Abstract
Epilepsy is a neurological disorder characterized by high morbidity, high recurrence, and drug resistance. Enhanced signaling through the excitatory neurotransmitter glutamate is intricately associated with epilepsy. Metabotropic glutamate receptors (mGluRs) are G protein-coupled receptors activated by glutamate and are key regulators of neuronal and synaptic plasticity. Dysregulated mGluR signaling has been associated with various neurological disorders, and numerous studies have shown a close relationship between mGluRs expression/activity and the development of epilepsy. In this review, we first introduce the three groups of mGluRs and their associated signaling pathways. Then, we detail how these receptors influence epilepsy by describing the signaling cascades triggered by their activation and their neuroprotective or detrimental roles in epileptogenesis. In addition, strategies for pharmacological manipulation of these receptors during the treatment of epilepsy in experimental studies is also summarized. We hope that this review will provide a foundation for future studies on the development of mGluR-targeted antiepileptic drugs.
Keywords: antiepileptic drugs, epileptogenesis, metabotropic glutamate receptors (mGluRs), signal pathways, therapeutic potentials
Introduction
Epilepsy is a devastating neurological and systemic disorder characterized by recurrent seizures that is likely to cause physical injury and death if not treated optimally. Epilepsy is estimated to affect about 65 million people worldwide, and its pathogenesis is thought to be related to a relative imbalance of excitatory and inhibitory neurotransmitters (Akyuz et al., 2021). Glutamate is present in the mammalian central nervous system (CNS) as an important excitatory neurotransmitter (Meldrum et al., 1999; Nicolo et al., 2019; Servaes et al., 2019) involved in the regulation of various neuronal and synaptic activities through the activation of two types of receptors, i.e. ionotropic and metabotropic glutamate receptors (mGluRs) (Luessen and Conn, 2022). Both receptor types are essential for the regulation of neuronal development, synaptic transmission, and overall brain function (Abd-Elrahman and Ferguson, 2022), therefore influencing sensory and motor processing, cognition, memory formation, pain, addiction, and behavior (Cleva and Olive, 2012; Guo et al., 2018; Seven et al., 2021; Liang et al., 2022). The mGluR family comprises membrane-bound proteins that transmit extracellular signals into cells; they have been shown to regulate, in addition to physiological processes, many aspects of tumor development and progression (Eddy et al., 2022). mGluRs are widely distributed on cell surfaces in all major regions of the brain, both in neuronal and non-neuronal cells, and regulate neuronal excitability and synaptic transmission primarily through the activation of G protein-regulated signal transduction mechanisms (Niswender and Conn, 2010; Pereira and Goudet, 2018; Alkadhi, 2021; Abd-Elrahman and Ferguson, 2022). The central importance of mGluRs in brain physiology and in neurological disorders such as stroke, epilepsy, schizophrenia, and neurodegenerative diseases is widely recognized (Maksymetz et al., 2017; Wang and Reddy, 2017; Fern and Matute, 2019; Roth, 2019; Zhang et al., 2019; Luo et al., 2023; Yeung and Kwakowsky, 2023).
Over time, it has become clear that mGluRs play an important role in epileptogenesis. In this review, we introduce and summarize the progress of research on mGluR signaling pathways, especially regarding how they affect the occurrence of epilepsy. We further provide a foundation for antiepileptic drug development by presenting an update on recent findings that substantiate the potential of mGluRs as drug targets.
Literature Search Strategy
A literature retrieval strategy was conducted on three online databases, i.e. PubMed, Web of Science, and ResearchGate. Full-text articles published in English from inception to January 5, 2023 were included in this narrative review. A combination of the following medical subject headings (MeSH) terms was used to maximize search specificity and sensitivity: “metabotropic glutamate receptors OR mGluRs”, “epileptogenesis”, “epilepsy”, “seizure disorder” and “epilepsy therapy”. The results were further screened by title and abstract, and only studies exploring the relationship between mGluRs and epilepsy were selected for this review.
Metabotropic Glutamate Receptor-Associated Signaling Pathways
mGluRs belong to the G-protein-coupled receptor superfamily. Eight mGluRs (mGluR1-8), each possessing seven structural transmembrane domains, have been identified, many of which presenting alternatively spliced isoforms. Structurally, mGluRs are characterized by a large extracellular N-terminal domain, which acts as a glutamate binding site, and a Venus flytrap domain (Abd-Elrahman and Ferguson, 2022; Zimmermann et al., 2022) linked to seven transmembrane α-helical domains through a cysteine-rich domain (Gregory and Goudet, 2021). VTFs exist as homodimers or heterodimers, with ligand binding influencing tight dimer interfaces to bring cysteine-rich domains closer together, resulting in a conformational change. Interactions between the cysteine-rich domain and the receptor’s second extracellular loop rearrange the seven transmembrane domain to initiate the signaling process (Akanuma et al., 2015; Akanuma, 2020).
The eight mGluR subtypes are conventionally divided into three subgroups based on sequence homology, post-receptor signaling connections, and pharmacology (Mao et al., 2022; Figure 1). Group I members (mGluR1 and mGluR5) are typically postsynaptically localized and are coupled to the Gq signaling pathway (Hermans and Challiss, 2001; Azam et al., 2022; Dahl et al., 2022), which regulates neuronal excitability by stimulating the Gαq/Gα subunit to induce phosphatidylinositol 4,5-bisphosphate hydrolysis via phospholipase C (PLC) activation (Eddy et al., 2022). Another key feature of Group I mGluRs is also their enhancing effect on glutamate N-methyl-D-aspartate (NMDA) receptor activation (Carey et al., 2022; Kovalenko et al., 2022).
Figure 1.
Classification of mGluRs.
Created with diagrams.net. mGluR: Metabotropic glutamate receptor.
In contrast, Group II (mGluR2 and mGluR3) and Group III (mGluR4, mGluR6, mGluR7, and mGluR8) members are both presynaptically and postsynaptically localized, are coupled to the Gi/Go signaling pathway, and signal through adenylate cyclase pathway inhibition in heterologous expression systems (Guo et al., 2018). Of note, Group II receptor activation inhibits Ca2+ channels and activates K+ channels to mediate presynaptic inhibition of neurotransmitter release (Kovalenko et al., 2022). A summary of relevant characteristics and pathophysiological actions of members in the three mGluR groups is shown in Table 1.
Table 1.
mGluRs groups, common agonists and antagonists and effects
| Receptor | Localization | Common agonist | Common antagonist | Convulsive seizures | Neurotransmitter release | Reference |
|---|---|---|---|---|---|---|
| mGlu1, mGlu5 | Postsynaptic neurons, presynaptic terminals, glial cells | Nonselective (1S, 3R)-ACPD, (R,S)-CHPG, (S)-3,5-dihydrox-yphenylglycine, L-quisqualic acid | (S)–MCPG, (T) AIDA, (U) (S)-4CPG | Proconvulsive | Enhancement | Thomsen and Dalby, 1998; Tang et al., 2009; Gregory and Goudet, 2021 |
| mGlu2, mGlu3 | Presynaptic membranes, postsynaptic membranes, glial cells | Nonselective (1S, 3R)-ACPD, DCG-IV, pomaglumetad (S)-4-carboxy-3-hydroxyphenylglycine | CPPG, (S)–MCPG, (T) L-serine-O-phosphate | Anticonvulsant | Reduction | Folbergrova et al., 2001; Alexander and Godwin, 2006; Gregory and Goudet, 2021 |
| mGlu4, mGlu6, mGlu7, mGlu8 | Presynaptic membranes, presynaptic membranes, glutamatergic and GABAergic neurons, glial cells | ACPT-1, cinnabarinic acid, L-serine-O-phosphate’ (RS)-PPG, cyclobutylene AP5 | CPPG, (S)–MCPG, (T) MSOP | Mixed responses | Reduction | Thomsen and Dalby, 1998; Folbergrova et al., 2001; Gregory and Goudet, 2021 |
(R, S)-PPG: (R,S)-4-phosphonophenylglycine; 1S,3R-ACPD: 1S,3R-1-aminocyclopentane-1,3-dicarboxylic acid; ACPT-1: (1S,3R,4S)-1-aminocyclopentane-1,2,4-tricarboxylic acid; AIDA: 1-aminoindan-1,5-dicarboxylic acid; CHPG: (R,S)-2-chloro-5-hydroxyphenylglycine; CPPG: α-cyclopropyl-4-phosphonophenylglycine; Cyclobutylene AP5: (RS)-1-amino-3-(phosphonomethylene) cyclobutene-carboxylic acid; DCG IV: (2S,2’R,3’R)-2-(2’,3’-dicarboxycyclopropyl)glycine; MCPG: α-methyl-4-carboxyphenylglycine; mGluR: metabotropic glutamate receptor; M-SOP: α-methylserine-O-phosphate; S-3,4-DCPG: (S)-3,4-dicarboxyphenylglycine. (S), (U), (T) represent the different relative configurations of the chiral centres.
The activation of mGluRs is modulated by some ions, notably Cl– (Tora et al., 2015) and Ca2+ (Zou et al., 2017), and exerts physiological functions through G protein-coupled or G protein-independent intracellular signaling (Ribeiro et al., 2017). Cl– behaves as a positive allosteric regulator of mGluRs, and is necessary for the activation of glutamate in many mGluRs. Similarly, extracellular Ca2+ play an essential regulatory role in the signaling pathway of mGluRs as allosteric regulators and second messengers of mGluRs Additionally, it is important to note that glutamate concentrations in the brain are primarily regulated by glial cells. Glial cells control glutamate uptake via glutamate transporters (GLUT receptors), followed by the conversion of glutamate to glutamine in a process known as the glutamate/glutamine cycle (Evans et al., 2022). Upon activation, mGluRs crucially influence synaptic transmission and plasticity by modulating cell excitability, ionic conductance, and neurotransmitter release (Gubellini et al., 2004), typically triggering multiple long-lasting intracellular signaling pathways (Bodzeta et al., 2021; Figure 2). Accordingly, several CNS pathologies, such as epilepsy and Alzheimer’s disease, may be associated with abnormal signaling through mGluRs (Edfawy et al., 2019; Carey et al., 2022).
Figure 2.
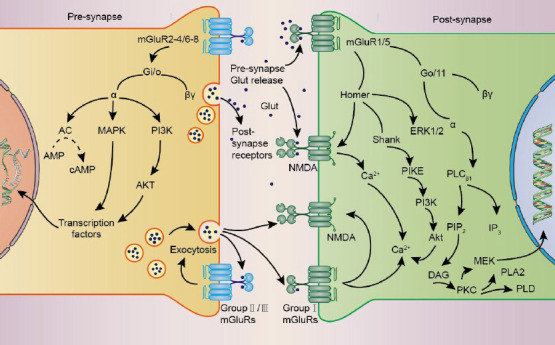
Signaling pathways mediated by mGluRs.
Schematic depiction of the synaptic localization of mGluRs and their signaling cascades. Relevant signaling pathways involve mainly MAPK, PI3K, Akt, PLC, IP3, mTOR, and ERK. A continuous line represents a physical interaction between elements, and the direction of signal conduction is indicated by arrows. Reduced activity is represented by dashed lines. Created with Adobe Illustrator (version 27.5). AMP: Adenosine monophosphate; ERK: extracellular signal-regulated kinase; GLUT: glucose transporters; IP3: inositol 1,4,5-trisphosphate; MAPK: mitogen-activated protein kinase; mGluRs: metabotropic glutamate receptors; mTOR: mechanistic target of rapamycin; NMDA: N-methyl-D-aspartatic acid; PI3K: phosphoinositide 3-kinase; PIKE: PI 3-kinase enhancer; PLC: phospholipase C.
A classical view is that Group I mGluRs couple to Gαq/11 and activate phospholipase Cβ1, leading to phosphoinositide hydrolysis for inositol 1,4,5-trisphosphate (InsP3) and diacylglycerol generation, and calcium mobilization and protein kinase C (PKC) activation (Niswender and Conn, 2010; Ribeiro et al., 2017). Group II and Group III mGluRs primarily couple to Gi/o proteins, with consequent downstream inhibition of adenylate cyclase activity leading to decreased cyclic adenosine monophosphate (cAMP) levels. In addition, Group III mGluR signaling through Gβγ subunits regulates ionic conductances in neurons, limiting presynaptic glutamate or gamma-aminobutyric acid (GABA) release by inhibiting voltage-dependent Ca2+ channels and promoting the activation of inwardly rectifying K+ channels (Schoepp, 2001; Niswender and Conn, 2010; Ribeiro et al., 2017; Vahidinia et al., 2021; Li et al., 2022).
Although the mGluR-associated second messenger system involved in phosphoinositide 3-kinase (PI3K)/Akt, mitogen-activated protein kinase (MAPK), nuclear factor kappa B (NF-κB), phospholipase C (PLC), mammalian target of rapamycin (mTOR), protein interacting with C kinase-1 (PICK1), and Ca2+/calmodulin-dependent protein kinase (Ca/CaM) signaling activation has been described in detail (Willard and Koochekpour, 2013; Dalley et al., 2018; Sadananda and Subramaniam, 2021), studies have identified new roles for mGluRs in other signaling pathways. For example, some Group I mGluR–mediated signaling pathways were shown to be associated with acid-sensing ion channel sensitization in dorsal root ganglion neurons; specifically, enhanced proton-gated currents induced by selective Group I mGluR agonism via (S)-3,5-dihydroxyphenylglycine (DHPG) were shown to fade after suppressing intracellular Gq/11, PLCβ, PKC, or PICK1 signaling (Gan et al., 2016).
Long-term potentiation (LTP) and long-term depression (LTD) of excitatory synaptic transmission are the two most common forms of synaptic plasticity (Malenka and Bear, 2004). In this regard, the role of mGluR1 in LTD signal processing has garnered considerable attention. LTD has been associated with numerous signaling cascades involving MAPK, protein tyrosine phosphatases, proteases, endocannabinoids, and PI3K/Akt-mTOR signaling (Gladding et al., 2009). By analyzing Ca2+ imaging results from Purkinje cells in slices of juvenile rat cerebella, Jin et al. showed that mGluR1-induced LTD results from activation of a slow excitatory post-synaptic current and PLC/IP3-mediated dendritic Ca2+ mobilization (Jin et al., 2007). Furthermore, it has been shown that mGluRs-induced LTD involves two interacting Ca2+ sensors, NCS-1 and PICK1 (Ghasemi et al., 2021).
In a recent study, Dasgupta et al. (2020) revealed that Group III mGluR4 and mGluR7 inhibition results in potentiation of LTP-resistant Schaffer collateral-CA2 synapses via ERK/MAPK signaling activation and striatal-enriched protein tyrosine phosphatase (STEP) downregulation, transforming a transient potentiation of the entorhinal cortical (EC)-CA2 synapses into a sustained, stable LTP pattern. Meanwhile, Kelly et al. (2009) discovered that in cerebellar stellate cells, GABA-dependent synaptic plasticity mediated by switching of GluR2-lacking Ca2+-permeable α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (CP-AMPA) receptor subunits and subsequent loss of Ca2+ permeability requires mGluR activation. This mechanism was shown to differ, in turn, from those described to mediate mGluR-induced LTD in CA1 neurons and parallel fiber-Purkinje cell synapses. These findings have significantly contributed to our understanding of the participation of mGluRs in various neurological disorders, including epilepsy, as discussed below.
Some neural circuit study tools contributed also to elucidate mGluR-related signaling pathways involved in the regulation of neuronal excitability. For example, Xiang et al. (2021) reported on mGluR2/mGluR4 heterodimers in brain slices through electrophysiology and optogenetic methods; these proteins are essential in excitatory synapse transmission within the rodent medial prefrontal cortex (mPFC) and may inhibit glutamatergic signaling at thalamus-mPFC synapses. These and several other techniques have greatly assisted precise localization of mGluR-related signaling pathways and are expected to promote this field’s development. A detailed picture of the multiple mGluRs-mediated signaling pathways will deepen our understanding of the molecular mechanisms underlying neuronal disorders and facilitate targeted drug development.
Potential Mechanisms Underlying Metabotropic Glutamate Receptor Regulation of Epileptogenesis
Group I mGluRs
Group I mGluR subtypes (mGluR1 and mGluR5) are primarily expressed postsynaptically but exist also in presynaptic terminals of GABA and glutamatergic neurons (Luessen and Conn, 2022). The roles of Group I mGluRs in epileptogenesis and epilepsy persistence have been studied for over two decades (Table 2). Focal cortical dysplasia is a substantial intractable epilepsy factor. Immunocytochemistry analysis of epilepsy patient samples showed that mGluR1α and mGluR5 are highly expressed in dysplastic neurons, suggesting that Group I mGluRs may trigger high epileptogenicity (Turati et al., 2022). In turn, analysis of mGluR expression in epileptic dentate gyrus granule cells from different mouse strains evidenced reduced excitatory group I mGluRs expression in correlation with a diminished epileptic phenotype (Chen et al., 2005; Lukawski et al., 2018; Celli et al., 2019). Attesting at the importance of glial mGluR activity in epilepsy, in mouse astrocytes mGluR5 expression is suppressed ~3 weeks after birth but its re-expression occurs during induction of epileptogenesis (Salvati and Beenhakker, 2019). More recently, it was also demonstrated that seizure-induced mGluR5 upregulation in reactive astrocytes is associated with increased TGF-β production (Mazzitelli et al., 2022).
Table 2.
Roles of Group I mGluRs in epileptogenesis
| Receptor | Mechanism | Signaling pathway | Model organism | Epilepsy inducer | Reference |
|---|---|---|---|---|---|
| mGluR5 | Initiation of fast ripples | phospholipase Cβ1 ↑ →intracellular Ca2+ ↑ | Male Wistar rats | KA (0.8 μg/0.5 μL) | Medina-Ceja and Garcia-Barba, 2017 |
| mGluR5 | Increased astrocytic glutamate uptake | Changes in synaptic contact: IP3 signaling↑→number of Schaffer collateral synapses↑→enhanced glutamate uptake/GLT-1 expression/PKC-dependent phosphorylation | Adult male and female mice | KA (2 mg/mL) | Umpierre et al., 2019 |
| mGluR5 | Synthesis of amyloid-beta protein precursor | mGluR5/FMRP signaling (influenced by Aβ/PrPC/mGluR5 interactions) | 3×Tg-AD mice | Auditory stimuli (5 min, high-pitched siren, 120 dB) | Kazim et al., 2017; Westmark, 2019 |
| mGluR1/5 | Initiating SWDs | mGluR↓→GAT-1 protein↓/GABA uptake↓→SWDs↑ | Male spontaneously epileptic WAG/Rij rats, age-matched Wistar rats, Sprague-Dawley rats | Celli et al., 2020 | |
| Male WAG/Rij rats, male non-epileptic control Wistar rats | Crans et al., 2020 | ||||
| mGluR5 | Decreasing tumor necrosis factor α expression in immune cells (microglia and macrophages) | Enhanced innate immune response→ proinflammatory cytokine secretion→seizure development | C57BL/6J inbred male mice | DA strain of TMEV | Hanak et al., 2019 |
| C57BL/6J inbred male mice, MyD88-deficient mice | 2 DA strain of TMEV | Kirkman et al., 2010 | |||
| mGluR1/5 | mGluR5-dependent calcium transients | Gq-PLC-IP3 signaling→ calcium transients/activity of PKC↑→epileptogenesis | Long Evans rats and genetic absence epilepsy rats from Strasbourg model | Neyer et al., 2016 | |
| mGluR1/5 | Eliciting epileptiform discharges | PLCβ1 signaling: Gαq/11 subunit→PLCβ pathway→IP3 | Ten-week-old F1 homozygous and wild-type littermates from crosses of C57BL/6J(N8)PLCβ1+/+ and 129S4/SvJae(N8)PLC+/− | Chuang et al., 2001 | |
| mGluR5 | NMDA receptor activation | Activated glutamatergic synapses/in vivo kindling→activated group I mGluRs→epileptogenesis | Male Wistar rats | Tramadol (150 mg/kg) | Valian et al., 2021 |
| NMDA receptor↑→epileptogenesis/maintenance of seizures | Male Wistar rats (7–8-wk-old) | Pilocarpine (10 mg/kg with 30 min intervals) | Kovalenko et al., 2022 | ||
| mGluR5 | Reducing CaMKII activity | Protein kinase C mediated CaMKII phosphorylation at Ser901→ CaMKII↓→ limbic epilepsy | Embryonic neurons; HEK293T cells | McNamara et al., 2006; Park et al., 2008 | |
| mGluR1/5 | Contributing to the paroxysmal burst of multiple unit activities in CA1 region | Exogenous activation of mGluR→extrasynaptic NMDAR/suprathreshold depolarization→ paroxysmal burst | P5–P7 Wistar rats | TBOA (DL-threo-β-benzyloxyaspartic acid, 100 μM) | Molinari et al., 2012 |
| mGluR1/5 | Elevation of spontaneous spike frequency in cortical neurons | Group I mGluRs↓→Anaphase-promoting complex/cyclosome↑/casein kinase I↑→ Mdm2 ↓→p53↑→proteasomal degradation of Akt | p53f/+-Emx1-Cre+ mouse neurons | Liu et al., 2020 | |
| mGluR5 | Co-localization with norbin in neurons of epileptic brain | Norbin↓→mGluR5 signaling↓/ CaMKII ↑ | Adult male Sprague-Dawley rats | Pilocarpine hydrochloride (30 mg/kg) | Xu et al., 2017 |
| mGluR1 | DNA methylation | Hypermethylation of mGluR1 gene→prolonged febrile seizure ↑ | Adult Sprague-Dawley rats | KA (1.83 mg/kg) | Alese and Mabandla, 2019 |
| mGluR1/5 | Altering efficacy of synaptic connections | ↑mGluR1α in TLE→intracellular calcium↑→ altered efficacy of synaptic connections | Male Sprague-Dawley rats/TLE patients | KA (12.5 mg/kg) | Blumcke et al., 2000 |
CaMKII: Calcium/calmodulin-dependent protein kinase II; FMRP: fragile X mental retardation protein; GLT-1: glutamate transporter-1; IP3: Inositol 1,4,5-trisphosphate; KA: kainic acid; mGluR: metabotropic glutamate receptor; NMDA: N-methyl-D-aspartatic acid; PKC: protein kinase C; PLC: phospholipase C; SWD: spike-and-wave discharges; TMEV: Theiler’s murine encephalomyelitis virus.
Although the association between high Group I mGluR expression and seizure susceptibility is well-established, a causal relationship has not yet been fully determined. It has been proposed that mGluR5 upregulation in surviving neurons is likely a consequence of seizure activity and may lead to hippocampal hyperexcitability in patients with pharmaco-resistant temporal lobe epilepsy (TLE), suggesting that mGluR5 signaling is a potential target for new antiepileptic drugs (Zimmermann et al., 2022). Along these lines, Sanon et al. reported that epileptiform burst discharges in hippocampal stratum oriens-alveus interneurons (O/A-INs) are partially mGluR5-dependent. Postsynaptic O/A-IN currents include different components, with mGluR5 contributing to slow and fast components and mGluR1α contributing to slow ones only. These findings indicate that differential mGluR1α and mGluR5 expression may influence cell type-specific epileptiform activity (Sanon et al., 2010; Govindaiah et al., 2018).
The development of pharmacological inhibitors of Group I mGluRs is a topic of great interest in epilepsy research and treatment. As early as 1995, McBain found that the mGluR antagonist (+)-2-methyl-4-carboxyphenylglycine reversibly abolished the periodic inward current elicited by high K+ in stratum oriens interneurons, thereby inhibiting epileptiform activity (McBain, 1995). Subsequently, Micheli reported on the development of 2-methyl-6-(phenylethynyl)-pyridine (MPEP), a selective, systemically active non-competitive mGluR5 antagonist with epilepsy treatment potential that effectively inhibited phosphoinositide hydrolysis (Micheli, 2000). Subsequently, Borowicz and collaborators proposed a new therapeutic approach focused on SIB 1893, a non-competitive Group I mGluRs antagonist, and conventional antiepileptic drug combinations (Borowicz et al., 2003, 2004). Aided by gene expression technology, Gass and Olive (2008) found that mGluR5 antagonism can mediate expressional changes in a wide range of genes. Their analyses showed that repeated administration of the mGluR5 antagonists MPEP and 3-[(2-methyl-1,3-thiazol-4-yl) ethynyl]-pyridine (MTEP) to rats influenced frontal cortex’s transcriptional profiling, differentially altering the expression of 63 genes.
Homer proteins are postsynaptic scaffolding proteins that act as molecular adaptors of Group I mGluRs and other proteins. Homer-1a expression during seizures can reduce seizure susceptibility, and intracellular Homer-1a protein injection reduces membrane excitability (Bockaert et al., 2021). Interactions between Homer proteins and mGluR1 and mGluR5 can fine-tune synaptic strength, thus modulating epileptic activity (Gurgone et al., 2022). Cavarsan et al. (2012) confirmed a significant increase in Homer-1a protein expression in the hippocampus, amygdala, and piriform and entorhinal cortices 24 hours after pilocarpine-induced epilepsy onset, coinciding with a significant decrease in mGluR5 protein expression in the same areas.
Substantial research has focused on the mechanisms linking Group I mGluR activities and epileptogenesis. D’Amore et al. (2013) reported that changes in mGluR5 expression might be central to the absence-seizure prone phenotype of WAG/Rij rats. This study showed that pharmacological mGluR5 activation reduced spike-and-wave discharge (SWD) number and duration in WAG/Rij rats, thus highlighting mGluR5 enhancers as potential drug candidates for treatment of absence epilepsy. Similarly, supporting a protective role for mGluR5 in TLE, Kandratavicius et al. proposed that mGluR5 upregulation in TLE may represent a postsynaptic adaptation to control hyperexcitability and excessive glutamate release (Kandratavicius et al., 2013). Moreover, Potter et al. (2013) concluded that increased mGluR5 contributes to epileptiform seizure activity and leads to LTD in the CA1 hippocampus in a mouse model of tuberous sclerosis complex (TSC). Interestingly, the pro-apoptotic effect of mGluR5 upregulation in glial cells, linked to intracellular Ca2+ overload and observed during brain hypoxia, epilepsy, and some neurodegenerative conditions, has been substantiated by several studies (Paquet et al., 2013; Hu et al., 2022).
Imaging technology has also advanced our knowledge regarding the involvement of Group I mGluRs in epilepsy. Choi et al. (2014) applied [11C]ABP688 micro positron emission tomography (PET)/CT to investigate mGluR5 expression in vivo, confirming regional mGluR5 non-displaceable binding potential (BPND) changes in a pilocarpine-induced epilepsy rat model. Using the same technique, DuBois et al. (2016) introduced in vivo evidence of reduced mGluR5 availability in focal cortical dysplasia in epilepsy patients, suggesting focal glutamatergic alterations in epilepsy-associated cortical developmental malformations. Applying also [11C]ABP688-PET to assess mGluR5 activity in mesial TLE patients, Lam et al. (2019) quantified [11C]ABP688 BPND and determined that mGluR5 was locally reduced in the epileptic hippocampal head and amygdala. This evidence likely reflected receptor internalization or conformational changes in response to excess extracellular glutamate, and suggested that mGluR5 is a potential therapeutic target for mesial TLE treatment. Using electrophysiology and two-photon microscopy, Ding et al. (2007) showed that in mice with pilocarpine-induced status epilepticus (SE), mGluR5 activation triggers astrocytic Ca2+ transients that promote neuronal hyperactivity and death by mediating the release of glial-derived glutamate and activating GluN2B-subunit containing NMDAR-mediated neuronal currents. The astrocyte hyperexcitability and altered glial transmission induced by this pathway are vital in controlling the excitability balance required to set lower seizure thresholds in epileptic circuits (Alcoreza et al., 2021).
Abnormal depolarization-induced suppression of excitation (DSE) was reported in animal epilepsy models. During chronic inflammatory pain, increased nociception due to reduced DSE in the anterior cingulate cortex may be associated with reduced mGluR5 protein levels and function (Guo et al., 2018). Extensive data have established mGluR1’s critical role in the transition of interictal bursts into ictal activity and development of sustained, synchronized discharges. Using a rat model, Postnikova et al. (2019) reported that NMDA-dependent LTP was inhibited during pentylenetetrazole (PTZ)-induced SE, and underwent a transient switching to mGluR1-dependent potentiation. One day after SE induction, the mGluR1 antagonist FTIDS completely blocked LTP in hippocampal slices from PTZ-treated animals, and mGluR1-dependent LTP was expressed postsynaptically without NMDA receptor activation. Thus, mGluR1 antagonists hold therapeutic promise for novel epilepsy syndrome treatments.
Drugs targeting mGluR1 and mGluR5 are considered to have great potential for the treatment of epilepsy (Anovadiya et al., 2012; Hanak et al., 2019; DuBois et al., 2021). Allosteric mGluR modulators are well studied and provide robust foundations for both basic research and therapeutic drug development. D’Amore et al. (2014) compared the effects of allosteric activators of mGluR1 and mGluR5 in the WAG/Rij rat models of absence epilepsy. They showed that the antiepileptogenic effect of VU0360172, a positive allosteric modulator (PAM) of mGluR5, largely persisted over time, with only minor tolerance signs. In contrast, that of mGluR1 PAM RO0711401 ceased over a relatively short period (D’Amore et al., 2014). These results hence support the development of mGluR5 PAMs as potential symptomatic agents for the treatment of chronic absence epilepsy (D’Amore et al., 2014; Celli et al., 2019; Brown et al., 2022). In subsequent experiments, some researchers locally injected VU0360172 and RO0711401 into the thalamus and cortex of WAG/Rij rats and found that both agents attenuated absence seizures by enhancing cortical GABAergic inhibition and reducing thalamic tonic GABAergic inhibition (D’Amore et al., 2015; Gedikli et al., 2023). Further research in this rat model concluded that VU0360172 elicited long-lasting antiabsence effects when used in conjunction with ethosuximide, the first-choice medication for the treatment of patients with absence seizures (D’Amore et al., 2016). Hanak et al. (2019) used a Theiler’s murine encephalomyelitis virus (TMEV)-induced TLE mouse model to demonstrate that VU0360172 reduced acute seizures and tumor necrosis factor α-producing microglia and macrophage abundance by three days post-infection. Furthermore, VU0360172 treatment did not alter viral antigen levels, suggesting that it does not affect viral clearance. In turn, Kelly et al. (2018) reported that treating a Tsc2 mutant mouse model with the mGluR5 PAM RO6807794 exacerbated seizures and hyperactivity. This finding suggests that mGluR5 inhibition might serve to effectively correct the disease’s core phenotype, characterized by seizures and hyperactivity.
Vijaya Prabhu and Singh (2019) developed an energy-optimized pharmacophore model to identify in the eMolecules database potent negative allosteric modulators (NAMs) of mGluR5 by obtaining variable conformation sites for docking targets. Afer validating the model through enrichment calculations, specific amino acid interactions with receptor metastable binding sites for the candidate compounds were analyzed via molecular dynamics simulations. Within the binding regions, TRP785 and TYR659 were identified as critical determinants of hydrophobic bonds, whereas SER809 critically influenced hydrogen bonding interactions.
The potential of plant-derived therapeutic agents to modulate mGluRR5 expression and/or activity has been suggested by some studies. Krishnakumar et al. (2015) reported that Bacopa monnieri extract ameliorated pilocarpine-induced TLE in rats at least in part by downregulating mGluR5 expression. Similarly, behavioral and physiological assays conducted by Li et al. (2017) showed that fermented Pu-erh tea significantly reduced mGluR5 transcription and translation and alleviated pilocarpine-induced epilepsy in rats .
Alese et al. (2020) demonstrated marked downregulation of mGluR5 expression in hippocampi from PTZ-treated rats with a history of LPS-induced prolonged febrile seizures compared to PTZ-treated controls without prolonged febrile seizure history, an effect probably related to increased glutamate concentrations in the epileptic brain. Of note, these experiments demonstrated also an association between epilepsy and depressive-like behavior regardless of background prolonged febrile seizure history.
There are however conflicting reports about whether mGluR5 antagonists can prevent epilepsy. Dyomina et al. (2022) showed that administration to rats of the selective mGluR5 antagonist MTEP during the latent phase of pilocarpine-induced SE did not prevent SE development nor symptoms, but completely prevented neuronal loss and partially attenuated astrogliosis in the hippocampus. They speculated that the neuroprotective effect of MTEP may result from short-term glial activation and enhanced glial glutamate reuptake secondary to prevention of excitatory amino-acid transporter 2 protein downregulation in astrocytes.
There is evidence that mGluR5 can also act in concert with other molecules and signaling pathways to affect epilepsy. For example, Wang et al. (2016) reported that CB1 receptor antagonist application early during PTZ-induced epileptogenesis after brain injury attenuated hyperexcitability by reversing mGluR5 overexpression in the hippocampus. Xu et al. (2017) demonstrated that norbin, a prominent endogenous mGluR5 signaling regulator co-expressed with mGluR5 in brain tissues, is downregulated in the temporal lobes of TLE patients and in the hippocampus of rats with pilocarpine-induced epilepsy. A connection between reduced norbin expression, possibly linked to mGluR activation, and epileptogenesis was therefore theorized. In turn, Medina-Ceja and Garcia-Barba (2017) found that blocking the mGluR5 receptor in KA-treated rats temporarily reduced the number of fast ripples (high-frequency oscillations of 250–600 Hz) associated with epilepsy.
The activities of mGluR1 and mGluR5 affect as well other proteins and pathways. Celli et al. (2020) described the regulation of thalamic GABA transmission by mGluR5 activation, indicated by increased GABA uptake in the thalamus of absence epileptic WAG/Rij rats. This was consistent with results from previous studies in various preclinical absence epilepsy models indicating that defective GABA uptake is associated with SWD pathophysiology (Yalcin, 2012; Kovacs et al., 2022). Drugs that enhance synaptic and extrasynaptic GABA levels, such as tiagabine and vigabatrin, exacerbate akinetic seizures and hold promise for treating akinetic epilepsy (Celli et al., 2020).
Crans et al. (2020) utilized a systemic kainic acid (KA) TLE model to demonstrate mGluR5 protein downregulation in the late latency and exponential growth phases post-SE, primarily in the hippocampal CA1 region. Furthermore, their data suggested a relationship between Homer-1b/c downregulation and seizure rate in the SE model, potentially indicating an endogenous neuroprotective mechanism. In addition, Di Cicco et al. determined that mGluR5 and Homer protein expression changes in symptomatic WAG/Rij rats significantly reduced mGluR1- and mGluR5-mediated LTD at Schaffer collateral-CA1 (SC-CA1) synapses, pointing that hippocampal mGluR5-dependent synaptic plasticity is associated with a pathological phenotype in these animals (Di Cicco et al., 2021). Previously, it had been suggested that mGluR1 and mGluR5 activation underly the attenuating effect of low-frequency stimulation on neuronal hyperexcitability produced by epileptiform activity in hippocampal CA1 pyramidal neurons (Neyman and Manahan-Vaughan, 2008). These findings corroborate long-term inhibition and shock absorption as low-frequency stimulation anti-epileptiform activity effects in vitro (Ghasemi et al., 2021). However, further studies are needed to fully elucidate the underlying cellular and molecular mechanisms involved in the antiepileptic effects of low-frequency stimulation.
Groups II–III mGluRs
Several studies corroborated that Group II and Group III mGluRs are located at glutamatergic neurons’ presynaptic ends and reduce glutamate release (Ure et al., 2006), which makes them potential targets for antiseizure drug development. However, compared with Group I mGluRs, relatively little research has focused on Groups II and III mGluRs in recent years (Tables 3 and 4). Several studies (Davidson et al., 2016; Bocchio et al., 2018; Li et al., 2020; Bodzeta et al., 2021; Miller et al., 2022) have explored Group II mGluRs’ influence on epilepsy and neuron excitability regulation. Das et al. examined mGluR changes in resected human hippocampus through immunohistochemical staining and western blotting (Das et al., 2012). Their study detected significant mGluR2 and mGluR3 upregulation and astrocyte activation in brain tissue from TLE patients, concluding that mGluR2 deregulation might play an essential role in TLE development. However, the authors failed to confirm whether this phenomenon is a compensatory mechanism to decrease neuronal activity.
Table 3.
Roles of Group II mGluRs in epileptogenesis
| Receptor | Mechanism | Signaling pathway | Model organism | Epilepsy inducer | Reference |
|---|---|---|---|---|---|
| mGluR2 | Astrogliosis, cellular hypertrophy, water homeostasis, inflammation, modulation of excitatory neurotransmission | GluR2 receptor and KA receptor ↓→Ca2+↑ | TLE patients | Das et al., 2012 | |
| mGluR2/3 | Excitatory synaptic transmission | Group II mGluRs↑→release of glutamate onto pyramidal neurons ↓→epileptogenesis | Pyramidal neurons of cortical layers 2–3 in acute slices derived from surgically removed cortical tissue of people with epilepsy | Bocchio et al., 2018 | |
| mGluR2/3 | Disruption of glutamate-mediated homeostasis of neuronal excitability | Drosophila GluRA mutations→PI3K activation↓, FOXO↑→neuronal excitability | Drosophila (UAS-PI3K DN and UAS-PI3K-CAAX transgenes) | Electrical stimulation (10 Hz) | Howlett et al., 2008; Lovisari et al., 2021 |
| mGluR2/3 | Involvement in the generation and propagation of epilepsy in the entorhinal cortex | LY354740→K+ conductance↑→Na+-permeable channel↓→neuronal excitability and epileptiform activity↓ | Sprague-Dawley rats | PTX (100 mM) | Zhang et al., 2015 |
| mGluR3 | Seizure-induced upregulation of mGluR3 | Mesial temporal lobe epilepsy→mGluR3↑→TGF-β↑ | Male Sprague-Dawley rats | Intermittent electrical stimulation (10-s trains of 50 Hz biphasic square pulses); max. stimulus intensity: 500 μA) | Aronica et al., 2000; Kovalenko et al., 2022; Celli et al., 2023 |
| mGluR2 | Synergistic interactions with the antiseizure drug levetiracetam and the positive allosteric modulator JNJ-46356479 | Adult male CF-1 mice | Electrical stimulation (6 Hz, 32 or 44 mA) | Metcalf et al., 2018 |
FoxO: Forkhead box O; LY354740: hexane-2,6-dicarboxylic acid; mGluR: metabotropic glutamate receptor; PI3K: phosphoinositide 3-kinase; PTX: Picro-toxin; TLE: temporal lobe epilepsy.
Table 4.
Roles of Group III mGluRs in epileptogenesis
| Receptor | Mechanism | Signaling pathway | Model organism | Epilepsy inducer | Reference |
|---|---|---|---|---|---|
| mGluR7 | Antagonism of mGluR7 | GIPC1↓→mGluR7↓→more and longer abnormal brain discharges in LFP | TLE patients and adult male C57BL/6 mice | KA (50 nL of a 20 mM solution) | Liu et al., 2022a |
| mGluR4 | mGluR4 knock-down | mGluR4↓→excitatory activity and seizure-associated vulnerability of hippocampal neurons↑→ severe seizure activity↑ | mGluR4-KO mice | Kumar et al., 2022 | |
| mGluR4, mGluR6, mGluR8 | Divergent patterns of associational-commissural-CA3 and SC-CA1 synapse depression in SE | mGluR4, mGluR6↓, mGluR8↑→synaptic depression→chronic epilepsy | Male Wistar rats | Pilocarpine hydrochloride (340 mg/kg) | Dammann et al., 2018 |
| Group III mGluRs | Excitotoxic action of 4-aminopyridine | Extracellular glutamate↑→mGluR III↑→seizure↓ | Male Wistar rats | 4-aminopyridine (7 mM) | Vera and Tapia, 2012 |
| Group III mGluRs | Neurotransmitter shift towards increased production of excitatory amino acids | mGluR III↓→GABA, alanine, taurine, and glutamine/GABA ratio↑→seizure | Adult male Wistar rats | PTZ (35 mg/kg) | Zhai et al., 2021; Zhao et al., 2022 |
| mGluR7 | Interaction between mGluR7 and PDZ proteins | Targeted mutation of the mGluR7a C terminus →disrupted interaction with PDZ proteins →EEG discharges | Adult Sprague-Dawley rats | Liu et al., 2022b; Tuduri et al., 2022 | |
| Group III mGluRs | Selective downregulation of group III mGluRs and dysregulated glutamatergic synaptic transmission | Elfn2 knockout→ selective downregulation of group III mGluRs and dysregulated glutamatergic synaptic transmission→seizure susceptibility↑ | HEK 293T/17 cells | Dunn et al., 2019 | |
| Group III mGluRs | Decreased expression of group III genes in the hippocampus and temporal cortex | Group III genes↓→mGluRs III expression ↓→epileptogenesis | Male Wistar rats | Pilocarpine (20–40 mg/kg) | Kovalenko et al., 2022 |
| mGluR7 | Interactions between extracellular leucine-rich repeat fibronectin domain-containing family proteins (Elfn1 and Elfn2) and mGluRs | Activation of mGluR7 by Elfn1→glutamate levels↓ | Elfn1/2-KO mice | Matsunaga and Aruga, 2021 |
EEG: Electroencephalography; GABA: gamma aminobutyric acid; KA: kainic acid; KO: knockout; LFP: local field potential; mGluRs: metabotropic glutamate receptors; TLE: temporal lobe epilepsy.
Evidence for such a negative feedback was obtained by experiments showing that mGluR2 gene mutation increases motor neuron excitability by disrupting autocrine glutamate-mediated negative feedback and preventing PI3K activation (Liu et al., 2021) These results suggest that neuron excitability disruption may induce CNS disorders, including epilepsy (Lima et al., 2020). The interactions between mGluRs and synaptic circuits within the human brain are garnering increasing attention. Bocchio et al. (2018) reported that most Group II mGluRs are autoreceptors that inhibit glutamate delivery to pyramidal cerebral cortex neurons to mediate presynaptic inhibition of excitatory transmission. Their study provided new methodological tactics for revealing antiseizure drug mechanisms.
Extensive research in rodent models has proven that inadequate glutamate uptake can induce seizures through mechanisms resembling those of some human epilepsy syndromes (Pajarillo et al., 2019). However, the cellular mechanisms linking glutamate transporter dysfunction with pathological cortical activity remain elusive. Shedding light on the involvement of amino acid transporters’ abnormalities in epilepsy, Molinari et al. (2012) showed that application of the amino acid transporter inhibitor TBOA to the CA1 region of hippocampal slices lead to insufficient extracellular glutamate buffering, which allowed Group I/II mGluRs and NMDAR interconversion. This interaction initiates hippocampal network hyperexcitability, promotes seizure-like activity generation during glutamate release, and reduces afterhyperpolarization currents.
In vivo experiments demonstrated that Group III mGluRs activation may aid in preventing and treating epilepsy through the excess extracellular glutamate spillover, excitotoxicity inhibition, and seizure suppression (Vera and Tapia, 2012; Lazo-Gomez and Tapia, 2016). However, a previous study had suggested that during seizure kindling, Group III mGluRs may mediate a shift in the balance between neurotransmitters toward increased excitatory amino acid production (Maciejak et al., 2009). Kovalenko et al. (2022) reported decreased Group III mGluR gene expression in the hippocampus and temporal cortex of rats during the latent and chronic stages of pilocarpine-induced epilepsy. Additionally, they showed that while most changes in mGluR expression detected in the latent phase were absent in the chronic phase, mGluR8 mRNA downregulation persisted during the latter. These finding led the authors to suggest that Group III mGluRs agonists are the most suitable targets for epilepsy prevention.
Several studies indicated that G-alpha-interacting protein (GAIP) is crucial for receptor transport and expression (Shaw et al., 2019; Liu et al., 2021, 2022b), but its role in epilepsy is still uncertain. Liu et al. (2022a) detected a reduction in GAIP interacting protein C-terminus 1 (GIPC1) expression in both TLE patients and in mice with KA-induced epilepsy. They further showed that GIPC1 colocalized with mGluR7 and its overexpression counteracted epileptogenesis through mGluR7 upregulation. Dammann et al. (2018) reported that mGluR4 and mGluR6 downregulation in associational-commissural-CA3 synapses occurred concomitantly with mGluR8 upregulation in SC-CA1 synapses in pilocarpine-treated epileptic mice, concluding that a bidirectional shift in the transcription of Group III mGluRs modulates synaptic depression in the hippocampus of chronically epileptic mice. Evidence for divergent roles for Group I and Group III mGluRs in epilepsy was in turn produced using pilocarpine-treated mGluR4-KO and mGluR1-overexpressing mice. For each mouse type, increased seizure frequency was observed, respectively, during the acute and the chronic stages of SE, while enhanced hippocampal neuronal loss was only evident in mGluR4-KO mice (Pitsch et al., 2007; Pohlentz et al., 2022). Meanwhile, the ongoing debate regarding mGluR3 expression levels in TLE patients (Berger et al., 2020; Peterson and Binder, 2020) highlights the need for further investigation in larger sample sizes, as well as more comprehensive basic research.
Protein interactions between extracellular leucine-rich repeat fibronectin domain-containing family proteins (Elfn1 and Elfn2) and Group III mGluRs have been described in recent studies (Matsunaga and Aruga, 2021). Elfn2-KO mice exhibit various behavioral abnormalities, including increased seizure susceptibility, hyperactivity, and anxiety. Dunn et al. (2019) showed that administering the mGluR4-selective PAM VU0155041 rescued these behavioral abnormalities in Elfn2-KO mice. Due to its low glutamate affinity, mGluR7 is speculated to act as a brake against excessive glutamate levels (Niswender and Conn, 2010), which may help explain increased seizure incidence in mGluR7-deficient animals (Sansig et al., 2001). In this regard, the Dunn et al. (2019) report pointed to mGluR7 downregulation as a determinant factor of the epilepsy-like phenotype of Elfn2-KO mice. Complementing mechanistic studies like those above with proteomic and genomic studies will further our understanding of the defining roles of mGluRs in epilepsy and foster the development of novel treatments.
Therapeutic Potential of Metabotropic Glutamate Receptors in Epilepsy
Epilepsy has become one of the most common neurological disorders. Although its exact etiology is still unclear, many researchers believe there is potential for antiepileptic treatment targeting mGluRs (Malik et al., 2017). mGluRs have three primary advantages in epilepsy-targeting drug development (Vijaya Prabhu and Singh, 2019; Dyomina et al., 2022; Witkin et al., 2022): (i) they generally modulate slow synaptic responses and have low inference in individual synaptic release events; (ii) they are only active under high neuronal activity conditions, which determines selectivity and thus favors their utility as drug targets; and (iii) the activation of mGluRs-related pathways usually produces long-lasting effects and can be further modulated by targeting associated G-proteins and downstream second messengers.
mGluR5 is a prominent calcium-dependent glutamate release mediator in astrocytes that promotes neuronal excitability. Elevated mGluR5 levels in hippocampal tissue of TLE patients suggest that its overexpression induces abnormal neuronal firing. In contrast, mGluR2 and mGluR3 are located both in astrocytes and in presynaptic terminals, and negatively regulate excitatory neurotransmission through alterations in intracellular cAMP levels. Intriguingly, the expression of both mGluR2 and mGluR3 is also significantly increased in the hippocampus of TLE patients, which may represent a compensatory response to counteract pathological neuronal activity (Das et al., 2012). In this regard, it has been shown that administration of a highly selective Group II mGluR agonist, (2R,4R)-4-aminopyrrolidine-2,4-dicarboxylate (2R,4R-APDC), significantly suppresses behavioral seizures in rats with pilocarpine-induced SE (Yao et al., 2015). This agent conferred not only anticonvulsant effects, but also neuroprotective ones, by reducing SE-induced neurogenesis in the dentate gyrus. Meanwhile, both 2R,4R-APDC and the Group III mGluR8 agonist (S)-3,4-DCPG were shown to provide neuroprotection in animal models of epilepsy by significantly reducing or completely eliminating superoxide anion formation in the brain (Folbergrova et al., 2012).
Group I mGluRs
Current studies on mGluR1 and mGluR5 mostly focus on the effects of their positive and negative modulators, to identify pharmacological strategies aimed at attenuating the pro-convulsive effects of these mGluRs (Anovadiya et al., 2012; Su et al., 2022). Preclinical studies have shown that Group I mGluR negative modulators are among the most efficacious antiepileptic drugs. Kelly et al. (2018) determined that NAM-mediated chronic mGluR5 inhibition corrected overactivity, reduced seizure frequency and duration, and elevated de novo synaptic protein synthesis in TSC model mice.
Dyomina et al. (2022) demonstrated potential neuroprotection for the selective mGluR5 antagonist MTEP, which prevented neuronal loss and excitotoxicity in pilocarpine-treated rats. However, the ability of MTEP to prevent epilepsy development and attenuate its symptoms and manifestations has not been investigated. The latter study suggested that it is essential to focus on the actions of epilepsy-specific target molecules when developing antiepileptic drugs, and that not all mGluR5 inhibitors will have equal efficacy against epilepsy. The authors pointed however to a therapeutic opportunity, by implying that specific Group I mGluR NAMs may aid in epilepsy treatments as part of drug combinations. Along those lines, a previous investigation had showed that MPEP, an mGluR5 NAM, reduced fast ripple events in KA-treated rats only during the first hour post-application, without altering oscillation frequency nor duration for each FR event (Medina-Ceja and Garcia-Barba, 2017). Meanwhile, Westmark et al. (2021) demonstrated that MPEP attenuated sensitivity to audiogenic-evoked seizures in a mouse model of fragile X syndrome mice, implying that NAMs effects may vary in relation to epileptogenesis mechanisms and types. These findings thus support the notion that Group I mGluR NAMs are promising antiepileptic drugs and thus merit more in-depth research.
Although Group I mGluR PAMs are often shown to cause epilepsy or to exacerbate the epileptic phenotype, there is evidence that they may instead be beneficial for epilepsy treatment. Celli et al. (2020) showed that systemic mGluR5 PAM VU0360172 injection increased GABA uptake both in the thalamus and in synaptosomes in vitro in the WAG/Rij model of absence epilepsy. D’Amore et al. (2014) had previously demonstrated that although both VU0360172 and RO0711401 (an mGluR1-selective PAM) were able to decrease SWD frequency, tolerance to RO0711401, but not VU0360172, developed after 3 days of treatment in WAG/Rij rats. Moreover, Hanak et al. (2019) noted that attenuated seizure activity after positive mGluR5 regulation was correlated with reduced brain levels of the proinflammatory cytokines IL-6 and tumor necrosis factor α in a TMEV-induced TLE mouse model. Thus, although there are conflicting conclusions and ongoing controversies, studying the therapeutic effects of Group I mGluR PAMs is still imperative as they have remarkable potential for epilepsy prevention and treatment.
mGluR-interacting proteins are also promising drug targets. For example, Homer proteins can physically connect glutamate receptors to intracellular calcium stores to regulate signaling cascades coupled to Group I mGluRs. In hippocampal cells, the scaffold protein tamalin regulates cell surface mGluR1a expression and the targeting of mGluR5 to neurons and may promote homodimerization of mGluRs (Pandey et al., 2020). Besides tamalin, calmodulin, and protein phosphatases 1 and 2, among others, were also shown to interact with mGluRs and regulate their effects (Enz, 2012).
Group II–III mGluRs
Many drugs based on Group II and III mGluRs were designed for treating neurological disorders including epilepsy. Currently available mGluR II agonists include (2R,4R)-APDC, DHPG, and (2S, 2’R, 3’R)-2-(2′,3′-dicarboxycyclopropyl)glycine, shown to alleviate audiogenic seizures in rats susceptible to hereditary epilepsy, and LY379268 and LY389795, shown to reduce absence seizures in lethargic (lh/lh) mutant mice (Thomsen et al., 1994; Attwell et al., 1998; Qian and Tang, 2016). Similarly, Zhang et al. (2015) reported that the selective Group II mGluR agonist LY354740 notably inhibited epileptiform activity in Horizontal brain slices from 13- to 20-day-old Sprague-Dawley rats. Their study further showed that application of the Gβγ inhibitor gallein significantly reduced LY354740-induced hyperpolarization of entorhinal neurons, indicating that mGluRs II activation reduced neuronal excitability via Gβγ. A study conducted by Yao et al. (2015) revealed that 2R,4R-APDC exerted a neuroprotective effect against hyperexcitability by reducing the amount of ectopic nascent dentate granule cells, which contributes to abnormal network reorganization in the adult rat hippocampus. However, elucidating the exact mechanism(s) responsible for this effect requires further investigation.
mGluRs III agonists, including L-(+)-2-amino-4-phosphonobutyric acid (Watanabe et al., 2011), L-serine-O-phosphate (Qian and Tang, 2016), (R, S)-4-phosphonophenylglycine (Selvam et al., 2018), and (1S,3R,4S)-1- aminocyclopentane-1,2,4-tricarboxylic acid (Selvam et al., 2018), have anticonvulsant actions in models of generalized epilepsy in mice. Unfortunately, relatively few antiepileptic drugs based on Group III mGluRs have been so far developed.
Limitations
This review has certain limitations. First, since epilepsy has attracted the attention of a large number of investigators in the field of neurosciences, there has been a wealth of research output over the years. Hence, in the process of literature retrieval and screening, researchers may inevitably miss some excellent or innovative studies. Additionally, in the summary of the signaling pathways involving mGluRs, it is difficult to provide an in-depth and exhaustive description due to the complex molecular mechanisms and various physicochemical factors involved. Moreover, the authors’ bias in selecting articles may also have an impact on the review.
Conclusion and Perspective
The mGluR family is vital in epilepsy onset and progression. Group I mGluRs (mGluR1 and mGluR5) are mainly involved in physiological G protein-coupled signaling pathway activities, intracellular calcium regulation, and neuronal firing. Group II (mGluR2 and mGluR3) and III (mGluR4, mGluR6, mGluR7, and mGluR8) mGluRs characteristically mediate adenylate cyclase activity inhibition and neurotransmitter release regulation. This review summarizes the involvement of the three mGluR groups in epilepsy, in the hope that it will further the identification of molecular markers and therapeutic drug targets. In light of the beneficial effects demonstrated for mGluR agonists and antagonists in preclinical models of epilepsy, their use as part of novel drug combination therapies may prove to be a highly effective clinical approach to optimize efficacy and delay or prevent drug resistance.
This review emphasized that factors related to the complex regulatory networks established between mGluRs and their numerous interacting proteins, mGluR agonist/antagonists dosages, and epilepsy stage may significantly influence therapeutic effects (Gregory and Goudet, 2021; Zhai et al., 2021). Thus, specific therapies targeting different pathogenic mechanisms also merit further investigation. Although several molecules, such as AMPA receptors, protein kinases, and mGluRs, are possible drug targets for epilepsy treatment, most related pharmacological interventions have only been animal-tested, and their potential use and safety in human epilepsy remain to be demonstrated in clinical trials.
Footnotes
Funding: This work was supported by the Natural Science Foundation of Hunan Province, No. 2021JJ30389 (to JG); the Key Research and Development Program of Hunan Province of China, Nos. 2022SK2042 (to LL) and 2020SK2122 (to ET).
Conflicts of interest: None of the authors has any conflict of interest to disclose.
Data availability statement: Not applicable.
C-Editor: Zhao M; S-Edior: Li CH; L-Editors: Li CH, Song LP; T-Editor: Jia Y
References
- 1.Abd-Elrahman KS, Ferguson SSG. Noncanonical metabotropic glutamate receptor 5 signaling in Alzheimer's disease. Annu Rev Pharmacol Toxicol. (2022);62:235–254. doi: 10.1146/annurev-pharmtox-021821-091747. [DOI] [PubMed] [Google Scholar]
- 2.Akanuma S, Sakurai T, Tachikawa M, Kubo Y, Hosoya K. Transporter-mediated L-glutamate elimination from cerebrospinal fluid:possible involvement of excitatory amino acid transporters expressed in ependymal cells and choroid plexus epithelial cells. Fluids Barriers CNS. (2015);12:11. doi: 10.1186/s12987-015-0006-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akanuma SI. Membrane transporters and their regulatory mechanisms at the brain and retinal barriers to establish therapies for refractory central nervous system diseases. Yakugaku Zasshi. (2020);140:1235–1242. doi: 10.1248/yakushi.20-00127. [DOI] [PubMed] [Google Scholar]
- 4.Akyuz E, Polat AK, Eroglu E, Kullu I, Angelopoulou E, Paudel YN. Revisiting the role of neurotransmitters in epilepsy:An updated review. Life Sci. (2021);265:118826. doi: 10.1016/j.lfs.2020.118826. [DOI] [PubMed] [Google Scholar]
- 5.Alcoreza OB, Patel DC, Tewari BP, Sontheimer H. Dysregulation of ambient glutamate and glutamate receptors in epilepsy:an astrocytic perspective. Front Neurol. (2021);12:652159. doi: 10.3389/fneur.2021.652159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alese OO, Mabandla MV. Transgenerational deep sequencing revealed hypermethylation of hippocampal mGluR1 gene with altered mRNA expression of mGluR5 and mGluR3 associated with behavioral changes in Sprague Dawley rats with history of prolonged febrile seizure. PLoS One. (2019);14:e0225034. doi: 10.1371/journal.pone.0225034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alese OO, Ngoupaye GT, Rakgantsho C, Mkhize NV, Zulu S, Mabandla MV. Glutamatergic pathway in depressive-like behavior associated with pentylenetetrazole rat model of epilepsy with history of prolonged febrile seizures. Life Sci. (2020);253:117692. doi: 10.1016/j.lfs.2020.117692. [DOI] [PubMed] [Google Scholar]
- 8.Alexander GM, Godwin DW. Metabotropic glutamate receptors as a strategic target for the treatment of epilepsy. Epilepsy Res. (2006);71:1–22. doi: 10.1016/j.eplepsyres.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 9.Alkadhi KA. NMDA receptor-independent LTP in mammalian nervous system. Prog Neurobiol. (2021);200:101986. doi: 10.1016/j.pneurobio.2020.101986. [DOI] [PubMed] [Google Scholar]
- 10.Anovadiya AP, Sanmukhani JJ, Tripathi CB. Epilepsy:Novel therapeutic targets. J Pharmacol Pharmacother. (2012);3:112–117. doi: 10.4103/0976-500X.95505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aronica E, van Vliet EA, Mayboroda OA, Troost D, da Silva FH, Gorter JA. Upregulation of metabotropic glutamate receptor subtype mGluR3 and mGluR5 in reactive astrocytes in a rat model of mesial temporal lobe epilepsy. Eur J Neurosci. (2000);12:2333–2344. doi: 10.1046/j.1460-9568.2000.00131.x. [DOI] [PubMed] [Google Scholar]
- 12.Attwell PJ, Singh Kent N, Jane DE, Croucher MJ, Bradford HF. Anticonvulsant and glutamate release-inhibiting properties of the highly potent metabotropic glutamate receptor agonist (2S,2'R, 3'R)-2-(2',3'-dicarboxycyclopropyl)glycine (DCG-IV) Brain Res. (1998);805:138–143. doi: 10.1016/s0006-8993(98)00698-2. [DOI] [PubMed] [Google Scholar]
- 13.Azam S, Jakaria M, Kim J, Ahn J, Kim IS, Choi DK. Group I mGluRs in therapy and diagnosis of Parkinson's disease:focus on mGluR5 subtype. Biomedicines. (2022);10:864. doi: 10.3390/biomedicines10040864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berger TC, Vigeland MD, Hjorthaug HS, Nome CG, Tauboll E, Selmer KK, Heuser K. Differential glial activation in early epileptogenesis-insights from cell-specific analysis of DNA methylation and gene expression in the contralateral hippocampus. Front Neurol. (2020);11:573575. doi: 10.3389/fneur.2020.573575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blumcke I, Becker AJ, Klein C, Scheiwe C, Lie AA, Beck H, Waha A, Friedl MG, Kuhn R, Emson P, Elger C, Wiestler OD. Temporal lobe epilepsy associated up-regulation of metabotropic glutamate receptors:correlated changes in mGluR1 mRNA and protein expression in experimental animals and human patients. J Neuropathol Exp Neurol. (2000);59:1–10. doi: 10.1093/jnen/59.1.1. [DOI] [PubMed] [Google Scholar]
- 16.Bocchio M, Lukacs IP, Stacey R, Plaha P, Apostolopoulos V, Livermore L, Sen A, Ansorge O, Gillies MJ, Somogyi P, Capogna M. Group II metabotropic glutamate receptors mediate presynaptic inhibition of excitatory transmission in pyramidal neurons of the human cerebral cortex. Front Cell Neurosci. (2018);12:508. doi: 10.3389/fncel.2018.00508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bockaert J, Perroy J, Ango F. The complex formed by group I metabotropic glutamate receptor (mGluR) and homer1a plays a central role in metaplasticity and homeostatic synaptic scaling. J Neurosci. (2021);41:5567–5578. doi: 10.1523/JNEUROSCI.0026-21.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bodzeta A, Scheefhals N, MacGillavry HD. Membrane trafficking and positioning of mGluRs at presynaptic and postsynaptic sites of excitatory synapses. Neuropharmacology. (2021);200:108799. doi: 10.1016/j.neuropharm.2021.108799. [DOI] [PubMed] [Google Scholar]
- 19.Borowicz KK, Piskorska B, Łuszczki J, Czuczwar SJ. Influence of SIB 1893, a selective mGluR5 receptor antagonist, on the anticonvulsant activity of conventional antiepileptic drugs in two models of experimental epilepsy. Pol J Pharmacol. (2003);55:735–740. [PubMed] [Google Scholar]
- 20.Borowicz KK, Łuszczki JJ, Czuczwar SJ. SIB 1893, a selective mGluR5 receptor antagonist, potentiates the anticonvulsant activity of oxcarbazepine against amygdala-kindled convulsions in rats. Pol J Pharmacol. (2004);56:459–464. [PubMed] [Google Scholar]
- 21.Brown J, Iacovelli L, Di Cicco G, Grayson B, Rimmer L, Fletcher J, Neill JC, Wall MJ, Ngomba RT, Harte M. The comparative effects of mGlu5 receptor positive allosteric modulators VU0409551 and VU0360172 on cognitive deficits and signalling in the sub-chronic PCP rat model for schizophrenia. Neuropharmacology. (2022);208:108982. doi: 10.1016/j.neuropharm.2022.108982. [DOI] [PubMed] [Google Scholar]
- 22.Carey C, Singh N, Dunn JT, Sementa T, Mendez MA, Velthuis H, Pereira AC, Pretzsch CM, Horder J, Hader S, Lythgoe DJ, Rotaru DG, Gee A, Cash D, Veronese M, Murphy D, McAlonan G. From bench to bedside:The mGluR5 system in people with and without Autism Spectrum Disorder and animal model systems. Transl Psychiatry. (2022);12:395. doi: 10.1038/s41398-022-02143-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cavarsan CF, Tescarollo F, Tesone-Coelho C, Morais RL, Motta FL, Blanco MM, Mello LE. Pilocarpine-induced status epilepticus increases Homer1a and changes mGluR5 expression. Epilepsy Res. (2012);101:253–260. doi: 10.1016/j.eplepsyres.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 24.Celli R, Santolini I, Van Luijtelaar G, Ngomba RT, Bruno V, Nicoletti F. Targeting metabotropic glutamate receptors in the treatment of epilepsy:rationale and current status. Expert Opin Ther Targets. (2019);23:341–351. doi: 10.1080/14728222.2019.1586885. [DOI] [PubMed] [Google Scholar]
- 25.Celli R, Wall MJ, Santolini I, Vergassola M, Di Menna L, Mascio G, Cannella M, van Luijtelaar G, Pittaluga A, Ciruela F, Bruno V, Nicoletti F, Ngomba RT. Pharmacological activation of mGlu5 receptors with the positive allosteric modulator VU0360172, modulates thalamic GABAergic transmission. Neuropharmacology. (2020);178:108240. doi: 10.1016/j.neuropharm.2020.108240. [DOI] [PubMed] [Google Scholar]
- 26.Celli R, Striano P, Citraro R, Di Menna L, Cannella M, Imbriglio T, Koko M, Euro Epinomics-Cogie C, De Sarro G, Monn JA, Battaglia G, Luijtelaar GV, Nicoletti F, Russo E, Leo A. mGlu3 Metabotropic glutamate receptors as a target for the treatment of absence epilepsy:preclinical and human genetics data. Curr Neuropharmacol. (2023);21:105–118. doi: 10.2174/1570159X20666220509160511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen J, Larionov S, Pitsch J, Hoerold N, Ullmann C, Elger CE, Schramm J, Becker AJ. Expression analysis of metabotropic glutamate receptors I and III in mouse strains with different susceptibility to experimental temporal lobe epilepsy. Neurosci Lett. (2005);375:192–197. doi: 10.1016/j.neulet.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 28.Chuang SC, Bianchi R, Kim D, Shin HS, Wong RK. Group I metabotropic glutamate receptors elicit epileptiform discharges in the hippocampus through PLCbeta1 signaling. J Neurosci. (2001);21:6387–6394. doi: 10.1523/JNEUROSCI.21-16-06387.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cleva RM, Olive MF. mGlu receptors and drug addiction. Wiley Interdiscip Rev Membr Transp Signal. (2012);1:281–295. doi: 10.1002/wmts.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crans RAJ, Daelemans S, Raedt R, Ciruela F, Stove CP. Kainic acid-induced status epilepticus decreases mGlu(5) receptor and phase-specifically downregulates Homer1b/c expression. Brain Res. (2020);1730:146640. doi: 10.1016/j.brainres.2019.146640. [DOI] [PubMed] [Google Scholar]
- 31.D'Amore V, Santolini I, van Rijn CM, Biagioni F, Molinaro G, Prete A, Conn PJ, Lindsley CW, Zhou Y, Vinson PN, Rodriguez AL, Jones CK, Stauffer SR, Nicoletti F, van Luijtelaar G, Ngomba RT. Potentiation of mGlu5 receptors with the novel enhancer, VU0360172 , reduces spontaneous absence seizures in WAG/Rij rats. Neuropharmacology. (2013);66:330–338. doi: 10.1016/j.neuropharm.2012.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.D'Amore V, Santolini I, Celli R, Lionetto L, De Fusco A, Simmaco M, van Rijn CM, Vieira E, Stauffer SR, Conn PJ, Bosco P, Nicoletti F, van Luijtelaar G, Ngomba RT. Head-to head comparison of mGlu1 and mGlu5 receptor activation in chronic treatment of absence epilepsy in WAG/Rij rats. Neuropharmacology. (2014);85:91–103. doi: 10.1016/j.neuropharm.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.D'Amore V, von Randow C, Nicoletti F, Ngomba RT, van Luijtelaar G. Anti-absence activity of mGlu1 and mGlu5 receptor enhancers and their interaction with a GABA reuptake inhibitor:Effect of local infusions in the somatosensory cortex and thalamus. Epilepsia. (2015);56:1141–1151. doi: 10.1111/epi.13024. [DOI] [PubMed] [Google Scholar]
- 34.D'Amore V, Raaijmakers RH, Santolini I, van Rijn CM, Ngomba RT, Nicoletti F, van Luijtelaar G. The anti-absence effect of mGlu5 receptor amplification with VU0360172 is maintained during and after antiepileptogenesis. Pharmacol Biochem Behav. (2016);146-147:50–59. doi: 10.1016/j.pbb.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 35.Dahl V, Helmbrecht H, Rios Sigler A, Hildahl K, Sullivan H, Janakiraman S, Jasti S, Nance E. Characterization of a mGluR5 knockout rat model with hallmarks of fragile X syndrome. Life (Basel) (2022);12:1308. doi: 10.3390/life12091308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dalley CB, Wroblewska B, Wolfe BB, Wroblewski JT. The role of metabotropic glutamate receptor 1 dependent signaling in glioma viability. J Pharmacol Exp Ther. (2018);367:59–70. doi: 10.1124/jpet.118.250159. [DOI] [PubMed] [Google Scholar]
- 37.Dammann F, Kirschstein T, Guli X, Muller S, Porath K, Rohde M, Tokay T, Kohling R. Bidirectional shift of group III metabotropic glutamate receptor-mediated synaptic depression in the epileptic hippocampus. Epilepsy Res. (2018);139:157–163. doi: 10.1016/j.eplepsyres.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 38.Danek J, Danacikova S, Kala D, Svoboda J, Kapoor S, Posusta A, Folbergrova J, Tauchmannova K, Mracek T, Otahal J. Sulforaphane ameliorates metabolic changes associated with status epilepticus in immature rats. Front Cell Neurosci. (2022);16:855161. doi: 10.3389/fncel.2022.855161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Das A, Wallace GCt, Holmes C, McDowell ML, Smith JA, Marshall JD, Bonilha L, Edwards JC, Glazier SS, Ray SK, Banik NL. Hippocampal tissue of patients with refractory temporal lobe epilepsy is associated with astrocyte activation, inflammation , and altered expression of channels and receptors. Neuroscience. (2012);220:237–246. doi: 10.1016/j.neuroscience.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dasgupta A, Lim YJ, Kumar K, Baby N, Pang KLK, Benoy A, Behnisch T, Sajikumar S. Group III metabotropic glutamate receptors gate long-term potentiation and synaptic tagging/capture in rat hippocampal area CA2. Elife. (2020);9:e55344. doi: 10.7554/eLife.55344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Davidson S, Golden JP, Copits BA, Ray PR, Vogt SK, Valtcheva MV, Schmidt RE, Ghetti A, Price TJ, Gereau RWt. Group II mGluRs suppress hyperexcitability in mouse and human nociceptors. Pain. (2016);157:2081–2088. doi: 10.1097/j.pain.0000000000000621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Di Cicco G, Marzano E, Iacovelli L, Celli R, van Luijtelaar G, Nicoletti F, Ngomba RT, Wall MJ. Group I metabotropic glutamate receptor-mediated long term depression is disrupted in the hippocampus of WAG/Rij rats modelling absence epilepsy. Neuropharmacology. (2021);196:108686. doi: 10.1016/j.neuropharm.2021.108686. [DOI] [PubMed] [Google Scholar]
- 43.DuBois JM, Mathotaarachchi S, Rousset OG, Sziklas V, Sepulcre J, Guiot MC, Hall JA, Massarweh G, Soucy JP, Rosa-Neto P, Kobayashi E. Large-scale mGluR5 network abnormalities linked to epilepsy duration in focal cortical dysplasia. Neuroimage Clin. (2021);29:102552. doi: 10.1016/j.nicl.2020.102552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dunn HA, Zucca S, Dao M, Orlandi C, Martemyanov KA. ELFN2 is a postsynaptic cell adhesion molecule with essential roles in controlling group III mGluRs in the brain and neuropsychiatric behavior. Mol Psychiatry. (2019);24:1902–1919. doi: 10.1038/s41380-019-0512-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dyomina AV, Kovalenko AA, Zakharova MV, Postnikova TY, Griflyuk AV, Smolensky IV, Antonova IV, Zaitsev AV. MTEP, a selective mGluR5 antagonist, had a neuroprotective effect but did not prevent the development of spontaneous recurrent seizures and behavioral comorbidities in the rat lithium-pilocarpine model of epilepsy. Int J Mol Sci. (2022);23:497. doi: 10.3390/ijms23010497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eddy K, Eddin MN, Fateeva A, Pompili SVB, Shah R, Doshi S, Chen S. Implications of a neuronal receptor family, metabotropic glutamate receptors, in cancer development and progression. Cells. (2022);11:2857. doi: 10.3390/cells11182857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Edfawy M, Guedes JR, Pereira MI, Laranjo M, Carvalho MJ, Gao X, Ferreira PA, Caldeira G, Franco LO, Wang D, Cardoso AL, Feng G, Carvalho AL, Peca J. Abnormal mGluR-mediated synaptic plasticity and autism-like behaviours in Gprasp2 mutant mice. Nat Commun. (2019);10:1431. doi: 10.1038/s41467-019-09382-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Enz R. Metabotropic glutamate receptors and interacting proteins:evolving drug targets. Curr Drug Targets. (2012);13:145–156. doi: 10.2174/138945012798868452. [DOI] [PubMed] [Google Scholar]
- 49.Evans EE, Mishra V, Mallow C, Gersz EM, Balch L, Howell A, Reilly C, Smith ES, Fisher TL, Zauderer M. Semaphorin 4D is upregulated in neurons of diseased brains and triggers astrocyte reactivity. J Neuroinflammation. (2022);19:200. doi: 10.1186/s12974-022-02509-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fern R, Matute C. Glutamate receptors and white matter stroke. Neurosci Lett. (2019);694:86–92. doi: 10.1016/j.neulet.2018.11.031. [DOI] [PubMed] [Google Scholar]
- 51.Folbergrova J, Haugvicova R, Mares P. Attenuation of seizures induced by homocysteic acid in immature rats by metabotropic glutamate group II and group III receptor agonists. Brain Res. (2001);908:120–129. doi: 10.1016/s0006-8993(01)02620-8. [DOI] [PubMed] [Google Scholar]
- 52.Folbergrova J, Otahal J, Druga R. Brain superoxide anion formation in immature rats during seizures:protection by selected compounds. Exp Neurol. (2012);233:421–429. doi: 10.1016/j.expneurol.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 53.Gan X, Wu J, Ren C, Qiu CY, Li YK, Hu WP. Potentiation of acid-sensing ion channel activity by peripheral group I metabotropic glutamate receptor signaling. Pharmacol Res. (2016);107:19–26. doi: 10.1016/j.phrs.2016.02.018. [DOI] [PubMed] [Google Scholar]
- 54.Gass JT, Olive MF. Transcriptional profiling of the rat frontal cortex following administration of the mGlu5 receptor antagonists MPEP and MTEP. Eur J Pharmacol. (2008);584:253–262. doi: 10.1016/j.ejphar.2008.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gedikli O, Akca M, Yildirim M. Investigating the mechanism of action of ginkgolides and bilobalide on absence seizures in male WAG/Rij rats. J Neurosci Res. (2023);101:866–880. doi: 10.1002/jnr.25166. [DOI] [PubMed] [Google Scholar]
- 56.Ghasemi Z, Naderi N, Shojaei A, Raoufy MR, Ahmadirad N, Barkley V, Mirnajafi-Zadeh J. Group I metabotropic glutamate receptors contribute to the antiepileptic effect of electrical stimulation in hippocampal CA1 pyramidal neurons. Epilepsy Res. (2021);178:106821. doi: 10.1016/j.eplepsyres.2021.106821. [DOI] [PubMed] [Google Scholar]
- 57.Gladding CM, Fitzjohn SM, Molnar E. Metabotropic glutamate receptor-mediated long-term depression:molecular mechanisms. Pharmacol Rev. (2009);61:395–412. doi: 10.1124/pr.109.001735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Govindaiah G, Kang YJ, Lewis HES, Chung L, Clement EM, Greenfield LJ, Jr, Garcia-Rill E, Lee SH. Group I metabotropic glutamate receptors generate two types of intrinsic membrane oscillations in hippocampal oriens/alveus interneurons. Neuropharmacology. (2018);139:150–162. doi: 10.1016/j.neuropharm.2018.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gregory KJ, Goudet C. International union of basic and clinical pharmacology. CXI. pharmacology, signaling, and physiology of metabotropic glutamate receptors. Pharmacol Rev. (2021);73:521–569. doi: 10.1124/pr.119.019133. [DOI] [PubMed] [Google Scholar]
- 60.Gubellini P, Pisani A, Centonze D, Bernardi G, Calabresi P. Metabotropic glutamate receptors and striatal synaptic plasticity:implications for neurological diseases. Prog Neurobiol. (2004);74:271–300. doi: 10.1016/j.pneurobio.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 61.Guo B, Wang J, Yao H, Ren K, Chen J, Yang J, Cai G, Liu H, Fan Y, Wang W, Wu S. Chronic inflammatory pain impairs mGluR5-mediated depolarization-induced suppression of excitation in the anterior cingulate cortex. Cereb Cortex. (2018);28:2118–2130. doi: 10.1093/cercor/bhx117. [DOI] [PubMed] [Google Scholar]
- 62.Gurgone A, Pizzo R, Raspanti A, Chiantia G, Devi S, Comai D, Morello N, Pilotto F, Gnavi S, Lupori L, Mazziotti R, Sagona G, Putignano E, Nocentini A, Supuran CT, Marcantoni A, Pizzorusso T, Giustetto M. mGluR5 PAMs rescue cortical and behavioural defects in a mouse model of CDKL5 deficiency disorder. Neuropsychopharmacology. (2022) doi: 10.1038/s41386-022-01412-3. doi:10.1038/s41386-022-01412-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hanak TJ, Libbey JE, Doty DJ, Sim JT, DePaula-Silva AB, Fujinami RS. Positive modulation of mGluR5 attenuates seizures and reduces TNF-alpha(+) macrophages and microglia in the brain in a murine model of virus-induced temporal lobe epilepsy. Exp Neurol. (2019);311:194–204. doi: 10.1016/j.expneurol.2018.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hermans E, Challiss RA. Structural, signalling and regulatory properties of the group I metabotropic glutamate receptors:prototypic family C G-protein-coupled receptors. Biochem J. (2001);359:465–484. doi: 10.1042/0264-6021:3590465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Howlett E, Lin CC, Lavery W, Stern M. A PI3-kinase-mediated negative feedback regulates neuronal excitability. PLoS Genet. (2008);4:e1000277. doi: 10.1371/journal.pgen.1000277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hu C, Chen C, Xia Y, Chen J, Yang W, Wang L, Chen DD, Wu YZ, Fan Q, Jia XX, Xiao K, Shi Q, Chen ZB, Dong XP. Different aberrant changes of mGluR5 and its downstream signaling pathways in the scrapie-infected cell line and the brains of scrapie-infected experimental rodents. Front Cell Dev Biol. (2022);10:844378. doi: 10.3389/fcell.2022.844378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jin Y, Kim SJ, Kim J, Worley PF, Linden DJ. Long-term depression of mGluR1 signaling. Neuron. (2007);55:277–287. doi: 10.1016/j.neuron.2007.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Luo XD, Xiang T, Li SJ, Ma MG, Chen ML, Wu Y. Activation of metabotropic glutamate receptor 1 regulates hippocampal CA1 region excitability in rats with status epilepticus by suppressing the HCN1 channel. Neural Regen Res. (2023);18:594–602. doi: 10.4103/1673-5374.350206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kandratavicius L, Rosa-Neto P, Monteiro MR, Guiot MC, Assirati JA, Jr, Carlotti CG, Jr, Kobayashi E, Leite JP. Distinct increased metabotropic glutamate receptor type 5 (mGluR5) in temporal lobe epilepsy with and without hippocampal sclerosis. Hippocampus. (2013);23:1212–1230. doi: 10.1002/hipo.22160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kazim SF, Chuang SC, Zhao W, Wong RK, Bianchi R, Iqbal K. Early-onset network hyperexcitability in presymptomatic Alzheimer's disease transgenic mice is suppressed by passive immunization with anti-human APP/Abeta antibody and by mGluR5 blockade. Front Aging Neurosci. (2017);9:71. doi: 10.3389/fnagi.2017.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kelly E, Schaeffer SM, Dhamne SC, Lipton JO, Lindemann L, Honer M, Jaeschke G, Super CE, Lammers SH, Modi ME, Silverman JL, Dreier JR, Kwiatkowski DJ, Rotenberg A, Sahin M. mGluR5 modulation of behavioral and epileptic phenotypes in a mouse model of tuberous sclerosis complex. Neuropsychopharmacology. (2018);43:1457–1465. doi: 10.1038/npp.2017.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kelly L, Farrant M, Cull-Candy SG. Synaptic mGluR activation drives plasticity of calcium-permeable AMPA receptors. Nat Neurosci. (2009);12:593–601. doi: 10.1038/nn.2309. [DOI] [PubMed] [Google Scholar]
- 73.Kirkman NJ, Libbey JE, Wilcox KS, White HS, Fujinami RS. Innate but not adaptive immune responses contribute to behavioral seizures following viral infection. Epilepsia. (2010);51:454–464. doi: 10.1111/j.1528-1167.2009.02390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kovács Z, Skatchkov SN, Szabó Z, Qahtan S, Méndez-González MP, Malpica-Nieves CJ, Eaton MJ, Kardos J, Héja L. Putrescine intensifies Glu/GABA exchange mechanism and promotes early termination of seizures. Int J Mol Sci. (2022);23:8191. doi: 10.3390/ijms23158191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kovalenko AA, Zakharova MV, Schwarz AP, Dyomina AV, Zubareva OE, Zaitsev AV. Changes in metabotropic glutamate receptor gene expression in rat brain in a lithium-pilocarpine model of temporal lobe epilepsy. Int J Mol Sci. (2022);23:2752. doi: 10.3390/ijms23052752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Krishnakumar A, Anju TR, Abraham PM, Paulose CS. Alteration in 5-HT₂C, NMDA receptor and IP3 in cerebral cortex of epileptic rats:restorative role of Bacopa monnieri. Neurochem Res. (2015);40:216–225. doi: 10.1007/s11064-014-1472-2. [DOI] [PubMed] [Google Scholar]
- 77.Kumar K, Banerjee Dixit A, Tripathi M, Dubey V, Siraj F, Sharma MC, Lalwani S, Chandra PS, Banerjee J. Transcriptomic profiling of nonneoplastic cortical tissues reveals epileptogenic mechanisms in dysembryoplastic neuroepithelial tumors. Funct Integr Genomics. (2022);22:905–917. doi: 10.1007/s10142-022-00869-1. [DOI] [PubMed] [Google Scholar]
- 78.Lam J, DuBois JM, Rowley J, González-Otárula KA, Soucy JP, Massarweh G, Hall JA, Guiot MC, Rosa-Neto P, Kobayashi E. In vivo metabotropic glutamate receptor type 5 abnormalities localize the epileptogenic zone in mesial temporal lobe epilepsy. Ann Neurol. (2019);85:218–228. doi: 10.1002/ana.25404. [DOI] [PubMed] [Google Scholar]
- 79.Lazo-Gomez R, Tapia R. Motor alterations induced by chronic 4-aminopyridine infusion in the spinal cord in vivo:role of glutamate and GABA receptors. Front Neurosci. (2016);10:200. doi: 10.3389/fnins.2016.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li C, Chai S, Ju Y, Hou L, Zhao H, Ma W, Li T, Sheng J, Shi W. Pu-erh tea protects the nervous system by inhibiting the expression of metabotropic glutamate receptor 5. Mol Neurobiol. (2017);54:5286–5299. doi: 10.1007/s12035-016-0064-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li Q, Jin R, Zhang S, Sun X, Wu J. Group II metabotropic glutamate receptor agonist promotes retinal ganglion cell survival by reducing neuronal excitotoxicity in a rat chronic ocular hypertension model. Neuropharmacology. (2020);170:108016. doi: 10.1016/j.neuropharm.2020.108016. [DOI] [PubMed] [Google Scholar]
- 82.Li SH, Abd-Elrahman KS, Ferguson SSG. Targeting mGluR2/3 for treatment of neurodegenerative and neuropsychiatric diseases. Pharmacol Ther. (2022);239:108275. doi: 10.1016/j.pharmthera.2022.108275. [DOI] [PubMed] [Google Scholar]
- 83.Liang X, Miao A, Zhang W, Li M, Xing Y. Effect of family integrated care on physical growth and language development of premature infants:a retrospective study. Transl Pediatr. (2022);11:965–977. doi: 10.21037/tp-22-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lima IVA, Bellozi PMQ, Batista EM, Vilela LR, Brandao IL, Ribeiro FM, Moraes MFD, Moreira FA, de Oliveira ACP. Cannabidiol anticonvulsant effect is mediated by the PI3Kgamma pathway. Neuropharmacology. (2020);176:108156. doi: 10.1016/j.neuropharm.2020.108156. [DOI] [PubMed] [Google Scholar]
- 85.Liu B, Nadeem U, Frick A, Alakaloko M, Bhide A, Thilaganathan B. Reducing health inequality in Black, Asian and other minority ethnic pregnant women:impact of first trimester combined screening for placental dysfunction on perinatal mortality. Bjog. (2022a);129:1750–1756. doi: 10.1111/1471-0528.17109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu DC, Soriano S, Yook Y, Lizarazo S, Eagleman DE, Tsai NP. Chronic activation of Gp1 mGluRs leads to distinct refinement of neural network activity through non-canonical p53 and Akt signaling. eNeuro. (2020);7 doi: 10.1523/ENEURO.0438-19.2020. ENEURO.0438-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liu Y, Wang Y, Yang J, Xu T, Tan C, Zhang P, Liu Q, Chen Y. G-alpha interacting protein interacting protein, C terminus 1 regulates epileptogenesis by increasing the expression of metabotropic glutamate receptor 7. CNS Neurosci Ther. (2022b);28:126–138. doi: 10.1111/cns.13746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu Y, Lou W, Chen G, Ding B, Kuang J, Zhang Y, Wang C, Duan S, Deng Y, Lu X. Genome-wide screening for the G-protein-coupled receptor (GPCR) pathway-related therapeutic gene RGS19 (regulator of G protein signaling 19) in bladder cancer. Bioengineered. (2021);12:5892–5903. doi: 10.1080/21655979.2021.1971035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lovisari F, Roncon P, Soukoupova M, Paolone G, Labasque M, Ingusci S, Falcicchia C, Marino P, Johnson M, Rossetti T, Petretto E, Leclercq K, Kaminski RM, Moyon B, Webster Z, Simonato M, Zucchini S. Implication of sestrin3 in epilepsy and its comorbidities. Brain Commun. (2021);3:fcaa130. doi: 10.1093/braincomms/fcaa130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Luessen DJ, Conn PJ. Allosteric modulators of metabotropic glutamate receptors as novel therapeutics for neuropsychiatric disease. Pharmacol Rev. (2022);74:630–661. doi: 10.1124/pharmrev.121.000540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lukawski K, Andres-Mach M, Czuczwar M, Luszczki JJ, Kruszynski K, Czuczwar SJ. Mechanisms of epileptogenesis and preclinical approach to antiepileptogenic therapies. Pharmacol Rep. (2018);70:284–293. doi: 10.1016/j.pharep.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 92.Maciejak P, Szyndler J, Turzynska D, Sobolewska A, Taracha E, Skorzewska A, Lehner M, Bidzinski A, Hamed A, Wislowska-Stanek A, Plaznik A. The effects of group III mGluR ligands on pentylenetetrazol-induced kindling of seizures and hippocampal amino acids concentration. Brain Res. (2009);1282:20–27. doi: 10.1016/j.brainres.2009.05.049. [DOI] [PubMed] [Google Scholar]
- 93.Maksymetz J, Moran SP, Conn PJ. Targeting metabotropic glutamate receptors for novel treatments of schizophrenia. Mol Brain. (2017);10:15. doi: 10.1186/s13041-017-0293-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Malenka RC, Bear MF. LTP and LTD:an embarrassment of riches. Neuron. (2004);44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 95.Malik R, Mehta P, Srivastava S, Choudhary BS, Sharma M. Structure-based screening, ADMET profiling, and molecular dynamic studies on mGlu2 receptor for identification of newer antiepileptic agents. J Biomol Struct Dyn. (2017);35:3433–3448. doi: 10.1080/07391102.2016.1257440. [DOI] [PubMed] [Google Scholar]
- 96.Mao LM, Bodepudi A, Chu XP, Wang JQ. Group I metabotropic glutamate receptors and interacting partners:an update. Int J Mol Sci. (2022);23:840. doi: 10.3390/ijms23020840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Matsunaga H, Aruga J. Trans-synaptic regulation of metabotropic glutamate receptors by Elfn proteins in health and disease. Front Neural Circuits. (2021);15:634875. doi: 10.3389/fncir.2021.634875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mazzitelli M, Presto P, Antenucci N, Meltan S, Neugebauer V. Recent advances in the modulation of pain by the metabotropic glutamate receptors. Cells. (2022);11:2608. doi: 10.3390/cells11162608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.McBain CJ. Hippocampal inhibitory neuron activity in the elevated potassium model of epilepsy. J Neurophysiol. (1995);73:2853–2863. doi: 10.1152/jn.1995.73.2.2853. [DOI] [PubMed] [Google Scholar]
- 100.McNamara JO, Huang YZ, Leonard AS. Molecular signaling mechanisms underlying epileptogenesis. Sci STKE. (2006);2006:r.e12. doi: 10.1126/stke.3562006re12. [DOI] [PubMed] [Google Scholar]
- 101.Medina-Ceja L, Garcia-Barba C. The glutamate receptor antagonists CNQX and MPEP decrease fast ripple events in rats treated with kainic acid. Neurosci Lett. (2017);655:137–142. doi: 10.1016/j.neulet.2017.06.056. [DOI] [PubMed] [Google Scholar]
- 102.Meldrum BS, Akbar MT, Chapman AG. Glutamate receptors and transporters in genetic and acquired models of epilepsy. Epilepsy Res. (1999);36:189–204. doi: 10.1016/s0920-1211(99)00051-0. [DOI] [PubMed] [Google Scholar]
- 103.Metcalf CS, Klein BD, Smith MD, Ceusters M, Lavreysen H, Pype S, Van Osselaer N, Twyman R, White HS. Potent and selective pharmacodynamic synergy between the metabotropic glutamate receptor subtype 2-positive allosteric modulator JNJ-46356479 and levetiracetam in the mouse 6-Hz (44-mA) model. Epilepsia. (2018);59:724–735. doi: 10.1111/epi.14005. [DOI] [PubMed] [Google Scholar]
- 104.Micheli F. Methylphenylethynylpyridine (MPEP) Novartis. Curr Opin Investig Drugs. (2000);1:355–359. [PubMed] [Google Scholar]
- 105.Miller B, Moreno N, Gutierrez BA, Limon A. Microtransplantation of postmortem native synaptic mGluRs receptors into Xenopus oocytes for their functional analysis. Membranes (Basel) (2022);12:931. doi: 10.3390/membranes12100931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Molinari F, Cattani AA, Mdzomba JB, Aniksztejn L. Glutamate transporters control metabotropic glutamate receptors activation to prevent the genesis of paroxysmal burst in the developing hippocampus. Neuroscience. (2012);207:25–36. doi: 10.1016/j.neuroscience.2012.01.036. [DOI] [PubMed] [Google Scholar]
- 107.Neyer C, Herr D, Kohmann D, Budde T, Pape HC, Coulon P. mGluR-mediated calcium signalling in the thalamic reticular nucleus. Cell Calcium. (2016);59:312–323. doi: 10.1016/j.ceca.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 108.Neyman S, Manahan-Vaughan D. Metabotropic glutamate receptor 1 (mGluR1) and 5 (mGluR5) regulate late phases of LTP and LTD in the hippocampal CA1 region in vitro. Eur J Neurosci. (2008);27:1345–1352. doi: 10.1111/j.1460-9568.2008.06109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nicolo JP, O'Brien TJ, Kwan P. Role of cerebral glutamate in post-stroke epileptogenesis. Neuroimage Clin. (2019);24:102069. doi: 10.1016/j.nicl.2019.102069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Niswender CM, Conn PJ. Metabotropic glutamate receptors:physiology, pharmacology , and disease. Annu Rev Pharmacol Toxicol. (2010);50:295–322. doi: 10.1146/annurev.pharmtox.011008.145533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pajarillo E, Rizor A, Lee J, Aschner M, Lee E. The role of astrocytic glutamate transporters GLT-1 and GLAST in neurological disorders:Potential targets for neurotherapeutics. Neuropharmacology. (2019);161:107559. doi: 10.1016/j.neuropharm.2019.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pandey S, Ramsakha N, Sharma R, Gulia R, Ojha P, Lu W, Bhattacharyya S. The post-synaptic scaffolding protein tamalin regulates ligand-mediated trafficking of metabotropic glutamate receptors. J Biol Chem. (2020);295:8575–8588. doi: 10.1074/jbc.RA119.011979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Paquet M, Ribeiro FM, Guadagno J, Esseltine JL, Ferguson SS, Cregan SP. Role of metabotropic glutamate receptor 5 signaling and homer in oxygen glucose deprivation-mediated astrocyte apoptosis. Mol Brain. (2013);6:9. doi: 10.1186/1756-6606-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Park S, Park JM, Kim S, Kim JA, Shepherd JD, Smith-Hicks CL, Chowdhury S, Kaufmann W, Kuhl D, Ryazanov AG, Huganir RL, Linden DJ, Worley PF. Elongation factor 2 and fragile X mental retardation protein control the dynamic translation of Arc/Arg3.1 essential for mGluR-LTD. Neuron. (2008);59:70–83. doi: 10.1016/j.neuron.2008.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pereira V, Goudet C. Emerging Trends in Pain Modulation by Metabotropic glutamate receptors. Front Mol Neurosci. (2018);11:464. doi: 10.3389/fnmol.2018.00464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Peterson AR, Binder DK. Astrocyte glutamate uptake and signaling as novel targets for antiepileptogenic therapy. Front Neurol. (2020);11:1006. doi: 10.3389/fneur.2020.01006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pitsch J, Schoch S, Gueler N, Flor PJ, der Putten van H, Becker AJ. Functional role of mGluR1 and mGluR4 in pilocarpine-induced temporal lobe epilepsy. Neurobiol Dis. (2007);26:623–633. doi: 10.1016/j.nbd.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 118.Pohlentz MS, Muller P, Cases-Cunillera S, Opitz T, Surges R, Hamed M, Vatter H, Schoch S, Becker AJ, Pitsch J. Characterisation of NLRP3 pathway-related neuroinflammation in temporal lobe epilepsy. PLoS One. (2022);17:e0271995. doi: 10.1371/journal.pone.0271995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Postnikova TY, Trofimova AM, Ergina JL, Zubareva OE, Kalemenev SV, Zaitsev AV. Transient switching of NMDA-dependent long-term synaptic potentiation in CA3-CA1 hippocampal synapses to mGluR(1)-dependent potentiation after pentylenetetrazole-induced acute seizures in young rats. Cell Mol Neurobiol. (2019);39:287–300. doi: 10.1007/s10571-018-00647-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Potter WB, Basu T, O'Riordan KJ, Kirchner A, Rutecki P, Burger C, Roopra A. Reduced juvenile long-term depression in tuberous sclerosis complex is mitigated in adults by compensatory recruitment of mGluR5 and Erk signaling. PLoS Biol. (2013);11:e1001627. doi: 10.1371/journal.pbio.1001627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Qian F, Tang FR. Metabotropic glutamate receptors and interacting proteins in epileptogenesis. Curr Neuropharmacol. (2016);14:551–562. doi: 10.2174/1570159X14666160331142228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ribeiro FM, Vieira LB, Pires RG, Olmo RP, Ferguson SS. Metabotropic glutamate receptors and neurodegenerative diseases. Pharmacol Res. (2017);115:179–191. doi: 10.1016/j.phrs.2016.11.013. [DOI] [PubMed] [Google Scholar]
- 123.Roth BL. Molecular pharmacology of metabotropic receptors targeted by neuropsychiatric drugs. Nat Struct Mol Biol. (2019);26:535–544. doi: 10.1038/s41594-019-0252-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sadananda G, Subramaniam JR. Absence of metabotropic glutamate receptor homolog(s) accelerates acetylcholine neurotransmission in Caenorhabditis elegans. Neurosci Lett. (2021);746:135666. doi: 10.1016/j.neulet.2021.135666. [DOI] [PubMed] [Google Scholar]
- 125.Salvati K, Beenhakker M. Astrocyte receptor rebirth. Epilepsy Curr. (2019);19:196–198. doi: 10.1177/1535759719844267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sanon NT, Pelletier JG, Carmant L, Lacaille JC. Interneuron subtype specific activation of mGluR1/5 during epileptiform activity in hippocampus. Epilepsia. (2010);51:1607–1618. doi: 10.1111/j.1528-1167.2010.02689.x. [DOI] [PubMed] [Google Scholar]
- 127.Sansig G, Bushell TJ, Clarke VR, Rozov A, Burnashev N, Portet C, Gasparini F, Schmutz M, Klebs K, Shigemoto R, Flor PJ, Kuhn R, Knoepfel T, Schroeder M, Hampson DR, Collett VJ, Zhang C, Duvoisin RM, Collingridge GL, van Der Putten H. Increased seizure susceptibility in mice lacking metabotropic glutamate receptor 7. J Neurosci. (2001);21:8734–8745. doi: 10.1523/JNEUROSCI.21-22-08734.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Schoepp DD. Unveiling the functions of presynaptic metabotropic glutamate receptors in the central nervous system. J Pharmacol Exp Ther. (2001);299:12–20. [PubMed] [Google Scholar]
- 129.Selvam C, Lemasson IA, Brabet I, Oueslati N, Karaman B, Cabaye A, Tora AS, Commare B, Courtiol T, Cesarini S, McCort-Tranchepain I, Rigault D, Mony L, Bessiron T, McLean H, Leroux FR, Colobert F, Daniel H, Goupil-Lamy A, Bertrand HO, et al. Increased potency and selectivity for Group III metabotropic glutamate receptor agonists binding at dual sites. J Med Chem. (2018);61:1969–1989. doi: 10.1021/acs.jmedchem.7b01438. [DOI] [PubMed] [Google Scholar]
- 130.Servaes S, Kara F, Glorie D, Stroobants S, Van Der Linden A, Staelens S. In vivo preclinical molecular imaging of repeated exposure to an N-methyl-d-aspartate antagonist and a glutaminase inhibitor as potential glutamatergic modulators. J Pharmacol Exp Ther. (2019);368:382–390. doi: 10.1124/jpet.118.252635. [DOI] [PubMed] [Google Scholar]
- 131.Seven AB, Barros-Alvarez X, de Lapeyriere M, Papasergi-Scott MM, Robertson MJ, Zhang C, Nwokonko RM, Gao Y, Meyerowitz JG, Rocher JP, Schelshorn D, Kobilka BK, Mathiesen JM, Skiniotis G. G-protein activation by a metabotropic glutamate receptor. Nature. (2021);595:450–454. doi: 10.1038/s41586-021-03680-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Shaw VS, Mohammadi M, Quinn JA, Vashisth H, Neubig RR. An interhelical salt bridge controls flexibility and inhibitor potency for regulators of G-protein signaling proteins 4, 8 , and 19. Mol Pharmacol. (2019);96:683–691. doi: 10.1124/mol.119.117176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Su LD, Wang N, Han J, Shen Y. Group 1 metabotropic glutamate receptors in neurological and psychiatric diseases:mechanisms and prospective. Neuroscientist. (2022);28:453–468. doi: 10.1177/10738584211021018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tang FR, Bradford HF, Ling EA. Metabotropic glutamate receptors in the control of neuronal activity and as targets for development of anti-epileptogenic drugs. Curr Med Chem. (2009);16:2189–2204. doi: 10.2174/092986709788612710. [DOI] [PubMed] [Google Scholar]
- 135.Thomsen C, Klitgaard H, Sheardown M, Jackson HC, Eskesen K, Jacobsen P, Treppendahl S, Suzdak PD. (S)-4-carboxy-3-hydroxyphenylglycine, an antagonist of metabotropic glutamate receptor (mGluR) 1a and an agonist of mGluR2, protects against audiogenic seizures in DBA/2 mice. J Neurochem. (1994);62:2492–2495. doi: 10.1046/j.1471-4159.1994.62062492.x. [DOI] [PubMed] [Google Scholar]
- 136.Thomsen C, Dalby NO. Roles of metabotropic glutamate receptor subtypes in modulation of pentylenetetrazole-induced seizure activity in mice. Neuropharmacology. (1998);37:1465–1473. doi: 10.1016/s0028-3908(98)00138-5. [DOI] [PubMed] [Google Scholar]
- 137.Tuduri P, Bouquier N, Girard B, Moutin E, Thouaye M, Perroy J, Bertaso F, Ster J. Modulation of hippocampal network oscillation by PICK1-dependent cell surface expression of mGlu3 receptors. J Neurosci. (2022);42:8897–8911. doi: 10.1523/JNEUROSCI.0063-22.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Turati J, Rudi J, Beauquis J, Carniglia L, Lopez Couselo F, Saba J, Caruso C, Saravia F, Lasaga M, Durand D. A metabotropic glutamate receptor 3 (mGlu3R) isoform playing neurodegenerative roles in astrocytes is prematurely up-regulated in an Alzheimer's model. J Neurochem. (2022);161:366–382. doi: 10.1111/jnc.15610. [DOI] [PubMed] [Google Scholar]
- 139.Umpierre AD, West PJ, White JA, Wilcox KS. Conditional knock-out of mGluR5 from astrocytes during epilepsy development impairs high-frequency glutamate uptake. J Neurosci. (2019);39:727–742. doi: 10.1523/JNEUROSCI.1148-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ure J, Baudry M, Perassolo M. Metabotropic glutamate receptors and epilepsy. J Neurol Sci. (2006);247:1–9. doi: 10.1016/j.jns.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 141.Vahidinia Z, Joghataei MT, Beyer C, Karimian M, Tameh AA. G-protein-coupled receptors and ischemic stroke:a focus on molecular function and therapeutic potential. Mol Neurobiol. (2021);58:4588–4614. doi: 10.1007/s12035-021-02435-5. [DOI] [PubMed] [Google Scholar]
- 142.Valian N, Sorayya M, Asadi S, Sherafati F, Ershad A, Savaheli S, Ahmadiani A. Preconditioning by ultra-low dose of tramadol reduces the severity of tramadol-induced seizure:Contribution of glutamate receptors. Biomed Pharmacother. (2021);133:111031. doi: 10.1016/j.biopha.2020.111031. [DOI] [PubMed] [Google Scholar]
- 143.Vera G, Tapia R. Activation of group III metabotropic glutamate receptors by endogenous glutamate protects against glutamate-mediated excitotoxicity in the hippocampus in vivo. J Neurosci Res. (2012);90:1055–1066. doi: 10.1002/jnr.23006. [DOI] [PubMed] [Google Scholar]
- 144.Vijaya Prabhu S, Singh SK. E-pharmacophore-based screening of mGluR5 negative allosteric modulators for central nervous system disorder. Comput Biol Chem. (2019);78:414–423. doi: 10.1016/j.compbiolchem.2018.12.016. [DOI] [PubMed] [Google Scholar]
- 145.Wang R, Reddy PH. Role of glutamate and NMDA receptors in Alzheimer's disease. J Alzheimers Dis. (2017);57:1041–1048. doi: 10.3233/JAD-160763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wang X, Wang Y, Zhang C, Liu C, Zhao B, Wei N, Zhang JG, Zhang K. CB1 receptor antagonism prevents long-term hyperexcitability after head injury by regulation of dynorphin-KOR system and mGluR5 in rat hippocampus. Brain Res. (2016);1646:174–181. doi: 10.1016/j.brainres.2016.05.055. [DOI] [PubMed] [Google Scholar]
- 147.Watanabe Y, Kaida Y, Fukuhara S, Takechi K, Uehara T, Kamei C. Participation of metabotropic glutamate receptors in pentetrazol-induced kindled seizure. Epilepsia. (2011);52:140–150. doi: 10.1111/j.1528-1167.2010.02764.x. [DOI] [PubMed] [Google Scholar]
- 148.Westmark CJ. Fragile X and APP:a decade in review, a vision for the future. Mol Neurobiol. (2019);56:3904–3921. doi: 10.1007/s12035-018-1344-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Westmark PR, Garrone B, Ombrato R, Milanese C, Di Giorgio FP, Westmark CJ. Testing Fmr1 (KO) phenotypes in response to GSK3 inhibitors:SB216763 versus AFC03127. Front Mol Neurosci. (2021);14:751307. doi: 10.3389/fnmol.2021.751307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Willard SS, Koochekpour S. Glutamate, glutamate receptors, and downstream signaling pathways. Int J Biol Sci. (2013);9:948–959. doi: 10.7150/ijbs.6426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Witkin JM, Pandey KP, Smith JL. Clinical investigations of compounds targeting metabotropic glutamate receptors. Pharmacol Biochem Behav. (2022);219:173446. doi: 10.1016/j.pbb.2022.173446. [DOI] [PubMed] [Google Scholar]
- 152.Xiang Z, Lv X, Lin X, O'Brien DE, Altman MK, Lindsley CW, Javitch JA, Niswender CM, Conn PJ. Input-specific regulation of glutamatergic synaptic transmission in the medial prefrontal cortex by mGlu2/mGlu4 receptor heterodimers. Sci Signal. (2021);14:eabd2319. doi: 10.1126/scisignal.abd2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Xu Y, Li Z, Yao L, Zhang X, Gan D, Jiang M, Wang N, Chen G, Wang X. Altered norbin expression in patients with epilepsy and a rat model. Sci Rep. (2017);7:13970. doi: 10.1038/s41598-017-13248-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yalcin O. Genes and molecular mechanisms involved in the epileptogenesis of idiopathic absence epilepsies. Seizure. (2012);21:79–86. doi: 10.1016/j.seizure.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 155.Yao H, Feng YB, Pang YJ, Xu JJ, Yu BX, Liu XP. Inhibitory effect of group II mGluR agonist 2R, 4R-APDC on cell proliferation in dentate gyrus in rats with epileptic seizure. Eur Rev Med Pharmacol Sci. (2015);19:2922–2927. [PubMed] [Google Scholar]
- 156.Yeung JH, Kwakowsky A. Metabotropic glutamate receptor 1 alpha:a unique receptor variant with variable implications for Alzheimer's disease pathogenesis. Neural Regen Res. (2023);18:2196–2197. doi: 10.4103/1673-5374.369109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zhai J, Zhou YY, Lagrutta A. Sensitivity, specificity and limitation of in vitro hippocampal slice and neuron-based assays for assessment of drug-induced seizure liability. Toxicol Appl Pharmacol. (2021);430:115725. doi: 10.1016/j.taap.2021.115725. [DOI] [PubMed] [Google Scholar]
- 158.Zhang H, Cilz NI, Yang C, Hu B, Dong H, Lei S. Depression of neuronal excitability and epileptic activities by group II metabotropic glutamate receptors in the medial entorhinal cortex. Hippocampus. (2015);25:1299–1313. doi: 10.1002/hipo.22437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zhang M, Liang LH, Lu YJ, Cao X. G protein-coupled receptor-associated sorting proteins:function and relevant disorders. Yi Chuan. (2020);42:713–724. doi: 10.16288/j.yczz.20-020. [DOI] [PubMed] [Google Scholar]
- 160.Zhang Z, Zhang S, Fu P, Zhang Z, Lin K, Ko JK, Yung KK. Roles of glutamate receptors in Parkinson's disease. Int J Mol Sci. (2019);20:4391. doi: 10.3390/ijms20184391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zhao X, Liang L, Xu R, Cheng P, Jia P, Bai Y, Zhang Y, Zhao X, Zheng X, Xiao C. Revealing the antiepileptic effect of alpha-asaronol on pentylenetetrazole-induced seizure rats using NMR-based metabolomics. ACS Omega. (2022);7:6322–6334. doi: 10.1021/acsomega.1c06922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zimmermann M, Minuzzi L, Aliaga Aliaga A, Guiot MC, Hall JA, Soucy JP, Massarweh G, El Mestikawy S, Rosa-Neto P, Kobayashi E. Reduced metabotropic glutamate receptor type 5 availability in the epileptogenic hippocampus:an in vitro study. Front Neurol. (2022);13:888479. doi: 10.3389/fneur.2022.888479. [DOI] [PMC free article] [PubMed] [Google Scholar]


