Abstract
The brain is, after the adipose tissue, the organ with the greatest amount of lipids and diversity in their composition in the human body. In neurons, lipids are involved in signaling pathways controlling autophagy, a lysosome-dependent catabolic process essential for the maintenance of neuronal homeostasis and the function of the primary cilium, a cellular antenna that acts as a communication hub that transfers extracellular signals into intracellular responses required for neurogenesis and brain development. A crosstalk between primary cilia and autophagy has been established; however, its role in the control of neuronal activity and homeostasis is barely known. In this review, we briefly discuss the current knowledge regarding the role of autophagy and the primary cilium in neurons. Then we review the recent literature about specific lipid subclasses in the regulation of autophagy, in the control of primary cilium structure and its dependent cellular signaling in physiological and pathological conditions, specifically focusing on neurons, an area of research that could have major implications in neurodevelopment, energy homeostasis, and neurodegeneration.
Keywords: autophagic flux, cholesterol, fatty acids, GPCR, lysosomal storage diseases, neurons, NPC1, phosphoinositides, primary cilium
Introduction
The brain is the organ with the highest lipid content in its structure, after adipose tissue. The brain is composed of different phospholipids, cholesterol, and sphingolipids that play structural and signaling roles (Svennerholm et al., 1997; Singh et al., 2009). During the life cycle, the brain suffers changes in the percentage of the major species of phospholipids and sphingolipids due to the specialization of some cells, such as myelination in neurons during development and loss of general lipid content with aging (Dawson, 2015). Changes also occur in age-associated neurodegenerative diseases such as Alzheimer’s disease (AD), where lipid changes have been observed in selective brain regions and the global brain lipid composition (Naudi et al., 2015; Mota-Martorell et al., 2022). Data in rodents also show changes in different lipids due to chronic feeding with a high fat diet (HFD) (Sighinolfi et al., 2021), especially plasmalogens, ceramides, and lysophospholipids in the hypothalamus, the main brain region involved in body weight control.
These changes in lipid content affect cellular processes that ultimately control neuronal differentiation, development, and metabolism. In this review, we will focus on the effect of lipids on (i) autophagy, an evolutionarily conserved process of recycling of cytosolic components required for neuronal homeostasis, and (ii) the primary cilium (PC), a cellular antenna with a key role in neuron differentiation and function. We will focus on these two aspects together, as a body of research has demonstrated that a crosstalk exists between autophagy and the PC in different cell types, as well as in neurons (Pampliega et al., 2013; Ávalos et al., 2017, 2022; Larsen and Moller, 2020; Claude-Taupin et al., 2022).
We will summarize recent developments in characterizing the role of different types of lipids in the control of neuronal autophagy as well as the emerging roles of lipids in determining PC composition and signaling. Although studies regarding the role of lipids in the control of PC in neurons are still limited, those discussed here show its relevance in neurons from neurodevelopment to neurodegeneration.
Search Strategy
The search has been performed between November 1, 2022 and March 1, 2023. The platforms used include: PubMed, Google Scholars, Google and Endnote. The search terms used were the following: Autophagy AND primary cilium AND neurons; lipids AND primary cilium AND neurons; neurodegenerative diseases AND autophagy AND lipids; neurodegenerative diseases AND primary cilium AND lipids. Homeostasis AND autophagy AND lipids. Homeostasis AND primary cilium AND lipids. NPC AND autophagy AND lipids. NPC AND primary cilium AND lipids. Alzheimer AND autophagy AND lipids. Alzheimer AND primary cilium AND lipids. Parkinson AND autophagy AND lipids. Parkinson AND primary cilium AND lipids. Caenorhabditis elegans AND lipids AND neurons. Caenorhabditis elegans AND primary cilium AND neurons. Caenorhabditis elegans AND autophagy AND neurons. phosphoinositides AND primary cilium AND autophagy in neurons.
Role of Autophagy in Neurons
Macroautophagy, hereafter autophagy, is a lysosome-dependent degradative process that occurs in every cell in the human body; it allows recycling of cytosolic components such as organelles, proteins, and lipids that maintains cell homeostasis (Dikic and Elazar, 2018). During autophagy, portions of the cytoplasm (proteins, organelles, and lipid droplets, among others) are enclosed in a double membrane structure called the autophagosome, whose formation relies on the activity of different ATG (autophagy-related) proteins (Figure 1). The molecular machinery required for this process has been reviewed elsewhere and will not be described here. For a detailed review of the autophagy process see Cao et al. (2021).
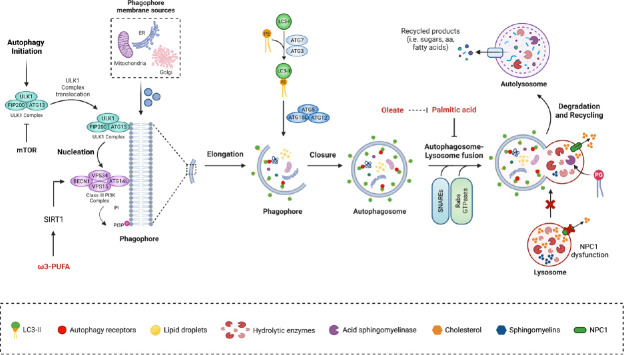
Figure 1.
Role of lipids as autophagy modulators in neurons.
The autophagic process is initiated by the activation of the ULK1 complex, which is negatively regulated by mTOR. Once activated, the ULK1 complex (ULK1, FIP200, and ATG13), translocates to the autophagy forming site, where it initiates phagophore nucleation by phosphorylating components of the Class III PI3K complex (BECN1, VPS34, VPS15, and ATG14L), promoting the recruitment of several ATGs that allow autophagosome formation. The elongation of the phagophore requires phospholipids that derive from different membranes, such as the endoplasmic reticulum, the Golgi apparatus, and the mitochondria. This step involves two ubiquitin-like conjugation systems, the ATG5-ATG12-ATG16L1 and the LC3-PE, which are required for proper phagophore membrane expansion and subsequent closure of the autophagosome. Fusion of the autophagosome with the lysosome requires the concerted action of several proteins to form the autolysosome, including small Rab GTPases and SNAREs, where hydrolytic enzymes degrade the enclosed material. Alterations in the autophagy-lysosomal degradation pathway have been described, depending on the accumulation of fatty acids to which cells are exposed. Oleate restores palmitic acid-mediated autophagic flux inhibition. Omega-3 polyunsaturated fatty acid (PUFA) increases autophagy through the activation of the deacetylase SIRT1. Mutations in NPC1, which mediates free cholesterol efflux from the lysosome, lead to accumulation of cholesterol and other lipids, impairing autophagy by decreasing autophagosome-lysosome fusion. Treatment with PG re-establishes autophagic flux in NPC1 deficient cells by enhancing the activity of the lysosomal enzyme acid sphingomyelinase. For more details, please refer to the text. ATG: Autophagy related proteins; BECN1: beclin-1; ER: endoplasmic reticulum; FIP200: focal adhesion kinase family interacting protein of 200 kDa; LC3: microtubule-associated protein 1A/1B-light chain 3; mTOR: mammalian target of rapamycin; NPC1: Niemann-Pick C1 protein; PE: phosphatidylethanolamine; PG: phosphatidylglycerol; PI3K: phosphatidylinositol 3-kinase; SIRT1: sirtuin-1; SNAREs: soluble N-ethylmaleimide sensitive factor attachment protein receptors; ULK1: unc-51-like kinase; VPS: vacuolar protein sorting. Created with BioRender.com.
Autophagy is particularly relevant in neurons, terminally differentiated cells that remove aggregated proteins and aged or defective organelles, maintaining their homeostasis and health throughout the lifetime of the organism (Stavoe and Holzbaur, 2019). Mitotic cells dilute damaged or accumulated proteins and organelles during cell division, a strategy that neurons cannot use, making them more dependent on basal autophagy than other cell types. Constitutive autophagy regulates neuronal homeostasis; its genetic inhibition, by deletion of core ATG proteins (ATG5 or ATG7), causes the accumulation of ubiquitin-positive inclusions and protein aggregates that lead to axonal and dendrite degeneration and neuronal cell death (Hara et al., 2006; Komatsu et al., 2006a, b, 2007; Lee et al., 2011) (for a recent review please see Stamatakou et al., 2020). Conditional knockout of Atg5 in neurons or depletion of ATG7 in the brain of mice leads to motor dysfunction and accumulation of polyubiquitinated aggregates, a hallmark of many neurodegenerative diseases caused by autophagy impairment. These mice also accumulate the proteins α-synuclein and Leucine-rich repeat kinase 2 (LRRK2) (Friedman et al., 2012), the two major components of the Lewy bodies encountered in Parkinson’s diseases (PD) (Spillantini et al., 1997).
Autophagy induction prevents neurodegeneration in models of AD or PD (Moreau et al., 2014). Autophagy plays different roles in neurons, depending on the neuronal subpopulation considered. While autophagy is required for the encoding of the cognitive function in hippocampal neurons (Vijayan and Verstreken, 2017), hypothalamic neurons regulates body weight by controlling food intake through the production of neuropeptides and leptin signaling (Quan et al., 2012; Oh et al., 2016). Autophagy is required in dopaminergic neurons for pre-synaptic neurotransmission and dopamine release (Hernandez et al., 2012), and in Purkinje cells, basal autophagy regulates axonal homeostasis (Komatsu et al., 2007). Despite these studies, the detailed molecular mechanisms that lead to the onset of neurodegeneration or body weight imbalance, when autophagy is inhibited, have not been completely elucidated yet.
The Primary Cilium in Neurons: from a Vestigial Structure to an Extra-Synaptic Signaling Center
The PC is a sensory organelle that is found in most mammalian cells and is highly conserved throughout eukaryotic evolution (Goetz and Anderson, 2010). PC is immobile and functions as a “cellular antenna” that extends from the cell surface to the extracellular medium (Praetorius and Spring, 2005). PC is enriched in different receptors, mainly G-protein coupled receptors (GPCRs) (Anvarian et al., 2019), which detect changes in the extracellular environment, activating intracellular responses (Kanamaru et al., 2022).
The PC is composed of a microtubule-based axoneme made up of a radial array of 9 microtubule doublets (9+0 structure) that emanates from the basal body and is surrounded by a portion of the cell membrane (Fisch and Dupuis-Williams, 2011). The ciliary membrane that surrounds the PC is continuous with the plasma membrane, however, it has exclusive lipids and receptors that activate ciliary signaling pathways dependent on receptors and downstream scaffold proteins that localize at the PC (Mansini et al., 2018; Anvarian et al., 2019; Wang et al., 2021). As such, the PC is considered as a signaling platform required for efficient signal transduction. See Klena and Pigino (2022) for a detailed review on PC structure.
The PC has been detected throughout the mammalian brain. Although the presence of PC in neurons was observed more than 100 years ago, its function has been the object of study only in the last two decades, identifying PC-dependent roles that highlight its relevance in brain-related physio-pathological processes. The PC is critical in cell-cell communication since early development by sensing extracellular signals that regulate Hedgehog (Hh) and Wingless pathways, finally controlling neuronal migration and interneuron placement, affecting brain development (Huangfu and Anderson, 2005; Higginbotham et al., 2012).
Recent studies show additional mechanisms involved in neuronal migration. Stoufflet and colleagues identified a PC-dependent cyclic adenosine 3′–5′ monophosphate/protein kinase A signaling pathway at the level of the centrosome that regulates neuronal migration from the embryo to the adult in mice (Stoufflet et al., 2020). In adults, PC also controls neuronal integrity and the maintenance of neuronal connectivity (Tereshko et al., 2021, 2022).
Different GPCRs have been identified on the surface of the PC in neurons (Handel et al., 1999; Brailov et al., 2000), suggesting that key responses such as somatostatin or serotonin-mediated signals are PC-dependent. Additional receptors that have been identified at the PC in neurons are the melanin-concentrating hormone-receptor-1 (Nino-Rivero et al., 2019; Diniz et al., 2020), leptin receptor (Seo et al., 2009) and insulin receptor (Ávalos et al., 2022), among others, unveiling a key role for the PC in the control of cell and body metabolism. Lack of the PC by deletion of the ciliary gene kinesin family member 3A in hypothalamic proopiomelanocortin neurons promotes food intake and reduces energy expenditure, driving obesity and glucose intolerance only if the PC is constitutively removed (Davenport et al., 2007; Lee et al., 2020). These results are consistent with the phenotype of a subgroup of ciliopathies, Bardet-Biedl syndrome and Alström syndrome, which are characterized by early onset of obesity, type 2 diabetes, and insulin resistance, with differences in severity depending on the ciliary gene carrying the mutation (Engle et al., 2021). Among the ciliary genes mutated in human obesity, of special relevance to the central nervous system are adenylate cyclase III (ACIII) and the melanocortin 4 receptor (MC4R) (Siljee et al., 2018). ACIII is almost ubiquitously localized on cilia in the central nervous system, and as such, used as a marker for PC in neurons (Bishop et al., 2007; Antal et al., 2017). Mice depleted of ACIII in the ventromedial hypothalamus are significantly more obese than wild types when fed a HFD (Yang et al., 2022). Conversely, humanized ACIII knock-in mice, which show longer PC in hypothalamic neurons, are resistant to HFD-induced obesity (Yang et al., 2022), suggesting that ciliary localization of ACIII in neurons plays an important role in the regulation of body weight. Moreover, MC4R is a critical regulator of energy homeostasis, and mutations in this receptor are the most common cause of monogenic obesity in humans (Ho and MacKenzie, 1999; Lubrano-Berthelier et al., 2003). Recent studies showed that MC4R also functions in the PC; lack of cilia in MC4R-expressing neurons of the paraventricular region of the hypothalamus impairs the anorexigenic function of the receptor by inhibiting adenylyl cyclase activity (Wang et al., 2021). Based on this research, the PC in hypothalamic neurons has recently been defined as “a hub for metabolic homeostasis” (for a recent review see Yang et al., 2021).
Nevertheless, the roles of the PC in neurons are not limited to neurogenesis and metabolism. Research has demonstrated that PC can affect spontaneous firing in rat neocortical pyramidal neurons (Tereshko et al., 2021) and that neurons through the PC can modulate axonal connectivity (Guo et al., 2019) and induce a nuclear signaling dependent on an axon-cilium synapse that regulates chromatin accessibility and therefore the post-neuronal epigenetic state (Sheu et al., 2022).
Human ciliopathies are associated with cognition defects; rodents deleted or depleted of proteins localized at the PC exhibit impairment in learning and memory (Berbari et al., 2014), effects that have been associated with the lack of the G-protein-coupling receptor somatostatin receptor-3 (Wang et al., 2011). Models of AD, as well as aged mice, also show alterations in neuronal cilia length and an impairment in ciliary dynamics (Chakravarthy et al., 2012; Kobayashi et al., 2022). Although these models do not clarify whether ciliary defects are a cause or effect of the disease, they suggest involvement of PC in AD and indicate that future research should be performed to assess the possible role of the PC in the etiology of neurodegenerative diseases (see Ma et al., 2022 for a recent review). Impaired autophagy is also a hallmark of AD; lack of autophagy in hypothalamic proopiomelanocortin neurons promotes obesity in mice, similar to the lack of PC, suggesting that a hitherto unknown crosstalk between autophagy and the PC might occur in these conditions.
Modulation of Autophagy by Lipids in the Brain
Changes in nutrients (i.e., carbohydrates, proteins) affect autophagy in different cellular populations. The role of lipids in autophagy regulation has been only partially elucidated, despite being a major component of the brain involved in cell structure and signaling. Lipid concentration should be tightly controlled to maintain neuronal function, as abnormal lipid metabolism leads to neurodegenerative diseases such as AD or PD (Mesa-Herrera et al., 2019; Chew et al., 2020). Neurodegenerative diseases such as AD, dementia with Loewy bodies, and PD share changes in the composition of lipid rafts, but the individual diseases differ considerably in terms of changes in specific lipid subclasses (Marin et al., 2017). Cholesterol has received the most attention among lipids in neurodegeneration (Dai et al., 2021) being involved in the survival and differentiation of axons and dendrites (Funfschilling et al., 2012). Cholesterol is synthesized by neurons during development, while in adults neurons rely only on external sources, suggesting that changes in the external amount of cholesterol can impact its levels and its metabolism and thus neuronal function (Vance, 2012).
Cholesterol regulates autophagy in different cell models (Suzuki et al., 2019; Shapira et al., 2021). In neurons, cholesterol accumulation and its role in autophagy have been mainly studied in the context of Niemann-Pick disease type C (NPC), a neurodegenerative lysosomal storage disorder characterized by lipid accumulation in endolysosome compartments due to a dysfunction in the protein NPC1, an endolysosomal lipid transporter (Torres et al., 2017). Different studies described that lack of NPC1 in neurons impairs autophagy. Liao and colleagues found that defective cholesterol metabolism inhibits autophagy-lysosome function and promotes the accumulation of dysfunctional and fragmented mitochondria, promoting neurodegeneration (Liao et al., 2007; Ordonez et al., 2012). Roney et al. (2021) showed that the elevated levels of cholesterol in lysosomal membrane sequestered proteins involved in lysosomal transport to the axon, namely kinesin-1 and Arl8-GTPase, which promote autophagosome accumulation into the axon. Later studies described that sphingomyelins are also increased, which are involved in autophagy impairment (Carsana et al., 2022). Interestingly, recent work demonstrated a mechanism that reverts the inhibition in the autophagic flux despite the lack of NPC1. Indeed, treatment with the glycerophospholipid phosphatidylglycerol improves the impairment in the autophagic flux, by enhancing the activity of the lysosomal enzyme acid sphingomyelinase (Ilnytska et al., 2021).
Changes in cellular concentration and distribution of cholesterol have been implicated in the pathogenesis of AD, with contradictory results depending on the model studied. High dietary cholesterol induces the accumulation and phosphorylation of the Tau protein, the microtubule-associated protein that forms insoluble filaments, with an impairment in autophagy (Wang et al., 2022). Conversely, intracellular cholesterol sequestration in the endosomal-lysosomal system in a mouse model overexpressing mutant human β-amyloid precursor protein (APP) in the absence of NPC1 causes increased levels of Aβ in the brain due to reduced endocytic-autophagic-lysosomal clearance (Maulik et al., 2015). Work by Roca-Agujetas et al. (2021) showed that high cholesterol blocks mitophagy in neuronal cell cultures and in vivo in mice expressing APP with the familial Alzheimer Swedish mutation (Mo/HuAPP695swe) and mutant presenilin 1 (PSEN1; PS1-dE9). In these conditions, despite the increase in PTEN induced kinase 1 (PINK1), a mitochondrial protein that promotes mitophagosome formation (Onishi et al., 2021), mitophagy flux is ultimately disrupted possibly due to endosome-lysosome fusion impairment caused by cholesterol.
Changes in cholesterol levels in the brain have also been observed in metabolic diseases such as type I diabetes, where the synthesis of cholesterol in the brain is reduced due to decreased activity of the protein Sterol regulatory element-binding protein 2 (SREBP2), which activates genes involved in cholesterol synthesis (Madison, 2016). Cholesterol reduction (20–31% decrease in cellular cholesterol content) in neuron-derived cells increases basal autophagy while reducing autophagy induction in response to glucose deprivation (Fukui et al., 2015). Accordingly, autophagy is inhibited in mice injected with a low dose of streptozotocin and fed with a HFD, where the cholesterol level in the hypothalamus is significantly higher than in control mice, as suggested by upregulation of the mammalian target of rapamycin and decrease in LC3-II (Jing et al., 2017). Intracellular lipid overload in PC12 neuronal cells caused by oleic acid treatment leads to cell damage, which is even worse when autophagy is inhibited chemically (Jing et al., 2017). Additional studies show that autophagy levels in neurons depend on the amount and type of fatty acids cells are exposed to. Research by our and other groups show that exposure to saturated fatty acids inhibits the autophagic flux in hypothalamic neurons by activation of the fatty acid receptor G-coupled receptor 40 and by impairment of autophagosome-lysosome fusion and endolysosomal dynamics (Portovedo et al., 2015; Hernandez-Caceres et al., 2019, 2020; Reginato et al., 2020; Ávalos et al., 2022; Espinosa et al., 2022). Oleate has been shown to restore the altered autophagic flux in response to palmitic acid overload in hypothalamic neurons (He et al., 2022). Omega-3 polyunsaturated fatty acid supplementation increases autophagy in neurons through the activation of the deacetylase Sirtuin-1, a known regulator of autophagy (Morselli et al., 2010), thus preventing traumatic brain injury-induced neuronal apoptosis (Chen et al., 2018). Changes in fatty acid concentrations regulate autophagy, at least in hypothalamic neurons, and through lipophagy; autophagy controls the amount of fatty acids in the brain, providing fatty acids to the brain in conditions of nutrient deprivation (Kaushik et al., 2011).
In conclusion, these studies highlight lipids as modulators of autophagy (Figure 1), with different outcomes depending on the lipid subclass and the level of saturation of fatty acids. Future research should focus on the specific mechanism by which lipids are modulating autophagy in the different pathophysiological settings and the different neuronal subtypes.
Lipid Composition of the Primary Cilium
The ciliary membrane is contiguous with the plasma membrane, yet it exhibits distinct protein and lipid composition, which are essential for PC formation and function (Verhey and Yang, 2016; Nachury and Mick, 2019). Previous studies focused on the composition of the lipid membrane of the PC in neurons are limited, hence we will first consider the information that exists in other cell types and discuss the relevance of these findings for neurons.
A major lipid class that regulates PC length in different cell types is the phosphatidylinositols (PtdIns), lipids composed of a glycerol backbone linked to two fatty acids and a phosphoinositol molecule (Balla, 2013). PtdIns, together with the enzymes that control their synthesis (oculocerebrorenal syndrome of Lowe protein (OCRL), type Iγ phosphatidylinositol 4-phosphate (PtdIns(4)P) 5-kinase (PIPKIγ), inositol polyphosphate-5-phosphatase E (INPP5E) and inositol polyphosphate-5-phosphatase B (INPP5B)), localize to discrete sub-compartments of the ciliary membrane, where they exert structural and signaling functions (Conduit and Vanhaesebroeck, 2020). PtdIns(4)P, the most abundant one, and PtdIns(4,5)P2 localize at the PC membrane, where they control Hedgehog signaling (Garcia-Gonzalo et al., 2015). PtdIns(4,5)P2, PIP3 (generated by phosphorylation of PtdIns(4,5)P2), and PtdIns(3,4)P2 are enriched at the transition zone at the ciliary base, where they regulate the stability of PC by balancing membrane turnover (Stilling et al., 2022). PtdIns(3)P, localized at the base of the PC, is associated with the pericentriolar recycling of the endocytic compartment (Conduit and Vanhaesebroeck, 2020). Recent results show that changes in ciliary PtdIns are a unifying point for PC membrane stability and turnover. Indeed, the paucity of PtdIns in the distal PC ensures ciliary stability in different mammalian cell lines (Stilling et al., 2022). Changes in PtdIns content also affect cargo trafficking within the PC, for example, deletion of the protein phosphatidylinositol 3-kinase (PI3K-C2α), which phosphorylates PtdIns and PtdIns(4)P, producing PtdIns(3)P (Franco et al., 2014), impairs the trafficking of transmembrane proteins into PC and reduces PC length in renal tubule-derived inner medullary collecting duct 3 cells, indicating that PI3K-C2α controls PC structure and function (Franco et al., 2016). Mutations in the protein inositol polyphosphate-5-phosphatase OCRL, which dephosphorylates PtdIns(4,5)P2 and regulates endocytosis, are associated with Lowe syndrome, a disease characterized by cognitive impairment, among other features. The levels of ciliary PtdIns(4,5)P2 are enhanced in fibroblasts derived from Lowe syndrome patients and mouse models, suggesting that changes in the amount of this PtdIns in the PC can promote Lowe’s disease by a still unknown mechanism (Prosseda et al., 2017). Mutations in INPP5B, which contributes to the regulation of ciliary PtdIns (Janne et al., 1998), cause two human ciliopathy syndromes with mental retardation (Bielas et al., 2009). INPP5B, a protein mutated in Joubert Syndrome (a neurodevelopmental disorder), controls PtdIns(4)P homeostasis; its deletion reduces ciliated cell number and cilia length Inpp5b zebrafish embryo morphants (Luo et al., 2013). INPP5B counteracts the activity of PIPKIγ, a centrosomal PtdIns(4)P 5-kinase, controlling axoneme growth in different cell lines (Xu et al., 2016). PtdIns(4)P levels at the ciliary base are essential for proper ciliogenesis, as they regulate the recruitment of different ciliary proteins, namely the centrosome protein of 164 kDa (CEP164), which recruits tau tubulin kinase 2, thus promoting PC growth (Xu et al., 2016). PtdIns(3)P defines PC length and is required for Rab11 recruitment, which controls the trafficking of PC proteins in MEF cells (Franco et al., 2014). The PtdIns(4)P/PtdIns(4,5)P2 balance controls receptor recruitment to PC and has a major impact on cilia signaling and stability in different cell lines (Phua et al., 2017). All these results indicate that PtdIns regulatory enzymes coordinate the homeostasis of PtdIns during ciliogenesis (see Table 1 for further details).
Table 1.
PC associated phosphatidylinositol metabolizing enzymes
| Enzyme | Substrate | Product | Localization of the enzyme in the PC | Role at the PC | Human relatad disorder | Reference |
|---|---|---|---|---|---|---|
| PI3K-C2α | PtdIns | PtdIns(3)P | Pericentriolar recycling endocytic compartment at the ciliary base | Modulate PC length and trafficking of ciliary proteins | Syndromic short stature, skeletal malformations, cataracts | Franco, 2014, 2016; Tiosano, 2019 |
| OCRL | PtdIns(4,5)P2 PIP3 | PtdIns(4)P PtdIns(3,4)P2 | Cilia axoneme and ciliary base | Assembly and maintenance of PC | Lowe syndrome | Luo, 2012; Prosseda, 2017 |
| INPP5B | PtdIns(4,5)P2 PIP3 | PtdIns(4)P PtdIns(3,4)P2 | Cilia axoneme | Regulates PC length and number | Joubert syndrome | Luo, 2013 |
| INPP5E | PtdIns(4,5)P2 PIP3 | PtdIns(4)P PtdIns(3,4)P2 | Ciliary transition zone | Controls cilia elongation and ciliary localization of phospholipids | Joubert syndrome | Bielas, 2009; Jacoby, 2009; Park, 2015 |
| PIPKIγ | PtdIns(4)P | PtdIns(4,5)P2 | Centrosome / cilia basal body | Regulates PC length and number | Lethal congenital contracture syndrome type 3 | Narkis, 2007; Xu, 2016 |
INPP5: Inositol polyphosphate-5-phosphatase; OCRL: oculocerebrorenal syndrome of Lowe protein; PC: primary cilium; PI3K-C2α: phosphatidylinositol 3-kinase type 2α; PIP3: phosphatidylinositol (3,4,5)-trisphosphate; PIPKIγ: type Iγ phosphatidylinositol 4-phosphate 5-kinase; PtdIns: phosphatidylinositol.
As indicated, most of the aforementioned studies were performed in non-neuronal cells, however, mutations in these proteins cause syndromes that produce cognitive impairment and neurodegeneration, indicating the importance of pursuing these studies in neuronal cells. A protein of relevance for PC function that is mainly expressed in the brain and retina (Mukhopadhyay et al., 2013) is the ciliary protein TULP3. TULP interacts with the founding tubby (TUB) family member in the PC (Badgandi et al., 2017), where they mediate the ciliary trafficking of GPCRs in a phosphoinositide 4,5-bisphosphate (PtdIns(4,5)P2)-dependent manner (Badgandi et al., 2017). DiTirro et al. (2019) show that compromised sensory signaling results in increased levels of PtdIns(4,5)P2 via TUB-1-dependent ciliary localization of the type I phosphatidylinositol 4-phosphate 5-kinase (PIP5K) PPK-1 of wild-type AWB neurons in Caenorhabditis elegans, confirming that further studies need to be performed in neurons to determine the relevance of this trafficking in these cells.
In Drosophila chordotonal neurons, the INPP5E homolog dINPP5E localizes to the ciliary transition zone, in a region surrounded by high PtdIns(4,5)P2 levels (Park et al., 2015), which suggests a possible regulation of PtdIns levels and ciliogenesis also in neurons. INPP5E controls ciliary localization of phospholipids in terminally developed mouse olfactory sensory neurons. Deletion of the phosphoinositide 5′-phosphatase gene Inpp5e in olfactory sensory neuron leads to a remodeling of PtdIns(4,5)P2, which correlates with cilia elongation; gene replacement of Inpp5e restores the localization phospholipids at the PC and PC function (Ukhanov et al., 2022). It was recently demonstrated that INPP5E interacts with the autophagy protein ATG16L1 in mouse embryonic fibroblasts, regulating the turnover of PtdIns in the PC. Moreover, a perturbation of trafficking of ATG16L1 to PC exhibits aberrant ciliary structures (Boukhalfa et al., 2021). These results evidence the existence of a crosstalk between PC and autophagy, which will be discussed in the next sections.
It is relevant to mention that other lipids have also been identified at the PC; however, their role in this cell compartment has been poorly studied. High levels of sterols and sphingolipids, including ceramide and raft-associated gangliosides GM1 and GM3, have been identified in PC of different cell types (Montesano, 1979; Souto-Padrón and de Souza, 1983; Kaneshiro et al., 1984; Chailley and Boisvieux-Ulrich, 1985; He et al., 2012). Flotillin-2, a lipid raft scaffold protein, was detected at the transition zone in epithelial cells (Schou et al., 2017). Lipid biosynthetic enzymes localize to distinct sub-ciliary compartments and locally modulate membrane lipid composition (Nechipurenko, 2020), but their functional impact is still unknown.
Taken together, these results suggest that the lipid composition of the PC membrane might be critical for PC composition and structure dynamics in neurons, which are essential for PC-dependent signaling. Further studies need to be performed to determine how distinct membrane lipid domains form and contribute to PC function, specifically in neurons, to identify possible new targets for the treatment of ciliopathies or additional brain diseases characterized by cognitive impairment and neurodegeneration.
Role of Lipids in the Regulation of Primary Cilium Signaling
An emerging theme is the role of ciliary lipids in controlling the localization of different receptors at the PC, modulating PC structure and thus PC-dependent signaling. The PC contains a unique composition of signal transduction elements, acting as a communication hub that transfers extracellular signals into intracellular responses, which are affected by the presence and concentration of ciliary lipids (Figure 2). Here, we will discuss the impact of lipids on PC signaling.
Figure 2.
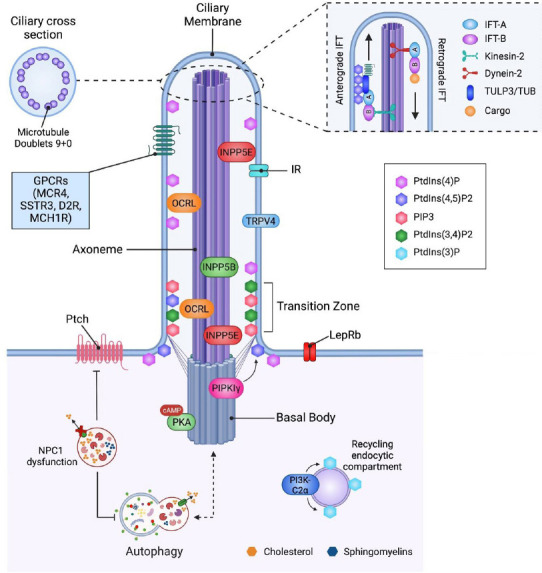
The interplay between primary cilium, lipids, and autophagy.
The ciliary membrane contains exclusive lipids and receptors, which activate ciliary signaling pathways dependent on receptors and downstream scaffold proteins that localize at the primary cilium (PC). IFT-B mediates the anterograde ciliary trafficking of proteins to the tip of the PC using kinesin-2 motors, and IFT-A, together with dynein-2 motors, allow the retrograde transport of ciliary cargo to the PC basal body. Different lipid species are restricted to specific subdomains of ciliary membrane, where they are tightly regulated by enzymes that localize in proximity to their substrates and products (denoted by curved arrows). These lipids are required to maintain PC structure and function. Lipids can also modulate cargo ciliary trafficking and PC-dependent signaling pathways such as hedgehog-Ptch signaling at the PC, which are negatively affected by cholesterol accumulation in the lysosome of NPC1-deficient neurons. Cholesterol accumulation is correlated with PC dysfunction induced by impaired autophagy in these neurons. Conversely, ciliary signaling pathway can modulate the autophagic process, suggesting a bidirectional crosstalk between autophagy and ciliogenesis. For more information, please refer to the text. Created with BioRender.com. cAMP: Cyclic adenosine 3′–5′ monosphosphate; D2R: dopaminergic D2-receptors; GPCRs: G-protein-coupled receptors; IFT: intraflagellar transport; INPP5: inositol polyphosphate-5-phosphatase; IR: insulin receptor; LepRb: leptin receptor; MC4R: melanocortin 4 receptor; MCHR1: melanin-concentrating hormone receptor-1; NPC1: Niemann-Pick C1 protein; OCRL: oculocerebrorenal syndrome of Lowe protein; PI3K-C2α: phosphatidylinositol 3-kinase type 2α; PIP3: phosphatidylinositol (3,4,5)-trisphosphate; PIPKIγ: type Iγ phosphatidylinositol 4-phosphate 5-kinase; PKA: protein kinase A; Ptch: Patched; PtdIns: phosphatidylinositol; SSTR3: somatostatin receptor-3; TRPV4: transient receptor potential cation channel subfamily V member 4; TUB: tubby protein; TULP3: tubby-like protein 3.
The transient receptor potential cation channel subfamily V member 4 (TRPV4) is a ciliary channel expressed in sensory neurons that contains cholesterol recognition motifs that can detect physical and chemical stimuli and adapt to environmental signals. Kumari et al. described that TRPV4 interacts with sterols that localize at the PC and function correctly (Bergdahl et al., 2003; Brazer et al., 2003; Kumari et al., 2015), indicating that cholesterol in the ciliary membrane is involved in the localization of ion channels at the PC. Cholesterol, together with glycosphingolipids, influences GPCRs function, modulating ligand affinity, G protein coupling and receptor oligomerization (Gahbauer and Böckmann, 2016). Several G protein-coupled receptors, including dopaminergic D2-receptors, are localized in the PC of mammalian neurons; lack of D2 dopaminergic input increases striatal PC length (Miyoshi et al., 2014; Figure 2). The activity of these longer cilia was not evaluated in this study, however, since a correlation between PC length and function has been demonstrated (Dummer et al., 2016), we can speculate that these longer cilia are dysfunctional, with a negative impact on PC-dependent signaling.
Lipids also interact with and modulate the activity of class A GPCRs, which localize at PC. This is the case of PtdIns(4,5)P2, which stabilizes class A GPCRs with the trimeric Gαsβγ subunits, modulating receptor function in different cell types (Yen et al., 2018). Ciliogenesis is also controlled by the lipogenic transcription factor sterol regulatory element-binding protein 1c (SREBP1c), which when constantly activated, blunts PC growth by affecting endosomal recycling and vesicular transport toward the PC. Importantly, this highly increases lysophosphatidylcholine and lysophosphatidylethanolamine levels (Gijs et al., 2015). Gijs et al. (2015) showed this mechanism in multiple cell lines, but they did not evaluate if it also occurs in neurons. However, SREBP1c is considered a critical regulatory molecule for lipid homeostasis in the brain, and its knockout causes memory impairment, a phenotype also seen in mice lacking the PC in the hippocampus (Rhee et al., 2016). An increase in lysophosphatidylcholine and lysophosphatidylethanolamine levels is associated with neurodegeneration (Law et al., 2019), suggesting that further investigation of ciliary SREBP1c in hippocampal neurons should be performed in the context of cognition.
Taken together, these studies demonstrate that lipids modulate PC-dependent signaling, impacting neuronal function.
Niemann-Pick Disease Type C: an Example of How Lipid Dysregulation Affects Primary Cilium Structure and Function in a Brain Disease
Impairment in PC function is increasingly recognized as a key event in many human diseases. These include ciliopathies, obesity, neurodegenerative diseases, and cancers, reflecting the importance of the PC in different physio-pathological scenarios (Youn and Han, 2018). A limited number of studies have evaluated if lipids affect PC structure or signaling in neurons, except NPC models. NPC, as mentioned, is caused by mutations in the NPC1 gene, encoding a transmembrane protein involved in the intracellular trafficking of cholesterol in the late endosomal/lysosomal system (Vanier, 2010). NPC1 has high sequence homology with Patched (Nusca et al., 2014; Zhang et al., 2018; Kowatsch et al., 2019), which localizes at the base of the PC (Goetz and Anderson, 2010), and is required for the Hh signaling pathway. Patched, by controlling the intracellular amount of cholesterol, determines the amount of Smoothened (Smo) at the PC. Oxysterols and cholesterol derivatives define the concentration of Smo at the PC and therefore its activation (Canterini et al., 2017). Canterini et al. (2017) determined that defective Hh signaling is responsible for abnormal morphogenesis of the cerebellum of NPC1-deficient mice and show that PC is shortened in NPC1 mouse model brains and fibroblasts from NPC1 patients, which correlates with a decrease in Hh signaling. These defects are prevented by treatment with 2-hydroxypropyl-β-cyclodextrin, an efficient cholesterol-lowering agent (Camargo et al., 2001), suggesting defective ciliogenesis is linked to cholesterol accumulation. This is consistent with the work of Lucarelli et al. (2019) who found alterations in PC number, length-frequency distribution, and tortuosity in the dorsal striatum in juvenile NPC1 mice (Lucarelli et al., 2019). The short and irregular cilia observed in NPC1 models possibly reflect a dysregulation of axonemal length, where mutant cilia undergo excessive fragmentation, a hypothesis that requires further investigation.
These studies that show the dependence of PC length and function on cholesterol concentration also suggest that deregulation of Hh signaling at the PC could occur in other lipid storage diseases. For example, the congenital malformations of Smith-Lemli-Opitz syndrome, which is due to mutations in the gene encoding the cholesterol biosynthetic enzyme 7-dehydrocholesterol reductase, are similar to those caused by aberrant Hh signaling (Kelley et al., 1996), suggesting that the PC could be involved in this disease. Thus, NPC and other lysosomal storage diseases could be considered ciliopathies, due to the overall disorganization of Hh signaling along with the shortening of PC. Recent studies have linked defects in genes encoding for ciliary proteins to various cerebellar disorders (Guemez-Gamboa et al., 2014) laying the basis for a new definition and classification of human ciliopathies.
Based on the literature showing the bidirectional crosstalk between autophagy and ciliogenesis (Pampliega et al., 2013), a possible mechanism for altered ciliogenesis in NPC disease is altered autophagy. The autophagic flux is impaired in NPC1-deficient cultured neurons, as indicated by the accumulation of autophagic vesicles (Meske et al., 2014); however, the direct or indirect connection with the PC in this disease has not been studied.
In conclusion, clinical neurological manifestations observed in human diseases associated with lysosomal dysfunction may be due to cilia abnormalities. We propose that additional diseases associated with lipid deregulation or accumulation in the lysosome would have an impact on the signaling pathways controlling the function and length of the PC.
Primary Cilium and Autophagy Crosstalk in Neurons
The literature we reviewed suggests overlap of the phenotypes of autophagy inhibition and PC depletion, in both scenarios showing neurodegeneration, impairment in cognition and dysregulated body metabolism. Consistent with this observation, compelling evidence has reported the presence of a bidirectional crosstalk between PC and autophagy, where autophagy controls ciliogenesis (Tang et al., 2013), and conversely, ciliary signaling pathways activate autophagy (Pampliega et al., 2013; Pampliega and Cuervo, 2016). The interplay between PC and autophagy occurs in different cell types and physio-pathological settings and by direct and indirect mechanisms (Boukhalfa et al., 2019; Claude-Taupin et al., 2022). The literature that focuses on the crosstalk between the PC and autophagy in the brain is limited, however, in neurons, ciliary activation of Smo directly enhances autophagy through the LKB1-AMPK pathway, which finally reduces the level of the centriole and centriolar satellite protein OFD1 in centriolar satellites. Although this event is required, it is not sufficient to induce ciliogenesis, which is activated by the Gαi-LGN-NuMA-dynein axis, increasing PC number and length (Akhshi and Trimble, 2021). Conversely, in a model of focal malformations of cortical development, an increase in OFD1 in centriolar satellites caused by a dysfunction in autophagy reduced ciliogenesis, which finally causes cortical dyslamination (Park et al., 2018). Mechanistically this effect depends on mutations in mammalian target of rapamycin, a master negative regulator of autophagy, which directly reduces autophagy, preventing autophagic degradation of OFD1 (Park et al., 2018). Although the mechanism has yet not been described in detail, OFD1 levels inversely correlate with ciliogenesis (Tang et al., 2013), unveiling a direct relationship between the control of protein levels by autophagy and PC number.
The inhibition of ciliogenesis due to autophagy impairment has been confirmed in other neuronal cellular models. It was recently reported that inhibition of autophagy by ATG5 depletion completely prevents ciliary elongation caused by treatment with mitochondrial respiratory complex-1 inhibitors in SH-SY5Y cells, a neuronal cell model (Bae et al., 2019). Our work confirms that genetic and chemical inhibition of autophagy inhibits ciliogenesis in hypothalamic neurons, showing that autophagy is required for efficient ciliogenesis even in neurons. Although we did not evaluate the mechanism that allows this crosstalk, we demonstrated that this functionally regulates insulin signaling (Ávalos et al., 2022).
Recent work also showed that, conversely, ciliogenesis can regulate autophagy by direct and indirect mechanisms. Bae et al. (2019) showed that enhanced ciliogenesis induced by mitochondrial stress in dopamine neurons indirectly promotes autophagy and neuronal survival in a PD model. This crosstalk between autophagy and PC could also occur in other neurodegenerative diseases such as Huntington’s disease (HD) (Tabrizi et al., 2020). A body of literature has shown a causal role for impaired autophagy in the development of HD. Autophagy dysfunction is the result of its abnormal induction, together with a failure in the recognition of the autophagic cargo and a reduced trafficking of autophagic vesicles (Martinez-Vicente et al., 2010; Martin et al., 2015). Importantly, PC are lost in HD, suggesting a possible connection between autophagy and PC in HD development. The mutated huntingtin has been shown to accumulate at the PC (Keryer et al., 2011; Nguyen et al., 2016); however, its involvement in the disease is still unknown, which warrants future studies to offer a potential new therapeutic avenue for HD.
These studies unveil a complex direct and indirect bidirectional crosstalk between autophagy and the PC in neurons, whose functional relevance has only begun to be understood.
Conclusions
In this review, we discussed the role of lipids on autophagy and the PC in neurons. We also highlighted the literature focusing on the crosstalk between this cell process and this cellular structure in neurons. There are similar phenotypes among the different models of PC and autophagy dysfunction in neurons, suggesting overlapping pathways. However, the available literature regarding this subject is limited, indicating the necessity to dissect the relevance and the relation of this crosstalk in neurons and the context of brain-related diseases, from neurodegeneration to metabolic dysfunctions. Similar phenotypes have been seen in neurodegenerative diseases such as AD and PD, in NPC and metabolic diseases, suggesting that dysfunction of the PC-autophagy crosstalk could be a common mechanism in a whole plethora of brain diseases. This indicates the need to focus on this axis in neurons to understand the detailed mechanisms that connect autophagy and the PC.
As highlighted throughout the manuscript, lipids represent another common point between autophagy and the PC, as they both impact autophagy and PC, with structural and signaling effects. Lipid-dependent signaling is essential for vesicle formation in autophagy, and lipid composition changes vesicle characteristics (i.e., fluidity or curvature), which can impact the autophagic flux. Lipid composition affects PC size, shape, composition, and therefore function. Thus, lipids are involved in the regulation of structural and functional aspects of the membrane that reciprocally influence each other, finally impacting the autophagy-PC interplay. Studying lipids is challenging; new tools to target subclasses of lipids in defined subcellular compartments should be developed to be able to attack specific questions such as determining the role of lipids at the PC without depleting the whole PC, which is the most common technique currently used. An additional unanswered question is how we can dissect the structural and signaling function of lipids in this context, as lipid-induced structural changes in autophagic vesicles or in PC structure finally affect protein interaction and signal transduction.
Despite the recent advances described in this review, many unanswered questions remain. While the role of autophagy in neurons during lifespan has been studied, understanding the role of the PC in neurons, mainly in adulthood, is still in its early ages. Unanswered open questions that remain include: How do lipids or changes in lipids composition affect PC-dependent signaling pathways? Is PC lipid composition the same from early life to adulthood and in the different neuronal subpopulations? These questions, which apparently only focus on the PC, can directly or indirectly impact autophagy and therefore neuronal homeostasis and survival.
Separately, lipids, autophagy, and the PC have been identified as causal in neurologic and metabolic impairment. However, the literature and the phenotype of diseases such as NPC, PD, and AD highly suggest a possible crosstalk between these separate elements, which to the best of our knowledge has not yet been evaluated. Thus, the study of a PC-autophagy axis, or conversely, an autophagy-PC signaling pathway regulated by lipids, could provide the foundation for the development of new therapeutic strategies for these diseases.
Acknowledgments
The authors thank everyone in the Morselli and Yañez laboratories for constructive discussions and criticisms.
Footnotes
Funding: This work was funded by grants from Fondo Nacional de Desarrollo Científico y Tecnológico, FONDECYT 1200499 to EM, 11200592 to MJY, 1211329 to AC; by the ANID PIA ACT172066 to EM and AC; by the ANID postdoctoral fellowship 3210630 to MPHC; by the ANID doctoral fellowship 21230122 to DPN; by the ANID doctoral fellowship 21211189 to PR; by the ANID doctoral fellowship by the ANID doctoral fellowship 21210611 to FDC.
Conflicts of interest: The authors declare no conflicts of interest.
Data availability statement: Not applicable.
C-Editors: Zhao M, Liu WJ, Qiu Y; T-Editor: Jia Y
References
- 1.Akhshi T, Trimble WS. A non-canonical Hedgehog pathway initiates ciliogenesis and autophagy. J Cell Biol. (2021);220:e202004179. doi: 10.1083/jcb.202004179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antal MC, Benardais K, Samama B, Auger C, Schini-Kerth V, Ghandour S, Boehm N. Adenylate cyclase type III is not a ubiquitous marker for all primary cilia during development. PLoS One. (2017);12:e0170756. doi: 10.1371/journal.pone.0170756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anvarian Z, Mykytyn K, Mukhopadhyay S, Pedersen LB, Christensen ST. Cellular signalling by primary cilia in development, organ function and disease. Nat Rev Nephrol. (2019);15:199–219. doi: 10.1038/s41581-019-0116-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ávalos Y, Peña-Oyarzun D, Budini M, Morselli E, Criollo A. New roles of the primary cilium in autophagy. Biomed Res Int. (2017);2017:4367019. doi: 10.1155/2017/4367019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ávalos Y, Hernández-Cáceres MP, Lagos P, Pinto-Nuñez D, Rivera P, Burgos P, Díaz-Castro F, Joy-Immediato M, Venegas-Zamora L, Lopez-Gallardo E, Kretschmar C, Batista-Gonzalez A, Cifuentes-Araneda F, Toledo-Valenzuela L, Rodriguez-Peña M, Espinoza-Caicedo J, Perez-Leighton C, Bertocchi C, Cerda M, Troncoso R, et al. Palmitic acid control of ciliogenesis modulates insulin signaling in hypothalamic neurons through an autophagy-dependent mechanism. Cell Death Dis. (2022);13:659. doi: 10.1038/s41419-022-05109-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Badgandi HB, Hwang SH, Shimada IS, Loriot E, Mukhopadhyay S. Tubby family proteins are adapters for ciliary trafficking of integral membrane proteins. J Cell Biol. (2017);216:743–760. doi: 10.1083/jcb.201607095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bae JE, Kang GM, Min SH, Jo DS, Jung YK, Kim K, Kim MS, Cho DH. Primary cilia mediate mitochondrial stress responses to promote dopamine neuron survival in a Parkinson's disease model. Cell Death Dis. (2019);10:952. doi: 10.1038/s41419-019-2184-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balla T. Phosphoinositides:tiny lipids with giant impact on cell regulation. Physiol Rev. (2013);93:1019–1137. doi: 10.1152/physrev.00028.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berbari NF, Malarkey EB, Yazdi SM, McNair AD, Kippe JM, Croyle MJ, Kraft TW, Yoder BK. Hippocampal and cortical primary cilia are required for aversive memory in mice. PLoS One. (2014);9:e106576. doi: 10.1371/journal.pone.0106576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bergdahl A, Gomez MF, Dreja K, Xu SZ, Adner M, Beech DJ, Broman J, Hellstrand P, Swärd K. Cholesterol depletion impairs vascular reactivity to endothelin-1 by reducing store-operated Ca2+entry dependent on TRPC1. Circ Res. (2003);93:839–847. doi: 10.1161/01.RES.0000100367.45446.A3. [DOI] [PubMed] [Google Scholar]
- 11.Bielas SL, Silhavy JL, Brancati F, Kisseleva MV, Al-Gazali L, Sztriha L, Bayoumi RA, Zaki MS, Abdel-Aleem A, Rosti RO, Kayserili H, Swistun D, Scott LC, Bertini E, Boltshauser E, Fazzi E, Travaglini L, Field SJ, Gayral S, Jacoby M, et al. Mutations in INPP5E, encoding inositol polyphosphate-5-phosphatase E, link phosphatidyl inositol signaling to the ciliopathies. Nat Genet. (2009);41:1032–1036. doi: 10.1038/ng.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bishop GA, Berbari NF, Lewis J, Mykytyn K. Type III adenylyl cyclase localizes to primary cilia throughout the adult mouse brain. J Comp Neurol. (2007);505:562–571. doi: 10.1002/cne.21510. [DOI] [PubMed] [Google Scholar]
- 13.Boukhalfa A, Miceli C, Avalos Y, Morel E, Dupont N. Interplay between primary cilia, ubiquitin-proteasome system and autophagy. Biochimie. (2019);166:286–292. doi: 10.1016/j.biochi.2019.06.009. [DOI] [PubMed] [Google Scholar]
- 14.Boukhalfa A, Roccio F, Dupont N, Codogno P, Morel E. The autophagy protein ATG16L1 cooperates with IFT20 and INPP5E to regulate the turnover of phosphoinositides at the primary cilium. Cell Rep. (2021);35:109045. doi: 10.1016/j.celrep.2021.109045. [DOI] [PubMed] [Google Scholar]
- 15.Brailov I, Bancila M, Brisorgueil MJ, Miquel MC, Hamon M, Verge D. Localization of 5-HT(6) receptors at the plasma membrane of neuronal cilia in the rat brain. Brain Res. (2000);872:271–275. doi: 10.1016/s0006-8993(00)02519-1. [DOI] [PubMed] [Google Scholar]
- 16.Brazer SC, Singh BB, Liu X, Swaim W, Ambudkar IS. Caveolin-1 contributes to assembly of store-operated Ca2+influx channels by regulating plasma membrane localization of TRPC1. J Biol Chem. (2003);278:27208–27215. doi: 10.1074/jbc.M301118200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Camargo F, Erickson RP, Garver WS, Hossain GS, Carbone PN, Heidenreich RA, Blanchard J. Cyclodextrins in the treatment of a mouse model of Niemann-Pick C disease. Life Sci. (2001);70:131–142. doi: 10.1016/s0024-3205(01)01384-4. [DOI] [PubMed] [Google Scholar]
- 18.Canterini S, Dragotto J, Dardis A, Zampieri S, De Stefano ME, Mangia F, Erickson RP, Fiorenza MT. Shortened primary cilium length and dysregulated Sonic hedgehog signaling in Niemann-Pick C1 disease. Hum Mol Genet. (2017);26:2277–2289. doi: 10.1093/hmg/ddx118. [DOI] [PubMed] [Google Scholar]
- 19.Cao W, Li J, Yang K, Cao D. An overview of autophagy:mechanism, regulation and research progress. Bull Cancer. (2021);108:304–322. doi: 10.1016/j.bulcan.2020.11.004. [DOI] [PubMed] [Google Scholar]
- 20.Carsana EV, Lunghi G, Prioni S, Mauri L, Loberto N, Prinetti A, Zucca FA, Bassi R, Sonnino S, Chiricozzi E, Duga S, Straniero L, Asselta R, Solda G, Samarani M, Aureli M. Massive accumulation of sphingomyelin affects the lysosomal and mitochondria compartments and promotes apoptosis in Niemann-Pick disease type A. J Mol Neurosci. (2022);72:1482–1499. doi: 10.1007/s12031-022-02036-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chailley B, Boisvieux-Ulrich E. Detection of plasma membrane cholesterol by filipin during microvillogenesis and ciliogenesis in quail oviduct. J Histochem Cytochem. (1985);33:1–10. doi: 10.1177/33.1.3965567. [DOI] [PubMed] [Google Scholar]
- 22.Chakravarthy B, Gaudet C, Menard M, Brown L, Atkinson T, Laferla FM, Ito S, Armato U, Dal Pra I, Whitfield J. Reduction of the immunostainable length of the hippocampal dentate granule cells'primary cilia in 3xAD-transgenic mice producing human Abeta(1-42) and tau. Biochem Biophys Res Commun. (2012);427:218–222. doi: 10.1016/j.bbrc.2012.09.056. [DOI] [PubMed] [Google Scholar]
- 23.Chen X, Pan Z, Fang Z, Lin W, Wu S, Yang F, Li Y, Fu H, Gao H, Li S. Omega-3 polyunsaturated fatty acid attenuates traumatic brain injury-induced neuronal apoptosis by inducing autophagy through the upregulation of SIRT1-mediated deacetylation of Beclin-1. J Neuroinflammation. (2018);15:310. doi: 10.1186/s12974-018-1345-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chew H, Solomon VA, Fonteh AN. Involvement of lipids in Alzheimer's disease pathology and potential therapies. Front Physiol. (2020);11:598. doi: 10.3389/fphys.2020.00598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Claude-Taupin A, Dupont N, Codogno P. Autophagy and the primary cilium in cell metabolism:What's upstream? Front Cell Dev Biol. (2022);10:1046248. doi: 10.3389/fcell.2022.1046248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Conduit SE, Vanhaesebroeck B. Phosphoinositide lipids in primary cilia biology. Biochem J. (2020);477:3541–3565. doi: 10.1042/BCJ20200277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dai L, Zou L, Meng L, Qiang G, Yan M, Zhang Z. Cholesterol metabolism in neurodegenerative diseases:molecular mechanisms and therapeutic targets. Mol Neurobiol. (2021);58:2183–2201. doi: 10.1007/s12035-020-02232-6. [DOI] [PubMed] [Google Scholar]
- 28.Davenport JR, Watts AJ, Roper VC, Croyle MJ, van Groen T, Wyss JM, Nagy TR, Kesterson RA, Yoder BK. Disruption of intraflagellar transport in adult mice leads to obesity and slow-onset cystic kidney disease. Curr Biol. (2007);17:1586–1594. doi: 10.1016/j.cub.2007.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dawson G. Measuring brain lipids. Biochim Biophys Acta. (2015);1851:1026–1039. doi: 10.1016/j.bbalip.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dikic I, Elazar Z. Mechanism and medical implications of mammalian autophagy. Nat Rev Mol Cell Biol. (2018);19:349–364. doi: 10.1038/s41580-018-0003-4. [DOI] [PubMed] [Google Scholar]
- 31.Diniz GB, Battagello DS, Klein MO, Bono BSM, Ferreira JGP, Motta-Teixeira LC, Duarte JCG, Presse F, Nahon JL, Adamantidis A, Chee MJ, Sita LV, Bittencourt JC. Ciliary melanin-concentrating hormone receptor 1 (MCHR1) is widely distributed in the murine CNS in a sex-independent manner. J Neurosci Res. (2020);98:2045–2071. doi: 10.1002/jnr.24651. [DOI] [PubMed] [Google Scholar]
- 32.DiTirro D, Philbrook A, Rubino K, Sengupta P. The Caenorhabditis elegans Tubby homolog dynamically modulates olfactory cilia membrane morphogenesis and phospholipid composition. Elife. (2019);8:e48789. doi: 10.7554/eLife.48789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dummer A, Poelma C, DeRuiter MC, Goumans MJ, Hierck BP. Measuring the primary cilium length:improved method for unbiased high-throughput analysis. Cilia. (2016);5:7. doi: 10.1186/s13630-016-0028-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Engle SE, Bansal R, Antonellis PJ, Berbari NF. Cilia signaling and obesity. Semin Cell Dev Biol. (2021);110:43–50. doi: 10.1016/j.semcdb.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Espinosa R, Gutierrez K, Rios J, Ormeno F, Yanten L, Galaz-Davison P, Ramirez-Sarmiento CA, Parra V, Albornoz A, Alfaro IE, Burgos PV, Morselli E, Criollo A, Budini M. Palmitic and stearic acids inhibit chaperone-mediated autophagy (CMA) in POMC-like neurons in vitro. Cells. (2022);11:920. doi: 10.3390/cells11060920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Franco I, Gulluni F, Campa CC, Costa C, Margaria JP, Ciraolo E, Martini M, Monteyne D, De Luca E, Germena G, Posor Y, Maffucci T, Marengo S, Haucke V, Falasca M, Perez-Morga D, Boletta A, Merlo GR, Hirsch E. PI3K class II alpha controls spatially restricted endosomal PtdIns3P and Rab11 activation to promote primary cilium function. Dev Cell. (2014);28:647–658. doi: 10.1016/j.devcel.2014.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Franco I, Margaria JP, De Santis MC, Ranghino A, Monteyne D, Chiaravalli M, Pema M, Campa CC, Ratto E, Gulluni F, Perez-Morga D, Somlo S, Merlo GR, Boletta A, Hirsch E. Phosphoinositide 3-kinase-C2alpha regulates polycystin-2 ciliary entry and protects against kidney cyst formation. J Am Soc Nephrol. (2016);27:1135–1144. doi: 10.1681/ASN.2014100967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Friedman LG, Lachenmayer ML, Wang J, He L, Poulose SM, Komatsu M, Holstein GR, Yue Z. Disrupted autophagy leads to dopaminergic axon and dendrite degeneration and promotes presynaptic accumulation of alpha-synuclein and LRRK2 in the brain. J Neurosci. (2012);32:7585–7593. doi: 10.1523/JNEUROSCI.5809-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fukui K, Ferris HA, Kahn CR. Effect of cholesterol reduction on receptor signaling in neurons. J Biol Chem. (2015);290:26383–26392. doi: 10.1074/jbc.M115.664367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Funfschilling U, Jockusch WJ, Sivakumar N, Mobius W, Corthals K, Li S, Quintes S, Kim Y, Schaap IA, Rhee JS, Nave KA, Saher G. Critical time window of neuronal cholesterol synthesis during neurite outgrowth. J Neurosci. (2012);32:7632–7645. doi: 10.1523/JNEUROSCI.1352-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gahbauer S, Böckmann RA. Membrane-mediated oligomerization of G protein coupled receptors and its implications for GPCR function. Front Physiol. (2016);7:494. doi: 10.3389/fphys.2016.00494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garcia-Gonzalo FR, Phua SC, Roberson EC, Garcia G, 3rd, Abedin M, Schurmans S, Inoue T, Reiter JF. Phosphoinositides regulate ciliary protein trafficking to modulate hedgehog signaling. Dev Cell. (2015);34:400–409. doi: 10.1016/j.devcel.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gijs HL, Willemarck N, Vanderhoydonc F, Khan NA, Dehairs J, Derua R, Waelkens E, Taketomi Y, Murakami M, Agostinis P, Annaert W, Swinnen JV. Primary cilium suppression by SREBP1c involves distortion of vesicular trafficking by PLA2G3. Mol Biol Cell. (2015);26:2321–2332. doi: 10.1091/mbc.E14-10-1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goetz SC, Anderson KV. The primary cilium:a signalling centre during vertebrate development. Nat Rev Genet. (2010);11:331–344. doi: 10.1038/nrg2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guemez-Gamboa A, Coufal NG, Gleeson JG. Primary cilia in the developing and mature brain. Neuron. (2014);82:511–521. doi: 10.1016/j.neuron.2014.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guo J, Otis JM, Suciu SK, Catalano C, Xing L, Constable S, Wachten D, Gupton S, Lee J, Lee A, Blackley KH, Ptacek T, Simon JM, Schurmans S, Stuber GD, Caspary T, Anton ES. Primary cilia signaling promotes axonal tract development and is disrupted in Joubert Syndrome-related disorders models. Dev Cell. (2019);51:759–774. doi: 10.1016/j.devcel.2019.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Handel M, Schulz S, Stanarius A, Schreff M, Erdtmann-Vourliotis M, Schmidt H, Wolf G, Hollt V. Selective targeting of somatostatin receptor 3 to neuronal cilia. Neuroscience. (1999);89:909–926. doi: 10.1016/s0306-4522(98)00354-6. [DOI] [PubMed] [Google Scholar]
- 48.Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, Yokoyama M, Mishima K, Saito I, Okano H, Mizushima N. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. (2006);441:885–889. doi: 10.1038/nature04724. [DOI] [PubMed] [Google Scholar]
- 49.He Q, Wang G, Dasgupta S, Dinkins M, Zhu G, Bieberich E. Characterization of an apical ceramide-enriched compartment regulating ciliogenesis. Mol Biol Cell. (2012);23:3156–3166. doi: 10.1091/mbc.E12-02-0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.He W, Tran A, Chen CT, Loganathan N, Bazinet RP, Belsham DD. Oleate restores altered autophagic flux to rescue palmitate lipotoxicity in hypothalamic neurons. Mol Cell Endocrinol. (2022);557:111753. doi: 10.1016/j.mce.2022.111753. [DOI] [PubMed] [Google Scholar]
- 51.Hernandez D, Torres CA, Setlik W, Cebrian C, Mosharov EV, Tang G, Cheng HC, Kholodilov N, Yarygina O, Burke RE, Gershon M, Sulzer D. Regulation of presynaptic neurotransmission by macroautophagy. Neuron. (2012);74:277–284. doi: 10.1016/j.neuron.2012.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hernandez-Caceres MP, Toledo-Valenzuela L, Diaz-Castro F, Avalos Y, Burgos P, Narro C, Pena-Oyarzun D, Espinoza-Caicedo J, Cifuentes-Araneda F, Navarro-Aguad F, Riquelme C, Troncoso R, Criollo A, Morselli E. Palmitic acid reduces the autophagic flux and insulin sensitivity through the activation of the free fatty acid receptor 1 (FFAR1) in the hypothalamic neuronal cell line N43/5. Front Endocrinol (Lausanne) (2019);10:176. doi: 10.3389/fendo.2019.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hernandez-Caceres MP, Cereceda K, Hernandez S, Li Y, Narro C, Rivera P, Silva P, Avalos Y, Jara C, Burgos P, Toledo-Valenzuela L, Lagos P, Cifuentes Araneda F, Perez-Leighton C, Bertocchi C, Clegg DJ, Criollo A, Tapia-Rojas C, Burgos PV, Morselli E. Palmitic acid reduces the autophagic flux in hypothalamic neurons by impairing autophagosome-lysosome fusion and endolysosomal dynamics. Mol Cell Oncol. (2020);7:1789418. doi: 10.1080/23723556.2020.1789418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Higginbotham H, Eom TY, Mariani LE, Bachleda A, Hirt J, Gukassyan V, Cusack CL, Lai C, Caspary T, Anton ES. Arl13b in primary cilia regulates the migration and placement of interneurons in the developing cerebral cortex. Dev Cell. (2012);23:925–938. doi: 10.1016/j.devcel.2012.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ho G, MacKenzie RG. Functional characterization of mutations in melanocortin-4 receptor associated with human obesity. J Biol Chem. (1999);274:35816–35822. doi: 10.1074/jbc.274.50.35816. [DOI] [PubMed] [Google Scholar]
- 56.Huangfu D, Anderson KV. Cilia and Hedgehog responsiveness in the mouse. Proc Natl Acad Sci U S A. (2005);102:11325–11330. doi: 10.1073/pnas.0505328102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ilnytska O, Lai K, Gorshkov K, Schultz ML, Tran BN, Jeziorek M, Kunkel TJ, Azaria RD, McLoughlin HS, Waghalter M, Xu Y, Schlame M, Altan-Bonnet N, Zheng W, Lieberman AP, Dobrowolski R, Storch J. Enrichment of NPC1-deficient cells with the lipid LBPA stimulates autophagy, improves lysosomal function, and reduces cholesterol storage. J Biol Chem. (2021);297:100813. doi: 10.1016/j.jbc.2021.100813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jacoby M, Cox JJ, Gayral S, Hampshire DJ, Ayub M, Blockmans M, Pernot E, Kisseleva MV, Compère P, Schiffmann SN, Gergely F, Riley JH, Pérez-Morga D, Woods CG, Schurmans S. INPP5E mutations cause primary cilium signaling defects, ciliary instability and ciliopathies in human and mouse. Nat Genet. (2009);41:1027–1031. doi: 10.1038/ng.427. [DOI] [PubMed] [Google Scholar]
- 59.Janne PA, Suchy SF, Bernard D, MacDonald M, Crawley J, Grinberg A, Wynshaw-Boris A, Westphal H, Nussbaum RL. Functional overlap between murine Inpp5b and Ocrl1 may explain why deficiency of the murine ortholog for OCRL1 does not cause Lowe syndrome in mice. J Clin Invest. (1998);101:2042–2053. doi: 10.1172/JCI2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jing YH, Zhang L, Gao LP, Qi CC, Lv DD, Song YF, Yin J, Wang DG. Autophagy plays beneficial effect on diabetic encephalopathy in type 2 diabetes:studies in vivo and in vitro. Neuro Endocrinol Lett. (2017);38:27–37. [PubMed] [Google Scholar]
- 61.Kanamaru T, Neuner A, Kurtulmus B, Pereira G. Balancing the length of the distal tip by septins is key for stability and signalling function of primary cilia. EMBO J. (2022);41:e108843. doi: 10.15252/embj.2021108843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kaneshiro ES, Matesic DF, Jayasimhulu K. Characterizations of six ethanolamine sphingophospholipids from Paramecium cells and cilia. J Lipid Res. (1984);25:369–377. [PubMed] [Google Scholar]
- 63.Kaushik S, Rodriguez-Navarro JA, Arias E, Kiffin R, Sahu S, Schwartz GJ, Cuervo AM, Singh R. Autophagy in hypothalamic AgRP neurons regulates food intake and energy balance. Cell Metab. (2011);14:173–183. doi: 10.1016/j.cmet.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kelley RL, Roessler E, Hennekam RC, Feldman GL, Kosaki K, Jones MC, Palumbos JC, Muenke M. Holoprosencephaly in RSH/Smith-Lemli-Opitz syndrome:does abnormal cholesterol metabolism affect the function of Sonic Hedgehog? Am J Med Genet. (1996);66:478–484. doi: 10.1002/(SICI)1096-8628(19961230)66:4<478::AID-AJMG22>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 65.Keryer G, Pineda JR, Liot G, Kim J, Dietrich P, Benstaali C, Smith K, Cordelières FP, Spassky N, Ferrante RJ, Dragatsis I, Saudou F. Ciliogenesis is regulated by a huntingtin-HAP1-PCM1 pathway and is altered in Huntington disease. J Clin Invest. (2011);121:4372–4382. doi: 10.1172/JCI57552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Klena N, Pigino G. Structural biology of cilia and intraflagellar transport. Annu Rev Cell Dev Biol. (2022);38:103–123. doi: 10.1146/annurev-cellbio-120219-034238. [DOI] [PubMed] [Google Scholar]
- 67.Kobayashi Y, Kohbuchi S, Koganezawa N, Sekino Y, Shirao T, Saido TC, Saito T, Saito Y. Impairment of ciliary dynamics in an APP knock-in mouse model of Alzheimer's disease. Biochem Biophys Res Commun. (2022);610:85–91. doi: 10.1016/j.bbrc.2022.04.050. [DOI] [PubMed] [Google Scholar]
- 68.Komatsu M, Kominami E, Tanaka K. Autophagy and neurodegeneration. Autophagy. (2006a);2:315–317. doi: 10.4161/auto.2974. [DOI] [PubMed] [Google Scholar]
- 69.Komatsu M, Waguri S, Chiba T, Murata S, Iwata J, Tanida I, Ueno T, Koike M, Uchiyama Y, Kominami E, Tanaka K. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature. (2006b);441:880–884. doi: 10.1038/nature04723. [DOI] [PubMed] [Google Scholar]
- 70.Komatsu M, Wang QJ, Holstein GR, Friedrich VL, Jr, Iwata J, Kominami E, Chait BT, Tanaka K, Yue Z. Essential role for autophagy protein Atg7 in the maintenance of axonal homeostasis and the prevention of axonal degeneration. Proc Natl Acad Sci U S A. (2007);104:14489–14494. doi: 10.1073/pnas.0701311104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kowatsch C, Woolley RE, Kinnebrew M, Rohatgi R, Siebold C. Structures of vertebrate patched and smoothened reveal intimate links between cholesterol and Hedgehog signalling. Curr Opin Struct Biol. (2019);57:204–214. doi: 10.1016/j.sbi.2019.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kumari S, Kumar A, Sardar P, Yadav M, Majhi RK, Kumar A, Goswami C. Influence of membrane cholesterol in the molecular evolution and functional regulation of TRPV4. Biochem Biophys Res Commun. (2015);456:312–319. doi: 10.1016/j.bbrc.2014.11.077. [DOI] [PubMed] [Google Scholar]
- 73.Larsen LJ, Møller LB. Crosstalk of Hedgehog and mTORC1 pathways. Cells. (2020);9:2316. doi: 10.3390/cells9102316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Law SH, Chan ML, Marathe GK, Parveen F, Chen CH, Ke LY. An updated review of lysophosphatidylcholine metabolism in human diseases. Int J Mol Sci. (2019);20:1149. doi: 10.3390/ijms20051149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lee CH, Song DK, Park CB, Choi J, Kang GM, Shin SH, Kwon I, Park S, Kim S, Kim JY, Dugu H, Park JW, Choi JH, Min SH, Sohn JW, Kim MS. Primary cilia mediate early life programming of adiposity through lysosomal regulation in the developing mouse hypothalamus. Nat Commun. (2020);11:5772. doi: 10.1038/s41467-020-19638-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lee S, Sato Y, Nixon RA. Primary lysosomal dysfunction causes cargo-specific deficits of axonal transport leading to Alzheimer-like neuritic dystrophy. Autophagy. (2011);7:1562–1563. doi: 10.4161/auto.7.12.17956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liao G, Yao Y, Liu J, Yu Z, Cheung S, Xie A, Liang X, Bi X. Cholesterol accumulation is associated with lysosomal dysfunction and autophagic stress in Npc1 -/- mouse brain. Am J Pathol. (2007);171:962–975. doi: 10.2353/ajpath.2007.070052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lubrano-Berthelier C, Cavazos M, Dubern B, Shapiro A, Stunff CL, Zhang S, Picart F, Govaerts C, Froguel P, Bougneres P, Clement K, Vaisse C. Molecular genetics of human obesity-associated MC4R mutations. Ann N Y Acad Sci. (2003);994:49–57. doi: 10.1111/j.1749-6632.2003.tb03161.x. [DOI] [PubMed] [Google Scholar]
- 79.Lucarelli M, Di Pietro C, La Sala G, Fiorenza MT, Marazziti D, Canterini S. Anomalies in dopamine transporter expression and primary cilium distribution in the dorsal striatum of a mouse model of Niemann-Pick C1 disease. Front Cell Neurosci. (2019);13:226. doi: 10.3389/fncel.2019.00226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Luo N, West CC, Murga-Zamalloa CA, Sun L, Anderson RM, Wells CD, Weinreb RN, Travers JB, Khanna H, Sun Y. OCRL localizes to the primary cilium:a new role for cilia in Lowe syndrome. Hum Mol Genet. (2012);21:3333–3344. doi: 10.1093/hmg/dds163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Luo N, Kumar A, Conwell M, Weinreb RN, Anderson R, Sun Y. Compensatory role of inositol 5-phosphatase INPP5B to OCRL in primary cilia formation in oculocerebrorenal syndrome of Lowe. PLoS One. (2013);8:e66727. doi: 10.1371/journal.pone.0066727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ma R, Kutchy NA, Chen L, Meigs DD, Hu G. Primary cilia and ciliary signaling pathways in aging and age-related brain disorders. Neurobiol Dis. (2022);163:105607. doi: 10.1016/j.nbd.2021.105607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Madison BB. Srebp2:A master regulator of sterol and fatty acid synthesis. J Lipid Res. (2016);57:333–335. doi: 10.1194/jlr.C066712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mansini AP, Lorenzo Pisarello MJ, Thelen KM, Cruz-Reyes M, Peixoto E, Jin S, Howard BN, Trussoni CE, Gajdos GB, LaRusso NF, Perugorria MJ, Banales JM, Gradilone SA. MicroRNA (miR)-433 and miR-22 dysregulations induce histone-deacetylase-6 overexpression and ciliary loss in cholangiocarcinoma. Hepatology. (2018);68:561–573. doi: 10.1002/hep.29832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Marin R, Fabelo N, Martin V, Garcia-Esparcia P, Ferrer I, Quinto-Alemany D, Diaz M. Anomalies occurring in lipid profiles and protein distribution in frontal cortex lipid rafts in dementia with Lewy bodies disclose neurochemical traits partially shared by Alzheimer's and Parkinson's diseases. Neurobiol Aging. (2017);49:52–59. doi: 10.1016/j.neurobiolaging.2016.08.027. [DOI] [PubMed] [Google Scholar]
- 86.Martin DD, Ladha S, Ehrnhoefer DE, Hayden MR. Autophagy in Huntington disease and huntingtin in autophagy. Trends Neurosci. (2015);38:26–35. doi: 10.1016/j.tins.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 87.Martinez-Vicente M, Talloczy Z, Wong E, Tang G, Koga H, Kaushik S, de Vries R, Arias E, Harris S, Sulzer D, Cuervo AM. Cargo recognition failure is responsible for inefficient autophagy in Huntington's disease. Nat Neurosci. (2010);13:567–576. doi: 10.1038/nn.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Maulik M, Peake K, Chung J, Wang Y, Vance JE, Kar S. APP overexpression in the absence of NPC1 exacerbates metabolism of amyloidogenic proteins of Alzheimer's disease. Hum Mol Genet. (2015);24:7132–7150. doi: 10.1093/hmg/ddv413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mesa-Herrera F, Taoro-Gonzalez L, Valdes-Baizabal C, Diaz M, Marin R. Lipid and lipid raft alteration in aging and neurodegenerative diseases:a window for the development of new biomarkers. Int J Mol Sci. (2019);20:3810. doi: 10.3390/ijms20153810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Meske V, Erz J, Priesnitz T, Ohm TG. The autophagic defect in Niemann-Pick disease type C neurons differs from somatic cells and reduces neuronal viability. Neurobiol Dis. (2014);64:88–97. doi: 10.1016/j.nbd.2013.12.018. [DOI] [PubMed] [Google Scholar]
- 91.Miyoshi K, Kasahara K, Murakami S, Takeshima M, Kumamoto N, Sato A, Miyazaki I, Matsuzaki S, Sasaoka T, Katayama T, Asanuma M. Lack of dopaminergic inputs elongates the primary cilia of striatal neurons. PLoS One. (2014);9:e97918. doi: 10.1371/journal.pone.0097918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Montesano R. Inhomogeneous distribution of filipin-sterol complexes in the ciliary membrane of rat tracheal epithelium. Am J Anat. (1979);156:139–145. doi: 10.1002/aja.1001560115. [DOI] [PubMed] [Google Scholar]
- 93.Moreau K, Fleming A, Imarisio S, Lopez Ramirez A, Mercer JL, Jimenez-Sanchez M, Bento CF, Puri C, Zavodszky E, Siddiqi F, Lavau CP, Betton M, O'Kane CJ, Wechsler DS, Rubinsztein DC. PICALM modulates autophagy activity and tau accumulation. Nat Commun. (2014);5:4998. doi: 10.1038/ncomms5998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Morselli E, Maiuri MC, Markaki M, Megalou E, Pasparaki A, Palikaras K, Criollo A, Galluzzi L, Malik SA, Vitale I, Michaud M, Madeo F, Tavernarakis N, Kroemer G. Caloric restriction and resveratrol promote longevity through the Sirtuin-1-dependent induction of autophagy. Cell Death Dis. (2010);1:e10. doi: 10.1038/cddis.2009.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mota-Martorell N, Andres-Benito P, Martin-Gari M, Galo-Licona JD, Sol J, Fernandez-Bernal A, Portero-Otin M, Ferrer I, Jove M, Pamplona R. Selective brain regional changes in lipid profile with human aging. Geroscience. (2022);44:763–783. doi: 10.1007/s11357-022-00527-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mukhopadhyay S, Wen X, Ratti N, Loktev A, Rangell L, Scales SJ, Jackson PK. The ciliary G-protein-coupled receptor Gpr161 negatively regulates the Sonic hedgehog pathway via cAMP signaling. Cell. (2013);152:210–223. doi: 10.1016/j.cell.2012.12.026. [DOI] [PubMed] [Google Scholar]
- 97.Nachury MV, Mick DU. Establishing and regulating the composition of cilia for signal transduction. Nat Rev Mol Cell Biol. (2019);20:389–405. doi: 10.1038/s41580-019-0116-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Narkis G, Ofir R, Landau D, Manor E, Volokita M, Hershkowitz R, Elbedour K, Birk OS. Lethal contractural syndrome type 3 (LCCS3) is caused by a mutation in PIP5K1C, which encodes PIPKI gamma of the phophatidylinsitol pathway. Am J Hum Genet. (2007);81:530–539. doi: 10.1086/520771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Naudi A, Cabre R, Jove M, Ayala V, Gonzalo H, Portero-Otin M, Ferrer I, Pamplona R. Lipidomics of human brain aging and Alzheimer's disease pathology. Int Rev Neurobiol. (2015);122:133–189. doi: 10.1016/bs.irn.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 100.Nechipurenko IV. The enigmatic role of lipids in cilia signaling. Front Cell Dev Biol. (2020);8:777. doi: 10.3389/fcell.2020.00777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nguyen KQ, Rymar VV, Sadikot AF. Impaired TrkB signaling underlies reduced BDNF-mediated trophic support of striatal neurons in the R6/2 mouse model of Huntington's disease. Front Cell Neurosci. (2016);10:37. doi: 10.3389/fncel.2016.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nino-Rivero S, Torterolo P, Lagos P. Melanin-concentrating hormone receptor-1 is located in primary cilia of the dorsal raphe neurons. J Chem Neuroanat. (2019);98:55–62. doi: 10.1016/j.jchemneu.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 103.Nusca S, Canterini S, Palladino G, Bruno F, Mangia F, Erickson RP, Fiorenza MT. A marked paucity of granule cells in the developing cerebellum of the Npc1(-/-) mouse is corrected by a single injection of hydroxypropyl-β-cyclodextrin. Neurobiol Dis. (2014);70:117–126. doi: 10.1016/j.nbd.2014.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Oh TS, Cho H, Cho JH, Yu SW, Kim EK. Hypothalamic AMPK-induced autophagy increases food intake by regulating NPY and POMC expression. Autophagy. (2016);12:2009–2025. doi: 10.1080/15548627.2016.1215382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ordonez MP, Roberts EA, Kidwell CU, Yuan SH, Plaisted WC, Goldstein LS. Disruption and therapeutic rescue of autophagy in a human neuronal model of Niemann Pick type C1. Hum Mol Genet. (2012);21:2651–2662. doi: 10.1093/hmg/dds090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pampliega O, Orhon I, Patel B, Sridhar S, Díaz-Carretero A, Beau I, Codogno P, Satir BH, Satir P, Cuervo AM. Functional interaction between autophagy and ciliogenesis. Nature. (2013);502:194–200. doi: 10.1038/nature12639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pampliega O, Cuervo AM. Autophagy and primary cilia:dual interplay. Curr Opin Cell Biol. (2016);39:1–7. doi: 10.1016/j.ceb.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Park J, Lee N, Kavoussi A, Seo JT, Kim CH, Moon SJ. Ciliary phosphoinositide regulates ciliary protein trafficking in Drosophila. Cell Rep. (2015);13:2808–2816. doi: 10.1016/j.celrep.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 109.Park SM, Lim JS, Ramakrishina S, Kim SH, Kim WK, Lee J, Kang HC, Reiter JF, Kim DS, Kim HH, Lee JH. Brain somatic mutations in MTOR disrupt neuronal ciliogenesis, leading to focal cortical dyslamination. Neuron. (2018);99:83–97. doi: 10.1016/j.neuron.2018.05.039. [DOI] [PubMed] [Google Scholar]
- 110.Phua SC, Chiba S, Suzuki M, Su E, Roberson EC, Pusapati GV, Schurmans S, Setou M, Rohatgi R, Reiter JF, Ikegami K, Inoue T. Dynamic remodeling of membrane composition drives cell cycle through primary cilia excision. Cell. (2017);168:264–279. doi: 10.1016/j.cell.2016.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Portovedo M, Ignacio-Souza LM, Bombassaro B, Coope A, Reginato A, Razolli DS, Torsoni MA, Torsoni AS, Leal RF, Velloso LA, Milanski M. Saturated fatty acids modulate autophagy's proteins in the hypothalamus. PLoS One. (2015);10:e0119850. doi: 10.1371/journal.pone.0119850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Praetorius HA, Spring KR. A physiological view of the primary cilium. Annu Rev Physiol. (2005);67:515–529. doi: 10.1146/annurev.physiol.67.040403.101353. [DOI] [PubMed] [Google Scholar]
- 113.Prosseda PP, Luo N, Wang B, Alvarado JA, Hu Y, Sun Y. Loss of OCRL increases ciliary PI(4,5)P(2) in Lowe oculocerebrorenal syndrome. J Cell Sci. (2017);130:3447–3454. doi: 10.1242/jcs.200857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Quan W, Kim HK, Moon EY, Kim SS, Choi CS, Komatsu M, Jeong YT, Lee MK, Kim KW, Kim MS, Lee MS. Role of hypothalamic proopiomelanocortin neuron autophagy in the control of appetite and leptin response. Endocrinology. (2012);153:1817–1826. doi: 10.1210/en.2011-1882. [DOI] [PubMed] [Google Scholar]
- 115.Reginato A, Siqueira BP, Miyamoto JE, Portovedo M, Costa SO, de Fante T, Rodrigues HG, Ignacio-Souza LM, Torsoni MA, Torsoni AS, Le Stunff H, Belsham DD, Milanski M. Acute effects of fatty acids on autophagy in NPY neurones. J Neuroendocrinol. (2020);32:e12900. doi: 10.1111/jne.12900. [DOI] [PubMed] [Google Scholar]
- 116.Rhee S, Kirschen GW, Gu Y, Ge S. Depletion of primary cilia from mature dentate granule cells impairs hippocampus-dependent contextual memory. Sci Rep. (2016);6:34370. doi: 10.1038/srep34370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Roca-Agujetas V, de Dios C, Abadin X, Colell A. Upregulation of brain cholesterol levels inhibits mitophagy in Alzheimer disease. Autophagy. (2021);17:1555–1557. doi: 10.1080/15548627.2021.1920814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Roney JC, Li S, Farfel-Becker T, Huang N, Sun T, Xie Y, Cheng XT, Lin MY, Platt FM, Sheng ZH. Lipid-mediated motor-adaptor sequestration impairs axonal lysosome delivery leading to autophagic stress and dystrophy in Niemann-Pick type C. Dev Cell. (2021);56:1452–1468. doi: 10.1016/j.devcel.2021.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Schou KB, Mogensen JB, Morthorst SK, Nielsen BS, Aleliunaite A, Serra-Marques A, Fürstenberg N, Saunier S, Bizet AA, Veland IR, Akhmanova A, Christensen ST, Pedersen LB. KIF13B establishes a CAV1-enriched microdomain at the ciliary transition zone to promote Sonic hedgehog signalling. Nat Commun. (2017);8:14177. doi: 10.1038/ncomms14177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Seo S, Guo DF, Bugge K, Morgan DA, Rahmouni K, Sheffield VC. Requirement of Bardet-Biedl syndrome proteins for leptin receptor signaling. Hum Mol Genet. (2009);18:1323–1331. doi: 10.1093/hmg/ddp031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Shapira KE, Shapira G, Schmukler E, Pasmanik-Chor M, Shomron N, Pinkas-Kramarski R, Henis YI, Ehrlich M. Autophagy is induced and modulated by cholesterol depletion through transcription of autophagy-related genes and attenuation of flux. Cell Death Discov. (2021);7:320. doi: 10.1038/s41420-021-00718-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sheu SH, Upadhyayula S, Dupuy V, Pang S, Deng F, Wan J, Walpita D, Pasolli HA, Houser J, Sanchez-Martinez S, Brauchi SE, Banala S, Freeman M, Xu CS, Kirchhausen T, Hess HF, Lavis L, Li Y, Chaumont-Dubel S, Clapham DE. A serotonergic axon-cilium synapse drives nuclear signaling to alter chromatin accessibility. Cell. (2022);185:3390–3407. doi: 10.1016/j.cell.2022.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sighinolfi G, Clark S, Blanc L, Cota D, Rhourri-Frih B. Mass spectrometry imaging of mice brain lipid profile changes over time under high fat diet. Sci Rep. (2021);11:19664. doi: 10.1038/s41598-021-97201-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Siljee JE, Wang Y, Bernard AA, Ersoy BA, Zhang S, Marley A, Von Zastrow M, Reiter JF, Vaisse C. Subcellular localization of MC4R with ADCY3 at neuronal primary cilia underlies a common pathway for genetic predisposition to obesity. Nat Genet. (2018);50:180–185. doi: 10.1038/s41588-017-0020-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Singh R, Kaushik S, Wang Y, Xiang Y, Novak I, Komatsu M, Tanaka K, Cuervo AM, Czaja MJ. Autophagy regulates lipid metabolism. Nature. (2009);458:1131–1135. doi: 10.1038/nature07976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Souto-Padrón T, de Souza W. Freeze-fracture localization of filipin-cholesterol complexes in the plasma membrane of Trypanosoma cruzi. J Parasitol. (1983);69:129–137. [PubMed] [Google Scholar]
- 127.Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. (1997);388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 128.Stamatakou E, Wrobel L, Hill SM, Puri C, Son SM, Fujimaki M, Zhu Y, Siddiqi F, Fernandez-Estevez M, Manni MM, Park SJ, Villeneuve J, Rubinsztein DC. Mendelian neurodegenerative disease genes involved in autophagy. Cell Discov. (2020);6:24. doi: 10.1038/s41421-020-0158-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Stavoe AKH, Holzbaur ELF. Autophagy in neurons. Annu Rev Cell Dev Biol. (2019);35:477–500. doi: 10.1146/annurev-cellbio-100818-125242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Stilling S, Kalliakoudas T, Benninghoven-Frey H, Inoue T, Falkenburger BH. PIP(2) determines length and stability of primary cilia by balancing membrane turnovers. Commun Biol. (2022);5:93. doi: 10.1038/s42003-022-03028-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Stoufflet J, Chaulet M, Doulazmi M, Fouquet C, Dubacq C, Metin C, Schneider-Maunoury S, Trembleau A, Vincent P, Caille I. Primary cilium-dependent cAMP/PKA signaling at the centrosome regulates neuronal migration. Sci Adv. (2020);6:eaba3992. doi: 10.1126/sciadv.aba3992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Suzuki A, Shim J, Ogata K, Yoshioka H, Iwata J. Cholesterol metabolism plays a crucial role in the regulation of autophagy for cell differentiation of granular convoluted tubules in male mouse submandibular glands. Development. (2019);146:dev178335. doi: 10.1242/dev.178335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Svennerholm L, Bostrom K, Jungbjer B. Changes in weight and compositions of major membrane components of human brain during the span of adult human life of Swedes. Acta Neuropathol. (1997);94:345–352. doi: 10.1007/s004010050717. [DOI] [PubMed] [Google Scholar]
- 134.Tabrizi SJ, Flower MD, Ross CA, Wild EJ. Huntington disease:new insights into molecular pathogenesis and therapeutic opportunities. Nat Rev Neurol. (2020);16:529–546. doi: 10.1038/s41582-020-0389-4. [DOI] [PubMed] [Google Scholar]
- 135.Tang Z, Lin MG, Stowe TR, Chen S, Zhu M, Stearns T, Franco B, Zhong Q. Autophagy promotes primary ciliogenesis by removing OFD1 from centriolar satellites. Nature. (2013);502:254–257. doi: 10.1038/nature12606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tereshko L, Gao Y, Cary BA, Turrigiano GG, Sengupta P. Ciliary neuropeptidergic signaling dynamically regulates excitatory synapses in postnatal neocortical pyramidal neurons. Elife. (2021);10:e65427. doi: 10.7554/eLife.65427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tereshko L, Turrigiano GG, Sengupta P. Primary cilia in the postnatal brain:subcellular compartments for organizing neuromodulatory signaling. Curr Opin Neurobiol. (2022);74:102533. doi: 10.1016/j.conb.2022.102533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Torres S, Balboa E, Zanlungo S, Enrich C, Garcia-Ruiz C, Fernandez-Checa JC. Lysosomal and mitochondrial liaisons in Niemann-Pick disease. Front Physiol. (2017);8:982. doi: 10.3389/fphys.2017.00982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ukhanov K, Uytingco C, Green W, Zhang L, Schurmans S, Martens JR. INPP5E controls ciliary localization of phospholipids and the odor response in olfactory sensory neurons. J Cell Sci. (2022);135:jcs258364. doi: 10.1242/jcs.258364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Vance JE. Dysregulation of cholesterol balance in the brain:contribution to neurodegenerative diseases. Dis Model Mech. (2012);5:746–755. doi: 10.1242/dmm.010124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Vanier MT. Niemann-Pick disease type C. Orphanet J Rare Dis. (2010);5:16. doi: 10.1186/1750-1172-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Verhey KJ, Yang W. Permeability barriers for generating a unique ciliary protein and lipid composition. Curr Opin Cell Biol. (2016);41:109–116. doi: 10.1016/j.ceb.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Vijayan V, Verstreken P. Autophagy in the presynaptic compartment in health and disease. J Cell Biol. (2017);216:1895–1906. doi: 10.1083/jcb.201611113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wang T, Zhang X, Wang Y, Liu W, Wang L, Hao L, Ju M, Xiao R. High cholesterol and 27-hydroxycholesterol contribute to phosphorylation of tau protein by impairing autophagy causing learning and memory impairment in C57BL/6J mice. J Nutr Biochem. (2022);106:109016. doi: 10.1016/j.jnutbio.2022.109016. [DOI] [PubMed] [Google Scholar]
- 145.Wang Y, Bernard A, Comblain F, Yue X, Paillart C, Zhang S, Reiter JF, Vaisse C. Melanocortin 4 receptor signals at the neuronal primary cilium to control food intake and body weight. J Clin Invest. (2021);131:e142064. doi: 10.1172/JCI142064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wang Z, Phan T, Storm DR. The type 3 adenylyl cyclase is required for novel object learning and extinction of contextual memory:role of cAMP signaling in primary cilia. J Neurosci. (2011);31:5557–5561. doi: 10.1523/JNEUROSCI.6561-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Xu Q, Zhang Y, Wei Q, Huang Y, Hu J, Ling K. Phosphatidylinositol phosphate kinase PIPKIγand phosphatase INPP5E coordinate initiation of ciliogenesis. Nat Commun. (2016);7:10777. doi: 10.1038/ncomms10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Yang D, Wu X, Wang W, Zhou Y, Wang Z. Ciliary type III adenylyl cyclase in the VMH is crucial for high-fat diet-induced obesity mediated by autophagy. Adv Sci (Weinh) (2022);9:e2102568. doi: 10.1002/advs.202102568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yang DJ, Hong J, Kim KW. Hypothalamic primary cilium:a hub for metabolic homeostasis. Exp Mol Med. (2021);53:1109–1115. doi: 10.1038/s12276-021-00644-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yen HY, Hoi KK, Liko I, Hedger G, Horrell MR, Song W, Wu D, Heine P, Warne T, Lee Y, Carpenter B, Pluckthun A, Tate CG, Sansom MSP, Robinson CV. PtdIns(4,5)P(2) stabilizes active states of GPCRs and enhances selectivity of G-protein coupling. Nature. (2018);559:423–427. doi: 10.1038/s41586-018-0325-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Youn YH, Han YG. Primary cilia in brain development and diseases. Am J Pathol. (2018);188:11–22. doi: 10.1016/j.ajpath.2017.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zhang Y, Bulkley DP, Xin Y, Roberts KJ, Asarnow DE, Sharma A, Myers BR, Cho W, Cheng Y, Beachy PA. Structural basis for cholesterol transport-like activity of the Hedgehog receptor patched. Cell. (2018);175:1352–1364. doi: 10.1016/j.cell.2018.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]