Abstract
In 1996, the Centers for Disease Control and Prevention recommended the use of a selective broth culture for the improved detection of genital tract or anorectal carriage of group B streptococci (GBS) in pregnant women. In order to verify this recommendation in our laboratory, we compared the sensitivity of Todd-Hewitt medium with gentamicin and nalidixic acid (SBM) with our current method of direct plating on blood agar medium containing neomycin and nalidixic acid (NNA). Five hundred consecutive cervicovaginal and anorectal specimens submitted for GBS culture were included in the study. Swabs were plated onto NNA and the swabs were immersed in SBM, followed by overnight incubation at 35°C. On the following day, the NNA plates were examined for colonies typical of GBS and the organisms were identified by the CAMP test or by latex agglutination. SBM cultures were subcultured onto blood agar and CNA agar plates, and the plates were reincubated for 24 h. Negative specimens from either medium were incubated for an additional 24 h and were examined again before finalization of the results. GBS were recovered from 78 specimens by both methods; from SBM only for 17 specimens (sensitivity, 86%) and from NNA only for 16 specimens (sensitivity, 85%). A moderate to heavy growth of Enterococcus faecalis was observed on plates containing NNA-positive, SBM-negative specimens. Competitive growth studies suggested that E. faecalis suppressed the growth potential of GBS in SBM. Our study suggests that direct plating on NNA, as a single method, is equivalent in sensitivity to SBM for the recovery of GBS, and the results are often available 24 h sooner. However, it appears that both direct plating and selective broth amplification techniques are required for the maximum level of identification of colonization with GBS in pregnant women.
Group B streptococci (GBS) continue to be the most important bacterial cause of neonatal morbidity and mortality in the United States, despite the availability of effective prophylactic antimicrobial therapy (5, 10). In 1996, the Centers for Disease Control and Prevention in conjunction with an extensive group of investigators with expertise in disease caused by GBS published guidelines designed to reduce the rate of intrapartum transmission of GBS from mother to child (5). These recommendations included two strategies based on either the identification of maternal GBS carriage through the use of late prenatal screening culture techniques or the recognition of certain predisposing risk factors prior to delivery. A significant finding in the algorithm of either strategy would trigger intrapartum antimicrobial prophylaxis which has previously been shown to reduce the incidence of early-onset disease caused by GBS by 69 to 86% (3).
The sensitivity of culture-based screening methods for the identification of maternal carriage of GBS depends on the timing of specimen collection, the source of the specimen, and the culture technique used by the microbiology laboratory. Optimally, specimens should be obtained as close to delivery as possible. Vaginal and rectal specimens have been shown to be superior to cervical specimens for the detection of carriers of GBS (8), while selective broth media have historically provided better sensitivity than solid media for the recovery of GBS from those sources (2, 7, 9). On the basis of an exhaustive review of the contemporary literature and the input of the select panel of consultants, the Centers for Disease Control and Prevention published recommendations designed to interrupt the perinatal transmission of disease caused by GBS (5). These recommendations included the use of culture techniques that would maximize the recovery of GBS from vaginal and rectal specimens and specified the use of a selective broth medium such as Todd-Hewitt broth supplemented with colistin and nalidixic acid (6) or gentamicin and nalidixic acid (1). By this protocol, specimens are inoculated into a selective broth medium and are incubated for 18 to 24 h, followed by subculture to blood agar plates (BAP). After an additional 18 to 24 h of incubation period, the BAP are examined for the presence of GBS. In order to conform to these guidelines, we initiated a comparison of the proposed culture protocol using Todd-Hewitt broth containing gentamicin and nalidixic acid with our current method of direct plating of specimens onto BAP containing neomycin and nalidixic acid. Five hundred consecutive vaginal, cervical, and rectal specimens submitted for culture for the detection of GBS were entered into the study, and the results of the comparison are presented herein.
MATERIALS AND METHODS
Five hundred consecutive specimens submitted to the Henry Ford Health System Microbiology Laboratory specifically for the detection of GBS were entered into the study. Specimens were received from 1 July 1997 through 5 December 1997 and consisted of 264 vaginal, 169 cervical, 65 rectal, and 2 rectal and vaginal specimens. Samples were collected on swabs and were transported to the laboratory in Amies medium with charcoal (Copan Diagnostics, Inc., New York, N.Y.) or Stuart’s medium (Baxter Healthcare Corp., Deerfield, Ill.). All specimens were inoculated onto blood agar plates containing 30 mg of neomycin per liter and 15 mg of nalidixic acid per liter (NNA; Becton Dickinson Microbiology Systems, Cockeysville, Md.), and the swabs were then submersed in Todd-Hewitt broth supplemented with 8 mg of gentamicin per liter and 15 mg of nalidixic acid per liter (SBM; Becton Dickinson) as described by Baker et al. (1). Both media were incubated overnight at 35°C, with 5% CO2 included for the plated specimens. On the following day, all specimens plated onto NNA were examined for colonies typical of GBS by laboratory personnel; if such colonies were present, they were identified by Gram staining, catalase reaction, and either the CAMP test or a particle agglutination assay (Streptex; MUREX, Kent, United Kingdom), depending on the number of isolated colonies available. Negative cultures were reincubated for an additional 18 to 24 h and were inspected again before finalization of the results. The results for the NNA cultures were entered into the laboratory information system and were not accessed by the investigators until the results for the broth cultures were available. After 18 to 24 h of incubation, the SBM cultures were subcultured onto a BAP and a CNA agar plate and were incubated for 18 to 24 h at 35°C with 5% CO2 prior to examination. As with the NNA plates, all SBM subcultures were examined for colonies typical of GBS, and the colonies were identified by the same methods. Negative subcultures were reincubated and were examined again on the following day.
RESULTS
GBS were recovered from 111 of the 500 specimens (22.2%) processed during the study period, including 34 of 169 (20.1%) cervical specimens, 56 of 264 (21.2%) vaginal specimens, and 21 of 65 (32.2%) rectal specimens, by one or both culture techniques. Of these, GBS were recovered from 78 specimens by both the direct plating and the broth amplification methods. GBS were isolated from SBM only for 17 specimens, while only NNA was positive for GBS for 16 specimens, for calculated sensitivities of 86 and 85%, respectively. Of those specimens for which GBS were recovered from subcultures of SBM, for 87 specimens GBS grew on both BAP and CNA, while for 6 and 2 specimens, GBS were recovered only on BAP and only on CNA, respectively.
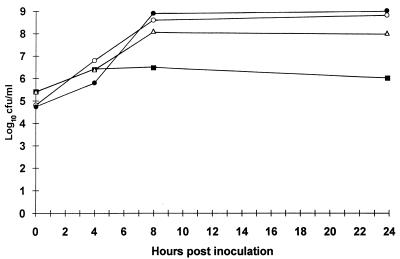
During the course of the study we noted that NNA-positive, SBM-negative specimens produced a moderate to heavy growth (often in pure culture) of Enterococcus species on subcultures of the SBM. The species of a number of isolates were determined, and all were identified as Enterococcus faecalis. In order to examine this phenomenon in greater detail, a separate experiment was performed in which equal inocula (approximately 105 CFU) of E. faecalis and strains of GBS recovered from one such specimen were introduced into SBM and the tubes were incubated at 35°C. Each organism was also inoculated into individual SBM tubes which served as growth controls. Samples were removed from the broth immediately after inoculation and after 4, 8, and 24 h of incubation. The samples were diluted and plated onto BAP for colony count determinations. The colonies of each strain could easily be identified on subcultures of the mixed SBM culture taken 0 and 4 h after inoculation. However, only rare colonies of GBS were recovered from the broth after 8 h of incubation, and colonies were completely absent from the subculture plate at 24 h. Colony counts showed an approximate 2 log10 reduction in the number of CFU of GBS/ml in the mixed culture taken after 8 and 24 h of incubation compared with the numbers of CFU in the SBM culture containing GBS only at the same sampling intervals (Fig. 1). A cell-free filtrate of a 24-h culture of E. faecalis grown in SBM was shown not to be inhibitory to the growth of GBS by a disk diffusion technique or by the direct addition of GBS grown in SBM to broth cultures (data not shown).
FIG. 1.
Growth of GBS in SBM over time either alone (▵) or in mixed culture with E. faecalis (■). Also shown are the results for the growth of E. faecalis in pure culture (○) or with GBS (•). In each case, SBM was seeded with equivalent inocula (∼105 CFU) of either one or both organisms.
DISCUSSION
The results of this study comparing the sensitivity of a selective broth amplification technique to that of a direct plating method for the detection of carriage of GBS in pregnant women provided evidence to support and extend current recommendations proposed by the Centers for Disease Control and Prevention (5). While SBM and NNA were found to be equally sensitive for the recovery of GBS from vaginal, cervical, and rectal specimens, neither was sufficiently sensitive in our hands to be supported as the sole medium for this purpose. When used in combination, the two media provided identifications of organisms from an additional 16 positive specimens that would have been missed if SBM had been used as the sole isolation medium. The results of our study differ somewhat from those presented in previous reports, which concluded that selective broth media are generally more sensitive than selective agar media for the recovery of GBS (2, 7, 9), although none of the previous studies evaluated NNA. In addition to providing improved sensitivity, the use of a direct plating method such as NNA generally provides an identification of GBS 24 h sooner than a broth method, which requires subculture after an overnight incubation. In this study, all GBS isolated from NNA were identified within 2 days of specimen receipt (depending upon the number of isolated colonies and the method of identification, i.e., latex agglutination or the CAMP test), whereas all SBM cultures required a minimum of 2 days and sometimes required 3 days for the identification of specimens positive for GBS. As for the type of solid medium to be used for subcultures of SBM, we found that BAP provided slightly superior recovery of GBS (97.9%) compared with CNA (93.7%), thus precluding the need for an additional selective medium in conjunction with the technique with SBM.
Similar to the results of Regan et al. (8), our data indicate that rectal and vaginal specimens provided equivalent or higher rates of GBS recovery than cervical specimens did, thus supporting the conclusion of the Centers for Disease Control and Prevention that pelvic examination and cervical visualization are unnecessary for the purpose of obtaining specimens for screening cultures (5).
Of interest was the observation that NNA-positive, SBA-negative cultures for GBS demonstrated moderate to heavy growth of Enterococcus species. For specimens for which the enterococci were examined further, all were identified as E. faecalis. Competitive overgrowth of GBS has previously been described with Staphylococcus aureus, but enterococci were not specifically mentioned (7). Experiments performed with isolates from one specimen showed that while the growth of the strain of GBS in SBM was not entirely inhibited in mixed culture with the strain of E. faecalis, the number of CFU per milliliter was reduced by approximately 2 log10 compared to the growth of GBS alone in SBM. The reason for the observed growth suppression of GBS by E. faecalis is unknown. The production of bacteriocins (enterocins) with a wide spectrum of activity against gram-positive bacteria including staphylococci, Clostridium species, lactobacilli, and Listeria species has been described for Enterococcus faecium (4). We could not, however, demonstrate the presence of inhibitory substances in cell-free filtrates of E. faecalis grown in SBM, which suggests that competitive overgrowth is likely responsible for the poor recovery of GBS from SBM when both organisms were present.
On the basis of the results of this study, we would encourage an extension of the current culture recommendation for the detection of carriage of GBS in pregnant women to include both a selective broth medium and a selective agar medium until accurate and rapid molecular methods of detection become commercially available. By this protocol, the selective agar would be examined initially after an overnight incubation. If colonial growth typical of that of GBS were not observed, the SBM would then be subcultured onto a BAP and examined for the presence of GBS on the following day. According to our data, this protocol would offer the potential of providing an identification of GBS 24 h sooner than selective broth methods for nearly 85% of specimens positive for GBS while increasing the overall sensitivity of the screening method by 14% by preventing the overgrowth of competing microorganisms.
REFERENCES
- 1.Baker C J, Clark D J, Barrett F F. Selective broth medium for isolation of group B streptococci. Appl Microbiol. 1973;26:884–885. doi: 10.1128/am.26.6.884-885.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker C J, Goroff D K, Alpert S L, Hayes C, McCormack W M. Comparison of bacteriological methods for the isolation of group B Streptococcus from vaginal cultures. J Clin Microbiol. 1976;4:46–48. doi: 10.1128/jcm.4.1.46-48.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bourbeau P P, Heiter B J, Figdore M. Use of Gen-Probe AccuProbe group B Streptococcus test to detect group B streptococci in broth cultures of vaginal-anorectal specimens from pregnant women: comparison with traditional culture method. J Clin Microbiol. 1997;35:144–147. doi: 10.1128/jcm.35.1.144-147.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casaus P, Nilsen T, Cintas L M, Nes I F, Hernandez P E, Holo H. Enterocin B, a new bacteriocin from Enterococcus faecium T136 which can act synergistically with enterocin A. Microbiology. 1997;143:2287–2294. doi: 10.1099/00221287-143-7-2287. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Prevention of perinatal group B streptococcal disease: a public health perspective. Morbid Mortal Weekly Rep. 1996;45(RR-7):1–24. [PubMed] [Google Scholar]
- 6.Jones D E, Friedl E M, Kanarek K S, Williams J K, Lim D V. Rapid identification of pregnant women heavily colonized with group B streptococci. J Clin Microbiol. 1983;18:558–560. doi: 10.1128/jcm.18.3.558-560.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gray B M, Pass M A, Dillon H C., Jr Laboratory and field evaluation of selective media for isolation of group B streptococci. J Clin Microbiol. 1979;9:466–470. doi: 10.1128/jcm.9.4.466-470.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Regan J A, Klebanoff M A, Nugent and the Vaginal Infections and Prematurity Study Group R P. The epidemiology of group B streptococcal colonization in pregnancy. Obstet Gynecol. 1991;77:604–610. [PubMed] [Google Scholar]
- 9.Wang E E L, Hammerberg O, Lyn P, Peng H, Hunter D, Richardson H. Rapid detection of group B streptococcal carriage in parturient women using a modified starch serum medium. Clin Invest Med. 1988;11:52–56. [PubMed] [Google Scholar]
- 10.Zangwill K M, Schuchat A, Wenger J D. Group B streptococcal disease in the United States, 1990: report from a multistate active surveillance system. Morbid Mortal Weekly Rep. 1992;41:25–32. [PubMed] [Google Scholar]