Abstract
Background:
The hepatoprotective effects of aspirin have been observed in individuals with viral hepatitis; however, its impact on the general population remains uncertain. Understanding the association between aspirin use and the development of liver diseases is crucial for optimizing preventive strategies.
Methods:
We identified individuals with aspirin use in the UK Biobank and the Penn Medicine Biobank, as well as propensity-score-matched controls. Outcome measures included new liver disease development, diagnosed by MRI or “International Classification of Diseases and Related Health Problems” coding, and incidences of gastrointestinal bleeding and ulcers.
Results:
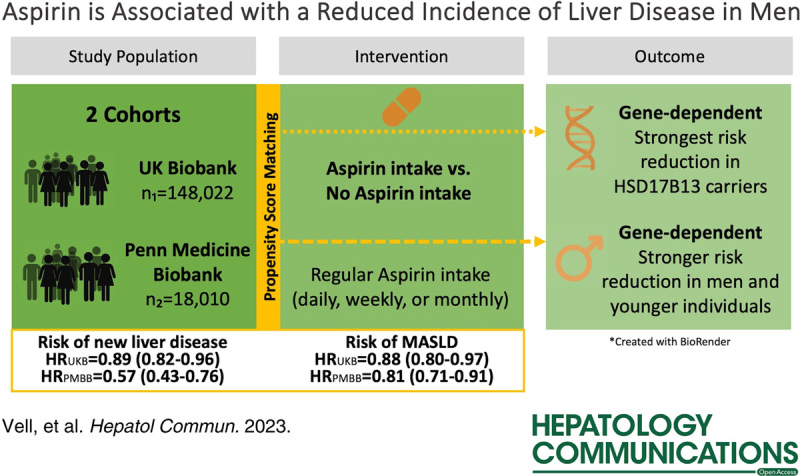
In the UK Biobank cohort, regular aspirin use was associated with an 11.2% reduction in the risk of developing new liver diseases during the average 11.84 ± 2.01-year follow-up period (HR=0.888, 95% CI = 0.819–0.963; p = 4.1 × 10-3). Notably, the risk of metabolic dysfunction-associated steatotic liver disease (ICD-10 K76.0) and MRI-diagnosed steatosis was significantly lower among aspirin users (HR = 0.882−0.911), whereas no increased risk of gastrointestinal bleeding or ulcers was observed. These findings were replicated in the Penn Medicine Biobank cohort, in which the protective effect of aspirin appeared to be dependent on the duration of intake. The greatest risk reduction for new liver disease development was observed after at least 1 year of aspirin use (HR = 0.569, 95% CI = 0.425−0.762; p = 1.6 × 10-4). Intriguingly, when considering general risk factors, only men exhibited a lower risk of MRI-confirmed or ICD-coded steatosis with aspirin use (HRs = 0.806−0.906), while no significant protective effect of aspirin was observed in females.
Conclusion:
This cohort study demonstrated that regular aspirin use was associated with a reduced risk of liver disease in men without an elevated risk of gastrointestinal bleeding or ulcers. Further investigation is warranted to elucidate potential sex-related differences in the effects of aspirin and to inform tailored preventive strategies for liver diseases.
INTRODUCTION
Although liver-related morbidity and, in particular, metabolic dysfunction-associated steatotic liver disease (MASLD) are pressing global health challenges, treatment options remain limited. A new approach involves the possibility of drug repurposing for personalized disease prevention.
Aspirin, also known as acetylsalicylic acid, is a widely used drug with analgesic, antipyretic, and anti-inflammatory effects.1,2 Recent studies have shown that regular aspirin use was significantly associated with a lower fibrosis index in individuals with suspected chronic liver disease.3 Likewise, regular aspirin intake reduced the risk of severe fibrosis in individuals with metabolic dysfunction-associated steatohepatitis4 in a time-dependent manner. A cross-sectional study also found that aspirin use was associated with a lower prevalence of MASLD.5 This benefit concerning MASLD is presumably triggered by the inhibition of lipid biosynthesis and anti-inflammatory properties of aspirin.6
Although previous data are encouraging, the use of aspirin for primary prevention in the general population has yet to be studied.
In this study, we hypothesized that aspirin may prevent liver diseases in the general population. To this end, we assessed the potential preventive effects of aspirin on the development of new liver diseases in a cohort of over 490,000 individuals recruited by the UK Biobank (UKB) and a separate cohort of 35,000 individuals recruited from a tertiary hospital system in Philadelphia, Pennsylvania, USA by the Penn Medicine Biobank (PMBB).
METHODS
UKB
The UKB dataset was accessed through a request for registration at UKB https://www.ukbiobank.ac.uk and was approved by the Northwest Multi-Center Research Ethics Committee (IRAS ID 299116). Baseline studies were conducted between 2006 and 2010. Death or the end of data collection in May 2021 was defined as the end of follow-up. Diagnoses were coded according to the “International Classification of Diseases and Related Health Problems” (ICD-10). Death data were collected using the National Death Registry, including the individual’s age and primary ICD-10 diagnosis that led to death. After providing informed consent for genotyping and data linkage of medical reports, a total of 502,511 volunteers aged from 37 to 73 were recruited. We excluded patients with preexisting liver disease or HCC at baseline, along with pathological alcohol consumption (>60g/d for men and >40g/d for women).7 Postmatching, we included 56,684 aspirin users and 91,338 nonusers.
Medication in UKB
Medications were registered through an interview, coded by numerical code, and assigned to the respective medication groups by our team (Table S1, http://links.lww.com/HC9/A522). A total of 4199 medications from 4382 records were included. Only medications taken regularly on a daily, weekly, or monthly basis were included in this category, which allowed us to assume that aspirin was administered on a regular schedule.
PMBB
The PMBB is a biobank of individuals who were enrolled from phlebotomy sites throughout the health system and is agnostic to the underlying diagnoses. The PMBB dataset was provided by the University of Pennsylvania Health System and was approved by an institutional review board (IRB ID 813913). Both the UKB and PMBB collected clinical data in accordance with the Declaration of Helsinki. Individuals provided informed consent to access their electronic health records (EHR) and collect health data. To identify diagnoses according to ICD-10 codes, we used health care records. Individual characteristics were extracted from the EHR. Similarly, PMBB recorded the death data and ICD-10 diagnoses leading to death through the EHR. The end of follow-up was defined as the death or end of data collection in December 2020. A total of 61,139 individuals aged from 18 to 102 were enrolled at the time of analysis. In accordance with the UKB, we excluded individuals with preexisting liver disease or HCC at baseline. Data on alcohol consumption were not available. There were 9005 aspirin users and 9005 nonusers after matching. In PMBB, we included single aspirin preparations.
Medication in PMBB
EHR access provided direct information regarding prescription medications, corresponding medication groups, and the date of the prescription. The duration of intake was calculated using the package size, number of prescriptions, and refills. We assessed a 30-package as the smallest prescribed package size unless otherwise specified. To analyze the duration of intake, we excluded patients whose intake lasted less than the period analyzed in the individual subset. In this manner, only the specific intake time or more was compared to patients who did not take aspirin at all. The follow-up times of patients with aspirin intake and controls were comparable.
Statistical analysis
Development of the propensity score model
To adjust for baseline characteristics that affected health status and, accordingly, the likelihood of aspirin use, we developed a propensity score for aspirin treatment.
Variables included sociodemographic, health, and medication risk factors known to influence the likelihood of taking aspirin. These included age, sex, body mass index, ethnicity, diabetes mellitus with or without the intake of insulin or biguanides, hypertension, ischemic heart disease, dyslipidemia with or without the intake of statins, and, as a factor for disease severity, the number of medications taken (available in UKB). By performing the Schoenfeld test for each variable included in the matching, we accounted for time-varying effects and ensured the reliability of our survival analysis. The analyses were performed in UKB at a ratio of 2. A ratio of 2 indicated that a maximum of 2 control individuals and a minimum of 1 control individual were matched to an individual taking aspirin. In PMBB, matching was performed at a ratio of 1:1, as there were more cases of aspirin use than controls. The multivariate model included 13 prognostic variables in the UKB and 11 variables in the PMBB. As a first estimate of the efficiency of propensity score matching, the standardized mean differences before and after propensity score matching were determined (Table 1). After matching, all variables were balanced (standardized mean differences < 0.1). Using a Cox regression model, we assessed the association between aspirin use and outcomes after adjusting for age, gender, body mass index, and number of medications (UKB only). A competing risk analysis was performed for specific liver diseases, integrating all other liver diseases except the one considered as a competing event.
TABLE 1.
Comparison of the basic characteristics of the matched cohort
| No Aspirin intake | Aspirin intake | |||
|---|---|---|---|---|
| (N=91,338) | (N=56,684) | Standardized mean difference before PS | Standardized mean difference after PS | |
| Patients without previously diagnosed liver disease in UKB | ||||
| Age (y) | 60.3±6.7 | 60.4±6.9 | 0.7 | 0.1 |
| Sex (% Women) | 44.2 | 42.0 | 0.4 | 0.0 |
| BMI (kg/m2) | 28.4±5.0 | 28.7±5.0 | 0.4 | 0.0 |
| Ethnicity (% White) | 94.6 | 94.2 | 0.0 | 0.0 |
| Number of medications | 4.6±3.5 | 5.3±3.1 | 1.2 | 0.0 |
| Diabetes mellitus type II (E11) | 10.4 | 15.0 | 0.5 | 0.0 |
| Arterial hypertension (I10) | 38.9 | 44.6 | 0.7 | 0.0 |
| Disorders of lipoprotein metabolism and other lipidemias (E78) | 19.8 | 25.2 | 0.6 | 0.0 |
| Patients without previously diagnosed liver disease in PMBB | (N=9005) | (N=9005) | — | — |
| Age (y) | 57.1±14.3 | 58.2±14.9 | 1.0 | 0.1 |
| Sex (% Women) | 50.8 | 48.9 | 0.4 | 0.0 |
| BMI (kg/m2) | 29.6±7.3 | 29.5±7.8 | 0.2 | 0.0 |
| Ethnicity (% White) | 67.7 | 69.0 | 0.1 | 0.0 |
| Diabetes mellitus type II (E11) | 20.0 | 23.2 | 0.5 | 0.0 |
| Arterial hypertension (I10) | 55.1 | 56.8 | 0.9 | 0.0 |
| Disorders of lipoprotein metabolism and other lipidaemias (E78) | 50.1 | 54.8 | 0.9 | 0.1 |
Note: Bold values are significant P values (<0.05).
Quantitative values are given as mean ± SD. Categorical variables are given as relative frequencies in percent (%).
Abbreviation: BMI, body mass index.
Primary outcome
As a primary outcome, we investigated new liver diseases (ICD-10 K70-K77) as a combined end point, with subsequent analysis of individual liver diseases. Diagnoses after baseline examination were considered as new liver disease. In the case of several diagnoses, the date of the first event was evaluated.
Secondary outcomes
As a secondary outcome, we regarded any event of ulcer development (ICD-10 K25-K28), gastritis or duodenitis (ICD-10 K29), or gastrointestinal bleeding (ICD-10 K92.2) in UKB. The diagnoses K25-K29 were summarized in the analysis. Furthermore, we repeated the analyses with an additional correction for the following factors: Mental and behavioral disorders related to alcohol (ICD-10 F10), smoking status, alcohol consumption in g/d, socioeconomic status (Townsend index)8, and dietary factors such as vegetable and fruit consumption per day and fish and meat consumption per week.
Sensitivity analyses
-
Genetic predisposition and common risk factors for liver disease
The UKB provided genetic analyses of 488,377 participants. We examined the impact of aspirin on UKB carriers of liver-associated gene variants. We considered the following gene variants in the sensitivity analyses: rs738409 patatin-like phospholipase domain-containing protein 3, rs58542926 transmembrane 6 superfamily member 2, rs72613567 hydroxysteroid 17-beta dehydrogenase 13 (HSD17B13), and rs2642438 mitochondrial amidoxime reducing component 1. In addition, we performed subanalyses based on age (</≥ 60 y) and in both genders.
-
Steatosis MRI data
UKB also performed liver MRI in 40,797 individuals to determine proton density fat fraction. Before matching, 35,556 liver MRIs were included. We defined steatosis as a liver fat fraction > 5%.9
Alternatives to aspirin
We investigated the effects of other antiplatelet and NSAIDs on primary end points.
Results were expressed as mean ± SD. The 95% CI were given in parentheses for the HRs. A p-value < 0.05 was considered significant. Statistical analyses were performed using R version 4.1.2 (R Foundation for Statistical Computing; Vienna, Austria), SPSS Statistics Version 27 (IBM; Armonk, NY, USA), and Prism Version 8.0.1. (GraphPad, LaJolla, CA, USA). The yEd Graph Editor version 3.21.1. was used for the flowchart. The graphical abstract was designed with the help of BioRender.
RESULTS
Aspirin is associated with protection against new liver disease and especially MASLD
The UKB cohort included 148,022 individuals postmatching—which included age, sex, body mass index, ethnicity, diabetes mellitus (insulin or biguanides intake), hypertension, ischemic heart disease, dyslipidemia (statin intake), and the number of medications taken—of whom 56,684 were taking aspirin regularly (Table 1, Table 2). In total, we recorded 1583 new liver diseases in nonusers and 962 new liver diseases in aspirin users during the 11.84-year follow-up (Figure 1). We examined the effect of aspirin on preventing newly diagnosed liver disease after baseline. Aspirin users had an 11.2% lower risk of being diagnosed with new liver disease (HR = 0.888, 95% CI = 0.819–0.963; p = 4.1×10-3, Table 2, Supplemental Figure S1, http://links.lww.com/HC9/A522).
TABLE 2.
Impact of aspirin intake in UKB on liver diseases, hepatocellular carcinoma, and liver-related mortality in A) all individuals without prior liver disease and B) in men
| (A) In all matched individuals | (B) In the male subgroup | |||||
|---|---|---|---|---|---|---|
| Event and treatment group | No. with event/total No. | HR (95% CI) | p | No. with event/total No. | HR (95% CI) | p |
| New liver diseasea | ||||||
| No aspirin intake | 1583/91,338 | 1.00 (reference) | — | 900/50,967 | 1.00 (reference) | — |
| Aspirin intake | 952/56,684 | 0.888 (0.819–0.963) | 4.1e–03 | 560/32,869 | 0.818 (0.735–0.911) | 2.4e–04 |
| Subdiagnosesb | ||||||
| Alcohol-associated liver disease (K70) |
122/56,684 | 0.97 (0.77–1.22) | 0.79 | 95/32,869 | 0.86 (0.66–1.11) | 0.24 |
| Toxic liver disease (K71) | 6/56,684 | 0.53 (0.21–1.32) | 0.17 | <5/32,869 | 0.50 (0.16–1.57) | 0.24 |
| Hepatic failure, not elsewhere classified (K72) |
86/56,684 | 1.01 (0.77–1.34) | 0.93 | 59/32,869 | 0.93 (0.66–1.30) | 0.67 |
| Chronic hepatitis, not elsewhere classified (K73) |
11/56,684 | 1.40 (0.61–3.22) | 0.43 | 6/32,869 | 1.29 (0.39–4.30) | 0.67 |
| Fibrosis and cirrhosis of the liver (K74) |
158/56,684 | 1.04 (0.84–1.27) | 0.74 | 91/32,869 | 0.14 (0.69–1.18) | 0.44 |
| Other inflammatory liver diseases (K75) |
128/56,684 | 1.06 (0.85–1.33) | 0.61 | 70/32,869 | 1.08 (0.79–1.48 | 0.64 |
| Other diseases of the liver (K76) |
716/56,684 | 0.882 (0.803–0.968) | 8.0e–03 | 411/32,869 | 0.806 (0.712–0.913) | 6.9e–04 |
| Liver disorders in diseases classified elsewhere (K77) |
5/56,684 | 3.89 (0.73–20.58) | 0.11 | <5/32,869 | 3.26 (0.302–35.23) | 0.33 |
| MRI-defined steatosis (>5% liver fat)c | ||||||
| No aspirin intake | 1755/5228 | 1.00 (reference) | — | 1220/3317 | 1.00 (reference) | — |
| Aspirin intake | 1084/3222 | 0.911 (0.843–0.985) | 2.0e–02 | 762/2042 | 0.906 (0.825–0.996) | 4.0e–02 |
Note: Bold values are significant P values (<0.05).
New liver disease is defined as new onset liver disease K70-K77 after baseline examination.
Subdiagnoses only refer to individuals taking aspirin, whereby the HRs and corresponding p values were consistently calculated in comparison to individuals not taking aspirin.
MRI data concerning the liver were only available in 8450 individuals after matching. There were 139,572 individuals who did not have MRI data available and, thus, were excluded from the analysis.
FIGURE 1.
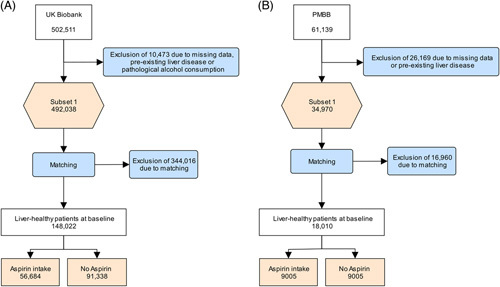
Flowchart of the UKB (A) and PMBB (B) cohorts. Abbreviations: PMBB, Penn Medicine Biobank; UKB, UK Biobank.
Among different liver diseases, especially K76 “other diseases of liver” showed a significant association with regular aspirin intake (HR = 0.882, 95% CI = 0.803–0.968; p = 8.0 × 10-3, Table 2). Since the ICD-10 diagnosis K76 includes the diagnosis of MASLD, we replicated the result in the liver MRI data of the UKB. The risk reduction for steatosis (> 5% liver fat) was 8.9% for aspirin users compared to nonusers (HR = 0.911, 95% CI = 0.843–0.985; p = 2.0 × 10-2, Table 2).
Impact of socioeconomic status and health risk factors
After additional adjustment for socioeconomic and health risk factors (ICD-10 F10, smoking status, alcohol consumption (g/d), socioeconomic status, and dietary factors), a similar association between aspirin use and liver protection was found. Aspirin users showed an 11.1% reduction in the risk of developing liver disease (HR = 0.889, 95% CI = 0.820–0.965; p = 4.9 × 10-3) (Supplemental Table S2, http://links.lww.com/HC9/A522). The risk of developing K76 was also reduced (HR = 0.884, 95% CI = 0.805–0.971; p = 1.0 × 10-2) (Supplemental Table S2, http://links.lww.com/HC9/A522).
Aspirin might have a strictly time-dependent effect
We then investigated whether aspirin could have a time-dependent effect. For this purpose, we used the PMBB, which, in contrast to the UKB, allows precise statements about the duration of intake. We included 9005 participants per group after matching, with comparable follow-up between the groups. While aspirin use for ≥ 30 days showed no significant association with the development of new liver disease, the association increased steadily from aspirin use of at least 90 days up to 360 days (Table 3). After ≥ 360 days, there was a significant association of 43.1% (HR = 0.569, 95% CI = 0.425–0.762; p = 1.6 × 10-4, Table 3).
TABLE 3.
Time-dependent impact of Aspirin intake on the development of liver disease in PMBB
| (A) In all matched individuals | (B) In the male subgroup | ||||||
|---|---|---|---|---|---|---|---|
| Event and treatment group | No. with event/total No.a | HR (95% CI) | p | Event and treatment group | No. with event/total No.a | HR (95% CI) | p |
| New liver diseases | — | — | — | New Liver diseases | — | — | — |
| No aspirin intake | 701/9005 | 1.00 (reference) | — | No Aspirin intake | 348/4426 | 1.00 (reference) | — |
| Aspirin intake | 883/9005 | 1.04 (0.94–1.15) | 0.46 | Aspirin intake | 428/4603 | 0.93 (0.81–1.08) | 0.35 |
| Time-dependent analysesb | — | — | — | Time-dependent analysesb | — | — | — |
| ≥30d | 867/8851 | 1.04 (0.94–1.15) | 0.47 | ≥30d | 421/4548 | 0.93 (0.81–1.07) | 0.33 |
| ≥90d | 130/1454 | 0.797 (0.659–0.964) | 2.0e–02 | ≥90d | 72/734 | 0.86 (0.67–1.11) | 0.25 |
| ≥180d | 78/909 | 0.710 (0.561–0.898) | 4.4e–03 | ≥180d | 44/470 | 0.77 (0.56–1.04) | 0.09 |
| ≥360d | 48/639 | 0.569 (0.425–0.762) | 1.6e–04 | ≥360d | 29/379 | 0.591 (0.406–0.860) | 6.0e–03 |
Note: Bold values are significant P values (<0.05).
The sensitivity analyses only refer to individuals taking NSAID, whereby the HRs and corresponding p values were consistently calculated in comparison to individuals not taking NSAID.
Liver disease included diagnoses K70-K77.
Association of NSAID or antiplatelet medication with primary outcomes
We hypothesized that the efficacy of aspirin might be a general therapeutic property of cyclooxygenase (COX) inhibitors and is related to its effect on platelets. We, therefore, investigated the effects of NSAIDs, ibuprofen, cyclooxygenase-2 inhibitors, and antiplatelet drugs on new liver diseases. No significant differences were found for any of these drug groups (Supplemental Table S3, http://links.lww.com/HC9/A522).
Bleeding and the occurrence of ulcers among Aspirin individuals
Aspirin users did not have a significantly higher risk of gastrointestinal bleeding than matched nonusers (HR = 0.904, 95% CI = 0.843–0.970; p = 4.7 × 10-3, Supplemental Table S4, http://links.lww.com/HC9/A522). Moreover, no significant increase was observed in the risk of ulcer development (HR = 0.812, 95% CI = 0.788–0.836; p = 9.9 × 10-300, Supplemental Digital Content, Table S4, http://links.lww.com/HC9/A522).
Hepatoprotective gene variants might be enhanced by aspirin
The UKB provided genomic data, which allowed us to investigate the interaction between the genetic risk of liver disease and aspirin use. In the sensitivity analysis for individuals with the known protective variant HSD17B13 rs72613567, we found a 12.6% risk reduction for heterozygous carriers of the minor allele taking aspirin, and for homozygotes, the reduction was as great as 31.9% (HR = 0.681, 95% CI = 0.489–0.948; p = 2.3 × 10-2, Table 4). This effect was replicated for heterozygous carriers of the minor allele mitochondrial amidoxime reducing component 1 rs2642438 (HR = 0.871, 95% CI = 0.765–0.992; p = 3.7 × 10-2). For patatin-like phospholipase domain-containing protein 3 rs738409 and transmembrane 6 superfamily member 2 rs58542926 carriers, no significant effects were observed (Table 4).
TABLE 4.
Different sensitivity analyses of aspirin intake on liver diseases in individuals without prior liver disease in UKBa
| No. with event/ Total No.a | HR (95% CI) | p | |
|---|---|---|---|
| New liver diseaseb | |||
| Menc | 560/32,869 | 0.818 (0.735–0.911) | 2.4e–04 |
| Womenc | 392/23,815 | 0.98 (0.86–1.11) | 0.70 |
| Age≥60 y | 630/36,432 | 0.91 (0.82–1.00) | 0.05 |
| Age<60 y | 322/20,252 | 0.859 (0.750–0.985) | 2.9e–02 |
| PNPLA3 rs738409 (wt) | 521/34,202 | 0.887 (0.795–0.989) | 3.1e–02 |
| PNPLA3 rs738409 (het) | 339/18,474 | 0.93 (0.81–1.06) | 0.26 |
| PNPLA3 rs738409 (hom) | 70/2555 | 0.83 (0.62–1.11) | 0.21 |
| TM6SF2 rs58542926 (wt) | 772/47,281 | 0.894 (0.818–0.978) | 1.5e–02 |
| TM6SF2 rs58542926 (het) | 141/7571 | 0.87 (0.71–1.08) | 0.21 |
| TM6SF2 rs58542926 (hom) | 11/300 | 1.08 (0.51–2.30) | 0.83 |
| HSD17B13 rs72613567 (wt) | 523/29,491 | 0.94 (0.84–1.05) | 0.30 |
| HSD17B13 rs72613567 (het) | 354/21,556 | 0.874 (0.766–0.997) | 4.5e–02 |
| HSD17B13 rs72613567 (hom) | 51/4045 | 0.681 (0.489–0.948) | 2.3e–02 |
| MTARC1 rs2642438 (wt) | 480/27,795 | 0.890 (0.795–0.997) | 4.5e–02 |
| MTARC1 rs2642438 (het) | 369/22,595 | 0.871 (0.765–0.992) | 3.7e–02 |
| MTARC1 rs2642438 (hom) | 80/4815 | 1.06 (0.79–1.43) | 0.68 |
Note: Bold values are significant P values (<0.05).
The sensitivity analyses only refer to individuals taking aspirin, whereby the HRs and corresponding p values were consistently calculated in comparison to individuals not taking aspirin.
New liver disease is defined as new onset liver disease K70-K77 after baseline examination.
The covariate sex was excluded.
Abbreviations: HSD17B13, hydroxysteroid 17-beta dehydrogenase 13; PNPLA3, patatin-like phospholipase domain-containing protein 3; MTARC1; mitochondrial amidoxime reducing component 1; TM6SF2, transmembrane 6 superfamily member 2.
Potential benefits of aspirin intake for men
We next examined common risk factors, such as age and sex. There was a significant risk reduction for individuals under 60 years of age (HR = 0.859, 95% CI = 0.750–0.985; p = 2.9 × 10-2). Strikingly, only men benefited from aspirin use (overall liver disease: HR = 0.818, 95% CI = 0.735–0.911; p = 2.4 × 10-4 and K76 “other diseases of liver” HR = 0.806, 95% CI = 0.712–0.913; p = 6.9 × 10-4, Table 2), while no effects of aspirin in females were seen (Table 4). In PMBB, this effect was replicated for men after ≥ 360 days of aspirin intake (HR = 0.591, 95% CI = 0.406–0.860; p = 6.0 × 10-3, Table 3).
Therefore, after establishing a connection between aspirin intake and liver disease protection, we analyzed the interaction between sex and aspirin in the UKB. We included aspirin intake, sex, and their interaction term (aspirin × sex) in a Cox-Grey proportional hazard model for liver disease development. Interestingly, including the interaction term yielded a significant result (p = 4.4 × 10-2). This indicates an interaction between sex and aspirin intake for liver disease prevention (Supplemental Table S5, http://links.lww.com/HC9/A522).
DISCUSSION
In 2 large population-based cohorts, the regular use of aspirin was associated with a significant decrease in new liver diseases, especially in men. This effect was strongly associated with the duration of aspirin use.
This finding is consistent with the results of previous studies. Aspirin was shown to reduce mortality from chronic liver disease10 and reduce the risk of liver fibrosis.3,4 Another study suggested differences between males and females and showed a reduced prevalence of MASLD in men but not in women taking aspirin.5
Our analyses showed that the effect of aspirin was not reproducible with antiplatelet medications or NSAIDs. These differences could be due to aspirins’ irreversible platelet inhibition (by inhibiting COX-11), which lasts as long as the platelets live (about 10 days), while other NSAIDs are reversible platelet inhibitors by blocking the COX-1 and cyclooxygenase-2 enzymes. Overexpression of COX significantly contributes to the pathogenesis of liver fibrosis by modulating inflammation,11 apoptosis,12 and senescence processes.13,14 In HCC cell lines, however, COX-independent mechanisms of aspirin could also be shown. Those included the inhibition of abnormal lipid metabolism during tumorigenesis,15 interference with the Wnt-ß-catenin signaling pathway,16 or inhibition of the extracellular signal-regulated kinase ERK1/2.17 In addition, lowering platelet activation is also associated with a reduced risk of metabolic dysfunction-associated steatohepatitis, indicating that aspirins’ antiplatelet effects could contribute to liver disease prevention.18–20 Presumably, it is an interplay of these processes, which could also explain the positive effect on a variety of different liver diseases.
An interesting question is why the hepatoprotective effect of aspirin only applies to males. Inflammation is a hallmark of liver disease development, and previous studies have revealed differences in prostaglandin synthesis between the sexes.21 Whereas human female neutrophils and macrophages produce more leukotrienes in an acute inflammatory reaction,22 male cells are more likely to produce prostaglandins.21 Due to a decreased production of leukotrienes from arachidonic acid in male cells,23 it can, therefore, be presumed that more arachidonic acid is present as a substrate for COX-enzymes to be used for the production of prostaglandins.21 Conversely, inhibition of COX-enzymes may have a more pronounced effect on levels of inflammatory prostaglandins in males than in females.
Moreover, other studies have shown a paradoxical attenuation of the antiplatelet effect of aspirin in response to epinephrine or ADP after 1 month of daily aspirin intake in women, which was not observed in men.24 Together, these data indicate important differences in the pathophysiological mechanisms, calling for sex-specific studies.
Another important aspect of our study was the analysis of genetic subgroups at risk of liver disease. Interestingly, sensitivity analyses showed that carriers of protective gene variants (both HSD17B13 rs7261356725 and mitochondrial amidoxime reducing component 1 rs264243826 allele carriers) benefited from additional aspirin intake. Especially in homozygous carriers of the HSD17B13 variant, nearly 3 times higher effect of aspirin than in the general population was observed, indicating that aspirin may act synergistically.
One potential downside to aspirin intake is side effects. Interestingly, no increase in ulcers or gastrointestinal bleeding was observed in aspirin users compared with matched controls. One reason for the lower incidence of ulcers could be that individuals with a higher susceptibility were not prescribed aspirin. This is supported by the low prescription rate of PPI among aspirin users (Supplemental Table S6, http://links.lww.com/HC9/A522). The low risk of bleeding could be due to the unequal distribution of other anticoagulants. Vitamin K antagonists were prescribed over 9 times as often in the nonuser group (Supplemental Table S6, http://links.lww.com/HC9/A522). Still, overall data on bleeding and aspirin are conflicting 27–30, so further analyses are necessary to provide reliable recommendations.
The unique strength of this study can be found in the use of 2 large individual cohorts complementary to each other. On the one hand, UKB allowed a detailed study of genetic risk, whereas PMBB permitted a time-dependent analysis of the results found in UKB. This cross-validation of the cohorts, the numerous sensitivity analyses, and the different end points in risk analyses minimized biases.
However, there are limitations to the study. One possible source of error in the UKB dataset might be drug misclassification, as no information was provided on dosage and the duration of intake, except that the listed drugs were taken regularly (daily, weekly, or monthly). The PMBB had a major advantage in that it specifically categorized medications based on their drug class and also provided information on both the duration and dose. Furthermore, we cannot completely exclude an immortal time bias for the time-dependent analyses of PMBB. We have performed a follow-up analysis comparing patients with aspirin intake to controls for this purpose, which shows balanced values (Supplemental Table S7, http://links.lww.com/HC9/A522). In addition, the report of alcohol use in UKB is based on a touchscreen interview, which might be limited in its reliability. However, self-reported alcohol in the UKB has been linked to known gene loci for alcohol use, supporting correct self-reporting.31 Nevertheless, our observational study is subject to possible confounding, and we attempted to account for this through various sensitivity analyses. We repeated our main analysis with additional corrections to account for confounding by health and socioeconomic risk factors, such as smoking status, diagnosis of mental and behavioral disorders related to alcohol (ICD-10 F10), Townsend index, or dietary factors. We further performed the Schoenfeld test to assess the proportional hazards assumption for each covariate included in the matching. Although some minor violations were found, we addressed this by implementing stratification and fitting separate Cox models for the variables with violations. This approach allows for time-varying effects and increases the reliability of our survival analysis (Supplemental Table S8, http://links.lww.com/HC9/A522). To address the limitation of reduced generalizability due to the relatively homogeneous ethnicity of the UK Biobank, we included the PMBB cohort, which offers greater ethnic diversity. However, it is essential to consider this restriction when interpreting our results, and further studies involving a more heterogeneous population should be conducted to validate and extend our findings. Finally, this study was based on ICD-10 diagnoses. Therefore, early disease stages might have remained undetected, or an incorrect group assignment might have occurred due to a lack of a timely diagnosis. However, we were able to reproduce the results for MASLD in a subgroup with liver MRI data.
In conclusion, we show that aspirin has a significant impact as a primary preventative measure in the general population, particularly in males.
Supplementary Material
AUTHOR CONTRIBUTIONS
Mara Sophie Vell analyzed the data overseen by Carolin Victoria Schneider. Carolin Victoria Schneider and Mara Sophie Vell had unrestricted access to all the data. Mara Sophie Vell, Carolin Victoria Schneider, and Kai Markus Schneider drafted the first version of the manuscript, and all authors reviewed and edited it. Mara Sophie Vell and Mara Sophie Vell had full access to all the data in the study and took responsibility for the integrity of the data and accuracy of the data analysis. All authors agreed to submit the manuscript, read and approve the final draft, and take full responsibility for its content, including the accuracy of the data and its statistical analysis. These data have not been made available to anyone before.
ACKNOWLEDGMENTS
This research has been conducted using the UK Biobank Resource under Application Number 71300. UK Biobank data were accessed by Mara Sophie Vell and Mara Sophie Vell Copyright © 2023, NHS England. Reused with the permission of the NHS England and/or UK Biobank. All rights reserved. This work uses data provided by patients and collected by the NHS as part of their care and support.
FUNDING INFORMATION
Carolin Victoria Schneider is supported by a grant from the Interdisciplinary Centre for Clinical Research within the Faculty of Medicine at the RWTH Aachen University (PTD 1-13/IA 532313) and the NRW Rueckkehr Programme of the Ministry of Culture and Science of the German State of North Rhine-Westphalia (MKW). Kai Markus Schneider is supported by the Federal Ministry of Education and Research (BMBF) and the Ministry of Culture and Science of the German State of North Rhine-Westphalia (MKW) under the Excellence strategy of the federal government and the Laender.
CONFLICTS OF INTEREST
Marina Serper received grants from Grifols. The remaining authors have no conflicts to report.
Footnotes
Abbreviations: EHR, electronic health records; HSD17B13, hydroxysteroid 17-beta dehydrogenase 13; ICD-10, International Classification of Diseases and Related Health Problems; MASLD, metabolic dysfunction-associated steatotic liver disease; MTARC1, mitochondrial amidoxime reducing component 1; PMBB, Penn Medicine Biobank; PNPLA3, patatin-like phospholipase domain-containing protein 3; SMD, standardized mean difference; TM6SF2, transmembrane 6 superfamily member 2.
Kai Markus Schneider and Carolin Victoria Schneider share the last authorship.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal’s website, http://www.hepcommjournal.com.
Contributor Information
Mara Sophie Vell, Email: mara.vell@rwth-aachen.de.
Arunkumar Krishnan, Email: dr.arunkumar.krishnan@gmail.com.
Kirk Wangensteen, Email: Wangensteen.kirk@mayo.edu.
Marina Serper, Email: Marinas2@pennmedicine.upenn.edu.
Katharina Sophie Seeling, Email: katharina.seeling@rwth-aachen.de.
Leonida Hehl, Email: leonida.hehl@rwth-aachen.de.
Miriam Daphne Rendel, Email: miriam.rendel@rwth-aachen.de.
Inuk Zandvakili, Email: Inuk.Zandvakili@Pennmedicine.upenn.edu.
Marijana Vujkovic, Email: vujkovic@pennmedicine.upenn.edu.
Eleonora Scorletti, Email: Eleonora.Scorletti@pennmedicine.upenn.edu.
Kate Townsend Creasy, Email: Kate.creasy@pennmedicine.upenn.edu.
Christian Trautwein, Email: ctrautwein@ukaachen.de.
Daniel James Rader, Email: rader@pennmedicine.upenn.edu.
Saleh Alqahtani, Email: salqaht1@jhmi.edu.
Kai Markus Schneider, Email: kmschneider@ukaachen.de.
Carolin Victoria Schneider, Email: cschneider@ukaachen.de.
REFERENCES
- 1. Vane JR, Botting RM. The mechanism of action of aspirin. Thromb Res. 2003;110:255–258. [DOI] [PubMed] [Google Scholar]
- 2. Arif H, Aggarwal S. Salicylic Acid (Aspirin). 2023 Jul 5. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan -. PMID: 30085574. [PubMed] [Google Scholar]
- 3. Jiang ZG, Feldbrügge L, Tapper EB, Popov Y, Ghaziani T, Afdhal N, et al. Aspirin use is associated with lower indices of liver fibrosis among adults in the United States. Aliment Pharmacol Ther. 2016;43:734–743. [DOI] [PubMed] [Google Scholar]
- 4. Simon TG, Henson J, Osganian S, Masia R, Chan AT, Chung RT, et al. Daily aspirin use associated with reduced risk for fibrosis progression in patients with nonalcoholic fatty liver disease. Clinical Gastroenterology and Hepatology. 2019;17:2776–2784.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shen H, Shahzad G, Jawairia M, Bostick RM, Mustacchia P. Association between aspirin use and the prevalence of nonalcoholic fatty liver disease: A cross-sectional study from the Third National Health and Nutrition Examination Survey. Aliment Pharmacol Ther. 2014;40:1066–1073. [DOI] [PubMed] [Google Scholar]
- 6. Han YM, Lee YJ, Jang YN, Kim HM, Seo HS, Jung TW, et al. Aspirin improves nonalcoholic fatty liver disease and atherosclerosis through regulation of the PPAR δ -AMPK-PGC-1 α pathway in dyslipidemic conditions. Biomed Res Int. 2020;2020:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stockwell T, Chikritzhs T, Holder H, Single E, Elena M, Jernigan D, et al. International Guide for Monitoring Alcohol Consumption and Harm. World Health Organization; 2000. [Google Scholar]
- 8. Townsend P, Phillimore P, Beattie A. Health and Deprivation: Inequality and the North (1st ed.). Routledge. 1988. 10.4324/9781003368885. [DOI]
- 9. Wilman HR, Kelly M, Garratt S, Matthews PM, Milanesi M, Herlihy A, et al. Characterisation of liver fat in the UK Biobank cohort. PLoS One. 2017;12:e0176867–S243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sahasrabuddhe V v, Gunja MZ, Graubard BI, Trabert B, Schwartz LM, Park Y, et al. Nonsteroidal anti-inflammatory drug use, chronic liver disease, and hepatocellular carcinoma. J Natl Cancer Inst. 2012;104:1808–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Motiño O, Agra N, Brea Contreras R, Domínguez-Moreno M, García-Monzón C, Vargas-Castrillón J, et al. Cyclooxygenase-2 expression in hepatocytes attenuates non-alcoholic steatohepatitis and liver fibrosis in mice. Biochim Biophys Acta Mol Basis Dis. 2016;1862:1710–1723. [DOI] [PubMed] [Google Scholar]
- 12. Niranjan R, Mishra KP, Thakur AK. Inhibition of Cyclooxygenase-2 (COX-2) initiates autophagy and potentiates mptp-induced autophagic cell death of human neuroblastoma cells, SH-SY5Y: an inside in the pathology of Parkinson’s Disease. Mol Neurobiol. 2018;55:8038–8050. [DOI] [PubMed] [Google Scholar]
- 13. Yang H, Xuefeng Y, Shandong W, Jianhua X. COX-2 in liver fibrosis. Clinica Chimica Acta. 2020;5060:196–203. [DOI] [PubMed] [Google Scholar]
- 14. Zdanov S, Toussaint O, Debacq-Chainiaux F. p53 and ATF-2 partly mediate the overexpression of COX-2 in H2 O2-induced premature senescence of human fibroblasts. Biogerontology. 2009;10:291–298. [DOI] [PubMed] [Google Scholar]
- 15. Yang G, Wang Y, Feng J, Liu Y, Wang T, Zhao M, et al. Aspirin suppresses the abnormal lipid metabolism in liver cancer cells via disrupting an NFκB-ACSL1 signaling. Biochem Biophys Res Commun. 2017;486:827–832. [DOI] [PubMed] [Google Scholar]
- 16. Yuan Z, Zhao J, Wang Z, Ren G, Zhang Z, Ma G. Effects of aspirin on hepatocellular carcinoma and its potential molecular mechanism. J BUON. 2020;25:981–986. [PubMed] [Google Scholar]
- 17. Abiru S, Nakao K, Ichikawa T, Migita K, Shigeno M, Sakamoto M, et al. Aspirin and NS-398 inhibit hepatocyte growth factor-induced invasiveness of human hepatoma cells. Hepatology. 2002;35:1117–1124. [DOI] [PubMed] [Google Scholar]
- 18. Malehmir M, Pfister D, Gallage S, Szydlowska M, Inverso D, Kotsiliti E, et al. Platelet GPIbα is a mediator and potential interventional target for NASH and subsequent liver cancer. Nat Med. 2019;25:641–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Iannacone M, Sitia G, Isogawa M, Marchese P, Castro MG, Lowenstein PR, et al. Platelets mediate cytotoxic T lymphocyte-induced liver damage. Nat Med. 2005;11:1167–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Iannacone M, Sitia G, Narvaiza I, Ruggeri ZM, Guidotti LG. Antiplatelet drug therapy moderates immune-mediated liver disease and inhibits viral clearance in mice infected with a replication-deficient adenovirus. Clinical and Vaccine Immunology. 2007;14:1532–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pace S, Rossi A, Krauth V, Dehm F, Troisi F, Bilancia R, et al. Sex differences in prostaglandin biosynthesis in neutrophils during acute inflammation. Sci Rep. 2017;7:3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rossi A, Pergola C, Pace S, Rådmark O, Werz O, Sautebin L. In vivo sex differences in leukotriene biosynthesis in zymosan-induced peritonitis. Pharmacol Res. 2014;87:1–7. [DOI] [PubMed] [Google Scholar]
- 23. Pergola C, Dodt G, Rossi A, Neunhoeffer E, Lawrenz B, Northoff H, et al. ERK-mediated regulation of leukotriene biosynthesis by androgens: A molecular basis for gender differences in inflammation and asthma. Proc Natl Acad Sci USA. 2008;105:19881–19886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Friede KA, Infeld MM, Tan RS, Knickerbocker HJ, Myers RA, Dubois LG, et al. Influence of sex on platelet reactivity in response to aspirin. J Am Heart Assoc. 2020;9:e014726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Abul-Husn NS, Cheng X, Li AH, Xin Y, Schurmann C, Stevis P, et al. A Protein-Truncating HSD17B13 Variant and Protection from Chronic Liver Disease. N Engl Jf Med. 2018;378:1096–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schneider C v, Schneider KM, Conlon DM, Park J, Vujkovic M, Zandvakili I, et al. A genome-first approach to mortality and metabolic phenotypes in MTARC1 p.Ala165Thr (rs2642438) heterozygotes and homozygotes. Med. 2021;2:851–863.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Simon TG, Duberg A-S, Aleman S, Chung RT, Chan AT, Ludvigsson JF. Association of aspirin with hepatocellular carcinoma and liver-related mortality. N Engl J Med. 2020;382:1018–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Memel ZN, Arvind A, Moninuola O, Philpotts L, Chung RT, Corey KE, et al. Aspirin use is associated with a reduced incidence of hepatocellular carcinoma: A Systematic Review and Meta-analysis. Hepatol Commun. 2021;5:133–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gaziano JM, Brotons C, Coppolecchia R, Cricelli C, Darius H, Gorelick PB, et al. Use of aspirin to reduce risk of initial vascular events in patients at moderate risk of cardiovascular disease (ARRIVE): A randomised, double-blind, placebo-controlled trial. The Lancet. 2018;392:1036–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li X, Wu S, Yu Y. Aspirin Use and the Incidence of Hepatocellular Carcinoma in Patients With Hepatitis B Virus or Hepatitis C Virus Infection: A Meta-Analysis of Cohort Studies. Front Med (Lausanne). 2021;7:569759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Clarke TK, Adams MJ, Davies G, Howard DM, Hall LS, Padmanabhan S, et al. Genome-wide association study of alcohol consumption and genetic overlap with other health-related traits in UK biobank (N=112117). Mol Psychiatry. 2017;22:1376–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]


