Abstract
In 2017, the Brazilian State of Amapá registered the first occurrence of visceral leishmaniosis (VL) in 17 dogs in the outskirts of the capital, Macapá. Given the lack of knowledge on phlebotomines in that area, this study aimed to survey the fauna of these Diptera. Sampling was performed using CDC light traps placed at ten sampling sites. The specimens captured were Evandromyia walkeri (n=237), Nyssomyia antunesi (n=65) and Bichromomyia flaviscutellata (n=6). The phlebotomine species composition resulted in low species diversity, and none of the main vectors of the etiological agent of VL were identified in the study area.
Keywords: Sand fly, leishmaniasis, diversity, Amazon
Resumo
Em 2017, o estado do Amapá registrou a primeira ocorrência de Leishmaniose Visceral (LV) em 17 cães, na periferia da capital, Macapá. Tendo em vista a falta de conhecimento sobre flebotomíneos nessa área, este trabalho teve como objetivo fazer um levantamento da fauna desses dípteros. A amostragem foi realizada usando-se armadilhas de luz CDC, colocadas em dez locais de amostragem. Os espécimes capturados foram Evandromyia walkeri (n=237), Nyssomyia antunesi (n=65) e Bichromomyia flaviscutellata (n=6). A composição de espécies de flebotomíneos resultou em baixa diversidade de espécies, e nenhum dos principais vetores do agente da LV foi identificado na área de estudo.
Palavras-chave: Flebótomos, leishmaniose, diversidade, Amazônia
Phlebotomine sand flies (Diptera: Psychodidae) are important vectors of human pathogens that have a great impact on public health, of which Leishmaniasis caused by Leishmania species is the most important (Alvar et al., 2012). Leishmaniasis is a neglected tropical disease caused by the digenetic protozoan parasite of the genus Leishmania. Leishmania parasites cause a wide spectrum of clinical manifestations, which includes cutaneous (CL), mucosal (ML) and visceral leishmaniasis (VL) (OPS, 2019).
At least 20 Leishmania species are known to cause human disease, transmitted by different phlebotomine sand fly species (Cecílio et al., 2022). In Brazil, the mainly etiological agent of VL is Leishmania infantum, where transmission occurs through the bite of the infected phlebotomine sand fly species Lutzomyia longipalpis, while CL has different vectors and etiologic agents, the principal vectors being Nyssomyia whitmani, Nyssomyia intermedia, Nyssomyia neivai, Migonemyia migonei and Psychodopygus wellcomei (OPS, 2019; Lainson & Rangel, 2005).
The first case of VL in a domestic dog in the Macapá municipality was reported in 2017 (Brasil, 2018). Since then, VL has been detected in dogs in Macapá and Mazagão. Despite the occurrence of canine cases and the possible establishment of an urban VL transmission cycle, there is a lack of knowledge regarding sand fly vectors. Studies on sand fly fauna and their distribution in the Macapá municipality are lacking with only one published report available so far on the sand fly fauna in that region (Cavalcante et al., 2021). The purpose of this study was to investigate the phlebotomine sand fly fauna in areas of canine infection caused by L. infantum in Macapá municipality of the state of Amapá, Brazil.
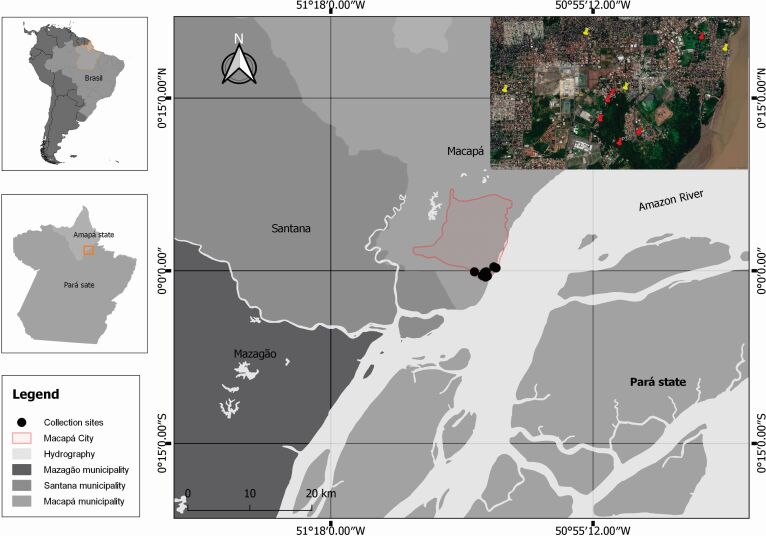
This study was conducted in an urban area of Macapá, Amapá, Brazil (0° 2' 4” N, 51° 3' 60” W). The municipality has an estimated population of 474, 706 inhabitants. According to the Köppen climate classification, the region’s climate is equatorial, hot, and humid, with two distinct seasons: rainy season from December to July and dry season from August to November. The vegetation of this area comprises the Cerrado and lowland forests (Brasil, 2008). It is worth emphasizing that these environments are undergoing a rapid and disorderly process of urban occupation.
Sampling locations were selected based on presumed sites of Canine Visceral Leishmaniasis (CVL) infection (Brasil, 2018). Thus, ten residences were selected in the Araxá, Zerão, Pedrinhas, and Jardim Marco Zero neighborhoods (Figure 1). Entomological captures were performed on three consecutive nights each month, from December 2017 to November 2018, corresponding to 4,320 trap-nights in the study period. CDC light traps were placed at 6:00 p.m. and removed at 6:00 a.m. in the following day (from dusk to dawn). Traps were placed 1.5 m above the ground level in a peridomicile environment, preferably near shelters for domestic animals. After triaging, the sand flies were stored in 70% alcohol, diaphanized with 10% potassium hydroxide (KOH) solution, and clarified with lactophenol. Finally, the specimens were mounted on slides with coverslips using a drop of Berlese. The taxonomic criteria and nomenclature were followed based on the description by Galati (2018).
Figure 1. Sampling sites where phlebotomines were captured with CDC light traps in the urban area of Macapá, Amapá, Brazil. Red pins show phlebotomine-positive locations. Source: Google Earth. Nov/2018.
In total, 308 sand fly specimens were captured, of which 127 (41.2%) were females and 181 (58.8%) males. The insects belonged to three species. Identification of species revealed Nyssomyia antunesi, Bichromomyia flaviscutellata and Evandromyia walkeri (Table 1).
Table 1. Abundance of sand fly species captured in the Municipality of Macapá, Amapá, Brazil, from December 2017 to November 2018.
| Species | |||||
|---|---|---|---|---|---|
| Month | Nyssomyia antunesi N (♀/♂) | Bichromomyia flaviscutellata N (♀/♂) | Evandromyia walkeri N (♀/♂) | Total N (♀/♂) | % |
| Dec/17 | 6 (3/3) | 1 (0/1) | 18 (11/7) | 25 (14/11) | 8.12 |
| Jan/18 | 5 (3/2) | 3 (3/0) | 15 (8/7) | 23 (14/9) | 7.47 |
| Feb/18 | 35 (21/14) | 0 | 139 (41/98) | 174 (62/112) | 56.49 |
| Mar/18 | 4 (2/2) | 0 | 2 (0/2) | 6 (2/4) | 1.95 |
| Apr/18 | 0 | 2 (0/2) | 1 (0/1) | 3 (0/3) | 1.00 |
| May/18 | 5 (4/1) | 0 | 9 (6/3) | 14 (10/4) | 4.55 |
| Jun/18 | 0 | 0 | 0 | 0 | 0.00 |
| Jul/18 | 0 | 0 | 1(1/0 | 1 (1/0) | 0.32 |
| Aug/18 | 3 (3/0) | 0 | 1(1/0) | 4 (4/0) | 1.00 |
| Sep/18 | 6 (5/1) | 0 | 17(6/11) | 23 (11/12) | 7.47 |
| Oct/18 | 1 (1/0) | 0 | 3(0/3) | 4 (1/3) | 1.30 |
| Nov/18 | 0 | 0 | 31(8/23) | 31(8/23) | 10.10 |
| Total | 65 (42/23) | 6 (3/3) | 237 (82/155) | 308 (127/181) | 100 |
| % | 21.10 | 1.95 | 76.95 | 100 |
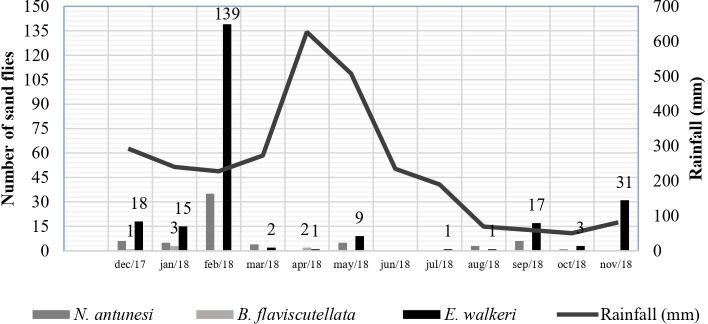
Among the ten capture sites, six (P2, P3, P4, P5, P6, and P9) were positive for phlebotomines (Figure 1), corresponding to the neighborhoods Marco Zero and Pedrinhas. The abundance and temporal fluctuations of sand flies can vary greatly from region to region (Guimarães et al., 2012). Vector distribution depends on environmental and biological factors like availability of hosts and weather conditions. In this study, the largest number of specimens was captured during the period of greatest precipitation, from December 2017 to July 2018 (245/308), corresponding to 80% of captures (Figure 2). Insect peaks were observed in the rainy seasons and in the months with less rain, such as November. In months with more cumulated rainfall, such as March, April and May, low densities of Evandromyia walkeri were found (Figure 2). On the other hand, February with the lowest accumulated precipitation represented the month with the highest density of this species together with N. antunesi. Thus, in this study, the highest number of specimens occurred in February corresponding to 56% (174/308) of the total number of sand flies captured in the state of Amapá, followed by November with 10% (31/308). None of the specimens were captured in June.
Figure 2. Number of captured sandflies accordingly to rainfall in Macapá, State of Amapá, from December 2017 to November 2018.
No association between rainfall and the abundance of sand flies was observed. These findings may be a result of the high rainfall fluctuations and difficulties in finding trends as there was no pattern. Although these meteorological variables may be related as predictor variables, they are not the only ones. Other variables may be determining factors in the densities of the sand flies in the area, such as microclimate, terrain, fauna, and vegetation.
Only one study has been conducted on the sand fly fauna in Macapá (Cavalcante et al., 2021). Our results corroborated with the same, in which there was little diversity of captured species and the largest number of specimens was captured during the period of greatest precipitation, with the males being more abundant than female specimens (Table 1). In this study, only three species were identified over the sampled year, indicating that species diversity was low, even though 77 phlebotomine species are known to occur in the State of Amapá (Galati, 2018).
The most abundant species found in this study was E. walkeri, contributing to 77% of the captured sand flies, mainly in the month of February in the rainy season. This species is widely distributed in both Central and South America and is often found in chicken coops (Young & Duncan, 1994). Although the epidemiological importance of E. walker is unknown, the species was recently found to be naturally infected with L. braziliensis DNA in Amazon region (De Ávila et al., 2018). The second-most abundant species, N. antunesi (21%) has tested positive for Leishmania DNA, suggesting its potential role as vector of Leishmania species (Pereira et al., 2019; Costa et al., 2021; Pimentel et al., 2022). In Central-Western Brazil, the pooled N. antunesi DNA sample was also found to be positive for L. chagasi (Thies et al., 2013). These facts emphasize the need for further investigation of this species in the context of local VL epidemiology. Bichromomyia flaviscutellata had the smallest number of captured specimens, which only occurred during the rainy season. It is rarely found in CDC light traps, as this species is considered highly zoophilic and predominates in forest areas, where it transmits Leishmania amazonensis at the soil level among rodents and marsupials (Ready et al., 1983). According to Rangel et al. (2018), B. flaviscutellata is a secondary vector of L. infantum among foxes, given that these canids are frequently found infected with L. amazonensis.
Although L. longipalpis was not found at the sampled sites in the present study, it was registered in the State of Amapá in 2013 in the municipality of Ferreira Gomes, 137 km from the state capital (Galardo et al., 2013). Therefore, this species may have been present in very small numbers, considering that the sampling effort undertaken in this study was unable to detect its presence. Another hypothesis is that the transmission of the VL agent involves alternative vectors, such as N. antunesi and/or B. flaviscutellata which, according to literature studies, has already been associated with L. chagasi life cycle under different circumstances.
Taken together, the phlebotomine entomofauna survey revealed low species diversity, along with the absence of the classical vector of L. chagasi the etiological agent of CVL in the study area. The registration of sand fly species as potential vectors of Leishmania in the Amazon region, such as B. flaviscutelata and E. walkeri, becomes a relevant factor in the transmission of Leishmania parasites in anthropic environments. With this, further efforts are required for better assessment of the possible epidemiological importance of the species captured in the surveyed area, with the main focus on Leishmania spp. detection.
Acknowledgements
The authors would like to thank the staff of the Entomology Center of the Municipal Health Secretary of the Municipality of Macapá for their help with the fieldwork. We thank Thiago Vasconcelos dos Santos for revising the manuscript.
Funding Statement
Financial support: This study was funded by a grant (no. 041/2018) awarded to L. A. Viana by the Amapá Research Support Foundation (Fundação de Amparo à Pesquisa do Amapá, FAPEAP).
Footnotes
How to cite: Pessoa LMB, Pinto EHC, Chaves TES, Rabelo GS, Brito AL, Zanini VM, et al. Phlebotomine sand flies (Psychodidae: Phlebotominae) in an area of canine infection caused by Leishmania infantum in the state of Amapá, eastern Amazon. Braz J Vet Parasitol 2023; 32(3): e002423. https://doi.org/10.1590/S1984-29612023054
Financial support: This study was funded by a grant (no. 041/2018) awarded to L. A. Viana by the Amapá Research Support Foundation (Fundação de Amparo à Pesquisa do Amapá, FAPEAP).
References
- Alvar J, Vélez ID, Bern C, Herrero M, Desjeux P, Cano J, et al. Leishmaniasis worldwide and global estimates of its incidence. PLoS One. 2012;7(5):e35671. doi: 10.1371/journal.pone.0035671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasil Governo alerta municípios para reforço na prevenção da Leishmaniose. 2018. [cited 2018 Nov 5]. Governo do Estado do Amapá (GEA) online. Available from: https://www.eap.ap.gov.br/noticia/1105/governo-alerta-municipios-para-reforco-na-prevencao-da-leishmaniose .
- Brasil . Macrodiagnóstico do Estado do Amapá: primeira aproximação do ZEE. 3rd. Macapá, Amapá: IEPA; 2008. Instituto de Pesquisas Científicas e Tecnológicas do Estado do Amapá (IEPA) [Google Scholar]
- Cavalcante KS, Júnior JR, Gama GS, Santos WM, Galardo AKR. Flebotomíneos (Diptera: Psychodidae) em uma área periurbana na cidade de Macapá, Amapá, Brasil. Braz J Anim Environ Re. 2021;4(2):2417–2426. doi: 10.34188/bjaerv4n2-070. [DOI] [Google Scholar]
- Cecílio P, Cordeiro-da-Silva A, Oliveira F. Sand flies: basic information on the vectors of leishmaniasis and their interactions with Leishmania parasites. Commun Biol. 2022;5(1):305. doi: 10.1038/s42003-022-03240-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa GS, Pereira AMP, Jr, Castro TS, Paulo PFM, Ferreira GEM, Medeiros JF. Sand fly fauna and molecular detection of Leishmania species and blood meal sources in different rural environments in western Amazon. Acta Trop. 2021;224:106150. doi: 10.1016/j.actatropica.2021.106150. [DOI] [PubMed] [Google Scholar]
- De Ávila MM, Brilhante AF, Souza CF, Bevilacqua PD, Galati EAB, Brazil RP. Ecology, feeding and natural infection by Leishmania spp. of phlebotomine sand flies in an area of high incidence of American tegumentary leishmaniasis in the municipality of Rio Branco, Acre, Brazil. Parasit Vectors. 2018;11(1):64. doi: 10.1186/s13071-018-2641-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galardo AKR, Galardo CD, Santana AA, Mendes JCC, Souza FRA, Duarte JP, et al. Primeira ocorrência de Lutzomyia (Lutzomyia) longipalpis Lutz & Neiva, 1912 (Diptera: Psychodidae: Phlebotominae) no Estado do Amapá, Brasil. Biota. 2013;3(2):179–183. doi: 10.18561/2179-5746/biotaamazonia.v3n2p179-183. [DOI] [Google Scholar]
- Galati EAB. In: Brazilian sand flies: biology, taxonomy, medical importance and control. Rangel EF, Shaw JJ, editors. Springer; Cham: 2018. Phlebotominae (Diptera, Psychodidae): classification, morphology, and terminology of adults and identification of American taxa. pp. 9–212. [DOI] [Google Scholar]
- Guimarães VCFV, Costa PL, Silva FJ, Silva KT, Silva KG, Araújo AIF, et al. Phlebotomine sandflies (Diptera: Psychodidae) in São Vicente Férrer, a sympatric area to cutaneous and visceral leishmaniasis in the State of Pernambuco, Brazil. Rev Soc Bras Med Trop. 2012;45(1):66–70. doi: 10.1590/S0037-86822012000100013. [DOI] [PubMed] [Google Scholar]
- Lainson R, Rangel EF. Lutzomyia longipalpis and the eco-epidemiology of American visceral leishmaniasis, with particular reference to Brazil: a review. Mem Inst Oswaldo Cruz. 2005;100(8):811–827. doi: 10.1590/S0074-02762005000800001. [DOI] [PubMed] [Google Scholar]
- OPS . Manual de procedimientos para vigilancia y control de las leishmaniasis en las Américas. Washington, DC: OPS; 2019. Organización Panamericana de la Salud. [Google Scholar]
- Pereira AM, Jr, Souza ABN, Castro TS, Silva MS, Paulo PFM, Ferreira GEM, et al. Diversity, natural infections, and blood meal sources of phlebotomine sand flies (Diptera, Psychodidae) in the western Brazilian Amazon. Mem Inst Oswaldo Cruz. 2019;114:e190170. doi: 10.1590/0074-02760190170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimentel AC, Uzcátegui YVS, Lima ACS, Silveira FT, Santos TV, Ishikawa EAY. Blood feeding sources of Nyssomyia antunesi (Diptera: Psychodidae): a suspected vector of Leishmania (Kinetoplastida: Trypanosomatidae) in the Brazilian Amazon. J Med Entomol. 2022;59(5):1847–1852. doi: 10.1093/jme/tjac108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangel EF, Lainson R, Carvalho BM, Costa SM, Shaw JJ. In: Brazilian sand flies: biology, taxonomy, medical importance and control. Rangel EF, Shaw JJ, editors. Springer; Cham: 2018. Sandfly vectors of American Cutaneous Leishmaniasis in Brazil. pp. 341–380. [DOI] [Google Scholar]
- Ready PD, Lainson R, Shaw JJ. Leishmaniasis in Brazil: XX. Prevalence of “enzootic rodent leishmaniasis” (Leishmania mexicana amazonensis), and apparent absence of “pian bois” (Le. braziliensis guyanensis), in plantations of introduced tree species and in other non-climax forests in eastern Amazônia. Trans R Soc Trop Med Hyg. 1983;77(6):775–785. doi: 10.1016/0035-9203(83)90288-2. [DOI] [PubMed] [Google Scholar]
- Thies SF, Ribeiro ALM, Michalsky EM, Miyazaki RD, Fortes-Dias CL, Fontes CJF, et al. Phlebotomine sandfly fauna and natural Leishmania infection rates in a rural area of the Cerrado (tropical savannah) in Nova Mutum, State of Mato Grosso in Brazil. Rev Soc Bras Med Trop. 2013;46(3):293–298. doi: 10.1590/0037-8682-0031-2013. [DOI] [PubMed] [Google Scholar]
- Young DG, Duncan MA. Guide to the identification and geographic distribution of Lutzomyia sand flies in Mexico, the West Indies, Central and South America (Diptera: Psychodidae). 54th. Gainesville: Associated Publishers; 1994. [DOI] [Google Scholar]