Abstract
Objective:
Adults living with HIV (ALWHIV) on antiretroviral therapy (ART) are at high risk of pneumococcal carriage and disease. To help evaluate carriage risk in African ALWHIV at least 4 years after infant pneumococcal conjugate vaccination introduction in 2011, we assessed association between pneumococcal carriage and potential risk factors.
Methods:
Nasopharyngeal swabs were collected from adults aged 18–40 years attending an ART clinic during rolling, cross-sectional surveys in Blantyre, Malawi between 2015 and 2019. We fitted generalized additive models to estimate the risk of sex, social economic status (SES), living with a child less than 5 years, and ART duration on carriage.
Results:
Of 2067 adults, median age was 33 years (range 28–37), 1427 (69.0%) were women, 1087 (61.4%) were in low–middle socioeconomic-status (SES), 910 (44.0%) were living with a child less than 5 years, and median ART duration was 3 years (range 0.004–17). We estimated 38.2 and 60.6% reductions in overall and vaccine-serotype carriage prevalence. Overall carriage was associated with low SES, living with a child less than 5 years and shorter duration on ART. By contrast, vaccine-type carriage was associated with living without a child less than 5 years and male sex.
Conclusion:
Despite temporal reductions in overall and vaccine-serotype carriage, there is evidence of incomplete vaccine-serotype indirect protection. A targeted-vaccination campaign should be considered for ALWHIV, along with other public health measures to further reduce vaccine-serotype carriage and therefore disease.
Keywords: antiretroviral, herd immunity, HIV, Malawi, pneumococcal carriage, pneumococcal conjugate vaccine, risk factors
Introduction
Pneumococcus is a common colonizer of the human nasopharynx, particularly in young children and populations with HIV [1]. Pneumococcal colonization is a prerequisite for transmission and the development of disease, including otitis media, sinusitis, pneumonia, meningitis, and bacteraemia [2]. The pneumococcus is associated with a large burden of disease in adults living with HIV (ALWHIV) compared with adults without HIV [3–5]. Adult HIV prevalence remains high (>10%) in many sub-Saharan African countries, with Malawi reporting a national prevalence of 10.6% [6–8]. The use of antiretroviral therapy (ART) has substantially increased survival and reduced the incidence of invasive pneumococcal disease (IPD) [9]. However, despite more than 85% of ALWHIV in Malawi receiving ART [10–12], ALWHIV remain at greater risk of IPD than adults without HIV [3].
Pneumococcal conjugate vaccines (PCVs) are widely used in infant schedules in low-income and middle-income countries (LMICs), generally targeting the most commonly invasive serotypes in this age group [1]. To date, in contrast to high-income settings, immunization of vulnerable adults with pneumococcal vaccines has neither been adopted in most LMICs such as Malawi, nor are pneumococcal vaccines available outside the Expanded Program on Immunization (EPI) [13]. In November 2011, Malawi introduced the 13-valent PCV (PCV13) into the national EPI using a three-primary-dose schedule without booster (3+0; one dose at 6, 10, and 14 weeks of age). Despite nearly 10 years of more than 80% PCV13 three-dose coverage among age-eligible children, there is evidence of a suboptimal reduction in both vaccine-serotype carriage prevalence and vaccine-serotype (VT)-IPD incidence in children and ALWHIV in Malawi [14–16]. Similar evidence of residual VT carriage prevalence is also reported in the Gambia and Mozambique after 5 and 2 years of implementation, respectively, [17] despite both countries also reporting more than 80% PCV three-dose coverage under a 3+0 schedule [18,19]. There is increasing evidence that the indirect protection offered by an infant PCV against VT carriage, especially in ALWHIV, is suboptimal [14,18].
The most effective strategy to reduce residual VT-IPD burden in ALWHIV depends on the factors shaping VT carriage and disease risk. Before introducing infant PCV in Malawi and South Africa, risk factors for IPD in ALWHIV included younger age, female sex, cotrimoxazole resistance, underlying medical conditions and living in a densely populated area [3,4]. Conversely, risk factors for carriage of any pneumococcal serotype included exposure to infants exposed to HIV [20,21], low socioeconomic status (SES), high density living in housing with inadequate ventilation and with intense social contacts [22–25]. Moreover, among Malawian ALWHIV, VT and non-VT (NVT) carriage prevalence was found to be higher in those on ART than not on ART [26,27].
In the PCV era, however, there are important gaps in our understanding of the relative importance of key factors for pneumococcal carriage and disease among ALWHIV. These include duration on ART, PCV vaccination status among children in the household, and SES. Following our recent data from Blantyre, Malawi showing high residual VT carriage and its determinants in PCV13-vaccinated and unvaccinated children and in ALWHIV [15,16], we extend the analysis to identify age-dependent and time-dependent risk factors for pneumococcal carriage in ALWHIV on ART using generalized additive modelling.
Methods
Study design
Blantyre spans 2025 km2, with an urban (population density 3334/km2) and rural (253/km2) population of approximately 800 000 and 451 000 people, respectively [28]. As described elsewhere [15], rolling, prospective cross-sectional pneumococcal nasopharyngeal (NP) carriage surveys were conducted between 29 June 2015 and 9 August 2019 in Blantyre to investigate temporal change of pneumococcal colonization in ALWHIV on ART. The majority (98.6%) of sampled individuals were on a first-line ART regimen containing either zidovudine, lamivudine and efavirenz; tenofovir, lamivudine and efavirenz or tenofovir, lamivudine and nevirapine [29]. Eight pneumococcal carriage surveys (each approximately 6 months in duration) were conducted, as per study protocol, from 3.6 to 7.9 years after infant PCV13 introduction into the EPI schedule. Thus, at least two carriage surveys per year except in 2019 when only one carriage survey was conducted. ALWHIV aged 18–40 years were recruited from the Queen Elizabeth Central Hospital (QECH) ART clinic in Blantyre using a systematic sampling approach. Exclusion from the study included being currently on treatment for tuberculosis, hospitalization within 2 weeks of recruitment and previously enrolled in the survey.
Nasopharyngeal sample collection and processing
A nasopharyngeal swab sample was collected from each participant and processed at the Malawi-Liverpool-Wellcome Programme laboratory, co-located to QECH, to ascertain the presence of pneumococci. Samples were collected and processed according to WHO guidelines [30]. Serotyping was done using latex agglutination, based on picking a single colony, to identify serotypes targeted by PCV13 (1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, 23F). Nontypeable and NVT isolates were both classified as NVT. Pneumococcal carriage was further evaluated using DNA microarray techniques, a technique which, in the case of co-carriage of multiple pneumococcal serotypes, differentiates all individual serotypes and reports relative abundance of each serotype in carriage [31–33]. Microarray was implemented only in surveys 1 through 4 and with samples having latex-confirmed pneumococcal carriage. Further details of sample processing was reported earlier [15,32].
Data collection and analysis
Participant data collected at recruitment included age, sex, cohabitation with a child less than 5 years, social economic status (SES), duration of ART use, CD4+ T-cell count, current ART regimen and cotrimoxazole use. A multiple imputation random forest-based method, using MissForest R package [34], was conducted to impute one (0.0005%), 297 (14.4%) and 537 (26.0%) missing data points on cohabitation with a child less than 5 years, SES and duration of ART use, respectively. Though reported in the descriptive analysis, CD4+ cell count was excluded from model-based analyses because 46% of its data points were missing, a proportion above the acceptable standard threshold for imputation [34]. Duration on ART was not used as a continuous variable because of data sparsity in age-stratified or time-stratified analyses but was categorized as short (<3 years) or long (≥3 years) duration based on (1) a previous study in rural Malawi, which showed strong evidence of high pneumococcal carriage during the first 2 years of ART use [26], and (2) the median value of ART duration in this study.
Individual fitted carriage prevalence estimates were categorized into 18–24, 25–29, 30–34 and 35–40-year age groups reflecting their distinct IPD incidence [3]. Time was stratified into year of survey initiation (2015, 2016, 2017, 2018 and 2019). Seasonality in carriage was captured using an indicator variable with values ranging from January to December based on NP sample collection month [26].
Generalized additive modelling framework
We used a generalized additive modelling (GAM) framework to fit to age-specific and time-specific trajectories of pneumococcal carriage, and allow flexibility in capturing nonlinear carriage dynamics. In brief, we used penalized B splines (P-splines) for the age and time spline smoothers to avoid knot selections, which usually introduce under-fitting and over-fitting biases when trading-off model fit to the data and the smoothness of the curve [35]. A penalized log-likelihood maximization was used to fit a nonparametric binomial model with complementary log–log link function defined by log-hazard of carriage as a function of the risk factors and a spline in age and time. No time-series autocorrelation structure was included in the model fits because ALWHIV were independently sampled without replacement and with no evidence to suggest strong autocorrelation across time.
Age-dependent and time-dependent carriage prevalence estimation
We modelled age-dependent and time-dependent carriage trajectories separately for overall (VT+NVT) and VT carriage as outcome variables for a set of potential risk factors. Because of reported poor immunogenicity and effectiveness of PCV13 against serotype 3 [36,37], we also modelled VT carriage without serotype 3 (VT-st3) to explore changes in carriage prevalence. A model with age and time smoothers and potential risk factors including sex, seasonality, duration on ART, cohabitation with a child less than 5 years old, and SES were fitted to the carriage data to estimate the overall or VT carriage prevalence and risk factor-specific effects on carriage prevalence dynamics.
A ‘gam’ function in the ‘mgcv’ R package facilitated model fitting [38], based on a model formulated as where Yi is a binomial outcome on whether an individual i is carrying pneumococcus (1) or not (0); g is the complementary log–log link function; π(ai,ti) is the carriage prevalence estimate for individuals of age (ai) at time (ti); η(ai,ti) is a nonparametric linear predictor as function of individual age and time, and a set of risk factors. The linear predictor on a predictor scale is further expanded using the equation , where β0 is a model intercept, Gi refers to individual risk factor category, βk is the risk factor coefficient, te(ai) and te(ti) denote tensor product P-spline of predictor age (ai) and time (ti).
The relative difference in carriage prevalence was computed by subtracting the GAM carriage prevalence estimate for each age or time category from the reference category and then dividing the difference by the reference category and then multiplying by 100%. The 95% confidence interval (95% CI) of the relative difference was estimated using , where ρ is the relative difference, δ1 and δ2 are the coefficient variations of the reference and comparator categories, respectively, and coefficient variation being standard deviation divided by the observed mean [39]. GAMs with and without interactions between age group or time and each independent risk factor on the overall and VT carriage prevalence were fitted and compared using Akaike information criterion (AIC), and results of these tests are presented in S1 Table. Given the model complexities, sensitivity analyses assesed factors that may affect carriage estimates, which included the impact on carriage of individual age group or survey, serotyping method, carriage autocorrection, model formulation and spline type. Detailed sensitivity methods and results are presented in Supplementary Material (S1 Text and S2 Text). Analyses were conducted in R v4.1.1 [40], with statistical significance set at P less than 0.05, and the code is publicly shared via GitHub [41].
Ethical approval
Ethical approval for this study was granted by the College of Medicine Research Ethics Committee, Kamuzu University of Health Sciences (P.02/15/1677), the Liverpool School of Tropical Medicine Research Ethics Committee (14.056) and the London School of hygiene and Tropical Medicine (26839). Individual written informed consent, including consent for publication, was obtained from each participant prior to study recruitment.
Results
Descriptive analysis
A total of 2067 ALWHIV aged 18–40 years were enrolled in the study between 29 June 2015 and 9 August 2019. Among adults with nonmissing data, 1427 (69%, n = 2,067) were women, 413 (23.3%, n = 1770) and 674 (38.1%, n = 1,770) were from low and middle SES households, respectively, 1156 (56.0%, n = 2066) were not living with a child less than 5 years, 1772 (98.5%, n = 1799) were on one of Malawi's first-line ART regimens, and 2010 (97.2%, n = 2067) were using prophylactic cotrimoxazole at recruitment. The median age was 33 years (IQR: 28–37, n = 2067), median CD4+ count was 252 cells/μl (IQR: 138–443, n = 1117), and median duration on ART at the time of study recruitment was 3.0 years, (range: 0–17, n = 1530) (Fig. 1).
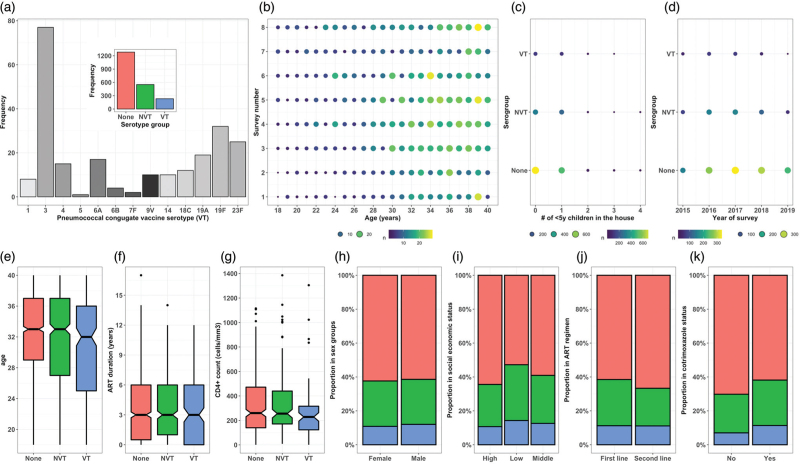
Fig. 1.
Demographics and clinical characteristics of participants using aggregated data across eight surveys.
(a) Frequency of each VT in carriage; insert shows frequency of VT, NVT and no carriage. (b) Number of adults in each annual age per survey with circle size proportional to total sample size. The number of adults with VT, NVT and no carriage living with (c) varying number of children less than 5 years or (d) across survey years. Notched box plots by serotype group representing participant distribution by (e) age, (f) duration on ART and (g) CD4+ count. Proportion of serotype group by (h) sex, (i) social economic status, (j) ART regimen and (k) Cotrimoxazole use.
Using survey-aggregated data, serotype 3 constituted 77 (33.2%, n = 232) of all VT serotypes identified. Survey-aggregated data showed that carriage prevalence was 784 (37.9%, n = 2067) for overall (VT+NVT) and 232 (11.2%, n = 2,067) for VT. It also showed that overall and VT carriage prevalence was 537 (37.7%, n = 1427) and 155 (10.9%, n = 1427) among women, 247 (38.6%, n = 640) and 77 (12%, n = 640) among men, 195 (47.2%, n = 413) and 59 (14.3%, n = 413) in low SES, 276 (40.9%, n = 674) and 85 (12.6%, n = 674) in middle SES and 243 (35.6%, n = 683), 73 (10.7%, n = 683) in high SES households, 361 (39.7%, n = 910) and 111 (12.2%, n = 910) in adults living with a child less than 5 years, 423 (36.6%, n = 1156) and 121 (10.5%, n = 1156) in adults living without a child less than 5 years, 682 (38.5%, n = 1778) and 199 (11.2%, n = 1778) in adults on a first-line ART regimen, nine (33.3%, n = 27) and three (11.1%, n = 27) in adults on second-line ART regimen, 767 (38.1%, n = 2010) and 228 (11.3%, n = 2010) in adults taking cotrimoxazole, 17 (29.8%, n = 57) and four (7%, n = 57) in adults not taking cotrimoxazole (Fig. 1).
Age-dependent and time-dependent carriage prevalence estimates
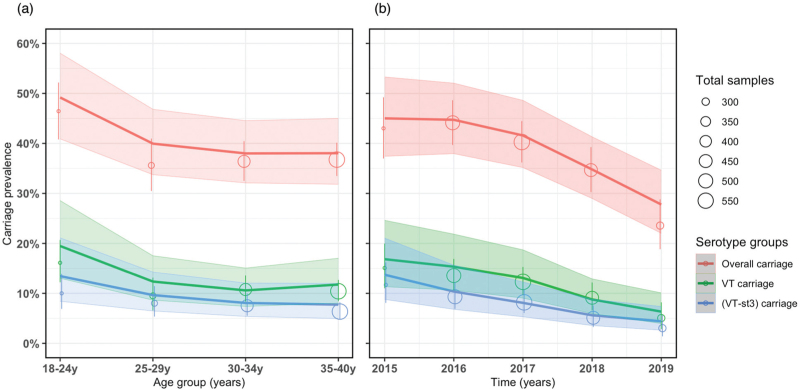
Our GAM predicted a significant reduction in overall and VT carriage prevalence with increasing age and time. Among older age categories, overall carriage prevalence was lower than the reference younger adults aged 18–24 years, with greatest reduction in adults aged 30–34 years (−22.8%, 95% CI −34.1 to −10.4). Likewise, VT carriage prevalence was lower in older than younger adults, with highest reduction in adults aged 30–34 years (−45.1%, 95% CI −61.8 to −25.6). Across time, we estimated lower overall (−38.2%, 95% CI −51.7 to −23.6) and VT (−60.6%, 95% CI −79.1 to −39.2) carriage prevalence in 2019 compared with 2015. In a sub-analysis, serotype 3 made up 22.6–34.7% (across age groups) and 18.9–38.2% (across time) of VT carriage prevalence (Table 1 and Fig. 2).
Table 1.
Age-dependent and time-dependent overall and VT carriage prevalence, and relative difference in fitted carriage prevalence among ALWHIV on ART, 2015–2019 in Blantyre, Malawi.
| Observed overall carriage n/N (%) | Modelleda overall carriage prevalence (95% CI) | Relative differenceb in overall carriage (95% CI) | Observed VT carriage n/N (%) | Modelleda VT carriage prevalence (95% CI) | Relative differenceb in VT carriage (95% CI) | |
| Age (years) | ||||||
| 18–24 | 144/310 (46.5) | 49.2 (40.9–58.0) | Reference | 50/310 (16.1) | 19.5 (13.0–28.6) | Reference |
| 25–29 | 120/337 (35.6) | 40.0 (33.8–46.8) | −18.7 (−32.3 to −4.1) | 32/337 (9.5) | 12.4 (8.7–17.6) | −36.4 (−58.0 to −11.4) |
| 30–34 | 213/585 (36.4) | 38.0 (32.1–44.5) | −22.8 (−34.1 to −10.4) | 63/585 (10.8) | 10.7 (7.4–15.1) | −45.1 (−61.8 to −25.6) |
| 35–40 | 307/835 (36.8) | 38.0 (31.8–45.0) | −22.8 (−33.3 to −11.2) | 87/835 (10.4) | 11.8 (8.1–17.1) | −39.4 (−55.9 to −19.9) |
| Year | ||||||
| 2015 | 114/265 (43.0) | 45.0 (37.4–53.3) | Reference | 40/265 (15.1) | 17.0 (11.4–24.8) | Reference |
| 2016 | 218/494 (44.1) | 44.7 (38.0–52.0) | −0.7 (−16.3 to 16.8) | 67/494 (13.6) | 15.3 (10.6–21.8) | −10.0 (−37.7 to 24.4) |
| 2017 | 226/561 (40.3) | 41.6 (35.2–48.6) | −7.6 (−22.1 to 8.7) | 69/561 (12.3) | 13.1 (9.1–18.8) | −22.9 (−46.9 to 6.8) |
| 2018 | 156/450 (34.7) | 34.8 (29.0–41.3) | −22.7 (−36.2 to −7.7) | 41/450 (9.1) | 8.8 (5.9–12.9) | −48.2 (−67.3 to −25.3) |
| 2019 | 70/297 (23.6) | 27.8 (22.0–34.6) | −38.2 (−51.7 to −23.6) | 15/297 (5.1) | 6.7 (4.2–10.6) | −60.6 (−79.1 to −39.2) |
Values in bold are statistically significant at P less than 0.05. 95% CI, 95% confidence interval; ALWHIV, adults living with HIV; ART, antiretroviral therapy; VT, PCV13 vaccine serotype.
Carriage prevalence was modelled by fitting a GAM to individual carriage trajectories adjusting for risk factors as described in Methods.
Relative difference was computed by subtracting a GAM carriage prevalence estimate from the reference category and then dividing the difference by the reference category and then multiplying by 100%.
Fig. 2.
Observed and fitted pneumococcal carriage prevalence curves using data from rolling, prospective cross-sectional surveys in Blantyre, Malawi 2015–2019.
Number of carriage samples per age group between 18 and 40 years (y) and survey time from 2015 to 2019 represented by open circles radius proportion to total sample size with corresponding confidence intervals (vertical lines). P-spline GAM fitted lines and confidence intervals (ribbons) for the (a) age-dependent and (B) time-dependent carriage prevalence stratified by overall carriage, vaccine serotypes (VT) carriage and VT carriage without serotype 3 (VT-st3).
Factors associated with overall carriage prevalence
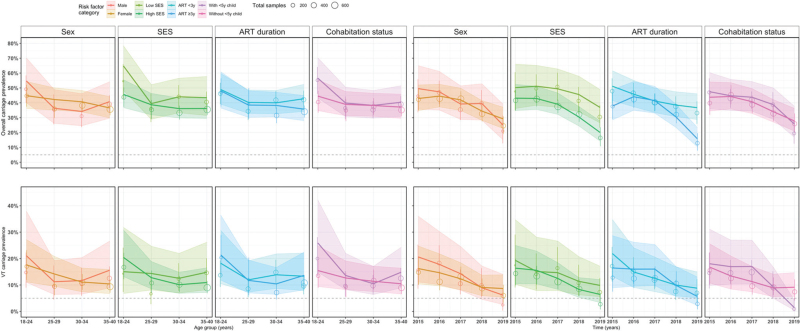
Overall carriage prevalence was only independently associated with SES, with adults in low SES having 22% higher overall carriage than those in high SES (21.9, 95% CI 1.6–43.7). In a subanalysis with age-stratification and time-stratification, our model predicted that being a younger (18–24 years) adult in low SES or living with a child aged less than 5 years was significantly associated with higher overall carriage prevalence. Significant associations with low SES and shorter ART duration were also seen with overall carriage. Overall carriage prevalence in younger adults was significantly higher by 42% for those in low vs. high SES (41.8, 95% CI 12.5–74.0) and 27% for those living with vs. those living without a child less than 5 years (27.2, 95% CI 0.4–57.4). Temporally, overall carriage prevalence was persistently higher by 50% in 2018 (49.5, 95% CI 13.4–89.8) and 84% in 2019 (83.6, 95% CI 20.5–167.4) in adults in the low vs. high SES, and higher by 35% in 2015 (35.4, 95% CI 0.9–77.2) and 131% in 2019 (130.8, 95% CI 43.2–255.4) in adults with shorter vs. longer duration on ART (Table 2 and Fig. 3, S1 Table).
Table 2.
Risk factors for age-dependent and time-dependent overall and VT with serotype 3 carriage prevalence, and the relative differences in the fitted carriage prevalence between the reference group and comparative groups among adults living with HIV on antiretroviral therapy, 2015–2019 in Blantyre, Malawi.
| % Relative differencea in age-dependent carriage prevalence (95% CI) | % Relative differencea in time-dependent carriage prevalence (95% CI) | |||||||||
| Risk factors | Overall % relative differencea (95% CI) | 18–24 years | 25–29 years | 30–34 years | 35–40 years | 2015 | 2016 | 2017 | 2018 | 2019 |
| Overall carriage | ||||||||||
| Female vs. male | −4.8 (−20.9 to 13.5) | −18.1 (−35.7 to 1.7) | 16.3 (−15.9 to 57.2) | 19.0 (−8.5 to 52.9) | −10.3 (−25.5 to 6.8) | −13.5 (−35.5 to 12.7) | −6.1 (−23.9 to 14.2) | 7.1 (−14.5 to 32.5) | −12.9 (−33.8 to 12.2) | 16.8 (−27.4 to 79.3) |
| Low vs. high SES | 21.9 (1.6–43.7) | 41.8 (12.5 to 74.0) | 2.1 (−26.0 to 32.9) | 22.2 (−2.8 to 49.2) | 19.9 (−1.8 to 42.9) | 16.5 (−16.2 to 52.2) | 19.3 (−5.4 to 45.8) | 26.7 (−0.1 to 55.2) | 49.5 (13.4 to 89.8) | 83.6 (20.5 to 167.4) |
| ART less than 3 years vs. ART at least 3 years | 11.5 (−6.7 to 31.5) | 1.9 (−20.8 to 28.4) | 4.7 (−23.9 to 40.6) | 4.4 (−15.7 to 26.8) | 19.9 (−0.4 to 41.8) | 35.4 (0.9–77.2) | 3.9 (−15.7 to 25.3) | −0.7 (−19.4 to 19.9) | 26.2 (−4.1 to 61.4) | 130.8 (43.2 to 255.4) |
| With vs. without child less than 5 years | 9.7 (−0.8 to 29.1) | 27.2 (0.4 to 57.4) | 2.6 (−22.9 to 31.8) | 0.3 (−19.5 to 22.1) | 8.9 (−9.2 to 28.5) | 8.7 (−18.4 to 39.5) | 1.1 (−17.9 to 22.2) | 7.4 (−12.4 to 29.4) | 13.8 (−14.3 to 44.7) | −7.6 (−41.8 to 31.8) |
| VT carriage | ||||||||||
| Female vs. male | −18.6 (−64.9 to 53.7) | −16.6 (−51.2 to 28.6) | 26.8 (−42.8 to 146.6) | −5.1 (−47.8 to 59.1) | −33.3 (−55.6 to −5.8) | −21.4 (−58.3 to 31.3) | −18.0 (−48.9 to 22.5) | −13.3 (−47.6 to 33.1) | 1.1 (−54.4 to 92.7) | 40.3 (−72.9 to 315.6) |
| Low vs. high SES | 6.0 (−61.0 to 91.2) | −26.5 (−67.6 to 19.6) | 12.5 (−46.6 to 85.4) | 23.8 (−32.4 to 90.8) | 35.8 (−14.5 to 93.2) | 17.7 (−47.0 to 94.6) | −3.2 (−48.7 to 47.9) | 17.6 (−38.2 to 80.9) | 37.3 (−39.2 to 133.2) | 53.1 (−48.0 to 229.1) |
| ART less than 3 years vs. ART at least 3 years | 0.0 (−55.1 to 77.4) | −14.5 (−49.6 to 32.3) | −15.3 (−63.7 to 65.3) | 33.7 (−19.3 to 102.1) | −2.9 (−36.4 to 35.0) | 32.7 (−26.2 to 114.5) | −6.9 (−42.9 to 36.2) | −21.9 (−51.5 to 13.6) | 1.0 (−49.2 to 67.6) | 54.4 (−53.7 to 255.6) |
| With vs. without child less than 5 years | 33.1 (−40.9 to 142.5) | −40.2 (−64.7 to −9.9) | −6.6 (−52.3 to 54.3) | 8.6 (−36.6 to 67.6) | −29.1 (−52.8 to −0.5) | 7.7 (−43.3 to 73.9) | 26.9 (−21.2 to 89.1) | 50.4 (−6.0 to 125.0) | 4.5 (−55.1 to 78.6) | −84.8 (−108.2 to −57.9) |
ART, antiretroviral therapy; ALWHIV, adults living with HIV; CI, confidence intervals; SES, Social Economic Status score based on a possession index, which is calculated as a sum of positive responses for household ownership of each of the 15 different functioning items, such as watch, radio, bank account, iron (charcoal), sewing machine (electric), mobile phone, CD player, fan (electric), bednet, mattress, bed, bicycle, motorcycle, car, and television. Middle and high SES were combined and named as high SES.
Relative difference was computed by subtracting a GAM carriage prevalence estimate of the reference category from the comparator category and then dividing the absolute difference by the reference category then multiplied by 100%.
Fig. 3.
P-spline generalized additive model: observed and fitted pneumococcal carriage prevalence curves for each potential risk factor category using data from rolling, prospective cross-sectional surveys in Blantyre, Malawi 2015–2019.
Nasopharyngeal samples across age groups from 18 to 40 years (y) and time represented by open circles. Circle radius is proportional to total sample size with corresponding confidence intervals (vertical lines). The coloured lines show P-spline GAM fitted model and confidence intervals for age-dependent and time-dependent carriage prevalence for overall carriage (plots from first row) and vaccine serotypes (VT) carriage (plots from second row) stratified by risk factor categories.
Factors associated with VT carriage prevalence
Sex, SES, ART duration and living with a child less than 5 years were not significantly associated with VT carriage prevalence. However, with age-stratification and time-stratification, our model of VT carriage outcome predicted that being a younger (18–24 years) or older (35–40 years) adult living without a child less than 5 years or being older male significantly increased VT carriage prevalence. Temporally, living without a child less than 5 years remained a significant predictor of higher carriage prevalence in 2019. Living without vs. with a child less than 5 years significantly increased VT carriage prevalence by 67% in younger adults 67.1 (95% CI 10.7–140.5) and 41% in older adults (95% CI 1.5–88.6). VT carriage prevalence was significantly higher in older men than women (50, 95% CI 7.2–100.7; Table 2 and Fig. 3, S1 Table).
Discussion
We used GAMs to estimate age-dependent and time-dependent overall (VT+NVT) and VT pneumococcal carriage prevalence and related risk factors in ALWHIV on ART. We analysed overall and VT carriage separately to take into account the effect of a high uptake infant PCV13 programme [42]. Overall and VT carriage declined with increasing age group and time, with VT carriage having a faster decline (faster still if serotype 3 was excluded from VT). Our models predicted higher overall carriage prevalence in younger adults from low SES and living with a child less than 5 years, as well as those with shorter duration on ART. Conversely, VT carriage prevalence was predominantly high in older males, and younger and older adults not living with a child less than 5 years. These findings suggest that the decline in VT carriage prevalence across time in ALWHIV on ART is in part because of VT indirect protection from vaccinated younger children, although it is imperfect in men or adults not living with younger children, who may potentially have different routes of pneumococcal acquisition outside the household. Accelerated temporal reduction in overall pneumococcal carriage suggests a combination of factors including the indirect effect of infant PCV13 vaccination, consequence of demographic change and general improvement in population-level immunity because of suppression of HIV viral load, and improved nutrition and access to healthcare [43–45], on the background of falling rates of pneumococcal disease, which were occurring before PCV13 introduction [46].
Our current analysis extends our previous observations that focused mainly on high residual VT carriage and its determinants in PCV13-vaccinated and unvaccinated children [15,16]. We now show substantially high overall and VT carriage prevalence in ALWHIV during the earlier (45 and 17%) than later (28 and 7%) years postinfant-PCV13 introduction. VT carriage declined faster than overall carriage, suggesting cumulative vaccine-induced community-level indirect protection from infant PCV vaccination [47–49]. The temporal reduction in VT carriage prevalence was even more marked when serotype 3 was excluded (and included as NVT), supporting accumulating evidence of the reduced effectiveness of PCV13 against serotype 3 [36,37].
Higher overall and VT carriage prevalences among younger than older adults reported in this study may suggest distinct high carriage acquisition risk in younger adults, partly supported by recent evidence of higher rates of skin-to-skin contacts between younger adults and with other age groups in urban Blantyre [50]. The shorter median duration on ART amongst the younger adults as shown in S6 Fig may contribute to this residual pneumococcal carriage through incomplete immune reconstitution at both the systemic and mucosal level [27,51,52].
Low SES neighbourhoods in urban Blantyre predominantly constitute high-density informal settlements, relatively larger households and low rates of formal employment [28]. Thus, substantial overall carriage prevalence in younger adults from low SES suggests that factors associated with low SES such as poorly ventilated and overcrowded houses with intense social contacts are reservoirs for pneumococcal carriage in the PCV13 era [16,28,50,53]. On the contrary, nondifferential VT carriage prevalence by household SES underlines an important role PCV vaccination plays to outweigh infection risks in poor settings. We uncover a phenomenon where adults living with children less than 5 years, mostly PCV13 recipients given the high (>90%) infant PCV13 vaccination coverage [54], have substantially lower VT but higher overall carriage prevalence suggesting some nonvaccine serotype (NVT) replacement in adults within households, in line with evidence from rural Malawi and South Africa [55,56].
In this setting, VT carriage prevalence was higher in older male than female adults. VT carriage acquisition between mothers and their infants has been demonstrated previously in Malawi and South Africa prior to infant-PCV introduction [20,21]. Thus, our finding aligns with recent evidence in the same setting showing strong intergenerational social mixing patterns between female individuals and their potentially PCV13-vaccinated younger children likely through parental or guardian roles [50]. This suggests that in the infant-PCV13 era, interruption of VT carriage transmission likely favours female individuals than male individuals.
Overall and VT carriage prevalence in ALWHIV on ART are heterogenous by age such that epidemiological models for carriage that incorporate ALWHIV should stratify for age for precise estimations. Our findings have policy implications in sub-Saharan African populations affected by HIV as persistent VT carriage in ALWHIV may imply continued risk of VT-IPD [14]. The indirect impact on VT carriage of alternative infant-PCV13 vaccine strategies, including two primary doses with a booster dose or double booster doses (i.e. 2+1 or 2+1+1), currently being tested to improve the control of childhood disease, should also be further evaluated in ALWHIV [18]. Indeed the 2+1 schedule, as implemented in South Africa, has generated indirect protection against IPD in unvaccinated older children and ALWHIV [48,57]. However, simply improving control of carriage in young children to indirectly protect vulnerable immunocompromised adults may be insufficient, particularly in the context of a high local force of infection and a rapid waning of vaccine-induced immunity [16,58]. Furthermore, we provide evidence of heterogeneity in VT carriage prevalence with males or adults living without less than 5-year-old child in their homes being at highest risk of VT carriage in the PCV era. Together, these data add weight to our viewpoint that as with many people living in high-income countries, targeted-pneumococcal vaccination should be considered in ALWHIV in LMICs.
We used a robust dataset with adequate samples to compute estimates for the overall, VT and risk factor-dependent carriage prevalence. Nonetheless, there were some limitations to our work, including limited data on risk factors such as viral load, use of tobacco, presence of other chronic comorbidities, adherence to ART and history of antibiotics, which may independently influence pneumococcal carriage dynamics [59]. However, population-level viral suppression has increased from 68% in 2015 to 87% in 2020 suggesting improved ART adherence [43]. In addition, latex agglutination method used in the main analysis for single serotype detection could underestimate our current prevalence estimates as compared with a more sensitive microarray method for multiple serotype detection as shown in Fig. S2. Finally, the systematic recruitment of ALWHIV may be prone to bias if a cyclical pattern (unnoticeable here) is present in the important characteristics of the individuals as they attend the ART clinic [60].
In conclusion, despite temporal reductions in overall pneumococcal carriage, the risk of VT carriage and, therefore, subsequent pneumococcal disease remains high in ALWHIV. Efficient infant PCV schedules that enhance indirect protection together with targeted-vaccination for ALWHIV should be considered, along with other public health measures to further reduce VT carriage and disease. These measures should be supported by robust surveillance to assess effectiveness and identify early evidence of vaccine escape.
Acknowledgements
We thank the individuals who participated in this study and the local authorities for their support. We are grateful to the study field teams (supported by Farouck Bonomali and Roseline Nyirenda). We are grateful to the hospitality of the QECH ART Clinic, led by Ken Malisita. Our thanks also extend to the MLW laboratory management team (led by Brigitte Denis) and the MLW data management team (led by the late Clemens Masesa whose contribution we wish to particularly acknowledge). D.T., K.C.J., J.O., S.F., N.F., R.S.H., and T.D.S. are supported by the National Institute for Health and Care Research (NIHR) Global Health Research Unit on Mucosal Pathogens and R is a NIHR Senior Investigator.
Role of the funding source: A project grant jointly funded by the UK Medical Research Council (MRC) and the UK Department for International Development (DFID) under the MRC/DFID Concordat agreement, also as part of the EDCTP2 programme supported by the European Union (Grant MR/N023129/1); and a recruitment award from the Wellcome (Grant 106846/Z/15/Z). The MLW Programme is supported by a Strategic Award from the Wellcome, UK. The National Institute for Health and Care Research (NIHR) Global Health Research Unit on Mucosal Pathogens is supported using UK aid from the UK Government (Grant 16/136/46). R.S.H. is funded by Bill & Melinda Gates Foundation (OPP1117653), a Wellcome Programme Grant (WT091909/B/10/Z), and NIHR Global Health Research Unit on Mucosal Pathogens using UK aid from the UK Government (16/136/46). S.F. is also supported by a Sir Henry Dale Fellowship jointly funded by the Wellcome Trust and the Royal Society (Grant 208812/Z/17/Z). The views expressed in this publication are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care. The funders had no role in study design, collection, analysis, data interpretation, writing of the report or in the decision to submit the article for publication. The corresponding author and senior authors had full access to the study data, and together, had final responsibility for the decision to submit for publication.
Author contributions: Conceptualization: D.T., S.F., N.F., T.D.S., R.S.H. Data curation: D.T., T.D.S., T.M. Formal analysis; D.T. and S.F. Funding acquisition: N.F., R.S.H., T.D.S. Investigation: D.T., T.M., A.K., J.M., C.B., T.D.S. Methodology: D.T. and S.F. Project administration: T.M., A.K., J.M., C.B., C.M., N.F., R.S.H., T.D.S. Resources: N.F., R.S.H., T.D.S. Software: D.T. Supervision: S.F., N.F., K.C.J., T.D.S. Validation: D.T., T.M., K.C.J., A.K., J.M., C.B., C.M., J.O., S.F., N.F., R.S.H., T.D.S. Visualization: D.T. Writing – original draft: D.T. Writing – review and editing: D.T., T.M., K.C.J., A.K., J.M., C.B., C.M., J.O., S.F., N.F., R.S.H. and T.D.S. All authors read and approved the final manuscript.
Data availability: An R script that was used to analyse the datasets is available in the GitHub repository.
This research was funded in whole, or in part, by the Wellcome Trust [Grant number 208812/Z/17/Z]. For the purpose of open access, the author has applied a CC BY public copyright licence to any Author Accepted Manuscript version arising from this submission.
Conflicts of interest
There are no conflicts of interest.
Supplementary Material
Footnotes
Supplemental digital content is available for this article.
References
- 1. WHO. WHO Pneumococcus vaccines position paper. 85–104. Available at: https://www.who.int/immunization/policy/position_papers/pneumococcus/en/ [Accessed 25 February 2019] [Google Scholar]
- 2.Simell B, Auranen K, Käyhty H, Goldblatt D, Dagan R, O’Brien KL. Pneumococcal Carriage Group. The fundamental link between pneumococcal carriage and disease. Exp Rev Vaccines 2012; 11:841–855. [DOI] [PubMed] [Google Scholar]
- 3.Bar-Zeev N, Mtunthama N, Gordon SB, Mwafulirwa G, French N. Minimum incidence of adult invasive pneumococcal disease in Blantyre, Malawi an Urban African Setting: a hospital based prospective cohort study. PLoS One 2015; 10:e0128738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meiring S, Cohen C, Quan V, de Gouveia L, Feldman C, Karstaedt A, et al. GERMS-SA. HIV infection and the epidemiology of invasive pneumococcal disease (IPD) in South African adults and older children prior to the introduction of a pneumococcal conjugate vaccine (PCV). PLoS One 2016; 11:e0149104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corcoran M, Vickers I, Mereckiene J, Murchan S, Cotter S, Fitzgerald M, et al. The epidemiology of invasive pneumococcal disease in older adults in the post-PCV era. Has there been a herd effect?. Epidemiol Infect 2017; 145:2390–2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dwyer-Lindgren L, Cork MA, Sligar A, Steuben KM, Wilson KF, Provost NR, et al. Mapping HIV prevalence in sub-Saharan Africa between 2000 and 2017. Nature 2019; 570:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.GBD 2017 HIV collaborators. Global, regional, and national incidence, prevalence, and mortality of HIV, 1980-2017, and forecasts to 2030, for 195 countries and territories: a systematic analysis for the Global Burden of Diseases, Injuries, and Risk Factors Study 2017. Lancet HIV 2019; 6:e831–e859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choko AT, MacPherson P, Webb EL, Willey BA, Feasy H, Sambakunsi R, et al. Uptake, accuracy, safety, and linkage into care over two years of promoting annual self-testing for HIV in Blantyre, Malawi: a community-based prospective study. PLoS Med 2015; 12:e1001873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Aalst M, Lötsch F, Spijker R, van der Meer JTM, Langendam MW, Goorhuis A, et al. Incidence of invasive pneumococcal disease in immunocompromised patients: a systematic review and meta-analysis. Travel Med Infect Dis 2018; 24:89–100. [DOI] [PubMed] [Google Scholar]
- 10.Harries A, Makombe S, Libamba E, Schouten E. Why did the scale-up of HIV treatment work?: a case example from Malawi. J Acquir Immune Defic Syndr 57: 2011: (Suppl 2): S64–S67. [DOI] [PubMed] [Google Scholar]
- 11.Harries AD, Ford N, Jahn A, Schouten EJ, Libamba E, Chimbwandira F, Maher D, et al. Act local, think global: how the Malawi experience of scaling up antiretroviral treatment has informed global policy. BMC Public Health 2016; 16:938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jahn A, Harries AD, Schouten EJ, Libamba E, Ford N, Maher D, Chimbwandira F. Scaling-up antiretroviral therapy in Malawi. Bulletin of the World Health Organization 2016; 94:772–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.VACFA. Immunization Schedules - Africa | Vaccines for Africa. Available at: http://www.vacfa.uct.ac.za/immunization-schedules-africa (2018). [Google Scholar]
- 14.Bar-Zeev N, Swarthout TD, Everett DB, Alaerts M, Msefula J, Brown C, et al. Impact and effectiveness of 13-valent pneumococcal conjugate vaccine on population incidence of vaccine and nonvaccine serotype invasive pneumococcal disease in Blantyre, Malawi, 2006–18: prospective observational time-series and case-control studies. Lancet Global Health 2021; 9:e989–e998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Swarthout TD, Fronterre C, Lourenço J, Obolski U, Gori A, Bar-Zeev N, et al. High residual carriage of vaccine-serotype Streptococcus pneumoniae after introduction of pneumococcal conjugate vaccine in Malawi. Nat Commun 2020; 11:2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lourenço J, Obolski U, Swarthout TD, Gori A, Bar-Zeev N, Everett D, et al. Determinants of high residual post-PCV13 pneumococcal vaccine-type carriage in Blantyre, Malawi: a modelling study. BMC Med 2019; 17: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klugman KP, Rodgers GL. Population versus individual protection by pneumococcal conjugate vaccination. Lancet 2019; 393:2102–2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thindwa D, Pinsent A, Ojal J, Gallagher KE, French N, Flasche S. Vaccine strategies to reduce the burden of pneumococcal disease in HIV-infected adults in Africa. Expert Rev Vaccines 2020; 19:1085–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. WHO | Pneumococcal conjugate 3rd dose (PCV3) immunization coverage. WHO. Available at: http://www.who.int/gho/immunization/pneumococcal/en/ [Accessed 16 December 2019] [Google Scholar]
- 20.Shiri T, Auranen K, Nunes MC, Adrian PV, van Niekerk N, de Gouveia L, et al. Dynamics of pneumococcal transmission in vaccine-naïve children and their HIV-infected or HIV-uninfected mothers during the first 2 years of life. Am J Epidemiol 2013; 178:1629–1637. [DOI] [PubMed] [Google Scholar]
- 21.Heinsbroek E, Tafatatha T, Chisambo C, Phiri A, Mwiba O, Ngwira B, et al. Pneumococcal acquisition among infants exposed to HIV in rural Malawi: a longitudinal household study. Am J Epidemiol 2016; 183:70–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.le Polain de Waroux O, Tafatatha T, Chisambo C, Phiri A, Mwiba O, Ngwira B, et al. Characteristics of human encounters and social mixing patterns relevant to infectious diseases spread by close contact: a survey in Southwest Uganda. BMC Infect Dis 2018; 18:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.le Polain de Waroux O, Flasche S, Kucharski AJ, Langendorf C, Ndazima D, Mwanga-Amumpaire J, et al. Identifying human encounters that shape the transmission of Streptococcus pneumoniae and other acute respiratory infections. Epidemics 2018; 25:72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neal EFG, Nguyen CD, Ratu FT, Dunne EM, Kama M, Ortika BD, et al. Factors associated with pneumococcal carriage and density in children and adults in Fiji, using four cross-sectional surveys. PLoS One 2020; 15:e0231041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neal EFG, Flasche S, Nguyen CD, Ratu FT, Dunne EM, Koyamaibole L, et al. Associations between ethnicity, social contact, and pneumococcal carriage three years post-PCV10 in Fiji. Vaccine 2020; 38:202–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heinsbroek E, Tafatatha T, Phiri A, Ngwira B, Crampin AC, Read JM, French N. Persisting high prevalence of pneumococcal carriage among HIV-infected adults receiving antiretroviral therapy in Malawi. AIDS 2015; 29:1837–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glennie SJ, Banda D, Gould K, Hinds J, Kamngona A, Everett DD, et al. Defective pneumococcal-specific Th1 responses in HIV-infected adults precedes a loss of control of pneumococcal colonization. Clin Infect Dis 2013; 56:291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. National Statistical Office Malawi. Malawi Population and Housing Census 2018. 1–311. Available at: http://www.nsomalawi.mw/index.php%3Foption%3Dcom_content%26view%3Darticle%26id%3D226:2018-malawi-population-and-housing-census%26catid%E2%80%89%3D%E2%80%898:reports%26Itemid%E2%80%89%3D%E2%80%896 [Accessed 20 June 2020] [Google Scholar]
- 29. Ministry of Health. Malawi Guidelines for Clinical Management of HIV in Children and Adults. 1–128. Available at: https://differentiatedservicedelivery.org/Portals/0/adam/Content/yb4xSSLvE0SW98_z7wTm_w/File/Malawi%20Clinical%20HIV%20Guidelines%202018%20(1).pdf [Accessed 20 April 2020] [Google Scholar]
- 30.Satzke C, Turner P, Virolainen-Julkunen A, Adrian PV, Antonio M, Hare KM, et al. WHO Pneumococcal Carriage Working Group. Standard method for detecting upper respiratory carriage of Streptococcus pneumoniae: updted recommendations from the World Health Organization Pneumococcal Carriage Working Group. Vaccine 2013; 32:165–179. [DOI] [PubMed] [Google Scholar]
- 31.Newton R, Hinds J, Wernisch L. Empirical Bayesian models for analysing molecular serotyping microarrays. BMC Bioinformatics 2011; 12:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Swarthout TD, Gori A, Bar-Zeev N, Kamng’ona AW, Mwalukomo TS, Bonomali F, et al. Evaluation of pneumococcal serotyping of nasopharyngeal-carriage isolates by latex agglutination, whole-genome sequencing (PneumoCaT), and DNA microarray in a high-pneumococcal-carriage-prevalence population in Malawi. J Clin Microbiol 2020; 59:e02103-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kamng’ona AW, Hinds J, Bar-Zeev N, Gould KA, Chaguza C, Msefula C, et al. High multiple carriage and emergence of Streptococcus pneumoniae vaccine serotype variants in Malawian children. BMC Infect Dis 2015; 15:234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stekhoven DJ, Bühlmann P. MissForest—nonparametric missing value imputation for mixed-type data. Bioinformatics 2012; 28:112–118. [DOI] [PubMed] [Google Scholar]
- 35.Eilers PHC, Marx BD. Flexible smoothing with B-splines and penalties. Stat Sci 1996; 11:89–102. [Google Scholar]
- 36.Linley E, Bell A, Gritzfeld JF, Borrow R. Should pneumococcal serotype 3 be included in serotype-specific immunoassays?. Vaccines 2019; 7:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Usuf E, Bottomley C, Bojang E, Cox I, Bojang A, Gladstone R, et al. Persistence of nasopharyngeal pneumococcal vaccine serotypes and increase of nonvaccine serotypes among vaccinated infants and their mothers 5 years after introduction of pneumococcal conjugate vaccine 13 in the Gambia. Clin Infect Dis 2019; 68:1512–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hens N, Shkedy Z, Aerts M, Faes C, Van Damme P, Beutels P, et al. Modeling infectious disease parameters based on serological and social contact data: a modern statistical perspective. New York: Springer Science & Business Media; 2012. [Google Scholar]
- 39.Kohavi R, Longbotham R, Sommerfield D, Henne RM. Controlled experiments on the web: survey and practical guide. Data Min Knowl Disc 2009; 18:140–181. [Google Scholar]
- 40. R Core Team (2018). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available at: https://www.r-project.org/ [Accessed 28 May 2019] [Google Scholar]
- 41. Thindwa, D. R code and data for Age- and time-dependent risk factors for pneumococcal carriage in HIV-positive adults on ART in the infant PCV era in Blantyre, Malawi. Available at: https://github.com/deusthindwa/Pneumo.carriage.adults.hiv.malawi [Accessed 15 December 2021] [Google Scholar]
- 42.Mvula H, Heinsbroek E, Chihana M, Crampin AC, Kabuluzi S, Chirwa G, et al. Predictors of uptake and timeliness of newly introduced pneumococcal and rotavirus vaccines, and of measles vaccine in rural Malawi: a population cohort study. PLoS One 2016; 11:e0154997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Malawi Ministry of Health. Malawi population-based HIV impact assessment 2020–21. 4. Available at: https://phia.icap.columbia.edu/malawi-summary-sheet-2/ [Accessed 21 July 2019] [Google Scholar]
- 44.Dovel K, Phiri K, Mphande M, Mindry D, Sanudi E, Bellos M, Hoffman RM. Optimizing test and treat in Malawi: healthcare worker perspectives on barriers and facilitators to ART initiation among HIV-infected clients who feel healthy. Global Health Action 2020; 13:1728830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. The Global Nutrition Report. The burden of malnutrition in Malawi. Available at: https://globalnutritionreport.org/resources/nutrition-profiles/africa/eastern-africa/malawi/ [Accessed 21 July 2019] [Google Scholar]
- 46.Everett DB, Mukaka M, Denis B, Gordon SB, Carrol ED, van Oosterhout JJ, et al. Ten years of surveillance for invasive Streptococcus pneumoniae during the era of antiretroviral scale-up and cotrimoxazole prophylaxis in Malawi. PLoS One 2011; 6:e17765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nzenze SA, Shiri T, Nunes MC, Klugman KP, Kahn K, Twine R, et al. Temporal changes in pneumococcal colonization in a rural African community with high HIV prevalence following routine infant pneumococcal immunization. Pediatr Infect Dis J 2013; 32:1270–1278. [DOI] [PubMed] [Google Scholar]
- 48.Nzenze SA, Madhi SA, Shiri T, Klugman KP, de Gouveia L, Moore DP, et al. Imputing the direct and indirect effectiveness of childhood pneumococcal conjugate vaccine against invasive pneumococcal disease by surveying temporal changes in nasopharyngeal pneumococcal colonization. Am J Epidemiol 2017; 186:435–444. [DOI] [PubMed] [Google Scholar]
- 49.Roca A, Hill PC, Townend J, Egere U, Antonio M, Bojang A, et al. Effects of community-wide vaccination with PCV-7 on pneumococcal nasopharyngeal carriage in the Gambia: a cluster-randomized trial. PLoS Med 2011; 8:e1001107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thindwa D, Jambo KC, Ojal J, MacPherson P, Dennis Phiri M, Pinsent A, et al. Social mixing patterns relevant to infectious diseases spread by close contact in urban Blantyre. Malawi Epidemics 2022; 40:100590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Glennie SJ, Sepako E, Mzinza D, Harawa V, Miles DJ, Jambo KC, et al. Impaired CD4 T cell memory response to Streptococcus pneumoniae precedes CD4 T cell depletion in HIV-infected Malawian adults. PLoS One 2011; 6:e25610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang L, Li Z, Wan Z, Kilby A, Kilby JM, Jiang W, et al. Humoral immune responses to Streptococcus pneumoniae in the setting of HIV-1 infection. Vaccine 2015; 33:4430–4436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dherani M, Heinsbroek E, Tafatatha T, Chartier R, Bruce N. Household air pollution and pneumococcal carriage in 6 months old children in Malawi – MSCAPE Study. ISEE Conference Abstracts. 2018. [Google Scholar]
- 54.Tsega A, Hausi H, Chriwa G, Steinglass R, Smith D, Valle M. Vaccination coverage and timely vaccination with valid doses in Malawi. Vaccine Rep 2016; 6:8–12. [Google Scholar]
- 55.Heinsbroek E, Tafatatha T, Phiri A, Swarthout TD, Alaerts M, Crampin AC, et al. Pneumococcal carriage in households in Karonga District, Malawi, before and after introduction of 13-valent pneumococcal conjugate vaccination. Vaccine 2018; 36:7369–7376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cohen C, von Mollendorf C, de Gouveia L, Lengana S, Meiring S, Quan V, et al. South African IPD Case-Control Study Group. Effectiveness of the 13-valent pneumococcal conjugate vaccine against invasive pneumococcal disease in South African children: a case-control study. Lancet Global Health 2017; 5:e359–e369. [DOI] [PubMed] [Google Scholar]
- 57.Madhi SA, Nunes MC. The potential impact of pneumococcal conjugate vaccine in Africa: Considerations and early lessons learned from the South African experience. Hum Vaccines Immunotherapeut 2016; 12:314–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Flasche S, Lipsitch M, Ojal J, Pinsent A. Estimating the contribution of different age strata to vaccine serotype pneumococcal transmission in the pre vaccine era: a modelling study. BMC Med 2020; 18:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thindwa D, Wolter N, Pinsent A, Carrim M, Ojal J, Tempia S, et al. Estimating the contribution of HIV-infected adults to household pneumococcal transmission in South Africa, 2016-2018: a hidden Markov modelling study. PLoS Computat Biol 2021; 17:e1009680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. PHAST. Methods of sampling from a population. Health Knowledge. Available at: https://www.healthknowledge.org.uk/public-health-textbook/research-methods/1a-epidemiology/methods-of-sampling-population [Accessed 15 January 2022] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.