Abstract
Background:
Difficulty of lead extraction does not track well with procedural complications, but several small retrospective studies have lead fibrosis on computed tomography as an important indicator of difficult lead extraction. The purpose of the present study was to apply a standardized gated cardiac computed tomography (CT) protocol to assess fibrosis and study it prospectively to examine the need for powered sheaths and risk outcomes.
Methods:
We performed a prospective, blinded, multicenter, international study at high-volume lead extraction centers and included patients referred for transvenous lead extraction with at least one lead with a dwell time >1 year and ability to receive a cardiac CT. The degree of fibrosis (as measured by amount of lead adherence to vessel wall) was graded on a scale of 1 to 4 by dedicated CT readers in 3 zones (vein entry to superior vena cava, superior vena cava, and right atrium to lead tip). The primary outcome of the study was number of extractions requiring powered sheaths at zone 2 for each fibrosis group.
Results:
A total of 200 patients were enrolled in the trial with 196 completing full gated CT and lead extraction analysis. The primary endpoint of powered sheath (laser and mechanical) sheath use was significantly higher in patients with higher fibrosis seen on CT (scores 3+4; 67.8%) at the zone 2 compared to patients with lower fibrosis (scores 1+2; 38.6%; P<0.001). There were 5 major complications with 3 vascular lacerations all occurring in zone 2 in the study.
Conclusions:
Gated, contrasted CT can predict the need for powered sheaths by identification of fibrosis but did not identify an absolute low-risk cohort who would not need powered sheaths.
Registration:
URL: https://www.clinicaltrials.gov; Unique identifier: NCT03772704.
Keywords: fibrosis; lead; risk; tomography; vena cava, superior
What Is Known?
Transvenous lead extraction has emerged as a less invasive alternative to open lead removal frequently requiring the use of specialized tools such as mechanical and laser sheaths.
Previous studies have examined patient comorbidities, age of indwelling leads, and types of leads as risk factors for predicting morbidity and mortality from transvenous lead extraction.
Small single-center retrospective studies have shown the utility of preoperative CT scans in predicting difficult transvenous lead extraction.
What the Study Adds
This was a prospective multicenter study that showed that a CT protocol can be helpful in predicting difficult lead extractions though with the limitation that it can not identify an absolute low-risk cohort who would not require use of powered sheaths.
See Editorial by Chrispin
Cardiac implantable electronic devices have increasingly been implanted over time given broader indications for pacing and defibrillation and an aging population with comorbidities.1 In addition to the rise in implantation, device and lead revision has also increased due in part to recall of leads such as the Fidelis and Riata leads and an increase in cardiac implantable electronic device–related infections over time.2,3 Lead extraction is performed not only for device and/or lead infection but also for malfunctioning or abandoned leads, venous occlusion that prevents the addition of another lead, lead or device thrombus with thromboembolic events, and patient preference.4 Although initially lead extraction was performed via open sternotomy, transvenous lead extraction has emerged as a less invasive alternative to lead removal frequently requiring the use of specialized tools such as mechanical and laser sheaths. Multicenter transvenous lead extraction studies have shown that transvenous lead extraction is successful in most cases with a 1% to 2% major complication rate, with the most significant being vascular injuries such as superior vena cava (SVC) tears.5,6
Previous studies have shown that significant predictors of 30-day mortality associated with lead extraction include increased age of the leads, end-stage renal disease, infection, low body mass index, and low left ventricular ejection fraction.7 It is unclear whether patient comorbidities such as diabetes, end-stage renal disease, left ventricular ejection fraction play more or less of an important role than the complexity of the lead and venous system (lead fibrosis interface) on lead extraction outcomes. Cardiac computed tomography (CT) has been increasingly used in preoperative planning for cardiac procedures. Preprocedure CT scans may be able to identify fibrotic tissue and venous stenosis which may aid in the procedure planning. Previous studies have shown that cardiac CT can identify difficult lead extractions measured by fluoroscopy and procedural time, laser sheath size, and need of femoral snares but as noted previously, these are limited to small retrospective, single-center studies.8–11 The purpose of the present study was to prospectively study whether uniformly collected and analyzed cardiac CT scans performed before lead extraction could identify patients with higher fibrosis scores needing increased use of powered sheaths.
Methods
The MILES (Multicenter Imaging in Lead Extraction Study) was a prospective, blinded, multicenter, international study performed at 5 experienced lead extraction centers (Cleveland Clinic, OH; Allina Health Minneapolis, MN; Duke University Medical Center Durham, NC; Northwell Hospital Manhasset, NY; University Heart Center Hamburg, Germany). This study was approved by the institutional review board at each of the 5 sites, and all patients participating in the study provided informed consent. The protocol of the study is available as Supplemental Material. Data and analysis of the study are available upon reasonable request to corresponding author.
Inclusion and Exclusion Criteria
The plan was targeted to enroll 200 patients referred for cardiac implantable electronic device lead extraction at one of the 5 sites with patients having at least one lead with a dwell time (time from implantation to removal) over 1 year. Other inclusion criteria included patient age of at least 18 years and an appropriate candidate for cardiac CT with contrast. Patients with atrial fibrillation with uncontrolled ventricular rates, which would not allow for proper ECG gating given very rapid heart rates, were excluded from the study.
CT Image Acquisition and Data Collection
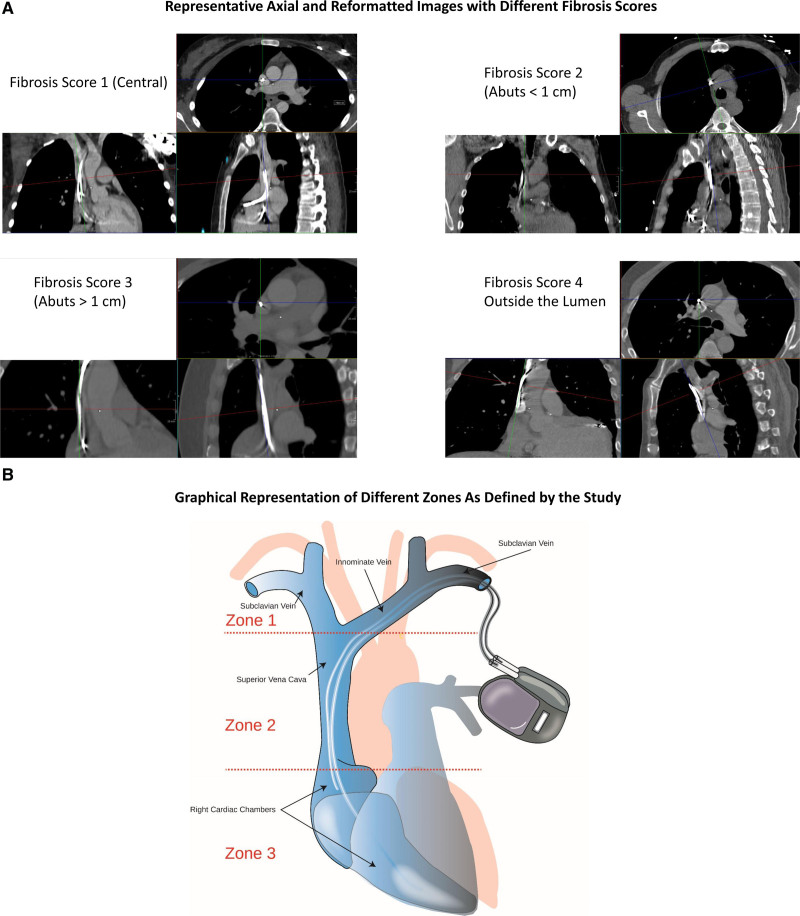
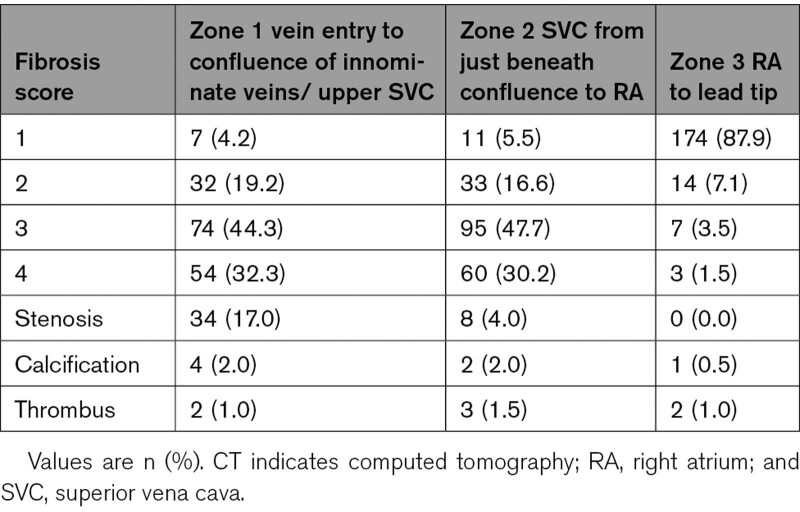
All patients planned to undergo an ECG-gated CT scan in supine position, with 70 to 150 mL of 3 to 4 mL/s injection rate of contrast with 64-MDCT or greater with ECG gating. CT scans were read by board-certified CT readers blinded to procedural details. Images were graded on quality (excellent, adequate, fair, poor) based on motion artifacts, beam hardening artifacts from the cardiac implantable electronic device, and contrast opacification of the SVC. Fibrosis score was used to describe the adherence of the lead relative to the lumen and wall of vessel and scored on a scale of 1 to 4 with 1 indicating lead within the contrast-enhanced lumen of the vessel without contact with the vessel wall, 2 indicating lead within the contrast-enhanced lumen of the vessel that abuts the wall of the vessel for a length <1 cm, 3 indicating lead within the contrast-enhanced lumen of the vessel that abuts the wall of the vessel for a length >1 cm, 4 indicating lead that is outside the contrast-enhanced lumen of the vessel (Figure 1A). These scores were evaluated in 3 zones of interest: zone 1 from the sheath to the brachiocephalic/SVC intersection, zone 2 from brachiocephalic/SVC interaction to where shadow of right atrium enlarges from the vertical lateral wall of the SVC, and zone 3 from where the right atrium enlarges from the vertical lateral wall of the SVC to the end of the lead (Figure 1B). Additional analyses on CT scan included stenosis of the vessel, calcification, anomalous anatomy, thrombus adherent to leads, microperforation/macroperforation of the lead tip, and relation of the lead with the tricuspid valve.
Figure 1.
Fibrosis scores and extraction zones. A, Axial and multiplanar reformatted images in representative patients with different fibrosis scores. B, Graphical representation of anatomical zones as defined by the study with presence of right atrial and right ventricular leads.
Lead Extraction Procedure
Lead extraction procedures were performed by lead extraction operators who were blinded to the CT grading performed by the radiologist. However, proceduralists were able to review studies before the procedure for planning purposes but did not have the graded scores as done by the imaging experts. Extraction procedures were done with the tools at the discretion of individual operators. Success was defined by complete lead removal or clinical success (achieved clinical goal but not complete removal of the lead) as previously defined.4 Operators graded ease/difficulty of extraction on a scale of 1 to 4 with 1 being easiest and 4 being most difficult. Major and minor complications of the procedure were defined by the Heart Rhythm Society 2017 consensus guidelines on lead management.4 All patients were followed for 30 days following the procedure to collect data on rehospitalization and mortality.
Primary and Secondary Outcomes
The primary outcome of the study was ease of sheath advancement defined by the use of powered sheath use (rotational mechanical or laser) over the leads in zone 2, from the brachiocephalic to the SVC. Sheath advancement over the other 2 zones, zone 1 and 3, were also studied. Additional outcomes included correlation between fibrosis score as graded by CT readers and extraction proceduralists. Secondary outcomes also included major and minor complications as defined by the Heart Rhythm Society 2017 consensus document, length of extraction, fluoroscopy time, mortality at 30 days, and procedural and clinical success of lead removal.
Statistical Analysis
Baseline CT scan and procedural characteristics were summarized using mean (standard deviation) for normally distributed continuous variables and median (interquartile range) for those with non-normal distribution, and frequency (percentage) for categorical variables. The association of the primary end point of power sheath and laser sheath with fibrosis score was examined using χ2 test (for fibrosis score 3 and 4 versus 1 and 2) and using Wilcoxon Rank Sum Test (for continuous fibrosis score). A multivariable logistic regression was performed as well to check their association controlling for key lead extraction covariates. The inter-rater agreement between raw fibrosis scores and raw lead extraction scores was measured by Cohen-weighted kappa statistic. Rank analysis of covariance (ANCOVA; Hettmansperger and McKean linear model aligned rank test) was implemented for assessing the relationship of higher fibrosis score with the clinical variables of duration of lead extraction and fluoroscopy, to accommodate both the nonparametric feature of the data and the need of adjusting for key covariates (Tables S1 and S2). Data were analyzed using the SAS Software (SAS Institute, Cary, NC) and the open-source software R (R Foundation for Statistical Computing, Vienna, Austria).
Results
Baseline Characteristics
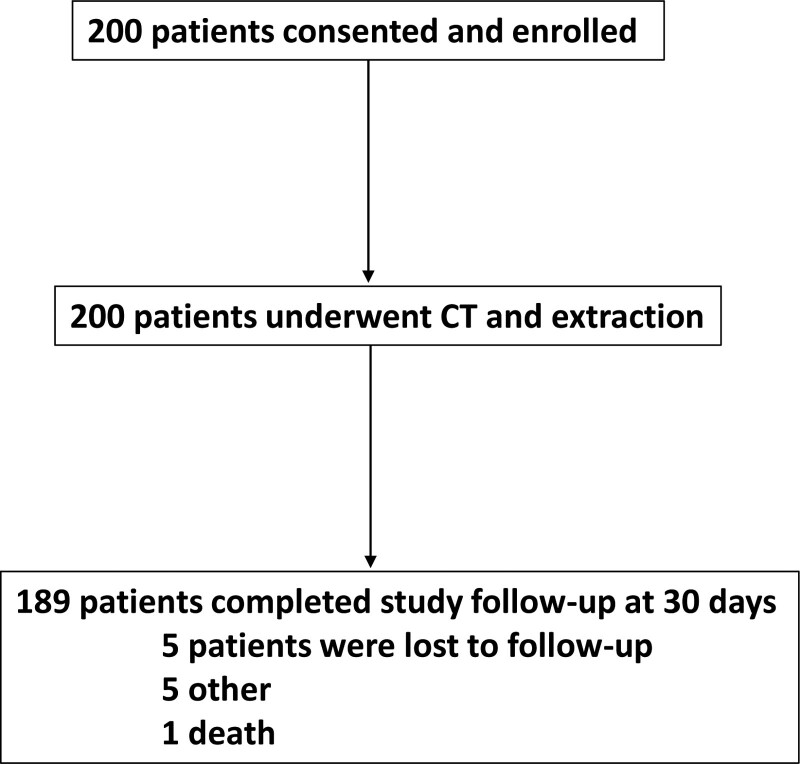
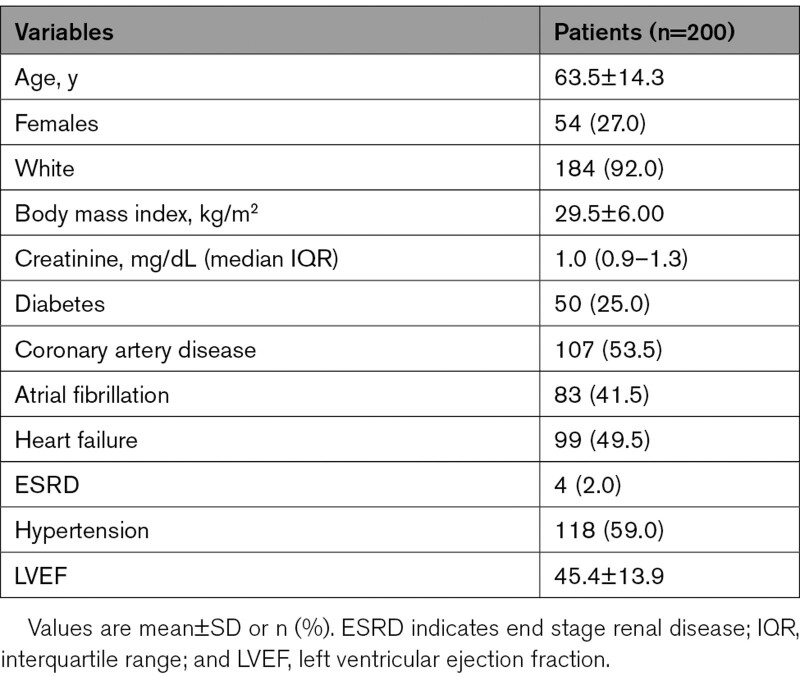
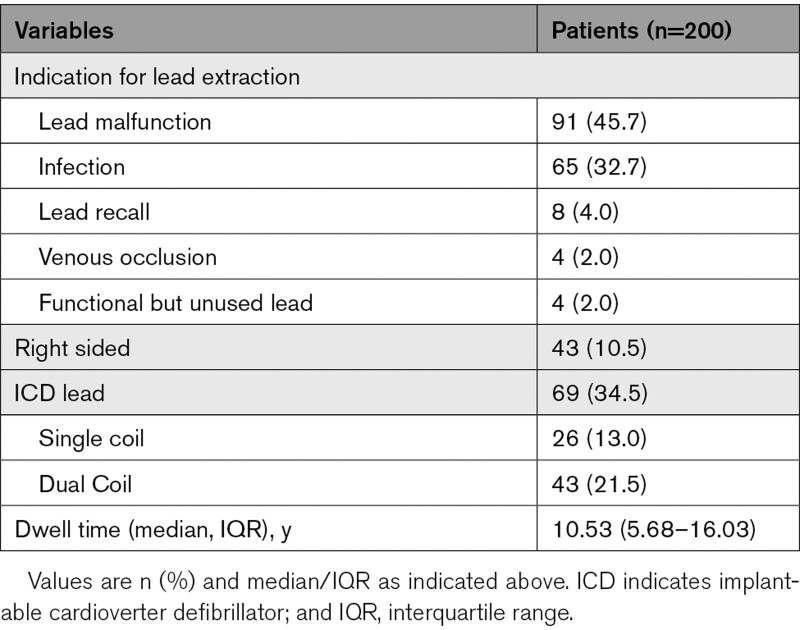
A total of 200 patients were enrolled from the 5 centers from December 11, 2018 to November 4, 2020 (Figure 2). Baseline characteristics of study participants are reported in Table 1. The mean age was 63.5±14.3 years and 27 % of participants were females. Ninety-nine (49.5%) patients had a diagnosis of heart failure, and the mean left ventricular ejection fraction of patients in the study was 45.4±13.9 %. The most common reason for lead extraction referral was lead malfunction (n=91) and infection (n=65). The mean number of indwelling leads was 2.4±0.9 leads with the median dwell time of leads of 10.53 (interquartile range, 5.68–16.03) years. There were 69 (34.5%) implantable cardioverter defibrillator leads removed in the study with majority being dual coil (Table 2).
Figure 2.
Flow diagram of enrollment and follow-up in the MILES (Multicenter Imaging in Lead Extraction Study). CT indicates computed tomography.
Table 1.
Baseline Medical History
Table 2.
Baseline Device and Lead Characteristics
Cardiac CT Scans
Cardiac CTs were read by physicians dedicated to cardiac radiology in 190 (95%) of the cases while the remaining 10 (5%) of cases were read by cardiologists or electrophysiologists. The readers graded most studies as adequate (52%) or excellent (30%) for allowing interpretation of lead adherence to the vessel wall. Only 7 (3.5%) studies were rated poorly for image interpretation. Frequencies of fibrosis score (1–4) in each of the zones and other imaging findings including stenosis, calcification, and thrombus are seen in Table 3. In zone 1, 7 (4.2%) had a fibrosis score of 1, 32 (19.2%) had a fibrosis score of 2, 74 (44.3%) had a fibrosis score of 3, and 54 (32.3%) had a fibrosis score of 4 with 34 (17.0%) with findings of stenosis, 4 (2.0%) with calcification, and 2 (1.0%) with thrombus adherent to the lead. In zone 2, 11 (5.5%) had a fibrosis score of 1, 33 (16.6%) had a fibrosis score of 2, 95 (47.7%) had a fibrosis score of 3, and 60 (30.2%) had a fibrosis score of 4 with 8 (4.0%) with findings of stenosis, 2 (2.0%) with calcification, and 3 (1.5%) with thrombus adherent to the lead. In zone 3, 174 (87.9%) had a fibrosis score of 1, 14 (7.1%) had a fibrosis score of 2, 7 (3.5%) had a fibrosis score of 3, and 3 (1.5%) had a fibrosis score of 4 with 1 (0.5%) having calcification and 2 (1.0%) having thrombus adherent to the lead.
Table 3.
ECG-Gated Cardiac CT Findings
Procedural Characteristics
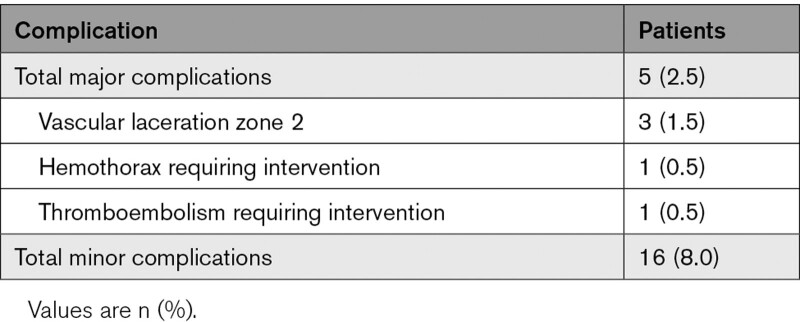
The study targeted removal of 395 total leads during the extraction procedure. The CT led to a change in plan to abandonment in 4 (1.0%) leads and surgical removal in 3 (0.8%) leads. Complete success of lead removal was achieved in 390 (94.9%) leads. There were 5 major complications with 3 vascular lacerations, of which all vascular lacerations happened in zone 2 (Table 4). There were 16 (8.0%) minor complications, of which the most common were hematoma requiring evacuation, venous thrombosis with intervention, bleeding with transfusion needed, worsening tricuspid valve regurgitation, and pulmonary embolism.
Table 4.
Major and Minor Complications
Follow-Up
Although 189 patients completed follow-up, there was 30-day data in 199 of the 200 patients enrolled in the study at mean follow-up time of 37.6 days. Sixteen (8.0%) were hospitalized after discharge from their lead extraction procedure, and all but 1 (0.5%) were alive at follow-up. The patient who died was a patient on chronic hemodialysis who had a hypoxic/hypercapnic respiratory arrest during a dialysis session and was transitioned to comfort care.
Primary End Point
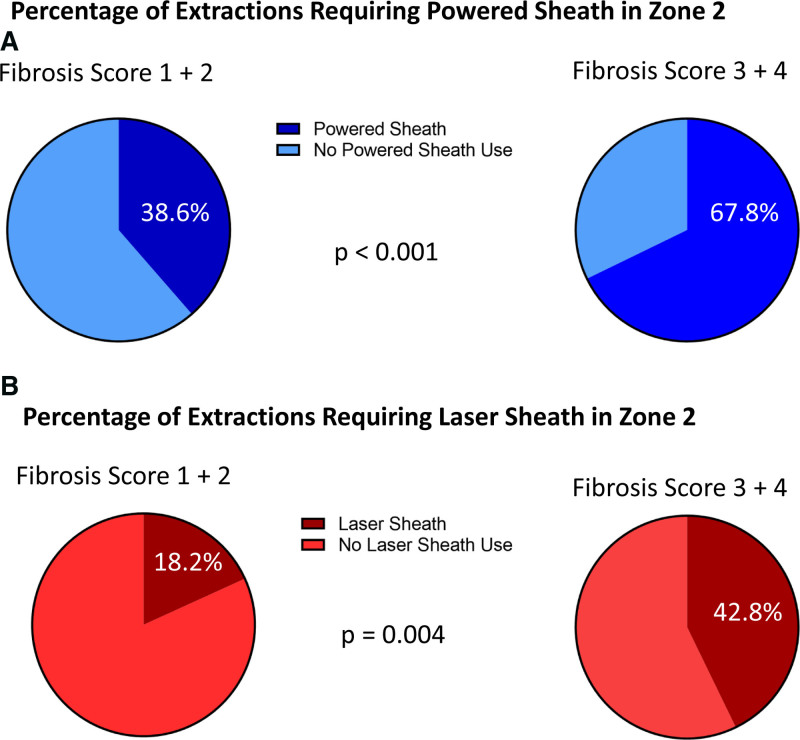
Total powered sheath use was statistically significantly higher in patients with higher fibrosis scores seen on CT (scores 3 and 4; 67.8%) in zone 2 compared with patients with lower fibrosis seen on CT (scores 1 and 2; 38.6%; P<0.001; Figure 3A). Laser sheaths, a subset of powered sheaths, use was also statistically significantly higher in patients with higher fibrosis on CT (scores 3&4; 42.8%) in zone 2 compared with patients with lower fibrosis seen on CT (scores 1 and 2; 18.2%; P=0.004; Figure 3B). If fibrosis scores are treated as continuous measures, power sheath use and laser sheath use are associated with higher fibrosis scores as well (P<0.001 and P=0.005, respectively). Logistic regression analysis demonstrated a significant positive association of power sheath with higher fibrosis (odds ratio, 2.78 [95% CI, 1.22–6.31], P=0.015 after controlling for number of leads to be extracted, log-transformed age of the oldest lead, centers, and patient age) and an almost significant association of laser sheath use with higher fibrosis (odds ratio, 2.33 [95% CI, 0.93–5.84]; P=0.073 after controlling for number of leads to be extracted, log-transformed age of the oldest lead, and centers).
Figure 3.
Extraction tools related to fibrosis score. A, Pie-chart representation of the powered sheath use in fibrosis score 1+2 vs fibrosis score 3+4. B, Pie-chart representation of the laser sheath use in fibrosis score 1+2 vs fibrosis score 3+4.
Secondary End Points
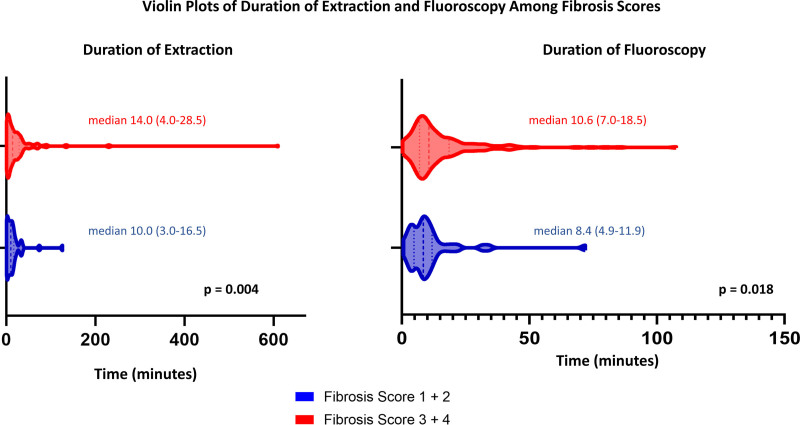
In zone 2, there was a slight but significant agreement between fibrosis score rated by the CT reader and the score given by the operator for the ease of extraction (weighted kappa estimate 0.21; P<0.001). The duration of extraction and fluoroscopy use was statistically significantly longer in those with fibrosis scores of 3+4 compared to those with lower fibrosis scores of 1+2 (duration of extraction: median (interquartile range) 14.0 [4.0–28.5] versus 10.0 minutes [3.0–16.5]; P=0.004; duration of fluoroscopy: 10.6 [7.0–18.5] versus 8.4 minutes [4.9–11.9]; P=0.018), after controlling for number of leads to be extracted in a rank ANCOVA model (Figure 4). In a multivariable rank ANCOVA model with important covariates and comorbidities including number of leads to be extracted, age of oldest lead, atrial fibrillation, previous smoking history, centers, and presence of SVC coil a higher fibrosis score was a marginally significant predictor of longer fluoroscopy time (P=0.053), although not related to longer extraction time (P=0.09). Fluoroscopy radiation exposure was statistically significantly greater when fibrosis score was higher (P=0.013) adjusting for age of oldest lead, body mass index, gender, atrial fibrillation, center, and presence of SVC coil. There was no significant correlation between fibrosis score and vascular complications (spearman coefficient of 0.064 with P=0.37) in zone 2, though the study was not powered to detect this given the low event rate and limited sample size.
Figure 4.
Violin plot representation of duration of extraction and fluoroscopy time in fibrosis score 1+2 vs fibrosis score 3+4. Thicker dashed lines represent median and thinner lines represent 25th and 75th percentile. Minimum and maximum represented by edges of the plot.
Discussion
This study was a prospective, multicenter clinical trial that assessed the effectiveness of ECG-gated cardiac CT in identifying difficult transvenous lead extractions, measured by the use of powered sheaths at the SVC level. The trial found a significant association between higher fibrosis scored by cardiac CT and need of powered sheaths.
Although transvenous lead extraction has increasingly become safer, the potential risk of major complication is still high and therefore frequently requires the use of a hybrid operating room and cardiothoracic surgical backup. To aid in the planning of extraction procedures, risk scores have been developed based on variables such as age of lead, age of patient, left ventricular ejection fraction, the experience of the operator, and diabetes that can help patients and providers weigh risks and benefits with lead extraction.6,7 However because catastrophic injuries during lead extraction occur infrequently, it is unclear whether these are comorbidities related to the patient or surrogate variables for other risk factors.
Fibrosis and lead adherence frequently occur after lead implantation making lead extraction difficult and vascular injury one of the most feared complications of a lead extraction procedure.12 Tarakji et al previously demonstrated that even if catastrophic vascular injuries are uncommon, microscopic venous injuries are very common due to dense fibrosis between the lead and the surrounding vasculature.13 In addition to the lead-vessel fibrosis, lead-to-lead adherence is critical and can make extractions more difficult in patients with multiple indwelling leads. Beaser et al14 was able to quantify lead-on-lead binding and with lead-vessel adherence show that patients with increased lead-on-lead binding had more difficult extractions, as measured by extraction time and laser pulses.
Other groups have studied invasive imaging modalities to examine transvenous lead extraction risk. Sadek et al15 used intracardiac echocardiography to define lead-to-vessel adherence and correlated that with difficulty of lead extraction, while Beaser et al14 used intravascular ultrasound to evaluate lead-to-vessel adherence and lead-to-lead binding to determine which patients required increased procedural complexity. The potential advantages of invasive imaging to define risk is a better evaluation of lead-to-lead binding which is not described by our study or previous studies and for the case of intracardiac echocardiography allows for a real-time evaluation for pericardial effusion, worsening left ventricular or right ventricular function, and worsening tricuspid regurgitation. The disadvantage of using intracardiac echocardiography or intravascular ultrasound is not only the invasive nature of the procedures but also the inability to tell patients' risk before having them on the operating table and the learning curve involved in study interpretation of these 2 modalities.
The current study standardized a protocol for imaging with CT that could be used by multiple centers for the evaluation of fibrosis by examining lead adherence to vessel wall noninvasively before extraction. This could aid in the planning stages by having the right equipment and personnel available to perform the extraction. Furthermore, imaging done before extraction can be incorporated into a risk score with other factors such as age of the lead and type of lead being extracted that can better inform electrophysiologists and patients about the possible risks associated with transvenous lead extraction. In fact, in a similar retrospective study done by Svennberg et al,11 the investigators found that CT may be better able to risk stratify difficult extractions in leads with dwell times less than 10 years. In those with leads >10 years, extraction difficulty may depend more on the lead age rather than CT findings.11 Although we sought to identify a low-risk group of patients that would not require the use of powered or laser sheath, this may have to do with operator preference and/or nature of the leads as the operators were blinded to the CT results. The protocol and study can better help inform patients about their individual risk with their given lead adherence and comorbidities and aid proceduralists and surgeons in whether leads should be extracted or capped, proper setting with surgical backup, and which tools or operators may be needed to perform the procedure safely. Although the study found a significant association between risk score and fluoroscopy, whether this is clinically relevant or relates to complications is unknown.
There are several limitations of the study that are worth mentioning. First, this study was standardized at 5 experienced extraction centers, and, therefore, the outcomes of the extraction may not represent the outcomes seen at other centers. Furthermore, the study was not powered to detect whether fibrosis could identify vascular complications given the low number that occur but rather identify surrogates of difficult extraction such as powered sheath or laser sheath use and procedural time. Information regarding the few patients who underwent CT scans but ultimately did not undergo lead extraction was not recorded or available. Lastly, operators’ differences in preferences as to the technique may dictate differences in primary and secondary outcomes.
Conclusions
Gated contrasted CT can predict a higher rate of powered sheath use in patients with higher fibrosis scores as patients with fibrosis scores of 3 and 4 required more use of powered sheaths compared to those with scores of 1 and 2. An absolute low-risk cohort who would not absolutely require powered sheaths as identified by CT was not seen in the study. However, CT does provide informative data that when combined with other data might identify low-risk patients with higher confidence than without using CT guidance.
Article Information
Sources of Funding
Philips investigator-initiated research grant, Colorado Springs, CO.
Disclosures
Dr Vatterott has received consultant fees from Medtronic, Boston Scientific, Cook Medical, and Philips. Dr Piccini has received honoraria and consultant fees from Abbott, Biotronik, Medtronic, and Philips. Dr Tarakji has received consultancy fees from Medtronic. Dr Francis is an employee of Philips. Dr Wilkoff has received consultant fees from Medtronic, Abbott, and Philips. The other authors report no conflicts.
Supplemental Material
Supplemental Methods
Tables S1–S2
Supplementary Material
Nonstandard Abbreviations and Acronyms
- CIEDs
- cardiac implantable electronic devices
- CT
- computed tomography
- MILES
- Multicenter Imaging in Lead Extraction Study
- SVC
- superior vena cava
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCEP.121.010779.
For Sources of Funding and Disclosures, see page 747.
Contributor Information
Divyang Patel, Email: divyangrpatel@gmail.com.
Pierce Vatterott, Email: pvatterott@msn.com.
Jonathan Piccini, Email: jonathan.piccini@duke.edu.
Laurence M. Epstein, Email: lepstein@northwell.edu.
Samer Hakmi, Email: s.hakmi@asklepios.com.
Imran Syed, Email: imranssyed@gmail.com.
Michael Bolen, Email: bolenm@ccf.org.
Paul Schoenhagen, Email: schoenp1@ccf.org.
Khaldoun G. Tarakji, Email: khaldoun.g.tarakji@medtronic.com.
Nathan Francis, Email: nathan.francis@philips.com.
Mingyuan Shao, Email: shaom@ccf.org.
Bruce L. Wilkoff, Email: wilkofb@ccf.org.
References
- 1.Zhan C, Baine WB, Sedrakyan A, Steiner C. Cardiac device implantation in the United States from 1997 through 2004: a population-based analysis. J Gen Intern Med. 2008;23:13–19. doi: 10.1007/s11606-007-0392-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brunner MP, Cronin EM, Jacob J, Duarte VE, Tarakji KG, Martin DO, Callahan T, Borek PP, Cantillon DJ, Niebauer MJ, et al. Transvenous extraction of implantable cardioverter-defibrillator leads under advisory--a comparison of Riata, Sprint Fidelis, and non-recalled implantable cardioverter-defibrillator leads. Heart Rhythm. 2013;10:1444–1450. doi: 10.1016/j.hrthm.2013.06.021 [DOI] [PubMed] [Google Scholar]
- 3.Voigt A, Shalaby A, Saba S. Rising rates of cardiac rhythm management device infections in the United States: 1996 through 2003. J Am Coll Cardiol. 2006;48:590–591. doi: 10.1016/j.jacc.2006.05.016 [DOI] [PubMed] [Google Scholar]
- 4.Kusumoto FM, Schoenfeld MH, Wilkoff BL, Berul CI, Birgersdotter-Green UM, Carillo R, Cha YM, Clancy J, Deharo JC, Ellenbogen KA, et al. 2017 HRS expert consensus statement on cardiovascular implantable electronic device lead management and extraction. Heart Rhythm. 2017;14:e503–e551. doi: 10.1016/j.hrthm.2017.09.001 [DOI] [PubMed] [Google Scholar]
- 5.Wilkoff BL, Byrd CL, Love CJ, Hayes DL, Sellers TD, Schaerf R, Parsonnet V, Epstein LM, Sorrentino RA, Reiser C. Pacemaker lead extraction with the laser sheath: results of the pacing lead extraction with the excimer sheath (Plexes) trial. J Am Coll Cardiol. 1999;33:1671–1676. doi: 10.1016/s0735-1097(99)00074-1 [DOI] [PubMed] [Google Scholar]
- 6.Wazni O, Epstein LM, Carrillo RG, Love C, Adler SW, Riggio DW, Karim SS, Bashir J, Greenspon AJ, DiMarco JP, et al. Lead extraction in the contemporary setting: the LExICon study: an observational retrospective study of consecutive laser lead extractions. J Am Coll Cardiol. 2010;55:579–586. doi: 10.1016/j.jacc.2009.08.070 [DOI] [PubMed] [Google Scholar]
- 7.Brunner MP, Cronin EM, Duarte VE, Chu Y, Tarakji KG, Martin DO, Callahan T, Cantillon DJ, Niebauer MJ, Saliba W, et al. Clinical predictors of adverse patient outcomes in an experience of more than 5000 chronic endovascular pacemaker and defibrillator lead extractions. Heart Rhythm. 2014;11:799–805. doi: 10.1016/j.hrthm.2014.01.016 [DOI] [PubMed] [Google Scholar]
- 8.Lewis RK, Pokorney SD, Greenfield RA, Hranitzky PM, Hegland D, Schroder JN, Lin SS, Milano C, Daubert JP, Smith PK, et al. Preprocedural ECG-gated computed tomography for prevention of complications during lead extraction. Pacing Clin Electrophysiol. 2014;37:1297–1305. doi: 10.1111/pace.12485 [DOI] [PubMed] [Google Scholar]
- 9.Ehieli WL, Boll DT, Marin D, Lewis R, Piccini JP, Hurwitz LM. Use of preprocedural mdct for cardiac implantable electric device lead extraction: frequency of findings that change management. AJR Am J Roentgenol. 2017;208:770–776. doi: 10.2214/AJR.16.16897 [DOI] [PubMed] [Google Scholar]
- 10.Patel D, Sripariwuth A, Abozeed M, Hussein AA, Tarakji KG, Wazni OM, Wilkoff BL, Schoenhagen P, Bolen MA. Lead location as assessed on cardiac computed tomography and difficulty of percutaneous transvenous extraction. JACC Clin Electrophysiol. 2019;5:1432–1438. doi: 10.1016/j.jacep.2019.07.018 [DOI] [PubMed] [Google Scholar]
- 11.Svennberg E, Jacobs K, McVeigh E, Pretorius V, Birgersdotter-Green U. Computed tomography-guided risk assessment in percutaneous lead extraction. JACC Clin Electrophysiol. 2019;5:1439–1446. doi: 10.1016/j.jacep.2019.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang TY, Baba N. Cardiac pathology of transvenous pacemakers. Am Heart J. 1972;83:469–474. doi: 10.1016/0002-8703(72)90037-3 [DOI] [PubMed] [Google Scholar]
- 13.Tarakji KG, Saliba W, Markabawi D, Rodriguez ER, Krauthammer Y, Brunner MP, Hussein AA, Baranowski B, Cantillon DJ, Kanj M, et al. Unrecognized venous injuries after cardiac implantable electronic device transvenous lead extraction. Heart Rhythm. 2018;15:318–325. doi: 10.1016/j.hrthm.2017.11.008 [DOI] [PubMed] [Google Scholar]
- 14.Beaser AD, Aziz Z, Besser SA, Jones CI, Jameria Z, Kannan A, Upadhyay GA, Broman MT, Ozcan C, Tung R, et al. Characterization of lead adherence using intravascular ultrasound to assess difficulty of transvenous lead extraction. Circ Arrhythm Electrophysiol. 2020;13:e007726. doi: 10.1161/CIRCEP.119.007726 [DOI] [PubMed] [Google Scholar]
- 15.Sadek MM, Cooper JM, Frankel DS, Santangeli P, Epstein AE, Marchlinski FE, Schaller RD. Utility of intracardiac echocardiography during transvenous lead extraction. Heart Rhythm. 2017;14:1779–1785. doi: 10.1016/j.hrthm.2017.08.023 [DOI] [PubMed] [Google Scholar]