Abstract
The myelin sheath is a lipoprotein-rich, multilayered structure capable of increasing conduction velocity in central and peripheral myelinated nerve fibers. Due to the complex structure and composition of myelin, various histological techniques have been developed over the centuries to evaluate myelin under normal, pathological or experimental conditions. Today, methods to assess myelin integrity or content are key tools in both clinical diagnosis and neuroscience research. In this review, we provide an updated summary of the composition and structure of the myelin sheath and discuss some histological procedures, from tissue fixation and processing techniques to the most used and practical myelin histological staining methods. Considering the lipoprotein nature of myelin, the main features and technical details of the different available methods that can be used to evaluate the lipid or protein components of myelin are described, as well as the precise ultrastructural techniques.
Keywords: fluorescence microscopy, histology, light microscopy, lipid histochemistry, metallographic techniques, myelin histochemistry, myelin immunohistochemistry, myelin structure & composition, myelin ultrastructural evaluation, tissue fixation & processing
Introduction
The study of the structure, genesis, and function of myelin began centuries ago with well-known authors such as Vesalius (1514–1564), van Leeuwenhoek (1632–1723), Schultze (1825–1874), Schwann (1810–1882), Virchow (1821–1902), Ranvier (1835–1922), and many others who contributed significantly to the knowledge (Boullerne, 2016). Currently, myelin is known to be a specialized lipoprotein-rich multilayered material produced by central or peripheral glial cells around the axons that form the myelinated nerve fibers. Myelin is a key structural and functional component of the nervous system, and its histological evaluation is crucial for establishing certain diagnoses and for specific research goals, such as nerve tissue regeneration (García-García et al., 2023). In this sense, this review aims to provide information on the basic structure of myelin, with special emphasis on the histological methods available to evaluate myelin structure and content in central or peripheral nervous system (PNS) tissue samples.
This review follows is a literature review structure and provides a narrative and descriptive summary of the findings and conclusions of previous research. It is a narrative but critical and comprehensive analysis of published literature on a specific topic or research question. This type of studies does not include a pre-defined search strategy as it is not a systematic review that follows a pre-stablished protocol with explicit inclusion and exclusion criteria and a comprehensive search strategy across multiple databases.
Basic structure of myelin
Myelin is the compaction of the plasma membrane of glial cells in a discontinuous spiral pattern over axons that form the myelinated central or peripheral nerve fibers. For this reason, these highly specialized and complex three-dimensional structures are also known as myelin sheaths. Functionally, myelin sheaths isolate axons from the surrounding environment, support saltatory electrical nerve impulse conduction, and significantly increase nerve conduction velocity (Salzer, 2015). Furthermore, myelin sheaths are also referred to as internodal segments because they are separated from each other by non-myelinated axonal segments, the nodes of Ranvier, where action potentials occur (Salzer, 2015). Ultrastructurally, the myelin sheaths can be divided into two distinct domains, compact and non-compact myelin. The compact myelin forms the bulk of the myelin sheath, while the non-compacted myelin can be found in the borders of the myelin sheath (paranodes) and in Schmidt-Lanterman incisions (Arroyo and Scherer, 2000). The compacted myelin sheaths are produced in a spiral pattern around the axons and generate two morphological features at the ultrastructural level, namely the main dense line and the intraperiodic line (Arroyo and Scherer, 2000; Colello, 2011; Figure 1). The dense line is the residual intracellular space left by the glial cell when the two opposing plasma membranes meet during rolling. Meanwhile, the intraperiodic line corresponds to the extracellular space between adjacent layers of the spiral. Here, the outer surfaces of the glial cell plasma membrane are tightly bound by the different outer protein coats of the original cell membrane (Siegel, 1999; Colello, 2011). This highly specialized and complex three-dimensional configuration of the glial cell plasma membrane is given by the lipid-protein interaction and the resulting structural stabilization.
Figure 1.
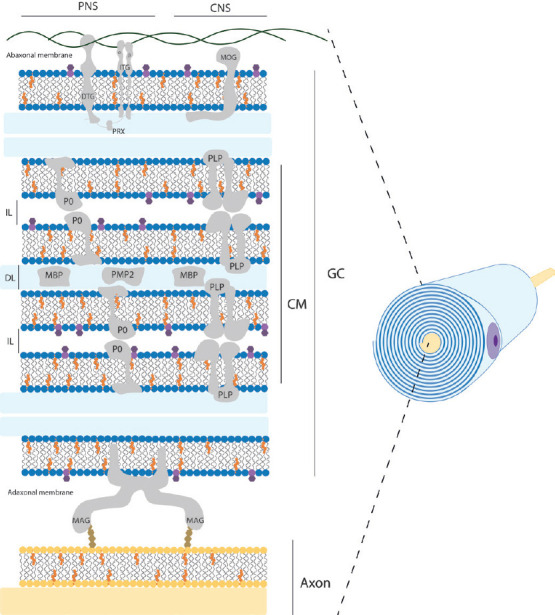
Schematic structural view of myelin sheath in the nervous system.
This representation shows a simplified distribution of the main components within myelin sheath in PNS and CNS. Residual intracellular space remaining of the glial cell is colored blue and corresponds to DL. The extracellular space between adjacent layers of the spiral corresponds to IL. Mayor lipid components are shown as cholesterol molecules in orange; phospholipids head in blue color; galactose residue of galactosylceramide molecule in dark purple. Mayor protein components are represented as CM, GC, DTG, ITG, PRX, MOG, P0, PLP, MBP, PMP2, and MAG. Note that cytoskeletal elements, other common proteins and cell organelles are not included for clarity. Original figure was created by Adobe Illustrator. CM: Compacted myelin; CNS: central nervous system; DL: mayor dense line; DTG: dystroglycan; GC: glial cell membrane; IL: intraperiod line; ITG: integrins; MAG: myelin associated glycoprotein; MBP: myelin basic protein; MOG: myelin oligodendrocyte glycoprotein; P0: myelin protein 0; PLP: proteolipid protein; PMP2: peripheral myelin protein 2; PNS: peripheral nerve system; PRX: periaxin.
Although myelin enhances action potential propagation in a similar manner in both the peripheral nervous system (PNS) and central nervous system (CNS), there are clear structural and biochemical differences between them (Figure 1). First, depending on its location, myelin is structurally produced by two different glial cells, oligodendrocytes and Schwann cells, in the CNS and PNS, respectively (Chato-Astrain et al., 2020a). In this sense, oligodendrocytes are able to myelinate multiple axon segments simultaneously, in contrast to Schwann cells, which can only myelinate a single axon segment from a neuron (Harty et al., 2019). Furthermore, the length of axonal segments generated varies depending on the glial type, with Schwann cells being 10 times longer than those generated by oligodendrocytes (Poitelon et al., 2020). It is important to note that the optic nerve is an exception and is not part of the PNS. This nerve belongs to the CNS because it embryologically arises with the other elements of the CNS, and therefore it is histologically composed of CNS neurons and glial cells, and it is completely covered by the meninges (Mills, 2007).
The molecular composition and deposition patterns also differ between the two systems. The general myelin composition in PNS and CNS is predominantly lipid (70–75% of its dry weight) with an unusual plasmatic membrane lipid composition compared to other cells. It is composed of cholesterol, phospholipids (e.g., sphingomyelin, plasmalogen), and glycolipids (e.g., galactosylceramide) in a ratio of approximately 4:4:2 (Norton and Poduslo, 1973; Nave and Werner, 2014; Poitelon et al., 2020). Interestingly, there is no significant difference in myelin lipid composition between the CNS and PNS, except for the predominance of glycolipids, specifically galactosyceramide in the CNS and sphingomyelin and phosphatidylcholine in the PNS (Poitelon et al., 2020). However, the remaining 25–30% of myelin protein content varies considerably (Kiernan, 2007; Jahn et al., 2009; Colello, 2011; Patzig et al., 2011). In fact, for each nervous system there are proteins specific for central or peripheral myelinated nerve fibers (Figure 1 and Table 1). Some examples of these specificities are the presence of myelin protein zero or periaxin in the PNS and myelin oligodendrocyte glycoprotein or claudin-11 in the CNS. It is important to note that the development of new protein detection techniques, such as liquid chromatography-mass spectrometry using an elevated collision energy mode of acquisition, has allowed the detection of numerous newly identified myelin-associated proteins, accounting for 65%, compared to 35% for all previously known myelin proteins combined (Jahn et al., 2009). Some of the new myelin-associated proteins detected in the CNS were: sirtuin, neurofascin, plasmolipin, among others (Jahn et al., 2009).
Table 1.
Comparison of the lipoprotein composition of myelin in central and peripheral nervous systems
| Lipid composition | CNSa | PNSb | Protein composition | CNSc | PNSd |
|---|---|---|---|---|---|
| Cholesterol | 46% | 41% | Myelin basic protein | 8% | 18% |
| Phospholipids | 33% | 42% | Myelin associated glycoprotein | 1% | 0.20% |
| - Plasmalogen | 13% | 13% | Cyclic nucleotide phosphodiesterase | 4% | 0.30% |
| - Phosphatidylcholine | 7% | 10% | Proteolipid protein | 17% | 0.20% |
| - Sphingomyelin | 6% | 13% | Myelin oligodendrocytes glycoprotein | 1% | – |
| - Glycerophosphatides | 7% | 7% | Claudin 11 | 1% | – |
| Glycolipids | 20% | 12% | Peripheral myelin protein 2 | – | 0.30% |
| -Cerebroside (galactosylceramide) | 17% | 10% | Myelin protein 0 | – | 45% |
| - Sulphatide | 3% | 1% | Periaxin | – | 16% |
| Other lipids | 1% | 5% | Other proteins | 68% | 20% |
Table was adapted from Poitelon et al. (2020) and Nave and Werner (2014). Data obtained from aNorton and Autilio (1966), bO’Brien et al. (1967), cJahn et al. (2009), and dSiems et al. (2020). CNS: Central nervous system; PNS: peripheral nervous system.
Why is it important to study myelin
In the field of neuroscience as well as in clinical practice, the assessment of myelin integrity or content is important. In fact, histological analysis of myelin has been instrumental in deciphering current knowledge and concepts about this highly specific key element (Boullerne, 2016). On the one hand, histology has provided the basis for studying the structure and even the composition of myelin at basic and advanced scientific levels. On the other hand, histological assessment of myelin still has an important clinical importance, providing the information to make an accurate diagnosis in patients affected by various diseases. In addition, myelin histology is widely used in a variety of research areas. Indeed, the identification of myelin by histological staining techniques allows to accurately determine the effectiveness or failure of certain experimental setups. For example, in nerve tissue engineering, myelin histology is a well-established quality control to detect differences in the degree of peripheral nerve regeneration using engineered substitutes (Chato-Astrain et al., 2018, 2020b). In addition, histological detection of myelin components plays a key role in the description and spatiotemporal study of different demyelination patterns caused by different pathological conditions, such as traumatic injury, autoimmune-mediated disorders, viral infections, metabolic or endocrine conditions, among others (Savaskan et al., 2009). These examples demonstrate the usefulness of histological analyses of myelin in clinical practice and biomedical research, as these methods are a valuable complement to clinical, proteomic, genetic, and even behavioral results.
Histological Techniques
Because of the importance of studying myelin, several methods have been developed to identify myelin. First, different approaches to fixation and processing of nerve tissue samples are reviewed. Second, some of the most useful and practical myelin detection techniques are commented on in detail.
Tissue fixation & processing
Although myelin can be observed in fresh material, for most applications the nervous tissue is fixed, sectioned and stained to obtain permanent histological specimens (Kiernan, 2007).
The first and most critical step in histology is the fixation (Sánchez-Porras et al., 2023), which aims to stabilize and preserve the structure and chemical composition of the tissue. Samples can be fixed by different mechanisms, the most used being chemical fixation, followed by physical techniques such as freezing.
In the case of myelin, the use of non-coagulant routine fixative solutions, such as formaldehyde or paraformaldehyde, only stabilizes the protein moieties by cross-linking, without interacting with most of the lipids present in its structure (Kiernan, 2007; Sánchez-Porras et al., 2023). Nevertheless, improved preservation of hydrophilic phospholipids has been obtained by adding calcium ions to an aqueous solution of formaldehyde (Baker’s formal-calcium) (Sánchez-Porras et al., 2023). Another chemical treatment often used for lipid stabilization is osmium tetroxide (OsO4) (Kiernan, 2007; García-García et al., 2023). This solution could be used as a primary fixative, but it is often used for post-fixation (after aldehyde fixation). OsO4 is reduced by unsaturated lipids generating black insoluble compounds (OsO2) (Kiernan, 2007), which provides a permanent and stable myelin stain. This method allows the assessment of myelinated fibers in cryosections, but is particularly useful in paraffin- and resin-embedded material (Raimondo et al., 2009; García-García et al., 2023).
Once fixed or preserved, and considering the aforementioned lipoprotein nature of myelin, it is possible to use techniques to label the lipid portion or its constituent proteins. Depending on the method chosen, it is important to select the correct processing technique, which can generally be divided into paraffin/resin embedding or freezing prior to sectioning. It is important to note that organic solvents such as xylene and alcohol (used for dehydration and clearing in tissue processing) extract most lipids (Sánchez-Porras et al., 2023). However, in the case of myelin, only those lipids that are covalently bound to protein (mainly phospholipids and other lipoproteins) are partially resistant to chemical extraction and can be identified in routinely prepared paraffin sections (Carriel et al., 2014b; García-García et al., 2023). Therefore, to avoid loss of material and to perform an accurate evaluation, lipid histochemistry is generally performed on frozen sections, which is particularly useful for detecting myelin degeneration by-products that contain hydrophobic esters rich in unsaturated fatty acids and cholesterol rather than the more hydrophilic sphingolipids and phospholipids (Kiernan, 2007). However, if the freezing process is not completed at a sufficient rate, the slow freezing often results in ice crystals that can permanently damage tissue architecture (Kiernan, 2008; Serrato et al., 2009; Cook and Warren, 2015). To minimize this undesirable effect, chemically pretreated (fixed and sucrose cryoprotected) tissue samples must be frozen using liquid nitrogen (–170°C) or precooled isopentane at –80°C (Kiernan, 2008; Serrato et al., 2009; Weiss et al., 2021). This procedure has been shown to improve tissue morphology and reduce damage due to ice crystal formation, thus providing a high quality cryosection suitable for most lipid histochemical methods (such as oil red and Sudan black) and other staining techniques such as immunofluorescence (Sánchez-Porras et al., 2023), which will be discussed later in this review. Therefore, to observe unaltered myelin sheaths microscopically, it is necessary to perform frozen sections or, alternatively, sections of embedded tissue that have been specially fixed to insolubilize lipids.
Histological assessment of myelin
After tissue fixation and sectioning, the morphologic characteristics of the myelin structure can be revealed by various staining techniques that highlight the different biochemical properties of the myelin sheath. Here we comment on some relatively simple methods for specific staining of myelin that could be used for diagnostic or research purposes (Table 2).
Table 2.
Preferred and recommendable applications of the different histological techniques to assess myelin sheaths
| Methods | Resin sections | Paraffin sections | Cryosections | CNS | PNS |
|---|---|---|---|---|---|
| Osmium tetroxide technique | HR | HR | S | S | HR |
| Solvent dyes | NR | *S | HR | HR | HR |
| - Oil red | |||||
| - Sudan (III, IV, black) | |||||
| Conventional Luxol fast blue (or KB) | NT | HR | HR | HR | HR |
| Myelin-collagen technique (MCOLL) | NT | HR | HR | HR | HR |
| Luxol fast blue–periodic acid–Schiff technique (LFB-PAS) | NT | HR | NT | HR | HR |
| FluoroMyelinTM | NR | NR | HR | HR | HR |
| Immunodetection techniques | NR | HR | HR | HR | HR |
| Metallographic techniques | HR | S | HR | HR | |
| Toluidine blue | HR | *NR | *NR | HR | HR |
| Transmission electron microscopy | Resin ultrathin sections | HR | HR |
*S: The positivity will depend on the degree of preservation of certain lipids after tissue fixation, processing and embedding and generally, the positive reaction is not optimal. *NR: Toluidine blue is often used on both paraffin and cryosections for the study of proteoglycans or metachromatic elements, but it will not stain myelin as it perfectly does on semithin sections where it is HR. CNS: Central nervous system; HR: highly recommended; KB: Klüver-Barrera; NR: not recommended; NT: not tested yet; PNS: peripheral nervous system; S: suitable.
Polarized light method
This method exploits the anisotropy of unmodified myelin, which results in an intrinsic birefringence of this structure. This anisotropy could be related to the high enrichment of birefringent or anisotropic phospholipids in the myelin sheath. However, this anisotropy is lost with degeneration because the products of myelin are triglycerides, which are isotropic and therefore easily distinguished from the intact myelin sheath by the polarizing microscope (Setterfield and Sutton, 1935; Prickett and Stevens, 1939). In addition, the presence of birefringent crystals in pathological nerve and other tissues has long been recognized and tentatively attributed to cholesterol and/or its esters (Kiernan, 2007), and their contrast could be enhanced by oil-soluble dyes. Although this method cannot specifically detect a lipid or protein element in the sheaths, it is able to detect myelin content and its structural status as a label-free method and is often used to analyze fresh material (Raine, 1984; Morgan et al., 2021).
Histochemical techniques
Here we briefly comment on some of the most commonly used histochemical techniques for myelin detection which are often based on the identification of the lipids which form main part of myelin sheaths.
The OsO4 staining, which frequently involves a post-fixation procedure and consequent staining, confers myelin stabilization, and provides a permanent black positive reaction for myelin as well as other lipids present in the tissue. OsO4 penetrates tissues slowly and, a strong oxidizing agent reacts with organic compounds and it is itself reduced into OsO2. This last molecule adheres to structures that have been in contact with OsO4. This reaction prevents myelin sheath swelling and its deposition imparts a dark color at light microscopy and electron opacity at transmission electron microscopy (TEM) (Kiernan, 2007). As a result, myelin is stained black, and the nodes of Ranvier are clearly visible (Figure 2). Schmidt-Lanterman incisures are paler V-shaped formations within the myelin, visible only within well-processed material at high magnifications being clearly visible in TEM analysis. In addition, other staining methods, such as Picrosirius or Masson’s trichome, can be combined with OsO4 staining to identify other important structures in nervous tissue (García-García et al., 2023). OsO4 technique can be applied to cryosections, but it is especially useful for paraffin or resin sections.
Figure 2.
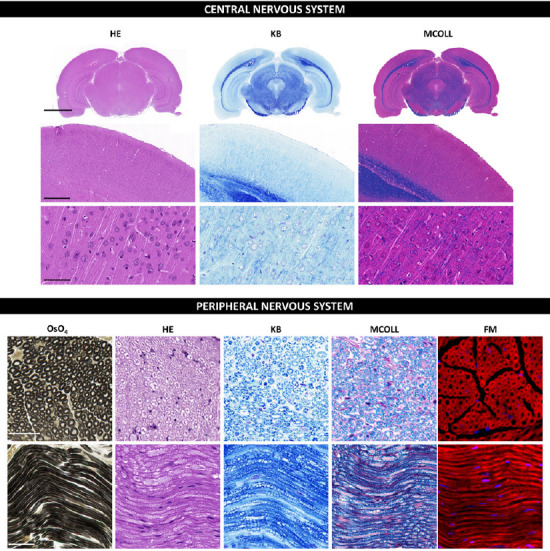
Histochemical staining techniques in central and peripheral nervous systems.
Representative coronal sections of rat encephalus, and cross and longitudinal sections of rat sciatic nerves stained by diverse histochemical methods. Here a comparison between the routine stain HE and specific myelin staining methods such as KB (or conventional LFB), MCOLL, OsO4 and FM staining is included. HE shows the typical histological pattern with nuclei stained blue and a pink contrast. Conventional KB reveals the myelin sheaths with an intense blue histochemical reaction and cellular elements in a light purple color. The MCOLL method provides a simultaneously and specific staining of myelin (blue) and fibrillar collagen fibers (red) with a cell nuclei contrast (blue/purple). OsO4 generates a permanent and stable myelin dark stain, which significantly facilitates the identification and evaluation of nerve fibers. FluoroMyelin™ stains myelin in red fluorescent color and nuclei were detected by DAPI (blue). All samples were fixed in formaldehyde and embedded in paraffin except FluoroMyelin™ ones, which were fixed in formaldehyde, cryopreserved and cryosectioned. Scale bars in images for central nervous system: low magnification: 3000 μm; medium magnification: 500 μm; higher magnification: 50 μm. Scale bars in images for peripheral nervous system: 50 μm cross and longitudinal sections. DAPI: 4′,6-Diamidino-2-phenylindole; FM: FluoroMyelin™; HE: hematoxylin and eosin; KB: Klüver-Barrera; LFB: Luxol fast blue; MCOLL: myelin-collagen method; OsO4: osmium tetroxide.
The solvent dyes, such as Sudan (III, IV and black) and Oil red O, are able to interact with the hydrophobic domains of lipids including myelin. These dyes are prepared in polar organic solvents and when they are in contact with lipids, they diffuse from their solution to the hydrophobic domains of the lipids present in the tissue. Dyes diffusion occurs because they are much more soluble in lipids than in the solvent used, for these reasons they are commonly known as solvent dyes or lysochromes (Kiernan, 2008). From all the “Sudan” dyes, the Sudan black B is a good option for myelin staining as it is the less hydrophobic Sudan dyes and stains most lipids. Indeed, Sudan Black B dye can interact better with the phosphor and sphingolipids of normal myelin resulting in a dark gray or blue-black myelin staining (Kiernan, 2007). These methods work in paraffin sections, but better results can be obtained in cryosections, especially when the samples were chemically fixed and cryoprotected (Sánchez-Porras et al., 2023).
The myelin-collagen (MCOLL) technique is a trichromatic, histochemical-based method designed to simultaneously stain myelin, collagen fibers and cell nuclei allowing to perform an integrated histological analysis (Carriel et al., 2011; Figure 2). This method combines the classical Luxol fast blue (LFB) myelin staining technique with picrosirius histochemical method for fibrillar collagens and Harris hematoxylin as nuclear contrast (Carriel et al., 2014a). LFB has largely been used for myelin detection since its introduction by Kluver and Barrera (1953) in fact this method is also know as KB technique (Figure 2). It was assumed that LFB stains specifically lipid domains, however, it is currently believed that LFB does not have any histochemical interaction with lipids. Indeed, the colored dye anion enters all parts of the tissue, but the basic amino acids of the myelin proteins may retain them in sites that are not easily reached by the differentiating solution staining myelin with a characteristic blue reaction (Kiernan, 2007). Then, after myelin stain, picrosirius is performed which is based on a strong anionic tetrakisazo dye called Sirius red F3B (Carriel et al., 2011). These dye molecules parallelly interact with cationic groups on the surface of the collagen, giving an intense red colorimetric reaction to the fibrillar collagen fibers in light microscopy (Trau et al., 1991). Moreover, picrosirius molecules increase the natural birefringence of these fibers allowing their select evaluation by polarized light microscopy. MCOLL technique can be conducted in cryosections, but it is especially useful in paraffin-embedded material (Chato-Astrain et al., 2023). In addition, there is another modification of the classical LFB technique, where it was combined with periodic acid-Schiff (PAS) histochemical method. This LFB-PAS method is especially useful to evaluate the demyelinating processes and also to identify the inflammatory activity. Indeed, the addition of PAS histochemical method allows to identify the cellular debris phagocyted by immunological cells, mainly by the microglia or foam cells, in some pathological conditions such as multiple sclerosis (Kuhlmann et al., 2017; Frosch et al., 2021).
The FluoroMyelinTM is a commercially available ready-to-use method to study myelin based on a fluorescent dye that can easily interact with myelin lipids. This component is a non-toxic water-soluble fluorescent dye with lipophilic chemical properties that primarily incorporate into the lipid portion of the myelin sheaths but also faintly label cellular membranes. FluoroMyelin technique is suitable to evaluate central or peripheral myelin in cryosections (direct frozen or formalin-fixed and cryoprotected samples) (Figure 2), cell cultures or in fresh or even living material. However, it is important to mention that this method does not work in paraffin-embedded tissues (García-García et al., 2023). This one-step method is fast and often used in combination with other immunofluorescence-based methods allowing to establish functional interactions in normal, degeneration or regeneration processes (Scott-Hewitt et al., 2018; Ciotu et al., 2023). FluoroMyelin™ is of advantage when information about the general myelination status is of interest, being a much shorter process than immunodetection technique and, thus, are independent of available myelin protein antibodies. However, its main disadvantages are the short-lifetime and its non-compatibility with paraffin-embedded samples (García-García et al., 2023). FluoroMyelin™ dye must be used in cryosection where it can be easily combined with other fluorochromes and immunofluorescences.
Metallographic techniques
Another way to detect myelin is using metallographic techniques after formaldehyde or paraformaldehyde tissue fixation. This method allows the amplification of a discrete binding or deposition of a gold or silver atom at a specific location in a histological section until they become visible in the light or electron microscope. Back in 1894, Camilo Golgi described a method that he called “reazione nera” that later on Santiago Ramón y Cajal adapted to avoid the problems encountered in staining myelinated neurons. The essence of the Golgi method consisted of the immersion of small pieces of nervous tissue into osmium-bichromic solution for several days. Then, the samples must be left in a fresh solution of silver nitrate for a few more days (de Castro et al., 2007). In recognition of their work on the structure of the nervous system, they were awarded the Nobel Prize in Physiology or Medicine. Since then, several modifications have been made to these methods and nowadays they can still be used for myelin staining. Now it is believed that this method takes advantage of the non-coagulant fixatives previously mentioned as it seems to cause a chemical build-up of “points of reduction” in the myelin membrane. These points are then flooded with silver ions contained in the developer, which will cause a build-up of clusters of metallic silver atoms on the spot. Further silver ions will bind the cluster and they will be reduced to metallic silver resulting in a visible silver grain (Larsen et al., 2003). Metallographic techniques are often applied in paraffin-embedded sections but can also be used in cryosections of tissues after formaldehyde fixation.
Immunodetection techniques
Myelin can also be detected by immunohistochemistry or immunofluorescence using antibodies that specifically recognize the myelin proteins. These are powerful techniques that exploit the specific binding between an antibody and antigen to detect and localize specific antigens in cells and tissue (Magaki et al., 2019). The antibody-mediated recognition focuses on myelin-specific proteins such as the specified in Table 1. The antigen detection could be either through chromogenic or fluorescents means in immunohistochemical or immunofluorescence staining respectively. Immunohistochemical staining usually combines a secondary antibody conjugated with the horseradish peroxidase enzyme that catalyzes the precipitation of a substrate, mostly 3,3′-diaminobenzidine, in insoluble colored precipitates at the antigen location site. Moreover, this process is normally accompanied by a slight hematoxylin counterstain that generates a tissue overview of the different histological structures. In immunofluorescence protocols, the secondary antibody is conjugated with a fluorochrome and normally counterstained with 4′,6-diamidino-2-phenylindole. These methods offer the possibility to detect different specific proteins (epitopes) and correlate them with different cellular or molecular processes proving highly valuable biological information. In addition, immunofluorescence techniques have the great advantage of allowing the simultaneous study, and even colocalization, of several molecules, either by combining them with fluorescent dyes or by using several specific antibodies against different target proteins, providing highly valuable information. From a technical point of view, immunofluorescence can be conducted in paraffin-embedded material, but better results can be obtained with fresh formaldehyde-fixed cryosections since the epitopes are better preserved (Scalia et al., 2017).
Semithin and ultrathin sections techniques
Myelin is usually detected, besides the light microscopy methods, with ultrastructural techniques based on TEM. Results from the preparation of the nervous tissue samples for TEM are significantly superior to those from light microscopy (Carriel et al., 2014a; Ronchi et al., 2014; Geuna, 2015). The technical procedure ensures appropriate fixation of the tissue (generally using glutaraldehyde), myelin preservation (due to the OsO4 postfixation), and the ability to create semithin and ultrathin sections that are transversally oriented, commonly obtained from resin and stained by toluidine blue dye. It has been reported that the selection of the embedding medium, resin or paraffin, can have an impact on nerve fiber size distribution in morphometrical analyses (Raimondo et al., 2009). Both semithin and ultrathin sections enable a highly precise, high-resolution, and quantitative evaluation of degeneration or regeneration profile in both CNS (spinal cord and brain) (Ek et al., 2010; Bondan et al., 2014) and PNS (Carriel et al., 2014a; Ronchi et al., 2023).
The semithin sections are especially useful to perform systematic counting of myelinated axons determining the effectiveness of different experimental approaches in tissue engineering (Raimondo et al., 2009; Chato-Astrain et al., 2020a; Ronchi et al., 2023). This systematic quantitative assessment offers a reproducible, accurate and objective evaluation of the regeneration profile in each case through the recording of mainly the number, size and shape parameters of myelinated fibers (Raimondo et al., 2009; Ronchi et al., 2014; Chato-Astrain et al., 2020a).
Finally, TEM allows the acquisition of high-resolution pictures at extracellular and intracellular levels, which is especially used in the description of peripheral nerves ultrastructural characteristics (Geuna et al., 2009). This technique enables not only a precise identification and quantification of myelinated fibers, but also the unmyelinated ones, improving the accuracy of the semithin section analyses (Ronchi et al., 2014; Chato-Astrain et al., 2020a). In addition, TEM analysis also allows to obtain all the aforementioned histomorphometrical parameters, including the unmyelinated/myelinated axon ratio, another crucial indicator of peripheral nerve regeneration, thanks to the high resolution of ultrathin section images (Lovati et al., 2018). Actually, both resin-based methods are considered gold standard techniques for the histological evaluation of myelin by several authors.
Limitations
In this review, we discussed the most commonly used methods to histologically evaluate the myelin content and it can provide valuable insights into the current state of the field. It attempted to summarize the advantages and limitations of each method and provide recommendations for choosing the appropriate method for a given research question. However, this review has some limitations that should be considered.
First, it is important to note that not all histological methods for myelin staining were included in this review and therefore, some methods that may be useful for certain research aims were not discussed here. Second, the results of studies that use different myelin staining methods could not be comparable due to differences in sensitivity, specificity, and wide range of other technical factors. Researchers should carefully consider the strengths and limitations of each method and choose the most appropriate technique based on their specific research aims taking into account that myelin is often affected by several technical factors such as fixation and subsequent tissue processing.
Researchers should consider that myelin can be evaluated by other molecular-based methods (mass spectrometry-liquid chromatography, gene expression analyses, etc.) obtaining more specific, semiquantitative, and even accurate information than by histological analysis. Indeed, myelin histological assessment should be complementary to other clinical, molecular and functional results, but not be used as the sole findings in an experimental setup or clinical study.
Conclusions
The study of the structure, genesis and function of myelin began centuries ago. Since then, numerous techniques have been developed for the histological identification of this important structure and, in fact, there are currently useful methods available for the assessment of myelin. In this review, we have discussed some of the most used techniques that can be applied not only for the clinical determination of specific diagnoses but also for the evaluation of the effectiveness of new treatments in the field of neuroscience. Myelin identification techniques are able to specifically detect some of the main components of this structure as it does the OsO4 technique, MCOLL histochemical technique or the toluidine blue semithin sections. However, staining technique must be selected in accordance with the previous tissue fixation and processing methods. Cryosection or paraffin-embedded tissue processing affects different structural elements of myelin, and thus it is important to be able to specifically detect the remaining molecules. In addition, the structural complexity of the sheaths makes it difficult to detect all myelin components using a single descriptive histological method that may include many technical limitations. In this sense, it is advisable to combine different histological techniques (histochemical, immunohistochemical and ultrastructural methods) to successfully study the structure and composition of myelin.
Acknowledgments
Authors are grateful to Prof. Ariane Ruyffelaert for her proofreading service.
Footnotes
Funding: This work was supported by the Spanish “Plan Nacional de Investigación Científica, Desarrollo e Innovación Tecnológica, Ministerio de Economía y Competitividad (Instituto de Salud Carlos III)”, Grant FIS PI20-0318 co-financed by “Fondo Europeo de Desarrollo Regional ERDF-FEDER European Union”; Grant P18-RT-5059 “Plan Andaluz de Investigación, Desarrollo e Innovación (PAIDI 2020), Consejería de Transformación Económica, Industria, Conocimiento y Universidades, Junta de Andalucía, España” (all to VC) and Grant PPJIA2022-19 “Ayudas del plan propio UGR 2022, Plan propio de investigación y transferencia, Universidad de Granada, España” (to JCA and ÓDGG).
Conflicts of interest: None declared.
Data availability statement: Not applicable.
C-Editors: Zhao M, Liu WJ, Yu J; T-Editor: Jia Y
References
- 2.Arroyo EJ, Scherer SS. On the molecular architecture of myelinated fibers. Histochem Cell Biol. (2000);113:1–18. doi: 10.1007/s004180050001. [DOI] [PubMed] [Google Scholar]
- 3.Bondan EF, Martins Mde F, Menezes Baliellas DE, Monteiro Gimenez CF, Castro Poppe S, Martha Bernardi M. Effects of propentofylline on CNS remyelination in the rat brainstem. Microsc Res Tech. (2014);77:23–30. doi: 10.1002/jemt.22308. [DOI] [PubMed] [Google Scholar]
- 4.Boullerne AI. The history of myelin. Exp Neurol. (2016);283:431–445. doi: 10.1016/j.expneurol.2016.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carriel V, Garzón I, Alaminos M, Cornelissen M. Histological assessment in peripheral nerve tissue engineering. Neural Regen Res. (2014a);9:1657–1660. doi: 10.4103/1673-5374.141798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carriel V, Alaminos M, Garzón I, Campos A, Cornelissen M. Tissue engineering of the peripheral nervous system. Expert Rev Neurother. (2014b);14:301–318. doi: 10.1586/14737175.2014.887444. [DOI] [PubMed] [Google Scholar]
- 7.Carriel VS, Aneiros-Fernandez J, Arias-Santiago S, Garzón IJ, Alaminos M, Campos A. A novel histochemical method for a simultaneous staining of melanin and collagen fibers. J Histochem Cytochem. (2011);59:270–277. doi: 10.1369/0022155410398001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chato-Astrain J, García-García ÓD, Campos F, Sánchez-Porras D, Carriel V. Basic nerve histology and histological analyses following peripheral nerve repair and regeneration. In: Phillips J, Hercher D, Hausner T, editors. Peripheral nerve tissue engineering and regeneration. Cham: Springer International Publishing; (2020a). pp. 1–37. [Google Scholar]
- 9.Chato-Astrain J, Roda O, Sánchez-Porras D, Miralles E, Alaminos M, Campos F, García-García ÓD, Carriel V. Peripheral nerve regeneration through nerve conduits evokes differential expression of growth-associated protein-43 in the spinal cord. Neural Regen Res. (2023);18:1852–1856. doi: 10.4103/1673-5374.363180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chato-Astrain J, Philips C, Campos F, Durand-Herrera D, García-García OD, Roosens A, Alaminos M, Campos A, Carriel V. Detergent-based decellularized peripheral nerve allografts:an in vivo preclinical study in the rat sciatic nerve injury model. J Tissue Eng Regen Med. (2020b);14:789–806. doi: 10.1002/term.3043. [DOI] [PubMed] [Google Scholar]
- 11.Chato-Astrain J, Campos F, Roda O, Miralles E, Durand-Herrera D, Sáez-Moreno JA, García-García S, Alaminos M, Campos A, Carriel V. In vivo evaluation of nanostructured fibrin-agarose hydrogels with mesenchymal stem cells for peripheral nerve repair. Front Cell Neurosci. (2018);12:501. doi: 10.3389/fncel.2018.00501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ciotu CI, Kistner K, Kaindl U, Millesi F, Weiss T, Radtke C, Kremer A, Schmidt K, Fischer MJM. Schwann cell stimulation induces functional and structural changes in peripheral nerves. Glia. (2023);71:945–956. doi: 10.1002/glia.24316. [DOI] [PubMed] [Google Scholar]
- 13.Colello RJ. Myelin. In: Kreutzer JS, DeLuca J, Caplan B, editors. Encyclopedia of clinical neuropsychology. New York, NY: Springer New York; (2011). pp. 1690–1691. [Google Scholar]
- 14.Cook DJ, Warren PJ. 3rd ed. Bloxham, Oxfordshire: Scion; (2015). Cellular pathology : introduction to techniques and applications. [Google Scholar]
- 15.de Castro F, López-Mascaraque L, De Carlos JA. Cajal:lessons on brain development. Brain Res Rev. (2007);55:481–489. doi: 10.1016/j.brainresrev.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 16.Ek CJ, Habgood MD, Callaway JK, Dennis R, Dziegielewska KM, Johansson PA, Potter A, Wheaton B, Saunders NR. Spatio-temporal progression of grey and white matter damage following contusion injury in rat spinal cord. PLoS One. (2010);5:e12021. doi: 10.1371/journal.pone.0012021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frosch M, Kremers N, Lisko K, Urbach H, Prinz M, Taschner CA. Freiburg neuropathology case conference :a 42-year-old patient with progressive neurological deficits, multiple brain lesions and accompanying affection of peripheral nerves. Clin Neuroradiol. (2021);31:529–535. doi: 10.1007/s00062-021-01021-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.García-García ÓD, Weiss T, Chato-Astrain J, Raimondo S, Carriel V. Staining methods for normal and regenerative myelin in the nervous system. Methods Mol Biol. (2023);2566:187–203. doi: 10.1007/978-1-0716-2675-7_15. [DOI] [PubMed] [Google Scholar]
- 19.Geuna S. The sciatic nerve injury model in pre-clinical research. J Neurosci Methods. (2015);243:39–46. doi: 10.1016/j.jneumeth.2015.01.021. [DOI] [PubMed] [Google Scholar]
- 20.Geuna S, Raimondo S, Ronchi G, Di Scipio F, Tos P, Czaja K, Fornaro M. Chapter 3:Histology of the peripheral nerve and changes occurring during nerve regeneration. Int Rev Neurobiol. (2009);87:27–46. doi: 10.1016/S0074-7742(09)87003-7. [DOI] [PubMed] [Google Scholar]
- 21.Harty BL, Coelho F, Pease-Raissi SE, Mogha A, Ackerman SD, Herbert AL, Gereau RWt, Golden JP, Lyons DA, Chan JR, Monk KR. Myelinating Schwann cells ensheath multiple axons in the absence of E3 ligase component Fbxw7. Nat Commun. (2019);10:2976. doi: 10.1038/s41467-019-10881-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jahn O, Tenzer S, Werner HB. Myelin proteomics:molecular anatomy of an insulating sheath. Mol Neurobiol. (2009);40:55–72. doi: 10.1007/s12035-009-8071-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kiernan JA. Histochemistry of staining methods for normal and degenerating myelin in the central and peripheral nervous systems. J Histotechnol. (2007);30:87–106. [Google Scholar]
- 24.Kiernan JA. 5th ed. Bloxham, UK: Scion; (2008). Histological and histochemical methods: theory and practice. [Google Scholar]
- 25.Kluver H, Barrera E. A method for the combined staining of cells and fibers in the nervous system. J Neuropathol Exp Neurol. (1953);12:400–403. doi: 10.1097/00005072-195312040-00008. [DOI] [PubMed] [Google Scholar]
- 26.Kuhlmann T, Ludwin S, Prat A, Antel J, Brück W, Lassmann H. An updated histological classification system for multiple sclerosis lesions. Acta Neuropathol. (2017);133:13–24. doi: 10.1007/s00401-016-1653-y. [DOI] [PubMed] [Google Scholar]
- 27.Larsen M, Bjarkam CR, Stoltenberg M, Sørensen JC, Danscher G. An autometallographic technique for myelin staining in formaldehyde-fixed tissue. Histol Histopathol. (2003);18:1125–1130. doi: 10.14670/HH-18.1125. [DOI] [PubMed] [Google Scholar]
- 28.Lovati AB, D'Arrigo D, Odella S, Tos P, Geuna S, Raimondo S. Nerve repair using decellularized nerve grafts in rat models. A review of the literature. Front Cell Neurosci. (2018);12:427. doi: 10.3389/fncel.2018.00427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Magaki S, Hojat SA, Wei B, So A, Yong WH. An introduction to the performance of immunohistochemistry. Methods Mol Biol. (2019);1897:289–298. doi: 10.1007/978-1-4939-8935-5_25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mills SE. 3rd ed. Lippincott Williams & Wilkins: Philadelphia; (2007). Histology for pathologists. [Google Scholar]
- 31.Morgan ML, Brideau C, Teo W, Caprariello AV, Stys PK. Label-free assessment of myelin status using birefringence microscopy. J Neurosci Methods. (2021);360:109226. doi: 10.1016/j.jneumeth.2021.109226. [DOI] [PubMed] [Google Scholar]
- 32.Nave KA, Werner HB. Myelination of the nervous system:mechanisms and functions. Annu Rev Cell Dev Biol. (2014);30:503–533. doi: 10.1146/annurev-cellbio-100913-013101. [DOI] [PubMed] [Google Scholar]
- 33.Norton WT, Autilio LA. The lipid composition of purified bovine brain myelin. J Neurochem. (1966);13:213–222. doi: 10.1111/j.1471-4159.1966.tb06794.x. [DOI] [PubMed] [Google Scholar]
- 34.Norton WT, Poduslo SE. Myelination in rat brain:changes in myelin composition during brain maturation. J Neurochem. (1973);21:759–773. doi: 10.1111/j.1471-4159.1973.tb07520.x. [DOI] [PubMed] [Google Scholar]
- 35.O'Brien JS, Sampson EL, Stern MB. Lipid composition of myelin from the peripheral nervous system. Intradural spinal roots. J Neurochem. (1967);14:357–365. doi: 10.1111/j.1471-4159.1967.tb09532.x. [DOI] [PubMed] [Google Scholar]
- 36.Patzig J, Jahn O, Tenzer S, Wichert SP, de Monasterio-Schrader P, Rosfa S, Kuharev J, Yan K, Bormuth I, Bremer J, Aguzzi A, Orfaniotou F, Hesse D, Schwab MH, Möbius W, Nave KA, Werner HB. Quantitative and integrative proteome analysis of peripheral nerve myelin identifies novel myelin proteins and candidate neuropathy loci. J Neurosci. (2011);31:16369–16386. doi: 10.1523/JNEUROSCI.4016-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poitelon Y, Kopec AM, Belin S. Myelin fat facts:an overview of lipids and fatty acid metabolism. Cells. (2020);9:812. doi: 10.3390/cells9040812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prickett CO, Stevens C. The polarized light method for the study of myelin degeneration as compared with the Marchi and Sudan III methods. Am J Pathol. (1939);15:241–250.7. [PMC free article] [PubMed] [Google Scholar]
- 39.Raimondo S, Fornaro M, Di Scipio F, Ronchi G, Giacobini-Robecchi MG, Geuna S. Chapter 5:Methods and protocols in peripheral nerve regeneration experimental research:part II-morphological techniques. Int Rev Neurobiol. (2009);87:81–103. doi: 10.1016/S0074-7742(09)87005-0. [DOI] [PubMed] [Google Scholar]
- 40.Raine CS. Morphology of Myelin and Myelination. In: Morell P, editor. Myelin. Boston, MA: Springer; (1984). pp. 1–50. [Google Scholar]
- 41.Ronchi G, Fregnan F, Muratori L, Gambarotta G, Raimondo S. Morphological methods to evaluate peripheral nerve fiber regeneration:a comprehensive review. Int J Mol Sci. (2023);24:1818. doi: 10.3390/ijms24031818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ronchi G, Jager SB, Vaegter CB, Raimondo S, Giacobini-Robecchi MG, Geuna S. Discrepancies in quantitative assessment of normal and regenerated peripheral nerve fibers between light and electron microscopy. J Peripher Nerv Syst. (2014);19:224–233. doi: 10.1111/jns.12090. [DOI] [PubMed] [Google Scholar]
- 43.Salzer JL. Schwann cell myelination. Cold Spring Harb Perspect Biol. (2015);7:a020529. doi: 10.1101/cshperspect.a020529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sánchez-Porras D, Bermejo-Casares F, Carmona R, Weiss T, Campos F, Carriel V. Tissue fixation and processing for the histological identification of lipids. Methods Mol Biol. (2023);2566:175–186. doi: 10.1007/978-1-0716-2675-7_14. [DOI] [PubMed] [Google Scholar]
- 45.Savaskan NE, Weinmann O, Heimrich B, Eyupoglu IY. High resolution neurochemical gold staining method for myelin in peripheral and central nervous system at the light- and electron-microscopic level. Cell Tissue Res. (2009);337:213–221. doi: 10.1007/s00441-009-0815-9. [DOI] [PubMed] [Google Scholar]
- 46.Scalia CR, Boi G, Bolognesi MM, Riva L, Manzoni M, DeSmedt L, Bosisio FM, Ronchi S, Leone BE, Cattoretti G. Antigen masking during fixation and embedding, dissected. J Histochem Cytochem. (2017);65:5–20. doi: 10.1369/0022155416673995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scott-Hewitt NJ, Folts CJ, Noble MD. Heterozygous carriers of galactocerebrosidase mutations that cause Krabbe disease have impaired microglial function and defective repair of myelin damage. Neural Regen Res. (2018);13:393–401. doi: 10.4103/1673-5374.228712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Serrato D, Nieto-Aguilar R, Garzón I, Roda O, Campos A, Alaminos M. Comparison of the effect of cryopreservation protocols on the histology of bioengineered tissues. Histol Histopathol. (2009);24:1531–1540. doi: 10.14670/HH-24.1531. [DOI] [PubMed] [Google Scholar]
- 49.Setterfield HE, Sutton TS. The use of polarized light in the study of myelin degeneration I. The appearance and progress of degeneration after tran-section of the sciatic nerve of the white rat. Anat Rec. (1935);61:397–411. [Google Scholar]
- 50.Siegel GJ. Lippincott Williams &Wilkins; (1999). Basic neurochemistry :molecular, cellular , and medical aspects. [Google Scholar]
- 51.Siems SB, Jahn O, Eichel MA, Kannaiyan N, Wu LMN, Sherman DL, Kusch K, Hesse D, Jung RB, Fledrich R, Sereda MW, Rossner MJ, Brophy PJ, Werner HB. Proteome profile of peripheral myelin in healthy mice and in a neuropathy model. Elife. (2020);9:e51406. doi: 10.7554/eLife.51406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Trau H, Dayan D, Hirschberg A, Hiss Y, Bubis JJ, Wolman M. Connective tissue nevi collagens. Study with picrosirius red and polarizing microscopy. Am J Dermatopathol. (1991);13:374–377. doi: 10.1097/00000372-199108000-00008. [DOI] [PubMed] [Google Scholar]
- 53.Weiss T, Taschner-Mandl S, Janker L, Bileck A, Rifatbegovic F, Kromp F, Sorger H, Kauer MO, Frech C, Windhager R, Gerner C, Ambros PF, Ambros IM. Schwann cell plasticity regulates neuroblastic tumor cell differentiation via epidermal growth factor-like protein 8. Nat Commun. (2021);12:1624. doi: 10.1038/s41467-021-21859-0. [DOI] [PMC free article] [PubMed] [Google Scholar]


