting AT2s in postnatal AT2s, as in adults (Figures 3B and 3C). Of note, in line with previous studies, we did not find the contribution of lineage-labeled AT2s to AT1s during postnatal development, providing proof of concept that AAV5 can be used for modulating gene expression specifically in AT2s in both adult and early postnatal stages (Figures 3B and 3C) (40, 41). In a second series of experiments, we administered AAV5-Cre to adult R26R-tdT mice intranasally to drive labeling of AT2s with tdTomato (Figures 3D and 3E). Third, we tested the versatility of AAV5-mediated transgenesis for simultaneous clonal cell labeling and cell ablation of AT2s. Currently, such studies are technically challenging because of the prolonged time needed both to breed complex alleles and to titrate cell ablation and labeling of the same cells. For our approach, we first administered tamoxifen to Sftpc-CreER;R26R-Rainbow homozygous mice to induce clonal labeling of AT2s with one or more of the 10 theoretically possible fluorescent protein combinations (42). This model allows for labeling of AT2s to near saturation by titrating tamoxifen dosing. By optimizing spectral delineation, we were able to reproducibly detect six different colors specifically in AT2s (Figures 3F and 3G). To achieve cell ablation, we intranasally administered AAV5-DTR virus to selectively ablate AT2s on diphtheria toxin (DT) administration. As expected, in DT-administered lungs, we observed very few residual AT2s marked by one of the six fluorescent colors (Figures 3G and 3H). Consistent with previous studies, after a single round of ablation followed by a 60-day recovery, we observed replenishment of AT2 cell numbers and single AT2 cell clones occupying large areas and encompassing multiple alveolar sacs (14, 15). These data thus provide proof of concept that AAV5-based transgenesis can be coupled with existing transgenic tools for multimodal manipulation of AT2s in vivo. Finally, in a fourth series of experiments, we evaluated the utility of AAV5-based transgene delivery for conditional loss of gene function. For this, we administered AAV5-Cre virus intranasally to Nkx2–1fl/fl;R26R-LSL-YFP mice in which expression of cre recombinase resulted in concomitant loss of Nkx2–1 and expression of lineage label (yellow fluorescent protein; YFP) in AT2s (Figure 3I). As expected, immunostaining revealed loss of NKX2–1 and gain of gastric markers (TFF2) in YFP+ lineage–labeled cells (Figure 3J). Taken together, the data from these four different approaches reveal that AAV5-mediated transgenesis can be a valuable tool for cell-lineage tracing, cell ablation, and conditional loss of gene function in AT2s during postnatal lung development and adult homeostasis and regeneration (Figure 3).
Figure 3.
AAV5-mediated efficient transgenesis in alveolar type-2 stem cells (AT2s) during postnatal lung development and in adults. (A) Schematic representation of experimental workflow for AAV5-Cre intranasal administration into R26R-LSL-TdTomato PN7 pups followed by lung collection at Postnatal Day (PN)21 and PN35. (B) Immunostaining for SFTPC (green), tdTomato (red), and AGER (gray) in lungs collected 2 weeks (PN21) and 4 weeks (PN35) postinfection. White boxed insets are enlarged single channels shown on right. Blue indicates DAPI staining of nuclei. Scale bars, 20 μm. (C) Quantification of lineage-labeled AT2s (SFTPC+, Tdt+) and AT1s (AGER+, TdT+) out of total tdTomato+ cells at indicated times. (D) Schematic representation of experimental workflow for intranasal administration of AAV5-Cre into R26R-LSL-TdTomato adult mice followed by lung collection and analysis. (E) Staining for SFTPC (green), tdTomato (red), and AGER (gray) in adult lungs collected on Day 10 after AAV5-Cre administration. White boxed insets are enlarged single channels shown on right. Blue indicates DAPI staining of nuclei. Scale bar, 20 μm. (F) Experimental workflow of viral-based AT2 ablation in Sftpc-CreER;R26R-Rw/Rw mice. (G) Representative maximum intensity projections (35 z-stacks) showing the density of AT2s in Sftpc-CreER;R26R-Rw/Rw mice with and without ablation at indicated times. Scale bars, 100 μm. (H) Quantification of AT2 cells in control and ablated lungs on Day 3 and Day 60 (n = 3 mice/condition). One-way ANOVA: Dunnett’s multiple comparisons test. **P = 0.0011 and NS refers to P = 0.1725. Data are presented as mean ± SEM. (I) Schematic representation of experimental design for intranasal administration of AAV5-Cre into R26R-LSL-YFP;Nxk2–1fl/fl mice followed by tissue collection and analysis. (J) Left: costaining for yellow fluorescent protein (YFP; green) and NKX2–1 (gray). Right: Costaining for YFP (green) and TFF2 (red). Blue indicates DAPI staining of nuclei. Scale bar, 20 μm. DT = diphtheria toxin; NS = not significant; Tmx = tamoxifen.
AAV6 Efficiently Infects Human and Mouse AT2 Organoids Ex Vivo
AT2s can be cultured and expanded in three-dimensional alveolospheres (hereinafter termed AT2 organoids), which are thus important tools for studying the self-renewal and differentiation of AT2s. We, therefore, sought to identify AAV serotypes that can efficiently infect AT2 organoids. First, as described previously, we purified murine AT2s (from Sftpc-creER; R26R-tdTomato mice) followed by incubation with all five aforementioned AAVs and culture in Matrigel (see Figure E3A) (43, 44). Human AT2s (HTII-280+ cells) were purified, plated on a Matrigel-coated surface, and cultured for 3 days followed by inoculation with the aforementioned five AAV serotypes for 48 hours (Figure 4A). We found efficient GFP expression in both human and mouse AT2s transduced with AAV6 but not other AAVs (Figures 4B, 4C, and E3B). This is in stark contrast with the in vivo experiments described earlier in which AAV5 efficiently transduced AT2s in murine lungs (Figure 1). Because our organoid cultures are composed of only AT2s, we reasoned that the presence of other cell types may influence AAV cell tropism. To test this, we cocultured AT2s (tdT+) with total lung cells followed by AAV5 or AAV6 inoculation (Figure E3C). Organoid cultures with AT2s alone served as controls. Our data revealed that AAV6 can infect AT2s when cultured alone or in the presence of other lung cells (Figures E3D and E3E). It is interesting that AAV5 was able to infect AT2s in the presence of other lung cells but not when AT2s were cultured alone (Figures E3D and E3E). Further immunostaining analyses for GFP and SFTPC revealed that human infected cells are, indeed, AT2s (Figures 4D and 4E). Together, our data show that AAV6 efficiently infects both human and mouse AT2s in organoids ex vivo.
Figure 4.
AAV6 infects murine and human AT2 organoids ex vivo. (A) Schematic showing experimental design used for infection of human AT2 cells with AAV serotypes. (B) Bright field and GFP (green) images of human organoids infected with indicated AAVs. Scale bars, 100 μm. (C) Quantification of AAV6-GFP infection efficiency (GFP+ organoids/total organoid number). Data are presented as mean ± SEM (n = 3). (D) Immunostaining for GFP (green), TdTomato (red), and SFTPC (gray) in mouse organoid infected with AAV6-GFP virus. Scale bar, 50 μm. (E) Staining for GFP (green) and SFTPC (red) in human organoid infected with AAV6-GFP virus. Scale bar, 20 μm. White boxed insets are enlarged single channels shown on right. Blue indicates DAPI staining of nuclei.
AAV-mediated Genome Engineering in AT2s Both In Vivo and Ex Vivo
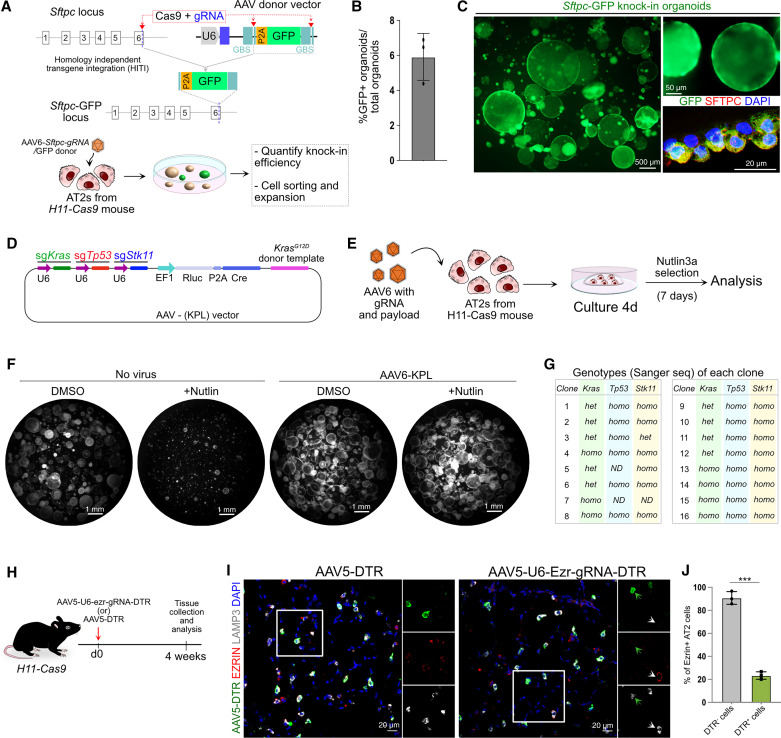
CRISPR-based genome engineering has been widely used for the modulation of stem cells in multiple tissues (1, 4, 6, 11, 36). We, therefore, tested whether AAV-mediated transgene delivery can be used for CRISPR-based genome editing in AT2s. First, we used a recently described homology independent targeting integration method to insert a P2A-GFP cassette into the murine Sftpc locus in AT2s (45). For this, we generated an AAV vector encoding guide RNA (gRNA) designed to target the 3′ end of the Sftpc locus and a “payload” containing P2A-GFP flanked by gRNA binding sites. Purified AT2s from H11-Cas9 mouse were infected with AAV-6-Sftpc-gRNA virus either in cell suspension or 2 days after cell seeding (Figure 5A). Successful gene editing was visualized by GFP expression in AT2 organoids. Quantification of GFP+ organoids revealed an ∼6% knockin efficiency (Figures 5B and 5C; see Figure E4A). To test whether reporter-expressing cells continue to propagate and express GFP, organoids were dissociated and purified using fluorescence-activated cell sorting followed by reseeding (Figure 5C). Passaged cells were able to form organoids and continue to express GFP reporter. Immunostaining revealed coexpression of GFP and AT2 marker SFTPC, indicating that these cells maintain AT2 characteristics (Figure 5C). We further confirmed the successful integration of the P2A-GFP cassette at the target site via Sanger sequencing (Figure E4B).
Figure 5.
AAV-mediated genome engineering in AT2s. (A) Schematic overview of GFP transgene integration at the Sftpc locus and experimental design for virus delivery into AT2 cells purified from H11-Cas9 mouse. (B) Quantification of knockin efficiency is presented as GFP+ organoids per total organoids. Data are presented as mean ± SEM (n = 3). (C) Low- and high-magnification images of organoids expressing GFP (green) introduced by AAV6-based gene delivery. Immunostaining for GFP (green) and SFTPC (red) in Sftpc-GFP knockin organoids. Scale bars: left panel, 500 μm; top right panel, 50 μm; bottom right panel, 20 μm, (D) Schematic map of AAV vector (AAV-KPL) to target known lung adenocarcinoma-associated genes: Kras, Trp53, and Stk11. (E) Overview of gene-editing experiment. AT2s purified from H11-Cas9 mice were infected with AAV6-KPL viral particles followed by culture, nutlin-3a selection, and analysis. (F) Representative images of uninfected and AAV6-KPL–infected organoid images treated with DMSO or Nutlin3a. Scale bars, 1 mm. (G) Table summarizing the results of Sanger sequencing (Sanger seq) of a total of 16 analyzed AT2 organoids. (H) Schematic representation of experimental design for intranasal administration of AAV5-DTR (control) or AAV-U6-ezr-gRNA-DTR followed by tissue collection and analysis. (I) Immunostaining for DTR (green), EZRIN (red), and LAMP3 (gray) in AAV5-DTR–and AAV-U6-ezr-gRNA-DTR virus–infected lungs. White boxed insets are enlarged single channels shown on right. White arrows depict uninfected AT2 cells expressing EZRIN. Green arrows depict EZRIN downregulation in infected AT2 cells. Blue indicates DAPI staining of nuclei. Scale bars, 20 μm. (J) Quantification of Ezrin knockout efficiency in AAV-U6-ezr-gRNA-DTR–infected lungs. Data are presented as mean ± SEM (n = 3). GBS = guide binding site; gRNA = guide RNA; het = heterozygous; homo = homozygous. Unpaired two-tailed t-test. ***P < 0.001.
To investigate the potential for multigene-targeted CRISPR-mediated gene editing, we used a previously described AAV vector (AAV-KPL) to target three known lung adenocarcinoma-associated genes: Kras, Trp53, and Stk11 (also known as Lkb1) (Figure 5D) (46). This construct was designed for simultaneous homology-mediated knockin of mutant KrasG12D, as well as random insertion and/or deletion events in the vicinity of guide binding sites within the Trp53 and Stk11 genomic loci. We generated AAV6-KPL viral particles and inoculated AT2s derived from H11-Cas9 mice and cultured them for 7 days (Figure 5E). To select Trp53 mutant cells, we used a previously described method in which treatment with Nutlin3a, an inhibitor of MDM2, allows the selective growth of Trp53 mutant but not wild-type cells (47). As expected, Nutlin3a treatment blocked the growth of control (no virus) but not AAV-KPL virus–treated cells (Figure 5F). Of note, we observed a slight increase in the colony-forming efficiency and colony size of AAV6-KPL–infected organoids, suggesting that gene-edited cells had a growth advantage. To evaluate genome editing efficiency, we manually picked individual organoids and expanded them separately, followed by genomic DNA isolation and genotyping of target loci. Sanger sequencing of PCR amplicons spanning the target loci revealed successful insertion of the targeting vector in the Kras locus (16/20) and high efficiency indels in one or both alleles of Trp53 (18/20) and Stk11 (16/20) (Figures 5G and E4C). These data collectively serve as a proof of concept that AAV6-mediated gene delivery in AT2s can be used for gene editing and disease modeling studies.
Finally, we evaluated AAV-mediated gene delivery for in situ editing of the AT2 genome in vivo. As a proof of concept, we targeted the Ezrin gene, which encodes an apical membrane protein in AT2s. We generated an AAV vector carrying Ezrin targeting gRNA and CMV-promoter–driven DT receptor (DTR) (hereinafter referred to as AAV-U6-Ezr-gRNA-DTR), which serves as a reporter for infected cells. An AAV5 vector that carries the DTR cassette but not gRNA (hereinafter referred to as AAV-DTR) served as a control (Figure 5H). We first tested the efficiency of two individual gRNAs targeting Ezrin (48) in AT2 cultures and selected gRNA-1 for further studies (see Figure E5). We then generated AAV5-DTR and AAV5-U6-Ezr-gRNA-DTR viral particles and administered them to H11-Cas9 mice intranasally; this was followed by lung collection at 4 weeks postinfection (Figure 5G). Immunostaining for DTR, EZRIN, and LAMP3 (marks AT2s) followed by quantification revealed selective loss of EZRIN in infected cells (DTR+) cells but not in uninfected cells (DTR−) or control virus (AAV5-DTR)–treated lungs (Figures 5I and 5J). In sum, AAV5- and AAV6-mediated gene delivery in AT2s can be used for gene editing in these cells in vivo and ex vivo, respectively.
Discussion
Here, we describe the efficient delivery of transgenes using natural AAV isolates in specific cell types of three different tissue lineages (epithelial, endothelial, and mesenchymal) of the lung alveolus. Specifically, we found that AAV5, AAV4, and AAV8 transduce alveolar epithelial stem cells, alveolar capillaries, and PDGFRA+ fibroblasts, respectively. Notably, we found that intranasal administration of AAV5 efficiently transduces AT2s, whereas no infected cells were found in alveolar tissues on retro-orbital administration of the same virus. Similarly, we found that AAV4 and AAV8 showed different cell tropism, depending on the route of administration. These differences are likely caused by the relative accessibility of receptors on host cells. Further, we found that AAV6, but not AAV5, was able to transduce AT2s in organoid cultures ex vivo. This further highlights the differences in AAV cell tropism and transduction efficiencies of the same target cells in vivo and ex vivo. It is interesting that coculture with total lung cells supported AAV5 infection in AT2s, suggesting that cells in the microenvironment may influence cell tropism.
Multiple studies have tested the delivery of transgenes into lung tissues using various viruses including lentivirus, adenovirus, bocavirus, and recombinant AAVs via intranasal, intratracheal, and systemic routes (23–26, 28–31). For the goal of targeting specific cell types, recent studies have used AAV5-mediated transgene delivery via intratracheal route in mice and found that it can efficiently transduce AT2s and airway epithelial cells, including secretory club cells and ciliated cells (36). Similarly, mutant AAV6.2FF transduces AT2s efficiently but also infects airway cells. A key finding in our work is that intranasal administration of AAV5 efficiently infects AT2s but not airway cells. The reason for the discrepancy between the study by Liang and colleagues (36) and our work is that we delivered AAV5 via the intranasal route, whereas they administered it intratracheally. Of note, we found that AAV5 also infects a small fraction of immune cells. Therefore, caution must be taken while modulating genes that may have a role in immune cells. Nevertheless, lack of AAV5 infection of airway epithelial cells makes this virus a particularly valuable tool for targeting transgene expression specifically in AT2s among all epithelial cells of the lung. We have also shown here that the AAV5 vector system can be used for other applications, including stem cell biology applications, including cre recombinase–mediated cell labeling. We also tested its utility in a sequential and combinatorial transgene modulation protocol designed to simultaneously label AT2s and ablate them for studying stem cell clonal dynamics. This combined approach not only allows saturation clonal labeling but also facilitates different degrees of AT2 ablation by titrating viral load as well as by the dosing of DT. This serves as a proof of concept for future studies that require combinatorial or sequential genetic modulation of multiple alleles. Although we have not tested the utility of AAV4 and AAV8 for gene modulation, we speculate that these viruses can be used for various modalities, as we have demonstrated for AAV5. In addition, recent reports have highlighted the utility of Bocaparvoviruses packaging AAV vector genomes in transducing ferret and human airways (29, 49, 50). Both Bocaviral and other AAV vectors will be the focus of future investigations.
In sum, AAV-mediated transgenesis can help potentially accelerate the investigation of stem cell dynamics and their gene regulatory networks in postnatal and adult lung tissues in vivo as well as in three-dimensional cultures ex vivo. Furthermore, we anticipate that these proof-of-concept studies will provide a roadmap for evaluating gene therapy and therapeutic genome editing approaches to treat lung disease in animal models and humans.
Acknowledgments
Acknowledgment
The authors thank Brigid Hogan for advice and critical reading of the manuscript and members of the Tata lab for fruitful discussions. The authors thank the Duke University BioRepository and Precision Pathology Center (BRPC) for providing human lung tissue.
Footnotes
Supported by a medical scientist training program fellowship (F30HL143911 to A.K.), a Pathways to Independence award (R00HL127181), and research grants (R01HL146557, R01HL160939, and R01HL153375) from the National Heart, Lung, and Blood Institute; by Cancer Center Support Grant P30CA014236 (to P.R.T.) from the National Cancer Institute; by Grants R01HL089221 and R01GM127708 (to A.A.) from the National Institutes of Health; and by funds from the Duke Regeneration Center (to P.R.T. and A.A.). This work was also partially supported by funds from the Whitehead Foundation. The BRPC received support from P30 CA014236 and UM1 CA239755.
Author Contributions: A.K. codesigned, conceived, and performed the experiments and analyzed data; Z.E. codesigned and produced viruses and analyzed data; S.K. performed gene editing in AT2 organoids, AAV transduction in mouse organoids, and immunostaining; L.M. performed immunostaining; H.K. performed gene editing in AT2 organoids; A.T. performed AAV transduction in human AT2 organoids and mice, image acquisition, and data analyses; and A.A. and P.R.T. codesigned, conceived, and supervised the work and cowrote the manuscript. All authors reviewed and edited the manuscript.
This article has a data supplement, which is accessible from this issue’s table of contents online at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2022-0424MA on June 13, 2023
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Alapati D, Zacharias WJ, Hartman HA, Rossidis AC, Stratigis JD, Ahn NJ, et al. In utero gene editing for monogenic lung disease. Sci Transl Med . 2019;11:eaav8375. doi: 10.1126/scitranslmed.aav8375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Flotte TR, Zeitlin PL, Reynolds TC, Heald AE, Pedersen P, Beck S, et al. Phase I trial of intranasal and endobronchial administration of a recombinant adeno-associated virus serotype 2 (rAAV2)-CFTR vector in adult cystic fibrosis patients: a two-part clinical study. Hum Gene Ther . 2003;14:1079–1088. doi: 10.1089/104303403322124792. [DOI] [PubMed] [Google Scholar]
- 3. Kang MH, van Lieshout LP, Xu L, Domm JM, Vadivel A, Renesme L, et al. A lung tropic AAV vector improves survival in a mouse model of surfactant B deficiency. Nat Commun . 2020;11:3929. doi: 10.1038/s41467-020-17577-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nelson CE, Hakim CH, Ousterout DG, Thakore PI, Moreb EA, Castellanos Rivera RM, et al. In vivo genome editing improves muscle function in a mouse model of Duchenne muscular dystrophy. Science . 2016;351:403–407. doi: 10.1126/science.aad5143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tran NT, Sommermann T, Graf R, Trombke J, Pempe J, Petsch K, et al. Efficient CRISPR/Cas9-mediated gene knockin in mouse hematopoietic stem and progenitor cells. Cell Rep . 2019;28:3510–3522.e5. doi: 10.1016/j.celrep.2019.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang Y, Li H, Min YL, Sanchez-Ortiz E, Huang J, Mireault AA, et al. Enhanced CRISPR-Cas9 correction of Duchenne muscular dystrophy in mice by a self-complementary AAV delivery system. Sci Adv . 2020;6:eaay6812. doi: 10.1126/sciadv.aay6812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tang Y, Yan Z, Engelhardt JF. Viral vectors, animal models, and cellular targets for gene therapy of cystic fibrosis lung disease. Hum Gene Ther . 2020;31:524–537. doi: 10.1089/hum.2020.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brown N, Song L, Kollu NR, Hirsch ML. Adeno-associated virus vectors and stem cells: friends or foes? Hum Gene Ther . 2017;28:450–463. doi: 10.1089/hum.2017.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Crowther AJ, Lim SA, Asrican B, Albright BH, Wooten J, Yeh CY, et al. An adeno-associated virus-based toolkit for preferential targeting and manipulating quiescent neural stem cells in the adult hippocampus. Stem Cell Reports . 2018;10:1146–1159. doi: 10.1016/j.stemcr.2018.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Doudna JA. The promise and challenge of therapeutic genome editing. Nature . 2020;578:229–236. doi: 10.1038/s41586-020-1978-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Goldstein JM, Tabebordbar M, Zhu K, Wang LD, Messemer KA, Peacker B, et al. In situ modification of tissue stem and progenitor cell genomes. Cell Rep . 2019;27:1254–1264.e7. doi: 10.1016/j.celrep.2019.03.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Joyeux L, Danzer E, Limberis MP, Zoltick PW, Radu A, Flake AW, et al. In utero lung gene transfer using adeno-associated viral and lentiviral vectors in mice. Hum Gene Ther Methods . 2014;25:197–205. doi: 10.1089/hgtb.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang D, Zhang F, Gao G. CRISPR-based therapeutic genome editing: strategies and in vivo delivery by AAV vectors. Cell . 2020;181:136–150. doi: 10.1016/j.cell.2020.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Barkauskas CE, Cronce MJ, Rackley CR, Bowie EJ, Keene DR, Stripp BR, et al. Type 2 alveolar cells are stem cells in adult lung. J Clin Invest . 2013;123:3025–3036. doi: 10.1172/JCI68782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Desai TJ, Brownfield DG, Krasnow MA. Alveolar progenitor and stem cells in lung development, renewal and cancer. Nature . 2014;507:190–194. doi: 10.1038/nature12930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hogan BLM, Barkauskas CE, Chapman HA, Epstein JA, Jain R, Hsia CCW, et al. Repair and regeneration of the respiratory system: complexity, plasticity, and mechanisms of lung stem cell function. Cell Stem Cell . 2014;15:123–138. doi: 10.1016/j.stem.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tata PR, Rajagopal J. Plasticity in the lung: making and breaking cell identity. Development . 2017;144:755–766. doi: 10.1242/dev.143784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Crossno PF, Polosukhin VV, Blackwell TS, Johnson JE, Markin C, Moore PE, et al. Identification of early interstitial lung disease in an individual with genetic variations in ABCA3 and SFTPC. Chest . 2010;137:969–973. doi: 10.1378/chest.09-0790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Morrell NW. Pulmonary hypertension due to BMPR2 mutation: a new paradigm for tissue remodeling? Proc Am Thorac Soc . 2006;3:680–686. doi: 10.1513/pats.200605-118SF. [DOI] [PubMed] [Google Scholar]
- 20. Finn JD, Smith AR, Patel MC, Shaw L, Youniss MR, van Heteren J, et al. A single administration of CRISPR/Cas9 lipid nanoparticles achieves robust and persistent in vivo genome editing. Cell Rep . 2018;22:2227–2235. doi: 10.1016/j.celrep.2018.02.014. [DOI] [PubMed] [Google Scholar]
- 21. Krishnamurthy S, Wohlford-Lenane C, Kandimalla S, Sartre G, Meyerholz DK, Théberge V, et al. Engineered amphiphilic peptides enable delivery of proteins and CRISPR-associated nucleases to airway epithelia. Nat Commun . 2019;10:4906. doi: 10.1038/s41467-019-12922-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mastorakos P, da Silva AL, Chisholm J, Song E, Choi WK, Boyle MP, et al. Highly compacted biodegradable DNA nanoparticles capable of overcoming the mucus barrier for inhaled lung gene therapy. Proc Natl Acad Sci USA . 2015;112:8720–8725. doi: 10.1073/pnas.1502281112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Aneja MK, Geiger JP, Himmel A, Rudolph C. Targeted gene delivery to the lung. Expert Opin Drug Deliv . 2009;6:567–583. doi: 10.1517/17425240902927841. [DOI] [PubMed] [Google Scholar]
- 24. Excoffon KJDA, Koerber JT, Dickey DD, Murtha M, Keshavjee S, Kaspar BK, et al. Directed evolution of adeno-associated virus to an infectious respiratory virus. Proc Natl Acad Sci USA . 2009;106:3865–3870. doi: 10.1073/pnas.0813365106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Körbelin J, Sieber T, Michelfelder S, Lunding L, Spies E, Hunger A, et al. Pulmonary targeting of adeno-associated viral vectors by next-generation sequencing-guided screening of random capsid displayed peptide libraries. Mol Ther . 2016;24:1050–1061. doi: 10.1038/mt.2016.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. van Lieshout LP, Domm JM, Rindler TN, Frost KL, Sorensen DL, Medina SJ, et al. A novel triple-mutant AAV6 capsid induces rapid and potent transgene expression in the muscle and respiratory tract of mice. Mol Ther Methods Clin Dev . 2018;9:323–329. doi: 10.1016/j.omtm.2018.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Limberis MP, Vandenberghe LH, Zhang L, Pickles RJ, Wilson JM. Transduction efficiencies of novel AAV vectors in mouse airway epithelium in vivo and human ciliated airway epithelium in vitro. Mol Ther . 2009;17:294–301. doi: 10.1038/mt.2008.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rindler TN, Brown KM, Stockman CA, van Lieshout LP, Martin EP, Weaver TE, et al. Efficient transduction of alveolar type 2 cells with adeno-associated virus for the study of lung regeneration. Am J Respir Cell Mol Biol . 2021;65:118–121. doi: 10.1165/rcmb.2021-0049LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yan Z, Feng Z, Sun X, Zhang Y, Zou W, Wang Z, et al. Human bocavirus type-1 capsid facilitates the transduction of ferret airways by adeno-associated virus genomes. Hum Gene Ther . 2017;28:612–625. doi: 10.1089/hum.2017.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yang MS, Park MJ, Lee J, Oh B, Kang KW, Kim Y, et al. Non-invasive administration of AAV to target lung parenchymal cells and develop SARS-CoV-2-susceptible mice. Mol Ther . 2022;30:1994–2004. doi: 10.1016/j.ymthe.2022.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zincarelli C, Soltys S, Rengo G, Rabinowitz JE. Analysis of AAV serotypes 1-9 mediated gene expression and tropism in mice after systemic injection. Mol Ther . 2008;16:1073–1080. doi: 10.1038/mt.2008.76. [DOI] [PubMed] [Google Scholar]
- 32. Kuzmin DA, Shutova MV, Johnston NR, Smith OP, Fedorin VV, Kukushkin YS, et al. The clinical landscape for AAV gene therapies. Nat Rev Drug Discov . 2021;20:173–174. doi: 10.1038/d41573-021-00017-7. [DOI] [PubMed] [Google Scholar]
- 33. Wang D, Tai PWL, Gao G. Adeno-associated virus vector as a platform for gene therapy delivery. Nat Rev Drug Discov . 2019;18:358–378. doi: 10.1038/s41573-019-0012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hildinger M, Auricchio A, Gao G, Wang L, Chirmule N, Wilson JM. Hybrid vectors based on adeno-associated virus serotypes 2 and 5 for muscle-directed gene transfer. J Virol . 2001;75:6199–6203. doi: 10.1128/JVI.75.13.6199-6203.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sen D, Balakrishnan B, Gabriel N, Agrawal P, Roshini V, Samuel R, et al. Improved adeno-associated virus (AAV) serotype 1 and 5 vectors for gene therapy. Sci Rep . 2013;3:1832. doi: 10.1038/srep01832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Liang SQ, Walkey CJ, Martinez AE, Su Q, Dickinson ME, Wang D, et al. AAV5 delivery of CRISPR-Cas9 supports effective genome editing in mouse lung airway. Mol Ther . 2022;30:238–243. doi: 10.1016/j.ymthe.2021.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chen OG, Mather SE, Brommel CM, Hamilton BA, Ehler A, Villacreses R, et al. Transduction of pig amall airway epithelial cells and distal lung progenitor cells by AAV4. Cells . 2021;10:1014. doi: 10.3390/cells10051014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shen S, Troupes AN, Pulicherla N, Asokan A. Multiple roles for sialylated glycans in determining the cardiopulmonary tropism of adeno-associated virus 4. J Virol . 2013;87:13206–13213. doi: 10.1128/JVI.02109-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Liu X, Luo M, Guo C, Yan Z, Wang Y, Engelhardt JF. Comparative biology of rAAV transduction in ferret, pig and human airway epithelia. Gene Ther . 2007;14:1543–1548. doi: 10.1038/sj.gt.3303014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Frank DB, Penkala IJ, Zepp JA, Sivakumar A, Linares-Saldana R, Zacharias WJ, et al. Early lineage specification defines alveolar epithelial ontogeny in the murine lung. Proc Natl Acad Sci U S A . 2019;116:4362–4371. doi: 10.1073/pnas.1813952116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gonzalez R, Leaffer D, Chapin C, Gillespie AM, Eckalbar W, Dobbs L. Cell fate analysis in fetal mouse lung reveals distinct pathways for TI and TII cell development. Am J Physiol Lung Cell Mol Physiol . 2019;317:L653–L666. doi: 10.1152/ajplung.00503.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tanaka T, Komai Y, Tokuyama Y, Yanai H, Ohe S, Okazaki K, et al. Identification of stem cells that maintain and regenerate lingual keratinized epithelial cells. Nat Cell Biol . 2013;15:511–518. doi: 10.1038/ncb2719. [DOI] [PubMed] [Google Scholar]
- 43. Katsura H, Sontake V, Tata A, Kobayashi Y, Edwards CE, Heaton BE, et al. Human lung stem cell-based alveolospheres provide insights into SARS-CoV-2-mediated interferon responses and pneumocyte dysfunction. Cell Stem Cell . 2020;27:890–904.e8. doi: 10.1016/j.stem.2020.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Konishi S, Tata A, Tata PR. Defined conditions for long-term expansion of murine and human alveolar epithelial stem cells in three-dimensional cultures. STAR Protoc . 2022;3:101447. doi: 10.1016/j.xpro.2022.101447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Suzuki K, Tsunekawa Y, Hernandez-Benitez R, Wu J, Zhu J, Kim EJ, et al. In vivo genome editing via CRISPR/Cas9 mediated homology-independent targeted integration. Nature . 2016;540:144–149. doi: 10.1038/nature20565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Platt RJ, Chen S, Zhou Y, Yim MJ, Swiech L, Kempton HR, et al. CRISPR-Cas9 knockin mice for genome editing and cancer modeling. Cell . 2014;159:440–455. doi: 10.1016/j.cell.2014.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Drost J, van Jaarsveld RH, Ponsioen B, Zimberlin C, van Boxtel R, Buijs A, et al. Sequential cancer mutations in cultured human intestinal stem cells. Nature . 2015;521:43–47. doi: 10.1038/nature14415. [DOI] [PubMed] [Google Scholar]
- 48. Doench JG, Fusi N, Sullender M, Hegde M, Vaimberg EW, Donovan KF, et al. Optimized sgRNA design to maximize activity and minimize off-target effects of CRISPR-Cas9. Nat Biotechnol . 2016;34:184–191. doi: 10.1038/nbt.3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yan Z, Keiser NW, Song Y, Deng X, Cheng F, Qiu J, et al. A novel chimeric adenoassociated virus 2/human bocavirus 1 parvovirus vector efficiently transduces human airway epithelia. Mol Ther . 2013;21:2181–2194. doi: 10.1038/mt.2013.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yan Z, Zou W, Feng Z, Shen W, Park SY, Deng X, et al. Establishment of a high-yield recombinant adeno-associated virus/human bocavirus vector production system independent of bocavirus nonstructural proteins. Hum Gene Ther . 2019;30:556–570. doi: 10.1089/hum.2018.173. [DOI] [PMC free article] [PubMed] [Google Scholar]