Acute myocardial infarction (MI) leads to the activation of a systemic inflammatory response which is imperative for tissue healing, but uncontrolled/prolonged inflammation is detrimental to cardiac functional recovery.1 Lysophosphatidic acid (LPA), regulates monocytosis and promotes inflammation in the setting of MI as we have recently reported.2 LPA can be inactivated by lipid phosphate phosphatases (LPPs) and one of these enzymes, LPP3 plays a protective role in vascular injury and tissue healing.3, 4 However, the role of myeloid LPPs in cardiac inflammation post-MI is poorly understood.
We generated a mouse model with myeloid-specific Plpp3 (gene encoding for LPP3) deletion (LysM-Plpp3Δ).4 Myeloid cells in this model lack the expression of LPP3 even when stimulated in vitro.4 There were no significant differences in cardiac function or inflammation at baseline (BSL, unoperated 6-week-old mice) (Fig. 1A, B, and C). LysM-Plpp3fl/fl and LysM-Plpp3Δ underwent MI (permanent LAD ligation) or sham surgery. Myeloid deletion of LPP3 was associated with a higher mean number of cardiac Ly6Chi monocytes (CD45+/Ly6G/C+/CD115+, p<0.01) and pro-inflammatory macrophages (CD45+/F4-80+/CD86+ cells, p<0.001 and CD45+/F4-80+/CD11b+ cells, p<0.001) compared to LysM-Plpp3fl/fl littermate controls (Fig. 1A). Increased monocytosis and bone marrow hematopoietic myeloid progenitor cell proliferation have been closely linked to intense atherogenesis5 and other inflammatory conditions. Flow cytometry analyses of BM cells isolated before and after MI revealed a significantly higher mean number of hematopoietic stem/progenitor cells (HSPCs), granulocyte-myeloid progenitors (GMPs), and common myeloid progenitors (CMPs) in LysM-Plpp3Δ, corresponding with the higher mean number of infiltrating myeloid cells in the myocardium (Fig. 1B). Together, these results demonstrate that myeloid-specific LPP3 deletion results in increased myeloid progenitor cell proliferation and an aggravated inflammatory response after MI.
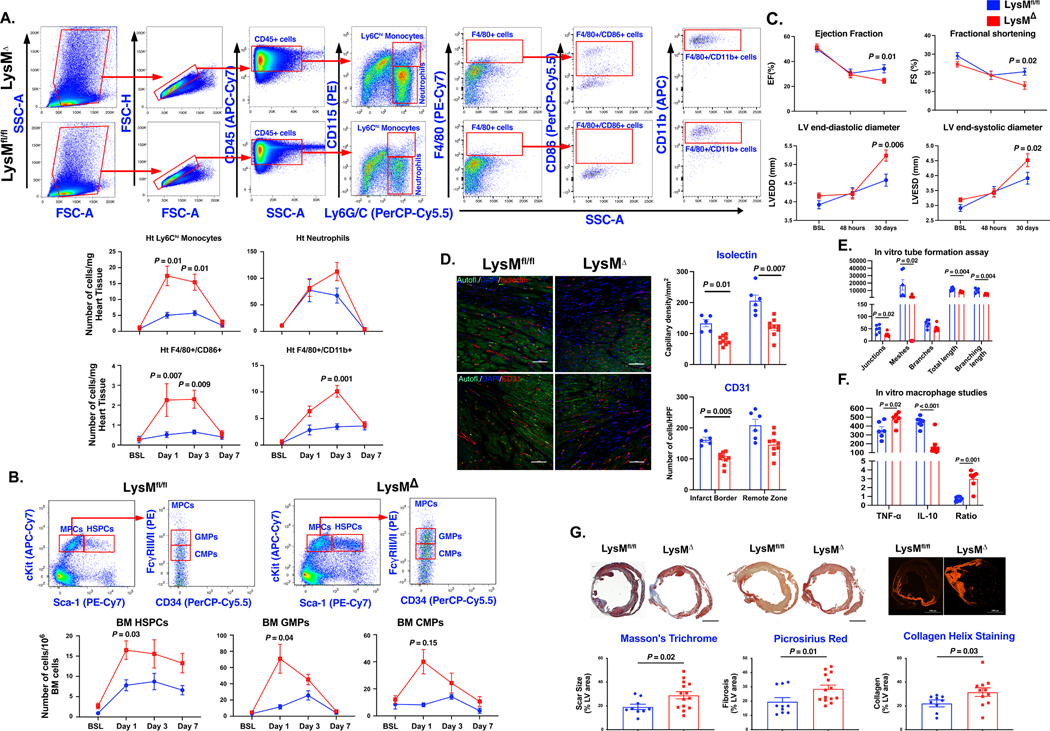
Figure 1. Myeloid deficiency of Plpp3 aggravates cardiac inflammatory response following acute myocardial ischemia.
A, Representative flow cytometry plots and analyses of cardiac inflammatory cells demonstrating elevated Ly6Chi monocytes, pro-inflammatory macrophages, and neutrophils, in LysM-Plpp3Δ mice after myocardial infarction (n=6–8 mice/group/time-point). B, Significant increase in the number of bone marrow HSPCs (Sca-1+/c-Kit+/Lin−), CMPs (Lin−/c-Kit+/Sca-1−/CD16/32−/CD34+), and GMPs (Lin−/c-Kit+/Sca-1−/CD16/32+/CD34+) in LysMΔ mice (n=6–8 mice/group/time-point). C, Echocardiography demonstrates significant deterioration in LV function and remodeling in LysM-Plpp3Δ (n=10–15 mice/group). D, Lower capillary density in mice with myeloid-specific LPP3 deletion (n=5–9 mice/group, scale bars represent 50 μm). E, HUVEC cells showing significant reduction in multiple parameters of tube formation assay when treated with LysM-Plpp3Δ BMDM supernatant (N = 3–5 technical repeats). F, LPS-stimulated LysM-Plpp3Δ BMDM exhibit exacerbated inflammatory response as demonstrated by the higher levels of TNF-α and lower expression of IL-10 (N = 3–5 technical repeats). G, Representative images of Masson’s trichrome, Picrosirius red staining, and Collagen Hybridizing Peptide Cy3 Conjugate (R-CHP) staining (upper panel) performed 30 days after LAD ligation, demonstrating a significant increase in scar size and fibrosis in LysM-Plpp3Δ mice (n=9–14 mice/group/analysis, scale bar = 2 mm). Throughout the figure, data are represented as mean ± SEM. Repeated analyses were conducted using repeated measures ANOVA, the Geisser-Greenhouse correction for unequal variance, and the Sidak posthoc test. Comparison between 2 groups was performed using the Mann-Whitney test.
We performed echocardiography at baseline, 48 hours, and 30 days post-MI. While both experimental groups showed similar cardiac function at baseline and comparable drop at 48 hours, we observed a significantly lower ejection fraction in LysM-Plpp3Δ group at 30 days after injury. Similarly, we observed worse cardiac remodeling, as evidenced by the higher end-systolic and end-diastolic left ventricular diameters, at long-term follow-up in LysM-Plpp3Δ mice (Fig. 1C). We found a significant decrease in capillary density (as assessed by Isolectin and CD31 staining) in LysM-Plpp3Δ mice suggesting a link between the exacerbated inflammatory response and reduced angiogenesis (Fig. 1D). Mechanistically, LPP3 deletion was associated with a reduction in pro-angiogenic secretome from bone marrow-derived macrophages (BMDMs) in vitro (Fig. 1E). Furthermore, BMDMs from LysM-Plpp3Δ mice showed exacerbated inflammatory response to LPS stimulation (Fig. 1F). Subsequently, we assessed the infarct size, fibrotic scar expansion, and collagen deposition and observed a significantly larger scar size and fibrotic area in LysM-Plpp3Δ mice compared to LysM-Plpp3fl/fl littermate controls at 30 days after MI (Fig. 1G).
Cardiac inflammation is detrimental to cardiac recovery after ischemic injury, and there are no clinically approved therapies to combat the ensuing heart failure. Myeloid-specific Plpp3 deletion increases the deleterious effects of inflammation on the ischemic myocardium. These effects could be related to cell-autonomous effects of LPP3 on myeloid cells or its non-cell-autonomous effects on other cell types which will need to be explored in future studies. Together, our studies have uncovered an important and previously unrecognized pathway orchestrating the immune response to MI and may represent a novel therapeutic approach to protect the heart from ischemic injury.
Acknowledgments
Dr. Abdel-Latif is supported by NIH Grant R01 HL124266.
Footnotes
None of the authors have any conflicts of interest/disclosures to report in relation to this manuscript.
References
- 1.Dutta P, Sager HB, Stengel KR, et al. Myocardial Infarction Activates CCR2(+) Hematopoietic Stem and Progenitor Cells. Cell stem cell. 2015;16:477–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tripathi H, Al-Darraji A, Abo-Aly M, et al. Autotaxin inhibition reduces cardiac inflammation and mitigates adverse cardiac remodeling after myocardial infarction. Journal of molecular and cellular cardiology. 2020;149:95–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Panchatcharam M, Salous AK, Brandon J, et al. Mice with targeted inactivation of ppap2b in endothelial and hematopoietic cells display enhanced vascular inflammation and permeability. Arterioscler Thromb Vasc Biol. 2014;34:837–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mueller PA, Yang L, Ubele M, et al. Coronary Artery Disease Risk-Associated Plpp3 Gene and Its Product Lipid Phosphate Phosphatase 3 Regulate Experimental Atherosclerosis. Arterioscler Thromb Vasc Biol. 2019;39:2261–2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murphy AJ, Akhtari M, Tolani S, et al. ApoE regulates hematopoietic stem cell proliferation, monocytosis, and monocyte accumulation in atherosclerotic lesions in mice. The Journal of clinical investigation. 2011;121:4138–49. [DOI] [PMC free article] [PubMed] [Google Scholar]