We read with great interest the study by Hegyi et al1 which reported that a commonly occurring haplotype spanning the PRSS1-PRSS2 locus (encoding human cationic and anionic trypsinogens) is associated with chronic pancreatitis. While PRSS1 is the major focus in many studies,2 3 PRSS2 is also a major trypsinogen isoform synthesised in human pancreas. In normal human, the PRSS1/PRSS2 ratio is approximately 2:1.4 Chronic alcoholism increases the risk of pancreatitis. Strikingly, in these patients, total trypsinogen secretion was increased with selective upregulation of PRSS2, reversing the PRSS1/PRSS2 ratio.5 In vitro studies have shown human PRSS2 more sensitive to autocatalytic degradation and with lower autoactivation than PRSS1.6 In test tube assays under conditions of intracellular pathological trypsinogen activation, mixtures of PRSS1 and PRSS2 with increasing ratios of PRSS2 had markedly decreased rates of trypsinogen activation and yields of active trypsin.6 These observations led to a hypothesis that upregulation of PRSS2 may play a protective role against pancreatitis.6
In order to elucidate the role of human PRSS2 in the pathogenesis of pancreatitis in vivo, transgenic mice expressing human PRSS2 were developed using a bacterial artificial chromosome containing the full length human PRSS2 gene (see online supplementary methods). Two lines of PRSS2 mice were developed. PRSS2 expression level was higher in line #2 than that in line #1. Focal spontaneous pancreatitis (area <10%) was observed in line #2 but not in line #1 mice (see online supplementary results and online supplementary figure 1). To further elucidate the increased sensitivity to pancreatitis by PRSS2 expression, control and PRSS2 line #1 mice were challenged with a single dose of caerulein (100 μg/kg) and acute pancreatitis was evaluated at 24 hours. We observed minimal pancreatitis in control C57BL6 mice; however, all PRSS2 mice developed severe pancreatic oedema (figure 1A) with elevated pancreatic oedema and serum amylase levels (figure 1B,C). Histological examination revealed more severe pancreatitis in PRSS2 mice compared to control mice (figure 1D). Using six doses of caerulein (100 μg/kg/hour), more severe pancreatitis, with massive acinar cell death and inflammatory cell infiltration, developed (online supplementary figure 2A,B). At day 7 and day 21 after a single caerulein induction, all control mice showed full recovery from acute pancreatitis. Strikingly, all PRSS2 mice progressed to chronic pancreatitis with pancreatic atrophy, acinar destruction, increased markers of fibrosis and inflammatory cell infiltration (figure 2, online supplementary figure 3). With high dose of caerulein, we observed similar changes in PRSS2 line #2 mice. Inhibition of trypsin ameliorated pancreatitis in these mice (online supplementary figure 4), supporting the idea that increased trypsin activity is key to the severity of pancreatitis.
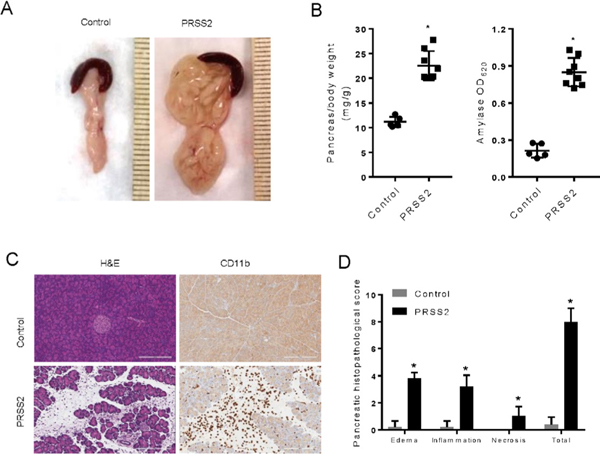
Figure 1.
Caerulein-induced severe acute pancreatitis in mice with transgenic expression of human PRSS2. (A) Wild type (WT) C57BL6 control and transgenic mice was injected with a single dose of caerulein (100 μg/kg) intraperitoneally. Macroscopic appearance of pancreas 24 hours after caerulein induction in control and PRSS2 line #1 mice was shown. (B) Pancreatic oedema and serum amylase were measured and compared in control and PRSS2 line #1 mice (n=5–8). (C) Pancreas histology (H&E) and CD11b-positive inflammatory cell staining. (D) Pancreatitis histopathological scores were evaluated.
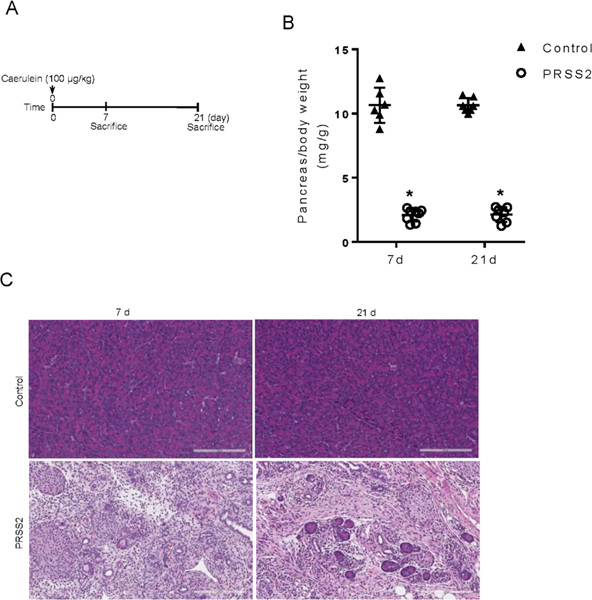
Figure 2.
Transgenic PRSS2 mice but not WT C57BL control mice developed chronic pancreatitis after one episode of acute pancreatitis. (A) Schema of pancreatitis induction and tissue harvest time points. (B) Measurements of pancreas size at day 7 and day 21 after acute pancreatitis induction (n=6–8). (C) Pancreas histology (H&E) at day 7 and day 21 after caerulein.
Collectively, our data demonstrated that increased human PRSS2 is in fact detrimental in pancreatitis. These findings are in agreement with a clinical study showing that a G191R variant of PRSS2 mitigates intrapancreatic trypsin activity and thereby protects against chronic pancreatitis in human.7 This study is of clinical importance in that the expression of PRSS2 surpasses PRSS1 and becomes the major isoform in the pancreas of human population with high risk of pancreatitis.5 It is believed that the evolutionary rationale for the existence of multiple trypsinogen isoforms is that their differences in inhibitor sensitivity may be advantageous for digestion of foods containing natural trypsin inhibitors.6 Therefore, inhibitors developed for pancreatitis therapy shall be tested for their sensitivity to all trypsin isoforms. Our novel animal model provides such an opportunity for testing the isoform-specific efficacy in vivo.
Supplementary Material
Funding
This work was supported by Grants Department of Defense DoD W81XWH-15-1-0257, National Institute of Health NIH r01 DK117910 and National Cancer Institute NCI K12 CA090628-18 and NCI P50 CA102701.
Footnotes
Competing interests None declared.
Patient consent for publication Not required.
Provenance and peer review Not commissioned; internally peer reviewed.
RefeRences
- 1.Hegyi E, Tóth AZ, Áron Vincze, et al. Alcohol-Dependent effect of Prss1-Prss2 haplotype in chronic pancreatitis. Gut 2020;69:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gui F, Zhang Y, Wan J, et al. Trypsin activity governs increased susceptibility to pancreatitis in mice expressing human Prss1r122H. J Clin Invest 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jancsó Z, Sahin-Tóth M. Mutation that promotes activation of trypsinogen increases severity of secretagogue-induced pancreatitis in mice. Gastroenterology 2019. doi: 10.1053/j.gastro.2019.11.020. [epub ahead of print: 18 Nov 2019]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guy O, Lombardo D, Bartelt DC, et al. Two human trypsinogens. Purification, molecular properties, and N-terminal sequences. Biochemistry 1978;17:1669–75. [DOI] [PubMed] [Google Scholar]
- 5.Rinderknecht H, Stace NH, renner IG. Effects of chronic alcohol abuse on exocrine pancreatic secretion in man. Dig Dis Sci 1985;30:65–71. [DOI] [PubMed] [Google Scholar]
- 6.Kukor Z, Toth M, Sahin-Toth M. Human anionic trypsinogen. properties of autocatalytic activation and degradation and implications in pancreatic diseases. Eur J Biochem 2003;270:2047–58. [DOI] [PubMed] [Google Scholar]
- 7.Witt H, Sahin-Tóth M, Landt O, et al. A degradation-sensitive anionic trypsinogen (Prss2) variant protects against chronic pancreatitis. Nat Genet 2006;38:668–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.