Introduction:
Acute lymphoblastic leukemia (ALL), the most common childhood malignancy, has excellent outcomes overall with contemporary therapy.(1) The combination of clinical features, sentinel genetic lesions, and day 8 peripheral blood and end of induction (EOI) bone marrow minimal residual disease (MRD) can be used to risk stratify patients based on risk of relapse.(2, 3) The Children’s Oncology Group (COG) retrospectively identified low risk (LR) subsets of standard-risk (SR) B-ALL patients with 5-year event-free survival (EFS) >95% and overall survival (OS) >97% treated with a 3-drug induction and post-induction intensification strategies with either intermediate dose methotrexate (ID MTX) without anthracyclines or alkylating agents (P9904), or a COG-modified Berlin-Frankfurt-Muenster (BFM) approach (CCG-1991).(2, 4, 5) COG AALL0932 was designed to prospectively confirm whether patients with low risk (LR) B-ALL could achieve a 5-year disease-free survival (DFS) of ≥95% with either of two regimens previously associated with outstanding DFS and overall survival (OS) and limited short- and long-term toxicity.
METHODS
AALL0932 enrolled patients with NCI SR (age 1–9.99 years, initial white blood cell count <50,000/microliter) B-ALL, all of whom received a 3-drug induction with dexamethasone, vincristine and pegaspargase.(6) MRD was assessed by multi-parameter flow cytometry performed in a central laboratory (prior to March 2017) or in a COG approved lab using a COG standardized analysis protocol (after March 2017). Following induction, LR patients were defined as those with favorable genetics (ETV6-RUNX1 fusion or trisomies of chromosomes 4 and 10); no steroid pre-treatment, unfavorable cytogenetics, Down syndrome (DS), central nervous system or testicular leukemia; and Day 8 peripheral blood (PB) and EOI bone marrow (BM) MRD <0.01% (Supplemental Table 1).(6) Consenting LR patients were randomized (1:1) to a P9904 based regimen that included 6 inpatient courses of ID MTX (1 g/m2/24 hours with leucovorin rescue) without alkylating agents or anthracyclines (Arm LR-M), or a CCG-1991-based outpatient regimen with reduced frequency vincristine/dexamethasone pulses given during maintenance at 12 instead of the 4 week intervals used in CCG-1991 (Arm LR-C). Therapy details are provided in Supplemental Tables 2, 3 and 4. As of May 2015, the LR randomization was closed to new study enrollees and subsequent patients classified as LR came off therapy at EOI.
AALL0932 (NCT01190930) was approved by the NCI Central institutional review board (IRB) and participating institution IRBs. Written informed consent and assent were obtained from a parent/guardian and where appropriate, patient, prior to therapy initiation and again at EOI. Toxicities were graded using the NCI Common Toxicity Criteria Version 4 (CTCAE4). Study data frozen 6/30/2019 are included in this report. Targeted toxicity rates were compared using Fisher’s exact tests. Disease-free (DFS) and overall OS rates were calculated from EOI randomization to first event (for DFS: relapse, second malignant neoplasm (SMN), or death) or censored at last follow-up. Median follow-up was calculated using the reverse Kaplan-Meier method.(7) The primary prospectively-defined aim for LR patients was to confirm that patients treated with either regimen would attain a 5-year DFS of ≥95%. With 603 LR patients randomized to the 2 arms, the DFS could be estimated for each arm with a maximum standard error of 2.9%. Survival rates were estimated by the method of Kaplan-Meier with 95% confidence intervals (CI) calculated using standard errors of Peto. Two-sided log-rank tests were used to compare DFS and OS between LR randomization groups.
Results and Discussion:
Demographics for the 9229 eligible (8890 non-DS) children with newly-diagnosed SR B-ALL enrolled in AALL0932 have been previously reported.(6) Post-induction, 8721 non-DS patients had sufficient data for risk classification and 1210 (13.9%) were LR; 448 of whom were risk-classified after the LR randomization was closed to accrual (Figure 1). Among 762 eligible LR patients, 603 (79.1%) consented to post-induction therapy and were randomized to LR-C (n= 302) or LR-M (n= 301); Demographics appear in Table 1. Compliance with protocol randomization was excellent with 95% of randomized patients (573) reaching the study endpoint.
Figure 1.
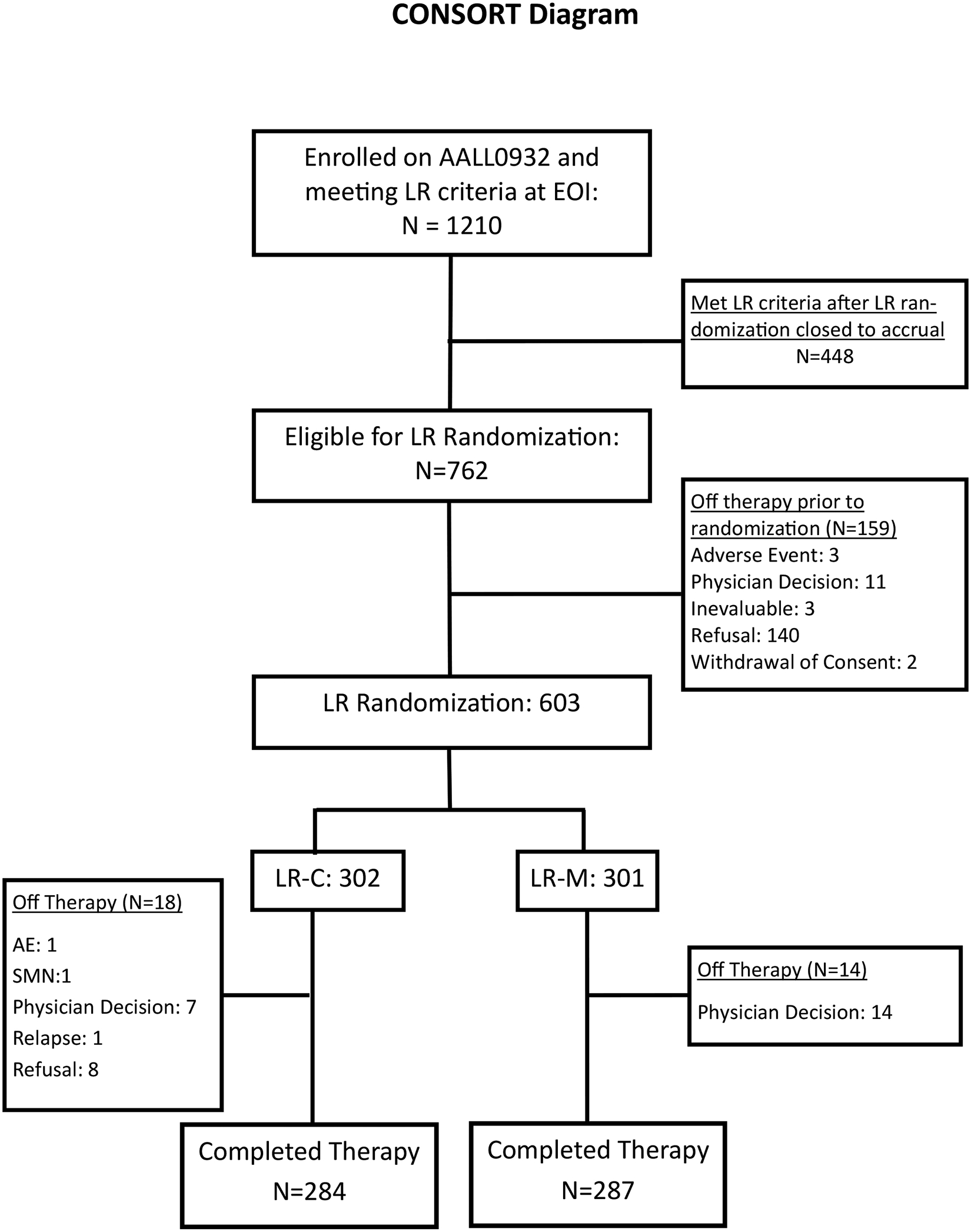
CONSORT Diagram of Low Risk ALL Patients Enrolled on COG AALL0932
Table 1.
Patient Characteristics
| Total LR Eligible (N=1210) |
LR-C1 (N=302) |
LR-M2 (N=301) |
Total LR Randomized (N=603) |
|
|---|---|---|---|---|
| Sex | ||||
| Female | 551 (45.5%) | 146 (48.3%) | 136 (45.2%) | 282 (46.8%) |
| Male | 659 (54.5%) | 156 (51.7%) | 165 (54.8%) | 321 (53.2%) |
| Race | ||||
| American Indian or Alaska | 12 (1.0%) | 2 (0.7%) | 2 (0.7%) | 4 (0.7%) |
| Asian | 52 (4.3%) | 10 (3.3%) | 13 (4.3%) | 23 (3.8%) |
| Black or African American | 65 (5.4%) | 16 (5.3%) | 18 (6.0%) | 34 (5.6%) |
| Multiple Races | 7 (0.6%) | 0 | 0 | 0 |
| Native Hawaiian or other | 8 (0.7%) | 2 (0.7%) | 1 (0.3%) | 3 (0.5%) |
| Unknown | 183 (15.1%) | 56 (18.5%) | 43 (14.3%) | 99 (16.4%) |
| White | 883 (73.0%) | 216 (71.5%) | 224 (74.4%) | 440 (73.0%) |
| Ethnicity | ||||
| Hispanic or Latino | 278 (23.0%) | 76 (25.2%) | 70 (23.2%) | 146 (24.2%) |
| Not Hispanic or Latino | 874 (72.2%) | 216 (71.5%) | 225 (74.8%) | 441 (73.1%) |
| Unknown | 58 (4.8%) | 10 (3.3%) | 6 (2.0%) | 16 (2.7%) |
| Age at diagnosis(years) | ||||
| <3 | 244 (20.2%) | 57 (18.9%) | 64 (21.3%) | 121 (20.1%) |
| 3–5 | 537 (44.4%) | 138 (45.7%) | 128 (42.5%) | 266 (44.1%) |
| 5–10 | 429 (35.4%) | 107 (35.4%) | 109 (36.2%) | 216 (35.8%) |
| Cytogenetics | ||||
| ETV6-RUNX1 ^ | 817 (67.5%) | 201 (66.6%) | 199 (66.1%) | 400 (66.3%) |
| Trisomies 4 & 10^ | 424 (35.0%) | 105 (34.8%) | 111 (36.9%) | 216 (35.8%) |
| WBC at diagnosis (/μL) | ||||
| <10,000 | 956 (79.0%) | 234 (77.4%) | 244 (81.1%) | 478 (79.3%) |
| 10,000–30,000 | 204 (16.9%) | 47 (15.6%) | 51 (16.9%) | 98 (16.2%) |
| 30,000–50,000 | 50 (4.1%) | 21 (7.0%) | 6 (2.0%) | 27 (4.5%) |
LR-M: Therapy based on P9904
LR-C: Therapy based on CCG 1991
Totals are greater than 1210 as 31 patients overall and 4 in LR-C and 9 in LR-M had both ETV6-RUNX1 and Trisomies 4 & 10
The 5-year DFS (±SE) for LR-C and LR-M were 98.5%±0.9% and 98.8%±0.8%, respectively (p=0.67; Figure 2A), with lower limits of two-sided 95% confidence intervals 96.8% (LR-C) and 97.1% (LR-M), each meeting the study objective of 5-year DFS ≥95%. The 5-year OS for both regimens was 100% (Figure 2B). Nine events occurred including 8 relapses (3 BM in each arm; one CNS on LR-C arm and one extramedullary on LR-M arm) and 1 SMN (LR-C).
Figure 2:
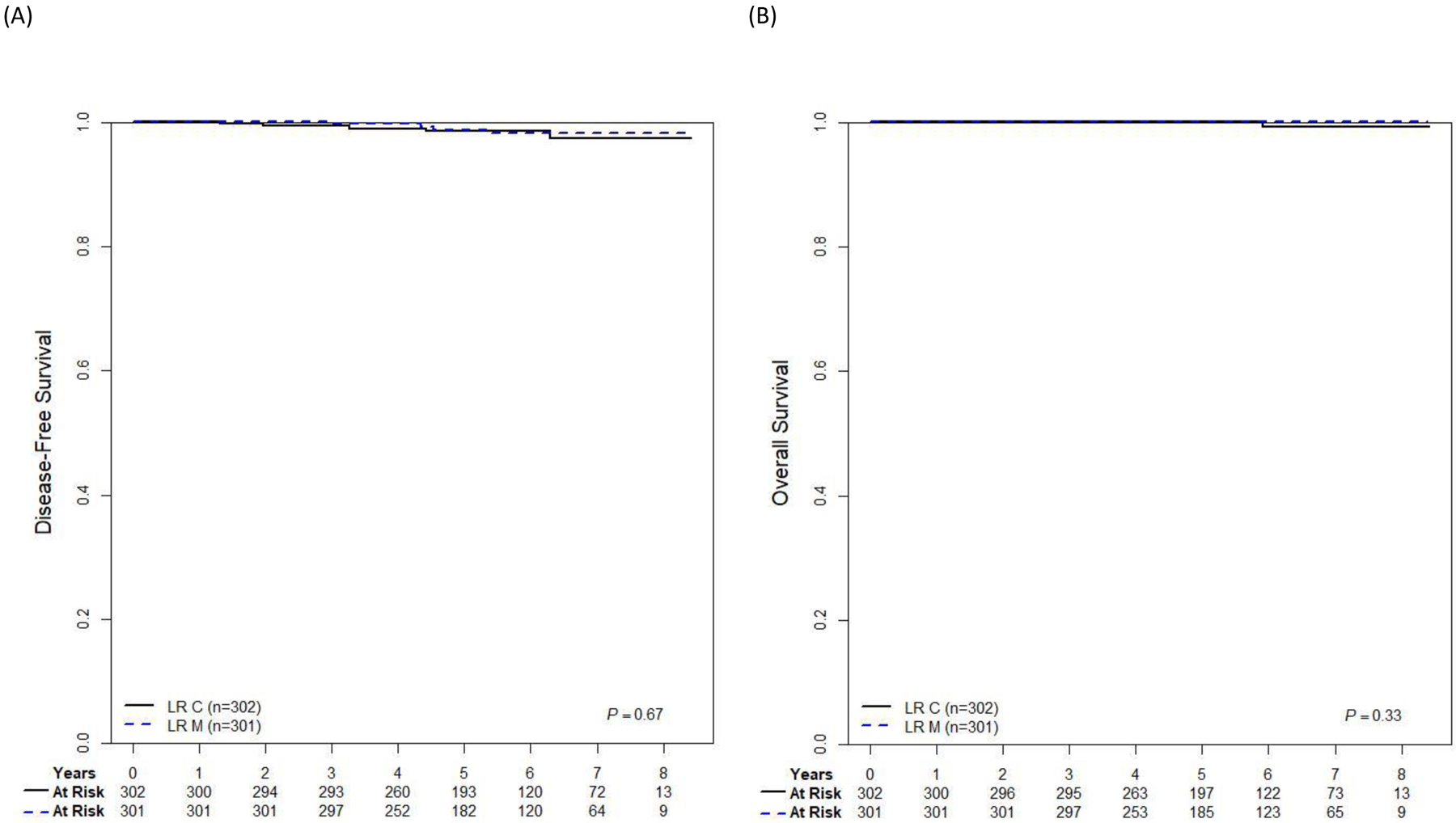
Survival in LR B-ALL patients from EOI by randomized treatment arm.
A Five-year disease-free survival. B Five-year overall survival.
Both regimens were well tolerated. When evaluating 14 previously-defined ALL targeted toxicities (6), the two arms had comparable and low incidences of toxicity except for higher incidences of grade 3–4 mucositis [12.9% vs. 6.3% (p=0.008)] and allergic reaction [2.3% vs. 0% (p=0.02)], on LR-C vs LR-M, respectively (Supplemental Table 5). There were no differences in acute neurotoxicity. These observed differences were likely attributed to escalating dose methotrexate without leucovorin rescue, doxorubicin, cyclophosphamide, cytarabine, and pegaspargase being included in post-induction phases of LR-C, but not LR-M.
Several important differences exist between the two regimens. LR-M included 6 inpatient hospitalizations to administer ID MTX, while LR-C did not include any scheduled hospitalizations but did include a delayed intensification phase. While data on the frequency of febrile neutropenia and unplanned hospitalizations were not collected on this trial, historically a significantly higher rate of febrile neutropenia/infectious complications has been seen in regimens that have included delayed intensification than those without.(4, 8, 9) The LR-C regimen included 75mg/m2 of anthracycline, which may impact long-term cardiac function(10). Prior studies have shown that even relatively low cumulative doses of anthracyclines can result in abnormal left ventricular fractional shortening or afterload.(11) A second potential benefit of LR-M is the omission of anthracyclines, cyclophosphamide, and thioguanine, which are associated with SMN risk.(12) One patient on LR-C and none on LR-M developed a SMN.
AALL0932 classified patients with favorable genetics as LR if Day 8 PB and day 29 BM MRD was <0.01%; 29% of ETV6-RUNX1 patients and 17% of trisomy 4+10 patients and 13.1% of the total study enrollment met these criteria. In contrast, the predecessor AALL0331 trial required Day 8 or 15 BM with <5% blasts by morphology and day 29 BM MRD <0.1% to be classified as LR, with Day 8 MRD collected for research purposes only(13, 14). On AALL0331, the 6-year DFS and OS rates for the low-risk cohort, treated very similarly to the AALL0932 LR-C regimen, were 94.7%±0.6% and 98.7%±0.3%, respectively but for the patients who met AALL0932 LR criteria, 6-year DFS and OS were 96.3% ± 1.4% and 98.6% ± 0.8%, respectively.(14) The current COG AALL1731 SR ALL (NCT03914625), uses a Day 8 PB MRD cutoff of <1%, but it is important to note that P9904 data suggests that patients with Day 8 PB MRD between 0.01–1% had worse outcome than those with Day 8 PB MRD <0.01% when LR-M therapy was used (M.D., Unpublished Data).
AIEOP-BFM ALL 2000, DCOG ALL10 and UKALL 2003 studied therapy reduction for lower risk patients, generally defined by MRD negativity and the absence of unfavorable cytogenetic abnormalities, but none prospectively incorporated favorable cytogenetics and the reduced therapy regimens tested were more intensive than either AALL0932 LR regimen.(8, 9, 15) Following a 4-drug induction, AIEOP-BFM ALL 2000 and DCOG ALL10 evaluated therapies with significant decreases in doses of drugs given during consolidation and delayed intensification while UKALL 2003 compared therapies with one versus two delayed intensification phases.(8, 9, 15) DCOG and UKALL 2003 reported successful treatment reduction in LR patient subsets.(8, 15) AIEOP-BFM ALL 2000 also demonstrated successful therapy reduction for patients with ETV6-RUNX1 fusion with an 8-year DFS of 94.5±1.7% vs 94.4±1.8% for reduced vs standard therapy, respectively.(9)
AALL0932 demonstrated that LR B-ALL patients can be prospectively identified at the end of a 3-drug induction that have an outstanding 5-year DFS of >98% and OS of 100% using either the ID MTX based LR-M or COG-modified BFM based LR-C regimen, with minimal short-term toxicity. This favorable B-ALL subgroup is almost certain to be cured with either of two lower intensity regimens, allowing physicians and families to select the treatment approach for a given child based on their preferences. Less stringent day 8 PB or day 29 BM MRD criteria might further expand this population of ultra-low risk patients. Given that age-matched controls of young children with LR ALL have 75 or more years of expected life, it is imperative to prospectively test further reductions in therapy for LR ALL patients to both prevent long-term adverse events and minimize the acute burden of therapy, including studies of short- and long-term quality of life. Treatment reductions to consider include elimination of specific phases of therapy, such as delayed intensification, or the substitution of immunotherapy for conventional chemotherapy.
Supplementary Material
Key points.
Stringent risk criteria can identify a favorable B-ALL subgroup with near universal cure with lower intensity therapy
Acknowledgements:
Special thanks to all the patients and their families as well as the participating institutions, and study staff.
Supported by:
Supported by grants from National Cancer Institute grants to the Children’s Oncology Group (U10 CA98543, U10 CA98413, U10 CA180886, U10 CA180899, U24 CA114766, 1U24-CA196173) and by research funding from St. Baldrick’s Foundation and BD Biosciences.
Disclosure of Conflicts of Interest:
RJS has been on an advisory board and received honoraria from Jazz Pharmaceuticals; ALA has been on an advisory board and received honoraria from Jazz Pharmaceuticals. JAK has stock in Johnson & Johnson common stock. PZM employment at ImmunoGen. MJB has been on an advisory board and received honoraria from Amgen and received honoraria from Blueprint Medicines; BW has served as a consultant for Amgen and had received research funding from Celgene/Juno, Kite, Novartis, and Wugen; NSKL has a family member who is a consultant for Medtronic and Boston scientific; MVR and her institution receive investigator-initiated funding from Servier; EAR has received institutional research funding from Pfizer and serves on a DSMB for Celgene/BMS;SPH owns common stock in Amgen and has received honoraria from Amgen, Jazz and Servier.
Footnotes
Presented in abstract form at the annual meeting of the American Society of Clinical Oncology, Chicago, IL, May 29, 2020.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health
Data Availability:
De-identified data will be submitted to and made available through the NCTN/NCORP Data Archive at https://nctn-data-archive.nci.nih.gov.
References
- 1.Pui CH, Yang JJ, Hunger SP, Pieters R, Schrappe M, Biondi A, et al. Childhood Acute Lymphoblastic Leukemia: Progress Through Collaboration. J Clin Oncol. 2015;33(27):2938–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borowitz MJ, Devidas M, Hunger SP, Bowman WP, Carroll AJ, Carroll WL, et al. Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia and its relationship to other prognostic factors: a Children’s Oncology Group study. Blood. 2008;111(12):5477–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hunger SP, Mullighan CG. Redefining ALL classification: toward detecting high-risk ALL and implementing precision medicine. Blood. 2015;125(26):3977–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Winick N, Martin PL, Devidas M, Shuster J, Borowitz MJ, Paul Bowman W, et al. Randomized assessment of delayed intensification and two methods for parenteral methotrexate delivery in childhood B-ALL: Children’s Oncology Group Studies P9904 and P9905. Leukemia. 2020;34(4):1006–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matloub Y, Bostrom BC, Hunger SP, Stork LC, Angiolillo A, Sather H, et al. Escalating intravenous methotrexate improves event-free survival in children with standard-risk acute lymphoblastic leukemia: a report from the Children’s Oncology Group. Blood. 2011;118(2):243–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Angiolillo AL, Schore RJ, Kairalla JA, Devidas M, Rabin KR, Zweidler-McKay P, et al. Excellent Outcomes With Reduced Frequency of Vincristine and Dexamethasone Pulses in Standard-Risk B-Lymphoblastic Leukemia: Results From Children’s Oncology Group AALL0932. J Clin Oncol. 2021;39(13):1437–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996;17(4):343–6. [DOI] [PubMed] [Google Scholar]
- 8.Pieters R, de Groot-Kruseman H, Van der Velden V, Fiocco M, van den Berg H, de Bont E, et al. Successful Therapy Reduction and Intensification for Childhood Acute Lymphoblastic Leukemia Based on Minimal Residual Disease Monitoring: Study ALL10 From the Dutch Childhood Oncology Group. J Clin Oncol. 2016;34(22):2591–601. [DOI] [PubMed] [Google Scholar]
- 9.Schrappe M, Bleckmann K, Zimmermann M, Biondi A, Moricke A, Locatelli F, et al. Reduced-Intensity Delayed Intensification in Standard-Risk Pediatric Acute Lymphoblastic Leukemia Defined by Undetectable Minimal Residual Disease: Results of an International Randomized Trial (AIEOP-BFM ALL 2000). J Clin Oncol. 2018;36(3):244–53. [DOI] [PubMed] [Google Scholar]
- 10.Lipshultz SE, Lipsitz SR, Sallan SE, Dalton VM, Mone SM, Gelber RD, et al. Chronic progressive cardiac dysfunction years after doxorubicin therapy for childhood acute lymphoblastic leukemia. J Clin Oncol. 2005;23(12):2629–36. [DOI] [PubMed] [Google Scholar]
- 11.Leger K, Slone T, Lemler M, Leonard D, Cochran C, Bowman WP, et al. Subclinical cardiotoxicity in childhood cancer survivors exposed to very low dose anthracycline therapy. Pediatr Blood Cancer. 2015;62(1):123–7. [DOI] [PubMed] [Google Scholar]
- 12.Hijiya N, Ness KK, Ribeiro RC, Hudson MM. Acute leukemia as a secondary malignancy in children and adolescents: current findings and issues. Cancer. 2009;115(1):23–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maloney KW, Devidas M, Wang C, Mattano LA, Friedmann AM, Buckley P, et al. Outcome in Children With Standard-Risk B-Cell Acute Lymphoblastic Leukemia: Results of Children’s Oncology Group Trial AALL0331. J Clin Oncol. 2020;38(6):602–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mattano LA Jr., Devidas M, Maloney KW, Wang C, Friedmann AM, Buckley P, et al. Favorable Trisomies and ETV6-RUNX1 Predict Cure in Low-Risk B-Cell Acute Lymphoblastic Leukemia: Results From Children’s Oncology Group Trial AALL0331. J Clin Oncol. 2021;39(14):1540–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vora A, Goulden N, Wade R, Mitchell C, Hancock J, Hough R, et al. Treatment reduction for children and young adults with low-risk acute lymphoblastic leukaemia defined by minimal residual disease (UKALL 2003): a randomised controlled trial. Lancet Oncol. 2013;14(3):199–209. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
De-identified data will be submitted to and made available through the NCTN/NCORP Data Archive at https://nctn-data-archive.nci.nih.gov.


