Abstract
Transition metal carbides and nitrides (MXenes) are crystal nanomaterials with a number of surface functional groups such as fluorine, hydroxyl, and oxygen, which can be used as carriers for proteins and drugs. MXenes have excellent biocompatibility, electrical conductivity, surface hydrophilicity, mechanical properties and easy surface modification. However, at present, the stability of most MXenes needs to be improved, and more synthesis methods need to be explored. MXenes are good substrates for nerve cell regeneration and nerve reconstruction, which have broad application prospects in the repair of nervous system injury. Regarding the application of MXenes in neuroscience, mainly at the cellular level, the long-term in vivo biosafety and effects also need to be further explored. This review focuses on the progress of using MXenes in nerve regeneration over the last few years; discussing preparation of MXenes and their biocompatibility with different cells as well as the regulation by MXenes of nerve cell regeneration in two-dimensional and three-dimensional environments in vitro. MXenes have great potential in regulating the proliferation, differentiation, and maturation of nerve cells and in promoting regeneration and recovery after nerve injury. In addition, this review also presents the main challenges during optimization processes, such as the preparation of stable MXenes and long-term in vivo biosafety, and further discusses future directions in neural tissue engineering.
Keywords: hydrogels, MXenes, nerve regeneration, neural cells, neural stem cells, organoids, spiral ganglion neurons
Introduction
Peripheral (PNS) and central (CNS) nervous system injuries can cause loss of motor and sensory function, greatly affecting patient quality of life. The PNS and CNS and have insufficient capacity to regenerate after injury; therefore, novel approaches are needed to reconstruct cellular structures and restore function. Neural tissue engineering (NET) has emerged as a promising approach for nervous system regeneration. I It is feasible to apply biomaterials to regulate the interactions with nerve cells and additionally use these signals to direct the development, maturation, proliferation, and differentiation of cells (Kim et al., 2012; Santos et al., 2016; Xiao et al., 2020). Various biomaterials have been used as substrates for neural reconstruction and neuron regeneration (Subramanian et al., 2009). In the development of the nervous system, electrical activity plays a marked role in regulating signal transmission and neuronal network activity. Therefore, electrical nanomaterials like graphene, MXenes, and their derivates have been extensively used to build microenvironments for nerve cells (Fabbro et al., 2016; Olabi et al., 2020; Xiao et al., 2022).
Two-dimensional (2D) transition metal carbides and nitrides (MXenes) are crystal nanomaterials with a number of surface functional groups like fluorine, hydroxyl, and oxygen, which can be used as carriers for proteins or drugs (Huang et al., 2018). MXenes have diverse structures and compositions, and their general formula is Mn+1XnTx, where M is the transition metal, X is the carbon or nitrogen, Tx is the surface terminations of the outer transition metal layer, and n ranges from 1 to 4 (Naguib et al., 2014; Anasori et al., 2017). MXenes have high conductivity, surface hydrophilicity, and excellent mechanical properties; the surfaces of MXenes can be easily modified and functionalized, as such MXenes can be used in various areas, such as environmental science (Rasool et al., 2016), physics (Kumar et al., 2017; Xia et al., 2018), and energy production (Anasori et al., 2017; Pang et al., 2019). Moreover, MXenes have considerable application prospects in biomedical engineering, such as in nanomedicine (Han et al., 2018), (electrochemical) biosensing (Wu et al., 2018), antibacterial treatments (Liu et al., 2011; Gao et al., 2022), diagnostic imaging (Dai et al., 2017), theranostics (Huang et al., 2018) and drug delivery system (Li et al., 2018).
The potential MXenes for inducing nerve regeneration include 2D MXene substrates and 3D MXene hydrogel systems also incorporating MXene nanosheets (Guo et al., 2022; Li et al., 2022; Liao et al., 2022; Xiao et al., 2022; Zhang et al., 2022). However, most studies have focused on the cellular level, and there are few in vivo studies. Herein, we comprehensively summarize the preparation and biocompatibility of MXenes and their regulation of nerve cells in MXene-based 2D and 3D culture systems. Finally, we discuss the challenges and future development directions to improve the preparation and biocompatibility of MXenes and applications of MXenes in NTE (Figure 1 and Table 1).
Figure 1.
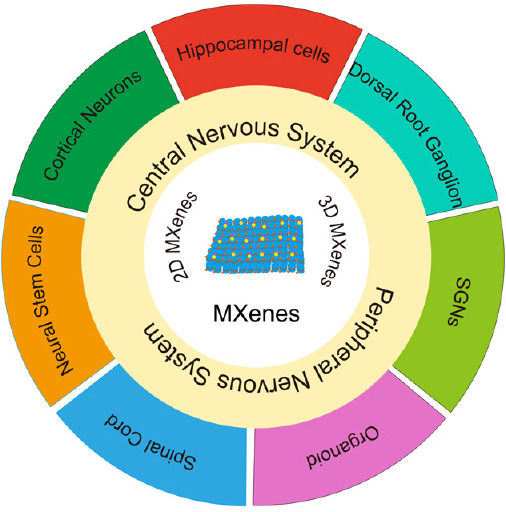
Applications of MXenes for neural tissue engineering and regeneration.
Application of two-dimensional and three-dimensional MXenes in peripheral and central nervous system. Investigations on the effects of MXenes on neural stem cells, cortical neurons, hippocampal cells, dorsal root ganglion, spiral ganglion neurons (SGNs), organoids, and spinal cord. Created using Adobe Illustrator CS6.
Table 1.
The applications of MXenes in neural tissue engineering
| Types | Models | In vitro/In vivo | Toxicity test | Key findings | Studies |
|---|---|---|---|---|---|
| Ti3C2Tx MXene | Neural stem cells (NSCs) | In vitro | Yes | Biocompatible, capable of supporting neuron growth and network formation; ensured normal growth of NSCs and enhanced proliferation, led to higher neuronal differentiation efficiency, promoted NSC maturation; promoted neuronal differentiation during strong depolarizations and increased synaptic release frequency, enhancing synaptic transmission. | Wang et al., 2021; Guo et al., 2022; Li et al., 2022 |
| Ti3C2 MXene | Primary cortical neurons | In vitro | Yes | Primary cortical neurons could adhere, grow, and form neuronal networks on Ti3C2 and polystyrene. This material provided a neural interface that has a high-resolution for neuroelectronic devices. | Driscoll et al., 2018 |
| Ti3C2Tx MXene | Dorsal root ganglion | In vitro | Yes | Both flakes and films could generate photothermal stimulation of dorsal root ganglion neurons even under the low incident energy densities. | Wang et al., 2021 |
| Ti3C2Tx MXene | Hippocampal cells | In vitro | Yes | This MXene preserved basic physiological functions in neuronal circuit development. | Xiao et al., 2022 |
| Ti3C2Tx MXene-Matrigel hydrogel | Spiral ganglion neurons | In vitro | Yes | 3D Ti3C2Tx MXene system could promote the formation of mature synapses and intercellular signal transmission in spiral ganglion neurons. | Liao et al., 2022 |
| Ti3C2Tx MXene-Matrigel hydrogel | Organoids | In vitro | Yes | Promoted their proliferation, maturation and formation of organoid hair cells, facilitated the establishment of neural connections between hair cells and spiral ganglion neurons, and increased the efficiency of synapse formation. | Zhang et al., 2022 |
| GelMA-MXene hydrogel | Spinal cord | In vitro and in vivo | Yes | Effectively repaired complete transection spinal cord injury and connections between the repaired and regenerated nerves. | Cai et al., 2022 |
Retrieval Strategy
An online search of the PubMed database was performed to retrieve articles published from inception until December 1, 2022. The following words and their combinations were used to maximize the specificity and sensitivity of the search: “MXenes, NTE, nerve, neuron, nerve regeneration, neural cells, neural stem cells, spiral ganglion neuron, hydrogels, organoid, and spinal cord”. The authors screened the related studies to identify potentially useful studies, first screening titles and abstracts and full texts using the keywords. Only studies addressing MXenes in relation to nerves were included to assess the research on the role of MXenes in NTE. There were no restrictions on language or study type.
Preparation and Properties of MXenes
Preparation of MXenes
MAX phases are a large family containing more than 50 members consisting of transition metal carbides, nitrides, or carbon nitrides (Naguib et al., 2011; Anasori, et al., 2017). The “M” is transition metal element, the “A” is a main family element, and the “X” is nitrogen or carbon (Figure 2A; Naguib et al., 2014). The basic formula can be expressed as M(n+1)AXn. Moreover, in the perspective of biomedical engineering, the most popular materials in this family are Ti3SiC2, Ti3C2Tx, and the multilayer Ti3C2 (Huang et al., 2022). MXenes are typically prepared using a top-down stripping method with selective stripping of the A element layer from the precursors to convert to an MAX phase or non-MAX phase (Naguib et al., 2014).
Figure 2.

Preparation and characterization of MXenes.
(A) Schematic representation of MAX phases and MXenes. (B) Characterization of MXenes. i) Scanning electron microscope (SEM) micrograph of the Ti3AlC2 showing a typical multilayer block structure. ii) SEM micrograph of multilayered Ti3AlC2 showing a multilayer structure in the shape of an accordion. iii) SEM micrograph of the two-dimensional delaminated ultrathin Ti3C2Tx MXene nanosheets after ultrasonic exfoliation. iv) Representative transmission electron microscopy image of the Ti3C2Tx MXene. The upper right corner is the SAED pattern. v, vi) X-ray diffraction and X-ray photoelectron spectroscopy patterns of Ti3AlC2 and Ti3C2Tx MXene, exhibiting the elimination of the Al layer after etching. Reprinted with permission from: (A) Naguib et al., 2014, Copyright © 2014 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim; (B) Dong et al., 2021, Copyright © 2021 American Chemical Society.
This method can be classified according to three aspects: the classes of precursors, the composition of the etchants, and the composition of delamination intercalants (Huang et al., 2018). There are two types of precursor materials used for the fabrication of MXenes: MAX phases and non-MAX phases. The target MXene determines which Mn+1AlXn with a metal composition is the effective MAX-phase precursor (like Ti3AlC2, Ti2AlC) (Naguib et al., 2011). Single layers or several layered stacks of MXenes can be achieved through selectively etching gel-like ion-binding layers (Alhabeb et al., 2017). Instead of pure metal layers, some non-MAX phase precursors, such as Zr3Al3C5, are etched with the metal-nonmetal layer from the precursor to obtain MXenes (Huang et al., 2018). Based on the composition of etchant, it can be classified into hydrofluoric acid-etching and non-hydrofluoric acid-etching. To avoid the harmfulness of hydrofluoric acid reagents and adapt these materials to biomedical applications, the reaction of the acid and fluoride or molten fluoride have been used to generate hydrofluoric acid to obtain selective etching (Ghidiu et al., 2016; Yu et al., 2017). In addition, the etchant mainly determines the surface termination of MXenes.
To produce monolayer MXenes, simple mechanical peeling is not efficient enough, and intercalators need to be used. Delamination intercalators mainly include metal ion intercalators (such as metal hydroxides or halide salts) and organic intercalators (such as TBAOH) (Alhabeb, et al., 2017; Yu et al., 2017). Dong et al. (2021) reported the procedure of converting multilayer Ti3AlC2 into the layered Ti3C2Tx MXene. MAX phases have a typical multilayer block structure, which is similar to an accordion structure, after selective etching and 2D nearly transparent ultrathin delaminates after ultrasonic exfoliation (Figure 2B; Dong et al., 2021). There are a few bottom-up synthesis methods of MXenes. Xu et al. (2015) synthesized ultra-thin α-Mo2C 2D crystals with a transverse size of up to 100 mm on Cu/Mo foil using chemical vapor deposition. They made W and Ta into ultra-thin WC and TaC crystals. The MXenes prepared using their method have a large lateral size. Compared with top-down manufacturing methods, the bottom-up synthesis methods usually start from small organic or inorganic molecules/atoms (Huang et al., 2018). They also have the advantages of manipulating the size distribution and morphology of MXenes, but the surface termination is less controllable. This may be due to the complex composition of MXenes.
In summary, the preparation of MXenes is usually achieved using top-down selective etching to strip the A elemental layer from the precursor MAX phase or non-MAX phase. However, suitable methods are still required for the bottom-up synthesis of MXenes.
Surface modification tactics for MXenes
Proper surface modification can enhance the performance of MXenes mainly using methods such as additive-mediated intercalation and additive-aided chemical modification (Zou et al., 2022). To date, there have been three main classes of mediated intercalations used for MXenes. (1) Molecules (e.g., DMSO and H2O) can increase the c-LP of Ti3C2Tx MXene (Wang et al., 2018). The water dispersion of MXenes can be effectively improved by polyethylene glycol (Xuan et al., 2016). A nanocomposite hydrogel (Ti3C2) composed of polyacrylamide and Ti facilitated drug delivery and release (Zhang et al., 2020b). The polymeric molecule polyvinyl alcohol can be used to regulate the thickness of MXenes through a vacuum filtration process (Ling et al., 2014). (2) Cations, including NH4+, H+, Li+, Na+, K+, Mg2+ and Al3+, under appropriate pH conditions (Lukatskaya et al., 2013), can be intercalated spontaneously between Ti3C2Tx MXene layers in aqueous solutions (Ghidiu et al., 2014; VahidMohammadi et al., 2019). Larger polyatomic cations, like +N2-phenyl-SO3H and [(CH3)3NR]+, were used to replace the intercalated Li/Na ions and increase the layer spacing of MXenes (Wang et al., 2016; Ghidiu et al., 2017). (3) As an intercalator, the organic base isopropylamine can form ammonium ions (r-NH3+) that can be inserted between the Nb2CTx layers (Mashtalir et al., 2015). Other organic bases, such as n-butylamine and choline hydroxide, can reduce the bond energy between the MXene layers (Naguib et al., 2015; Yu et al., 2017). Chemical modification is also effective. The introduced MXene surface terminal groups include halogen terminal (–F, –Cl, –Br), chalcogen terminal (–OH, –O, –S, –Se, –Te) and imino (–NH) groups (Agresti et al., 2019; Velusamy et al., 2019; Deysher et al., 2020; Kamysbayev et al., 2020; VahidMohammadi et al., 2021).
Properties of MXenes
The Ti3C2Tx MXene consists of the transition metals nitride and carbide. It has good electrothermal, mechanical, and chemical properties. The structure, composition, surface, and interlayer chemistry of MXenes can determine their physical and (electro)chemical properties. Unlike other 2D materials, the electric properties of MXenes rely on the M, X, and their surface ends (Huang et al., 2018). Because of defects in their surface ends, the synthesis process affects their electronic properties (Anasori et al., 2016). MXenes have optical properties such as light absorption, emission, and scattering (Maleski et al., 2021). MXenes display surface plasmon modes among the visible and near infrared ranges (El-Demellawi et al., 2018; Hantanasirisakul and Gogotsi, 2018) and exhibit strong absorption in the ultraviolet range (Han et al., 2020). The structure, M and X sites, and surface terminations are main factors influencing their optical properties. Studies on the mechanical properties of MXenes have used monolayers of Ti3C2Tx and Nb4C3Tx (Lipatov et al., 2018). Ti3C2Tx MXene films show a high tensile strength (Zhang et al., 2020a), indicating high fracture toughness (VahidMohammadi et al., 2021).
MXenes show good hydrophilicity, surface flexibility, high metal conductivity, biocompatibility, and other characteristics, with an extensive scope of possible uses, including in nanomedicine (Lin et al., 2018), biosensors (Hroncekova et al., 2020; Ramanavicius and Ramanavicius, 2020), biological imaging (Yu et al., 2017; Song et al., 2020), antimicrobial therapy (Rasool et al., 2017), and therapeutic diagnostics (Fu et al., 2019).
Biocompatibility of MXenes
MXenes have already shown great promise in biomedical applications, although their biocompatibility and potential toxicity must be taken into consideration. The biocompatibility of MXenes has been reported in a variety of cell types. Guo et al. (2022) dispersed the Ti3C2Tx MXene with abundant surface functional groups on tissue culture polystyrene (TCPS) and cultured neural stem cells (NSCs) on the material after coating it with laminin to investigate regulatory effects on the cell survival and behavior. The researchers found that these cells were cultured on both the Ti3C2Tx MXene and TCPS forms with stable adhesion, additionally exhibiting extensive spreading of their terminal extensions (Figure 3Ai). Histology of living and dead NSCs (Figure 3Aii) showed that Ti3C2Tx MXenes had good biocompatibility and were an excellent neural interface material. Although the NSCs on these two materials had similar proliferative abilities, the Ti3C2Tx MXene film showed more efficient neuronal differentiation (Figure 4A). Moreover, compared with TCPS substrates, cells on Ti3C2Tx MXenes had longer neurites, more branches and tips, and were more active, mature, and more highly differentiated, implying that Ti3C2Tx MXene was an efficient interface for NSC-derived neurons.
Figure 3.

Biocompatibility of MXene films for neuronal growth .
(A) Scanning electron microscope (SEM) and fluorescence images of neural stem cells (NSCs). i) SEM images of NSCs. ii) Fluorescence immunohistochemistry of live and dead NSCs. The cytoplasm of live cells was marked green, and the nuclei in dead cells were marked red. Scale bar, 50 μm. (B) Representative immunofluorescence images of nerve cells cultured on Ti3C2 for 7 days in vitro. Neurons, anti-Tuj1 (red); synapses, anti-synapsin-1 (green); nuclei, 4′,6-diamidino-2-phenylindole (DAPI; blue). A magnified view of the white-boxed region in panel i is shown in ii–iv, and a representation of panel iv is shown in v and vi. The use of arrows and boxes highlights the network formation. The scale bars for i, iv, and vi are 100, 50, and 25 μm, respectively. Reprinted with permission from: (A) Guo et al., 2022, Copyright © 2022 Acta Materialia Inc. Published by Elsevier Ltd. (B) Driscoll et al., 2018, Copyright © 2018 American Chemical Society. TCPS: Tissue culture polystyrene
Figure 4.

Effects of MXenes on neural stem cell differentiation.
(A) Representative fluorescence images of neurons from neural stem cells (NSCs) after 7 days of differentiation on tissue culture polystyrene (TCPS) and MXene film. Neurons, anti-Tuj1 (red); nuclei, 4′,6-diamidino-2-phenylindole (DAPI; blue). (B) Under the strong depolarization condition, Ti3C2Tx MXene selectively enhanced the peak value of NSC-derived neurons. i) Representative spontaneous spikes of neural cells (TCPS: black; Ti3C2Tx MXene: red). ii) Frequency of spontaneous spikes. iii) Representative voltage responses evoked. iv) Number of spikes (10–100 pA). (C) Representative fluorescence microscopy images of neurons. Neurons, anti-Tuj1 (red); mature neurons, anti-MAP2 (green); GABAergic neurons, anti-GABA (green); presynaptic VGLUT1, anti-VGLUT1 (green). Scale bar, 50 μm. Reprinted with permission from: (A) Guo et al., 2022, Copyright © 2022 Acta Materialia Inc. Published by Elsevier Ltd.; (B) Li et al., 2022, Copyright © licensed under a Creative Commons Attribution 4.0 International License (http://creativecommons.org/lisceses/by/4.0/); (C) Xiao et al., 2022, reproduced with permission from Royal Society Chemistry, Copyright © 2022 Copyright Clearance Center.
Driscoll et al. (2018) performed nerve recordings in vivo using Ti3C2 MXenes and reported good biocompatibility with immortalized tumor cell lines and the rat brain. They investigated the cytotoxicity of Ti3C2 films on primary cortical neurons, and immunocytochemistry results exhibited a widespread network on both substrates (Figure 3B; Driscoll et al., 2018). Their results demonstrated that the primary cortical neurons could adhere to, grow on, and form neuronal networks on Ti3C2 and TCPS. This material provided a high-resolution neural interface for neuroelectronic devices. This research can greatly broaden the potential applications of the MXene families for neuroscientific research. Zhang et al. (2019a) implanted Ti3C2Tx MXenes into Sparague-Dawley rats and found that MXenes were actively taken up via cell-mediated phagocytosis. In another study, a Ti3C2Tx MXene film showed no cytotoxicity to NSCs (Zhang et al., 2022), and no cytotoxicity was detected in nerve tissue (Vural et al., 2020; Wu et al., 2020). The above studies show that MXenes are biocompatible and are capable of supporting neuron growth and network formation (Wang et al., 2021; Guo et al., 2022).
Applications of MXenes in Neural Cells
2D MXenes
Among biomedical materials, MXenes show a special potential in regulating the fate of stem cells (Driscoll et al., 2018; Guo et al., 2022; Li et al., 2022). Because of their unique surface structure—which can be functionalized and has great electrical conductivity—MXenes have been used to offer an efficient and appropriate physiochemical environment (VahidMohammadi et al., 2021).
A great challenge in exploring complex neuronal functions is the modulation of neuronal activity (Pisanello et al., 2016; Zimmerman and Tian, 2018). In the family of MXenes, Ti3C2Tx MXene can be used for optical modulation of neuronal electrical activity with a high spatiotemporal resolution (Wang et al., 2021). In addition to the aforementioned applications, Wang et al. (2021) measured the photothermal performance of Ti3C2Tx MXene at a single-flake level and observed that both the flakes and films could generate photothermal stimulation of dorsal root ganglion neurons, even under low incident energy densities. This method can be combined with biomedical and tissue engineering to build practical neural therapy models (Wang et al., 2019).
Electrical activity is involved with numerous aspects of early neuronal development (Spitzer, 2006), meaning that effective electrical stimulation (ES) can be a good way to regulate the behavior of excitatory cells, such as in nerve regeneration (Zhang et al., 2007; Ghasemi-Mobarakeh et al., 2009). In previous studies, the ES mode that effectively enhanced functional recovery usually employed high-frequency sine wave signals or low-frequency pulse wave signals (Guo et al., 2021, 2022). Several studies applying ES have been conducted in mammalian animal models to promote peripheral nerve regeneration (Capogrosso et al., 2016; Song et al., 2016; Bonizzato et al., 2021; Roh et al., 2022). ES has been reported to play a crucial role in inducing a suitable stem cell response, which greatly affects the proliferation, self-renewal, and differentiation of stem cells (Thrivikraman et al., 2018). Guo et al. (2022) reported that ES could enhance the function of NSCs. The combination of stem cell therapy and biological materials towards the treatment of various neurodegenerative diseases is promising. Therefore, it is necessary to determine whether MXene films can be used as an effective interface for electrical transmission.
Li et al. (2022) reported that ES coupled with Ti3C2Tx MXene could ensure normal growth of NSCs and markedly enhance proliferation in NSCs, which also exhibited higher neuronal differentiation efficiency, implying that Ti3C2Tx MXenes can promote the maturation of NSCs. Ion channels are known to be essential for the fate and function of nerve cells (Yin et al., 2019). Li et al. (2022) investigated effects of MXenes on ion channels, synaptic connections between cells, and neural network formation in newborn neurons using patch clamp electrophysiology (Figure 4B). There was no significant difference in voltage-gated Na+/K+ currents when cells were in close contact with Ti3C2Tx MXenes, but the amplitude of the voltage-gated Ca2+ current was selectively increased, which may account for longer neurites. Further, Ti3C2Tx MXene promoted neuronal differentiation during strong depolarizations and increased the frequency of synaptic release, thereby enhancing synaptic transmission. In addition, Xiao et al. (2022) applied an uncoated Ti3C2Tx MXene to explore the development of hippocampal cells. Using electrophysiological recordings, they tested the effects of uncoated Ti3C2Tx MXenes on cultured neuronal cells and the activity of neuronal microcircuits; this MXene was suggested to preserve basic physiological functions in neuronal circuit development (Figure 4C). Their work makes it possible to construct neural repair devices that can be applied in research, diagnosis, and therapy.
3D MXenes
Effects of 3D MXene on organoids
Organoids are mini-organs with various cell types that are derived from stem cells of various tissues and organs, such as embryonic stem cells or induced pluripotent stem cells (Huch and Koo, 2015; Nengzhuang et al., 2022). They have highly similar cellular compositions and physiological characteristics to real organs, providing marked advances in research on nervous system development and disease (Di Lullo and Kriegstein, 2017). Cochlear organoids (Cochlear-Orgs) have been successfully formed in 3D in vitro using induced pluripotent stem cells and embryonic stem cells (Lee et al., 2017; McLean et al., 2017). However, difficulties remain in the simulation of extracellular environments (Gjorevski et al., 2016; Roccio et al., 2018). To better simulate the cellular environment, Zhang et al. (2022) combined Ti3C2Tx MXene nanomaterials with Matrigel to establish a co-culture system between Cochlear-Orgs and spiral ganglion neurons (SGNs) in vitro. The 3D hydrogel containing a certain concentration of Ti3C2Tx MXene was appropriate for Cochlea-Orgs and had the ability to promote the formation, maturation, and proliferation of organoid hair cells (Figure 5A; Zhang et al., 2022). In addition, they demonstrated that this 3D Ti3C2Tx MXene could facilitate the establishment of neural connections between hair cells and SGNs, and it increased the efficiency of synapse formation.
Figure 5.

MXene-Matrigel regulates the formation of neural networks in hair cells and spiral ganglion neurons.
(A) Co-cultured cochlea organoids and modiolus formed synapse-like contacts. Neurons, anti-Tuj1 (red); hair cells (HCs), anti-Myosin 7a (Myo7a) (green); supporting cells, anti-Sox2 (blue). i–iii) bright field images and representative immunofluorescence images of co-cultured cochlea organoids and modiolus. The representative region is highlighted in iii. Scale bar, 100 μm. (B) 3D Ti3C2Tx MXene with electroacoustic stimulation (EAS) positively regulated the growth of spiral ganglion neurons (SGNs). i) 3D Ti3C2Tx MXene combined with EAS promoted the functional maturation of SGNs. ii–iv) Side view and SEM images of Matrigel and 3D Ti3C2Tx MXene hydrogel. Scale bar is 20 μm. v, vi) Representative immunofluorescence images of SGNs and its growth cones. Scale bar, 5 μm. Reprinted with permission from: (A) Zhang et al., 2022, Copyright © 2022 The Authors. Advanced Science published by Wiley-VCH GmbH; licensed under a Creative Commons Attribution 4.0 International License (http://creativecommons.org/lisceses/by/4.0/); (B) Liao et al., 2022, Copyright © 2022 The Authors. Published by American Chemical Society; licensed under a Creative Commons Attribution 4.0 International License (http://creativecommons.org/lisceses/by/4.0/).
Effects of 3D MXenes on SGNs
The culture of neural cells remains restricted in our ability to regulate the survival, regeneration, and function of SGNs. Low-frequency bidirectional postoperative electrical stimulations has been found to stimulate axonal regeneration in neural cells and to reduce neuron degeneration (Kopelovich et al., 2013). Cochlear implants can convert sound information into electrical signals, which can be precisely delivered to targeting functional SGNs (Ramsden et al., 2012). This may serve as an effective means of stimulation. Liao et al. (2022) constructed a 3D electroacoustic stimulation system that consists of a cochlear implant device and conductive Ti3C2Tx MXene-Matrigel hydrogel. The 3D Ti3C2Tx MXene hydrogel positively regulated the growth and maturation of SGNs (Figure 5B). In addition, the SGNs exhibited a greater number of synapses and higher calcium oscillation frequencies, suggesting that the 3D Ti3C2Tx MXene system could promote the formation of mature synapses and intercellular signal transmission. This research showed the potential value of MXenes in promoting the regeneration and recovery of the auditory nerve after injury.
Effects of 3D MXenes on spinal cord
The spinal cord orchestrates walking and movements, and spinal cord injury can interrupt pathways from the CNS to the lumbar spinal cord (Kathe et al., 2022), leading to some severe neurological disorders such as chronic pain, impaired mobility, autonomic dysfunction, and paralysis (Tate et al., 2020; Zipser et al., 2022). In strategies to repair the spinal cord after injury, the reconnection of injured axons and regenerated neurons and the improvement of the microenvironment are the preferred methods (Zipser et al., 2022). Cai et al. (2022) fabricated a GelMA-MXene hydrogel nerve conduit based on a microgroove hydrogel film. In vitro experiments showed that this 3D MXene system could positively regulate NSC differentiation, direct NSC proliferation, and effectively repair the spinal cord after complete transection by increasing connections between the repaired and regenerated nerves. Therefore, this NSC-loaded 3D Ti3C2Tx MXene hydrogel system with a microgroove structure is a potential therapeutic strategy to treat spinal cord injury. However, to the best of our knowledge, there have been no other reports on the efficacy of MXenes in spinal cord injury. Therefore, more comprehensive research and exploration are needed on this aspect in the future.
Limitations
This review had several limitations that should be noted. First, the rapid publication rate of studies on MXenes in NTE means that new results and perspectives are frequently shown. Second, the studies reviewed here were based solely on cells or animal experiments, and there were no clinical trials, posing a high risk of bias or imprecision in the effects shown. Third, this review focused only on articles published in English, and it may lack comprehensive relevant international data.
Conclusions and Future Perspectives
Despite their unique properties, MXenes have received extensive attention in biomedical applications in recent years, including biosensors (Zhang et al., 2019b; Chia et al., 2020), antibacterial treatments (Mao et al., 2020; Yang et al., 2021), bioimaging (Xue et al., 2017; Soleymaniha et al., 2019), diagnostics (Dai et al., 2017; Lin et al., 2018) and therapeutics (Szuplewska et al., 2019). This review summarized the progress of research on using MXenes in nerve regeneration. The toxicity of MXenes in different nerve cells have been analyzed to evaluate the effects and potential applications of MXenes in neurobiology, expecting to provide a certain reference value to design safer MXenes. Although the biocompatibility of Ti3C2Tx MXene was confirmed in some neural cells, such as NSCs, SGNs, hippocampal cells, immortalized tumor cell lines, rat brain and spinal cord, most studies were based on cell experiments (Driscoll et al., 2018). The long-term biosafety of MXenes remains unclear and has not been systematically evaluated. This is essential for further neurobiological applications of MXenes. There are still many problems to be solved, such as chronic toxicity, biodistribution, immunocompetence, and other effects of MXenes in small- and large-animal models. Therefore, it is crucial to establish an effective animal model.
In terms of preparation methods, most MXenes are synthetized using top-down etching, which is difficult to control in experimental conditions and the stability of the obtained products needs to be improved. The preparation method, surface modifications, and material size are the key factors affecting the effects of MXenes on nerve regeneration. In the future, more synthesis methods, such as bottom-up synthesis, need to be explored for preparing more stable MXenes with controllable morphology, structure, and properties. Moreover, the properties of MXenes should be further detailed to optimize their applications in neurobiology.
Regarding the applications of MXenes in neuroscience, previous studies are still limited and mainly studied the utility of MXenes for nerve repair and regeneration at a limited cellular level. Further studies are needed to clarify the regulatory mechanisms of MXenes for directly interfacing with targeted neural cells (Fabbro, et al., 2016; Pampaloni et al., 2018). To sufficiently reveal the interplay of the nervous system and MXenes, it is necessary to strictly regulate the dimensions of the material, its components, and its surface functional groups. The material stress characteristics and further application status of 3D MXene systems also need further exploration. In addition, it is vital to establish animal models of nerve injuries to explore implantation routes and functional verification of MXenes in vivo, so as to provide further research basis for clinical applications.
In conclusion, due to their non-toxicity to some types of nerve cells, MXenes have the ability to promote neuronal proliferation, differentiation and regeneration and are materials with excellent biomedical carrier properties. MXenes show considerable application potential in biomedicine, especially in nerve repair and regeneration. The combination of MXenes and other functional materials, such as graphene, Matrigel, and methacrylated gelatin, make multifunctional biomedical applications possible. Overall, MXene-based materials will play an active role in nerve regeneration and repair applications.
Footnotes
Funding: This work was supported by grants from the National Key R&D Program of China, Nos. 2021YFA1101300, 2021YFA1101803, 2020YFA0112503; the National Natural Science Foundation of China, Nos. 82030029, 81970882, 92149304; Science and Technology Department of Sichuan Province, No. 2021YFS0371 (all to RC).
Conflicts of interest: The authors declare no conflicts of interests.
Data availability statement: Not applicable.
C-Editor: Zhao M; S-Editor: Li CH; L-Editor: Song LP; T-Editor: Jia Y
References
- 1.Agresti A, Pazniak A, Pescetelli S, Di Vito A, Rossi D, Pecchia A, Auf der Maur M, Liedl A, Larciprete R, Kuznetsov DV, Saranin D, Di Carlo A. Titanium-carbide MXenes for work function and interface engineering in perovskite solar cells. Nat Mater. (2019);18:1228–1234. doi: 10.1038/s41563-019-0478-1. [DOI] [PubMed] [Google Scholar]
- 2.Alhabeb M, Maleski K, Anasori B, Lelyukh P, Clark L, Sin S, Gogotsi Y. Guidelines for synthesis and processing of two-dimensional titanium carbide (Ti3C2Tx MXene) Chem Mater. (2017);29:7633–7644. [Google Scholar]
- 3.Anasori B, Lukatskaya MR, Gogotsi Y. 2D metal carbides and nitrides (MXenes) for energy storage. Nat Rev Mater. (2017);2:1–17. [Google Scholar]
- 4.Anasori B, Shi C, Moon EJ, Xie Y, Voigt CA, Kent PRC, May SJ, Billinge SJL, Barsoum MW, Gogotsi Y. Control of electronic properties of 2D carbides (MXenes) by manipulating their transition metal layers. Nanoscale Horiz. (2016);1:227–234. doi: 10.1039/c5nh00125k. [DOI] [PubMed] [Google Scholar]
- 5.Bonizzato M, James ND, Pidpruzhnykova G, Pavlova N, Shkorbatova P, Baud L, Martinez-Gonzalez C, Squair JW, DiGiovanna J, Barraud Q, Micera S, Courtine G. Multi-pronged neuromodulation intervention engages the residual motor circuitry to facilitate walking in a rat model of spinal cord injury. Nat Commun. (2021);12:1925. doi: 10.1038/s41467-021-22137-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cai J, Zhang H, Hu Y, Huang Z, Wang Y, Xia Y, Chen X, Guo J, Cheng H, Xia L, Lu W, Zhang C, Xie J, Wang H, Chai R. GelMA-MXene hydrogel nerve conduits with microgrooves for spinal cord injury repair. J Nanobiotechnology. (2022);20:460. doi: 10.1186/s12951-022-01669-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Capogrosso M, Milekovic T, Borton D, Wagner F, Moraud EM, Mignardot JB, Buse N, Gandar J, Barraud Q, Xing D, Rey E, Duis S, Jianzhong Y, Ko WK, Li Q, Detemple P, Denison T, Micera S, Bezard E, Bloch J, Courtine G. A brain-spine interface alleviating gait deficits after spinal cord injury in primates. Nature. (2016);539:284–288. doi: 10.1038/nature20118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chia HL, Mayorga-Martinez CC, Antonatos N, Sofer Z, Gonzalez-Julian JJ, Webster RD, Pumera M. MXene titanium carbide-based biosensor:strong dependence of exfoliation method on performance. Anal Chem. (2020);92:2452–2459. doi: 10.1021/acs.analchem.9b03634. [DOI] [PubMed] [Google Scholar]
- 9.Dai C, Chen Y, Jing X, Xiang L, Yang D, Lin H, Liu Z, Han X, Wu R. Two-dimensional tantalum carbide (MXenes) composite nanosheets for multiple imaging-guided photothermal tumor ablation. ACS Nano. (2017);11:12696–12712. doi: 10.1021/acsnano.7b07241. [DOI] [PubMed] [Google Scholar]
- 10.Deysher G, Shuck CE, Hantanasirisakul K, Frey NC, Foucher AC, Maleski K, Sarycheva A, Shenoy VB, Stach EA, Anasori B, Gogotsi Y. Synthesis of Mo(4)VAlC(4) MAX phase and two-dimensional Mo(4)VC(4) MXene with five atomic layers of transition metals. ACS Nano. (2020);14:204–217. doi: 10.1021/acsnano.9b07708. [DOI] [PubMed] [Google Scholar]
- 11.Di Lullo E, Kriegstein AR. The use of brain organoids to investigate neural development and disease. Nat Rev Neurosci. (2017);18:573–584. doi: 10.1038/nrn.2017.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dong J, Luo S, Ning S, Yang G, Pan D, Ji Y, Feng Y, Su F, Liu C. MXene-coated wrinkled fabrics for stretchable and multifunctional electromagnetic interference shielding and electro/photo-thermal conversion applications. ACS Appl Mater Interfaces. (2021);13:60478–60488. doi: 10.1021/acsami.1c19890. [DOI] [PubMed] [Google Scholar]
- 13.Driscoll N, Richardson AG, Maleski K, Anasori B, Adewole O, Lelyukh P, Escobedo L, Cullen DK, Lucas TH, Gogotsi Y, Vitale F. Two-dimensional Ti(3)C(2) MXene for high-resolution neural interfaces. ACS Nano. (2018);12:10419–10429. doi: 10.1021/acsnano.8b06014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.El-Demellawi JK, Lopatin S, Yin J, Mohammed OF, Alshareef HN. Tunable multipolar surface plasmons in 2D Ti(3)C(2) T (x) MXene flakes. ACS Nano. (2018);12:8485–8493. doi: 10.1021/acsnano.8b04029. [DOI] [PubMed] [Google Scholar]
- 15.Fabbro A, Scaini D, Leon V, Vazquez E, Cellot G, Privitera G, Lombardi L, Torrisi F, Tomarchio F, Bonaccorso F, Bosi S, Ferrari AC, Ballerini L, Prato M. Graphene-based interfaces do not alter target nerve cells. ACS Nano. (2016);10:615–623. doi: 10.1021/acsnano.5b05647. [DOI] [PubMed] [Google Scholar]
- 16.Fu Q, Zhu R, Song J, Yang H, Chen X. Photoacoustic imaging:contrast agents and their biomedical applications. Adv Mater. (2019);31:1805875. doi: 10.1002/adma.201805875. [DOI] [PubMed] [Google Scholar]
- 17.Gao Y, Dong Y, Yang S, Mo A, Zeng X, Chen Q, Peng Q. Size-dependent photothermal antibacterial activity of Ti(3)C(2)T(x) MXene nanosheets against methicillin-resistant Staphylococcus aureus. J Colloid Interface Sci. (2022);617:533–541. doi: 10.1016/j.jcis.2022.03.032. [DOI] [PubMed] [Google Scholar]
- 18.Ghasemi-Mobarakeh L, Prabhakaran MP, Morshed M, Nasr-Esfahani MH, Ramakrishna S. Electrical stimulation of nerve cells using conductive nanofibrous scaffolds for nerve tissue engineering. Tissue Eng Part A. (2009);15:3605–3619. doi: 10.1089/ten.TEA.2008.0689. [DOI] [PubMed] [Google Scholar]
- 19.Ghidiu M, Lukatskaya MR, Zhao MQ, Gogotsi Y, Barsoum MW. Conductive two-dimensional titanium carbide 'clay'with high volumetric capacitance. Nature. (2014);516:78–81. doi: 10.1038/nature13970. [DOI] [PubMed] [Google Scholar]
- 20.Ghidiu M, Halim J, Kota S, Bish D, Gogotsi Y, Barsoum MW. Ion-exchange and cation solvation reactions in Ti3C2 MXene. Chem Mater. (2016);28:3507–3514. [Google Scholar]
- 21.Ghidiu M, Kota S, Halim J, Sherwood AW, Nedfors N, Rosen J, Mochalin VN, Barsoum MW. Alkylammonium cation intercalation into Ti3C2 (MXene):effects on properties and ion-exchange capacity estimation. Chem Mater. (2017);29:1099–1106. [Google Scholar]
- 22.Gjorevski N, Sachs N, Manfrin A, Giger S, Bragina ME, Ordóñez-Morán P, Clevers H, Lutolf MP. Designer matrices for intestinal stem cell and organoid culture. Nature. (2016);539:560–564. doi: 10.1038/nature20168. [DOI] [PubMed] [Google Scholar]
- 23.Guo R, Liao M, Ma X, Hu Y, Qian X, Xiao M, Gao X, Chai R, Tang M. Cochlear implant-based electric-acoustic stimulation modulates neural stem cell-derived neural regeneration. J Mater Chem B. (2021);9:7793–7804. doi: 10.1039/d1tb01029h. [DOI] [PubMed] [Google Scholar]
- 24.Guo R, Xiao M, Zhao W, Zhou S, Hu Y, Liao M, Wang S, Yang X, Chai R, Tang M. 2D Ti(3)C(2)T(x)MXene couples electrical stimulation to promote proliferation and neural differentiation of neural stem cells. Acta Biomater. (2022);139:105–117. doi: 10.1016/j.actbio.2020.12.035. [DOI] [PubMed] [Google Scholar]
- 25.Han M, Maleski K, Shuck CE, Yang Y, Glazar JT, Foucher AC, Hantanasirisakul K, Sarycheva A, Frey NC, May SJ, Shenoy VB, Stach EA, Gogotsi Y. Tailoring electronic and optical properties of MXenes through forming solid solutions. J Am Chem Soc. (2020);142:19110–19118. doi: 10.1021/jacs.0c07395. [DOI] [PubMed] [Google Scholar]
- 26.Han X, Jing X, Yang D, Lin H, Wang Z, Ran H, Li P, Chen Y. Therapeutic mesopore construction on 2D Nb(2)C MXenes for targeted and enhanced chemo-photothermal cancer therapy in NIR-II biowindow. Theranostics. (2018);8:4491–4508. doi: 10.7150/thno.26291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hantanasirisakul K, Gogotsi Y. Electronic and optical properties of 2D transition metal carbides and nitrides (MXenes) Adv Mater. (2018);30:1804779. doi: 10.1002/adma.201804779. [DOI] [PubMed] [Google Scholar]
- 28.Hroncekova S, Bertok T, Hires M, Jane E, Lorencova L, Vikartovska A, Tanvir A, Kasak P, Tkac J. Ultrasensitive Ti(3)C(2)T(X) MXene/chitosan nanocomposite-based amperometric biosensor for detection of potential prostate cancer marker in urine samples. Processes (Basel) (2020);8:580. doi: 10.3390/pr8050580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang H, Dong C, Feng W, Wang Y, Huang B, Chen Y. Biomedical engineering of two-dimensional MXenes. Adv Drug Deliv Rev. (2022);184:114178. doi: 10.1016/j.addr.2022.114178. [DOI] [PubMed] [Google Scholar]
- 30.Huang K, Li Z, Lin J, Han G, Huang P. Two-dimensional transition metal carbides and nitrides (MXenes) for biomedical applications. Chem Soc Rev. (2018);47:5109–5124. doi: 10.1039/c7cs00838d. [DOI] [PubMed] [Google Scholar]
- 31.Huch M, Koo BK. Modeling mouse and human development using organoid cultures. Development. (2015);142:3113–3125. doi: 10.1242/dev.118570. [DOI] [PubMed] [Google Scholar]
- 32.Kamysbayev V, Filatov AS, Hu H, Rui X, Lagunas F, Wang D, Klie RF, Talapin DV. Covalent surface modifications and superconductivity of two-dimensional metal carbide MXenes. Science. (2020);369:979–983. doi: 10.1126/science.aba8311. [DOI] [PubMed] [Google Scholar]
- 33.Kathe C, Skinnider MA, Hutson TH, Regazzi N, Gautier M, Demesmaeker R, Komi S, Ceto S, James ND, Cho N, Baud L, Galan K, Matson KJE, Rowald A, Kim K, Wang R, Minassian K, Prior JO, Asboth L, Barraud Q, et al. The neurons that restore walking after paralysis. Nature. (2022);611:540–547. doi: 10.1038/s41586-022-05385-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim H, Cooke MJ, Shoichet MS. Creating permissive microenvironments for stem cell transplantation into the central nervous system. Trends Biotechnol. (2012);30:55–63. doi: 10.1016/j.tibtech.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 35.Kopelovich JC, Cagaanan AP, Miller CA, Abbas PJ, Green SH. Intracochlear electrical stimulation suppresses apoptotic signaling in rat spiral ganglion neurons after deafening in vivo. Otolaryngol Head Neck Surg. (2013);149:745–752. doi: 10.1177/0194599813498702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kumar H, Frey NC, Dong L, Anasori B, Gogotsi Y, Shenoy VB. Tunable magnetism and transport properties in nitride MXenes. ACS Nano. (2017);11:7648–7655. doi: 10.1021/acsnano.7b02578. [DOI] [PubMed] [Google Scholar]
- 37.Lee JH, Tammela T, Hofree M, Choi J, Marjanovic ND, Han S, Canner D, Wu K, Paschini M, Bhang DH, Jacks T, Regev A, Kim CF. Anatomically and functionally distinct lung mesenchymal populations marked by Lgr5 and Lgr6. Cell. (2017);170:1149–1163. doi: 10.1016/j.cell.2017.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li Y, Hu Y, Wei H, Cao W, Qi Y, Zhou S, Zhang P, Li H, Li GL, Chai R. Two-dimensional Ti(3)C(2)T(x) MXene promotes electrophysiological maturation of neural circuits. J Nanobiotechnology. (2022);20:398. doi: 10.1186/s12951-022-01590-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li Z, Zhang H, Han J, Chen Y, Lin H, Yang T. Surface nanopore engineering of 2D MXenes for targeted and synergistic multitherapies of hepatocellular carcinoma. Adv Mater. (2018);30:1706981. doi: 10.1002/adma.201706981. [DOI] [PubMed] [Google Scholar]
- 40.Liao M, Hu Y, Zhang Y, Wang K, Fang Q, Qi Y, Shen Y, Cheng H, Fu X, Tang M, Sun S, Gao X, Chai R. 3D Ti3C2Tx MXene-matrigel with electroacoustic stimulation to promote the growth of spiral ganglion neurons. ACS Nano. (2022);16:16744–16756. doi: 10.1021/acsnano.2c06306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin H, Wang Y, Gao S, Chen Y, Shi J. Theranostic 2D Tantalum Carbide (MXene) Adv Mater. (2018);30:1703284. doi: 10.1002/adma.201703284. [DOI] [PubMed] [Google Scholar]
- 42.Ling Z, Ren CE, Zhao MQ, Yang J, Giammarco JM, Qiu J, Barsoum MW, Gogotsi Y. Flexible and conductive MXene films and nanocomposites with high capacitance. Proc Natl Acad Sci U S A. (2014);111:16676–16681. doi: 10.1073/pnas.1414215111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lipatov A, Lu HD, Alhabeb M, Anasori B, Gruverman A, Gogotsi Y, Sinitskii A. Elastic properties of 2D Ti3C2Tx MXene monolayers and bilayers. Sci Adv. (2018);4:eaat0491. doi: 10.1126/sciadv.aat0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu S, Zeng TH, Hofmann M, Burcombe E, Wei J, Jiang R, Kong J, Chen Y. Antibacterial activity of graphite, graphite oxide, graphene oxide, and reduced graphene oxide:membrane and oxidative stress. ACS Nano. (2011);5:6971–6980. doi: 10.1021/nn202451x. [DOI] [PubMed] [Google Scholar]
- 45.Lukatskaya MR, Mashtalir O, Ren CE, Dall'Agnese Y, Rozier P, Taberna PL, Naguib M, Simon P, Barsoum MW, Gogotsi Y. Cation intercalation and high volumetric capacitance of two-dimensional titanium carbide. Science. (2013);341:1502–1505. doi: 10.1126/science.1241488. [DOI] [PubMed] [Google Scholar]
- 46.Maleski K, Shuck CE, Fafarman AT, Gogotsi Y. The broad chromatic range of two-dimensional transition metal carbides. Adv Opt Mater. (2021);9:2001563. [Google Scholar]
- 47.Mao L, Hu SM, Gao YH, Wang L, Zhao WW, Fu LN, Cheng HY, Xia L, Xie SX, Ye WL, Shi ZJ, Yang G. Biodegradable and electroactive regenerated bacterial cellulose/MXene (Ti3C2Tx) composite hydrogel as wound dressing for accelerating skin wound healing under electrical stimulation. Adv Healthc Mater. (2020);9:e2000872. doi: 10.1002/adhm.202000872. [DOI] [PubMed] [Google Scholar]
- 48.Mashtalir O, Lukatskaya MR, Zhao MQ, Barsoum MW, Gogotsi Y. Amine-assisted delamination of Nb2C MXene for Li-ion energy storage devices. Adv Mater. (2015);27:3501–3506. doi: 10.1002/adma.201500604. [DOI] [PubMed] [Google Scholar]
- 49.McLean WJ, Yin X, Lu L, Lenz DR, McLean D, Langer R, Karp JM, Edge ASB. Clonal expansion of Lgr5-positive cells from mammalian cochlea and high-purity generation of sensory hair cells. Cell Rep. (2017);18:1917–1929. doi: 10.1016/j.celrep.2017.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Naguib M, Kurtoglu M, Presser V, Lu J, Niu J, Heon M, Hultman L, Gogotsi Y, Barsoum MW. Two-dimensional nanocrystals produced by exfoliation of Ti3 AlC2. Adv Mater. (2011);23:4248–4253. doi: 10.1002/adma.201102306. [DOI] [PubMed] [Google Scholar]
- 51.Naguib M, Mochalin VN, Barsoum MW, Gogotsi Y. 25th anniversary article:MXenes:a new family of two-dimensional materials. Adv Mater. (2014);26:992–1005. doi: 10.1002/adma.201304138. [DOI] [PubMed] [Google Scholar]
- 52.Naguib M, Unocic RR, Armstrong BL, Nanda J. Large-scale delamination of multi-layers transition metal carbides and carbonitrides “MXenes”. Dalton Trans. (2015);44:9353–9358. doi: 10.1039/c5dt01247c. [DOI] [PubMed] [Google Scholar]
- 53.Nengzhuang W, Jiaming S, Minghua LIU, Long MA, Lina QIN, Xuemei GE, Hongli YAN. A brief history of testicular organoids:from theory to the wards. J Assist Reprod Genet. (2022);39:1423–1431. doi: 10.1007/s10815-022-02529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Olabi AG, Wilberforce T, Sayed ET, Elsaid K, Rezk H, Abdelkareem MA. Recent progress of graphene based nanomaterials in bioelectrochemical systems. Sci Total Environ. (2020);749:141225. doi: 10.1016/j.scitotenv.2020.141225. [DOI] [PubMed] [Google Scholar]
- 55.Pampaloni NP, Lottner M, Giugliano M, Matruglio A, D'Amico F, Prato M, Garrido JA, Ballerini L, Scaini D. Single-layer graphene modulates neuronal communication and augments membrane ion currents. Nat Nanotechnol. (2018);13:755–764. doi: 10.1038/s41565-018-0163-6. [DOI] [PubMed] [Google Scholar]
- 56.Pang J, Mendes RG, Bachmatiuk A, Zhao L, Ta HQ, Gemming T, Liu H, Liu Z, Rummeli MH. Applications of 2D MXenes in energy conversion and storage systems. Chem Soc Rev. (2019);48:72–133. doi: 10.1039/c8cs00324f. [DOI] [PubMed] [Google Scholar]
- 57.Pisanello F, Sileo L, De Vittorio M. Micro- and nanotechnologies for optical neural interfaces. Front Neurosci. (2016);10:70. doi: 10.3389/fnins.2016.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ramanavicius S, Ramanavicius A. Progress and insights in the application of MXenes as new 2D nano-materials suitable for biosensors and biofuel cell design. Int J Mol Sci. (2020);21:9224. doi: 10.3390/ijms21239224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ramsden JD, Gordon K, Aschendorff A, Borucki L, Bunne M, Burdo S, Garabedian N, Grolman W, Irving R, Lesinski-Schiedat A, Loundon N, Manrique M, Martin J, Raine C, Wouters J, Papsin BC. European bilateral pediatric cochlear implant forum consensus statement. Otol Neurotol. (2012);33:561–565. doi: 10.1097/MAO.0b013e3182536ae2. [DOI] [PubMed] [Google Scholar]
- 60.Rasool K, Helal M, Ali A, Ren CE, Gogotsi Y, Mahmoud KA. Antibacterial Activity of Ti(3)C(2)Tx MXene. ACS Nano. (2016);10:3674–3684. doi: 10.1021/acsnano.6b00181. [DOI] [PubMed] [Google Scholar]
- 61.Rasool K, Mahmoud KA, Johnson DJ, Helal M, Berdiyorov GR, Gogotsi Y. Efficient antibacterial membrane based on two-dimensional Ti(3)C(2)T(x) (MXene) nanosheets. Sci Rep. (2017);7:1–11. doi: 10.1038/s41598-017-01714-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Roccio M, Perny M, Ealy M, Widmer HR, Heller S, Senn P. Molecular characterization and prospective isolation of human fetal cochlear hair cell progenitors. Nat Commun. (2018);9:1–14. doi: 10.1038/s41467-018-06334-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Roh J, Schellhardt L, Keane GC, Hunter DA, Moore AM, Snyder-Warwick AK, Mackinnon SE, Wood MD. Short-duration, pulsatile , electrical stimulation therapy accelerates axon regeneration and recovery following tibial nerve injury and repair in rats. Plast Reconstr Surg. (2022);149:681e–690e. doi: 10.1097/PRS.0000000000008924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Santos T, Boto C, Saraiva CM, Bernardino L, Ferreira L. Nanomedicine approaches to modulate neural stem cells in brain repair. Trends Biotechnol. (2016);34:437–439. doi: 10.1016/j.tibtech.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 65.Soleymaniha M, Shahbazi MA, Rafieerad AR, Maleki A, Amiri A. Promoting role of MXene nanosheets in biomedical sciences:therapeutic and biosensing innovations. Adv Healthc Mater. (2019);8:e1801137. doi: 10.1002/adhm.201801137. [DOI] [PubMed] [Google Scholar]
- 66.Song J, Sun B, Liu S, Chen W, Zhang Y, Wang C, Mo X, Che J, Ouyang Y, Yuan W, Fan C. Polymerizing pyrrole coated poly (l-lactic acid-co-epsilon-caprolactone) (PLCL) conductive nanofibrous conduit combined with electric stimulation for long-range peripheral nerve regeneration. Front Mol Neurosci. (2016);9:117. doi: 10.3389/fnmol.2016.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Song M, Pang SY, Guo F, Wong MC, Hao J. Fluoride-free 2D niobium carbide MXenes as stable and biocompatible nanoplatforms for electrochemical biosensors with ultrahigh sensitivity. Adv Sci (Weinh) (2020);7:2001546. doi: 10.1002/advs.202001546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Spitzer NC. Electrical activity in early neuronal development. Nature. (2006);444:707–712. doi: 10.1038/nature05300. [DOI] [PubMed] [Google Scholar]
- 69.Subramanian A, Krishnan UM, Sethuraman S. Development of biomaterial scaffold for nerve tissue engineering:Biomaterial mediated neural regeneration. J Biomed Sci. (2009);16:108. doi: 10.1186/1423-0127-16-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Szuplewska A, Kulpinska D, Dybko A, Jastrzebska AM, Wojciechowski T, Rozmyslowska A, Chudy M, Grabowska-Jadach I, Ziemkowska W, Brzozka Z, Olszyna A. 2D Ti2C (MXene) as a novel highly efficient and selective agent for photothermal therapy. Mater Sci Eng C Mater Biol Appl. (2019);98:874–886. doi: 10.1016/j.msec.2019.01.021. [DOI] [PubMed] [Google Scholar]
- 71.Tate DG, Wheeler T, Lane GI, Forchheimer M, Anderson KD, Biering-Sorensen F, Cameron AP, Santacruz BG, Jakeman LB, Kennelly MJ, Kirshblum S, Krassioukov A, Krogh K, Mulcahey MJ, Noonan VK, Rodriguez GM, Spungen AM, Tulsky D, Post MW. Recommendations for evaluation of neurogenic bladder and bowel dysfunction after spinal cord injury and/or disease. J Spinal Cord Med. (2020);43:141–164. doi: 10.1080/10790268.2019.1706033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Thrivikraman G, Boda SK, Basu B. Unraveling the mechanistic effects of electric field stimulation towards directing stem cell fate and function:a tissue engineering perspective. Biomaterials. (2018);150:60–86. doi: 10.1016/j.biomaterials.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 73.VahidMohammadi A, Mojtabavi M, Caffrey NM, Wanunu M, Beidaghi M. Assembling 2D MXenes into highly stable pseudocapacitive electrodes with high power and energy densities. Adv Mater. (2019);31:e1806931. doi: 10.1002/adma.201806931. [DOI] [PubMed] [Google Scholar]
- 74.VahidMohammadi A, Rosen J, Gogotsi Y. The world of two-dimensional carbides and nitrides (MXenes) Science. (2021);372:eabf1581. doi: 10.1126/science.abf1581. [DOI] [PubMed] [Google Scholar]
- 75.Velusamy DB, El-Demellawi JK, El-Zohry AM, Giugni A, Lopatin S, Hedhili MN, Mansour AE, Fabrizio ED, Mohammed OF, Alshareef HN. MXenes for plasmonic photodetection. Adv Mater. (2019);31:e1807658. doi: 10.1002/adma.201807658. [DOI] [PubMed] [Google Scholar]
- 76.Vural M, Zhu H, Pena-Francesch A, Jung H, Allen BD, Demirel MC. Self-assembly of topologically networked protein-Ti(3)C(2)T(x) MXene composites. ACS Nano. (2020);14:6956–6967. doi: 10.1021/acsnano.0c01431. [DOI] [PubMed] [Google Scholar]
- 77.Wang BX, Zhou AG, Liu FF, Cao JL, Wang LB, Hu QK. Carbon dioxide adsorption of two-dimensional carbide MXenes. J Adv Ceram. (2018);7:237–245. [Google Scholar]
- 78.Wang HB, Zhang JF, Wu YP, Huang HJ, Li GY, Zhang X, Wang ZY. Surface modified MXene Ti3C2 multilayers by aryl diazonium salts leading to large-scale delamination. Appl Surf Sci. (2016);384:287–293. [Google Scholar]
- 79.Wang K, Frewin CL, Esrafilzadeh D, Yu C, Wang C, Pancrazio JJ, Romero-Ortega M, Jalili R, Wallace G. High-performance graphene-fiber-based neural recording microelectrodes. Adv Mater. (2019);31:1805867. doi: 10.1002/adma.201805867. [DOI] [PubMed] [Google Scholar]
- 80.Wang Y, Garg R, Hartung JE, Goad A, Patel DA, Vitale F, Gold MS, Gogotsi Y, Cohen-Karni T. Ti(3)C(2)T(x) MXene flakes for optical control of neuronal electrical activity. ACS Nano. (2021);15:14662–14671. doi: 10.1021/acsnano.1c04431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wu L, Lu X, Dhanjai, Wu ZS, Dong Y, Wang X, Zheng S, Chen J. 2D transition metal carbide MXene as a robust biosensing platform for enzyme immobilization and ultrasensitive detection of phenol. Biosens Bioelectron. (2018);107:69–75. doi: 10.1016/j.bios.2018.02.021. [DOI] [PubMed] [Google Scholar]
- 82.Wu W, Ge H, Zhang L, Lei X, Yang Y, Fu Y, Feng H. Evaluating the cytotoxicity of Ti(3)C(2) MXene to neural stem cells. Chem Res Toxicol. (2020);33:2953–2962. doi: 10.1021/acs.chemrestox.0c00232. [DOI] [PubMed] [Google Scholar]
- 83.Xia Y, Mathis TS, Zhao MQ, Anasori B, Dang A, Zhou Z, Cho H, Gogotsi Y, Yang S. Thickness-independent capacitance of vertically aligned liquid-crystalline MXenes. Nature. (2018);557:409–412. doi: 10.1038/s41586-018-0109-z. [DOI] [PubMed] [Google Scholar]
- 84.Xiao M, Ulloa Severino FP, Iseppon F, Cheng G, Torre V, Tang M. 3D free-standing ordered graphene network geometrically regulates neuronal growth and network formation. Nano Lett. (2020);20:7043–7051. doi: 10.1021/acs.nanolett.0c02107. [DOI] [PubMed] [Google Scholar]
- 85.Xiao M, Li X, Pifferi S, Pastore B, Liu Y, Lazzarino M, Torre V, Yang X, Menini A, Tang M. 2D MXene interfaces preserve the basal electrophysiology of targeted neural circuits. Nanoscale. (2022);14:10992–11002. doi: 10.1039/d2nr01542k. [DOI] [PubMed] [Google Scholar]
- 86.Xu C, Wang L, Liu Z, Chen L, Guo J, Kang N, Ma XL, Cheng HM, Ren W. Large-area high-quality 2D ultrathin Mo2C superconducting crystals. Nat Mater. (2015);14:1135–1141. doi: 10.1038/nmat4374. [DOI] [PubMed] [Google Scholar]
- 87.Xuan J, Wang Z, Chen Y, Liang D, Cheng L, Yang X, Liu Z, Ma R, Sasaki T, Geng F. Organic-base-driven intercalation and delamination for the production of functionalized titanium carbide nanosheets with superior photothermal therapeutic performance. Angew Chem Int Ed Engl. (2016);55:14569–14574. doi: 10.1002/anie.201606643. [DOI] [PubMed] [Google Scholar]
- 88.Xue Q, Zhang HJ, Zhu MS, Pei ZX, Li HF, Wang ZF, Huang Y, Huang Y, Deng QH, Zhou J, Du SY, Huang Q, Zhi CY. Photoluminescent Ti3C2 MXene quantum dots for multicolor cellular imaging. Adv Mater. (2017) doi: 10.1002/adma.201604847. doi:10.1002/adma.201604847. [DOI] [PubMed] [Google Scholar]
- 89.Yang C, Luo Y, Lin H, Ge M, Shi J, Zhang X. Niobium carbide mxene augmented medical implant elicits bacterial infection elimination and tissue regeneration. ACS Nano. (2021);15:1086–1099. doi: 10.1021/acsnano.0c08045. [DOI] [PubMed] [Google Scholar]
- 90.Yin S, Liu J, Kang Y, Lin Y, Li D, Shao L. Interactions of nanomaterials with ion channels and related mechanisms. Br J Pharmacol. (2019);176:3754–3774. doi: 10.1111/bph.14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yu X, Cai X, Cui H, Lee SW, Yu XF, Liu B. Fluorine-free preparation of titanium carbide MXene quantum dots with high near-infrared photothermal performances for cancer therapy. Nanoscale. (2017);9:17859–17864. doi: 10.1039/c7nr05997c. [DOI] [PubMed] [Google Scholar]
- 92.Zhang J, Fu Y, Mo A. Multilayered titanium carbide mxene film for guided bone regeneration. Int J Nanomedicine. (2019a);14:10091–10103. doi: 10.2147/IJN.S227830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang JZ, Kong N, Uzun S, Levitt A, Seyedin S, Lynch PA, Qin S, Han MK, Yang WR, Liu JQ, Wang XG, Gogotsi Y, Razal JM. Scalable manufacturing of free-standing, strong Ti3C2Tx MXene films with outstanding conductivity. Adv Mater. (2020a);32:e2001093. doi: 10.1002/adma.202001093. [DOI] [PubMed] [Google Scholar]
- 94.Zhang P, Yang XJ, Li P, Zhao Y, Niu QJ. Fabrication of novel MXene (Ti(3)C(2))/polyacrylamide nanocomposite hydrogels with enhanced mechanical and drug release properties. Soft Matter. (2020b);16:162–169. doi: 10.1039/c9sm01985e. [DOI] [PubMed] [Google Scholar]
- 95.Zhang R, Liu J, Li Y. MXene with great adsorption ability toward organic dye:an excellent material for constructing a ratiometric electrochemical sensing platform. ACS Sens. (2019b);4:2058–2064. doi: 10.1021/acssensors.9b00654. [DOI] [PubMed] [Google Scholar]
- 96.Zhang Z, Rouabhia M, Wang Z, Roberge C, Shi G, Roche P, Li J, Dao LH. Electrically conductive biodegradable polymer composite for nerve regeneration:electricity-stimulated neurite outgrowth and axon regeneration. Artif Organs. (2007);31:13–22. doi: 10.1111/j.1525-1594.2007.00335.x. [DOI] [PubMed] [Google Scholar]
- 97.Zhang Z, Gao S, Hu YN, Chen X, Cheng C, Fu XL, Zhang SS, Wang XL, Che YW, Zhang C, Chai RJ. Ti(3) C(2) T(x) MXene composite 3D hydrogel potentiates mTOR signaling to promote the generation of functional hair cells in cochlea organoids. Adv Sci (Weinh) (2022);9:e2203557. doi: 10.1002/advs.202203557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zimmerman JF, Tian B. Nongenetic optical methods for measuring and modulating neuronal response. ACS Nano. (2018);12:4086–4095. doi: 10.1021/acsnano.8b02758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zipser CM, Cragg JJ, Guest JD, Fehlings MG, Jutzeler CR, Anderson AJ, Curt A. Cell-based and stem-cell-based treatments for spinal cord injury:evidence from clinical trials. Lancet Neurol. (2022);21:659–670. doi: 10.1016/S1474-4422(21)00464-6. [DOI] [PubMed] [Google Scholar]
- 100.Zou J, Wu J, Wang Y, Deng F, Jiang J, Zhang Y, Liu S, Li N, Zhang H, Yu J, Zhai T, Alshareef HN. Additive-mediated intercalation and surface modification of MXenes. Chem Soc Rev. (2022);51:2972–2990. doi: 10.1039/d0cs01487g. [DOI] [PubMed] [Google Scholar]


