Abstract
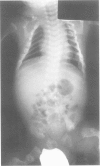
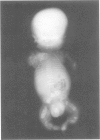
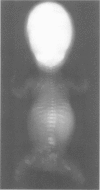
Perinatal lethal osteogenesis imperfecta is the result of heterozygous mutations of the COL1A1 and COL1A2 genes that encode the alpha 1(I) and alpha 2(I) chains of type I collagen, respectively. Point mutations resulting in the substitution of Gly residues in Gly-X-Y amino acid triplets of the triple helical domain of the alpha 1(I) or alpha 2(I) chains are the most frequent mutations. They interrupt the repetitive Gly-X-Y structure that is mandatory for the formation of a stable triple helix. Most babies have their own private de novo mutation. However, the recurrence rate is about 7% owing to germline mosaicism in one parent. The mutations act in a dominant negative manner as the mutant pro alpha chains are incorporated into type I procollagen molecules that also contain normal pro alpha chains. The abnormal molecules are poorly secreted, more susceptible to degradation, and impair the formation of the extracellular matrix. The collagen fibres are abnormally organised and mineralisation is impaired. The severity of the clinical phenotype appears to be related to the type of mutation, its location in the alpha chain, the surrounding amino acid sequences, and the level of expression of the mutant allele.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barsh G. S., Roush C. L., Bonadio J., Byers P. H., Gelinas R. E. Intron-mediated recombination may cause a deletion in an alpha 1 type I collagen chain in a lethal form of osteogenesis imperfecta. Proc Natl Acad Sci U S A. 1985 May;82(9):2870–2874. doi: 10.1073/pnas.82.9.2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman J. F., Chan D., Mascara T., Rogers J. G., Cole W. G. Collagen defects in lethal perinatal osteogenesis imperfecta. Biochem J. 1986 Dec 15;240(3):699–708. doi: 10.1042/bj2400699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman J. F., Lamande S. R., Dahl H. H., Chan D., Mascara T., Cole W. G. A frameshift mutation results in a truncated nonfunctional carboxyl-terminal pro alpha 1(I) propeptide of type I collagen in osteogenesis imperfecta. J Biol Chem. 1989 Jul 5;264(19):10960–10964. [PubMed] [Google Scholar]
- Bateman J. F., Lamande S. R., Hannagan M., Moeller I., Dahl H. H., Cole W. G. Chemical cleavage method for the detection of RNA base changes: experience in the application to collagen mutations in osteogenesis imperfecta. Am J Med Genet. 1993 Jan 15;45(2):233–240. doi: 10.1002/ajmg.1320450216. [DOI] [PubMed] [Google Scholar]
- Bateman J. F., Moeller I., Hannagan M., Chan D., Cole W. G. Characterization of three osteogenesis imperfecta collagen alpha 1(I) glycine to serine mutations demonstrating a position-dependent gradient of phenotypic severity. Biochem J. 1992 Nov 15;288(Pt 1):131–135. doi: 10.1042/bj2880131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonadio J., Ramirez F., Barr M. An intron mutation in the human alpha 1(I) collagen gene alters the efficiency of pre-mRNA splicing and is associated with osteogenesis imperfecta type II. J Biol Chem. 1990 Feb 5;265(4):2262–2268. [PubMed] [Google Scholar]
- Bonadio J., Saunders T. L., Tsai E., Goldstein S. A., Morris-Wiman J., Brinkley L., Dolan D. F., Altschuler R. A., Hawkins J. E., Jr, Bateman J. F. Transgenic mouse model of the mild dominant form of osteogenesis imperfecta. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7145–7149. doi: 10.1073/pnas.87.18.7145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner R. E., Vetter U., Nerlich A., Wörsdorfer O., Teller W. M., Müller P. K. Osteogenesis imperfecta: insufficient collagen synthesis in early childhood as evidenced by analysis of compact bone and fibroblast cultures. Eur J Clin Invest. 1989 Apr;19(2):159–166. doi: 10.1111/j.1365-2362.1989.tb00211.x. [DOI] [PubMed] [Google Scholar]
- Byers P. H. Brittle bones--fragile molecules: disorders of collagen gene structure and expression. Trends Genet. 1990 Sep;6(9):293–300. doi: 10.1016/0168-9525(90)90235-x. [DOI] [PubMed] [Google Scholar]
- Byers P. H., Tsipouras P., Bonadio J. F., Starman B. J., Schwartz R. C. Perinatal lethal osteogenesis imperfecta (OI type II): a biochemically heterogeneous disorder usually due to new mutations in the genes for type I collagen. Am J Hum Genet. 1988 Feb;42(2):237–248. [PMC free article] [PubMed] [Google Scholar]
- Byers P. H., Wallis G. A., Willing M. C. Osteogenesis imperfecta: translation of mutation to phenotype. J Med Genet. 1991 Jul;28(7):433–442. doi: 10.1136/jmg.28.7.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bächinger H. P., Morris N. P., Davis J. M. Thermal stability and folding of the collagen triple helix and the effects of mutations in osteogenesis imperfecta on the triple helix of type I collagen. Am J Med Genet. 1993 Jan 15;45(2):152–162. doi: 10.1002/ajmg.1320450204. [DOI] [PubMed] [Google Scholar]
- Chessler S. D., Wallis G. A., Byers P. H. Mutations in the carboxyl-terminal propeptide of the pro alpha 1(I) chain of type I collagen result in defective chain association and produce lethal osteogenesis imperfecta. J Biol Chem. 1993 Aug 25;268(24):18218–18225. [PubMed] [Google Scholar]
- Chu M. L., Gargiulo V., Williams C. J., Ramirez F. Multiexon deletion in an osteogenesis imperfecta variant with increased type III collagen mRNA. J Biol Chem. 1985 Jan 25;260(2):691–694. [PubMed] [Google Scholar]
- Cohen-Solal L., Zylberberg L., Sangalli A., Gomez Lira M., Mottes M. Substitution of an aspartic acid for glycine 700 in the alpha 2(I) chain of type I collagen in a recurrent lethal type II osteogenesis imperfecta dramatically affects the mineralization of bone. J Biol Chem. 1994 May 20;269(20):14751–14758. [PubMed] [Google Scholar]
- Cohn D. H., Starman B. J., Blumberg B., Byers P. H. Recurrence of lethal osteogenesis imperfecta due to parental mosaicism for a dominant mutation in a human type I collagen gene (COL1A1). Am J Hum Genet. 1990 Mar;46(3):591–601. [PMC free article] [PubMed] [Google Scholar]
- Cohn D. H., Zhang X., Byers P. H. Homology-mediated recombination between type I collagen gene exons results in an internal tandem duplication and lethal osteogenesis imperfecta. Hum Mutat. 1993;2(1):21–27. doi: 10.1002/humu.1380020105. [DOI] [PubMed] [Google Scholar]
- Cole W. G., Campbell P. E., Rogers J. G., Bateman J. F. The clinical features of osteogenesis imperfecta resulting from a non-functional carboxy terminal pro alpha 1(I) propeptide of type I procollagen and a severe deficiency of normal type I collagen in tissues. J Med Genet. 1990 Sep;27(9):545–551. doi: 10.1136/jmg.27.9.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole W. G., Chow C. W., Rogers J. G., Bateman J. F. The clinical features of three babies with osteogenesis imperfecta resulting from the substitution of glycine by arginine in the pro alpha 1(I) chain of type I procollagen. J Med Genet. 1990 Apr;27(4):228–235. doi: 10.1136/jmg.27.4.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole W. G., Patterson E., Bonadio J., Campbell P. E., Fortune D. W. The clinicopathological features of three babies with osteogenesis imperfecta resulting from the substitution of glycine by valine in the pro alpha 1 (I) chain of type I procollagen. J Med Genet. 1992 Feb;29(2):112–118. doi: 10.1136/jmg.29.2.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantine G., McCormack J., McHugo J., Fowlie A. Prenatal diagnosis of severe osteogenesis imperfecta. Prenat Diagn. 1991 Feb;11(2):103–110. doi: 10.1002/pd.1970110205. [DOI] [PubMed] [Google Scholar]
- Fertala A., Westerhausen A., Morris G., Rooney J. E., Prockop D. J. Two cysteine substitutions in procollagen I: a glycine replacement near the N-terminus of alpha 1(I) chain causes lethal osteogenesis imperfecta and a glycine replacement in the alpha 2(I) chain markedly destabilizes the triple helix. Biochem J. 1993 Jan 1;289(Pt 1):195–199. doi: 10.1042/bj2890195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lightfoot S. J., Holmes D. F., Brass A., Grant M. E., Byers P. H., Kadler K. E. Type I procollagens containing substitutions of aspartate, arginine, and cysteine for glycine in the pro alpha 1 (I) chain are cleaved slowly by N-proteinase, but only the cysteine substitution introduces a kink in the molecule. J Biol Chem. 1992 Dec 15;267(35):25521–25528. [PubMed] [Google Scholar]
- Mackay K., Byers P. H., Dalgleish R. An RT-PCR-SSCP screening strategy for detection of mutations in the gene encoding the alpha 1 chain of type I collagen: application to four patients with osteogenesis imperfecta. Hum Mol Genet. 1993 Aug;2(8):1155–1160. doi: 10.1093/hmg/2.8.1155. [DOI] [PubMed] [Google Scholar]
- Marini J. C., Lewis M. B., Wang Q., Chen K. J., Orrison B. M. Serine for glycine substitutions in type I collagen in two cases of type IV osteogenesis imperfecta (OI). Additional evidence for a regional model of OI pathophysiology. J Biol Chem. 1993 Feb 5;268(4):2667–2673. [PubMed] [Google Scholar]
- Mottes M., Gomez Lira M. M., Valli M., Scarano G., Lonardo F., Forlino A., Cetta G., Pignatti P. F. Paternal mosaicism for a COL1A1 dominant mutation (alpha 1 Ser-415) causes recurrent osteogenesis imperfecta. Hum Mutat. 1993;2(3):196–204. doi: 10.1002/humu.1380020308. [DOI] [PubMed] [Google Scholar]
- Nicholls A. C., Osse G., Schloon H. G., Lenard H. G., Deak S., Myers J. C., Prockop D. J., Weigel W. R., Fryer P., Pope F. M. The clinical features of homozygous alpha 2(I) collagen deficient osteogenesis imperfecta. J Med Genet. 1984 Aug;21(4):257–262. doi: 10.1136/jmg.21.4.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niyibizi C., Bonadio J., Byers P. H., Eyre D. R. Incorporation of type I collagen molecules that contain a mutant alpha 2(I) chain (Gly580-->Asp) into bone matrix in a lethal case of osteogenesis imperfecta. J Biol Chem. 1992 Nov 15;267(32):23108–23112. [PubMed] [Google Scholar]
- Pereira R., Khillan J. S., Helminen H. J., Hume E. L., Prockop D. J. Transgenic mice expressing a partially deleted gene for type I procollagen (COL1A1). A breeding line with a phenotype of spontaneous fractures and decreased bone collagen and mineral. J Clin Invest. 1993 Feb;91(2):709–716. doi: 10.1172/JCI116252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruchno C. J., Cohn D. H., Wallis G. A., Willing M. C., Starman B. J., Zhang X. M., Byers P. H. Osteogenesis imperfecta due to recurrent point mutations at CpG dinucleotides in the COL1A1 gene of type I collagen. Hum Genet. 1991 May;87(1):33–40. doi: 10.1007/BF01213088. [DOI] [PubMed] [Google Scholar]
- Raghunath M., Bruckner P., Steinmann B. Delayed triple helix formation of mutant collagen from patients with osteogenesis imperfecta. J Mol Biol. 1994 Feb 25;236(3):940–949. doi: 10.1006/jmbi.1994.1199. [DOI] [PubMed] [Google Scholar]
- Rao V. H., Steinmann B., de Wet W., Hollister D. W. Decreased thermal denaturation temperature of osteogenesis imperfecta mutant collagen is independent of post-translational overmodifications of lysine and hydroxylysine. J Biol Chem. 1989 Jan 25;264(3):1793–1798. [PubMed] [Google Scholar]
- Rose N. J., Mackay K., Byers P. H., Dalgleish R. A Gly859Ser substitution in the triple helical domain of the alpha 2 chain of type I collagen resulting in osteogenesis imperfecta type III in two unrelated individuals. Hum Mutat. 1994;3(4):391–394. doi: 10.1002/humu.1380030411. [DOI] [PubMed] [Google Scholar]
- Rose N. J., Mackay K., De Paepe A., Steinmann B., Punnett H. H., Dalgleish R. Three unrelated individuals with perinatally lethal osteogenesis imperfecta resulting from identical Gly502Ser substitutions in the alpha 2-chain of type I collagen. Hum Genet. 1994 Nov;94(5):497–503. doi: 10.1007/BF00211014. [DOI] [PubMed] [Google Scholar]
- Schnieke A., Harbers K., Jaenisch R. Embryonic lethal mutation in mice induced by retrovirus insertion into the alpha 1(I) collagen gene. 1983 Jul 28-Aug 3Nature. 304(5924):315–320. doi: 10.1038/304315a0. [DOI] [PubMed] [Google Scholar]
- Sharony R., Browne C., Lachman R. S., Rimoin D. L. Prenatal diagnosis of the skeletal dysplasias. Am J Obstet Gynecol. 1993 Sep;169(3):668–675. doi: 10.1016/0002-9378(93)90641-u. [DOI] [PubMed] [Google Scholar]
- Sillence D. O., Senn A., Danks D. M. Genetic heterogeneity in osteogenesis imperfecta. J Med Genet. 1979 Apr;16(2):101–116. doi: 10.1136/jmg.16.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacey A., Bateman J., Choi T., Mascara T., Cole W., Jaenisch R. Perinatal lethal osteogenesis imperfecta in transgenic mice bearing an engineered mutant pro-alpha 1(I) collagen gene. Nature. 1988 Mar 10;332(6160):131–136. doi: 10.1038/332131a0. [DOI] [PubMed] [Google Scholar]
- Stöss H., Freisinger P. Collagen fibrils of osteoid in osteogenesis imperfecta: morphometrical analysis of the fibril diameter. Am J Med Genet. 1993 Jan 15;45(2):257–257. doi: 10.1002/ajmg.1320450220. [DOI] [PubMed] [Google Scholar]
- Sztrolovics R., Glorieux F. H., Travers R., van der Rest M., Roughley P. J. Osteogenesis imperfecta: comparison of molecular defects with bone histological changes. Bone. 1994 May-Jun;15(3):321–328. doi: 10.1016/8756-3282(94)90295-x. [DOI] [PubMed] [Google Scholar]
- Tromp G., Prockop D. J. Single base mutation in the pro alpha 2(I) collagen gene that causes efficient splicing of RNA from exon 27 to exon 29 and synthesis of a shortened but in-frame pro alpha 2(I) chain. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5254–5258. doi: 10.1073/pnas.85.14.5254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter U., Eanes E. D., Kopp J. B., Termine J. D., Robey P. G. Changes in apatite crystal size in bones of patients with osteogenesis imperfecta. Calcif Tissue Int. 1991 Oct;49(4):248–250. doi: 10.1007/BF02556213. [DOI] [PubMed] [Google Scholar]
- Vogel B. E., Doelz R., Kadler K. E., Hojima Y., Engel J., Prockop D. J. A substitution of cysteine for glycine 748 of the alpha 1 chain produces a kink at this site in the procollagen I molecule and an altered N-proteinase cleavage site over 225 nm away. J Biol Chem. 1988 Dec 15;263(35):19249–19255. [PubMed] [Google Scholar]
- Wallis G. A., Kadler K. E., Starman B. J., Byers P. H. A tripeptide deletion in the triple-helical domain of the pro alpha 1(I) chain of type I procollagen in a patient with lethal osteogenesis imperfecta does not alter cleavage of the molecule by N-proteinase. J Biol Chem. 1992 Dec 15;267(35):25529–25534. [PubMed] [Google Scholar]
- Wallis G. A., Starman B. J., Zinn A. B., Byers P. H. Variable expression of osteogenesis imperfecta in a nuclear family is explained by somatic mosaicism for a lethal point mutation in the alpha 1(I) gene (COL1A1) of type I collagen in a parent. Am J Hum Genet. 1990 Jun;46(6):1034–1040. [PMC free article] [PubMed] [Google Scholar]
- Westerhausen A., Kishi J., Prockop D. J. Mutations that substitute serine for glycine alpha 1-598 and glycine alpha 1-631 in type I procollagen. The effects on thermal unfolding of the triple helix are position-specific and demonstrate that the protein unfolds through a series of cooperative blocks. J Biol Chem. 1990 Aug 15;265(23):13995–14000. [PubMed] [Google Scholar]
- Williams E. M., Nicholls A. C., Daw S. C., Mitchell N., Levin L. S., Green B., MacKenzie J., Evans D. R., Chudleigh P. A., Pope F. M. Phenotypical features of an unique Irish family with severe autosomal recessive osteogenesis imperfecta. Clin Genet. 1989 Mar;35(3):181–190. doi: 10.1111/j.1399-0004.1989.tb02926.x. [DOI] [PubMed] [Google Scholar]
- Willing M. C., Cohn D. H., Byers P. H. Frameshift mutation near the 3' end of the COL1A1 gene of type I collagen predicts an elongated Pro alpha 1(I) chain and results in osteogenesis imperfecta type I. J Clin Invest. 1990 Jan;85(1):282–290. doi: 10.1172/JCI114424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willing M. C., Cohn D. H., Starman B., Holbrook K. A., Greenberg C. R., Byers P. H. Heterozygosity for a large deletion in the alpha 2(I) collagen gene has a dramatic effect on type I collagen secretion and produces perinatal lethal osteogenesis imperfecta. J Biol Chem. 1988 Jun 15;263(17):8398–8404. [PubMed] [Google Scholar]