Abstract
Introduction:
Alcohol cessation improves mortality in alcohol-associated liver disease (ALD), but few ALD patients will engage in treatment. We aimed to demonstrate the feasibility and acceptability of a mobile health intervention to increase alcohol use disorder (AUD) treatment among ALD patients.
Methods:
We conducted a pilot randomized controlled trial (September 2020 to June 2022) at a single tertiary care center in adults with any stage of ALD, past 6-month drinking, and no past-month AUD treatment. Sixty participants were randomized 1:1 to a mobile health application designed to increase AUD treatment engagement through preference elicitation and matching to treatment and misconception correction. Controls received enhanced usual care. The primary outcomes were feasibility (recruitment and retention rates) and acceptability. Exploratory outcomes were AUD treatment engagement and alcohol use, measured by Timeline Followback. Outcomes were measured at 3 and 6 months.
Results:
Baseline characteristics were balanced. The recruitment rate was 46%. Retention was 65% at 6 months. The intervention was highly acceptable to participants (91% were mostly/very satisfied; 95% felt that the intervention matched them well to AUD treatment). Secondary outcomes showed increased AUD treatment at 6 months in the intervention group (intent-to-treat: 27.3% vs. 13.3%, OR 2.3, 95% CI, 0.61–8.76). There was a trend toward a 1-level or greater reduction in World Health Organization (WHO) drinking risk levels in the intervention group (OR 2.25, 95% CI, 0.51–9.97).
Conclusions:
A mobile health intervention for AUD treatment engagement was highly feasible, acceptable, and produced promising early outcomes, with improved AUD treatment engagement and alcohol reduction in ALD patients.
INTRODUCTION
Alcohol-associated liver disease (ALD) is a rising cause of liver-related mortality, with 40% increase in incidence over recent years and a 3.4% rise in mortality over the past 15 years.1,2 Patients with ALD often present late in their disease course with many having alcoholic hepatitis or cirrhosis complications at the time of diagnosis.3 Despite decades of research in medical treatments to arrest the disease course, alcohol cessation remains the most effective treatment modality for improving mortality and morbidity for ALD patients. Alcohol use disorder (AUD) treatment, even just a single session of behavioral therapy, has been associated with decreases in hepatic decompensation, 30-day readmissions, 30-day alcohol relapse, and, critically, short-term and long-term mortalities.4–6 While greater negative consequences, including health-related consequences, often increase AUD treatment engagement rates, only 10%–15% of patients with advanced ALD (cirrhosis or acute alcoholic hepatitis) access AUD treatment after their initial diagnosis.4,5
Interventions to improve referral to AUD treatment for patients who use alcohol in broader medical settings (eg, emergency departments, inpatient wards, trauma units, and primary care clinics) have been largely ineffective in linking patients to alcohol use treatment.7–10 Few studies have focused on AUD treatment engagement interventions specifically for ALD patients with advanced liver disease. Several studies suggest potential reasons for the lack of treatment engagement among ALD patients. Many patients with advanced ALD have more advanced AUD.11 Unique personal and structural barriers, such as inaccurate relapse risk perception or the physical stigma of liver disease, play a role in the lack of engagement among ALD patients,12 in addition to more commonly cited barriers among all AUD patients, such as feeling that they do not need treatment or lacking transportation or access to treatment.13–17 Many patients with ALD have misconceptions about alcohol use, AUD treatment, and liver disease that may represent targets for interventions to overcome barriers to treatment.12 Such misconceptions include, but are not limited to, beliefs that hard liquor that harms the liver beer is safe or the absence of cravings means that there is no risk of relapse.
In addition, prior studies of AUD treatment in patients with ALD found that patient factors related to treatment preference and the concept of alcohol addiction contribute to a lack of engagement.18 These findings suggest that poor engagement could be improved by incorporating patient preferences into alcohol treatment referrals. Patients with AUD want their treatment preferences to be considered,19 and incorporating patient preferences in treatment choice reduces drop-out rates, increases treatment engagement, and improves outcomes in a range of behavioral interventions, including for alcohol use.20–26 Despite this, no intervention to overcome treatment barriers and facilitate AUD treatment engagement based on patient preferences exists for ALD patients.
We sought to perform a pilot, randomized controlled clinical trial to evaluate the feasibility and acceptability of a novel, online behavioral intervention to increase AUD treatment engagement. Our primary hypothesis was that the deployment of a behavioral intervention in the context of hepatology care would be feasible and acceptable. Our secondary hypothesis was that a behavioral intervention designed to address 2 potential AUD treatment barriers (misconceptions about treatment, ALD, and alcohol use; and lack of integration of patient preferences into AUD treatment choice) would improve AUD treatment engagement rates among patients with ALD.
METHODS
Setting and participants
We conducted a single-center pilot randomized controlled clinical trial with a planned enrollment of 60 patients with ALD at a tertiary care center. A study sample size of 60 participants (30 intervention and 30 control) was chosen to provide data on the feasibility and acceptability of the intervention. Inclusion criteria were adults (above age 18 y) with any stage of ALD as determined by labs, imaging, biopsies, and/or clinical or procedural notes (see Supplemental Data, http://links.lww.com/HC9/A387, for full diagnostic criteria and clinical trial protocol); recent alcohol use of any amount within the past 6 months as determined by chart review, patient interview at the time of enrollment, and/or alcohol biomarker positivity on lab review; and no AUD treatment engagement in the past month. Patients were excluded if they were critically ill with <1 week of life expectancy, cognitively impaired (due to hepatic encephalopathy or other cognitive disorder determined by Mini-Mental Status Exam score <23), and unable to speak English. Potential patients were identified by screening inpatient ward census and outpatient clinic lists using the electronic health record for a diagnosis of ALD. After initial screening, research staff examined each chart to confirm the ALD diagnosis (see Supplemental Data, http://links.lww.com/HC9/A387, for diagnostic criteria). Potential participants were approached in-person in the clinic or on inpatient wards. Due to the COVID pandemic, an option for virtual recruitment was added where participants identified by clinic records but were receiving a virtual visit were phoned to complete screening questions for recency of alcohol use and AUD treatment. For those recruited remotely, only participants with access to either a smartphone or computer to enable completion of the intervention were included. To expand recruitment, before any enrollment, the inclusion criteria were expanded to include all stages of ALD. AUD treatment was defined as at least 1 instance of formal help-seeking for alcohol reduction or cessation including professional mental health or addiction counseling of any type; attendance at community-based alcohol recovery groups (eg, Alcoholics Anonymous, SMART [Self-Management and Recovery Training] Recovery, and Celebrate Recovery) and community-based church groups primarily focused on alcohol abstinence or reduction; residential alcohol treatment; intensive outpatient programs; alcohol relapse prevention medications prescribed for the purpose of reducing alcohol cravings; and any telehealth version of the above options. Informed consent was obtained at the time of enrollment. Adverse events were assessed throughout the study duration. This study was conducted in accordance with the Declarations of Helsinki and Istanbul. The University of Michigan Institutional Review Board (HUM 00174946) approved this study, and written consent was given in writing by all participants, http://links.lww.com/HC9/A385.
Study design
The overall study design is illustrated in Figure 1. After consenting for enrollment, participants were randomized. Computer-generated blocks with stratification for sex (male/female) and recruitment site (inpatient/outpatient) were used to randomize participants. Stratification for sex ensures equal representation of sexes given potential differences in AUD treatment engagement and known sex differences in alcohol’s effect on liver health. Stratification for the recruitment site ensures a balanced representation of ALD severity, given that hospitalized patients are expected to have more advanced liver disease. Blinding was not possible due to the nature of the intervention. A single research coordinator administered the intervention, recorded data, and assessed outcomes.
FIGURE 1.

Study design. Abbreviations: AUD, alcohol use disorder.
Intervention development
The intervention is a single session, online web application consisting of 2 modules, designed based on principles of the Health Belief Model27 and the Health Action Process Approach28,29 conceptual model of behavior change. The 2 modules are a misconception correction module and a treatment matching module. The misconception correction module included 10 true/false questions, drawn from prior studies12 evaluating the presence of misconceptions about alcohol use, AUD treatment, and liver disease. The intervention was developed following a 2-year-long process in which preliminary data regarding misconceptions and preferences about alcohol use, liver disease, and AUD treatment12 were transformed into paper-based surveys and iteratively presented to 11 men and 6 women with alcohol-associated cirrhosis or alcoholic hepatitis and recent drinking within past 6 months to determine face and content validity (Supplemental Figure S1, http://links.lww.com/HC9/A383). Paper mock-ups of app questions were presented to participants, and semistructured interviews were conducted with all 17 participants evaluated. Interview questions focused on if the mock-up contents are clear and easy to understand, helpful (and if so, how or how not helpful), how they might use the information in the tool to make a decision regarding alcohol use treatment, what information should be presented differently, what is missing, and any additional information that participants wanted to tell us regarding the mock-up. App question language was iteratively changed in response to the interviews until the final product was felt to be easily understood by participants.
In the online app, participants completed the 2 modules sequentially, with the misconception module first followed by the treatment preference matching module. After answering each question in the misconception module, participants were shown an immediate didactic screen providing the correct answer and reasoning (Figure 2A). The treatment preference module asked participants to rate, on a 5-point Likert scale, their preferences on 17 different components of AUD treatment (eg, one-on-one vs. group therapy, cost, presence, or absence of a licensed mental health or substance use professional, and presence or absence of family members in therapy). Each point on the Likert scale corresponded with a value (−2 to +2), and certain questions were weighted more heavily (eg, questions about if a participant would or would not want family or spouse/partner involved in treatment). An algorithm then calculated the participant’s top 3 AUD treatment options based on the components that they rated highest. The final screen showed participants a summary of their top AUD treatment choices and why they were matched to those treatment choices based on their preferences and values (Figure 2B). Participants entered their zip code and were provided with a list of locally available AUD treatment resources, drawn from the Substance Abuse and Mental Health Services Association Treatment Locator (www.findtreatment.samhsa.gov). The control group received enhanced usual care, which consisted of a brochure describing available alcohol use treatment services.
FIGURE 2.

(A) Representative screen showing misconception answers and didactic screen. (B) Representative screen showing the final output of the intervention and patient’s preferred treatments, nearby locations for AUD treatment, and preferences regarding the cost of treatment. Abbreviations: AUD, alcohol use disorder.
Intervention delivery
Participants could access the intervention either through the web or as an application on their smartphone, tablet, or personal computer. Participants enrolled in-person completed the intervention in the clinic at the time of enrollment, while those enrolled by phone completed the intervention on their own device after a link to the intervention website was sent to them through text message or email. For a period of 2 months in late 2020, the study was paused as a defect in data transfer from the app to the secure database was discovered, which resulted in the loss of 5 intervention participants’ app data. These participants were witnessed to have completed the app by study staff, and secondary outcome data were analyzed based on their assigned group as in intent-to-treat. Three additional participants, for a total of 8 participants, who were remotely recruited had a lack of app data transfer but completed all other surveys and outcome measures. After the correction of the data transfer defect, no further data loss occurred.
Outcomes and measures
The primary outcomes were feasibility, determined by recruitment and retention rates, and acceptability determined by the System Usability Scale and participant satisfaction surveys (Supplemental Data: Survey Instruments, http://links.lww.com/HC9/A387). The System Usability Scale is a validated and reliable scale consisting of 10 questions related to the user’s perceptions of a given technology. Scores of 68 or higher are considered average/better-than-average.30 Secondary outcomes were AUD treatment engagement (defined as above) and alcohol use, defined as at least a 1-level reduction in the World Health Organization drinking risk level.31 Drinking risk levels were determined using the 30-day Timeline Follow-back (TLFB).32,33 Timeline Follow-back was administered in-person or through phone (at the initial study visit) and through follow-up phone calls at 3 and 6 months. Additional secondary alcohol use outcomes calculated from the TLFB include percent drinking days and percent heavy drinking days. A drinking day was defined as a day when drinking of any amount occurred, while a heavy drinking day was defined as any day where alcohol use was >4 drinks per day for women or 5 drinks per day for men using the National Institute on Alcohol Abuse and Alcoholism standard definitions of a drink.34
Secondary outcomes and exploratory measures were collected at baseline, 3 and 6 months (Supplemental Figure S1 and Supplemental Table S1, http://links.lww.com/HC9/A384), with 6 months being the primary outcome endpoint. Additional measures, including the Stages of Change Readiness and Treatment Eagerness Scale for Alcohol (SOCRATES-8A),35 the Alcohol Use Disorders Identification Test-10 (AUDIT-10),36 and the Short Inventory of Problems (SIP),37 were collected at baseline, 3 and 6 months. The SOCRATES-8A measure contains 3 submeasures: recognition (indicating the degree of participant recognition of the problem), Ambivalence (how much a participant wonders if their alcohol use is a problem), and Taking Steps (whether or not a participant is taking steps to make a positive change to their drinking). Ranges for scores and interpretation are presented in Supplemental Data: Survey Instruments, http://links.lww.com/HC9/A387. Data recorded for clinical care, including liver chemistries (aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, and total bilirubin), International Normalized Ratio, and alcohol biomarkers (urinary ethyl glucuronide, urinary ethyl sulfate, phosphatidylethanol, and blood alcohol levels), were abstracted from the medical record if available but were not ordered for study purposes. Data were collected at baseline, 3 and 6 months, where available, with a 1-month window around the study timepoints. Where available lab data was complete, the Model for End-Stage Liver Disease-Sodium scores were calculated.
Participants in the intervention group were invited to take part in a qualitative interview at the end of study follow-up to determine their perceptions of the intervention and its effectiveness, and any changes that they might suggest. We aimed to recruit a 15% subsample, divided equally between responders and nonresponders (5 in each group). The interview guide was determined by a priori study goals requiring information from participants about app effectiveness, app performance, improvements needed, and barriers to AUD treatment encountered. A study coordinator experienced in performing qualitative interviews performed all interviews and recorded field notes and audio recordings. Audio recordings were transcribed verbatim, and all qualitative data were coded by the authors (Jessica L. Mellinger and Haila Asefah) using grounded theory to determine broad themes and findings related to the app’s effectiveness, changes needed, and barriers to AUD treatment experienced by participants. All enrolled participants were incentivized as follows: $30 for an enrollment visit, $30 for a 3-month follow-up, and $40 for a 6-month follow-up for a total of $100 for participation in all assessment points of the study. If a participant did not complete a visit, they did not receive the incentive payment for that visit.
Statistical methods
Results were analyzed based on intention-to-treat. Per-protocol analyses are also reported for comparison. Generalized estimating equations with robust sandwich estimators were used for secondary outcomes (AUD treatment engagement and alcohol use) with Wald 95% CIs after transformation to ORs. Logistic regression was used to compare World Health Organization (WHO) risk level reduction at 6 months between intervention and control. Missing data for intention-to-treat analyses were calculated considering the worst-case scenario as follows: subjects who had missing AUD treatment engagement data were treated as having NOT engaged in treatment, and subjects with data for AUD treatment engagement at 3 months were treated as having engaged by 6 months whether or not their data was missing at the final 6-month endpoint. Those with missing alcohol use data through TLFB at either 3 or 6 months were treated as having made no change in their alcohol use at those timepoints. Participants were considered retained at 3 and 6 months if they had at least 1 data point for secondary outcomes or measures (AUD treatment engagement, alcohol use, and survey data) at that timepoint. Summary scores for survey measures were calculated if 50% or more of the data were available for the survey measure. Data were analyzed using R version 4.2.2.
RESULTS
Baseline characteristics
Patients were recruited from September 23, 2020, until January 30, 2022, and followed until June 30, 2022. Baseline characteristics of the 60 recruited patients were balanced between the intervention and control groups, except for marital status and employment (Table 1). There were more single participants in the control group than the intervention group (67% vs. 31%). The intervention group had a greater number of unemployed (33% vs. 4%) and employed (30% vs. 15%) participants than the control group. Self-reported last drink (60 vs. 61 d for intervention vs. control) and stage of ALD (77% vs. 67% for advanced stage ALD [cirrhosis or alcoholic hepatitis]) were well-balanced. Model for End-Stage Liver Disease-Sodium scores were available to calculate for 18 participants in the intervention group and 22 participants in the control group at baseline and were similar [intervention: 13.6 (SD 8.25) and control: 16.7 (SD 9.4)]. Liver chemistries, sodium, and creatinine levels were available at baseline in 45 participants and were likewise similar between groups (Supplemental Tables, http://links.lww.com/HC9/A384).
TABLE 1.
Baseline characteristics of intervention and control cohorts
| Characteristic | Intervention (n=30) | Control (n=30) |
|---|---|---|
| Age in years | 46.8 (11.3) | 50.0 (13.4) |
| Race, n (%) | ||
| White | 24 (80) | 27 (90) |
| Black | 3 (10) | 1 (3.3) |
| Native American | 1 (3.3) | 0 (0) |
| Asian | 0 (0) | 1 (3.3) |
| -Unknown/declined to answer | 2 (6.7) | 1 (3.3) |
| Ethnicity, n (%) | ||
| Hispanic/Latino | 2 (6.7) | 1 (3.3) |
| Non-Hispanic/Latino | 28 (93) | 29 (97) |
| Marital status, n (%) | ||
| Single | 9 (31) | 20 (67) |
| Married | 10 (34) | 6 (20) |
| Divorced | 6 (21) | 3 (10) |
| Unknown/declined to answer | 4 (14) | 1 (3) |
| Ever had children | 16 (57) | 10 (35) |
| Highest level of education, n (%) | ||
| High school/GED | 4 (15) | 4 (15) |
| Undergraduate | 6 (23) | 7 (26) |
| Graduate degree or higher | 0 | 3 (11) |
| Other | 6 (23) | 2 (7) |
| Unknown/declined to answer | 10 (39) | 11 (41) |
| Employment status, n (%) | ||
| Unemployed | 9 (33) | 1 (4) |
| Employed | 8 (30) | 4 (15) |
| On Disability | 1 (4) | 2 (7) |
| Retired | 1 (4) | 4 (15) |
| Unknown/declined to answer | 8 (29) | 16 (59) |
| ALD severity, n (%) | ||
| AC or AAH or both | 23 (77) | 20 (67) |
| Alcohol-associated steatosis | 7 (23) | 10 (33) |
| For cirrhosis (n=37) , n (%) | ||
| Compensated | 3 (15) | 4(23) |
| Decompensated | 17 (85) | 13(77) |
| Variceal bleeding | 8 (27) | 8 (27) |
| Ascites | 15 (50) | 13 (43) |
| HE | 10 (33) | 11 (37) |
| Self-reported last drink (days before enrollment) | 60 (11.2) | 61 (12.4) |
Note: Data presented as number (%) or mean (SD).
Abbreviations: AAH, alcohol-associated hepatitis; AC, alcohol-associated cirrhosis; ALD, alcohol-associated liver disease; GED, general educational development.
Feasibility
Recruitment and retention figures are shown in Figure 3. Of 276 potentially eligible participants, 130 met full eligibility criteria and were able to be approached for enrollment and 60 (46%) consented to enrollment (30 male and 30 female; 30 inpatient and 30 outpatient recruitment sites). Outpatient recruitment rates were higher than inpatient (14% declined in outpatient vs. 38% of inpatients), which was almost entirely due to inpatient participants feeling too sick, with a minority not interested or not giving a reason. The major reasons for the lack of recruitment among outpatients were lack of time and lack of interest. Three participants died during the study (2 before the 3-month assessment and 1 before the 6-month assessment), all of whom were in the control group. In all 3 cases, death occurred in hospice due to progressive liver disease complications. No deaths were related to study procedures. At 3 months, 50 (87%) participants were retained. At 6 months, 37 (65%) were retained.
FIGURE 3.
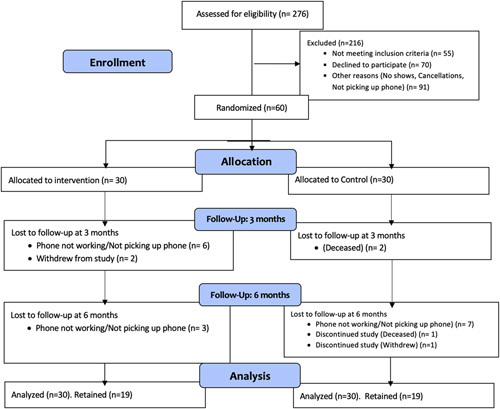
CONSORT study diagram.
Acceptability
The app was highly acceptable to participants. The average System Usability Scale score across the available 22 intervention participants was 70, corresponding to an above-average usability scale. Overall satisfaction with the app was high, with 20 participants (91%) being mostly or very satisfied with the app. Twenty-one participants (95%) felt that the app matched them somewhat or very well to the AUD treatment that they preferred. Eighteen participants (82%) felt that they had some to a lot of improvement in their AUD treatment knowledge, and 19 (86%) felt that they had some to a lot of improvement in their liver knowledge. Twenty participants (91%) felt that the app was just the right length, while 2 (8%) felt that it was too long. Twenty-one participants (95%) would recommend the app to others. Participants had a median of seven (range 4–10) correct answers out of 10 total questions in the true/false misconception correction module.
Secondary outcomes and measures
AUD treatment engagement at 6 months was higher in the intervention cohort compared with the control cohort (27.3% vs. 13.3%, OR 2.3, 95% CI, 0.61–8.76); see Figure 4. The most common types of treatment chosen were one-on-one therapy (n=8) and Alcoholics Anonymous or other group meetings (whether in-person or online) (n=6). Other types of treatment included medications (n=1) and inpatient rehabilitation (n=1). Subjects often used more than 1 modality, most commonly Alcoholics Anonymous/group meetings and individual one-on-one therapy. In addition, inpatients were much more likely to engage in AUD treatment compared with outpatients (33.3% inpatient vs. 6.7% outpatient engaged in AUD treatment, p=0.01) In a simple logistic regression model controlling for intervention status, inpatient enrollment was associated with higher rates of AUD treatment engagement (OR 6.9, 95% CI, 1.3–35.6). Secondary alcohol use and survey measures are shown in Table 2. Intent-to-treat analysis of alcohol use measured by WHO drinking risk level showed a trend toward increased odds for a 1-level or greater reduction in the intervention group (OR 2.25, 95% CI, 0.51–9.97). The average WHO drinking risk level reduction was 0.75 in the intervention cohort versus 0.19 in the control cohort. Similarly, there were trends toward reductions in percent drinking days and percent heavy drinking days in the intervention group compared with the control group (Figure 5 and Table 2). In the treated group, the average reduction in percent drinking days between baseline and 6 months was 12.3% versus 1.7% in controls. With respect to percent heavy drinking days, the treated group saw a 15.5% reduction at 6 months versus a 0.3% reduction in the control cohort. Survey scores at 3 and 6 months for SOCRATES, AUDIT-10, and SIP-2r were missing in >50% of the participants, largely due to time constraints (Supplemental Tables, http://links.lww.com/HC9/A384).
FIGURE 4.

Alcohol treatment engagement rates at 6 months.
TABLE 2.
Baseline and 6-month alcohol use and survey responses. Reported as mean (SD)
| Baselinea | 6-moa | |||
|---|---|---|---|---|
| Alcohol use variable | Intervention | Control | Intervention | Control |
| SOCRATESb recognition | 25 (9) | 26 (7) | 25 (11) | 25 (7) |
| SOCRATESb ambivalence | 13 (5) | 13 (4) | 14 (6) | 13 (5) |
| SOCRATESb taking steps | 31 (8) | 29 (8) | 32 (11) | 31 (7) |
| AUDIT-10 | 16 (11) | 18 (10) | 14 (12) | 9 (6) |
| SIP-2r total | 16 (15) | 18 (15) | 11 (14) | 3 (5) |
| Timeline follow-back total | 87 (192) | 72 (191) | 13 (33) | 29 (75) |
| Total alcohol use (g/mo) | 41 (89) | 33 (89) | 6 (15) | 14 (35) |
| WHO drinking risk level | 1.03 (1.48) | 1.03 (1.45) | 0.38 (0.7) | 0.56 (1.1) |
| Percent drinking days | 24 (38) | 25 (38) | 13 (33) | 19 (38) |
| Percent abstinent days | 76 (38) | 75 (38) | 87 (33) | 81 (38) |
| Percent heavy drinking days | 18 (35) | 17 (35) | 1 (3) | 6 (24) |
Complete cases only used. Missing values: baseline: SOCRATES 8%, AUDIT-10 10%, and SIP-2r 4%. 6 months: TLFB: 13.5%, SOCRATES 51%; AUDIT-10: 56%; and SIP-2r 54%. Because all other drinking measures are calculated based on the TLFB, the remaining drinking measures had 13.5% missing values as well.
SOCRATES = Stages of Change Readiness and Treatment Eagerness Scale. Abbreviations: AUDIT-10, Alcohol Use Disorders Identification Test-10; SIP, Short Inventory of Problems; TLFB, Timeline Follow-back.
FIGURE 5.

Average percent reduction in drinking outcomes from baseline to 6 months.
Qualitative data
Eight participants in the intervention group agreed to a qualitative interview on the Michigan Alcohol Improvement Network- Alcohol Reduction and Treatment Trial (MAIN-ART) at a 6-month follow-up. One man and 7 women completed the interviews (2 who engaged in AUD treatment and 6 who did not). One participant was Black, and the remainder were White. The average duration of abstinence at enrollment was 114 days (range 7–180 d). Three had alcohol-associated steatosis, 1 had alcohol-associated hepatitis, and 4 had decompensated cirrhosis. Participants were generally quite satisfied with the app. The most common reason for not engaging in AUD treatment was a perceived lack of need for alcohol use treatment, citing they felt that they could quit on their own, despite having recently drinking. One participant who did engage in AUD treatment felt that the app helped him to “cool down” (ie, reduce) his alcohol use. While those who did not engage in AUD treatment often felt that they did not need treatment, several felt that the app was helpful in other ways. One participant wanted to engage in AUD treatment but felt that having someone to talk to about how to navigate treatment initiation would have been helpful. Another participant wanted to engage in AUD treatment but had difficulty navigating treatment access due to a lack of transportation or insurance coverage for transportation. One participant interviewed was not in treatment due to a perceived lack of need for help with alcohol use but was looking for general therapy as a means of “sorting out the way I am thinking and living the best life I [can],” http://links.lww.com/HC9/A386.
DISCUSSION
In this pilot randomized, controlled trial of a novel online behavioral intervention to improve AUD treatment engagement among participants with ALD, recruitment, and retention data provided evidence for high feasibility. Exploratory outcomes of AUD treatment engagement showed promising 2fold or greater increases in treatment engagement among the intervention group in both intent-to-treat and per-protocol analyses. Similarly, alcohol use outcomes showed favorable trends toward abstinence in the intervention group with greater reductions in WHO drinking risk levels compared with the control group.
Low engagement in AUD treatment is a well-described problem in both the general AUD population and the ALD population.5,38,39 AUD treatment is essential for improving outcomes in ALD patients and preventing disease progression.40,41 While there are multiple interventions to improve alcohol cessation,42–45 including several studies of alcohol cessation interventions in liver disease patients,46 there are fewer interventions designed specifically to improve AUD treatment engagement. A recent meta-analysis of brief interventions for referral to AUD treatment in the general medical population, which were largely implemented in primary care, general medical inpatient, or emergency departments, found no evidence for effectiveness (RR 1.08, 95% CI, 0.92–1.28).10 None of the studies in this meta-analysis, however, included ALD patients specifically, and only one was a mobile health intervention.9 In addition, all focused primarily, if not exclusively, on motivational interviewing and motivational enhancement as the primary behavioral mechanism for change. By contrast, the MAIN-ART intervention included motivational language but also capitalized on correcting unique cognitive misconceptions about alcohol use and liver health, as well as assessing patient preferences and matching them to AUD treatment.
In the liver disease population, recent efforts to leverage the hepatology clinic as a site for treatment through colocating and integrating AUD and ALD care have shown promising early results though prospective data are lacking.11 The liver clinic may be a particularly promising site for more assertive AUD treatment engagement interventions because ALD patients are frequently alarmed by the sudden deterioration of their health and, thus, motivated by their liver disease to reduce or stop alcohol use.12 Integrating AUD assessment with liver disease diagnosis was shown, in a small study of an inpatient ALD consult service, to increase referrals to outpatient multidisciplinary ALD care.47 In studies of alcohol cessation interventions delivered in an integrated fashion with hepatology care, alcohol reduction has been demonstrated in some studies46,48 but not in others.49 In a recent study of integrated, colocated alcohol treatment provided to hepatitis C patients in addition to Screening, Brief Intervention and Referral to Treatment (SBIRT), participants in both the SBIRT only and SBIRT plus integrated alcohol treatment arm had increased rates of alcohol cessation at 6 months though there was no difference between groups.49 Because highly integrated and colocated AUD and ALD services are resource-intensive, such interventions are not always possible in all locations. The MAIN-ART intervention, by contrast, provides hepatology clinicians the means to improve AUD treatment engagement even in the absence of integrated, colocated ALD clinics. Such a tool represents another means of integrating AUD care with liver care and maybe a more acceptable, flexible, and scalable tool for those seeking ways to improve AUD/ALD treatment.50
Our study adds to the growing literature on providing AUD treatment to patients with ALD in several ways. A tailored, behavioral mobile health intervention developed specifically for AUD treatment engagement in ALD patients is novel, and we have shown that it is feasible and acceptable. Our intervention was designed based on an evidence-based conceptual model of behavior change, the Health Belief Model and the Health Action Process Approach, and targeted barriers to AUD treatment drawn from our own experience and literature specific to the ALD population.12 This ALD-specific tailoring may be one reason why both AUD treatment engagement and alcohol reduction were greater in the intervention cohort. A unique aspect of our intervention is the algorithm matching patients to AUD treatment based on their preferences. Large-scale trials of alcohol treatment matching (such as COMBINE51 and Project MATCH52) excluded patients with advanced ALD patients and did not match patients to AUD treatment based on patient preferences but rather on investigator preferences. A trial of post-transplant alcohol use treatment failed because patients felt that their preferences for treatment had not been taken into account and because misconceptions about AUD treatment had not been addressed.53 Our findings that over 90% of the intervention group felt that they were matched to their AUD treatment support the idea that we captured the patients’ preferences and the matching algorithm worked. Although we failed to show a statistically significant reduction in alcohol use, we did show a favorable trend, and reduction in alcohol use was not the primary outcome of this small proof-of-concept pilot trial. Moreover, several participants indicated in qualitative interviews that the intervention helped them reduce alcohol use, sometimes despite not engaging in AUD treatment. Our findings support the idea that patient preferences for AUD treatment should be assessed and used in decisions about treatment choices. In addition, our pilot trial results support the conduct of a larger, well-powered trial to determine feasibility and retention in more diverse ALD patients, clinical settings, and the efficacy of the app in reducing alcohol use. Given that there are multiple barriers to AUD treatment, a sequential, multiple assignment, randomized trial with a second-stage intervention designed to overcome some of the barriers that we have identified might further increase AUD treatment engagement than the MAIN-ART intervention alone.
Patients with acute alcohol-associated hepatitis and advanced ALD can be challenging to recruit and retain in studies.53,54 Our overall recruitment rate was high, but recruitment among inpatients was lower, suggesting that alternative approaches are necessary for these very sick patients, such as approaching them after they have been discharged from the hospital, when they are more medically stable and able to devote at least some cognitive energy to the challenging and complex psychological effort required for AUD treatment. Inpatients were, however, much more likely to engage in AUD treatment compared with outpatients, suggesting that the inpatient wards are a golden motivational opportunity for helping ALD patients engage in AUD treatment.
Limitations
There were several limitations. The small size of the pilot trial did not allow for full power to determine differences in AUD treatment engagement or alcohol use outcomes. Challenges with data transfer occurred, which were corrected but resulted in the loss of 5 subjects’ app data. Future studies should include regular audits to ensure that errors are detected early and reconciled promptly. We were unable to correlate data regarding treatment preferences from the app to actual treatment engagement, but future studies will include this correlation and collection of data on barriers to AUD treatment. Survey fatigue resulted in a high degree of missing follow-up surveys for SOCRATES, AUDIT-10, and SIP surveys, as patients declined to spend the time to complete these surveys. Future studies should use multimodal assessment strategies, such as email, text messaging, and phone calls, or spread the administration of follow-up surveys at different timepoints or use a wider window for each timepoint. Prioritizing the most important surveys to be administered first at each timepoint and focusing on the surveys that generate the most meaningful information should also be considered particularly in larger studies. Patients without internet access or smartphones would not be able to access the app. Offering the app during routine hepatology visits, in addition to remote, virtual recruitment, allows those without access to the necessary technology to access the app as well. Qualitative data should be interpreted with caution due to the lower number of patients interviewed (13% of the total sample). Future, larger studies of the intervention should include a larger cohort for qualitative interviewing.
CONCLUSIONS
As the fields of hepatology and liver transplantation move toward greater integration with mental health specialists, AUD treatment engagement in ALD patients can be enhanced with tailored, online interventions designed to overcome key cognitive barriers to treatment and with preference-sensitive AUD treatment matching. Larger, fully powered trials to test the app in a more diverse and generalizable cohort will be needed to confirm the feasibility and demonstrate efficacy. Interventions designed to assess and overcome additional attitudinal and logistic barriers to AUD treatment will also be needed to improve rates of alcohol cessation and reduce mortality in ALD patients.
Supplementary Material
Acknowledgments
DATA AVAILABILITY STATEMENT
On request to Michigan Medicine, Ann Arbor, MI, USA (jmelling@med.umich.edu).
MAIN-ART trial cohort (NCT 04473482) was registered at ClinicalTrials.gov NCT 04473482, and the full protocol is available at the following link: https://clinicaltrials.gov/ct2/show/NCT04473482.
Michigan Medicine Institutional Review Board approval initially received on 3/10/2020 (HUM 00174946).
AUTHOR CONTRIBUTIONS
Study concept and design: Jessica L. Mellinger and Fred Blow. Acquisition of data: Jessica L. Mellinger and Haila Asefah. Analysis and interpretation of data: Jessica L. Mellinger, Sarah Medley, Kelley M. Kidwell, and Fred Blow. Drafting of the manuscript: Jessica L. Mellinger. Critical revision of the manuscript for important intellectual content: Jessica L. Mellinger, Kelley M. Kidwell, Anne C. Fernandez, G. Scott Winder, Anna S. F. Lok, and Fred Blow. Statistical analysis: Sarah Medley, Kelley M. Kidwell, and Jessica L. Mellinger. Study supervision: Jessica L. Mellinger and Fred Blow.
FUNDING INFORMATION
Jessica L. Mellinger was supported by NIAAA K23 023666.
CONFLICTS OF INTEREST
G. Scott Winder consults for Alexion. The remaining authors have no conflicts to report.
Footnotes
Abbreviations: AAH, alcohol-associated hepatitis; AC, alcohol-associated cirrhosis; ALD, alcohol-associated liver disease; AUD, alcohol use disorder; AUDIT-10, Alcohol Use Disorders Identification Test-10; GED, general educational development; MAIN-ART, Michigan Alcohol Improvement Network-Alcohol Reduction and Treatment Trial; SBIRT, Screening, Brief Intervention and Referral to Treatment; SIP, Short Inventory of Problems; SOCRATES, Stages of Change Readiness and Treatment Eagerness Scale for Alcohol; TLFB, Timeline Follow-back.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal's website, www.hepcommjournal.com.
Contributor Information
Jessica L. Mellinger, Email: jmelling@med.umich.edu.
Sarah Medley, Email: medley@umich.edu.
Kelley M. Kidwell, Email: kidwell@umich.edu.
Haila Asefah, Email: asefah@med.umich.edu.
G. Scott Winder, Email: gwinder@med.umich.edu.
Anne C. Fernandez, Email: acfernan@med.umich.edu.
Anna S. F. Lok, Email: aslok@umich.edu.
Fred Blow, Email: fredblow@med.umich.edu.
REFERENCES
- 1. Tapper EB, Parikh ND. Mortality due to cirrhosis and liver cancer in the United States, 1999-2016: Observational study. BMJ. 2018;362:k2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mellinger JL, Shedden K, Winder GS, Tapper E, Adams M, Fontana RJ, et al. The high burden of alcoholic cirrhosis in privately insured persons in the United States. Hepatology. 2018;68:872–82. [DOI] [PubMed] [Google Scholar]
- 3. Shah ND, Ventura-Cots M, Abraldes JG, Alboraie M, Alfadhli A, Argemi J, et al. Alcohol-related liver disease is rarely detected at early stages compared with liver diseases of other etiologies worldwide. Clin Gastroenterol Hepatol. 2019;17:2320–29. e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mellinger JL, Fernandez A, Shedden K, Winder GS, Fontana RJ, Volk ML, et al. Gender disparities in alcohol use disorder treatment among privately insured patients with alcohol-associated cirrhosis. Alcohol Clin Exp Res. 2019;43:334–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rogal S, Youk A, Zhang H, Gellad WF, Fine MJ, Good CB, et al. Impact of alcohol use disorder treatment on clinical outcomes among patients with cirrhosis. Hepatology. 2020;71:2080–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Peeraphatdit T, Ahn JC, Choi DH, Allen AM, Simonetto DA, Kamath PS, et al. A Cohort study examining the interaction of alcohol consumption and obesity in hepatic steatosis and mortality. Mayo Clin Proc. 2020;95:2612–20. [DOI] [PubMed] [Google Scholar]
- 7. Saitz R, Palfai TP, Cheng DM, Horton NJ, Freedner N, Dukes K, et al. Brief intervention for medical inpatients with unhealthy alcohol use: a randomized, controlled trial. Ann Intern Med. 2007;146:167–76. [DOI] [PubMed] [Google Scholar]
- 8. Kuchipudi V, Hobein K, Flickinger A, Iber FL. Failure of a 2-hour motivational intervention to alter recurrent drinking behavior in alcoholics with gastrointestinal disease. J Stud Alcohol. 1990;51:356–60. [DOI] [PubMed] [Google Scholar]
- 9. Bischof G, Grothues JM, Reinhardt S, Meyer C, John U, Rumpf HJ. Evaluation of a telephone-based stepped care intervention for alcohol-related disorders: A randomized controlled trial. Drug Alcohol Depend. 2008;93:244–51. [DOI] [PubMed] [Google Scholar]
- 10. Glass JE, Hamilton AM, Powell BJ, Perron BE, Brown RT, Ilgen MA. Specialty substance use disorder services following brief alcohol intervention: A meta-analysis of randomized controlled trials. Addiction. 2015;110:1404–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mellinger JL, Winder GS, Fernandez AC, Klevering K, Johnson A, Asefah H, et al. Feasibility and early experience of a novel multidisciplinary alcohol-associated liver disease clinic. J Subst Abuse Treat. 2021;130:108396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mellinger JL, Scott Winder G, DeJonckheere M, Fontana RJ, Volk ML, Lok ASF, et al. Misconceptions, preferences and barriers to alcohol use disorder treatment in alcohol-related cirrhosis. J Subst Abuse Treat. 2018;91:20–7. [DOI] [PubMed] [Google Scholar]
- 13. Chartier KG, Miller K, Harris TR, Caetano R. A 10-year study of factors associated with alcohol treatment use and non-use in a U.S. population sample. Drug Alcohol Depend. 2016;160:205–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xu J, Rapp RC, Wang J, Carlson RG. The multidimensional structure of external barriers to substance abuse treatment and its invariance across gender, ethnicity, and age. Subst Abus. 2008;29:43–54. [DOI] [PubMed] [Google Scholar]
- 15. Xu J, Wang J, Rapp RC, Carlson RG. The multidimensional structure of internal barriers to substance abuse treatment and its invariance across gender, ethnicity, and age. J Drug Issues. 2007;37:321–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gilbert PA, Pro G, Zemore SE, Mulia N, Brown G. Gender differences in use of alcohol treatment services and reasons for nonuse in a national sample. Alcohol Clin Exp Res. 2019;43:722–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Green CA. Gender and use of substance abuse treatment services. Alcohol Res Health. 2006;29:55–62. [PMC free article] [PubMed] [Google Scholar]
- 18. Weinrieb RM, Van Horn DHA, Lynch KG, Lucey MR. A randomized, controlled study of treatment for alcohol dependence in patients awaiting liver transplantation. Liver Transpl. 2011;17:539–47. [DOI] [PubMed] [Google Scholar]
- 19. Friedrichs A, Silkens A, Reimer J, Kraus L, Scherbaum N, Piontek D, et al. Role preferences of patients with alcohol use disorders. Addict Behav. 2018;84:248–54. [DOI] [PubMed] [Google Scholar]
- 20. McCrady BS, Epstein EE, Cook S, Jensen NK, Ladd BO. What do women want? Alcohol treatment choices, treatment entry and retention. Psychol Addict Behav. 2011;25:521–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Swift JK, Callahan JL. The impact of client treatment preferences on outcome: A meta-analysis. J Clin Psychol. 2009;65:368–81. [DOI] [PubMed] [Google Scholar]
- 22. Adamson SJ, Sellman JD, Dore GM. Therapy preference and treatment outcome in clients with mild to moderate alcohol dependence. Drug Alcohol Rev. 2005;24:209–16. [DOI] [PubMed] [Google Scholar]
- 23. Brown TG, Seraganian P, Tremblay J, Annis H. Matching substance abuse aftercare treatments to client characteristics. Addict Behav. 2002;27:585–604. [DOI] [PubMed] [Google Scholar]
- 24. McKay JR, Alterman AI, McLellan AT, Snider EC, O'Brien CP. Effect of random versus nonrandom assignment in a comparison of inpatient and day hospital rehabilitation for male alcoholics. J Consult Clin Psychol. 1995;63:70–8. [DOI] [PubMed] [Google Scholar]
- 25. McKay JR, Drapkin ML, Van Horn DHA, Lynch KG, Oslin DW, DePhilippis D, et al. Effect of patient choice in an adaptive sequential randomization trial of treatment for alcohol and cocaine dependence. J Consult Clin Psychol. 2015;83:1021–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hell ME, Miller WR, Nielsen B, Mejldal A, Nielsen AS. The impact of free choice in alcohol treatment. Primary outcomes of the self-match study. Drug Alcohol Depend. 2021;221:108587. [DOI] [PubMed] [Google Scholar]
- 27. Skinner CTJ, Champion VL. Glanz KRB, Viswanath K. The Health Belief Model. Health Behavior: Theory Research and Practice. Jossey-Bass; 2015:75–94. [Google Scholar]
- 28. Schwarzer R. Models of health behaviour change: Intention as mediator or stage as moderater? Psychol Health. 2008;23:259–63. [DOI] [PubMed] [Google Scholar]
- 29. Zhang CQ, Zhang R, Schwarzer R, Hagger MS. A meta-analysis of the health action process approach. Health Psychol. 2019;38:623–37. [DOI] [PubMed] [Google Scholar]
- 30. Lewis JR, Sauro J. K M. The factor structure of the system usability scale. Human Centered Design HCD 2009 Lecture Notes in Computer Science. Springer; 2009. [Google Scholar]
- 31. Witkiewitz K, Heather N, Falk DE, Litten RZ, Hasin DS, Kranzler HR, et al. World Health Organization risk drinking level reductions are associated with improved functioning and are sustained among patients with mild, moderate and severe alcohol dependence in clinical trials in the United States and United Kingdom. Addiction. 2020;115:1668–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hjorthøj CR, Hjorthøj AR, Nordentoft M. Validity of timeline follow-back for self-reported use of cannabis and other illicit substances-—ystematic review and meta-analysis. Addict Behav. 2012;37:225–33. [DOI] [PubMed] [Google Scholar]
- 33. Hoeppner BB, Stout RL, Jackson KM, Barnett NP. How good is fine-grained Timeline Follow-back data? Comparing 30-day TLFB and repeated 7-day TLFB alcohol consumption reports on the person and daily level. Addict Behav. 2010;35:1138–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mellinger J, Winder GS, Fernandez AC. Measuring the alcohol in alcohol-associated liver disease: Choices and challenges for clinical research. Hepatology. 2021;73:1207–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Miller WR, Tonigan JS. Assessing drinkers’ motivation for change: The Stages of Change Readiness and Treatment Eagerness Scale (SOCRATES). Psychol Addict Behav. 1996;10:81–9. [Google Scholar]
- 36. Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption--II. Addiction. 1993;88:791–804. [DOI] [PubMed] [Google Scholar]
- 37. Kiluk BD, Dreifuss JA, Weiss RD, Morgenstern J, Carroll KM. The Short Inventory of Problems - revised (SIP-R): Psychometric properties within a large, diverse sample of substance use disorder treatment seekers. Psychol Addict Behav. 2013;27:307–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. McElroy LM, Likhitsup A, Scott Winder G, Saeed N, Hassan A, Sonnenday CJ, et al. Gender disparities in patients with alcoholic liver disease evaluated for liver transplantation. Transplantation. 2020;104:293–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bourdon JL, Tillman R, Francis MW, Dick DM, Stephenson M, Kamarajan C, et al. Characterization of service use for alcohol problems across generations and sex in adults with alcohol use disorder. Alcohol Clin Exp Res. 2020;44:746–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vannier AGL, Przybyszewski EM, Shay J, Patel SJ, Schaefer E, Goodman RP, et al. Psychotherapy for alcohol use disorder is associated with reduced risk of incident alcohol-associated liver disease. Clin Gastroenterol Hepatol. 2022;21:1571–80.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vannier AGL, Shay JES, Fomin V, Patel SJ, Schaefer E, Goodman RP, et al. Incidence and progression of alcohol-associated liver disease after medical therapy for alcohol use disorder. JAMA Netw Open. 2022;5:e2213014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hutton A, Prichard I, Whitehead D, Thomas S, Rubin M, Sloand E, et al. mHealth interventions to reduce alcohol use in young people: A systematic review of the literature. Compr Child Adolesc Nurs. 2020;43:171–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kruse CS, Lee K, Watson JB, Lobo LG, Stoppelmoor AG, Oyibo SE. Measures of effectiveness, efficiency, and quality of telemedicine in the management of alcohol abuse, addiction, and rehabilitation: Systematic review. J Med Internet Res. 2020;22:e13252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Song T, Qian S, Yu P. Mobile health interventions for self-control of unhealthy alcohol use: Systematic review. JMIR Mhealth Uhealth. 2019;7:e10899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Carreiro S, Newcomb M, Leach R, Ostrowski S, Boudreaux ED, Amante D. Current reporting of usability and impact of mHealth interventions for substance use disorder: A systematic review. Drug Alcohol Depend. 2020;215:108201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Khan A, Tansel A, White DL, Kayani WT, Bano S, Lindsay J, et al. Efficacy of psychosocial interventions in inducing and maintaining alcohol abstinence in patients with chronic liver disease: A systematic review. Clin Gastroenterol Hepatol. 2016;14:191–202. e1-4; quiz e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fomin V, Marshall C, Tsai S, Goodman R, Schaefer E, Luther J. Creation of an inpatient alcohol liver service improves early liver disease detection in patients with alcohol use disorder. Clin Gastroenterol Hepatol. 2022;21:1646–8. [DOI] [PubMed] [Google Scholar]
- 48. Patel YA, Yao J, Proeschold-Bell RJ, Niedzwiecki D, Goacher E, Muir AJ. Reduced alcohol use is sustained in patients provided alcohol-related counseling during direct-acting antiviral therapy for hepatitis C. Dig Dis Sci. 2021;66:2956–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Proeschold‐Bell RJ, Evon DM, Yao J, Niedzwiecki D, Makarushka C, Keefe KA, et al. A randomized controlled trial of an integrated alcohol reduction intervention in patients with hepatitis C infection. Hepatology. 2020;71:1894–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Winder GS, Fernandez AC, Mellinger JL. Integrated care of alcohol-related liver disease. J Clin Exp Hepatol. 2022;12:1069–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Greenfield SF, Pettinati HM, O’Malley S, Randall PK, Randall CL. Gender differences in alcohol treatment: an analysis of outcome from the COMBINE study. Alcohol Clin Exp Res. 2010;34:1803–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Project MATCH secondary a priori hypotheses. Project MATCH Research Group. Addiction. 1997;92:1671–98. [PubMed] [Google Scholar]
- 53. Weinrieb RM, Van Horn DHA, McLellan AT, Alterman AI, Calarco JS, O’Brien CP, et al. Alcoholism treatment after liver transplantation: Lessons learned from a clinical trial that failed. Psychosomatics. 2001;42:110–6. [DOI] [PubMed] [Google Scholar]
- 54. Comerford M, Lourens S, Liangpunsakul S, Chalasani NP, Sanyal AJ, Shah VH, et al. Challenges in patient enrollment and retention in clinical studies for alcoholic hepatitis: Experience of the TREAT Consortium. Alcohol Clin Exp Res. 2017;41:2000–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
On request to Michigan Medicine, Ann Arbor, MI, USA (jmelling@med.umich.edu).
MAIN-ART trial cohort (NCT 04473482) was registered at ClinicalTrials.gov NCT 04473482, and the full protocol is available at the following link: https://clinicaltrials.gov/ct2/show/NCT04473482.
Michigan Medicine Institutional Review Board approval initially received on 3/10/2020 (HUM 00174946).


