Abstract
Objectives
The primary objective of our study was to evaluate the effectiveness of renal cell carcinoma (RCC) screening in renal transplant (RT) recipients.
Design
Single-centre retrospective study.
Setting and participants
1998 RT recipients who underwent RT at Memorial Hermann Hospital (MHH) Texas Medical Center (TMC) between 1 January 1999 and 31 December 2019 were included and we identified 16 patients (0.8%) with RCC. An additional four patients with RCC who underwent RT elsewhere but received follow-up at MHH TMC were also included. Subject races included white (20%), black (50%), Hispanic (20%) and Asian (10%).
Outcome measures
The RCC stage at diagnosis and outcomes were compared between patients who were screening versus those who were not.
Results
We identified a total of 20 patients with post-RT RCC, 75% of whom were men. The median age at diagnosis was 56 years. RCC histologies included clear cell (75%), papillary (20%) and chromophobe (5%). Patients with post-RT RCC who had screening (n=12) underwent ultrasound or CT annually or every 2 years, whereas eight patients had no screening. All 12 patients who had screening had early-stage disease at diagnosis (stage I (n=11) or stage II (n=1)) and were cured by nephrectomy (n=10) or cryotherapy (n=2). In patients who had no screening, three (37.5%) had stage IV RCC at diagnosis and all of whom died of metastatic disease. There was a statistically significant difference in RCC-specific survival in patients who were screened (p=0.01) compared with those who were not screened.
Conclusion
All RT recipients who had RCC diagnosed based on screening had early-stage disease and there were no RCC-related deaths. Screening is an effective intervention in RT recipients to reduce RCC-related mortality.
Keywords: kidney tumours, renal transplantation, end stage renal failure
STRENGTHS AND LIMITATIONS OF THIS STUDY.
The key strength of this study is the large number of patients who were screened for renal cell carcinoma (RCC) after renal transplant (RT), which is a topic that is not well studied in existing literature.
A major limitation of this study is the small number of patients who developed RCC after an RT.
There is a possibility of selection bias since certain patients were omitted due to paucity of information in medical records.
Due to the retrospective nature of this study, confounding variables may exist which could lead to bias.
This study is susceptible to a time period bias due to the long time period from which data were included.
Introduction
Renal transplantation is the mainstay of treatment for patients with end-stage renal disease (ESRD). Better immunosuppressive strategies have significantly improved renal allograft survival, but post-transplant malignancies remain a major obstacle to improving long-term survival.1 Renal transplant (RT) recipients have a fivefold to sixfold increased risk of developing renal cell carcinoma (RCC) compared with the general population.2 The risk factors for RCC in the general population include smoking, male sex, hypertension, family history, acquired cystic disease, longer duration of dialysis and African American race.2 3 The additional contributing factors in RT recipients include immunosuppression, oncogenic viruses and changes in immune surveillance.1 2 4 Most cases of RCC post-RT arise in the native kidney; however, these cancers also rarely arise from the allograft. Modern immunosuppression has significantly reduced the incidence of post-RT acute and chronic rejections. Data also show that 60–80% of patients survive for >10 years after their first transplant.5 With the reduction in mortality in RT recipients, the risk of developing malignancies associated with immunosuppression may increase.
The role of screening in reducing gastric and colorectal cancer mortality in RT recipients has been clearly demonstrated; however, the role of screening for RCC remains controversial and is not incorporated into the standard of care for these patients.6–8 Moreover, there have been very few studies examining the effectiveness of RCC screening in RT recipients. In this retrospective study, we evaluated the role of screening for early detection of RCC in RT recipients at our institution.
Methods
Design and setting
This is a retrospective observational study where we reviewed the medical records of patients aged 18–75 years who underwent kidney transplantation at Memorial Hermann Hospital (MHH), Texas Medical Center between 1 January 1999 and 31 December 2019.
Exclusion criteria
Patients were excluded if they developed a malignancy within 30 days of a transplant under the assumption that the malignancy was present prior to transplantation. Patients were also excluded if there was a pre-transplant history of active malignancy within 5 years or if there was a documented death of a patient from unknown cause within 1 year of transplant. Lastly, patients were excluded if there were incomplete medical records for analysis including unreported malignancy status or incomplete information on immunosuppression. All clinical data for the RT recipients were collected from our departmental electronic database.
Data collection
The following data were collected: baseline demographic variables, cause of ESRD, duration of dialysis prior to RT, type of RT (cadaveric vs living donor), immunosuppression regimen, site of RCC (native kidney vs transplanted kidney), time from RT to diagnosis of RCC, stage and Fuhrman grade of RCC, tumour histology, RCC treatment and RCC-related mortality. Tumours were staged according to the American Joint Committee on Cancer Staging Manual, eighth edition. The histological diagnosis of the patients was based on the diagnosis made during initial biopsy/resection.
The RT recipients who developed RCC were identified and divided into two groups: those who received post-transplant screening for RCC in the form of imaging (ultrasonography or CT) and those who did not receive any screening for RCC. The decision to pursue screening and determining the frequency of screening for RCC was based on the discretion of the provider caring for the patient and there was no clear screening institutional protocol implemented. The stage at diagnosis of RCC and RCC-related mortality were compared between these two groups. Patients with incomplete medical records or follow-up were excluded from the data set.
Statistical analysis
Descriptive statistics are presented in percentages, medians and IQRs. Continuous variable analysis was performed with Kruskal-Wallis one-way analysis of variance and t-test for non-normal and normal distribution, respectively. As for categorical variables, χ2 or Fisher’s exact test was used. The Kaplan-Meier method and the log-rank test were used to compare survival rates between the two groups. Statistical significance was set at a p≤0.05. Data were analysed using GraphPad Prism V.9.0.2.
Patient and public involvement
None.
Results
A total of 1998 patients were identified who underwent RT between 1 January 1999 and 31 December 2019. Among the 1998 patients, 16 (0.8%) developed RCC after RT. Baseline characteristics are provided in table 1. Forty-seven patients with RCC in the setting of RT were identified and 22 patients were excluded due to their history of pre-transplant RCC. Five patients without adequate data were excluded. An additional four patients with post-RT RCC who underwent transplantation elsewhere but received follow-up at MHH were also included. Therefore, a total of 20 patients with post-RT RCC were identified. Most of the patients had a cadaveric renal transplant (74.3%). Among the 1998 patients, 1799 (90%) underwent RT alone, and of those who underwent simultaneous transplant, 146 (7.3%) received a pancreas, 41 (2.05%) received a liver and 12 (0.60%) received a heart in addition to RT. Immunosuppressive regimens consisted of a calcineurin inhibitor, mycophenolate mofetil, mammalian target of rapamycin inhibitor and glucocorticoids. In addition, antilymphocyte globulin was used for induction until 2003; after that, this was replaced by basiliximab.
Table 1.
Baseline characteristics of renal transplant (RT) recipients diagnosed with renal cell carcinoma (RCC)
| Characteristic | Value |
| Age, mean (range), years | 52 (18–83) |
| Sex, n | |
| Male | 15 |
| Female | 5 |
| Type of renal transplant | |
| DDKT, n | 2 |
| LDKT, n | 3 |
| Both DDKT and LDKT | 15 |
| Patients who had more than one kidney transplant, n | 8 |
| Patients who developed RCC after first kidney transplant in native kidney | 2 |
| Patients who developed RCC after first kidney transplant in allograft | 3 |
| Duration of dialysis prior to transplant, median (range), months | 36 (1–120) |
| Time from RT to diagnosis of RCC, median (range), months | 96 (16–312) |
| Site of RCC | |
| Native kidney | 12 |
| Transplanted kidney | 8 |
| TNM staging of RCC | |
| Stage I | 14 |
| Stage II | 1 |
| Stage III | 2 |
| Stage IV | 3 |
| Histology of RCC | |
| Clear cell | 15 |
| Papillary | 4 |
| Chromophobe | 1 |
| Treatment for malignancy | |
| Nephrectomy alone | 18 |
| Cryotherapy | 2 |
| Systemic chemotherapy/immunotherapy | 3 |
DDKT, deceased donor kidney transplantation; LDKT, living donor kidney transplantation; TNM, tumour, node, metastases.
The median age of identified patients with RCC was 56 years. Subject races included white (20%), black (50%), Hispanic (20%) and Asian (10%). The most common causes of ESRD were diabetes and hypertension. Eight patients (40%) received a second RT for initial graft failure (mainly from chronic allograft nephropathy), five of whom had a history of RCC after their first transplant. The median duration of dialysis prior to RT was 36 months (range: 1–120 months).
The clinical profile of RT recipients with post-transplant RCC is provided in table 2. For the 20 RT recipients who developed RCC, the median time to RCC diagnosis post-RT was 96 months (range: 16–312 months). A smoking history was noted in eight patients (40%), and six patients (30%) were obese (body mass index >30); four patients (70%) had a history of systemic hypertension. None of the patients had a family history of RCC. RCC histologies included clear cell (75%), papillary (20%) and chromophobe (5%) RCC. RCC after RT was diagnosed in the native kidney in 12 patients (60%) and in the allograft in 8 patients (40%). Table 3 compares the histology of the tumours, immunosuppressive regimen and the management of patients with native versus allograft RCC.
Table 2.
Clinical profile of renal transplant (RT) recipients with post-transplant renal cell carcinoma (RCC)
| Age range (years) (sex) | Duration of dialysis prior to RT (months) | Post-RT RCC screening | Stage | Histology | Site of RCC | Treatment | Graft function | Survival |
| 60–69 (F) | 12 | Yes | I | Clear cell | Allograft | Transplant nephrectomy | Failed | Alive |
| 50–59 (M) | 6 | Yes | I | Papillary | Native | Nephrectomy | Failed | Alive |
| 40–49 (M) | 72 | Yes | I | Chromophobe | Native | Nephrectomy | Preserved | Alive |
| 10–19 (M) | 1 | No | I | Papillary | Allograft | Transplant nephrectomy | Failed | Alive |
| 80–89 (M) | 12 | Yes | I | Papillary | Native | Percutaneous cryotherapy | Preserved | Alive |
| 50–59 (F) | 36 | Yes | I | Clear cell | Native | Nephrectomy | Preserved | Alive |
| 60–69 (F) | 84 | Yes | I | Clear cell | Allograft | Transplant nephrectomy | Failed | Alive |
| 40–49 (M) | 72 | No | IV | Clear cell | Native | Immunotherapy | Failed | Died from metastatic RCC |
| 60–69 (M) | 72 | Yes | II | Clear cell | Native | Nephrectomy | Failed | Alive |
| 30–39 (M) | 24 | Yes | I | Clear cell | Native | Nephrectomy | Failed | Alive |
| 60–69 (M) | 60 | Yes | I | Clear cell | Native | Nephrectomy | Preserved | Alive |
| 50–59 (M) | 48 | No | I | Clear cell | Allograft | Transplant nephrectomy | Failed | Died in 2021 from COVID-19 |
| 60–69 (F) | 72 | Yes | I | Clear cell | Allograft | Cryotherapy | Brief dialysis support followed by renal recovery | Alive |
| 20–29 (M) | 36 | No | III | Clear cell | Native | Nephrectomy | Failed | Alive |
| 60–69 (M) | 24 | Yes | I | Clear cell | Native | Nephrectomy | Failed | Died from gastric cancer |
| 30–39 (M) | 4 | No | IV | Papillary | Allograft | Transplant nephrectomy with pelvic lymph nephrectomy | Preserved | Died from metastatic RCC |
| 40–49 (M) | 120 | Yes | I | Clear cell | Allograft | Transplant nephrectomy | Failed | Died in 2018 from sepsis |
| 60–69 (M) | 36 | No | III | Clear cell | Native | Nephrectomy | Preserved | Alive |
| 50–59 (F) | 12 | No | I | Clear cell | Allograft | Transplant nephrectomy | Failed | Alive |
| 50–59 (M) | 72 | No | IV | Clear cell | Native | Sunitinib | Preserved | Died in 2012 from metastatic RCC |
Table 3.
Comparison of patients with RCC in the native kidney versus allograft RCC
| RCC in native kidney (n=12) | RCC in allograft (n=8) | P value | |
| Gender | |||
| Male, n | 11 | 4 | 0.1089 |
| Female, n | 1 | 4 | |
| Histology | |||
| Clear cell | 9 | 6 | >0.9999 |
| Papillary | 2 | 2 | >0.9999 |
| Chromophobe | 1 | 0 | >0.9999 |
| Type of immunosuppression | |||
| Tacrolimus, MMF, prednisone | 9 | 5 | 0.6424 |
| Ciclosporin, prednisone | 3 | 3 | 0.6424 |
| Sirolimus | 2 | 4 | 0.1611 |
| Treatment for malignancy | |||
| Nephrectomy alone | 9 | 7 | |
| Cryotherapy | 1 | 1 | |
| Systemic chemotherapy/ immunotherapy | 2 | 0 | |
MMF, mycophenolate mofetil; RCC, renal cell carcinoma.
Patients with post-RT RCC were divided into those who underwent regular screening (n=12) and those who did not (n=8). The regular screening group (n=12) underwent ultrasound or CT annually or every 2 years (annual=9, every 2 years=3). All patients who received regular screening had early-stage disease at presentation: stage I (n=11) or stage II (n=1). All patients who had RCC detected by screening underwent nephrectomy (n=10) or cryotherapy (n=2). Dialysis was resumed in four patients (20%) after definitive treatment for RCC. One patient who underwent cryotherapy for RCC in the allograft developed acute kidney injury requiring dialysis support for a few weeks and then recovered renal function. Two patients received a second RT and had no evidence of RCC recurrence. There was no RCC-related mortality in patients who underwent regular screening. One patient diagnosed with stage I RCC in the native kidney was diagnosed simultaneously with early-stage bladder cancer and underwent transurethral resection of the bladder tumour. He was diagnosed with gastric cancer 6 months later and was treated with immunotherapy. He developed acute graft rejection and went back on dialysis. He later died from metastatic gastric cancer.
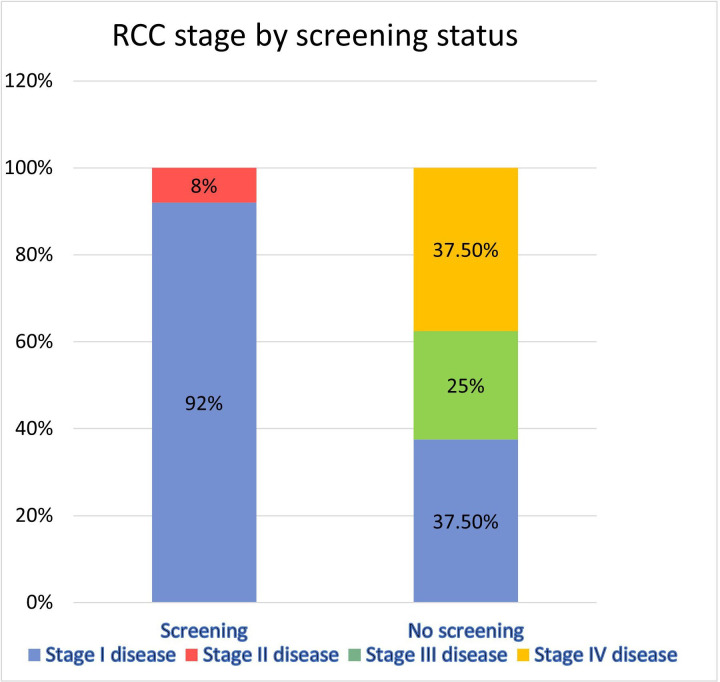
In patients who had no screening after RT (n=8), three (37.5%) had stage I, two (25%) had stage III and three (37.5%) had stage IV RCC (figure 1). All three patients with stage IV RCC died from metastatic disease. The mean interval from diagnosis of metastatic RCC to death was 7.3 months. One of the patients with metastatic RCC was treated with carboplatin and paclitaxel, one received sunitinib and the third was treated with immune checkpoint inhibitors and a VEGF/TKI (vascular endothelial growth factor/tyrosine kinase inhibitor) agent.
Figure 1.
RCC stage at the time of diagnosis in patients who underwent screening versus no screening. RCC, renal cell carcinoma.
Patients who received screening had earlier-stage disease than those who were not screened (p=0.001). The mean age in the screening group was 59.25 years compared with 41.38 in the no screening group (p=0.007). The mean duration of dialysis in patients who received screening was 43.5 months compared with 35.8 months in those who were not screened (p=0.62). The mean time from RT to diagnosis of RCC in patients who received screening was 105.3 months compared with 102 months in those who were not screened (p=0.9).
Among the 20 patients with post-RT RCC, 4 developed second primary malignancies (breast cancer (n=1), prostate cancer (n=1), post-transplant lymphoproliferative disorder (n=1) and gastric cancer (n=1)). Except for the patient with gastric cancer, the other three patients are alive and in remission from their malignancies.
At 12 months from the time of diagnosis of RCC, the RCC-related mortality was 0% in the screening group, compared with 37.5% in patients who had no screening. There was a statistically significant difference in RCC-specific survival in patients who were screened (p=0.01) when compared with those who did not receive screening (figure 2). However, no statistically significant difference was noted in the overall survival of the two groups (HR for death: 0.32; 95% CI 0.05 to 2.09; p=0.11). No difference in RCC-related survival was noted between patients with native versus allograft RCC (p=0.8).
Figure 2.
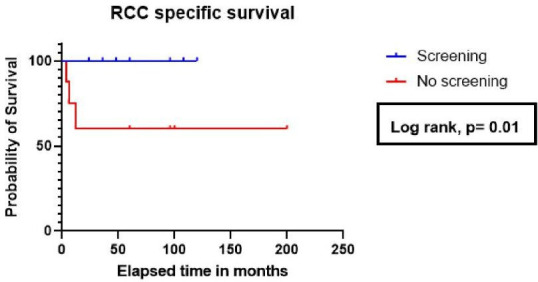
RCC-specific survival by screening status post-renal transplant. RCC, renal cell carcinoma.
Discussion
The incidence of RCC has increased steadily over the past three decades.9–11 RCC is about twice as common in men than in women, and it is more common in African Americans. The duration of dialysis prior to RT is an important risk factor: 60–80% of patients undergoing renal replacement therapy for ≥4 years develop acquired cystic kidney disease, and 15–20% of these cases may transform into multifocal RCC.12 Although clear cell RCC tends to be the most common histology, the risk of papillary RCC is significantly higher in RT recipients than in the non-transplant population.2 Interestingly, an increased risk of RCC is also observed in other solid organ transplant recipients (SOTRs).13 It is possible that in addition to immunosuppression and other risk factors, more frequent imaging in SOTRs could also be contributing to the higher detection rate of renal tumours.
The 5-year survival rate of stage 1 RCC is 93%.14 However, early detection of this malignancy is challenging since early-stage RCC can remain relatively asymptomatic given its retroperitoneal location. Most RCCs are slow growing and have a preclinical phase lasting for 3.7–5.8 years. When patients present with symptomatic disease such as flank pain or haematuria, they often already have local advanced or metastatic RCC.14 The proposed benefit of screening is based on the hypothesis that early detection and treatment of malignant kidney tumours within the preclinical phase would lead to better survival outcomes. Klein et al showed that routine ultrasound surveillance of native kidneys in the pre-transplant population would allow for early detection of RCC.15 Hence, screening can potentially identify patients with early-stage disease, and this has been identified by experts as an area of unmet need.16
RCC in RT recipients poses unique challenges. Immunotherapy, one of the pillars of RCC treatment, carries a significant risk of graft rejection. In the general population, patients with intermediate or high risk of recurrence of RCC are treated with adjuvant pembrolizumab after nephrectomy.17 However, it is important to note that adjuvant immunotherapy is not an option in the post-RT setting. Similarly, in RT recipients with metastatic RCC, immunotherapy carries a similar risk of graft rejection, and it is unclear whether immunotherapy provides a clear survival advantage.18 In this study, among the three patients who were diagnosed with metastatic RCC, one was treated with carboplatin and paclitaxel, one received sunitinib and the third was treated with immune checkpoint inhibitors and a VEGF/TKI agent. All three patients eventually died from metastatic cancer.
The treatment for RCC can adversely affect allograft function. Screening may allow the identification of smaller tumours that can be successfully treated with minimally invasive nephron-sparing surgeries or cryotherapy, which would lead to less morbidity. In our study, the two patients in the screening group who developed RCC in their allografts were successfully treated with cryotherapy.
There are conflicting recommendations from major society guidelines on the role of screening in this population. The European Association of Urologists recommends annual screening for early detection of RCC.19 However, the American Society of Transplantation and Kidney Disease Improving Global Outcomes guidelines do not recommend screening for RCC in RT recipients, as it is not backed by high-quality evidence. Moreover, there is no consensus on the optimum screening modality. Ultrasound is an attractive, less expensive, easily available imaging modality with a sensitivity of 82–83% and specificity of 98–99%. However, ultrasound is operator dependent and may not be sensitive enough to detect early lesions, especially in obese patients and those with acquired cystic kidney disease.20 Contrast-enhanced CT might be a better screening tool in kidneys with acquired cysts, although the cost, risk of contrast nephropathy and radiation exposure remain a major concern.14 21 Similar to lung cancer screening, the potential role of low-dose CT scanning for screening purposes should be evaluated further.
Given the escalating cost of healthcare delivery, the cost-effectiveness of any screening modality should be an important consideration. Wong et al compared the cost versus benefit of ultrasound screening for RCC in RT recipients (n=1000) and determined that although it may reduce RCC-related mortality by up to 25%, routine screening may not be cost-effective.22 However, two recent studies have shown otherwise. Using the Markov model, Roizman et al proposed that screening for RCC in the general population using ultrasonography could be a cost-effective option.23 Another study used a similar model and suggested that RCC screening in the general population may be cost-effective in males. This cost-effectiveness was not demonstrated in females due to a lower prevalence of RCC.24 Additional research into the cost-effectiveness of screening is important. However, the cost-effectiveness of screening in post-RT patients has yet to be evaluated at all, highlighting the need for prospective data evaluating the benefit of RCC screening in RT recipients.
Another important consideration for RCC screening is to follow an individualised approach focusing on certain high-risk subgroups of RT recipients. Our study population had several high-risk features for RCC: 75% were men, 50% were African Americans, 40% had a smoking history, 30% were obese, 70% had hypertension and 45% had acquired cystic kidney disease. All of these factors are independently associated with a higher risk of RCC.25 26 Some of the RCC cases were detected incidentally on imaging done for other reasons. Although one may argue that routine screening in all RT recipients may not be feasible and cost-effective, we strongly believe that screening is a highly effective strategy in reducing RCC-related mortality in the high-risk RT population. The optimal frequency of imaging is unknown; however, in our study, it was clear that patients who had imaging annually or every 2 years had a clear RCC-specific mortality benefit compared with patients who had no screening.
The major strength of this study is the inclusion of a large number of patients who underwent RT. Although the sample size of patients who developed RCC after RT was small, it is important to note that there was an RCC-specific survival benefit noted in patients who underwent screening for RCC compared with those who did not, which was statistically significant. The incorporation of routine screening resulted in the diagnosis of early-stage RCC which did positively affect survival outcomes.
Given the non-randomised retrospective nature of this study, a major limitation includes potential selection bias as patients with incomplete key data recorded in medical records were excluded. Given that data were collected for a period of 20 years, the data are not immune to a period time bias due to the development and changes in immunosuppression regimens. This may have influenced the long-term incidence of RCC in this group of patients. The retrospective nature of this study also allows for the influence of potential confounding factors that could not be adequately measured which can also allow for a misclassification bias. Our study may lack external validity given that the sample size is not representative of the general renal transplant population. Our study included 75% men and 50% African Americans. Hence, the benefits of screening in an ultra-high-risk population cannot be generalised to all RT recipients.
Despite these limitations, we believe that this study offers valuable insight into an aspect of the management of RT recipients that has not been studied well. Given improvement in immunosuppression regimens and longer survival of patients who have undergone RT, it is important to consider the potential for increasing incidence of malignancies such as RCC in these patients. It is important to further delve into research looking to improve the outcomes of this cohort of patients.
Conclusion
RT recipients have a significantly increased risk of RCC. Screening in the form of ultrasonography and/or CT every year or every 2 years appears to be an effective tool for early detection of RCC. Further research is required to identify high-risk patients who will benefit from screening, and prospective data are required to evaluate the cost-effectiveness and utility of screening in patients who have undergone RT.
Supplementary Material
Footnotes
BY and AS contributed equally.
Contributors: BY—data collection, analysis and writing the manuscript, guarantor. AS—data collection, analysis and writing the manuscript, guarantor. HK—data collection, critically reviewing the manuscript for intellectual content. AD—critically reviewing the manuscript for important intellectual content. NM—critically reviewing the manuscript for important intellectual content.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
This study was approved by the University of Texas (UT) Health Science Center Institutional Review Board (IRB) (UT Health IRB number HSC-MS-21-0751).
References
- 1.Rama I, Grinyó JM. Malignancy after renal transplantation: the role of immunosuppression. Nat Rev Nephrol 2010;6:511–9. 10.1038/nrneph.2010.102 [DOI] [PubMed] [Google Scholar]
- 2.Karami S, Yanik EL, Moore LE, et al. Risk of renal cell carcinoma among kidney transplant recipients in the United States. Am J Transplant 2016;16:3479–89. 10.1111/ajt.13862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim CS, Han K-D, Choi HS, et al. Association of hypertension and blood pressure with kidney cancer risk: A nationwide population-based cohort study. Hypertension 2020;75:1439–46. 10.1161/HYPERTENSIONAHA.120.14820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Danpanich E, Kasiske BL. Risk factors for cancer in renal transplant recipients. Transplantation 1999;68:1859–64. 10.1097/00007890-199912270-00008 [DOI] [PubMed] [Google Scholar]
- 5.Dahle DO, Skauby M, Langberg CW, et al. Renal cell carcinoma and kidney transplantation: A narrative review. Transplantation 2022;106:e52–63. 10.1097/TP.0000000000003762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collins MG, Teo E, Cole SR, et al. Screening for colorectal cancer and advanced colorectal Neoplasia in kidney transplant recipients: cross sectional prevalence and diagnostic accuracy study of Faecal Immunochemical testing for Haemoglobin and colonoscopy. BMJ 2012;345:e4657. 10.1136/bmj.e4657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee I-S, Kim T-H, Kim Y-H, et al. Clinical significance of gastric cancer surveillance in renal transplant recipients. World J Surg 2012;36:1806–10. 10.1007/s00268-012-1605-1 [DOI] [PubMed] [Google Scholar]
- 8.Wong G, Li MWY, Howard K, et al. Health benefits and costs of screening for colorectal cancer in people on dialysis or who have received a kidney transplant. Nephrol Dial Transplant 2013;28:917–26. 10.1093/ndt/gfs490 [DOI] [PubMed] [Google Scholar]
- 9.Saad AM, Gad MM, Al-Husseini MJ, et al. Trends in renal-cell carcinoma incidence and mortality in the United States in the last 2 decades: A SEER-based study. Clin Genitourin Cancer 2019;17:46–57. 10.1016/j.clgc.2018.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Znaor A, Lortet-Tieulent J, Laversanne M, et al. International variations and trends in renal cell carcinoma incidence and mortality. Eur Urol 2015;67:519–30. 10.1016/j.eururo.2014.10.002 [DOI] [PubMed] [Google Scholar]
- 11.King SC, Pollack LA, Li J, et al. Continued increase in incidence of renal cell carcinoma, especially in young patients and high grade disease: United States 2001 to 2010. J Urol 2014;191:1665–70. 10.1016/j.juro.2013.12.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scandling JD. Acquired cystic kidney disease and renal cell cancer after transplantation: time to rethink screening Clin J Am Soc Nephrol 2007;2:621–2. 10.2215/CJN.02000507 [DOI] [PubMed] [Google Scholar]
- 13.Engels EA, Pfeiffer RM, Fraumeni JF, et al. Spectrum of cancer risk among US solid organ transplant recipients. JAMA 2011;306:1891. 10.1001/jama.2011.1592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fenton JJ, Weiss NS. Screening computed tomography. Cancer 2004;100:986–90. 10.1002/cncr.20055 Available: http://doi.wiley.com/10.1002/cncr.v100:5 [DOI] [PubMed] [Google Scholar]
- 15.Klein JA, Gonzalez SA, Fischbach BV, et al. Routine Ultrasonography surveillance of native kidneys for renal cell carcinoma in kidney transplant candidates. Clin Transplant 2016;30:946–53. 10.1111/ctr.12769 [DOI] [PubMed] [Google Scholar]
- 16.Jones J, Bhatt J, Avery J, et al. The kidney cancer research priority-setting partnership: identifying the top 10 research priorities as defined by patients, Caregivers, and expert Clinicians. Can Urol Assoc J 2017;11:379–87. 10.5489/cuaj.4590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choueiri TK, Tomczak P, Park SH, et al. Adjuvant Pembrolizumab after nephrectomy in renal-cell carcinoma. N Engl J Med 2021;385:683–94. 10.1056/NEJMoa2106391 [DOI] [PubMed] [Google Scholar]
- 18.Venkatachalam K, Malone AF, Heady B, et al. Poor outcomes with the use of Checkpoint inhibitors in kidney transplant recipients. Transplantation 2020;104:1041–7. 10.1097/TP.0000000000002914 [DOI] [PubMed] [Google Scholar]
- 19.Dunnick NR. Renal cell carcinoma: staging and surveillance. Abdom Radiol (NY) 2016;41:1079–85. 10.1007/s00261-016-0692-0 [DOI] [PubMed] [Google Scholar]
- 20.Rossi SH, Klatte T, Usher-Smith J, et al. Epidemiology and screening for renal cancer. World J Urol 2018;36:1341–53. 10.1007/s00345-018-2286-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diana P, Klatte T, Amparore D, et al. Screening programs for renal cell carcinoma: a systematic review by the EAU young academic Urologists renal cancer working group. World J Urol 2022;41:929–40. 10.1007/s00345-022-03993-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong G, Chapman JR, Craig JC. Cancer screening in renal transplant recipients: what is the evidence. Clin J Am Soc Nephrol 2008;3:S87–100. 10.2215/CJN.03320807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roizman S, Leshno M, Haifler M, et al. A cost-benefit analysis for Sonographic screening for renal tumors. JCO 2018;36:665. 10.1200/JCO.2018.36.6_suppl.665 [DOI] [Google Scholar]
- 24.Rossi SH, Klatte T, Usher-Smith JA, et al. A decision analysis evaluating screening for kidney cancer using focused renal ultrasound. Eur Urol Focus 2021;7:407–19. 10.1016/j.euf.2019.09.002 [DOI] [PubMed] [Google Scholar]
- 25.Sanfilippo KM, McTigue KM, Fidler CJ, et al. Hypertension and obesity and the risk of kidney cancer in 2 large cohorts of US men and women. Hypertension 2014;63:934–41. 10.1161/HYPERTENSIONAHA.113.02953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chow W-H, Dong LM, Devesa SS. Epidemiology and risk factors for kidney cancer. Nat Rev Urol 2010;7:245–57. 10.1038/nrurol.2010.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request.