Abstract
The pace of locomotor development is a critical component of lifetime evolutionary fitness. Developmental researchers often divide species into two broad categories based on functional competence at birth: precocial infants who can independently stand and locomote soon after birth versus altricial infants who are either incapable of independent movement or can only do so in a rudimentary manner. However, investigating the lower level neuromotor and biomechanical traits that account for perinatal variation in motor development is complicated by the lack of experimental control inherent to all comparative analyses. Precocial and altricial animals often differ along a host of dimensions that can obfuscate the specific factors controlling motor development per se. Here, we propose an alternative approach of examining locomotor development in a nominally precocial species—the domestic pig (Sus scrofa)—in which gestation length has been experimentally manipulated, thereby creating “functionally altricial” cohorts for comparison. We have used standard biomechanical testing to evaluate balance and locomotor performance in preterm pigs born at 94% full-term gestation (N = 29 individuals) and compared these data to a similar dataset on age-matched full-term piglets (N = 15 individuals). Static balance tests showed that preterm pigs were characterized by increased postural sway, particularly in the fore-aft (anteroposterior) direction. Locomotor analyses showed that preterm piglets tended to take shorter, more frequent strides, use higher duty factors, and preferentially choose gait patterns that ensured they were supported by at least three limbs during most of the stride cycle, though differences between preterm and full-term animals were often modulated by variation in locomotor speed. Morphometric analysis showed no differences in relative extensor muscle mass between preterm and full-term animals, suggesting that neurological immaturity might be more determinant of preterm piglet motor dysfunctions than musculoskeletal immaturity per se (though much work remains to be done to fully document the neuromotor phenotype of the preterm infant pig model). In many ways, the postural and locomotor deficits shown by the preterm piglets paralleled the locomotor phenotype of altricial mammals. Overall, our study demonstrates the utility of a “within-species” design for studying the biomechanical correlates and neuromotor basis of evolutionary variation in motor skill at birth.
The pace of locomotor development is a critical component of evolutionary fitness over an animal’s life history. The ability for a growing individual to feed itself, evade predation, disperse from a natal group, and find a mate—in short, become an ecologically independent, reproductive adult—is predicated on its ability to first move independently and second achieve the performance capacity necessary for accomplishing these varied biological functions (Carrier 1996; Herrel and Gibb 2006; Young et al. 2022). Historically, researchers interested in the pace of avian and mammalian development have divided species into two broad categories based on functional competence at birth. Precocial animals are born with open eyes, are sufficiently feathered or furred to thermoregulate, can ingest solid foods, and can move independently. Altricial animals, by contrast, are born with closed eyes (or even fused eyelids), are relatively naked and unable to thermoregulate, depend on caregivers for food processing (or, in the case of mammals, maternal lactation), and are either unable to move independently or do so in a rudimentary manner (Fox 1964; Derrickson 1992; Starck and Ricklefs 1998; Muir 2000). Though both avian and mammalian species can be found at extreme ends of the precocial-altricial species dichotomy—e.g., extremely precocial brush turkeys (Jackson et al. 2009) and wildebeest (Pennycuick 1975) versus extremely altricial owls (Köppl et al. 2005) and rats (Jamon and Clarac 1998)—most taxa exist at intermediate positions along this dichotomy (Derrickson 1992; Starck and Ricklefs 1998), showing variable rates of functional development at birth depending on the system (e.g., sensory versus motor; Grand,1992) or even region of the body (e.g., forelimbs versus hindlimbs; Carrier and Leon 1990; Pflieger et al. 1996; Dial and Carrier 2012).
Why are precocial animals able to locomote independently within hours after birth whereas altricial animals can take weeks, months, or even longer to do so? At a fundamental level, independent locomotion requires a developing animal to do two things: (1) produce coordinated, alternating limb movements to facilitate progression (i.e., develop a gait) and (2) maintain postural equilibrium against self-induced and external perturbations to stability (Muir 2000; Shumway-Cook et al. 2023). The requisite neural circuitry and muscle strength to produce alternating limb movements appear to develop relatively early, even in species with altricial rates of locomotor development, such as rats and nonhuman primates (Bradley 1990; Gramsbergen 1998; Muir 2000). For example, fetal rats still in utero spontaneously produce bouts of synchronized limb movements that can be quantitatively similar to patterns of interlimb coordination required for postnatal gait (Kleven et al. 2004). Similarly, newborn vervet monkeys (Chlorocebus aethiops) will produce coordinated bouts of “air-stepping” movements when suspended above the ground (Vilensky et al. 1989), several months prior to the onset of independent locomotion (Hurov 1982; Vilensky and Gankiewicz 1989). In contrast, insufficient vestibulospinal control and weak limb extensor muscles appear to limit postural stability in altricial infants (Peters 1983; Clarac et al. 1998; Gramsbergen 1998), suggesting that postural development may be a bottleneck on locomotor maturity overall (Kernell 1998). As such, postural skills are typically mastered prior to locomotor skills in species with protracted locomotor development (e.g., humans and other primates; Negayama et al. 1983; Adolph et al. 2008).
However, investigating the lower-level morphological and biomechanical traits that might account for variation in postural—and thus locomotor—development across altricial and precocial species can be complicated by the lack of experimental control inherent to all comparative analyses across species (Felsenstein 1985; Garland and Adolph 1994). Precocial and altricial animals often differ along a host of dimensions that can obfuscate the factors controlling motor development. We propose that an alternative approach may be to examine locomotor development in a nominally precocial species in which gestation lengths have been experimentally manipulated, thereby creating “functionally altricial” cohorts for comparison. This “within-species” design bypasses the problem unexplained phylogenetic variation that can confound multispecies comparisons of precocial and altricial taxa, perhaps facilitating a more accurate understanding of the lower-level factors that determine the pace of locomotor development across the precocial-altricial spectrum.
Specific aim and hypotheses
We present the results of set of three integrated studies focusing on postural and locomotor development in preterm and full-term infant domestic pigs (Sus scrofa domesticus). As artiodactyls, pigs are nominally a precocial species (Graves 1984; Martin et al. 2015; Vanden Hole et al.2017b). However, prior research has shown that postnatal development in infant pigs delivered approximately one week preterm (∼94% full gestation length) broadly mimics the developmental profile of more altricial species. Overall, pigs born at 7 days preterm are physiologically similar to human infants born at 30–32 weeks of gestation (Eiby et al. 2013; Sangild et al. 2013). In other words, approximately 20%–25% of critical maturational events occur in the last week of pig gestation. Preterm piglets open their eyes, begin to stand, and begin walking at later ages than full-term piglets (Andersen et al. 2016; Nielsen et al. 2018).
The primary aim of this study was to establish the degree to which the preterm infant pig could serve as a within species model system, in which to explore some of the fundamental determinants of locomotor development across precocial and altricial mammals. Using a combination of standard kinetic, kinematic, and morphometric analyses, we tested three hypotheses and associated predictions:
H1. Preterm birth will compromise static balance performance in preterm piglets.
H1.P1. Periods of uninterrupted, independent stance will be significantly shorter in duration in preterm piglets than in full-term pigs.
H1.P2. During periods of quiet stance, preterm piglets will exhibit significantly greater magnitudes of postural sway than full-term piglets, as quantified by the amplitude of the whole-body center of pressure (CoP) displacement.
H1.P3. During periods of quiet stance, preterm piglets will exhibit significantly greater mean CoP speed than full-term piglets, reflecting continuous postural adjustment to maintain balance in the preterm infants.
H2. Preterm birth will compromise dynamic stability during locomotion, necessitating several changes to spatiotemporal gait kinematics to promote stability.
H2.P1. Compared to full-term piglets, preterm piglets will exhibit shorter, more frequent strides, higher duty factors, and will choose footfall patterns that facilitate broader polygons of support across the stride cycle.
H3. Preterm birth will compromise musculoskeletal development.
H3.P1. For their body mass, preterm piglets will have significantly reduced limb extensor muscle mass relative to full-term piglets.
Methods
General animal husbandry
Infant pigs were delivered via Caesarean section at either term (115 days) or seven days preterm (∼94% gestation) using standard aseptic technique (see Ballester et al. 2018 for further description of surgical procedures). Newborn pigs were placed in incubators until they reached stable temperature and oxygenation. Once stable, piglets were placed in housing pens and monitored 24h/day during the first week of life, after which we followed standard methods for laboratory infant pig care (German et al. 1998). Infants were trained to drink formula (Solustart Pig Milk Replacement, Land o’ Lakes, Arden Mills, MN, USA) from bottles fitted with artificial lamb nipples (NASCO Farm and Ranch, Fort Atkinson, WI), providing a ready means of motivating locomotion, and other behaviors during data collection. Pigs were euthanized at 1–1.5 months of age via pentobarbital overdose (>200 mg/kg). All live animal procedures received prior approval by the NEOMED Institutional Animal Care and Use Committee (protocol #17–04-071).
We studied two cohorts of preterm piglets (total N: 29 individuals) and two cohorts of full-term piglets (total N: 15 individuals). Specific details of the samples used in each study are detailed below.
Study 1: Static balance performance in preterm and full-term piglets
Data collection and processing. Our sample for this study included 14 preterm and six full-term piglets. Data were collected at two time points, once perinatally (i.e., 2 days for preterm pigs and 3 days for full-term pigs) and once at approximately two weeks of age (i.e., 16 days for preterm pigs and 11 days for full-term pigs), collecting one trial per individual at each time point. Unfortunately, most of the preterm piglets in this cohort contracted a fatal bacterial infection during the first week of life and died soon thereafter, reducing our sample size to a single individual at the older time point. We thus focus on the overall main effects of birth status (collapsing across age group) and age group (collapsing across birth status), eschewing interaction tests between birth status and age. More details on these analytical decisions are provided in the section on Statistical Analyses below.
Static balance performance was quantified by measuring CoP deviations while piglets stood quadrupedally during bouts of bottle feeding (i.e., stabilography; Nauwelaerts et al. 2013). Pigs stood on two adjacent HE6 × 6–16 small animal force platforms (AMTI, Watertown, MA, USA; 15 × 15 cm in area) while feeding from a bottle (Supplementary Online Video S1). Though the contact between the pig’s mouth and the nipple of the bottle meant that the infants were not completely mechanically isolated, the high compliance of the rubber nipple and the small area of contact would provide only limited postural support at best. Force platforms were covered with stairway tread tape to ensure traction (Safety-Walk Slip Resistant Tread, 3M Corp., Minneapolis, MN, USA). Trials were ended after two minutes or earlier if the animal refused to cooperate. Experiments were recorded using a single camera (Xcitex XC-2; Xcitex Inc., Woburn, MA, USA) operating at 10 hz. Forces were sampled at 1000 Hz. Videos and force data were recorded synchronously using ProCapture software (Xcitex, Inc., Woburn, MA, USA).
During postprocessing, we used Xcitex ProAnalyst motion analysis software to identify periods of quiet stance where the animal’s body weight was supported entirely by the force platform. Raw forces (i.e., Fx,Fy,Fz) and moments (i.e., Mx, My, and Mz) from each force platform during periods of quiet stance were then exported to MATLAB (R2022b; Mathworks, Natick, MA, USA) for subsequent processing. CoP position in the X and Y directions (i.e., CoPx and CoPy) were calculated as
 |
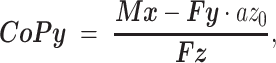 |
where az0 represents the thickness of the force plate top piece (i.e., the top plane offset; equal to 8.9 mm for the HE 6 × 6). Because the global coordinate system was set to that of the first force platform, the calculated CoPy coordinates for the second force platform were offset by 15.875 cm to account for the spatial offset between platforms. Finally, combined CoP coordinates across the platforms were calculated as the average of the instantaneous X and Y coordinates from each plate, weighted by the corresponding vertical force magnitude.
From these data, we extracted several measures of static balance performance, including total stance duration, fore-aft CoP position amplitude, mediolateral CoP position amplitude, overall CoP area, and mean CoP speed over the stance period. Given significant variation in stance durations between preterm and full-term infants (see Results section below) we only analyzed the central 10 sec of data for any trials greater than 10 sec. This ensured that summary measurers of CoP displacement and speed were not biased by the longer trial durations of the full-term piglets. Because body mass significantly differed between preterm and full-term piglets (see “Results” section below), all performance measures were adjusted for body size. Variable definitions and formulae for size adjustment are summarized in Table 1.
Table 1.
Performance metrics used in Studies 1 and 2.
| Variable1 | Definition | Scaling factor2 |
|---|---|---|
| Study 1: Static balance performance in preterm and full-term piglets | ||
| Stance duration | Total duration of periods of unaided support on the force platforms during the 120s trial. | (lg−1)−1/2 |
| Fore-aft CoP amplitude | Y-axis length of a 90% confidence ellipse fit to the CoP coordinates across the stance duration. | l−1 |
| Mediolateral CoP amplitude | X-axis length of a 90% confidence ellipse fit to the CoP coordinates across the stance duration. | l−1 |
| CoP area | Area of a 90% confidence ellipse fit to the CoP coordinates across the stance duration. | l−2 |
| Mean CoP speed | First derivative of a quintic smoothing spline function fit to the CoP position data (tolerance of 1mm2; Walker [1998]). CoP trajectories were decimated to 5% of the original sampling rate to avoid velocity spikes. CoP velocity was calculated separately in the X and Y directions, with overall CoP speed calculated as the resultant magnitude of these two vectors. | (gl)−1/2 |
| Study 2: Locomotor performance in preterm and full-term piglets | ||
| Speed | Unsigned mean of the first derivative of a quintic smoothing spline function fit to the fore-aft position of the tip of animal’s nose throughout the stride (tolerance of 1mm2). | (gl)−1/2 |
| Stride length | Total distance travelled by the midpoint of shoulder and hip position during the stride. | l−1 |
| Stride frequency | Number of stride cycles per second. | (gl−1)−1/2 |
| Mean duty factor | Proportion of a stride cycle that a given limb is in contact with the substrate (i.e., stance phase), averaged across all four limbs. | – |
| Support periods | Percentage of a stride cycle during which the animal is supported by combinations of one, two, three, or four limbs. | – |
Variables quantifying balance performance are in the top, whereas those characterizing locomotor performance are in the bottom group.
Scaling factor represents the quantity by which the original measure was multiplied for size adjustment (g = gravitational acceleration, l = fundamental animal length, defined here as the cube root of body mass). Formulae follow the conventions of Hof (1996). Because duty factors and support periods are proportions, size adjustment is unnecessary.
Study 2: Locomotor performance in preterm and full-term piglets
Data collection and processing. Our sample for this study included 15 preterm and nine full-term piglets. Data were collected at various ages the first two weeks of life, including postnatal days 2, 3, 8, and 15 for the preterm piglets and postnatal days 1, 2, 3, 4, 7, 11, and 15 for the full-term piglets. Sampling was less frequent for preterm piglets due to the greater fragility of this sample. Piglets were coaxed to cross short runways (∼2m in total length) at self-selected speeds using positive reinforcement (i.e., presentation of a nursing bottle; Supplementary Online Video S2). We collected a minimum of five trials per individual per day. We recorded locomotor activity using 2–4 high-speed cameras (XC-2; Xcitex Inc.), with 1–2 cameras positioned on either side of the runway. Videos were recorded at 100 frames per second and synchronized using Xcitex ProCapture.
Synchronized videos were imported into Xcitex ProAnalyst for subsequent processing of spatiotemporal gait kinematics. We first triaged all trials in which the piglets stopped, changed direction, or were otherwise not moving a continuous, steady rate of speed. This resulted in a final dataset of 247 strides (100 preterm; 147 full-term), including 1–11 strides per individual per experiment (median = 4 strides per experiment). Our methods for coding quadrupedal gait kinematics have been thoroughly described in previous publications (e.g., Young,2012; Chadwell and Young 2015; Young et al. 2016; Dunham et al. 2018; Schapker et al. 2022) and will be only briefly summarized here. Individual strides were identified by sequential contacts of a reference limb, chosen to ensure the animal was moving at a steady speed during the coded stride. To isolate stance and swing periods for each limb over the stride, ProAnalyst software was used to mark the touchdown and liftoff frame of each foot during the stride interval, where touchdown was defined as the first frame, in which a limb contacted the runway following a swing period and liftoff was defined as the first frame, in which limb did not contact the runway following a stance period. Animal displacement during the stride was coded by tracking the horizontal position of the tip of the animal’s nose, using the dimensions of the runway to calibrate apparent pixel displacements from the video into real world units (i.e., meters). From these data, we calculated several metrics of locomotor performance, including average speed, stride length, stride frequency, mean duty factor, and limb support proportions. The definitions of each of these variables and the formulae used for size adjustment when necessary are summarized in Table 1.
Study 3: Limb extensor muscle growth in preterm and full-term piglets
We sampled muscle masses from 15 cadaveric specimens, including seven preterm piglets (age: 3–40 days; mass at death: 0.82–10.2 kg) and eight full-term piglets (postnatal ages 7–41 days; mass at death: 1.2–10.2 kg). All samples were obtained from individuals that had been euthanized for reasons unrelated to this study. Whole cadavers were stored frozen (−20°C) prior to tissue collection. We systematically dissected eight muscles from each cadaver, including extensors at the elbow (m. triceps brachii), hip (m. biceps femoris, m. semimembranosus, and m. semitendinosus), knee (m. rectus femoris, m. vastus lateralis, and m. vastus medialis), and ankle (m. gastrocnemius). Muscles were blotted dry and removed from their free tendons prior to weighing them using an electronic balance (Mettler Toledo, Columbus, OH, USA: accurate to 0.001 g).
Statistical analyses
Developmental changes in body mass across the full sample of preterm and full-term piglets included in Studies 1 and 2 were analyzed using standard least squares analysis of covariance, specifying birth status (preterm versus full-term) as a categorical factor and postnatal age as the covariate.
Variation in static balance performance (Study 1) was analyzed using mixed effects two-way analyses of variance, specifying birth status and age group (i.e., perinatal versus older) as fixed main effects and individual animal as a random effect in the model. As described above, given the dearth of preterm pigs in the older age group, the models were fit with main effects only, eschewing tests of interaction between birth status and age group.
Variation in locomotor performance (Study 2) associated with birth status, postnatal age, and speed was analyzed using mixed-effects analyses of covariance (ANCOVA), specifying birth status as a fixed factor, age and speed as fixed covariates, and individual animal as a random factor. For each test, we first fit the full model that included all interactions between birth status and age/speed. We then simplified each model by removing non-significant factor by covariate interactions. Though none of the birth status by age interactions were significant, birth status by speed interactions were significant for several models. In these cases, we tested for differences between preterm and term piglets at the minimum, mean, and maximum values of the overlapping range of dimensionless speeds common to both groups (minimum value: 0.215; mean value: 0.358; maximum value: 0.678).
Finally, variation in limb extensor muscle mass between preterm and full-term infants (Study 3) was tested using standard least squares analysis of covariance, specifying birth status as the factor and body mass at death as the covariate.
Most continuous variables were logarithmically transformed (base e) prior to analyses to improve normality. Raw limb support percentages approximated a normal distribution and were thus left unadjusted. Post hoc pairwise comparisons were carried out using Tukey tests, with error term degrees of freedom adjusted using the Welch–Satterthwaite approximation to mitigate heteroscedasticity between groups. For all pairwise post-hoc comparisons, we calculated Cohen’s d as a measure of effect size (Quinn and Keough 2002), and followed Sawilosky’s (2009) categorical “rules of thumb” for categorizing effects as very small (d ≤ 0.2), small (d = 0.2–0.5), moderate (d = 0.5–0.8), large (d = 0.8–1.2), very large (d = 1.2–2.0), and huge (d ≥ 2.0). Statistical analyses were carried out in R (R Core Team 2022), supplemented by the add-on packages car (Fox and Weisberg 2019), dplyr (Wickham et al. 2022), effects (Fox and Weisberg 2018), emmeans (Lenth 2023), lme4 (Bates et al. 2015), and lmerTest (Kuznetsova et al. 2017). Results of all statistical analyses are described in Supplementary Tables S1–S7.
Results
Body mass differences between preterm and full-term piglets
Body mass significantly increased with postnatal age in both preterm and full-term infant pigs (Supplementary Table S1; Fig. 1). However, preterm piglets gained mass with age at a significantly slower rate than full-term piglets (preterm growth rate: 39 g/day [95% confidence interval, CI: 34.7, 43.8 g/day]; full-term growth rate: 73 g/day [95% CI: 70.4, 75.6 g/day]). Although preterm pigs were always significantly smaller than full-term pigs at any given age, the magnitude of the difference increased at older ages (compare test at postnatal day 1 versus postnatal day 15 in Supplementary Table S1). Given these trends, all static balance and locomotor performance metrics were adjusted for individual body size, as summarized in the “Material and methods” section above.
Fig. 1.

Developmental changes in overall body mass in preterm and full-term piglets over the first month of life. Data encompass measurements of all 29 infants included in Studies 1 and 2. Trend lines indicate fits from the mixed-effects ANCOVA model. Gray triangles with dashed trend lines represent preterm piglets whereas white circles with solid trend lines represent full-term piglets.
Study 1: Static balance performance in preterm and full-term piglets
Preterm birth compromised some, but not all, measures of static balance performance (Fig. 2). Full-term piglets could stand for longer durations than preterm piglets (P < 0.001, huge effect). Preterm pigs also demonstrated greater fore-aft CoP amplitudes and greater mean CoP speeds (all P ≤ 0.042, large effects). In contrast, neither mediolateral CoP amplitudes nor overall CoP areas differed according to birth status (all P ≥ 0.220, small to moderate effects).
Fig. 2.
Static balance performance in preterm (PT) and full-term (FT) piglets. Box plots show variation in stance duration (A), fore-aft CoP amplitude (B), mediolateral CoP amplitude (C), CoP area (D), and mean CoP speed (E) according to birth status and age. In each box plot, dark lines represent the median of the distribution, boxes extend across the interquartile range (IQR) and whiskers extend to ±150% of the IQR, or the extremes of the distribution, whichever is closer to the median value. Open circles identify outlier data points beyond 150% of the IQR. Dot plots to the right of each box plot show individual data points in each group. The large white diamond indicates the preterm piglet that was sampled for both age groups. The y-axis of each plot is on a logarithmic scale. All variables were made dimensionless to adjust for size differences between preterm and full-term piglets (see Table 1 for formulae).
Mixed-effects analyses of variance showed several significant age group effects as well. Stance durations significantly increased with age (P = 0.030, large effect), whereas, fore-aft and mediolateral CoP amplitudes and overall CoP area decreased at older ages (all P ≤ 0.049, large to very large effects). In contrast, mean CoP speed did not differ among age groups (P = 0.142, moderate effect).
Study 2: Locomotor performance in preterm and full-term piglets
Across age, preterm pigs moved at significantly higher dimensionless speeds than full-term pigs (Supplementary Table S3; Fig. 3; P = 0.014, large effect), whereas neither preterm nor full-term pigs significantly changed dimensionless speed with increasing age (P = 0.870).
Fig. 3.

Influence of postnatal age on dimensionless speed in preterm and full-term piglets. Symbols represent average dimensionless speed over a single trial for an individual animal. Trend lines indicate fits from the mixed-effects ANCOVA model. Gray triangles with dashed trend lines represent preterm piglets whereas white circles with solid trend lines represent full-term piglets. The y-axis is on a logarithmic scale.
Both preterm and full-term pigs showed significant age-related changes in dimensionless stride lengths and frequencies after controlling for the effects of dimensionless speed, moving with relatively longer but less frequent strides at older ages (Supplementary Table S4, Figs. 4A and C; all P ≤ 0.042). Controlling for age, results showed that piglets increased dimensionless speed by increasing both dimensionless stride length and frequency (Table 5, Figs. 4B and D; all P < 0.001). However, both measures showed significant birth status by dimensionless speed interactions (all P ≤ 0.006), indicating that preterm and full-term piglets differed in strategies for speed modulation. Specifically, dimensionless stride length increased with speed at a faster rate in full-term piglets (preterm slope: 0.34 [95% CI: 0.275, 0.405], full-term slope: 0.455 [95% CI: 0.402, 0.509]), whereas dimensionless stride frequencies increased with speed at a faster rate in preterm piglets (preterm slope: 0.68 [95% CI: 0.608, 0.748], full-term slope: 0.55 [95% CI: 0.496, 0.604). As a result, controlling for the effects of age and dimensionless speed, full-term piglets showed significantly greater dimensionless stride lengths at the maximum overlapping speed (P = 0.004, very large effect), whereas preterm piglets showed significantly greater dimensionless stride frequencies at the mean and maximum values of the overlapping speed range (P ≤ 0.004, large to huge effects).
Fig. 4.
Influence of postnatal age and dimensionless speed on stride length and stride frequency in preterm and full-term piglets. Panels summarize the results of the mixed-effects ANCOVA models. Each panel shows the independent relationship between dimensionless stride length (A and B) or frequency (C and D) and either postnatal age controlling for the effects of speed (A and C) or dimensionless speed controlling for the effects of age (B and D). Gray triangles with dashed trend lines represent preterm piglets whereas white circles with solid trend lines represent full-term piglets. Dimensionless stride lengths, frequencies, and speeds are plotted on logarithmic scales.
Preterm and full-term pigs decreased mean duty factors with increasing dimensionless speed (Supplementary Table S5; Fig. 5B; P < 0.001). However, there was a significant interaction between birth status and dimensionless speed (P = 0.002), such that mean duty factor decreased with speed at a higher rate in preterm pigs than full-term pigs (preterm slope: −0.17 [95% CI: −0.205, −0.125], full-term slope: −0.08 [95% CI: −0.119, −0.051]). As a result, preterm piglets moved with significantly higher mean duty factors at the minimum and mean values of the overlapping dimensionless speed range (Fig 5B; all P ≤ 0.008, moderate to very large effects), whereas mean duty factors were similar across piglets at the highest overlapping dimensionless speeds (P = 0.436, small effect). Controlling for the effects of dimensionless speed, neither preterm nor full-term pigs showed age-related changes in mean duty factor (Supplementary Table S5; Fig. 5A).
Fig. 5.
Influence of postnatal age and dimensionless speed on mean duty factor in preterm and full-term piglets. Panels summarize the results of the mixed-effects ANCOVA model. Each panel shows the independent relationship between mean duty factor and either postnatal age controlling for the effects of speed (A) or dimensionless speed controlling for the effects of age (B). Gray triangles with dashed trend lines represent preterm piglets whereas white circles with solid trend lines represent full-term piglets. The y-axis of each plot is on a logarithmic scale. Mean duty factor and dimensionless speed are plotted on logarithmic scales.
Estimated marginal means from mixed-effects ANCOVA models showed that, at the mean value of dimensionless speed, both preterm and full-term piglets primarily supported themselves on two or three limbs during quadrupedal locomotion, with the percentage of bipedal support averaging 46% (95% CI: 42.3%, 50.0%) in preterm pigs and 54% (95% CI: 49.4%, 57.6%) in full-term pigs, and the percentage of tripedal support averaging 47.0% (95% CI: 43.0%, 51.0%) in preterm pigs and 33% (95% CI: 29.0%, 37.7%) in full-term pigs. In contrast, full quadrupedal support averaged 7% (95% CI: 5.4%, 9.5%) in preterm pigs and 12% (95% CI: 10.2%, 13.8%) in full-term pigs, and unipedal support only 0.05% (95% CI: −0.56%, 0.67%) in preterm pigs and 1.2% (95% CI: 0.60%, 1.73%) in full-term pigs. A whole-body aerial phase (i.e., period of no limb support) was only seen in the fastest stride recorded in the full-term pigs.
Though support patterns showed no significant age-related effects, piglets did alter support patterns with increasing dimensionless speed, significantly increasing bipedal and unipedal support while decreasing quadrupedal and tripedal support (Supplementary Table S6; Fig. 6; all P < 0.001). However, percentages of both tripedal and bipedal support showed significant birth status by dimensionless speed interactions (all P ≤ 0.011). As dimensionless speed increased, preterm pigs showed a more pronounced decrease in the percentage of tripedal support (preterm slope: −43% [95% CI: −52.3%, −34.2%]; full-term slope −25% [95% CI: −33.2%, −17.9%]) and a more pronounced increase in the percentage of bipedal support (preterm slope: 48% [95% CI: 39.0%, 56.6%]; full-term slope 33% [95% CI: 25.6%, 40.4%]). As a result, at the minimum and mean values of the overlapping speed range, percentages of tripedal support were significantly greater in preterm piglets (all P < 0.001, large to huge effects) whereas percentages of bipedal support were significantly greater in full-term piglets (all P ≤ 0.013; moderate to very large effects). Additionally, controlling for dimensionless speed, full-term pigs showed significantly greater percentages of both quadrupedal and unipedal support (all P ≤ 0.014, small to moderate effects). However, as noted above, support by four limbs or a single limb was relatively rare during piglet quadrupedal gait.
Fig. 6.
Influence of postnatal age and dimensionless speed on limb support percentages in preterm and full-term piglets. Panels summarize the results of the mixed-effects ANCOVA models. Each panel shows a partial residual plot showing the independent relationship between percentage of tripedal support (A and B) or bipedal support (C and D) and either postnatal age controlling for the effects of speed (A and C) or dimensionless speed controlling for the effects of age (B and D). Gray triangles with dashed trend lines represent preterm piglets whereas white circles with solid trend lines represent full-term piglets. Dimensionless speed is plotted on a logarithmic scale.
Study 3: Extensor muscle growth in preterm and full-term piglets
Extensor muscle mass scaled to overall body mass with an exponent of 1.18 [95% CI: 1.082, 1.287], significantly greater than the isometric expectation of 1.00 (Supplementary Table S7; Fig. 7). Ordinary least squares analysis of covariance showed no significant difference in elevation between preterm and full-term piglets (P = 0.595, Cohen’s d: −0.284, small effect), indicating that birth status had little influence on relative proportions of extensor muscle mass. The mass of most individual extensor muscles also scaled to body mass with positive allometry (Supplementary Table 7), though the quadriceps muscles scaled with isometry in full-term piglets, vastus lateralis scaled with negative allometry in preterm piglets, and triceps brachii scaled with isometry across the infant pig sample.
Fig. 7.

Ontogenetic allometry of extensor muscle mass in preterm and full-term piglets. The summed mass of eight forelimb and hindlimb extensor muscles is plotted against total body mass in a sample of 8 preterm and 7 full-term pigs, ranging in postnatal age from 3–41 days. The solid trend line represents an ordinary least-squares for to the data, whereas the dashed trend line represents the isometric expectation that extensor muscles mass should scale linearly with body mass (i.e., Mb1.0). Gray triangles represent preterm piglets whereas white circles represent full-term piglets. Data are plotted on log-log axes.
Discussion
As artiodactyls, pigs are nominally a precocial species, capable of expressing mature locomotor kinematics within 2–8 h after birth (Martin et al. 2015; Vanden Hole et al.2017). Our data demonstrate that late preterm birth at 94% of full-term gestation significantly interrupts this developmental trajectory. Many of the deviations we observed between preterm and full-term infants mimic the broad locomotor phenotype of developmentally altricial animals.
Effects of preterm birth on body mass and postnatal growth rate
We found that preterm piglets were significantly smaller than full-term animals throughout the first two postnatal weeks, replicating the results of several previous studies of the preterm piglet model (Eiby et al. 2013; Andersen et al. 2016; Adjerid et al. 2021). Preterm human infants are also born significantly smaller than full-term peers (Roggero et al. 2009). Similarly, viviparous altricial mammals tend to produce relatively smaller infants than precocial mammals (Derrickson 1992). The relatively small birth masses of preterm infants and altricial mammals likely reflects a similar shift of overall “growth resources” toward postnatal life (Derrickson 1992; Gaillard et al. 1997; Starck and Ricklefs 1998; Halley 2017).
Effects of preterm birth on static balance
As predicted, preterm piglets were characterized by significantly greater fore-aft postural sway than full-term piglets, though the amplitude of mediolateral sway did not differ by birth status. As a result, though average CoP area (proportional to total postural sway) was greater in preterm piglets (Fig. 2), this difference was not significant. In a study of postural development in newborn foals (Equus ferus caballus), Nauwelaerts et al. (2013) found that CoP deviations were initially much greater in the fore-aft direction than mediolaterally, a difference they attribute to the longer craniocaudal base of support of the infant horses providing the center of mass greater room to migrate within a stable base of support. In a similar manner, the infant pigs in our study may have been prioritizing mediolateral control over fore-aft control.
We also found that overall CoP speed was greater in preterm piglets. Previous studies of developmental changes in CoP speed during stance in growing horses and humans have interpreted the rapid CoP movements of young individuals as indicative of a feed-forward “open-loop” strategy of postural control, where forceful, ballistic contractions of multiple muscles are constantly required to adjust balance from one moment to the next. This strategy contrasts to the “closed-loop” postural control seen at older ages, where CoP speeds are tightly regulated and feedback from visual, vestibular, and somatosensory systems is used to constantly modulate postural reflexes in real-time (Riach and Starkes 1994; Nauwelaerts et al. 2013). Overall, dependence on feed-forward, open-loop postural control is thought be indicative of incomplete sensorimotor control in young animals. Prior research on neural development in infant pigs has found that preterm birth compromises cerebellar growth and cerebral white matter myelination (Buddington et al. 2018; Nielsen et al. 2018; Chizhikov et al. 2020). The reduced sensorimotor integration and slower nerve transmission speeds that likely result from these deficits may further compromise preterm animals’ ability to prospectively modulate posture. Confirmation that preterm piglets were using more of an “open-loop” strategy for balance control would require additional physiological testing, including electromyography of postural muscles to establish the temporal association between muscle contractions and online balance adjustments.
In summary, preterm birth significantly compromised static balance performance in infant pigs. The impact of preterm birth on static balance is perhaps best represented by the extremely short stance durations of the preterm pigs. On average, perinatal preterm piglets were only able to achieve 11% of the stance durations of similarly aged full-term infants (i.e., 4 sec in preterm piglets versus 36 sec in full-term infants; Fig. 2A). The limited postural abilities of the newborn pigs mimics that of altricial animals who are often unable to independently support their body weight for several days, weeks, or months after birth (Fox 1964; Hildebrand,1967a; Peters 1983; Jamon and Clarac 1998).
However, it is also important to acknowledge that most of the preterm piglets in our sample later contracted a fatal bacterial infection after initial balance testing. We cannot exclude the possibility that some of the preterm animals may have been ill during data collection, perhaps further compromising balance performance. Having said this, none the animals showed visible symptoms or pathologies at the time of testing.
Effects of preterm birth on locomotor performance
Given the primacy of postural development in setting the pace of locomotor development (Gramsbergen 1998; Kernell 1998; Muir 2000), we expected the postural instability of preterm piglets to significantly impact their locomotor development as well. Surprisingly, preterm pigs moved at significantly greater dimensionless speeds than full-term pigs across the entire age range sampled in our study. Given that the animals were permitted to self-select their speed across the runway, and a multitude of factors might have affected their choice, we cannot fully account for this difference. Nevertheless, we do note that several human studies have indicated that moving more quickly can increase dynamic stability (particularly in the mediolateral direction), though results have been variable and can be prone to methodological variation (Buzzi and Ulrich 2004; Bruijn et al. 2009; Stenum et al. 2014). Further research would be needed to test this hypothesis, including the kinematic measures of sustained treadmill locomotion that would be required to quantitatively measure dynamic stability (Bruijn et al. 2013).
After adjusting for the influence of locomotor speed, we found multiple kinematic differences between preterm and full-term piglets. In general, the observed differences supported our predictions that preterm birth would necessitate several changes to spatiotemporal gait kinematics to compensate for reduced postural stability. First, preterm and full-term piglets differed in their strategy for speed modulation, with preterm pigs preferentially increasing dimensionless stride frequency, whereas full-term pigs preferentially increased dimensionless stride length. As a result, at the fastest speeds common to both groups, preterm pigs exhibited significantly shorter, more frequent strides. The few previous studies of preterm pig locomotor kinematics have also found a similar tendency toward shorter, more frequent strides in preterm animals (Nielsen et al. 2018; Vanden Hole et al.2021). Broadly, increasing speed by increasing stride frequency is thought to be the energetically more expensive strategy, due to the greater power demands of quickly activating muscle force to support body weight and of driving the limb at beyond its natural pendular frequency during swing phase (Kram and Taylor 1990; Pontzer 2005). However, increasing speed via greater stride lengths necessitates increased mechanical work as the CoM undergoes increased deceleration and acceleration at each step (Usherwood 2013). It may be that preterm pigs are more limited in their capacity to produce mechanical work than mechanical power per se. Along these lines, an interesting area for future research would be to compare the metabolic cost of transport in preterm and full-term piglets.
Second, at all but the fastest speeds, preterm piglets walked with higher duty factors than full-term piglets and preferentially selected footfall patterns that ensured support by at least three limbs during most of the gait cycle. Both adjustments should enhance stability by promoting increased durations of substrate contact throughout a stride (Young 2023). Broadly, the gait adjustments seen in preterm pigs mimic the slow, shuffling gaits of newborn mammals with altricial locomotor abilities. Like preterm piglets, young rats, cats, dogs, and non-human primates tend to use short, frequent strides, relatively high duty factors, and broad polygons of support (Hildebrand,1967;1968; Peters 1983; Westerga and Gramsbergen 1990; Jamon and Clarac 1998; Young,2009; 2012 )
Proximate causes of motor delays in preterm infants and altricial animals
Performance testing showed that preterm birth significantly impacted newborn piglet balance and locomotor function, resulting in a broadly “altricial” phenotype in a mammal that is nominally precocial at birth. Preterm human children also show quantitative deficits in motor skill performance (Bolk et al. 2018), including difficulty with both static and dynamic balance tasks (Fallang and Hadders-Algra 2005; Dusing et al. 2014) and walking with a relatively choppy, ataxic gait (Albesher et al. 2019; Bisi et al. 2022). Deficits in motor skill performance can be long-lasting, extending into late childhood and adolescence (Maitra et al. 2014; Tripathi and Dusing 2015; Evensen et al. 2020).
At a gross level, the motor delays of preterm infants and altricial animals are undoubtedly related to gestation length. For their body size, precocial species have significantly longer gestation durations than altricial species (Martin and MacLarnon 1985; Starck and Ricklefs 1998). Longer gestation lengths permit precocial species to shift more of brain and muscle growth to prenatal development; peak growth velocities for both brain size and overall body mass typically occur during prenatal development in precocial taxa but postnatally in altricial taxa (Gaillard et al. 1997; Halley 2017). As a result, precocial animals are typically born with relatively larger brains and greater muscle mass than altricial species (Starck and Ricklefs 1998; Barton and Capellini 2011). In a similar fashion, preterm birth has been shown to be associated with a small, relatively unmyelinated brain characterized by poorly developed cortical, subcortical, and cerebellar motor processing centers (Hüppi et al. 1998; Limperopoulos et al. 2005; Padilla et al. 2015; Nielsen et al. 2018), and a relatively weak muscular system with limited endurance (Schloon et al. 1979; Owen-Jones et al. 2020). Even among full-term human infants, longer gestations durations are associated with increased performance on cognitive and motor assessments during early childhood (Espel et al. 2014).
What lower-level biomechanical or neurological factors best account the motor delays of preterm infants or altricial species? Newborn mammals typically have relatively less muscle mass than adults, with total muscle mass increasing by 20%–25% relative to total body mass during postnatal growth (Grand,1977; 1983;1992; Grand and Barboza 2001; Goldspink 1980a). Precocial taxa, however, exhibit much less of a proportional deficit in muscularity than altricial taxa (Grand,1992; Dearolf et al. 2000). In the preterm infant pig model, we found that extensor muscle mass scaled to body mass with significant positive allometry, indicating that younger piglets follow the typical mammalian pattern of being proportionally less muscular than older animals. However, accounting for differences in body mass, we found that extensor muscle mass did not differ between preterm and full-term piglets. Recent research from our laboratory has also shown that quantitative measures of limb bone bending strength also do not significantly vary between preterm and full-term piglets (Magrini et al. 2023). Overall, these data suggest that the perinatal pig musculoskeletal development is relatively impervious to small variations in gestation length.
However, muscle performance is determined by factors other than muscle mass alone. Many infant mammals characterized by altricial locomotor abilities are born with a preponderance of Type II (“fast-twitch”) fibers in muscles that are critical for maintaining limb posture (e.g., soleus) (Rubinstein and Kelly 1978; Jouffroy and Medina 1996; von Mering and Fischer 1999; Dearolf et al. 2000; Bewick et al. 2004; Goldspink 1980b). By contrast, more precocial infants show a relatively adult-like mix of Type II and Type I (i.e., “slow twitch”) fibers, suggesting a greater ability for sustained locomotor activity (Dubowitz 1965; von Mering and Fischer 1999; Dearolf et al. 2000). There is some evidence from clinical human studies that preterm birth can also limit the proportion of Type I muscle fibers in locomotor muscles (Schloon et al. 1979). A relative paucity of Type I muscle fibers might also compromise the ability of preterm and altricial infants to utilize “closed-loop” strategies for balance control, as such a strategy likely requires continuous low-speed, low-amplitude CoP adjustments to maintain balance with set parameters.
Finally, several previous studies have noted specific aspects of central and peripheral nervous system maturation that appear to be associated with the onset of more mature postural and locomotor performance in altricial animals. Broadly, the pace of postnatal motor development in altricial animals is associated with the rate of myelination of critical motor tracts in the central nervous system, and the growth, differentiation, and pruning of both motoneurons and afferent inputs in the peripheral nervous system (Fox 1964; Westerga and Gramsbergen 1990; Gramsbergen 1998; Muir 2000). Similarly, because oligodendrocytes differentiate late in development, preterm birth results in a relatively unmyelinated, and thus poorly coordinated, central nervous system (Duerden et al. 2013; Eiby et al. 2013).
Age-related changes in performance
Several of the balance and locomotor performance metrics we examined showed evidence of significant age-related changes, even in full-term (i.e., precocial) piglets. Measures of postural sway, including fore-aft amplitude, mediolateral amplitude, and total CoP area, decreased with age in piglets. Similarly, total stance duration during the static balance tests also increased with age. Overall, these data suggest that balance improves with age in newborn piglets. Similarly, Nauwelaerts et al. (2013) found that CoP amplitudes and speed during quiet stance decreased over the first 1–3 months of age in newborn foals, a pattern they attribute to increasing strength and coordination of the limb muscles.
We also observed age-related changes in piglet locomotor performance. Controlling for dimensionless speed, we found that both preterm and full-term piglets increased dimensionless stride lengths and decreased dimensionless stride frequencies with increasing age. Vanden Hole et al. (2017) also found that full-term pigs tend to walk with relatively short strides at young ages, though they only tested infant pigs through 4 days of postnatal age. Similarly, Muir et al. (1996) and Schilling (2005) found ontogenetic increases in stride lengths in precocial chicks (Gallus gallus domesticus) and guinea pigs (Cavia porcellus), respectively. Overall, these data reinforce our understanding of the precocial-altricial axis as a continuum, rather than a dichotomy.
Conclusions
Preterm birth at 94% term gestation length significantly affected both static balance and locomotor kinematics in perinatal pigs. In several ways, the postural and locomotor deficits shown by the preterm piglets parallel the locomotor phenotype of altricial mammals, though motor abilities of the nominally precocial preterm infant pigs were still more advanced than the extremely limited movement of completely altricial eutherian mammals (e.g., laboratory rats or kittens). Available data suggest that preterm piglet motor deficits may be more closely associated with neurological immaturity than musculoskeletal immaturity per se, though much work remains to be done to fully document the neuromotor phenotype of the preterm infant pig model. Documenting the developmental coordination among the musculoskeletal, neurological, and biomechanical factors that constrain or promote motor performance would be a particularly fruitful area for future research. Overall, this study demonstrates the utility of a “within-species” design for studying the biomechanical correlates and neuromotor basis of evolutionary variation in motor skill at birth.
Supplementary Material
Acknowledgement
We thank Dr. Randall Buddington for his input and advice on working with and caring for preterm pigs. We thank Dr. Stanley Dannemiller, the staff of the NEOMED Comparative Medicine Unit, and the members of the German Lab at NEOMED for their assistance with pig care and husbandry during these studies. The members of the NEOMED Musculoskeletal Research Focus Area Journal Club provided input on previous presentations of this research.
Notes
From the symposium “Biology at birth: the role of infancy in providing the foundation for lifetime success” presented at the annual meeting of the Society for Integrative and Comparative Biology, January 16–March 31, 2023.
Contributor Information
Jesse W Young, School of Biomedical Sciences, Kent State University, Kent OH 44242, USA; Department of Anatomy and Neurobiology, Northeast Ohio Medical University (NEOMED), Rootstown OH 44272, USA.
Christopher J Mayerl, Department of Biological Sciences, Northern Arizona University, Flagstaff AZ 86011, USA.
Alekhya Mannava, Department of Anatomy and Neurobiology, Northeast Ohio Medical University (NEOMED), Rootstown OH 44272, USA.
Claire Lewis, Department of Anatomy and Neurobiology, Northeast Ohio Medical University (NEOMED), Rootstown OH 44272, USA.
Tianhui Fan, Department of Anatomy and Neurobiology, Northeast Ohio Medical University (NEOMED), Rootstown OH 44272, USA.
Manas Nair, Department of Anatomy and Neurobiology, Northeast Ohio Medical University (NEOMED), Rootstown OH 44272, USA.
Christopher Mamone, Department of Anatomy and Neurobiology, Northeast Ohio Medical University (NEOMED), Rootstown OH 44272, USA.
Nicole M Schapker, School of Biomedical Sciences, Kent State University, Kent OH 44242, USA; Department of Anatomy and Neurobiology, Northeast Ohio Medical University (NEOMED), Rootstown OH 44272, USA.
Angela M Mossor, School of Biomedical Sciences, Kent State University, Kent OH 44242, USA; Department of Anatomy and Neurobiology, Northeast Ohio Medical University (NEOMED), Rootstown OH 44272, USA.
Rebecca Z German, School of Biomedical Sciences, Kent State University, Kent OH 44242, USA; Department of Anatomy and Neurobiology, Northeast Ohio Medical University (NEOMED), Rootstown OH 44272, USA.
Conflict of interest statement
The authors collectively have no conflicts of interest to declare.
Funding
This work was supported by the National Institutes of Health [NIH R01 HD96881 to R.Z.G.]
Data availability statement
The data underlying this study are available from the Figshare repository at https://dx.doi.org/10.6084/m9.figshare.22141919.
References
- Adjerid K, Mayerl CJ, Gould FD, Edmonds CE, Stricklen BM, Bond LE, German RZ.. 2021. Does birth weight affect neonatal body weight, growth, and physiology in an animal model?. PLoS One. 16: e0246954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolph KE, Robinson SR, Young JW, Gill-Alvarez F.. 2008. What is the shape of developmental change?. Psychol Rev. 115: 527–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albesher RA, Spittle AJ, McGinley JL, Dobson FL.. 2019. Gait characteristics of children born preterm. NeoReviews. 20: e397–408. [DOI] [PubMed] [Google Scholar]
- Andersen AD, Sangild PT, Munch SL, van der Beek EM, Renes IB, Ginneken Cv, Greisen GO, Thymann T.. 2016. Delayed growth, motor function and learning in preterm pigs during early postnatal life. Am J Physiol Regul Integr Comp Physiol. 310: R481–92. [DOI] [PubMed] [Google Scholar]
- Ballester A, Gould F, Bond L, Stricklen B, Ohlemacher J, Gross A, DeLozier K, Buddington R, Buddington K, Danos N, German R.. 2018. Maturation of the coordination between respiration and deglutition with and without recurrent laryngeal nerve lesion in an animal model. Dysphagia. 33: 627–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton RA, Capellini I.. 2011. Maternal investment, life histories, and the costs of brain growth in mammals. Proc Natl Acad Sci. 108: 6169–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D, Mächler M, Bolker B, Walker S.. 2015. Fitting linear mixed-effects models using lme4. J Stat Softw. 67:1–48. [Google Scholar]
- Bewick GS, Reid B, Jawaid S, Hatcher S, Shanley L.. 2004. Postnatal emergence of mature release properties in terminals of rat fast- and slow-twitch muscles. Eur J Neurosci. 19:2967–76. [DOI] [PubMed] [Google Scholar]
- Bisi MC, Fabbri M, Cordelli DM, Stagni R.. 2022. Gait performance in toddlers born preterm: a sensor based quantitative characterization. Comput Methods Programs Biomed. 106808. [DOI] [PubMed]
- Bolk J, Farooqi A, Hafström M, Åden U, Serenius F.. 2018. Developmental coordination disorder and its association with developmental comorbidities at 6.5 years in apparently healthy children born extremely preterm. JAMA pediatrics. 172: 765–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley NS. 1990. Animal models offer the opportunity to acquire a new perspective on motor development. Phys Ther. 70:776–87. [DOI] [PubMed] [Google Scholar]
- Bruijn SM, Meijer OG, Beek PJ, Van Dieen JH.. 2013. Assessing the stability of human locomotion: a review of current measures. J R Soc Interface. 10: 20120999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijn SM, van Dieėn JH, Meijer OG, Beek PJ.. 2009. Is slow walking more stable?. J Biomech. 42: 1506–12. [DOI] [PubMed] [Google Scholar]
- Buddington RK, Chizhikov VV, Iskusnykh IY, Sable HJ, Sable JJ, Holloway ZR, Blumenfeld Katzir T, Van der Merwe M, Yakimkova T, Buddington KK.. 2018. A phosphatidylserine source of docosahexanoic acid improves neurodevelopment and survival of preterm pigs. Nutrients. 10: 637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzzi UH, Ulrich BD.. 2004. Dynamic stability of gait cycles as a function of speed and system constraints. Motor Control. 8: 241–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrier DR. 1996. Ontogenetic limits on locomotor performance. Physiol Zool. 69:467–88. [Google Scholar]
- Carrier DR, Leon LR.. 1990. Skeletal growth and function in the California gull (Larus californicus). J Zool London. 222:375–89. [Google Scholar]
- Chadwell BA, Young JW.. 2015. Angular momentum and arboreal stability in common marmosets (Callithrix jacchus). Am J Phys Anthropol. 156:565–76. [DOI] [PubMed] [Google Scholar]
- Chizhikov D, Buddington RK, Iskusnykh IY.. 2020. Effects of phosphatidylserine source of docosahexaenoic acid on cerebellar development in preterm pigs. Brain Sciences. 10: 475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarac F, Vinay L, Cazalets J-R, Fady J-C, Jamon M.. 1998. Role of gravity in the development of posture and locomotion in the neonatal rat. Brain Res Rev. 28: 35–43. [DOI] [PubMed] [Google Scholar]
- Dearolf J, McLellan W, Dillaman R, Frierson D Jr, Pabst D. 2000. Precocial development of axial locomotor muscle in bottlenose dolphins (Tursiops truncatus). J Morphol. 244: 203–15. [DOI] [PubMed] [Google Scholar]
- Derrickson EM. 1992. Comparative reproductive strategies of altricial and precocial eutherian mammals. Funct Ecol. 6:57–65. [Google Scholar]
- Dial TR, Carrier DR.. 2012. Precocial hindlimbs and altricial forelimbs: partitioning ontogenetic strategies in mallards (Anas platyrhynchos). J Exp Biol. 215:3703–10. [DOI] [PubMed] [Google Scholar]
- Dubowitz V. 1965. Enzyme histochemistry of skeletal muscle. J Neurol Neurosurg Psychiatry. 28: 516–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duerden EG, Taylor MJ, Miller SP.. 2013: Brain development in infants born preterm: looking beyond injury. Semin Pediatr Neurol. 20: 65–74. [DOI] [PubMed] [Google Scholar]
- Dunham NT, McNamara A, Shapiro LJ, Hieronymus TL, Young JW.. 2018. A user’s guide for the quantitative analysis of substrate characteristics and locomotor kinematics in free-ranging primates. Am J Phys Anthropol. 167:569–84. [DOI] [PubMed] [Google Scholar]
- Dusing SC, Izzo TA, Thacker LR, Galloway JC.. 2014. Postural complexity differs between infant born full term and preterm during the development of early behaviors. Early Hum Dev. 90: 149–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiby YA, Wright LL, Kalanjati VP, Miller SM, Bjorkman ST, Keates HL, Lumbers ER, Colditz PB, Lingwood BE.. 2013. A pig model of the preterm neonate: anthropometric and physiological characteristics. PLoS One. 8: e68763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espel EV, Glynn LM, Sandman CA, Davis EP.. 2014. Longer gestation among children born full term influences cognitive and motor development. PLoS One. 9: e113758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evensen KAI, Ustad T, Tikanmäki M, Haaramo P, Kajantie E.. 2020: Long-term motor outcomes of very preterm and/or very low birth weight individuals without cerebral palsy: a review of the current evidence. Semin Fetal Neonatal Med. 25: 101116. [DOI] [PubMed] [Google Scholar]
- Fallang B, Hadders-Algra M.. 2005. Postural behavior in children born preterm. Neural Plast. 12:175–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenstein J. 1985. Phylogeneies and the comparative method. Am Nat. 125:1–25. [Google Scholar]
- Fox J, Weisberg S.. 2018. Visualizing fit and lack of fit in complex regression models with predictor effect plots and partial residuals. J Stat Softw. 87:1–27. [Google Scholar]
- Fox J, Weisberg S.. 2019. An R Companion to Applied Regression. Thousand Oaks (CA): Sage. [Google Scholar]
- Fox MW. 1964. A phylogenetic analysis of behavioral neuro-ontogeny in precocial and nonprecocial mammals. Can J Comp Med Vet Sci. 28:197–202. [PMC free article] [PubMed] [Google Scholar]
- Gaillard J-M, Pontier D, Allaine D, Loison A, Herve J-C, Heizman A.. 1997. Variation in growth form and precocity at birth in eutherian mammals. Proc R Soc London Series B Biol Sci. 264: 859–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland JT, Adolph SC.. 1994. Why not to do two-species comparative studies: limitations on inferring adaptation. Physiol Zool. 67: 797–828. [Google Scholar]
- German R, Crompton A, Thexton A.. 1998. The coordination and interaction between respiration and deglutition in young pigs. J Comp Physiol A. 182: 539–47. [DOI] [PubMed] [Google Scholar]
- Goldspink DF. 1980a. Physiological factors influencing protein turnover and muscle growth in mammals. In: Goldspink DF, editor. Development and Specialization of Skeletal Muscle. Cambridge: Cambridge University Press. 65–89. [Google Scholar]
- Goldspink G. 1980b. Growth of muscle. In: Goldspink DF, editor. Development and Specialization of Skeletal Muscle. Cambridge: Cambridge University Press. p. 19–35. [Google Scholar]
- Gramsbergen A. 1998. Posture and locomotion in the rat: independent or interdependent development?. Neurosci Biobehav Rev. 22:547–53. [PubMed] [Google Scholar]
- Grand TI. 1977. Body weight: its relation to tissue composition, segment distribution, and motor function. II. Development of Macaca mulatta. Am J Phys Anthropol. 47: 241–8. [DOI] [PubMed] [Google Scholar]
- Grand TI. 1983. The anatomy of growth and its relation to locomotor capacity in Macaca. In: Eisenberg JF, Kleiman DG, editors. Advances in the Study of Mammalian Behavior. Shippensburg (PA): American Society of Mammalogists. p. 5–23. [Google Scholar]
- Grand TI. 1992. Altricial and precocial mammals: a model of neural and muscular development. Zoo Biol. 11: 3–15. [Google Scholar]
- Grand TI, Barboza PS.. 2001. Anatomy and development of the koala, Phascolarctos cinereus, an evolutionary perspective on the superfamily Vombatoidea. Anat Embryol (Berl). 203:211–23. [DOI] [PubMed] [Google Scholar]
- Graves H. 1984. Behavior and ecology of wild and feral swine (Sus scrofa). J Anim Sci. 58: 482–92. [Google Scholar]
- Halley AC. 2017. Minimal variation in eutherian brain growth rates during fetal neurogenesis. Proc R Soc B Biol Sci. 284: 20170219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrel A, Gibb AC.. 2006. Ontogeny of performance in vertebrates. Physiol Biochem Zool. 79:1–6. [DOI] [PubMed] [Google Scholar]
- Hildebrand M. 1967. Symmetrical gaits of primates. Am J Phys Anthropol. 26:119–30. [Google Scholar]
- Hildebrand M. 1968. Symmetrical gaits of dogs in relation to body build. J Morphol. 124:353–60. [DOI] [PubMed] [Google Scholar]
- Hüppi PS, Warfield S, Kikinis R, Barnes PD, Zientara GP, Jolesz FA, Tsuji MK, Volpe JJ.. 1998. Quantitative magnetic resonance imaging of brain development in premature and mature newborns. Ann Neurol Official J Am Neurol Child Neurol Soc. 43: 224–35. [DOI] [PubMed] [Google Scholar]
- Hurov JR. 1982. Diagonal walking in captive infant vervet monkeys. Am J Primatol. 2:211–3. [DOI] [PubMed] [Google Scholar]
- Jackson BE, Segre P, Dial KP.. 2009. Precocial development of locomotor performance in a ground-dwelling bird (Alectoris chukar): negotiating a three-dimensional terrestrial environment. Proc R Soc B Biol Sci. 276: 3457–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamon M, Clarac F.. 1998. Early walking in the neonatal rat: a kinematic study. Behav Neurosci. 112: 1218–28. [DOI] [PubMed] [Google Scholar]
- Jouffroy FK, Medina MF.. 1996. Developmental changes in the fibre composition of elbow, knee, and ankle extensor muscles in cercopithecid monkeys. Folia Primatol (Basel). 66:55–67. [DOI] [PubMed] [Google Scholar]
- Kernell D. 1998. The final common pathway in postural control—developmental perspective. Neurosci Biobehav Rev. 22:479–84. [DOI] [PubMed] [Google Scholar]
- Kleven GA, Lane MS, Robinson SR.. 2004. Development of interlimb movement synchrony in the rat fetus. Behav Neurosci. 118(4):835. [DOI] [PubMed] [Google Scholar]
- Köppl C, Futterer E, Nieder B, Sistermann R, Wagner H.. 2005. Embryonic and posthatching development of the barn owl (Tyto alba): reference data for age determination. De Dyn Am Anat. 233: 1248–60. [DOI] [PubMed] [Google Scholar]
- Kram R, Taylor CR.. 1990. Energetics of running: a new perspective. Nature. 346:265–7. [DOI] [PubMed] [Google Scholar]
- Kuznetsova A, Brockhoff PB, Christensen RH.. 2017. lmerTest package: tests in linear mixed effects models. J Stat Softw. 82: 1–26. [Google Scholar]
- Lenth RV. 2023. emmeans: estimated Marginal means, aka Least-Squares means. R package version 1.8.4-1. 15 Jan. 2023; https://CRAN.R-project.org/package=emmeans.
- Limperopoulos C, Soul JS, Gauvreau K, Huppi PS, Warfield SK, Bassan H, Robertson RL, Volpe JJ, du Plessis AJ.. 2005. Late gestation cerebellar growth is rapid and impeded by premature birth. Pediatrics. 115: 688–95. [DOI] [PubMed] [Google Scholar]
- Magrini S, Mossor AM, German RZ, Young JW.. 2023. Developmental factors influencing bone quality in precocial mammals: an infant pig model. J Anat. DOI: 10.1111/joa.13848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maitra K, Park HY, Eggenberger J, Matthiessen A, Knight E, Ng B.. 2014. Difficulty in mental, neuromusculoskeletal, and movement-related school functions associated with low birthweight or preterm birth: a meta-analysis. Am J Occup Ther. 68: 140–8. [DOI] [PubMed] [Google Scholar]
- Martin JE, Ison SH, Baxter EM.. 2015. The influence of neonatal environment on piglet play behaviour and post-weaning social and cognitive development. Appl Anim Behav Sci. 163:69–79. [Google Scholar]
- Martin RD, MacLarnon AM.. 1985. Gestation period, neonatal size and maternal investment in placental mammals. Nature. 313:220–3. [Google Scholar]
- Muir GD. 2000. Early ontogeny of locomotor behaviour: a comparison between altricial and precocial animals. Brain Res Bull. 53: 719–26. [DOI] [PubMed] [Google Scholar]
- Muir GD, Gosline JM, Steeves JD.. 1996. Ontogeny of bipedal locomotion: walking and running in the chick. J Physiol London. 493: 589–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauwelaerts S, Malone SR, Clayton HM.. 2013. Development of postural balance in foals. Vet J. 198:e70–4. [DOI] [PubMed] [Google Scholar]
- Negayama K, Kondo K, Itoigawa N.. 1983. Development of locomotor behavior in infant Japanese macaques (Macaca fuscata). Ann Sci Nat Zool. 5:169–80. [Google Scholar]
- Nielsen CH, Brandt AB, Thymann T, Obelitz-Ryom K, Jiang P, Hole CV, van Ginneken C, Pankratova S, Sangild PT.. 2018. Rapid postnatal adaptation of neurodevelopment in pigs born late preterm. Dev Neurosci. 40: 586–600. [DOI] [PubMed] [Google Scholar]
- Owen-Jones Z, Perrochon A, Hermand E, Ponthier L, Fourcade L, Borel B. 2020. Evolution of muscular oxygenation during a walking test in preterm children. J Pediatr. 227:142–8. [DOI] [PubMed] [Google Scholar]
- Padilla N, Alexandrou G, Blennow M, Lagercrantz H, Ådén U.. 2015. Brain growth gains and losses in extremely preterm infants at term. Cereb Cortex. 25: 1897–905. [DOI] [PubMed] [Google Scholar]
- Pennycuick CJ. 1975. On the running of the gnu (Connochaetes taurinus) and other animals. J Exp Biol. 63:775–99. [Google Scholar]
- Peters SE. 1983. Postnatal development of gait behavior and functional allometry in the domestic cat (Felis catus). J Zool London. 199:461–86. [Google Scholar]
- Pflieger JF, Cassidy G, Cabana T.. 1996. Development of spontaneous locomotor behaviors in the opossum, Monodelphis domestica. Behav Brain Res. 80: 137–43. [DOI] [PubMed] [Google Scholar]
- Pontzer H. 2005. A new model predicting locomotor cost from limb length via force production. J Exp Biol. 208:1513–24. [DOI] [PubMed] [Google Scholar]
- Quinn GP, Keough MJ.. 2002. Experimental Design and Data Analysis for Biologists. Cambridge: Cambridge University Press. [Google Scholar]
- Team R Core. 2022. R: A Language and Environment for Statistical Computing. 4.2.2, “Innocent and Trusting” editor, Vienna: R Foundation for Statistical Computing. [Google Scholar]
- Riach C, Starkes J.. 1994. Velocity of centre of pressure excursions as an indicator of postural control systems in children. Gait Posture. 2: 167–72. [Google Scholar]
- Roggero P, Giannì ML, Amato O, Orsi A, Piemontese P, Morlacchi L, Mosca F.. 2009. Is term newborn body composition being achieved postnatally in preterm infants?. Early Hum Dev. 85: 349–52. [DOI] [PubMed] [Google Scholar]
- Rubinstein NA, Kelly AM.. 1978. Myogenic and neurogenic contributions to the development of fast and slow twitch muscles in rat. Dev Biol. 62:473–85. [DOI] [PubMed] [Google Scholar]
- Sangild PT, Thymann T, Schmidt M, Stoll B, Burrin DG, Buddington RK.. 2013. Invited review: the preterm pig as a model in pediatric gastroenterology. J Anim Sci. 91: 4713–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawilowsky SS. 2009. New effect size rules of thumb. J Mod Appl Stat Methods. 8: 597–9. [Google Scholar]
- Schapker NM, Chadwell BA, Young JW.. 2022. Robust locomotor performance of squirrel monkeys (Saimiri boliviensis) in response to simulated changes in support diameter and compliance. J Exp Zool A Ecol Integr Physiol. 337:417–33. [DOI] [PubMed] [Google Scholar]
- Schilling N. 2005. Ontogenetic development of locomotion in small mammals—a kinematic study. J Exp Biol. 208:4013–34. [DOI] [PubMed] [Google Scholar]
- Schloon H, Schlottmann J, Lenard H, Goebel H.. 1979. The development of skeletal muscles in premature infants. Eur J Pediatr. 131: 49–60. [DOI] [PubMed] [Google Scholar]
- Shumway-Cook A, Woollacott MH, Rachwani J, Santamaria V.. 2023. Motor Control: Translating Research into Clinical Practice. Philadelphia (PA): Wolters Kluwer. [Google Scholar]
- Starck JM, Ricklefs RE.. 1998. Patterns of development: the altricial-precocial spectrum. In: Starck JM, Ricklefs RE, editors. Avian Growth and Development. New York (NY): Oxford University Press. p. 3–30. [Google Scholar]
- Stenum J, Bruijn SM, Jensen BR.. 2014. The effect of walking speed on local dynamic stability is sensitive to calculation methods. J Biomech. 47: 3776–9. [DOI] [PubMed] [Google Scholar]
- Tripathi T, Dusing S.. 2015. Long-term neurodevelopmental outcomes of infants born late preterm: a systematic review. Res Rep Neonatol. 5:91–111. [Google Scholar]
- Usherwood JR. 2013. Constraints on muscle performance provide a novel explanation for the scaling of posture in terrestrial animals. Biol Lett. 9: 20130414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanden Hole C, Ayuso M, Aerts P, Van Cruchten S, Thymann T, Sangild PT, Van Ginneken C.. 2021. Preterm birth affects early motor development in pigs. Front Pediatrics. 9:1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanden Hole C, Goyens J, Prims S, Fransen E, Ayuso Hernando M, Van Cruchten S, Aerts P, Van Ginneken C. 2017. How innate is locomotion in precocial animals? A study on the early development of spatio-temporal gait variables and gait symmetry in piglets. J Exp Biol. 220:2706–16. [DOI] [PubMed] [Google Scholar]
- Vilensky JA, Gankiewicz E.. 1989. Early development of locomotor behavior in vervet monkeys. Am J Primatol. 17:11–25. [DOI] [PubMed] [Google Scholar]
- Vilensky JA, Wilson P, Gankiewicz E, Townsend DW.. 1989. An analysis of air-stepping in normal infant vervet monkeys. J Mot Behav. 21:429–56. [DOI] [PubMed] [Google Scholar]
- von Mering F, Fischer MS.. 1999. Fibre type regionalization of forelimb muscles in two mammalian species, Galea musteloides (Rodentia, Caviidae) and Tupaia belangeri (Scandentia, Tupaiidae), with comments on postnatal myogenesis. Zoomorphology. 119:117–26. [Google Scholar]
- Westerga J, Gramsbergen A.. 1990. The development of locomotion in the rat. Dev Brain Res. 57: 163–74. [DOI] [PubMed] [Google Scholar]
- Wickham H, François R, Henry L, Müller K.. 2022. dplyr: a grammar of data manipulation. R package version 1.0.10: 15 Jan. 2023, https://CRAN.R-project.org/package=dplyr
- Young JW. 2009. Substrate determines asymmetrical gait dynamics in marmosets (Callithrix jacchus) and squirrel monkeys (Saimiri boliviensis). Am J Phys Anthropol. 138:403–20. [DOI] [PubMed] [Google Scholar]
- Young JW. 2012. Gait selection and the ontogeny of quadrupedal walking in squirrel monkeys (Saimiri boliviensis). Am J Phys Anthropol. 147:580–92. [DOI] [PubMed] [Google Scholar]
- Young JW. 2023. Convergence of arboreal locomotor specialization: morphological and behavioral solutions for movement on narrow and compliant supports. In: Bels VL, Russell AP, editors. Convergent Evolution: Animal Form and Function. New York (NY): Springer Nature. p. 289–322. [Google Scholar]
- Young JW, Foster AD, Russo GA, Smith GA, Butcher MT.. 2022. Only the good die old? Ontogenetic determinants of locomotor performance in eastern cottontail rabbits (Sylvilagus floridanus). Integr Org Biol. 4. obab037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Stricklen BM, Chadwell BA.. 2016. Effects of support diameter and compliance on common marmoset (Callithrix jacchus) gait kinematics. J Exp Biol. 219:2659–72. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this study are available from the Figshare repository at https://dx.doi.org/10.6084/m9.figshare.22141919.






