Abstract
Objectives
Clearing secretions from the airway can be difficult for people with chronic obstructive pulmonary disease (COPD). Mucus clearance devices (MCDs) are an option in disease management to help with this, but healthcare provider awareness and knowledge about them as well as current clinical practice in Saudi Arabia are not known.
Design
A cross-sectional online survey consisting of four themes; demographics, awareness, recommendations and clinical practice, for MCDs with COPD patients.
Setting
Saudi Arabia.
Participants
1188 healthcare providers including general practitioners, family physicians, pulmonologists, nursing staff, respiratory therapists and physiotherapists.
Primary outcome measures
Healthcare providers’ level of awareness about MCDs, and the identification of current clinical practices of COPD care in Saudi Arabia.
Results
1188 healthcare providers (44.4% female) completed the survey. Regarding devices, 54.2% were aware of the Flutter, 23.8% the Acapella and 5.4% the positive expiratory pressure mask. 40.7% of the respondents identified the Acapella, and 22.3% the Flutter as first choice for COPD management. 75% would usually or always consider their use in COPD patients reporting daily difficulty clearing mucus, whereas 55.9% would sometimes or usually consider the use of MCDs with COPD patients who produced and were able to clear mucus with cough. In clinical practice, 380 (32%) of the respondents would prescribe MCDs, 378 (31.8%) would give MCDs without prescriptions, 314 (26.4%) would not provide them at all and 116 (9.8%) would only advise patients about them.
Conclusion
Healthcare providers are aware of the existence of MCDs and their benefits for sputum clearance and believe that MCDs are beneficial for sputum clearance in some COPD patients.
Keywords: respiratory medicine (see thoracic medicine); pulmonary disease, chronic obstructive; chronic airways disease
STRENGTHS AND LIMITATIONS OF THIS STUDY.
The sample size in this study was 1188 healthcare practitioners, which represented both physicians and non-physicians from different geographical locations in Saudi Arabia.
The data were collected using a validated questionnaire about preference of mucus clearance devices (MCDs).
The study was unable to capture actual usage of MCDs in clinical practice due to unavailable prescribing data.
The study included the common options for airway clearance therapy for chronic obstructive pulmonary disease but there are many other MCDs available.
Introduction
Mucus clearance is defined as the removal of secretions from the airway, including by coughing or using an adjunct device.1–3 Clearing mucus is one of the most crucial goals in chronic obstructive pulmonary disease (COPD) management.4 5 When coughing is ineffective in clearing mucus, secretions accumulate in the airways and cause infections resulting in patient deterioration. Mucus clearance devices (MCDs) are proposed as an alternative option to aid people with COPD in airway clearance.4 5 Despite, the traditional therapeutic approaches of mucus clearance, there are different MCDs available in the market to aid airway clearance; however, little is known about their short-term or long-term effects on clinical outcomes.6 The handheld MCD is a small portable device which is activated by the patient exhaling against a resistance valve. This process creates vibrations which keeping the airway open. These vibrations facilitate the movement of mucus, making it simpler to expel.7 Literature presented a variety of mucus devices (eg, Flutter (Allergan, Dublin, Ireland), Acapella (Smiths-Medical, Dublin, Ohio, USA), Lung Flute (Medical Acoustics, Buffalo, New York, USA), RC-Cornet (Cegla Medical Technology, Montabaur, Germany) and Aerobika (Monaghan Medical, Plattsburgh, New York, USA).6
Recent systematic reviews and retrospective prescribing data related to using MCDs in people with COPD suggest that they can improve clinical outcomes and health-related quality of life.3 6 8 Although there has been an incremental effort in the use of MCDs in clinics, the use rate of these devices, as well as the attitudes and perceptions of using them from the perspective of healthcare practitioners (HCPs) have not been evaluated in clinical practice.8 This may be due to a lack of awareness about MCDs and their advantages for managing COPD, a practice gap where these devices are not considered to be a viable alternative to pharmaceuticals or a lack of standards and guidelines concerning adopting the use of MCDs in routine clinical practice.3 A randomised clinical trial of regularly used MCDs with sputum producers in COPD patients showed that they can reduce coughing frequency, improve cough-related quality of life and enhance mucus expectorations.9 Another double-blind randomised clinical trial using MCDs with COPD patients found that they improved maximum inspiratory pressure.10
Across the world, the perceived usefulness of MCDs in COPD management is lacking among HCPs.11–13 In Saudi Arabia, guidelines for COPD care were established in 2014 but are still premature and need further amendments.13 As recent evidence indicates, there are a number of challenges in formulating, structuring and expanding COPD care services in the kingdom, including a lack of awareness about national guidelines, a lack of hospital capacity and a lack of trained healthcare professionals.13 14 A cross-sectional study involving 44 physicians concluded that 65.5% of HCPs appeared unaware of the COPD management guidelines.15 Also, our group previously reported that the lack of experienced staff as well as insufficient knowledge were considered to be significant barriers in COPD management in Saudi Arabia.16 Furthermore, neither international nor local COPD management guidelines emphasised the existence of MCDs as a non-pharmacological treatment for excessive mucus production.13 17–19 To fill this gap, it is important to identify the levels of awareness of MCDs and the routine care of prescribing adjunct sputum devices. Accordingly, this study aims to assess HCPs’ level of awareness about MCDs and COPD management, and to identify current clinical practices related to their use in COPD in Saudi Arabia.
Methods
Study design
The survey was conducted using an online platform (Survey Monkey) between 1 August and 31 December 2022.
Questionnaire
The survey was originally developed and validated by a team of respiratory medicine experts including assessment of face and content validity.3 This survey had been used in COPD clinical studies before and was only available in English (online supplemental file). The online survey (SurveyMonkey.com) consisted of four themes, demographics, awareness, recommendations and clinical practices, for MCDs with COPD patients. The questionnaire focused on the assessment of MCD use with COPD patients, including levels of awareness and clinical practices. We defined MCDs as any physical device used to assist in mucus clearance.20 COPD exacerbation was defined as any deterioration in the symptoms requiring additional treatment.17 The participant could answer the multiple-choice questions using a 5-point Likert scale (ie, ‘always’, ‘usually’, ‘sometimes’, ‘rarely’, ‘never’). The summary and aim of the study and information about the principal investigator were presented to participants before they began filling out the questionnaire. The survey did not collect any personal information. The participants were asked whether they agreed to participate or not. On completing the survey, the following additional statement was provided: ‘By answering ‘yes’ or ‘no’ to the survey questions, you give your consent for your anonymous data to be used for research purposes’. If the participant answered ‘yes’ the page opened to the survey, and if they responded ‘no’, they exited the survey. Approximately 10–15 min were needed to complete the survey.
bmjopen-2023-074849supp001.pdf (1.1MB, pdf)
bmjopen-2023-074849supp002.pdf (85.5KB, pdf)
Data collection
The questionnaires were distributed online. Professional bodies managing respiratory diseases were invited to participate in the data collection. These included the Saudi Society of Family and Community Medicine, the Saudi Thoracic Society (STS), the Saudi Society of Respiratory Care the Saudi Physical Therapy Association and the Saudi Nurses Association. These bodies posted the survey via their social networks (LinkedIn, Twitter, WhatsApp and Telegram) to reach a wider audience of Saudi HCPs. In addition, five authorities from five different medical centres in five different Saudi Arabian provinces contributed to the data collection to ensure countrywide sample representation as well as to guarantee that all of Saudi Arabia’s geographical regions were covered. The targeted population in this study were HCPs who worked with COPD patients, and this was stated clearly in the consent form as well as the invitation to this study.
Sample size calculation
Study participants were recruited using convenience sampling techniques. A primary focus of the study was to reach general practitioners, family physicians, pulmonologists, nursing staff, respiratory therapists and physiotherapists who manage patients with COPD. Due to the exploratory nature of this study, a sample size calculation was not required.
Statistical analysis
The analysis was performed using the SPSS (V.26). Percentages and frequencies were used to report categorical variables. A χ2 test was used to determine the statistically significant difference between categorical variables. Statistical significance was considered if the p<0.05.
Patient and public involvement
No patients involved.
Results
Overall, 1188 HCPs (44.4% female) completed the online survey between 1 August and 31 December 2022. Most of the respondents (75%) worked in government hospitals, while 14.5% worked in rehabilitation centres, and 10.5% worked in primary care clinics. Most of the participants had a bachelor’s degree (68.4%), and 55 (4.6%) of them had completed residency or fellowship programmes. Respiratory therapists accounted for 30% of the participants, followed by family physicians (19.3%), and nurses (15.6%). The majority of respondents had 3–4 (34.8%) or 5–6 (28.1%) years of clinical experience in caring for individuals with COPD, while 22.8% had 1–2 years (table 1).
Table 1.
Characteristics of the study participants
| Gender | Frequency (%) |
| Male | 661 (55.6%) |
| Female | 527 (44.4%) |
| Age | |
| 20–30 | 699 (58.8%) |
| 31–40 | 329 (27.7%) |
| 41–50 | 114 (9.6%) |
| 51–60 | 38 (3.2%) |
| >60 | 8 (0.75%) |
| Nationality | |
| Saudi | 1023 (86.1%) |
| Non-Saudi | 165 (13.9%) |
| Medical centres | |
| Governmental/private hospitals | 891 (75.0%) |
| Rehabilitation centres | 172 (14.5%) |
| Primary care clinics | 125 (10.5%) |
| Geographical location | |
| Central Region | 184 (15.5%) |
| Eastern Region | 218 (18.4%) |
| Northern Region | 122 (10.3%) |
| Southern Region | 452 (38.0%) |
| Western Region | 212 (17.8%) |
| Academic and clinical qualifications | |
| Associate diploma | 105 (8.8%) |
| Bachelor’s degree | 812 (68.4%) |
| Master’s degree | 159 (13.4%) |
| Medical Board Residency/Fellowship | 55 (4.6%) |
| PhD degree | 56 (4.7%) |
| Role (profession) | |
| General physicians | 135 (11.4%) |
| Family physicians | 229 (19.3%) |
| Pulmonary physicians | 98 (8.2%) |
| Nursing staff | 185 (15.6%) |
| Respiratory therapists | 356 (30%) |
| Physiotherapists | 67 (5.6%) |
| Others | 118 (9.9%) |
| Years of experience with COPD patients | |
| 1–2 | 271 (22.8%) |
| 3–4 | 413 (34.8%) |
| 5–6 | 341 (28.7%) |
| 7–8 | 76 (6.4%) |
| >8 | 87 (7.3%) |
Data are presented as frequencies and percentages.
COPD, chronic obstructive pulmonary disease.
Awareness of MCDs
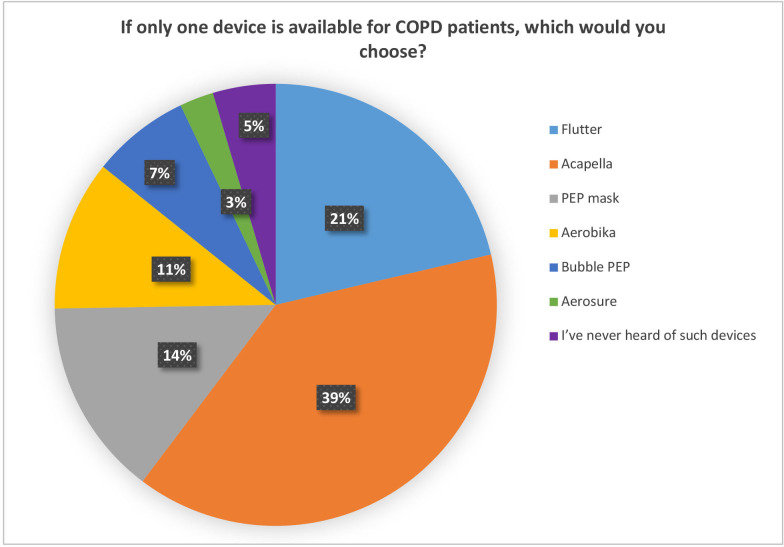
The second theme in the survey dealt with awareness regarding MCDs. 54.2% of the respondents were aware of Flutter and 23.8% of Acapella devices, followed by 5.4% for the positive expiratory pressure (PEP) mask. For COPD care, 40.7% of the respondents chose Acapella, and 22.3% chose Flutter as their preferred device; these are the most commonly prescribed MCDs. As an option for COPD care, 15.1% of the respondents chose PEP mask, 11.5% chose Aerobika, 7.5% chose Bubble PEP and 2.6% chose Aerosure (figure 1).
Figure 1.
Mucus clearance device preference (n=1188). COPD, chronic obstructive pulmonary disease; PEP, positive expiratory pressure.
Recommending MCDs for COPD management in clinical practice
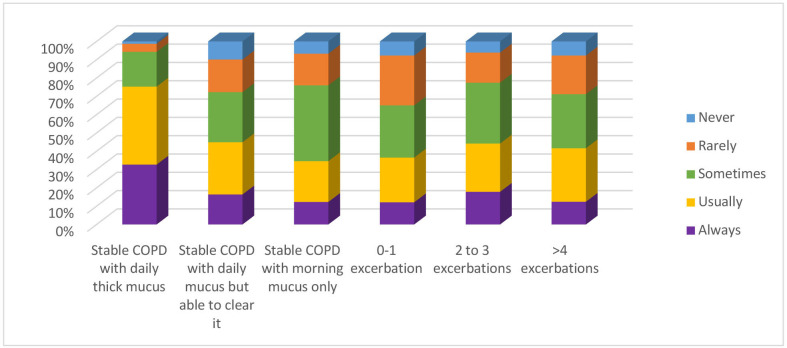
The third theme in the survey dealt with recommending MCDs for COPD management in clinical practice. Of the respondents, 75% said they would usually or always consider the use of an MCD with a COPD patient who had daily difficulty clearing mucus, whereas 55.9% of the respondents said they would sometimes or usually consider the use of an MCD with a COPD patient who produced the mucus and was able to clear it with a cough. Of the respondents, 63% said they would sometimes or usually consider the use of an MCD with a COPD patient who produced mucus in the morning only.
When the HCPs were asked about how often they would recommend using an MCD for COPD patients with exacerbations, there was a range in their responses. 51.6% said they would rarely or sometimes consider using an MCD for a COPD who had exacerbations 0–1 times per year, 59.7% would sometimes or usually consider using an MCD for a COPD patient who had 2–3 exacerbations per year and 58.7% of the HCPs would sometimes or usually consider using an MCD with a COPD patient who had >4 exacerbations (figure 2).
Figure 2.
Threshold to consider use of mucus clearance devices for chronic obstructive pulmonary disease (COPD) management (n=1188).
Clinical practice for using MCDs
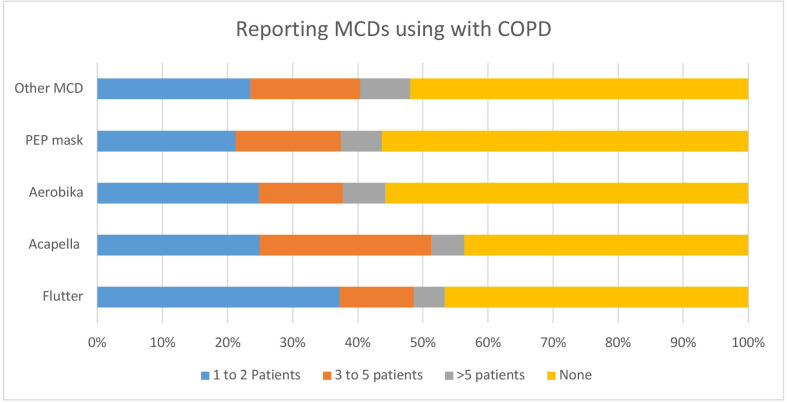
When the participants were asked about how many patients with COPD had started on MCDs in the last 6 months, 441 (37.1%) of the respondents had started Flutter, 297 (25%) of the respondents started Acapella, 295 (24.8%) started Aerobika and 253 (21.3%) started a PEP mask in at least one COPD patient (table 2).
Table 2.
Clinical practice for using MCDs in the last 6 months (n=1188)
| MCDs | Frequencies for using MCDs | |||
| 1–2 patients | 3–5 patients | >5 patients | None | |
| Flutter | 441 (37.1%) | 134 (11.3%) | 56 (4.7%) | 557 (46.4%) |
| Acapella | 297 (25.0%) | 313 (26.3%) | 60 (5.1%) | 518 (43.6%) |
| Aerobika | 295 (24.8%) | 153 (12.9%) | 77 (6.5%) | 663 (55.8%) |
| PEP mask | 253 (21.3%) | 191 (16.1%) | 75 (6.3%) | 669 (56.3%) |
| Other MCDs | 279 (23.5%) | 201 (16.9%) | 91 (7.7%) | 617 (51.9%) |
Data are presented as frequencies and percentages.
MCDs, mucus clearance devices; PEP, positive expiratory pressure.
In providing MCDs in clinical practice, 380 (32%) of the respondents said they would prescribe MCDs, 378 (31.8%) said they would give MCDs without prescriptions, 314 (26.4%) would not provide them at all and 116 (9.8%) would only advise patients about them (figure 3). Most of the respondents prescribed or recommended MCDs for COPD patients based on the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guideline for COPD (20.1%), followed by the STS (STS) guidelines (20%) (online supplemental table).
Figure 3.
Clinical practice for using MCDs in the last 6 months (n=1188). COPD, chronic obstructive pulmonary disease; MCDs, mucus clearance devices; PEP, positive expiratory pressure.
Discussion
This is the first Saudi national study to report the use of MCDs in clinical practice. The results demonstrate that awareness about MCDs in clinical practice exists in general but there are differences in preferences for device among HCPs as well as around the threshold of symptoms where a device would be recommended. Flutter and Acapella were the most frequently prescribed devices compared with other MCDs in COPD management. Among all the participants, using MCDs were accepted in such management but there were different responses regarding the use of MCDs with exacerbated patients. The data on prescribing MCDs revealed that the Acapella and Flutter devices were favoured in the clinical setting. The treatment recommendation for COPD was based on the GOLD guidelines.
In clinical settings, patients with COPD with persistent productive coughs are common but there are few steps taken to deal with this.6 21 Our results demonstrate that awareness about assisting COPD patients with MCDs is present among HCPs in Saudi Arabia but there are differences in their responses regarding the role of MCDs in treating COPD. This is perhaps because of the lack of evidence that emphasises the importance of using non-pharmacological treatment in COPD management.8 13 In addition, MCDs have received less attention as a treatment for stable and exacerbated COPD patients. This is, perhaps, owing to a lack of knowledge13 15 16 or the lack of adopting guideline recommendations about the potential role of MCDs in COPD management.15
HCPs had a strong preference for Flutter and Acapella for mucus clearance. But with COPD management, Acapella, particularly, was the most favoured device. This is consistent with a survey that was carried out previously in the UK concerning MCDs for COPD patients.3 In that research, HCPs were more likely to use Acapella for COPD management compared with other MCDs.3
Current evidence supports the use of both Acapella and Flutter as common options for airway clearance therapy for COPD, but there are many other MCDs available.22–24 It is the case that MCDs receive less attention in clinical practice because of the lack of awareness about their effectiveness in COPD management. However, evidence is still emerging to support their use in this management.9 10 For example, a recent randomised clinical trial of using Acapella treatment versus the active cycle of breathing technique in stable COPD patients over 3 months yielded promising results. The study demonstrated significant values for the regular use of these devices. After 3 months of regular use of the Acapella in stable COPD patients, cough-related quality of life, as well as mucus clearance, significantly improved.8 9
In COPD management, increased mucus clearance and the control of symptoms via MCDs is a desirable goal, and clinicians must consider this in treating COPD patients.22 25 Our analysis has revealed that recommendations for MCDs for COPD patients were following different guidelines to those being used to prescribe them. This is an indicator that clinical practice is missing the best practice strategy by not recommending MCDs.15 16 26 However, it must be remembered that domestic clinical practice guidelines cannot be generalised to fit all clinical centres and hospitals in Saudi Arabia as there are other aspects to be considered, such as maturity of COPD care in the kingdom as well as the cost and availability of the devices.13 16 27
Our findings show that, in general, most clinicians would give MCDs to COPD patients with or without prescriptions. This is attributed to the fact that these devices, like any other non-pharmacological treatments, have fewer contraindications compared with pharmacological treatment.5 In addition, managing COPD and controlling symptoms require a bundle of treatments, of which MCDs are but one.28
At the clinician level, family physicians numbered the fewest clinicians in terms of providing MCDs for COPD patients. This may be because of the physicians’ generally limited perception concerning the benefits of non-pharmacological treatments, including MCDs in COPD management. This was explored by Aldhahir et al who reported the perceptions of Saudi Arabian physicians concerning non-pharmacological treatment for COPD.16 A lack of experience and lack of enough information were considered to be challenges in clinical practice.16 Perhaps real-time clinical data on MCD prescriptions would give us a clearer picture of their use in clinical settings.
Our findings show that recommending MCDs is usually driven by medical judgement rather than clinical guidelines. Similarities have been found regarding MCD prescribing in different parts of the world.5 24 29 This may be attributed to the growing clinical evidence regarding MCD effectiveness in COPD management.8 In Saudi Arabia, there are a limited number of advanced COPD clinics that provide comprehensive COPD management, including MCD training.16 27 30 The use of MCDs, like any other airway clearance technique, needs training for both patients and HCPs.31 The establishment of telehealth approaches to deliver training, conduct follow-ups with patients, and to monitor adherence to MCD guidelines has already been proposed.9 This approach was found suitable and effective during the COVID-19 outbreak for demonstrating, instructing, and following up with COPD patients who used MCDs.9 32 33
According to this study, MCD preference could be driven by their availability at the clinical centre or the features of the MCD itself. For example, Acapella devices have certain mechanical advantages, such as being gravity-independent, which allows the patient to use the device in any position.34 This field of research is growing globally and there are always new devices that provide the same functions and help COPD patients with sputum clearance. Future research may focus on comparing these devices one-to-one to further inform the medical guidelines, as well as help reach a clinical consensus.
As this is the first national survey about MCDs in Saudi Arabia, several lessons have been learnt from this research. First, we have found that the perceived benefits of MCDs among clinicians vary. Second, it seems that medical judgement and recommendations guide the application of MCDs rather than the clinical guidelines. At present, the clinical guidelines for COPD management in Saudi Arabia still neglect the use of MCDs. Third, there is still insufficient data related to the use of MCDs compared with mucolytics or medications. It is hoped that the data from this study will inform the current practice regarding MCDs in general, as well as with COPD patients, as an option in clinical practice.
Strengths and limitations
This study has several strengths. First, it is the first national Saudi cross-sectional study to explore and report on MCDs use in clinical practice. Second, the participants in this study were from multiple clinical centres, and they were all dealing with COPD, thus, offering extended validity for the results presented here. These results could serve as a baseline for future work in this growing field of the evaluation of MCD use. However, they must be interpreted with caution. The survey focused on four MCDs while there are many more in use in clinical practice. Even though our sample covers HCPs from multiple backgrounds, we may not have captured the full response to and all the perceptions of others regarding the use of MCDs in clinical practice. It would be helpful if future research compared our data with clinical or prescription data.
Conclusion
HCPs are aware of the existence of MCDs and their benefits for sputum clearance. HCPs believe that MCDs are beneficial in sputum clearance with stable and exacerbated COPD patients. However, real-time clinical data recording the use of MCDs is lacking, and further efforts are required to explore the actual usage of MCDs.
Supplementary Material
Footnotes
Twitter: @saeedmordy, @DrAbdulelah1989, @COPDdoc
Correction notice: This article has been corrected since it was first published. Trial registration number has been removed from the article.
Contributors: SMA and NSH were responsible for the conceptualisation, designing, obtaining ethical approval for the study, and wrote the first version of the manuscript. AA, YMA, AAAR, ASA and RAS did the acquisition of the data and data validity. AHA, AMA, AMOA, JSA, AMZ, STJ and BAA planned and run statistical analysis of the data and interpreted data. SMA, NSH, AA, YMA, AAAR, ASA, RAS, AHA, AMA, AMOA, JSA, AMZ, STJ and BAA contributed to and approved the final version of this manuscript. SMA is responsible for the overall content as guarantor and he has full responsibility for the work and/or the conduct of the study, had access to the data, and controlled the decision to publish.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s)
Ethics approval
The Institutional Review Board approval for the study was obtained from Umm Al-Qura University, ID number HAPO-02-K-012-2022-09-1205.
References
- 1.Chung KF, McGarvey L, Song W-J, et al. Cough hypersensitivity and chronic cough. Nat Rev Dis Primers 2022;8:45. 10.1038/s41572-022-00370-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hughes R, Rapsomaniki E, Janson C, et al. Frequent productive cough: symptom burden and future exacerbation risk among patients with asthma and/or COPD in the NOVELTY study. Respiratory Medicine 2022;200:106921. 10.1016/j.rmed.2022.106921 [DOI] [PubMed] [Google Scholar]
- 3.Barker R, Laverty AA, Hopkinson NS. Adjuncts for Sputum clearance in COPD: clinical consensus versus actual use. BMJ Open Respir Res 2017;4:e000226. 10.1136/bmjresp-2017-000226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tse J, Wada K, Wang Y, et al. Impact of oscillating positive Expiratory pressure device use on post-discharge hospitalizations: a retrospective cohort study comparing patients with COPD or chronic Bronchitis using the Aerobika® and Acapella® devices. Int J Chron Obstruct Pulmon Dis 2020;15:2527–38. 10.2147/COPD.S256866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bourbeau J, McIvor RA, Devlin HM, et al. Oscillating positive Expiratory pressure (OPEP) device therapy in Canadian respiratory disease management: review, care gaps and suggestion for use. Canad J Resp Crit Care Sleep Med 2019;3:233–40. 10.1080/24745332.2018.1558426 [DOI] [Google Scholar]
- 6.Alghamdi SM, Barker RE, Alsulayyim ASS, et al. Use of oscillatory positive Expiratory pressure (OPEP) devices to augment Sputum clearance in COPD: a systematic review and meta-analysis. Thorax 2020;75:855–63. 10.1136/thoraxjnl-2019-214360 [DOI] [PubMed] [Google Scholar]
- 7.Svenningsen S, Paulin GA, Sheikh K, et al. Oscillatory positive Expiratory pressure in chronic obstructive pulmonary disease. COPD 2016;13:66–74. 10.3109/15412555.2015.1043523 [DOI] [PubMed] [Google Scholar]
- 8.Lewis A, Osadnik CR. Changing practice by changing pressures: a role for oscillating positive Expiratory pressure in chronic obstructive pulmonary disease. Thorax 2023;78:113–5. 10.1136/thorax-2022-219451 [DOI] [PubMed] [Google Scholar]
- 9.Alghamdi SM, Alsulayyim AS, Alasmari AM, et al. Oscillatory positive Expiratory pressure therapy in COPD (O-COPD): a randomised controlled trial. Thorax 2023;78:136–43. 10.1136/thorax-2022-219077 [DOI] [PubMed] [Google Scholar]
- 10.Daynes E, Greening N, Singh SJ. Randomised controlled trial to investigate the use of high-frequency airway Oscillations as training to improve dyspnoea (tide) in COPD. Thorax 2022;77:690–6. 10.1136/thoraxjnl-2021-217072 [DOI] [PubMed] [Google Scholar]
- 11.Cooper L, Johnston K, Williams M. Australian airway clearance services for adults with chronic lung conditions: A national survey. Chron Respir Dis 2023;20:14799731221150435. 10.1177/14799731221150435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rose L, McKim D, Leasa D, et al. Monitoring cough effectiveness and use of airway clearance strategies: a Canadian and UK survey. Respir Care 2018;63:1506–13. 10.4187/respcare.06321 [DOI] [PubMed] [Google Scholar]
- 13.Khan JH, Lababidi HMS, Al-Moamary MS, et al. The Saudi guidelines for the diagnosis and management of COPD. Ann Thorac Med 2014;9:55–76. 10.4103/1817-1737.128843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aldhahir AM, Alghamdi SM, Alqahtani JS, et al. Pulmonary rehabilitation for COPD: A narrative review and call for further implementation in Saudi Arabia. Ann Thorac Med 2021;16:299–305. 10.4103/atm.atm_639_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alsubaiei ME, Frith PA, Cafarella PA, et al. COPD care in Saudi Arabia: physicians' awareness and knowledge of guidelines and barriers to implementation. Int J Tuberc Lung Dis 2017;21:592–5. 10.5588/ijtld.16.0656 [DOI] [PubMed] [Google Scholar]
- 16.Aldhahir AM, Alqahtani JS, Alghamdi SM, et al. Physicians’ attitudes, beliefs and barriers to a pulmonary rehabilitation for COPD patients in Saudi Arabia: a cross-sectional study. Healthcare 2022;10:904. 10.3390/healthcare10050904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2007;176:532–55. 10.1164/rccm.200703-456SO [DOI] [PubMed] [Google Scholar]
- 18.Qaseem A, Wilt TJ, Weinberger SE. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American college of physicians, American college of chest physicians. Ann Intern Med 2011;155:179. 10.7326/0003-4819-155-3-201108020-00008 [DOI] [PubMed] [Google Scholar]
- 19.Bourne S, DeVos R, North M, et al. Online versus face-to-face pulmonary rehabilitation for patients with chronic obstructive pulmonary disease: randomised controlled trial. BMJ Open 2017;7:e014580. 10.1136/bmjopen-2016-014580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Osadnik CR, McDonald CF, Holland AE. Airway clearance techniques in acute exacerbations of COPD: a survey of Australian Physiotherapy practice. Physiotherapy 2013;99:101–6. 10.1016/j.physio.2012.01.002 [DOI] [PubMed] [Google Scholar]
- 21.Hull JH, Langerman H, Ul-Haq Z, et al. Burden and impact of chronic cough in UK primary care: a Dataset analysis. BMJ Open 2021;11:e054832. 10.1136/bmjopen-2021-054832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Belli S, Prince I, Savio G, et al. Airway clearance techniques: the right choice for the right patient. Front Med (Lausanne) 2021;8:544826. 10.3389/fmed.2021.544826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Daynes E, Jones AW, Greening NJ, et al. The use of airway clearance devices in the management of chronic obstructive pulmonary disease. A systematic review and meta-analysis of randomized controlled trials. Ann Am Thorac Soc 2021;18:308–20. 10.1513/AnnalsATS.202005-482OC [DOI] [PubMed] [Google Scholar]
- 24.Westerdahl E, Osadnik C, Emtner M. Airway clearance techniques for patients with acute exacerbations of chronic obstructive pulmonary disease: physical therapy practice in Sweden. Chron Respir Dis 2019;16:1479973119855868. 10.1177/1479973119855868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Osadnik CR, McDonald CF, Jones AP, et al. Airway clearance techniques for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2012;2012:CD008328. 10.1002/14651858.CD008328.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alsubaiei ME, Cafarella PA, Frith PA, et al. Current care services provided for patients with COPD in the eastern province in Saudi Arabia: a descriptive study. Int J Chron Obstruct Pulmon Dis 2015;10:2379–91. 10.2147/COPD.S89456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aldhahir AM, Alqahtani JS, AlDraiwiesh IA, et al. Healthcare providers’ attitudes, beliefs and barriers to pulmonary rehabilitation for patients with chronic obstructive pulmonary disease in Saudi Arabia: a cross-sectional study. BMJ Open 2022;12:e063900. 10.1136/bmjopen-2022-063900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boutou AK, Tanner RJ, Lord VM, et al. An evaluation of factors associated with completion and benefit from pulmonary rehabilitation in COPD [BMJ open respiratory research 2014;1:e000051]. BMJ Open Resp Res 2014;1:e000051. 10.1136/bmjresp-2014-000051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yohannes AM, Connolly MJ. A national survey: percussion, vibration, shaking and active cycle breathing techniques used in patients with acute exacerbations of chronic obstructive pulmonary disease. Physiotherapy 2007;93:110–3. 10.1016/j.physio.2006.07.003 [DOI] [Google Scholar]
- 30.Alqahtani JS. Prevalence, incidence, morbidity and mortality rates of COPD in Saudi Arabia: trends in burden of COPD from 1990 to 2019. PLoS One 2022;17:e0268772. 10.1371/journal.pone.0268772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ides K, Vissers D, De Backer L, et al. Airway clearance in COPD: need for a breath of fresh air? A systematic review. COPD 2011;8:196–205. 10.3109/15412555.2011.560582 [DOI] [PubMed] [Google Scholar]
- 32.Alghamdi SM, Alqahtani JS, Aldhahir AM. Current status of Telehealth in Saudi Arabia during COVID-19. J Fam Community Med 2020;27:208. 10.4103/jfcm.JFCM_295_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.MDPI . Digital health platforms in Saudi Arabia: determinants from the COVID-19 pandemic experience. Health Care (Don Mills) 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poncin W, Reychler G, Liistro M, et al. Comparison of 6 oscillatory positive Expiratory pressure devices during active Expiratory flow. Respir Care 2020;65:492–9. 10.4187/respcare.07271 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2023-074849supp001.pdf (1.1MB, pdf)
bmjopen-2023-074849supp002.pdf (85.5KB, pdf)
Data Availability Statement
Data are available upon reasonable request.