Abstract
Purpose
In the United States, AMD is a leading cause of low vision that leads to central vision loss and has a high co-occurrence with hearing loss. The impact of central vision loss on the daily functioning of older individuals cannot be fully addressed without considering their hearing status. We investigated the impact of combined central vision loss and hearing loss on spatial localization, an ability critical for social interactions and navigation.
Methods
Sixteen older adults with central vision loss primarily due to AMD, with or without co-occurring hearing loss, completed a spatial perimetry task in which they verbally reported the directions of visual or auditory targets. Auditory testing was done with eyes open in a dimly lit room or with a blindfold. Twenty-three normally sighted, age-matched, and hearing-matched control subjects also completed the task.
Results
Subjects with central vision loss missed visual targets more often. They showed increased deviations in visual biases from control subjects as the scotoma size increased. However, these deficits did not generalize to sound localization. As hearing loss became more severe, the sound localization variability increased, and this relationship was not altered by coexisting central vision loss. For both control and central vision loss subjects, sound localization was less reliable when subjects wore blindfolds, possibly due to the absence of visual contextual cues.
Conclusions
Although central vision loss impairs visual localization, it does not impair sound localization and does not prevent vision from providing useful contextual cues for sound localization.
Keywords: spatial localization, vision impairment, hearing impairment, cross-modal perception
Low vision affects 5.7 million people in the United States, with more than 70% over age 65.1 Low vision is frequently accompanied by hearing loss (dual sensory impairment [DSI]) in older adults. It is estimated that for people over the age of 65, 50% of the individuals with low vision also have hearing loss.2 DSI introduces unique functional challenges that need special rehabilitation approaches to address the joint impact of vision and hearing impairment, but these have received very little attention.3–6 In particular, the most common form of vision loss that co-occurs with hearing loss is AMD, which is also a leading cause of low vision in the United States.7–9 Advanced AMD is accompanied by central vision loss, including decreased acuity, decreased contrast sensitivity, and field loss. Past literature has consistently shown the impact of central vision loss on reading and mobility.10 However, the impact of central vision loss on the everyday functioning of these individuals cannot be fully addressed without considering the individual's hearing status.
The current article deals with spatial localization, an important ability for both safe navigation and social interactions. Spatial localization refers to judgement of the direction and distance of people and objects around us. Although other senses such as haptic, olfaction, and proprioception can also provide location information and are increasingly important for people with sensory impairment, vision and hearing remain the most important senses for the spatial localization of targets beyond arm length.11 Here, we recruited subjects with combined central vision loss and hearing loss, central vision loss only, hearing loss only, and controls with normal vision (NV) and hearing to perform a spatial perimetry task in which they verbally report the directions of visual or auditory targets. In an earlier report, we validated this task in testing older individuals and showed that it is a sensitive test that reveals age-related changes in visual and auditory localization.12 Our goal is to build a theoretical understanding of how central vision loss and hearing loss interact during the localization of visual and auditory targets.
A first question to be answered by this study is the impact of central vision loss on egocentric visual localization. Central vision is critical for orientation and mobility tasks, and central vision loss affects the individuals’ performance on mobility tasks such as obstacle course navigation and street crossing decision-making.13–15 Here we focus on the egocentric localization of simple visual targets, which is critical for judging the directions of obstacles in relation to oneself in real-life tasks. Egocentric representation of external space is a basis for more complex tasks such as forming cognitive maps.16 AMD often results in central scotomas, and the patients often adapt by developing a preferred retinal locus (PRL) as their new fovea.17,18 Due to these characteristics of central vision loss, we asked how the scotoma size and PRL sites influence visual localization performance.
Our second question involves the cross-modal impact of central vision loss on sound localization. There has been extensive research into the impact of blindness (ie, lack of form vision), either congenital or acquired in early life, on various sound localization tasks (eg, reference19,20); however, research into the impact of vision loss with residual form-vision on sound localization is sparse,21–25 and to our knowledge there has been no study looking into the interaction between vision and hearing impairment on sound localization. It is tempting to predict the impact of partial vision loss on sound localization based on two theories proposed in the case of blindness. The compensation theory proposes that blind individuals develop superior auditory abilities to compensate for their vision loss,26 whereas the deficit theory proposes that blind individuals have deficits in sound localization because vision plays a fundamental role in calibrating auditory spatial perception.27 However, it is not straightforward to apply these theories to the case of impaired central vision with retained peripheral vision.
Here we explore the impact of central vision loss on sound localization from two perspectives. First, hearing impairment alone can affect sound localization performance.28–30 Does central vision loss pose an additional impact on sound localization that interacts with the impact of hearing impairment? If this is true, we would expect to see a quantitatively different relationship between sound localization performance and hearing threshold among subjects with NV and those with coexisting central vision loss. Second, sound localization has been found to be more precise and accurate when normally sighted subjects are not blindfolded, suggesting a benefit of visual context on sound localization.31–33 Is this important benefit of vision retained for people with central vision loss?
Methods
Subjects
Thirty-nine subjects (25 women, aged 59–94 years) were recruited from the Retiree Volunteer Center at the University of Minnesota, the Vision Loss Resources in Minneapolis, and the Minnesota Laboratory for Low Vision Research. Twenty-three subjects had no known vision disorders (NV group) with normal hearing or age-related sensorineural hearing loss. Sixteen subjects had central vision loss due to macular disorders (vision impairment [VI] group) with normal hearing or age-related sensorineural hearing loss. The presence and types of hearing loss were obtained from the self-reports of each subject. Sensorineural hearing loss was confirmed by a typical audiometric result that showed increasing loss from low to high frequencies. According to the International Organization for Standardization, it is considered age appropriate if an individual shows normal thresholds (≤20 dB hearing level [HL]) at low frequencies (125–2 kHz) but mild hearing loss at high frequencies (4 k–8 kHz).34 Normal cognitive status was verified by the Mini-Mental State Examination (score of >24).
This study was approved by the University of Minnesota Institutional Review Board and followed the Declaration of Helsinki. Consent forms were acquired from all subjects before their participation in this study.
Vision and Hearing Screening
Subjects completed vision screening tests with their most up-to-date prescriptions, if any. Visual acuity was assessed by the Lighthouse Distance Visual Acuity chart.35 All subjects in the NV group had normal visual acuity with an average of –0.01 logMAR. For the VI group, acuity ranged from −0.02 to 1.20 logMAR, averaging 0.50 logMAR, which was significantly worse acuity than the NV group, F(1,34) = 32.5, P < 0.001. Note that some subjects in the VI group had relatively good acuity because they were at an early stage of AMD or had a ring scotoma with spared fovea.
Contrast sensitivity was assessed by the Pelli-Robson Contrast Sensitivity Chart Low-Vision Version.36 Subjects in the NV group had contrast sensitivity equal to or better than 1.65 logCS, which corresponds with the last line on the chart. For the VI group, the contrast sensitivity ranged from 0.45 to 1.65 logCS, averaging 1.24 logCS. The VI group had significantly worse contrast sensitivity than the NV group, F(1,34) = 21.3, P < 0.001.
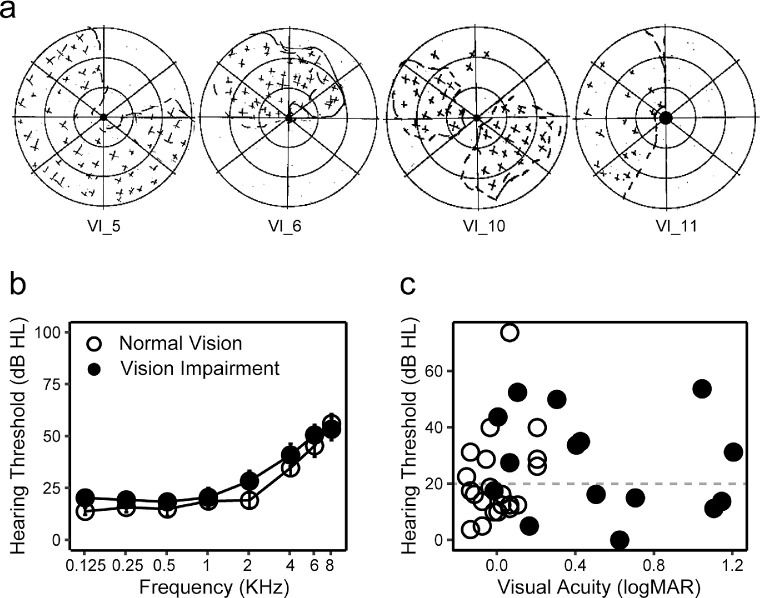
For the VI group, the status of central visual field and the location of the binocular PRL was assessed with the California Central Visual Field Test (Precision Vision, Woodstock, IL).17 Among the 16 subjects in the VI group, 5 had intact foveal fixation, 2 had ring scotoma and used the central island for fixation, 3 adopted PRLs inferior to their scotoma, 4 adopted a PRL to the left, and 2 adopted a PRL to the right of their scotoma (all in visual field coordinates). Scotoma width was obtained as the visual angle extending from the left-most to the right-most edges of the scotoma. For subjects with ring scotomas, the spared fovea was subtracted from the total width. Examples of California Central Visual Field Test results are shown in Figure 1a.
Figure 1.
Vision and hearing status. (a) Examples of the California Central Visual Field Test results from four subjects with central scotomas. The shaded areas represent scotomas. (b) Audiogram of VI (dots) and NV (circles) groups. Pure tone thresholds (dB HL) were plotted as a function of sound frequencies (kHz). (c) Scatter plot showing distributions of acuity (logMAR, x axis) and hearing thresholds (dB HL, y axis) for each individual subject. Dots and circles represent VI and NV groups, respectively.
Hearing thresholds were tested at 125, 250, 500, 1k, 2k, 4k, 6k, and 8 kHz by pure-tone audiometry using an air conduction audiometer. Five subjects in the NV group and four subjects in the VI group were hearing aid users. Figure 1b shows the average hearing thresholds for each group at each tested frequency. The pure-tone average (PTA) was calculated across 0.5 to 4.0 kHz frequencies according to World Health Organization standards. Using a PTA of ≤20 dB HL as the criterion, 10 subjects in the NV group and 8 subjects in the VI group had impaired hearing thresholds. Note that subjects meet the criterion of a PTA of ≤20 dB HL across 0.5 to 4.0 kHz may still have mild loss at higher frequencies as it is typical for older subjects.34 The PTA ranged from 3.8 to 73.8 dB HL in the NV group and 0 to 53.8 dB HL in the VI group. There was no significant difference in the PTA between the two groups, F(1,34) = 0.30; P = 0.59. Asymmetry of hearing was quantified as the difference between the PTA in the two ears. Only one subject had an asymmetry of >20 dB HL.
A summary of individual subject characteristics in the VI group is provided in Table. Figure 1b visualizes the distribution of hearing threshold against visual acuity in each group.
Table.
Summary of Vision and Hearing Characteristics for Subjects in the VI Group.
| ID | Age (Years) | Diagnosis | Acuity | Contrast Sensitivity | Scotoma Width (°) | PRL Site | Hearing Threshold (Left)† | Hearing Threshold (Right) | Hearing Aids |
|---|---|---|---|---|---|---|---|---|---|
| VI_1 | 87 | AMD* | 0.50 | 1.65 | 4.5 | R | 16.25 | 27.50 | No |
| VI_2 | 68 | AMD | 0.22 | 1.65 | NA | C | 16.25 | 22.50 | No |
| VI_3 | 83 | AMD | −0.02 | 1.65 | NA | C | 17.50 | 21.25 | No |
| VI_4 | 66 | Macular hole | 0.62 | 1.35 | 3.1 | Inferior | 0 | 6.25 | No |
| VI_5 | 56 | Stargardt | 1.14 | 0.45 | 15.0 | L | 13.75 | 22.5 | No |
| VI_6 | 63 | Stargardt | 0.16 | 1.35 | 12.5 | Inferior | 5.00 | 8.75 | No |
| VI_7 | 71 | AMD | 1.10 | 1.35 | 7.5 | Inferior | 20.00 | 11.25 | No |
| VI_8 | 56 | Pseudoxanthoma Elasticum | 0.70 | 1.35 | 10.0 | Central Island | 16.25 | 15.00 | No |
| VI_9 | 83 | AMD | 0.42 | 0.45 | 7.5 | L | 38.75 | 35.00 | No |
| VI_10 | 91 | AMD | 0.30 | 1.35 | 14.3 | Central Island | 68.75 | 50.00 | Yes |
| VI_11 | 89 | AMD | 1.04 | 0.45 | 7.5 | L | 53.75 | 55.00 | No |
| VI_12 | 94 | AMD | 0.10 | 1.50 | 4.3 | C | 52.50 | 60.00 | Yes |
| VI_13 | 84 | AMD | 0 | 1.65 | NA | C | 52.50 | 43.75 | Yes |
| VI_14 | 75 | AMD | 0.4 | 1.20 | 6.4 | R | 41.25 | 33.75 | No |
| VI_15 | 77 | AMD | 0.06 | 1.65 | NA | C | 35.00 | 27.50 | Yes |
| VI_16 | 75 | Stargardt | 1.20 | 1.05 | 12.5 | L | 31.25 | 36.25 | No |
AMD.
This is the PTA across 0.5–4.0 kHz.
Spatial Localization Task
The apparatus and procedure of the spatial localization task were described in more detail in Xiong et al.12 Subjects were seated in the center of a semi-anechoic chamber, surrounded by 19 speakers and a video projection system arranged in a semi-circle with a 1.5-m radius. The direction and intensity of the speakers were calibrated by positioning a microphone at the center of the semicircle and analyzing the received sound spectrum. The projector screens were acoustically transparent and occluded the speakers.
Auditory stimuli were pink noise bursts with a frequency band of 200 to 8000 Hz. The stimuli lasted 200 ms (with 50-ms onset/offset ramps), with an intensity of 60 dB SPL. Visual stimuli were bright disks 3° in diameter, 200 ms in duration, and with a contrast of 90%. Subjects wore their habitual glasses, contact lenses, and hearing aids in the spatial localization task, if any.
In a vision-only condition, subjects performed the task while only visual stimuli were presented. In an audition-only condition, subjects wore a blindfold while localizing auditory stimuli. In an audition no-blindfold condition, subjects localized the auditory stimulus without wearing a blindfold. In this condition, the projector screen had a dark background without any visual stimuli. However, the layout of the room and the screen–floor boundaries were visible under the dim light of the projectors.
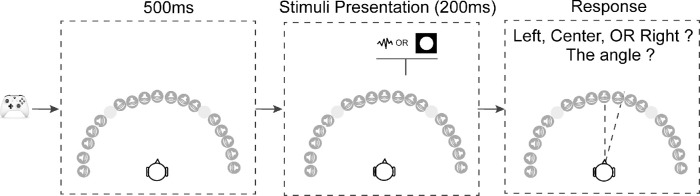
In each trial, the stimulus was presented randomly at 1 of 17 locations in steps of 10°, ranging from −90° to 90° azimuth (Fig. 2). The −40° and 40° locations were omitted because these locations were at the intersection between the screens where visual stimuli could not be presented properly. The subjects verbally reported the direction of the stimulus, first by reporting its orientation (left, right, or center), then by estimating its location angle (0° to 90°). Subjects were instructed to be as precise as possible. If the subject couldn't see or hear the stimuli, they were allowed to report the trial as a miss. Subjects were asked to keep their head and gaze straight ahead during testing.
Figure 2.
Schematic of the trial structure. Subjects used an Xbox controller to initiate each trial. After the key press there was a 500-ms delay, followed by a stimulus presented from one of the 17 locations. In the vision-only condition, a white disk was presented for 200 ms; in two audition conditions (with or without blindfold) pink noise was presented for 200 ms. After the target presentation, the subjects needed to verbally report the location of the stimuli, first responding left or right, then responding with an angle relative to directly in front of the subject. The −40° and 40° locations were omitted because these locations were at the intersection between the screens where visual stimuli cannot be presented properly.
Subjects first viewed or listened to demos of the visual and auditory stimuli played in order from the left most direction to the right most direction or vice versa. They then completed five practice trials for each condition. In the actual testing, subjects completed two blocks per condition in random order. In each block, each of the 17 directions was tested two times in random order. No feedback was given in either practice or testing.
Data Analysis
Analyses were performed using R software.37 Three key localization parameters were obtained for each subject in each condition.
-
•
The miss rate was calculated as the percentage of trials reported as a miss at each location.
-
•
Bias (signed error) was calculated as the mean signed deviation between the reported and actual location (Bias = Reported locations – Actual locations), representing both the magnitude and direction of any response errors. Overshooting happens when a target is reported as being more peripheral than its actual location, and undershooting happens when a target is reported as being more central than its actual location.
-
•
Variability was calculated as the standard deviation of the responses across the four trials at each location for each condition, with smaller variability representing higher precision.
In the analyses of miss rates and variability, we grouped the 17 locations into 5 bins corresponding with left far periphery (−90° to −60°), left near periphery (−50° to −20°), center (−10° to 10°), right near periphery (20° to 50°), and right far periphery (60° to 90°) (Fig. 3a). Binning was used to both increase the statistical power and to examine the impact of scotoma characteristics and PRL sites on localization in different spatial fields.
Figure 3.
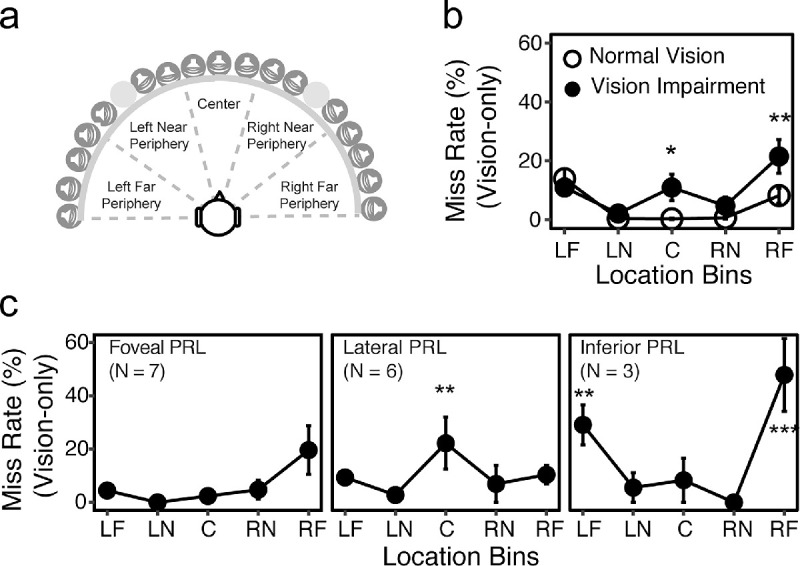
Miss rate. (a) An illustration of how the 17 locations were separated into five bins corresponding with −90° to −60° (left far periphery [LF]), −50° to −20° (left near periphery [LN]), −10° to 10° (center [C]), 20° to 50° (right near periphery [RN]), 60° to 90° (right far periphery [RF]). (b) Average miss rates across the five bins for each group. The NV and VI groups are plotted as open and closed symbols, respectively. The asterisks represent significant difference between the two groups. (c) Miss rates in the vision-only condition at each bin for each PRL subgroup. The asterisks represent significant difference from the central PRL group. In (b) and (c), the error bars represent standard errors. *** P < 0.001; **P < 0.05; *P < 0.01.
Linear mixed effect (LME) models38 were constructed to examine the differences between the NV and VI groups or among the test conditions. Condition (vision-only, audition-only, audition no-blindfold), group, and bins (left far periphery, left near periphery, center, right near periphery, right far periphery; as described elsewhere in this article) were included as fixed factors, and subject was included as a random factor. For all LME models, significant main effects of the fixed factors were examined by the ANOVA function. Post hoc analyses were conducted with Bonferroni correction (“emmeans” package39). In the cases of absence of a group effect or its interaction with other factors, data from both groups were merged in the post-hoc analysis.
Linear regression models were constructed to examined which vision factors (acuity, contrast sensitivity, PRL sites, and scotoma width) and/or hearing factors (threshold, binaural asymmetry, and use of hearing aids) played significant roles in determining each of the three main localization parameters. Because there was a significant age difference between the two groups (averaging 69 years in the NV group and 76 years in the VI group; P = 0.017), we included age as a covariate in all analyses.
Results
Miss Rate
The miss rate is a parameter of interest because it may directly reflect the field loss characteristics and PRL sites of the VI group. We found that the misses were reported exclusively in the vision-only condition, averaging 10.5% of the trials in the VI group and 5.4% in the NV group.
We grouped the 17 target locations into five bins (see Methods and Fig. 3a) and compared the miss rates between groups (Fig. 3b). LME analysis confirmed significant main effects of group, F(1,36) = 4.79; P = 0.035; bin, F(4,148) = 10.76, P < 0.001; and a significant interaction, F(4,148) = 3.28; P = 0.013. Post hoc analyses showed that the VI group had significantly higher miss rates not only in the central bin (averaging 0.3% for the NV group vs 10.8% for the VI group; P = 0.010), but also in the right far peripheral bin (averaging 8.3% for the NV group and 21.3% for the VI group; P = 0.001). There were no group differences in the other bins.
To further explore whether the patterns of misses were consistent with the subjects’ central vision status and PRL sites, the 16 subjects in the VI group were separated into subgroups based on their PRL sites: foveal PRL (ie, using fovea for fixation; n = 7), lateral PRL (n = 6), and inferior PRL (n = 3). Left and Right PRLs were merged into the Lateral subgroup because there was no significant difference in their patterns of misses.
As shown in Figure 3c, the three PRL subgroups showed different patterns of misses across the five bins. Subjects with foveal PRLs had a similar pattern of misses to the NV group, with the misses primarily occurring in far peripheral locations. The lateral PRL subgroup had significantly higher miss rates than the foveal PRL subgroup only at the center bin (21.3% vs 1.2%; P = 0.007), consistent with their central vision loss. In contrast, the inferior PRL subgroup showed higher miss rates than the foveal and lateral PRL subgroups in far peripheral locations (30.1% vs 3.3% and 8.5% at the left far peripheral bin [P = 0.004 and P = 0.040], and 48.8% vs 18.5% and 9.5% at the right far peripheral bins [all P < 0.001]).
Localization Bias
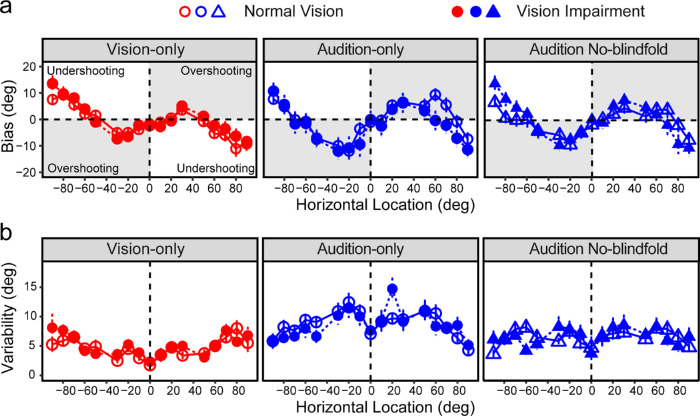
Figure 4a shows the group average biases as a function of the horizontal direction for each group and in each condition. Both groups showed overshooting biases in central locations and undershooting biases in peripheral locations in all three conditions.
Figure 4.
Localization bias and variability. (a) Localization biases across the 17 locations in each condition. (b) Localization variability across the 17 locations in each condition. The two groups are illustrated by open (NV) and closed (VI) symbols, respectively. The error bars represent standard errors.
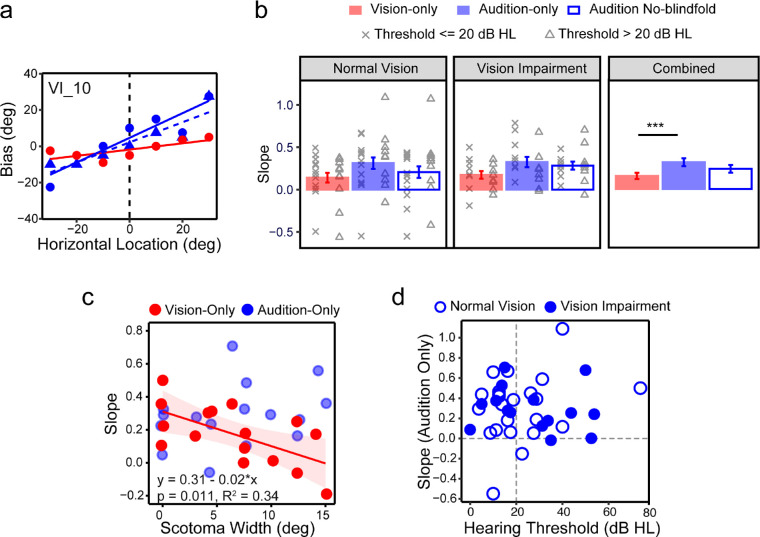
To explore whether central vision loss may alter the localization biases in central space, we quantified the overshooting by linear regression analyses on the biases as a function of spatial locations across −30° to 30°, where the overshooting was most prominent.12,40 As shown in the example in Figure 5a, the slope of the linear regression represents the magnitude of overshooting, and the intercept with the y-axis represents the bias in perceived straight-ahead.
Figure 5.
Localization bias. (a) An example from subject VI_10 showing how the overshooting in central space is quantified using linear regressions. The slopes of the regression lines represent the magnitude of overshooting. (b) Bar plots showing average slopes for each condition for NV group (left), VI group (center), and both groups combined (right). Individual slopes were also plotted overlaying each bar plot, with crosses representing subjects with hearing thresholds of ≤20 dB HL, and triangles representing subjects with hearing thresholds of >20 dB HL. (c) Scatter plot showing the correlation between the slope in the vision-only (red circles) and audition-only (blue circles) conditions and the width of scotomas (in degrees). Regression line and the equation for the vision-only condition are also noted. (d) Scatter plot showing the slopes in the audition-only condition as a function of hearing thresholds. Open and solid circles represent NV and VI groups, respectively. The error bars represent standard errors. ***P < 0.001.
The intercepts in all three conditions were close to zero, indicating accurate perception of egocentric straight-ahead. Therefore, the following analyses primarily focus on the slope. LME analysis on the slopes showed a significant effect of condition, F(2, 74) = 7.45; P = 0.001, but no significant main effect of group or their interaction. As shown in Figure 5b, the slopes in the vision-only condition averaged 0.14 (NV) and 0.18 (VI). In the audition-only condition the slopes averaged 0.31 (NV) and 0.33 (VI), significantly larger (steeper) than the vision-only condition (P < 0.001). The audition no-blindfold condition had intermediate slopes that averaged 0.21 (NV) and 0.29 (VI), which were not significantly different from either the vision-only or the audition-only condition. Figure 5b also shows individual slopes with crosses and triangles representing subjects with hearing thresholds of ≤20 dB HL and >20 dB HL, respectively. There was no significant difference between these subgroups in any of the three conditions.
Within the VI group there was a significant impact of central scotoma on the magnitude of overshooting in the vision-only condition. The slope decreased as the scotoma width increased, F(1,14) = 8.61; P = 0.011 (Fig. 5c), which explained 34% of the variations in the slopes. For example, subject VI_2 with an intact central field had a slope of 0.5, indicating that they may report a 10° visual target as 15°. In contrast, subject VI_5 with a scotoma of 15° width had a slope of −0.19, indicating that they may report a 10° visual target as 8°, showing undershooting instead. We asked whether the slopes in either audition condition was also correlated with the scotoma width. We did not find such correlation (see Fig. 5c for the auditory-only condition).
For the slopes in the audition-only condition, hearing threshold, binaural asymmetry, and use of hearing aids were not significant predictors. Figure 5d plots the slopes in the audition-only condition as a function of hearing thresholds for the NV and VI groups. The figure illustrates a lack of correlation between the slopes and hearing thresholds and a highly similar distribution of the slopes in the NV and VI groups, which indicates a lack of cross-modal impact of VI on sound localization bias.
Localization Variability
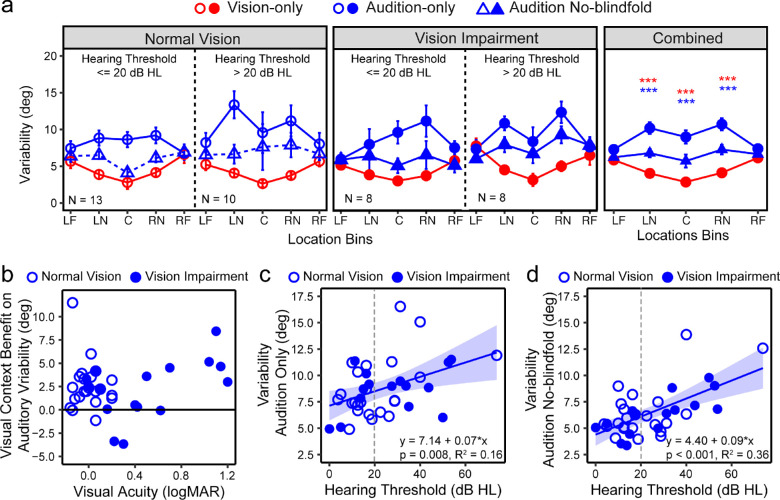
Greater localization variability represents lower precision. Figure 4b shows the group average variability at each of the 17 directions in each condition, for both groups. In Figure 6a, the variabilities were grouped into the five location bins. In addition, Figure 6a provides separate plots for subjects whose hearing thresholds were ≤20 dB HL and >20 dB HL. We report the results based on the NV and VI groups and then the impacts of hearing loss.
Figure 6.
Localization variability. (a) Variability (in degrees) across the five bins at each condition for the NV group (left), VI group (middle), and all groups combined (right). In each group, results were plotted separately for subjects whose hearing thresholds ≤20 dB HL and >20 dB HL. The error bars represent standard errors. The five bins correspond with −90° to −60° (left far periphery [LF]), −50° to −20° (left near periphery [LN]), −10° to 10° (center [C]), 20° to 50° (right near periphery [RN]), and 60° to 90° (right far periphery [RF]). (d) The visual context benefit on auditory variability, calculated as the difference between the variabilities in the audition-only and audition no-blindfold conditions, as a function of visual acuity. (c and d). The overall variability across all locations in the audition-only (b) and audition no-blindfold (c) conditions as functions of hearing thresholds. Open and closed circles represent the NV and VI groups, respectively. A vertical dashed lines mark out 20 dB HL. Regression lines and the equations are also noted. This is the overall benefit across all five bins. ***P < 0.001.
LME modeling showed significant main effects of condition, F(2,39) = 51.92, P < 0.001, and bin, F(4,66) = 3.39; P = 0.014, and an interaction between them, F(8,402) = 8.62, P < 0.001. There was no main effect of group (NV vs VI) or its interaction with other terms. The visual variability increased from center to peripheral space (P < 0.001), whereas the variability in the audition-only condition decreased from central to peripheral space (P < 0.001). The comparisons between the vision-only and audition-only conditions showed a significant vision advantage (smaller variability) in central and near peripheral bins (all P < 0.001), but not in far peripheral bins.
Comparing the two auditory conditions, there was a significant decrease in variability when localizing sound without blindfolds in the central and near peripheral bins (all P < 0.001), but not the far peripheral bins. We consider this difference a result of a visual context effect that provides spatial references for sound localization. We calculated the difference between the two auditory conditions to facilitate the comparison between the NV and VI groups. Figure 6b shows the visual contextual benefit as a function of visual acuity. There was no group effect or any impact of acuity or other vision parameters on the calculated difference (all P > 0.05).
For both the NV and VI groups and in both auditory conditions, we observed higher auditory variabilities for subjects whose hearing thresholds were >20 dB HL (ie, people with clinically defined hearing impairment). Averaged across all bins, for both auditory conditions, the variabilities increased with the hearing thresholds, and the presence of central vision loss did not quantitatively alter this impact (Figs. 6c and d).
Discussion
Our study shows that central vision loss affected visual localization by increasing misses and altering the patterns of overshooting bias in the central field. However, this impact did not affect sound localization. Although hearing loss increased the variability in sound localization, this effect was not affected by coexisting central vision loss. Moreover, across the levels of low vision tested in this study, vision continues to have a beneficial role for sound localization when comparing the audition-only and audition no-blindfold conditions.
Vision Impairment and Visual Localization
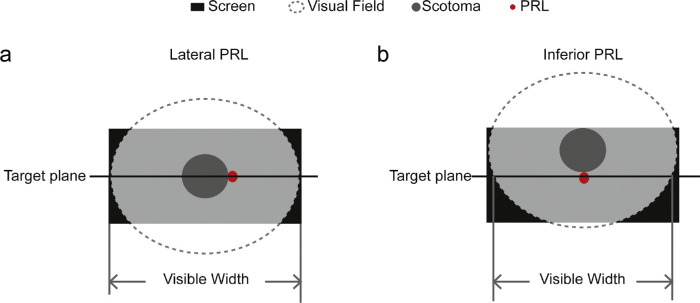
Our results showed evidence that central vision loss affected visual localization differently depending on the preferred retinal location a person used. Subjects with Lateral PRLs showed more misses in the central bins, indicating that they may have faced straight-ahead using their original fovea as the reference (Fig. 7a), which is functionally more plausible than using a lateral reference, due to the importance of horizontal head and eye directions in tasks such as straight-ahead walking. However, subjects with inferior PRLs showed significantly more misses in the far peripheral bins. They may have shifted their gaze upward as an adaptation because it does not interfere with the horizontal head and eye direction, and it can also be beneficial for monitoring the path ahead in navigation tasks.41,42 As illustrated in Figure 7b, such vertical shifts would lead to more misses at far peripheral locations as shown in our results. Inferior PRLs have historically been reported to be less common than lateral PRLs,17,43 which was also the case with our sample. Given our small sample, we acknowledge that a strong conclusion about the effects of PRL location on localization cannot be made before more subjects are tested.
Figure 7.
Hypothetical strategies for facing straight-ahead. The black square represents the test screen. A transparent ellipse with dashed outline that overlays on top of the screen represents the visual field. A gray patch at the center of the visual field represents a hypothetical scotoma. A red dot next to the scotoma represents a newly developed PRL, either lateral (a) or inferior (b) to the scotoma. (a) When there is a lateral PRL, subjects face straight-ahead with their original fovea; therefore, the visible horizontal range of the screen equals the maximum width of the visual field. When there is an inferior PRL, subjects moved their scotoma upwards to use the inferior PRL to look straight-ahead; therefore, the visible horizontal range of the screen is smaller than the maximum width of the visual field. The more upward the subjects look, the more constrained the visible horizontal range gets.
In addition to more misses, central vision loss also affects the biases in visual localization. Past literature has reported distortions in the visual representation of space in patients with peripheral field loss44,45 or hemianopia.46 Here we report for the first time that central vision loss is also associated with distortions in visual space perception. Although subjects with NV consistently show an expansion in the perceived central space, such expansion decreases as the scotoma size increases. Expansion in central space may be relevant to the higher functional significance of central vision than peripheral vision in many daily tasks. As scotoma size increases, the use of central vision decreases, which may have led to the decreased magnitude of expansion shown in our data.
Vision Impairment and Sound Localization
The lack of cross-modal impact of VI on sound localization is consistent with the work of Kolarik et al. (2013),21 who reported no impact of partial vision loss on auditory distance judgment. However, ours and Kolarik's results seem to conflict with both the compensation and deficit theories in the literature on blind sound localization.26,27 As we noted elsewhere in this article, it is not straightforward to directly apply these theories to cases with partial vision loss when people still have functional vision. Instead, we propose that only when vision becomes less reliable than hearing in a spatial task will we expect a cross-modal impact of vision loss on sound localization. This hypothesis is consistent with the sensory integration theories,47,48 in which people determine their dominance sense based on the relative reliability of two different senses.
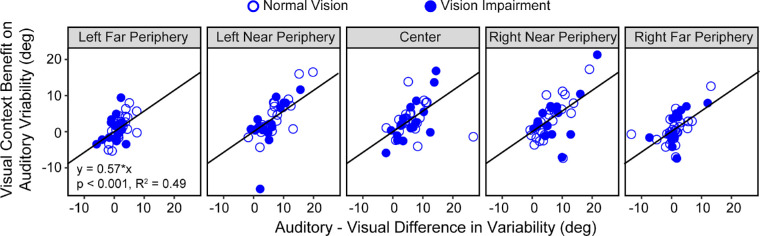
Our findings are consistent with this hypothesis. First, central vision loss did not affect the localization performance under the vision-only condition; therefore, it was not surprising to see an absence of cross-modal impact on the auditory-only condition. Further, the absence of a visual contextual benefit during auditory localization at far peripheral bins was consistent with the absence of an advantage of visual over auditory localization at far peripheral bins. In an exploratory analysis, we found that there were significant correlations between the magnitude of the vision advantage and the magnitude of visual contextual benefit (Fig. 8).
Figure 8.
Visual context benefit. Scatter plots showing the correlation between the visual context benefit, calculated as the differences in variability between the audition-only and audition no-blindfold conditions, and the audiovisual differences, calculated as the difference in variability between the audition-only and vision-only conditions. Correlation results for the five bins were plotted in separate panels. Data from the NV and VI groups are plotted as open and closed symbols, respectively. Regression lines and the corresponding equation are also noted in the plot.
This hypothesis also provides a possible explanation for the previous findings in the literature. Visual localization may be less reliable than auditory localization when using a different report method (eg, using eye, hand, or head pointing),23,49 or in a different task,21,22,23 which will then predict a cross-modal impact on the auditory localization in these designs. This realization points to the importance of evaluating the spatial performance in both visual and auditory modalities to have a comprehensive understanding of the impact of partial VI on sound localization.
Practical Considerations, Limitations, and Future Directions
Spatial localization tasks like ours have been used in the literature for different purposes. We adapted the test for individuals with vision and/or hearing loss who are often older in age. The verbal report method is intended to avoid motor errors and fatigue effects. The number of trials was decided based on pilot testing to limit the test duration. We found that this test is valid in showing the independent and joint impact of vision and hearing loss on localization.
We have a total sample size of 39 subjects. Looking at vision alone, 16 subjects had central field deficits and 23 subjects had NV. Looking at hearing alone, 18 subjects had hearing loss (pure tone thresholds ≥20 dB) and 21 subjects had normal hearing. Sample sizes similar to ours have been used frequently in previous behavioral studies to investigate the impacts of vision loss alone or hearing loss alone on spatial tasks.14,21,23,29 However, because of our unique interest in both vision and hearing loss, this sample size was not sufficient to support comparison across the four combinations of vision and hearing status: controls, vision loss only, hearing loss only, and DSI. Although we deal with this limitation by treating vision loss as categorical and hearing loss as continuous variables, it remains a significant limitation of our study in its generalizability to a broader combination of vision and hearing loss. This issue unique to behavioral research on DSI needs to be addressed for future studies.
Our small sample size was also not ideal for evaluating the impact of PRL site on localization, but their mild to intermediate acuity loss of ≤1.2 logMAR is representative of the specific pathologies of AMD. AMD is not a blinding eye disease and rarely leads to more severe acuity loss. Moreover, as treatments such as anti-VEGF become widely received, the progression of acuity loss is becoming slower. Among patients with AMD who seek low vision services, 80% have an acuity of >20/200 (1.0 logMAR, personal communication with Dr. Donald Fletcher, Envision Vision Rehabilitation Center, Wichita, KS). Therefore, our sample is representative of the range of acuities seen in patients with AMD.
People with central vision loss and hearing loss simultaneously face deficits in visual and auditory localization, including increased misses of visual targets, distortion in the visual space, and increased variability in sound localization. These localization deficits may be prominent and interact more explicitly in daily activities, such as street crossing and navigating busy environments. A positive message from our study is that visual context remains beneficial for sound localization, which may be especially important for these individuals. Our findings speak to the importance of taking both impairments into consideration when developing rehabilitation plans for individuals with DSI. In ongoing studies, we are comparing the spatial localization abilities measured by this task and the performances in real-world spatial localization tasks and extending our test to a broader range of vision and hearing conditions.
Acknowledgments
Supported by grants from the National Institutes of Health (K99/R00 EY030145 to Y-ZX and R01 EY002934 to GEL). Pilot testing for this study was supported by an internal grant from the Center for Applied and Translational Sensory Sciences at the University of Minnesota. DAA was supported by the NSF NRT Fellowship DGE-17348915.
Disclosure: Y.-Z. Xiong, None; D.A. Addleman, None; N.A. Nguyen, None; P. Nelson, None; G.E. Legge, None
References
- 1. Chan T, Friedman DS, Bradley C, Massof R.. Estimates of incidence and prevalence of visual impairment, low vision, and blindness in the United States. JAMA Ophthalmol . 2018; 136(1): 12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fuller SD, Mudie LI, Siordia C, Swenor BK, Friedman DS. Nationwide prevalence of self-reported serious sensory impairments and their associations with self-reported cognitive and functional difficulties. Ophthalmology. 2018; 125(4): 476–485. [DOI] [PubMed] [Google Scholar]
- 3. Brabyn JA, Schneck ME, Haegerstrom-Portnoy G, Lott LA.. Dual sensory loss: overview of problems, visual assessment, and rehabilitation. Trends Amplif . 2007; 11: 219–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Koutsoklenis A, Papadopoulos K.. Auditory cues used for wayfinding in urban environments by individuals with visual impairments. J Vis Impair Blind . 2011; 105(10): 703–714. [Google Scholar]
- 5. Wittich W, Southall K, Johnson A.. Usability of assistive listening devices by older adults with low vision. Disabil Rehabil Assist Technol . 2016; 11(7): 564–571. [DOI] [PubMed] [Google Scholar]
- 6. St-Amour L, Jarry J, Wittich W.. The audibility of low vision devices with speech output used by older adults with dual sensory impairment. Optom Vis Sci . 2019; 96(5): 345–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Klein R, Cruickshanks KJ, Klein BEK, Nondahl DM, Wiley T.. Is age-related maculopathy related to hearing loss? Epidemiology and Biostatistics. 1998; 116: 360–365. [DOI] [PubMed] [Google Scholar]
- 8. Chia EM, Mitchell P, Rochtchina E, Foran S, Golding M, Wang JJ.. Association between vision and hearing impairments and their combined effects on quality of life. Arch Ophthalmol . 2006; 124: 1465–1470. [DOI] [PubMed] [Google Scholar]
- 9. Ghasemi H, Pourakbari MS, Entezari M, Yarmohammadi ME.. Association of age related macular degeneration and age related hearing impairment. J Ophthalmic Vis Res . 2016; 11(1): 54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Legge GE, Chung STL.. Low vision and plasticity: implications for rehabilitation. Annu Rev Vis Sci . 2016; 2(10): 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Long RG, Giudice NA.. Establishing and maintaining orientation for mobility. In Blasch B.B., Wiener W.R., & Welsh R.W. (Eds.), Foundations of Orientation and Mobility . 3rd ed. New York: American Foundation for the Blind; 2010; 1: 45–62. [Google Scholar]
- 12. Xiong Y-Z, Addleman DA, Nguyen NA, Nelson PB, Legge GE.. Visual and auditory spatial localization in younger and older adults. Front Aging Neurosci. 2022; 14: 838194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Marron JA, Bailey IL.. Visual factors and orientation-mobility performance. Am J Optom Physiol Opt. 1982; 59(5): 413–426. [DOI] [PubMed] [Google Scholar]
- 14. Hassan SE, Snyder BD.. Street-crossing decision-making: a comparison between patients with age-related macular degeneration and normal vision. Invest Ophthalmol Vis Sci . 2012; 53(10): 6137–6144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kuyk T, Elliott JL.. Visual factors and mobility in persons with age-related macular degeneration. J Rehabil Res Dev . 1999; 36: 303–312. [PubMed] [Google Scholar]
- 16. Burgess N. Spatial memory: how egocentric and allocentric combine. Trends Cogn Sci . 2006; 10(12): 551–557. [DOI] [PubMed] [Google Scholar]
- 17. Fletcher DC, Schuchard RA, Watson G.. Relative locations of macular scotomas near the PRL: effect on low vision reading. J Rehabil Res Dev . 1999; 36: 356–364. [PubMed] [Google Scholar]
- 18. Fletcher DC, Schuchard RA, Renninger LW.. Patient awareness of binocular central scotoma in age-related macular degeneration. Optom Vis Sci . 2012; 89: 1395–1398. [DOI] [PubMed] [Google Scholar]
- 19. Gori M, Amadeo MB, Campus C.. Spatial metric in blindness: behavioural and cortical processing. Neurosci Biobehav Rev . 2020; 109: 54–62. [DOI] [PubMed] [Google Scholar]
- 20. Sabourin CJ, Merrikhi Y, Lomber SG.. Do blind people hear better? Trends Cogn Sci . 2022; 26(11): 999–1012. [DOI] [PubMed] [Google Scholar]
- 21. Kolarik AJ, Cirstea S, Pardhan S.. Evidence for enhanced discrimination of virtual auditory distance among blind listeners using level and direct-to-reverberant cues. Exp Brain Res. 2013; 224: 623–633. [DOI] [PubMed] [Google Scholar]
- 22. Kolarik AJ, Raman R, Moore BC, Cirstea S, Gopalakrishnan S, Pardhan S.. Partial visual loss affects self-reports of hearing abilities measured using a modified version of the Speech, Spatial, and Qualities of Hearing Questionnaire. Front Psychol. 2017; 8: 561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ahmad H, Setti W, Campus C, Capris E, Facchini V, Sandini G, Gori M.. The sound of scotoma: audio space representation reorganization in individuals with macular degeneration. Front Integr Neurosci. 2019; 13(44): 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kolarik AJ, Moore BCJ, Cirstea S, et al.. Partial visual loss disrupts the relationship between judged room size and sound source distance. Exp Brain Res . 2020; 240: 81–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kolarik AJ, Raman R, Moore BCJ, et al.. The accuracy of auditory spatial judgments in the visually impaired is dependent on sound source distance. Sci Rep. 2022; 10: 7169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Voss P, Collignon O, Lassonde M, Lepore F. Adaptation to sensory loss. Wiley Int Rev Cogn Sci. 2010; 1: 308–328. [DOI] [PubMed] [Google Scholar]
- 27. Jones B. Spatial perception in the blind. Br J Psychol. 1975;66: 461–472. [DOI] [PubMed] [Google Scholar]
- 28. Lorenzi C, Gatehouse S, Lever C.. Sound localization in noise in hearing-impaired listeners. J. Acoust. Soc. Am. 1999; 105: 3454–3463. [DOI] [PubMed] [Google Scholar]
- 29. Best V, Carlile S, Kopco N, van Schaik A.. Localization in speech mixtures by listeners with hearing loss. J. Acoust. Soc. Am. 2011; 129: EL210–EL215. [DOI] [PubMed] [Google Scholar]
- 30. Akeroyd MA. An overview of the major phenomena of the localization of sound sources by normal-hearing, hearing-impaired, and aided listeners. Trends Hear . 2014; 9(18): 2331216514560442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Voss P. Auditory spatial perception without vision. Front Psychol . 2016; 7: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tabry V, Zatorre RJ, Voss P.. The influence of vision on sound localization abilities in both the horizontal and vertical planes. Front Psychol . 2013; 4: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lovelace EA, Anderson DM.. The role of vision in sound localization. Percept Mot Skills. 1993; 77(3 Pt 1): 843–850. [DOI] [PubMed] [Google Scholar]
- 34. International Organization for Standardization [ISO]. Acoustics: statistical Distribution of Hearing Thresholds as a Function of Age. ISO 7029. Geneva: International Organization for Standardization; 2000. [Google Scholar]
- 35. Ferris FL III, Kassoff A, Bresnick GH, Bailey I. New visual acuity charts for clinical research. Am J Ophthalmol . 1982; 94: 91–96. [PubMed] [Google Scholar]
- 36. Pelli DG, Robson JG, Wilkins AJ.. The design of a new letter chart for measuring contrast sensitivity. Clin Vis Sci . 1988; 2: 187–199. [Google Scholar]
- 37. R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing. 2005. [Google Scholar]
- 38. Pinheiro J, Bates D. Mixed-Effects Models in S and S-PLUS. New York: Springer;2000. [Google Scholar]
- 39. Piepho HP. An algorithm for a letter-based representation of all pairwise comparisons. J Comput Graph Stat. 2004; 13(2): 456–466. [Google Scholar]
- 40. Dobreva M, O'Neill WE, Paige GD. Influence of aging on human sound localization. J Neurophysiol . 2011; 105(5): 2471–2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Legge G. Driving with central field loss. JAMA Ophthalmol . 2013; 131(3): 393–395. [DOI] [PubMed] [Google Scholar]
- 42. Bronstad PM, Albu A, Bowers AR, Goldstein R, Peli E.. Driving with central visual field loss II: how scotomas above or below the preferred retinal locus (PRL) affect hazard detection in a driving simulator. PLoS One . 2015; 10(9): e0136517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Verezen CA, Hoyng CB, Meulendijks CF, Keunen JE, Klevering BJ.. Eccentric gaze direction in patients with central field loss. Optom Vis Sci . 2011; 88(10): 1164–1171. [DOI] [PubMed] [Google Scholar]
- 44. Temme LA, Maino JH, Noell WK.. Eccentricity perception in the periphery of normal observers and those with retinitis pigmentosa. Am J Optom Physiol Optics . 1985; 62(11): 736–743. [DOI] [PubMed] [Google Scholar]
- 45. Fortenbaugh FC, Hicks JC, Turano KA.. The effect of peripheral visual field loss on representations of space: evidence for distortion and adaptation. Invest Ophthalmol Vis Sci. 2008; 49: 2765–2772. [DOI] [PubMed] [Google Scholar]
- 46. Lewald J, Kentridge RW, Peters S, Tegenthoff M, Heywood CA, Hausmann M.. Auditory-visual localization in hemianopia. Neuropsychology. 2013; 27(5): 573–582. [DOI] [PubMed] [Google Scholar]
- 47. Ernst MO, Banks MS. Humans integrate visual and haptic information in a statistically optimal fashion. Nature. 2002; 415: 429–433. [DOI] [PubMed] [Google Scholar]
- 48. Garcia SE, Jones PR, Reeve EI, Michaelides M, Rubin GS, Nardini M.. Multisensory cue combination after sensory loss: audio-visual localization in patients with progressive retinal disease. J Exp Psychol Hum Percept Perform. 2017; 43(4): 729–740. [DOI] [PubMed] [Google Scholar]
- 49. Lessard N, Pare M, Lepore F, Lassonde M.. Early-blind human subjects localize sound sources better than sighted subjects. Nature. 1998; 395: 278–280. [DOI] [PubMed] [Google Scholar]