Abstract
Background:
Open tibial fractures have a high risk of infection that can lead to severe morbidity. Antibiotics administered locally at the site of the open wound are a potentially effective preventive measure, but there are limited data evaluating aminoglycoside antibiotics. The objective of this study was to assess the feasibility of a clinical trial to test the efficacy of local gentamicin in reducing the risk of fracture-related infection after open tibial fracture.
Methods:
This study is a single-center, pilot, masked, randomized controlled trial conducted at the Muhimbili Orthopaedic Institute. Participants were randomized intraoperatively after wound closure to receive gentamicin solution or normal saline solution injected at the fracture site. Follow-ups were completed at 2 weeks, 6 weeks, 3 months, 6 months, 9 months, and 1 year postoperatively. The primary feasibility outcomes were the rate of enrollment and retention. The primary clinical outcome was the occurrence of fracture-related infection.
Results:
Of 199 patients screened, 100 eligible patients were successfully enrolled and randomized over 9 months (11.1 patients/month). Complete data were recorded at baseline and follow-up for >95% of cases. The rate of follow-up at 6 weeks, 3 months, 6 months, 9 months, and 1 year were 70%, 68%, 69%, 61%, and 80%, respectively. There was no difference in adverse events or any of the measured primary and secondary outcomes.
Conclusion:
This pilot study is among the first to evaluate locally administered gentamicin in open tibial fractures. Results indicate a rigorous clinical trial with acceptable rates of enrollment and follow-up to address this topic is possible in this setting.
Key Words: fracture related infection, open tibia fracture, local antibiotics
1. Introduction
The global incidence of fractures and fracture-related disability has grown exponentially in recent years.[1] Although partly due to aging populations, lower extremity injuries in the setting of high-energy trauma continue to be a leading cause of global fracture burden.[2] Tibial fractures are the most common lower extremity fracture[3] and most likely to be open.[4] The generally accepted standard of care for open fractures includes timely systemic antibiotic prophylaxis, tetanus booster, wound debridement, and fracture stabilization.[5,6] Despite these measures, infection has been shown to occur in up to 40% of cases.[5,7,8] Fracture-related infection (FRI) can be especially challenging to manage because of the ability of many FRI-causing bacteria to produce biofilms that provide effective protection from host immunity and antibiotics.[9] FRI places a significant burden on both patients and the health care system because it increases patient mortality, delays healing, decreases functional outcomes and health-related quality of life, and often requires prolonged hospitalization or reoperation, increasing the cost of care.[7,10–12]
Although timely intravenous (IV) antibiotic prophylaxis is widely accepted and used in open fracture management, high rates of infection have been shown to persist despite their use.[7,13,14] IV antibiotic concentration at the surgical site is limited by systemic toxicity and devascularization at the time of injury, leading to compromised local blood supply.[8,14] Local antibiotics administered intraoperatively and applied directly into the traumatic wound may confer additional protection against infection.[15–17] Antibiotics applied directly into the wound may achieve high local concentrations with lower systemic levels and thereby decrease systemic toxicity, biofilm creation, and bacterial resistance while working synergistically with IV antibiotics to further decrease the risk of SSI.[9,12–14,18] However, results of studies on the topic have been mixed. A recent meta-analysis found a decreased incidence of infection in both spine and trauma surgeries, but this effect was absent with more rigorous study designs.[19]
Aminoglycoside gentamicin has been proposed as a promising and lower cost alternative to vancomycin for local application in low-resource settings. For example, in Tanzania, one 80 mg vial of liquid gentamicin costs five cents, which is over 100-fold cheaper than vancomycin powder, which costs $20 per gram. In addition, aminoglycosides are broad-spectrum antibiotics that cover gram-negative and many gram-positive bacteria, including both staphylococcus aureus and coagulase-negative staphylococcus, the 2 most common bacteria in FRI.[20] Reduced rates of infection have been observed after local gentamicin injection in an animal model,[21] and one large retrospective cohort study found a 50% reduction in the odds of deep and superficial infection after prophylactic local injection of aminoglycosides for open tibial fractures compared with no local antibiotics.[13] Although promising, the latter study's limitations are numerous, including retrospective, nonrandomized design, absence of masking, and variability in treatment protocols and definitions of infection. There remains a clear gap in knowledge regarding the efficacy of locally administered gentamicin in preventing FRI in open fractures.
This study is a pilot study to evaluate the feasibility of a masked, randomized, placebo-controlled trial to assess the efficacy of intraoperative, locally applied gentamicin to prevent FRI in open tibial fractures.[22] We hypothesized that the study would be feasible for the rate of enrollment, follow-up, and data completeness.
2. Methods
The Pilot Masked, Randomized Controlled Trial Evaluating Locally-applied Gentamicin versus Saline in Open Tibia Fractures (pGO-Tibia) trial was conducted at the Muhimbili Orthopaedic Institute (MOI) in Dar es Salaam, Tanzania, in partnership with the Institute for Global Orthopaedics and Traumatology (IGOT) at the University of California, San Francisco (UCSF). MOI is a tertiary referral hospital in the largest city in Tanzania.[23]
The study protocol was previously published[22] in accordance with the Standard Protocol Items: Recommendation for Interventional Trials (SPIRIT)[24] and Consolidated Standards of Reporting Trials (CONSORT)[25] guidelines for randomized pilot and feasibility trials and was registered at ClinicalTrials.gov (NCT03559400).[26] The pGO-Tibia trial received funding from the Orthopaedic Trauma Association International Grant, the Hellman Fellows Fund as part of the UCSF Hellman Fellowship Program, and the UCSF Department of Orthopaedic Surgery. Ethical approval was obtained by the National Institute of Medical Research, Tanzania (Ref#: NIMR/HQ/R.8a/Vol. IX/2958), and the UCSF Human Subjects Research Internal Review Board (IRB# 17-23950).
All adult patients who presented to the MOI emergency department with an open tibial fracture between November 2019 and August 2020 were screened for study eligibility. Prophylactic systemic ceftriaxone was administered, and serum creatinine levels and radiographs were obtained for all screened patients, regardless of eligibility status per best practice guidelines and the MOI protocol.[27] Inclusion criteria included patients 18 years and older with a Gustilo-Anderson I, II, or IIIA open tibia shaft fracture (OTA/AO type 42). Patients injured >48 hours before presentation or >7 days before surgery were excluded (Table 1).
TABLE 1.
| Inclusion Criteria | Exclusion Criteria |
| 1. Skeletally mature patients (>18 years old) | 1. Time from injury to presentation >48 h |
| 2. Open tibial shaft fracture meeting the following criteria: | 2. Time from injury to surgery >7 d |
| OTA/AO Type 42 | 3. Aminoglycoside allergy |
| Primarily closable wound | 4. GA IIIB or IIIC open fractures |
| GA I, II, or IIIA | 5. Bilateral open tibial fractures |
| 6. Severe brain (GCS<12) or spinal cord injury | |
| 7. Severe vascular injury | |
| 8. Severe burns (>10% TBSA or >5% TBSA with full thickness or circumferential injury) | |
| 9. Pathologic fracture | |
| 10. History of active limb infection, ipsilaterally | |
| 11. Unlikely to complete follow-up |
OTA/AO, Orthopaedic Trauma Association; GA, Gustilo-Anderson; GCS, Glasgow Coma Scale; TBSA, total body surface area.
After obtaining written, informed consent, participants were initially managed with irrigation and debridement in the operating theater. Definitive skeletal stabilization was performed using internal (hand-reaming SIGN IM nail) or external (monoplanar) fixation at the discretion of the treating surgeon. At the conclusion of the procedure, an attempt was made to close the wound primarily. If this could not be achieved without excessive tension based on surgeon judgment, the patient was excluded from the study. If primary closure without excessive tension was successful, participants were individually randomized using a web-based randomization tool (Research Electronic Data Capture, REDCap) to receive either local injection of gentamicin or saline control. The gentamicin dose was determined using previously published data.[13] The study solution consisted of 80-mg aqueous gentamicin (Sichuan Long March Pharmaceutical Co, Ltd, Leshan, Sichuan Province, China) diluted in 40 mL of saline (2 mg/mL). The intervention was administered at the fracture site using a 22-gauge needle through an anteromedial approach until the solution filled the wound cavity and extravasation was seen or a maximum of 40 mL was administered.[13,22] A minimum of 5 mL of the study solution was injected. The control solution followed the same procedure, but consisted of 40 mL of plain saline (Otsuka Pharmaceutical India Private Limited, Ahmedabad, India). Study solutions were prepared and stored in accordance with proper guidelines, and quality control of the solutions was monitored by the Muhimbili University of Health and Allied Sciences (MUHAS) microbiology laboratory.[22] A protocol for solution administration was tested on a cadaveric model, and then a video was created for training purposes to standardize the administration procedure among surgeons. Both control and gentamicin solutions were clear in color and odorless.
Radiographic and serum creatine data were regularly obtained and analyzed to monitor potential adverse events associated with gentamicin use per study protocol.[22] An independent adjudication committee of nontreating surgeons assessed all adverse events. The committee included 2 US orthopaedic trauma surgeons and 1 Tanzanian orthopaedic trauma surgeon. In addition, an independent data safety and monitoring committee (DSMC), comprising both American and Tanzanian members, convened a minimum of every 6 months to monitor recruitment, retention, data quality, and patient safety.[22]
All study participants, care providers, research coordinators involved in data collection, adjudication committee members, and data analysts were masked to the treatment group. Only 2 research nurses at the study site involved with solution preparation and 2 research staff at the coordinating center were unmasked to treatment assignment.
The pGO-Tibia trial was designed with feasibility outcomes as well as planned primary and secondary outcomes as outlined in the study protocol.[22] Feasibility outcomes included enrollment rate, retention rate, and data completeness in preparation for the definitive trial. The planned primary end point was the presence of a fracture-related infection (FRI). Planned secondary end points included suggestive FRI criteria, the occurrence of a nonunion or unplanned reoperation, health-related quality of life as measured by the EQ-5D score administered in Swahili, radiographic healing as measured by the mRUST score, and clinical healing as measured by the Function IndeX for Trauma (FIX-IT) assessment.[22]
All data for the study were collected, stored, and analyzed using REDCap, a secure web-based software designed to support data collection and management for research studies.[28,29] Baseline clinical, demographic, and socioeconomic data were obtained for all patients on enrollment. Study participants returned to the clinic for follow-up at 2 weeks, 6 weeks, 3 months, 6 months, 9 months, and 1 year after surgery for clinical evaluation and outcomes assessment.[22] In addition, radiographs were obtained at each follow-up and assessed for signs of healing as determined by using the Modified Radiographic Union Score for Diaphyseal Tibial Fractures (mRUST).[30]
All statistical analyses were conducted using Stata (College Station, TX: StataCorp LLC). Feasibility outcomes were summarized using descriptive statistics. The primary clinical outcome of occurrence of FRI was analyzed as a time-to-event outcome using a Cox proportional hazards model. Secondary outcomes were assessed using the Fischer exact test and Student t-test for binary and continuous outcomes, respectively.
3. Results
3.1. Demographics, Mechanism, Injury Classification, and Treatment
Of 199 patients screened, 100 were eligible and consented to be enrolled in the study (Fig. 1). The mean age was 33 years, and most participants (80%) were male. Road traffic accidents were the most common mechanism of injury (85%), followed by falls (5%). Most of the open fractures were Gustilo-Anderson type IIIA and were treated with either intramedullary nailing (53%) or external fixation (46%) (Table 2).
Figure 1.
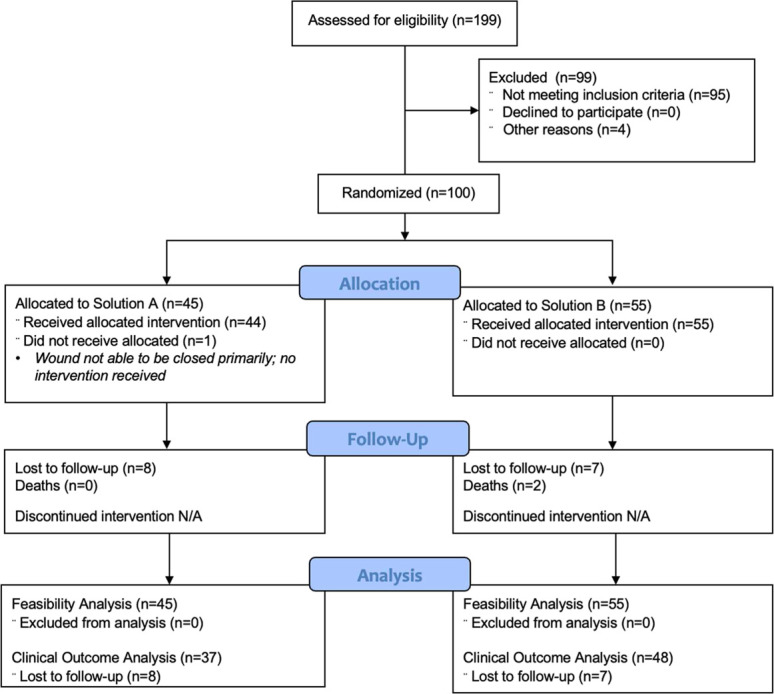
Consort flow diagram for the pGO-Tibia study.
TABLE 2.
Demographics, Mechanism, Injury Classification, and Treatment
| All Patients | Saline | Gentamicin | P | Test | |
| N | 100 | 55 | 45 | ||
| Age, mean (SD) | 33.58 (12) | 32.54 (11) | 34.86 (14) | 0.35 | Two sample t test |
| Sex | 0.14 | Fisher exact | |||
| Male | 80 (80.0) | 47 (85) | 33 (73) | ||
| Female | 20 (20.0) | 8 (15) | 12 (27) | ||
| BMI, mean (SD) | 25.01 (3.8) | 24.66 (3.6) | 25.43 (4) | 0.33 | Two sample t test |
| Smoking status | 0.80 | Fisher exact | |||
| Current smoker | 17 (17) | 9 (16) | 8 (18) | ||
| Former smoker | 5 (5) | 2 (4) | 3 (7) | ||
| Nonsmoker | 78 (78) | 44 (80) | 34 (76) | ||
| Alcohol consumption | 0.68 | Fisher exact | |||
| No | 58 (59) | 30 (57) | 28 (62) | ||
| Yes | 40 (41) | 23 (43) | 17 (38) | ||
| Diabetes status | 0.25 | Fisher exact | |||
| Yes | 1 (1) | 1 (2) | 0 (0) | ||
| No | 96 (96) | 51 (93) | 45 (100) | ||
| Unknown/never tested | 3 (3) | 3 (5) | 0 (0) | ||
| HIV/AIDS status | 1.00 | Fisher exact | |||
| No | 91 (91) | 50 (91) | 41 (91) | ||
| Unknown/never tested | 9 (9.0) | 5 (9) | 4 (9) | ||
| Educational attainment | 0.097 | Fisher exact | |||
| None | 3 (3) | 0 (0) | 3 (7) | ||
| Standard I-VII | 44 (44) | 22 (40) | 22 (49) | ||
| Form 1-4 | 42 (42) | 24 (44) | 18 (40) | ||
| Form 5 and 6 | 1 (1) | 1 (2) | 0 (0) | ||
| College or university | 10 (10) | 8 (15) | 2 (4) | ||
| Working for pay | 0.84 | Fisher exact | |||
| No | 58 (58) | 31 (56) | 27 (60) | ||
| Yes | 42 (42) | 24 (44) | 18 (40) | ||
| Mechanism of injury | 0.66 | Fisher exact | |||
| Road traffic injury | 84 (85) | 47 (87) | 37 (82) | ||
| Fall | 5 (5) | 3 (6) | 2 (4) | ||
| Crush injury | 5 (5) | 3 (6) | 2 (4) | ||
| Gunshot | 2 (2) | 0 (0) | 2 (4) | ||
| Other | 3 (3) | 1 (2) | 2 (4) | ||
| Gustilo-Anderson classification | 0.19 | Fisher exact | |||
| I | 3 (3) | 0 (0) | 3 (7) | ||
| II | 22 (22) | 13 (24) | 9 (20) | ||
| IIIA | 75 (75) | 42 (76) | 33 (73) | ||
| Type of fixation | 0.83 | Fisher exact | |||
| Intramedullary nail | 53 (53) | 30 (55) | 23 (51) | ||
| External fixation | 46 (46) | 24 (44) | 22 (49) | ||
| Cast | 1 (1) | 1 (2) | 0 (0) |
3.2. Enrollment and Retention
Enrollment took place over a period of 9 months, and the rate of enrollment was 11.1 per month. However, enrollment was paused between April 15 and May 14, 2020, because of the coronavirus pandemic. Follow-up was continued throughout that period to monitor for adverse events, per guidelines from the Tanzanian National Institute for Medical Research (NIMR). The trial resumed enrollment on May 14, 2020, after receiving written approval for the trial to continue from NIMR. If the 1-month pause was excluded, the enrollment rate was 12.5 patients per month (Table 3). The rate of follow-up at 6 weeks, 3 months, 6 months, 9 months, and 1 year were 70%, 68%, 69%, 61%, and 80%, respectively (Table 3). Retention, which was defined as either completion of 1 year of follow-up or experiencing a primary event, was 85%.
TABLE 3.
Enrollment, Retention, and Data Completeness
| Time Point | Follow-Up Rate | Data Completeness | |
| Enrollment rate: 12.5 patients monthly | |||
| 6 Weeks | 70% | 92% | |
| 3 Months | 68% | 100% | |
| 6 Months | 69% | 97% | |
| 9 Months | 61% | 97% | |
| 1 Year | 80% | 98% | |
3.3. Data Completeness
Complete data for most variables were recorded preoperatively and postoperatively for 100% of cases. Preoperative and postoperative radiograph data were recorded for over 96% of cases. Complete data on adverse events were recorded for 98% of patients at the 2-week time point and 92% at the 6-week time point (Table 3). All data including adverse event screening, return to work, EQ-5D, FIX-IT, and radiographic data were recorded for 100% of patients at 3 months, >97% at 6 months and 9 months, and 98% at 52 weeks (Table 3).
3.4. Quality Control: Surgeon Masking, Solution Quality, and Solution Administration
Surgeons guessed which solution the patient had received incorrectly in 51% of cases, correctly in 45% of cases, and with no guess in 4% of cases. The quality and efficacy of the gentamicin solution given was tested monthly during enrollment from February to July 2020. In all cases, the results were consistent with the masking key and prior results. On average, 18 mL of fluid was administered before fluid extravasated from the wound, for a mean gentamicin dose of 36 mg.
3.5. Safety
In the gentamicin group, there were 5 cases (11%) of mild-to-moderate acute kidney injury, which was defined as a serum creatinine of 0.3–1.0 above baseline creatinine. In the saline group, there were 4 instances (7%) of mild-to-moderate acute kidney injury. There were no instances of severe or persistent kidney injury (Fig. 2).
Figure 2.
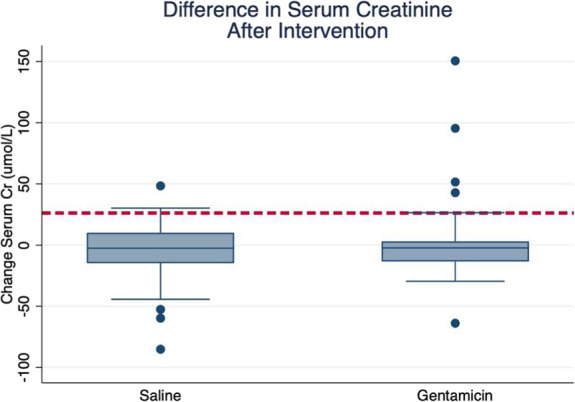
Change in serum creatinine by treatment group. The dashed red line represents a 25 umol/L rise in serum creatine, which was defined as an AKI in our study. AKI, acute kidney injury.
3.6. Clinical Outcomes
The rate of FRI was 24% in the gentamicin group and 15% in the saline group (hazard ratio 1.40; 95% CI; 0.87–2.25). The rate of suggestive FRI in the gentamicin group was 6.6% and 9.0% in the saline group. The rate of nonunion was 15.5% and 10.9% in the gentamicin and saline groups, respectively. Fracture-related reoperation occurred in 6.6% of the gentamicin group and 1.8% of the saline group. Trends in EQ-5D, mRUST, and FIX-IT were similar between the 2 groups over time (Figs. 3–5).
Figure 3.
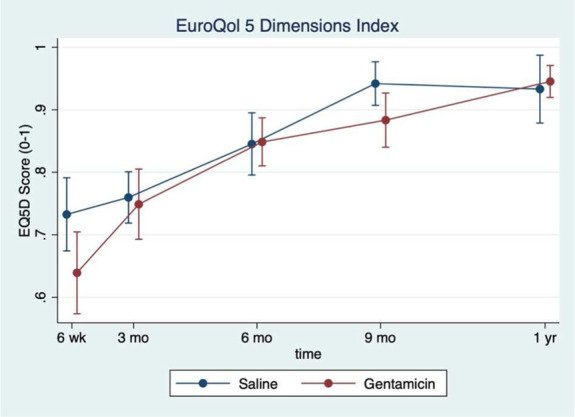
EuroQol 5 Dimensions (EQ-5D) index over time by treatment group. The mean EQ-5D score plotted over time at each follow-up time point. The saline group is represented by the blue dots while the gentamicin group is represented by the red dots.
Figure 5.
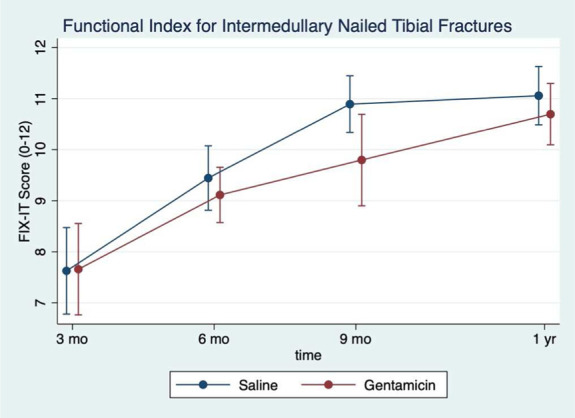
Functional IndeX for intermedullary nailed tibial fractures (FIX-IT) score by treatment group. The mean FIX-IT score plotted over time at each follow-up time point. The saline group is represented by the blue dots while the gentamicin group is represented by the red dots.
Figure 4.
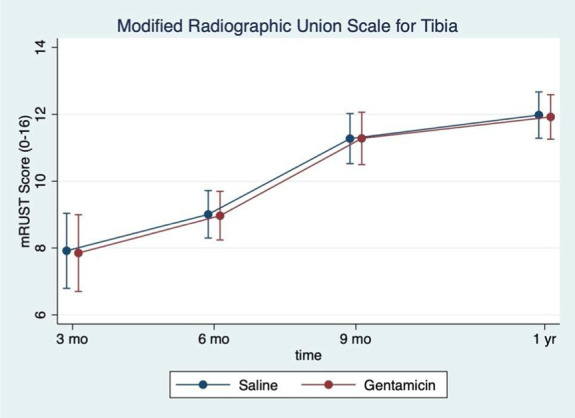
Modified Radiographic Union Scale for Tibia (mRUST) by treatment group. The mean mRUST score plotted over time at each follow-up time point. The saline group is represented by the blue dots while the gentamicin group is represented by the red dots.
4. Discussion
This study aimed to assess the feasibility of a randomized controlled trial to evaluate the efficacy of local gentamicin in preventing fracture-related infection after open tibial fractures. This study enrolled 100 patients over an 8-month period and achieved a follow-up rate approaching 80%, suggesting that an acceptable rate of enrollment and retention are possible in this setting. This represents the first randomized clinical trial evaluating locally applied gentamicin for open tibial fractures and the first clinical trial with this degree of methodologic rigor for any intervention for open tibial fractures in a low-income country.
The sample size and follow-up observed in the pGO-Tibia study compares favorably alongside some of the largest trials conducted on open tibial fractures in Europe and North America (Table 4). This trial alongside a prior trial of fixation methods conducted at MOI illustrates the high trauma volume and efficiency of enrollment possible at this single center.[31] While the 1-year follow-up rate is lower than that in previous studies, this follow-up rate does not include any telephone follow-up, which was common in other studies, but may not be effective in identifying FRI. In addition, we believe the COVID-19 pandemic may have negatively affected both enrollment and follow-up compared with the previous study at MOI. Nonetheless, we believe these results support the pursuit of a definitive clinical trial evaluating local antibiotics that is adequately powered to address the primary outcome.
TABLE 4.
Published Clinical Trials Reporting Outcomes of Open Tibial Shaft Fractures
| Studies | Total Sample | Open Tibia Sample | Number Centers | Duration (Years) | Enrolled/Center | Enrolled/Center/Year | Follow-Up Rate |
| FLOW trial [10] | 2447 | 912 | 41 | 4 | 22 | 6 | 90% |
| SPRINT trial [11] | 1226 | 392 | 29 | 5 | 13 | 3 | 93% |
| MOI IMN v. Ex-fix [12] | 240 | 240 | 1 | 1.3 | 240 | 180 | 92.1% |
| TRUST trial [13] | 501 | 114 | 43 | 4.5 | 3 | <1 | 96% |
| LEAP study [14] | 569 | 173 | 8 | 3 | 22 | 7 | 96% |
| INFINITI [15] | 768 | 162 | 9 | NR | 18 | NR | 98% |
| Alberta cohort [16] | 791 | 140 | 3 | 8 | 47 | 6 | 94% |
The previous randomized trial conducted by the coordinating center and MOI is represented in bold.
Few randomized trials have been published evaluating the efficacy of local antibiotics. The VANCO trial evaluated the efficacy of locally administered vancomycin powder in preventing surgical site infections for high-risk closed and open fractures. The study enrolled 1036 patients from 36 centers over the course of 3 years and achieved a follow-up rate of 94.6%. Although the effect size was nearly 50% in favor of vancomycin, the difference in primary outcome was not statistically significant.[14] The TOBRA trial is a follow-up trial that will assess the efficacy of local vancomycin plus tobramycin versus vancomycin alone.
This study had several limitations. Because it was a pilot study and only included 100 participants, it was predictably underpowered to detect any statistically significant differences in FRI between the control and intervention groups. In the definitive trial, which began enrollment in the fall of 2022, the sample size will be sufficiently powered to detect differences in our primary outcome considering an approximately 80% follow-up rate. If the follow-up rate is less than 80% in the definitive trial, there is the possibility that the trial may miss a meaningful impact. Another limitation of this study is that many patients did not get the full dose of gentamicin. Each syringe was filled with 40 mL of gentamicin solution, which equated to 80 mg of local gentamicin. On average, less than half the dose was administered, resulting in an average dose of approximately 36 mg of gentamicin. This dose is far lower than the 1200 mg-dose of tobramycin being used in the TOBRA trial despite equivalent weight-based dosing for gentamicin and tobramycin.[14] In the definitive trial, the dose will be concentrated to 80 mg of gentamicin in 5 mL of saline to ensure that all participants receive the full dose without extravasation.
5. Conclusions
This study supports the feasibility of a masked randomized trial in Tanzania to evaluate the efficacy of local gentamicin to prevent fracture-related infection after open tibial fractures. If successful, the definitive trial has the potential to establish the efficacy of a simple, low-cost intervention to reduce the burden of open fractures in both high and low-income settings.
Footnotes
The pGO-Tibia trial received funding from the Orthopaedic Trauma Association International Grant, the Hellman Fellows Fund as part of the UCSF Hellman Fellowship Program, and the UCSF Department of Orthopaedic Surgery.
The authors have no conflicts of interest to disclose
Contributor Information
Billy T. Haonga, Email: bhaonga@gmail.com.
Patrick Ngunyale, Email: patrickdeogratias541@yahoo.com.
Ericka P. von Kaeppler, Email: ericka.vonkaeppler.research@outlook.com.
Claire A. Donnelley, Email: claire.donnelley@yale.edu.
Nae Y. Won, Email: naeyeonwon@gmail.com.
Edmund N. Eliezer, Email: ndalama@yahoo.com.
Kelsey Brown, Email: kelsey_brown@brown.edu.
Michael Flores, Email: michael.flores@yale.edu.
Patricia Rodarte, Email: patricia_rodarte@brown.edu.
Mayur Urva, Email: murva@student.nymc.edu.
Abigail Cortez, Email: abigailcortez@mednet.ucla.edu.
Travis Porco, Email: travis.porco@ucsf.edu.
Saam Morshed, Email: saam.morshed@ucsf.edu.
David W. Shearer, Email: david.shearer@ucsf.edu.
References
- 1.Wu AM, Bisignano C, James SL, et al. Global, regional, and national burden of bone fractures in 204 countries and territories, 1990–2019: a systematic analysis from the Global Burden of Disease Study 2019. Lancet Healthy Longev. 2021;2:e580–e592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roser M, Ritchie H. Burden of Disease. Our World in Data, 2016. Available at: https://ourworldindata.org/burden-of-disease. Accessed September 21, 2021. [Google Scholar]
- 3.Thompson JH, Koutsogiannis P, Jahangir A. Tibia Fractures Overview. StatPerals Publishing, 2021. Available at. http://www.ncbi.nlm.nih.gov/books/NBK513267/. Accessed November 18, 2021. [PubMed] [Google Scholar]
- 4.Gustilo RB, Anderson JT. Prevention of infection in the treatment of one thousand and twenty-five open fractures of long bones: retrospective and prospective analyses. J Bone Joint Surg Am. 1976;58:453–458. [PubMed] [Google Scholar]
- 5.Kohlprath R, Assal M, Uçkay I, et al. Open fractures of the tibia in the adult: surgical treatment and complications. Rev Med Suisse. 2011;7:2484–2488. [PubMed] [Google Scholar]
- 6.Dellinger EP, Miller SD, Wertz MJ, et al. Risk of infection after open fracture of the arm or leg. Arch Surg. 1988;123:1320–1327. [DOI] [PubMed] [Google Scholar]
- 7.Harris AM, Althausen PL, Kellam J, et al. , Lower Extremity Assessment Project (LEAP) Study Group. complications following limb-threatening lower extremity trauma. J Orthop Trauma. 2009;23:1–6. [DOI] [PubMed] [Google Scholar]
- 8.Barei DP, Nork SE, Mills WJ, et al. Complications associated with internal fixation of high-energy bicondylar tibial plateau fractures utilizing a two-incision technique. J Orthop Trauma. 2004;18:649–657. [DOI] [PubMed] [Google Scholar]
- 9.Cancienne JM, Burrus MT, Weiss DB, et al. Applications of local antibiotics in orthopedic trauma. Orthop Clin North Am. 2015;46:495–510. [DOI] [PubMed] [Google Scholar]
- 10.Kirkland KB, Briggs JP, Trivette SL, et al. The impact of surgical-site infections in the 1990s: attributable mortality, excess length of hospitalization, and extra costs. Infect Control Hosp Epidemiol. 1999;20:725–730. [DOI] [PubMed] [Google Scholar]
- 11.Starr AJ. Fracture repair: successful advances, persistent problems, and the psychological burden of trauma. J Bone Joint Surg Am. 2008;90:132–137. [DOI] [PubMed] [Google Scholar]
- 12.Metsemakers WJ, Kuehl R, Moriarty TF, et al. Infection after fracture fixation: current surgical and microbiological concepts. Injury. 2018;49:511–522. [DOI] [PubMed] [Google Scholar]
- 13.Lawing CR, Lin FC, Dahners LE. Local injection of aminoglycosides for prophylaxis against infection in open fractures. J Bone Joint Surg Am. 2015;97:1844–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Major Extremity Trauma Research Consortium (METRC), et al. Effect of intrawound vancomycin powder in operatively treated high-risk tibia fractures: a randomized clinical Trial. JAMA Surg. 2021;156:e207259. [DOI] [PubMed] [Google Scholar]
- 15.Hanssen AD. Local antibiotic delivery vehicles in the treatment of musculoskeletal infection. Clin Orthop. 2005;(437):91–96. [DOI] [PubMed] [Google Scholar]
- 16.Henry SL, Galloway KP. Local antibacterial therapy for the management of orthopaedic infections. Pharmacokinetic considerations. Clin Pharmacokinet. 1995;29:36–45. [DOI] [PubMed] [Google Scholar]
- 17.Antoci V, Adams CS, Hickok NJ, et al. Antibiotics for local delivery systems cause skeletal cell toxicity in vitro. Clin Orthop. 2007;462:200–206. [DOI] [PubMed] [Google Scholar]
- 18.Buttaro MA, Gimenez MI, Greco G, et al. High active local levels of vancomycin without nephrotoxicity released from impacted bone allografts in 20 revision hip arthroplasties. Acta Orthop. 2005;76:336–340. [PubMed] [Google Scholar]
- 19.Kim EK, Donnelley CA, Tiee M, et al. Prophylactic topical antibiotics in fracture repair and spinal fusion. Adv Orthop. 2021;2021:1949877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edson RS, Terrell CL. The aminoglycosides. Mayo Clin Proc. 1999;74:519–528. [DOI] [PubMed] [Google Scholar]
- 21.Cavanaugh DL, Berry J, Yarboro SR, et al. Better prophylaxis against surgical site infection with local as well as systemic antibiotics. An in vivo study. J Bone Joint Surg Am. 2009;91:1907–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.von Kaeppler EP, Donnelley C, Ali SH, et al. A study protocol for a pilot masked, randomized controlled trial evaluating locally-applied gentamicin versus saline in open tibia fractures (pGO-Tibia) in Dar es Salaam. Tanzan Pilot Feasibility Stud. 2021;7:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ibrahim J, Liu M, Yusi K, et al. Conducting a randomized controlled trial in Tanzania: Institute for Global Orthopaedics and Traumatology and the Muhimbili Orthopaedic Institute. J Orthop Trauma. 2018;32:S47–S51. [DOI] [PubMed] [Google Scholar]
- 24.Chan AW, Tetzlaff JM, Altman DG, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med. 2013;158:200–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eldridge SM, Chan CL, Campbell MJ, et al. Consort 2010 statement: extension to randomised pilot and feasibility trials. BMJ. 2016;355:i5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.University of California, Randomized Controlled Trial Evaluating Locally-Applied Gentamicin Versus Saline in Open Tibia Fractures. San Francisco, CA. A Pilot Masked, 2021. Accessed October 3, 2021. https://clinicaltrials.gov/ct2/show/NCT03559400 [Google Scholar]
- 27.Hoff WS, Bonadies JA, Cachecho R, et al. East Practice Management Guidelines Work Group: update to practice management guidelines for prophylactic antibiotic use in open fractures. J Trauma. 2011;70:751–754. [DOI] [PubMed] [Google Scholar]
- 28.Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inf. 2019;95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inf. 2009;42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mitchell SL, Obremskey WT, Luly J, et al. Inter-rater reliability of the modified radiographic union score for diaphyseal tibial fractures with bone defects. J Orthop Trauma. 2019;33:301–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haonga BT, Liu M, Albright P, et al. Intramedullary nailing versus external fixation in the treatment of open tibial fractures in Tanzania: results of a randomized clinical trial. J Bone Joint Surg Am. 2020;102:896–905. [DOI] [PMC free article] [PubMed] [Google Scholar]


