Abstract
THOC6 is the genetic basis of autosomal recessive THOC6 Intellectual Disability Syndrome (TIDS). THOC6 facilitates the formation of the Transcription Export complex (TREX) tetramer, composed of four THO monomers. The TREX tetramer supports mammalian mRNA processing that is distinct from yeast TREX dimer functions. Human and mouse TIDS model systems allow novel THOC6-dependent TREX tetramer functions to be investigated. Biallelic loss-of-functon(LOF) THOC6 variants do not influence the expression and localization of TREX members in human cells, but our data suggests reduced binding affinity of ALYREF. Impairment of TREX nuclear export functions were not detected in cells with biallelic THOC6 LOF. Instead, mRNA mis-splicing was observed in human and mouse neural tissue, revealing novel insights into THOC6-mediated TREX coordination of mRNA processing. We demonstrate that THOC6 is required for regulation of key signaling pathways in human corticogenesis that dictate the transition from proliferative to neurogenic divisions that may inform TIDS neuropathology.
INTRODUCTION
Intellectual disability (ID) is a clinical feature of neurodevelopmental disorders characterized by limitations in cognitive ability and adaptive behavior1. In recent years, genetic etiologies of syndromic ID have become increasingly heterogeneous due to broad use of exome-based genetic testing2–6. Monogenetic etiologies are an important basis of syndromic ID, with many following an autosomal-recessive mode of inheritance7. THOC6 Intellectual Disability Syndrome (TIDS; OMIM# 613680) is one such recessive ID due to biallelic THOC6 variants8–18. Individuals with TIDS exhibit moderate to severe syndromic ID with microcephaly, d evelopmental delay, and multi-organ involvement19.
THOC6 is a subunit of the six member THO (suppressors of the transcriptional defects of hpr1 delta by overexpression) complex, which serves as a core component of the transcription/export (TREX) complex20. TREX is critical for licensing export factors required for nuclear pore docking and export of mRNA from the nucleus to the cytoplasm21–24. While this is a conserved function of TREX, there are notable species differences in TREX composition that mirror the evolutionary complexity of mRNA processing requirements. In yeast, TREX is a dimer composed of two five-subunit THO monomers, with THOC6 being the notable exception. Yeast TREX monomers dimerize via the coiled coil domains of Thp2 and Mft1, the yeast orthologs of THOC5 and THOC725. In humans, TREX is a tetramer, where dimers of THO monomers are tethered by THOC625. The increased size and molecular complexity of the mammalian TREX tetramer correlates with increased mRNA processing demands that have evolved in organisms with higher transcriptome complexity and mRNP composition, namely expression of long genes with high levels of complex splicing patterns26. For example, introns comprise ~24% of mammalian genomes27. On the level of gene organization, introns are longer and are present in >95% of all human genes28–30. By contrast, introns constitute only 5% of yeast genes, are short relative to the gene length, and mostly limited to one per gene29–32. Thus, alternative splicing is rare in yeast, but plays a major role in gene expression in mammals, especially humans.
The conservation of THO dimer functions in mammalian cells is an open question. THOC1, THOC3, THOC5, and THOC7 exhibit high probability of loss-of-function intolerance (pLI) in gnomAD and have not been identified as the genetic basis of developmental disorders, suggesting conserved THO components are likely embryonic lethal. THOC2 is the genetic basis of an X-linked neurodevelopmental disorder33. Depletion of THO components THOC1-THOC5 and THOC7 lead to strong nuclear export defects34. This leads to the speculation that dimers retain mRNP functions in mammals, and that THOC6-dependent tetramer functions enhance the efficiency and coordination of these activities. This would also explain the tissue sensitivity in TIDS, where development is disrupted in tissues that disproportionately express long genes and exhibit elevated isoform diversity.
Unlike the dimer, the TREX tetramer recruits functionally diverse auxiliary factors, that have no yeast orthologs. DDX39A, CHTOP, UIF, LUZP4, POLDIP3, ZC3H11A, ERH, ZC3H18, SRRT, and NCBP3, complex with tetramer TREX and participate in the step of mRNP processing and export, including mRNA 5’ capping, splicing, and 3’ end processing to create a messenger ribonucleoprotein complex (mRNP) capable of translocating through the nuclear pore complex into the cytoplasm22,35,36. Increased TREX complex complexity may represent greater mRNA processing functionality as the complexity of mRNP processing evolved36,37. Likewise, the larger complex would allow TREX to serve as a mRNA chaperone to prevent formation of DNA-RNA hybrid or R-loop structures, with the emergence of longer transcripts with elevated splicing that can promote genome instability38,39.
THOC6 evolved as a scaffolding protein to create a larger TREX complex, that has implication for mRNA processing coordination in metazoans relative to yeast. Despite the neurological features of TIDS, there is a lack of research in neural cells, cells that undergo extensive changes in mRNA processing during differentiation40. Using a series of THOC6 models with clinically relevant pathogenic alleles, we assess essential functions of TREX tetramers functions in mRNA processing in neural development. This experimental approach will also allow THOC6 dependent tetramer function to be evaluated relative to dimer functions. First, we contribute to the THOC6 pathogenic allele series and TIDS clinical phenotypic spectrum, extending the total number of reported THOC6 variants in TIDS to 20 and the total number of reported affected individuals to 34. Second, we generated a Thoc6 mouse model and human induced pluripotent stem cell (iPSC)-derived cell culture models to investigate shared pathogenic mechanisms of mammalian THOC6. Given the high penetrance of microcephaly in TIDS, we focus on mRNA processing/export changes and accompanying phenotypes that occur during cortical development by analyzing primary mouse and dorsal forebrain fated human organoids. We propose a model of unproductive selective mRNA processing/export from partial TREX disruption due to loss-of-function THOC6 alleles leading to dysregulation of proliferation and differentiation. Our findings reveal a broader supportive role across mRNA processing within the context of THOC6 variants than has previously been attributed to THO.
RESULTS
Biallelic missense and nonsense THOC6 variants are the genetic basis of TIDS
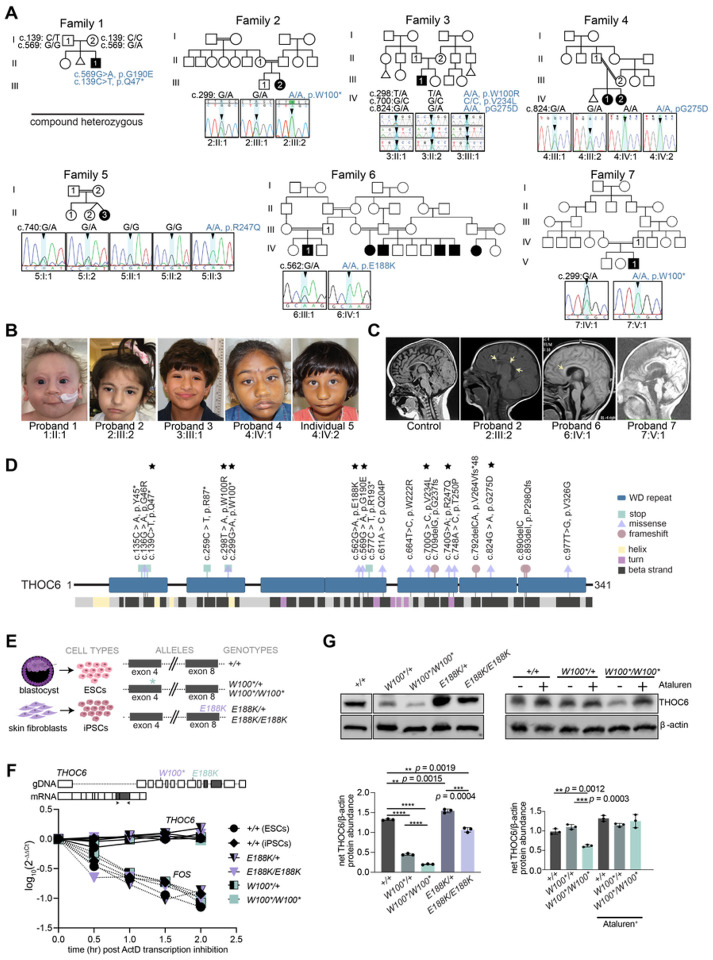
While initially detected in Hutterite populations11, a growing number of pathogenic biallelic THOC6 variants are being discovered across the globe in individuals of diverse ancestry8–10,12–18,41. As part of an ongoing effort to determine the genetic etiologies of syndromic ID, we discovered nine THOC6 variants by exome-based genetic testing (Figure 1A). We confirmed six recurrent variants at W100, G190, V234, and G275 amino acids and identified three novel alleles (p.Q47*, p.E188K, and p.R247Q). The clinical phenotype associated with THOC6 variants affirm penetrance of the core clinical features of TIDS, namely global developmental delay, moderate to severe ID and facial dysmorphisms (Figure 1B). Variable expressivity of cardiac and renal malformations, structural brain abnormalities with and without seizures, urogenital defects, recurrent infections, and feeding complications were also noted, clinical features that highlight the multiorgan involvement of this developmental syndrome (Figure 1C). Detailed clinical summaries for all individuals are provided in Table S1 and S2.
Figure 1. Biallelic pathogenic variants in THOC6 cause syndromic intellectual disability.
(A) Pedigree drawings of segregating TIDS phenotypes in families 1–7, with generations listed on the left-hand side. Females are represented as circles and males are denoted by squares. Miscarriages are denoted by small triangles. Affected family members are indicated by solid black coloring while unaffected are unfilled. Consanguineous partnerships are represented by double lines. Chromatograms from Sanger sequencing of THOC6 confirmation of genotypes are provided for each tested family member in families 1–7. (B) Facial photographs of Probands 1–4 and Individual 5. (C) Sagittal brain MRI showing corpus callosum dysgenesis (Probands 2, 6, & 7) and cortical and cerebellar atrophy (Proband 6) compared to control (left). (D) Canonical THOC6 protein map consisting of 341 amino acids. WD40 repeat domains 1–7 are denoted by purple rectangles. Location of known pathogenic variants are annotated relative to linear protein map (top) and secondary structure (below). Variants reported in present study are distinguished by a black star. Missense (blue triangle), nonsense (red square), and frameshift (green circle). (E) Schematic of patient and control-derived human cell types and respective genotypes. (F) Decay of THOC6 mRNA (solid line) following ActD transcriptional inhibition compared to FOS mRNA decay (dotted line) in human ESC/iPSCs across genotypes. Values calculated relative to GAPDH reference mRNA. (G) Western blot of human ESC/iPSCs indicating reduced THOC6 protein expression in THOC6W100*/W100* iPSCs compared to unaffected controls. Confirmation of readthrough by ataluren treatment (30 mM). Abundance quantifications relative to b-actin control (right). Data represented as mean ±SEM. P-value, two-tailed unpaired t test. ****, p-value <0.0001.
The novel THOC6 variants are representative of previously described nonsense and missense variants that contribute equally to the severity of TIDS phenotypes. We describe a nonsense THOC6 c.139C>T, (p.Q47*) variant in exon 2 of proband 1, a missense c.740G>A, (p.R247Q) variant in exon 11 of proband 5, and a missense c.562G>A, (p.E188K) variant in proband 6 (Figures 1A and 1C). THOC6 is comprised of seven WD40 repeat domains (Figure 1D) that form a β-propeller structure when folded. These novel variants, like other clinically relevant THOC6 variants, map to the WD40 repeats that comprise the beta strand structural regions of THOC6 (Figure 1D). The p.Q47* nonsense variant represents a cluster of three THOC6 variants in the first WD40 repeat that exhibit a consistent genotype-phenotype correlation for both recessive nonsense and missense variants. The same trend is observed for the novel missense variants that are localized to subsequent THOC6 WD40 domains, which support a loss-of-function (LOF) pathogenic mechanism for both THOC6 variant types.
A LOF mechanism is also implicated by the clinical consistency observed between biallelic inheritance of pathogenic THOC6 haplotypes and biallelic inheritance of a single haplotype variant alone. Biallelic inheritance of a triple-variant haplotype (TVH), THOC6 c.[298T>A;700G>C;824G>A], (p. [W100R;V234L;G275D]) has been reported in seven individuals with clinical features of TIDS9,13,17,42. The TVH segregates as a founder haplotype in individuals of European ancestry42. In comparison, a homozygous THOC6 c.824G>A; p.G275D variant was identified in siblings with classic TIDS in family 4 who are of South Asian ancestry. This finding provides additional evidence for the pathogenicity of the TVH THOC6 c.824G>A; p.G275D variant but does not negate the predicted pathogenicity of the corresponding W100R or V234L TVH variants. Pathogenicity of p.W100R or p.V234L are supported by variants detected in WD40 repeats 2 and 4 (Figure 1D). Comparing biallelic inheritance of TVH and THOC6 c.824G>A; p.G275D suggests a single THOC6 variant in both alleles are sufficient to comprehensively disrupt THOC6, a baseline deficiency not exacerbated by accumulation of additional LOF variants.
THOC6 variants show mRNA stability with differential effect on protein abundance
To investigate the genetic mechanism of THOC6, the impact of on mRNA nonsense mediated decay (NMD) and protein expression on pathogenicity was tested in embryonic stem cells (ESCs) and iPSCs, collectively referred to as human pluripotent stem cells (hPSCs). hPSCs were reprogrammed from two individuals with TIDS (6:IV:1, THOC6E188K/E188K and 7:V:2, THOC6W100*/W100*) and their respective unaffected heterozygous parent (6:IV:2, THOC6E188K/+ and 7:V:1, THOC6 W100*/+) (Figure 1E), preserving the shared genetic background between affected and unaffected conditions. Consistent with a LOF mechanism, reduction in protein expression due to mRNA nonsense mediated decay was predicted. However, THOC6 mRNA transcripts remain relatively stable between genotypes, as assessed by Actinomycin D treatment where transcription is inhibited, compared to the unstable mRNA, FOS, that is quickly degraded (Figure 1F)43. This finding is consistent regardless of THOC6 variant, with the c.299G>A, (p.W100*) and c.562G>A, (p.E188K) variants exhibiting similar decay rates as wildtype transcripts (Figure S1C and S1D). This finding could reflect defective NMD from failure of THOC6-affected mRNA to be exported to the cytoplasm. Nevertheless, the impact on protein expression is divergent between nonsense and missense variants. Significant reductions in THOC6 abundance were detected in THOC6W100*/W100* and THOC6E188K/E188K iPSCs relative to the THOC6+/+ control, with the most remarkable reduction for THOC6W100*/W100*. Significant abundance differences were also noted for heterozygous unaffected iPSCs relative to wildtype controls (Figure 1G). Full-length THOC6 in THOC6W100*/W100* samples represent a minority readthrough product. Increased frequency of this rare event was promoted by treatment with 30 mM Ataluren, which extends translation by skipping premature termination codons, leading to an increase in THOC6 detectable by Western blot in THOC6W100*/W100* iPSCs (Figure 1G). No truncated product was observed by Western blotting in THOC6W100*/W100* and THOC6W100*/+ iPSCs, suggesting THOC6 reduction is due to rapid degradation of an unstable, truncated protein. Stable expression from missense THOC6 alleles suggests variant THOC6 is likely functionally inactive.
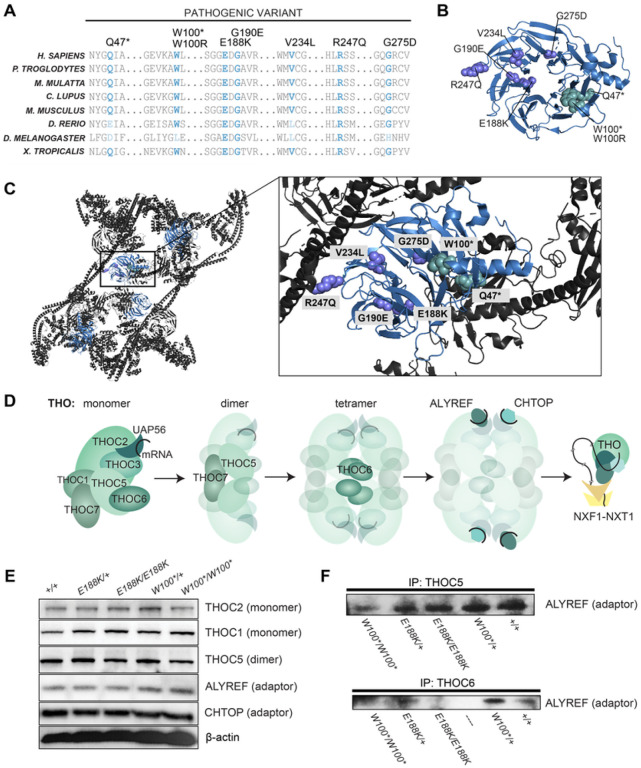
THOC6 variants interfere with TREX functions
Based on the solved crystal structure25, LOF THOC6 variants are predicted to impair TREX tetramer formation. The WD40 repeat domains of THOC6 form beta strands predicted to provide the structural interface for TREX core tetramer formation. Pathogenic THOC6 variants disrupt residues conserved in mammals, though several are not conserved across other metazoan species, mirroring TREX composition variability across species and the evolving function for THOC6 in TREX (Figure 2A). Evaluation of the TREX crystal structure indicates that pathogenic THOC6 variants are positioned at the TREX core tetrameric interface, where THOC5-THOC7 interaction is responsible for dimerization and THOC6-THOC5 interaction tetramerizes the complex (Figure 2B, 2C, and 2D). The allelic series of THOC6 LOF variants implicate a pathogenic mechanism where LOF missense variants are predicted to perturb THOC6 β-propeller folding and/or interactions with THOC5 and THOC7 that are required for TREX tetramer assembly, and nonsense variants would produce a similar outcome due to low protein abundance25. Likewise, THOC6 variants do not alter the protein abundance of other THO/TREX members (Figure 2E). Consistent with normal abundance of functional THO protein, subcellular localizations at nuclear speckle domain were observed by immunohistochemistry (Figures S1E and S1F). Conversely, ALYREF association with the THO subcomplex is diminished in THOC6E188K/E188K and THOC6W100*/W100* as assessed by co-immunoprecipitation with THOC5 and THOC6 in patient-derived iPSC lines (Figure 2F). These findings suggest a THOC6-dependent association of ALYREF to THO, with implications for the affinity of other adaptors due to the potential disruption of TREX tetramer formation.
Figure 2. Genetic mechanism of biallelic pathogenic THOC6 variants.
(A) Amino acid alignment showing conservation of affected residues for pathogenic variants in present clinical study. Variants mapped to THOC6 folded β-propeller structure (B) and THO/TREX complex (C). (D) Schematic of TREX core tetrameric assembly mediated by THOC6 with functional implications for mRNA processing and export based on published crystal structure25. (E) Steady-state protein abundance for THO/TREX complex members and (F) ALYREF abundance following co-immunoprecipitation with THOC5 and THOC6 across genotypes.
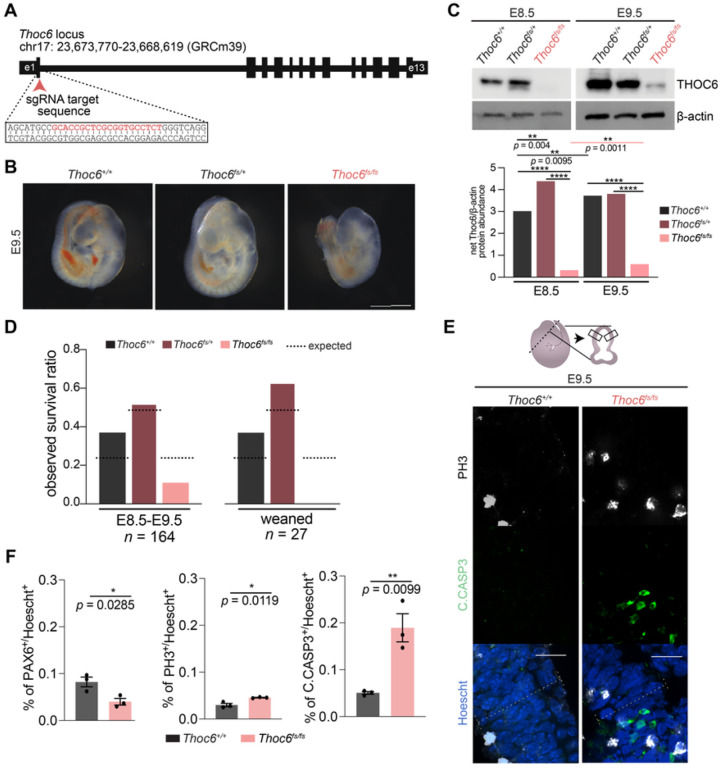
Thoc6 is required for mouse embryogenesis
To investigate Thoc6 pathogenic mechanisms in mammalian neural development in vivo, we used CRISPR/Cas9 genome editing to introduce an insertion variant in mouse Thoc6 exon 1 that resulted in a premature termination codon predicted to ablate Thoc6 expression (p.P6Lfs*8, herein referred to as Thoc6fs) (Figure 3A). Thoc6+/fs male and female mice do not display phenotypic abnormalities, but their intercrosses yielded no homozygous offspring. Analysis at selected embryonic days (E) of gestation confirmed Thoc6fs/fs littermates die in utero. Differences in embryonic morphology between wildtype (WT) and Thoc6fs/fs embryos were noted starting at E7.5, the earliest day of analysis. By E9.5, Thoc6fs/fs embryos were smaller with delayed development; however, the difference in the developing neocortex was particularly pronounced (Figure 3B). No body turning differences were observed. Consistent with embryonic lethality, THOC6 was undetectable in E8.5 Thoc6fs/fs mouse embryos relative to control littermates (Figure 3C). In E9.5 Thoc6fs/fs embryos, a developmental timepoint with high Thoc6 expression, Thoc6 is detectable by Western blot at greatly diminished levels (Figure 3C). Embryonic lethality was confirmed by E11.5, indicating one functional allele of Thoc6 is essential for mouse embryonic development (Figures 3C and 3D).
Figure 3. Generation of Thoc6fs/fs mouse model.
(A) CRISPR/Cas9 editing strategy to introduce frameshift variants in mouse Thoc6. (B) Representative images of isolated Thoc6+/+, Thoc6fs/+, and Thoc6fs/fs whole embryos at E9.5 prior. Scale bar: 50 μm. (C) Western blot analysis with quantifications of E8.5 and E9.5 mouse embryos showing increased expression during development. Ablation of Thoc6 protein in Thoc6fs/fs is observed at E8.5, with presence of band suggesting read-through product at E9.5. β-actin, loading control. (D) Litter ratio analysis for E8.5–9.5 (left) and weaned (right) Thoc6fs/fs mice. Ratios are consistent with embryonic lethality of homozygous frameshift mice. n = 164 mice (left); n = 27 mice (right). (E) Immunostaining of markers PH3 and C.CASP3 in E9.5 mouse forebrain. Illustration highlights sectioning and quantification approach. (F) Quantifications of fractions of PAX6, PH3, and C.CASP3-expressing cells in E9.5 neuroepithelium. Measurements were combined from one rostral and one caudal section (from two lateral segments depicted by solid black boxes in E) per three embryo replicates per genotype. Data shown as mean ±SEM. Significance, two-tailed t test.
Forebrain tissues of E8.5-E10.5 Thoc6 littermates were characterized to identify neurodevelopmental changes in Thoc6fs/fs pups. Immunohistochemistry of the telencephalic vesicles revealed a consistently thinner neuroepithelium (PAX6) in Thoc6fs/fs compared to Thoc6+/+ littermate controls (Figures 3E,3F, S2B, and S2D). While E9.5 neuroepithelium showed a relative increase in mitotically active cells (PH3), widespread apoptosis (Cleaved Caspase-3 (C.CASP3)) was noted (Figures 3E and 3F). These findings are consistent with proliferative defects noted in Thoc6fs/fs tissue, suggesting THOC6 is important for corticogenesis.
Global mRNA export is not altered in THOC6 models of human in vitro neural development
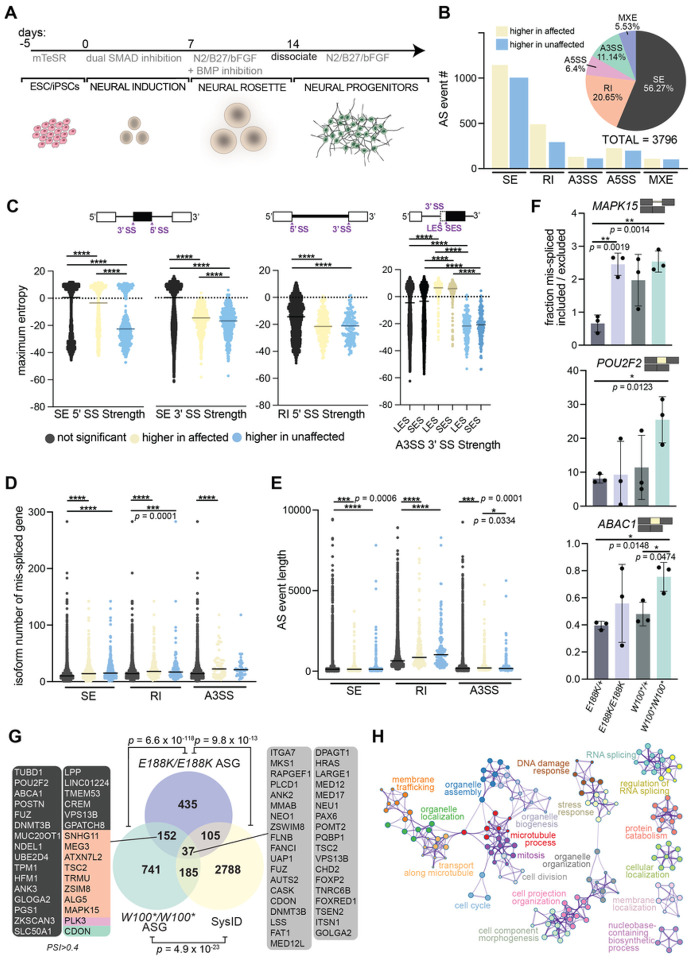
Given the prominent link between the THO subcomplex and RNA export in current literature, we first sought to investigate the impact of THOC6 variants on RNA nuclear export functions in human neural progenitor cells (hNPCs) differentiated from hPSCs (Figure 4A). NPCs are the embryonic cell population frequently implicated in the developmental mechanism of primary microcephaly, a TIDS clinical feature. Defects in mRNA export are typically observed as differential accumulation of polyadenylated (polyA+) mRNA in the nucleus, enriched at nuclear speckle domains44. Standard oligo-dT fluorescent in situ hybridization (FISH) was performed on hNPCs to visualize polyA+ mRNA signal in nuclear and cytoplasmic cellular fractions (Figures S3A, S3B, and S3C). Comparison of the nuclear-to-cytoplasmic (N/C) polyA+ signal intensity ratios across genotypes indicates a slight reduction in THOC6 affected samples, suggesting a trend towards nuclear reduction in affected hNPCs relative to THOC6+/+ and heterozygous unaffected control hNPCs (Figure S3C). The significance of this modest export finding is evident when the N/C polyA+ signal intensity ratios are compared to THOC6+/+ hNPCs treated with wheat germ agglutinin (WGA), a potent inhibitor of all nuclear pore transport and the positive control for export changes45 (Figure S3A), Relative to all THOC6 genotypes, WGA treated hNPCs have a significantly higher N/C ratio (Figure S3C) attributed to strong polyA+ mRNA accumulation in the nucleus (Figure S3B). Although bulk mRNA export is largely unaltered in THOC6-affected hNPCs, a THOC6 dependent tetramer TREX export cannot be ruled out for specific polyA+ or in mRNP processing functions upstream of export—TREX functions that have not been explored in hNPCs.
Figure 4. Characterization of alternative splicing events in THOC6 affected hNPCs.
(A) Differentiation protocol to derive human neural progenitor cells from affected and unaffected ESC/iPSCs. (B) Combined rMATS summary results for AS events in THOC6W100*/W100* and THOC6E188K/E188K hNPCs relative to THOC6W100*/+ control hNPCs. Event type (pie chart) and inclusion status (bar chart). Yellow, higher inclusion in affected. Blue, higher inclusion in unaffected. (C) Significant splice site strength score differences at mis-spliced events in affected hNPCs based on maximum entropy model. (D) transcript number per AS gene and (E) AS event length in THOC6W100*/W100 and THOC6E188K/E188K vs. THOC6W100*/+ NPCs. (F) RT-PCR AS validations of SE (ABAC1, POU2F2) and RI (MAPK15) events in three additional biological replicates of hNPCs per genotype with quantified mis-spliced ratios. Data shown as mean ±SEM. P-values (C-F), two-tailed unpaired t test. ****, p = < 0.0001. (G) Venn diagram of overlap of THOC6 W100*/W100* and THOC6E188K/E188K AS genes and all syndromic intellectual disability genes included in the SysID database. Overlap significance tested by Fisher’s exact test. ASG, alternatively spliced genes. Metascape analysis on combined significant mis-spliced events (FDR <0.05) in THOC6E188K/E188K and THOC6W100*/W100* NPCs (H).
THOC6 depletion reveals TREX function in pre-mRNA splicing in hNPCs
Proper mRNP processing, including mRNA splicing, is required for TREX-dependent RNA nuclear export, linking these steps in mRNA biogenesis. Co-transcriptional recruitment of TREX to the 5’ end of maturing mRNPs coupled with described TREX associations with splicing factors46 led us to investigate features of mRNA processing for vulnerability to loss of THOC6. To capture RNA processing differences caused by loss of THOC6 function, we performed RNA-sequencing (RNAseq) on ribosomal (r)RNA-depleted RNA extracted from wildtype and heterozygous unaffected and homozygous affected hNPCs (Table S4). Principal components analysis (PCA) of RNAseq data demonstrates reproducibility across, and distinct transcriptomic differences between the affected hNPC replicates compared to the unaffected control hNPC replicates (Figure S4A). Genotype driven differential expression and splicing changes were assessed. To investigate a THOC6-dependent role for TREX in splicing, comparative splicing analysis was carried out using the rMATS pipeline on biallelic THOC6E188K/E188K and THOC6W100*/W100* samples versus heterozygous controls (Table S5)47. A combined total of 3,796 significant alternative splicing (AS) events were detected in affected hNPCs, representing the major AS types: skipped/cassette exon (SE), alternative 5’ splice site (A5SS), alternative 3’ splice site (A3SS), retained intron (RI), and mutually exclusive exon (MXE). The most overrepresented AS events observed in affected cells were SE (56%, 2136 of 3796) and RIs (21%, 784 of 3796). The high frequency of RIs is notable and unique relative to splicing defects identified in LOF models of other splicing factors, as well as for normal splicing patterns in neural development (Figure 4B)48–53. AS events in affected hNPCs show comparable inclusion and exclusion of AS junctions, with a slight trend towards inclusion due in part to the high frequency of RIs (Figure 4B). SE and RI splicing events occur by distinct molecular mechanisms, mediated by EJC pathways. Detection of defects in both splicing categories suggests the THO tetramer serves as a molecular platform for coordinating complex splicing events, as opposed to regulation of a specific subset of splicing events controlled by association with and function of individual RNA splicing factors. In agreement with this finding, we did not find consistent motif enrichment for specific RNA-binding proteins at AS junctions. These findings implicate a novel role for THOC6-dependent TREX splicing in mRNP processing in hNPCs.
Since AS motif enrichment analysis of THOC6-affected and control hNPCs transcriptomic data did not reveal trans-regulatory elements responsible for the differential splicing patterns, it was posited that cis-elements may underlie these differences. A maximum entropy model that assesses short sequence motif distributions was used to test the strength of the donor (5’) and acceptor (3’) of AS events54. A general trend towards weaker splice sites were detected at differential SE, RI, and A3SS events in affected cells (unpaired two-tailed t test, Figure 4C). The SE, RI, and A3SS events were enriched in genes with a disproportionately high number of isoforms that show dependence on weak, alternative/cryptic splice sites to facilitate isoform diversity (Figure 4D)55. RI events in affected hNPCs also had weaker splice sites compared to controls, suggesting that THOC6 deficiency induces mis-splicing at weak splice sites. In addition, the AS SE, RI, and A3SS events in affected THOC6 hNPCs impacted exons/introns that are significantly longer than nonsignificant events (Figure 4E). Likewise, the length of introns retained in RI events were significantly longer, with a 1.4-fold increase in length quantified for significant RI events (P= <0.0001, unpaired two-tailed t test, Figure 4E). Lastly, no positional bias was observed for AS events (Figure S4D and S4F). To validate our bioinformatic analysis, AS inclusion trends in select, top, shared events were validated by qRT-PCR, demonstrating a high correlation (unpaired two-tailed t test, Figure 4F). Together, the detected RNA processing signature across diverse SE and RI events at weak splices sites suggest impaired splicing fidelity from loss of THOC6.
To investigate the role of RNA misprocessing in ID pathology, we intersected our AS events with the genes that are known to cause syndromic ID, deposited in the SysID database (SysIDdb). 152 genes with significant AS events included or excluded in >10% of transcripts in hNPCS were detected in nonsense and missense affected genotypes (Figure 4G). 185 AS genes in THOC6W100*/W100* and 105 AS genes in THOC6E188K/E188K hNPCs are known genes causative for syndromic ID represented in the SysIDdb (Figure 4G). Aberrantly spliced ID genes were identified in both THOC6 affected genotypes, consistent with a role for THOC6 in ID. 37 ID genes (1.3% of SysIDdb) are AS in both affected genotypes, identifying genes for shared mechanisms that may preferentially contribute to TIDS pathology. To identify biological mechanisms implicated by THOC6-dependent AS, biological pathway enrichment analysis was performed on mis-spliced genes in affected cells. Genes with differential splicing were significantly enriched for functions in RNA splicing, cell projection organization, membrane trafficking, organelle organization, mitosis cell cycle, and DNA damage response (Figure 4H). RNA processing is tightly controlled by feedback loops (e.g., auto-repression by poison exons or intron retention), which would explain how effects on cis elements may lead to changes in trans factors (i.e., AS events in splicing regulatory factors).
THOC6-dependent mRNP processing is required for hNPC proliferation and differentiation
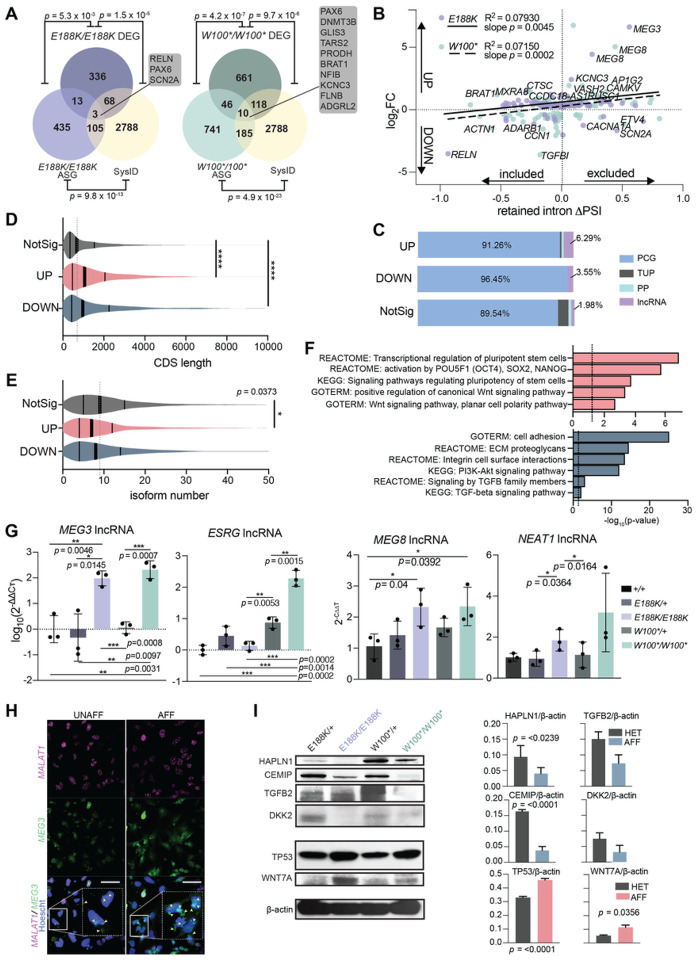
Alternative mRNA processing events, such as SE and RI in mRNA splicing, can impact gene expression through different mechanisms; isoform ratio differences versus inclusion of premature termination codons that initiate NMD. To investigate the impact of AS events on expression, we performed differential gene expression analysis on THOC6E188K/E188K versus THOC6W100*/+, and THOC6W100*/W100* versus THOC6W100*/+ dyads (Figure S5B). Among the 336 differentially expressed genes (DEGs) in THOC6E188K/E188K hNPCs, only 13 had splicing defects (p = 5.3×10‒3, Fisher’s exact test) compared to 46 mis-spliced DEGs (of 661 DEGs, p = 4.2×10‒7, Fisher’s exact test) in THOC6W100*/W100* hNPCs, indicating a subtle effect of mis-splicing on expression (Figure 5A). Notably, there are nearly double the number of AS genes (ASGs) (435 E188K; 741 W100*) and DEGs (336 E188K; 661 W100*) in THOC6W100*/W100* hNPCs, suggesting that the nonsense genotype has a greater impact on splicing and gene expression (Figure 5A). Relevant for TIDS pathology, 20% (68 of 336; p = 1.5×10‒5, Fisher’s exact test) of THOC6E188K/E188K DEGs and 18% (118 of 661; p = 9.7×10‒6, Fisher’s exact test) of THOC6W100*/W100* DEGs are syndromic ID genes, which conveys important information pertinent for understanding the pathogenic mechanisms of TIDS (Figure 5A).
Figure 5. Differential expression analysis in affected hNPCs.
(A) Venn diagram of gene overlap of THOC6 W100*/W100* and THOC6E188K/E188K affected genes and all syndromic intellectual disability genes included in the SysID database. Overlap significance tested by Fisher’s exact test. DEG, differentially expressed genes; ASG, alternatively spliced genes. (B) Linear regression analysis of log2foldchange and D percent transcripts spliced in (PSI) for significant retained intron events in affected cells. Purple dots indicate THOC6E188K/E188K hits and green dots indicate THOC6 W100*/W100*. Best fit line, R2, and slope p-value for THOC6E188K/E188K (solid line) and THOC6 W100*/W100* (dotted line). (C) Percentage of gene type by condition for combined DEGs in affected hNPCs. NotSig, not significant; Up, upregulated. Down, downregulated; lncRNA, long non-coding RNA; PP, processed pseudogene; TUP, transcribed unprocessed pseudogene; PCG, protein coding gene. Violin plots of coding sequence (CDS) length (D) and isoform number (E) of combined DEGs in affected cells compared to non-significant genes. (F) DAVID biological pathway enrichment analysis of combined upregulated genes (top, red) and downregulated genes (bottom, blue) in THOC6 affected hNPCs. (G) qPCR relative abundance quantifications (2‒ΔΔCt) for MEG3, ESRG, MEG8, and NEAT1 in hNPCs. Three technical replicates of three biological replicates per genotype. (H) RNA FISH probing for MEG3 and MALAT1 in affected and unaffected hNPCs. Cell inset showing MEG3 expression and localization differences with yellow arrows in merged image. Scale bar 50 mm. UNAFF, unaffected (W100*/+); AFF, affected (W100*/W100*). (I) Protein abundance of top downregulated and upregulated genes across genotypes. Genes labeled with log2FC > 1 or < −1 and PSI > 0.1 or < −0.1. Data shown as mean ±SEM. Significance, two-tailed unpaired t test.
Retained intron AS events are often subject to NMD in protein-coding genes (PCGs), while intron inclusion in lncRNAs alters nuclear export and conformation. Given the high number of RI events detected in THOC6 affected hNPCs, we tested the correlation between differential expression and RI events. For this analysis, gene-level mRNA abundance fold-change in affected hNPCs was correlated to the change in intron inclusion within transcripts from PCGs or non-coding RNA loci, referred to as percentage spliced-in (DPSI). We observed a trend in both THOC6-affected genotypes that lower gene expression correlates with greater intron inclusion (slope, p = 0.0045 for THOC6W100*/W100*and p = 0.0002 for THOC6E188K/E188K, simple linear regression) (Figure 5B). Conversely, the quadrant representing differential intron exclusion and elevated expression was prominently represented by lncRNAs, where intron exclusion is implicated in impaired lncRNA function. The three significantly dysregulated ASGs represented in both affected genotypes were MEG3, PAX6, and POSTN. Consistent with the observed trend between RI events and expression, analysis of all DEGs revealed that PCGs make up the largest portion of DEGs (with a greater portion, 96.45% affected in downregulated genes), while the portion of lncRNAs is highest in upregulated genes (6.29%; compared to 1.98% of non-significant genes), reflecting the molecular differences between these distinct mRNA subtypes.
Additional mRNA characteristics that may account for a portion of the observed differential expression are gene length and isoform number. In affected hNPCs, significantly more transcripts from long genes with on average of less than 10 annotated isoforms were identified compared to non-significant genes (Figures 5D and 5E). The trend towards DEGs with fewer transcript isoforms in affected hNPCs suggest alternatively spliced transcripts are more stable in affected cells (Figure 5C). These findings again reflect a requirement for the larger THOC6-dependent TREX tetramer complex function in facilitating mRNP processing of long mRNAs with high expression in brain.
To identify biological pathways predicted to contribute to THOC6 neuropathology, DAVID analysis was performed to identify biological categories defined by DEGs inTHOC6 affected hNPCs (Figure 5D). Downregulated genes are enriched in integrin cell adhesion, extracellular matrix interactions, PI3K-AKT signaling, and TGF-b signaling pathways, which are critical for brain development (Figure 5F). PI3K-AKT/mTOR signaling regulates cortical NPC proliferation, differentiation, and apoptosis56,57. Over 30 genes attributed to the PI3K-AKT/mTOR signaling pathway were downregulated in affected cells, accounting for the significant enrichment (p = <1 × 10‒13). HAPLN1, MYC, BMPR1B, DCN, FBN1, INHBA, ID4, THBS1, TGFB2, DEGs enriched in the TGF-b signaling pathway (p = <0.001), have direct implications for TGF-b signaling in neural induction, differentiation, and NPC fate specification in TIDS developmental mechanisms58,59. Complementary pathways enriched with upregulated DEGs implicate multipotency (OCT4, PAX6), proliferation, neuron differentiation and WNT signaling pathways (p = <1 × 10‒6, p = <0.001, p = <0.001, and p = <0.001, respectively). WNT signaling is known to promote NPC self-renewal expansion during corticogenesis60,61. Shared dysregulation of mTOR, TGF-b, and WNT signaling, coupled with upregulation of multipotency factors in affected genotypes, suggests defects in hNPC multipotency and neural differentiation underlie TIDS pathogenesis.
To identify transcription factor networks dysregulated in THOC6-affected hNPCs, transcription factor motif enrichment analyses was performed. Significant enrichment of MEF2, LHX3, and SRF target genes was observed in heterozygous controls compared to both affected genotypes (Figure S5D). Using a second analysis tool, ChEA3, differential expression of SOX, FEZF, FOX, and GLI target genes, and downregulation of HEYL, TWIST, FOX, MEOX2, PRRX2, and MKX target genes were enriched in affected hNPCs (Figure S5E). These transcription factor networks are important for neuronal differentiation and fate specification62–64, concordant with GSEA findings. Together, these results suggest that gene expression programs that modulate timing of the switch from neural proliferation to differentiation are altered in TIDS.
To refine specific candidate genes implicated in shared TIDS neuropathology, DEGs between affected THOC6 genotypes were intersected. 12 genes were upregulated and 117 were downregulated in affected hNPCs, with notable lncRNAs represented. Significant enrichment was detected in Integrin 1 pathway and extracellular matrix protein interaction networks (Figure S5C). Using mRNA obtained from three additional replicate differentiations of hNPCs per genotype, significant upregulation of MEG3, MEG8, ESRG, and NEAT1 lncRNAs was confirmed by qRT-PCR (Figures 5G). RNA FISH confirmed increased expression of MEG3 in affected hNPCs compared to controls, with elevated signal observed in both nuclear and cytoplasmic fractions (Figure 5H). Upregulation of functional lncRNAs NEAT1 and MEG3 has been linked to activation of WNT activation and suppression of TGF-b signaling, respectively65,66. Concordant with these findings, the protein level of WNT and TGF-b signaling components in THOC6-affected hNPCs exhibit a corresponding differential up- and down-expression relative to controls. Specifically, WNT signaling components WNT7A and TP53 showed increased protein expression, with higher abundance detected in affected hNPCs (Figure 5I). TGF-b pathway proteins HAPLN1 and TGFB2 also showed reduced protein expression in affected hNPCs together with high CEMIP and DKK2 (Figure 5I). We propose that loss of THOC6 leads to lncRNA-mediated dysregulation of key developmental signaling pathways which has implications for the balance of proliferation and differentiation during neural development.
Apoptotic upregulation and retained intron enrichment in Thoc6fs/fs E9.5 mouse forebrain
To investigate the conserved THOC6-dependent TREX functions that account for divergent phenotypic outcomes between mammalian models, mRNP processing was assessed in E9.5 mouse brain using complementary RNAseq experiments to those performed in hNPCs (Figures 6A and S6A). Three biological replicates were analyzed per genotype (Thoc6+/+, Thoc6fs/+, Thoc6fs/fs). Fewer significant AS events (FDR <0.05) were detected in Thoc6fs/fs E9.5 brain than in affected hNPCs, but the pattern of AS events was recapitulated, with the majority of AS events categorized as SE (45%) and RI (26%) (Figure 6B). Greater than 40 PSI was quantified in the Thoc6fs/fs transcriptome and retained and excluded intron events in Cenpt, Admts6, and Fam214b were validated (Figures 6C and S6B). Maximum entropy model analysis of splice junctions revealed significantly weaker 3’ splice site strengths for SE events and weaker 5’ splice sites associated with RI events in the Thoc6fs/fs mouse model. While this signature of splice site weakness is more modest in mouse than in human THOC6 models, these findings suggest a conserved role of THOC6-dependent TREX tetramer in coordinating mRNA processing that precedes TREX export functions (Figures 6D and S6C).
Figure 6. Characterization of mRNA processing defects in Thoc6fs/fs mouse E9.5 forebrain.
(A) Cartoon of E9.5 mouse forebrain total RNA sample preparation. (B) rMATS summary results for AS events in Thoc6fs/fs E9.5 forebrain. Event type (pie chart) and inclusion status (bar chart). Yellow, higher inclusion in Thoc6fs/fs. Blue, higher inclusion in Thoc6+/+. (C) Quantifications from RT-PCR validating top AS events Cenpt, Admts6, Fam214b in 2–4 biological replicates. Significance, two-tailed unpaired t test. Data are mean ±SEM. (D) Significant splice site strength score differences at mis-spliced events in Thoc6fs/fs samples based on maximum entropy model. (E) RT-qPCR validations of Thoc6, Wnt7a, Islr2, Ier3, Kcnt2, Anax2 mRNA abundance on two additional biological replicates of E9.5 forebrain per genotype. Three technical replicates analyzed per sample. Significance, two-tailed unpaired t test. Data are mean ±SEM. (F) DAVID analysis showing significantly enriched biological pathways among upregulated genes (top, magenta) and downregulated genes (bottom, blue) in Thoc6fs/fs E9.5 forebrain. (G) Venn diagram of overlap of DEG in affected hNPCs and Thoc6fs/fs mouse E9.5 forebrain. Overlap significance tested by Fisher’s exact test.
Notably, biological pathway and network enrichment analysis of AS genes identified mRNA processing, pre-miRNA processing, de-adenylation of mRNA, central nervous system development, forebrain development, multicellular growth, response to oxidative stress, cytoskeletal organization, and neuron projection (Figure S6D) — several of the biological categories associated with hNPCs ASGs. These shared findings suggest selective conservation of mRNP processing mechanisms by THOC6 in mouse and human forebrain.
To assess the correlation between THOC6 mRNP processing defects and expression, differential expression analysis of Thoc6fs/fs forebrain mRNA sequencing data was performed compared to Thoc6+/+ controls. Of note, Thoc6 mRNA is downregulated (two-fold, p=<0.0001), consistent with NMD (Figures 6E and S6F). In this model, 5x more genes were upregulated (144 genes) than downregulated (27 genes). Nevertheless, downregulated genes may covey important pathology. First, downregulated genes functionally converge on neurogenesis, proliferation, and differentiation pathways (Figure 6F). Upregulated genes are implicated in the hypoxic response, HIF-1 signaling pathway, and glycolysis—biological categories indicative of increased apoptosis in affected cells (Figure 6F). To investigate if altered transcription factor networks contribute to pathway dysregulation, we performed GSEA transcription factor motif enrichment analysis. HIF1, NRSF, SMAD3, and STAT3 target genes were enriched in Thoc6fs/fs E9.5 forebrain (Figure S6G). HIF-1 and STAT3 can induce apoptosis in response to hypoxia67,68, which is consistent the observed elevation of apoptosis in Thoc6fs/fs E9.5 neuroepithelium (Figures 3E and 3F). SMAD3 signaling is activated by TGF-b to promote cortical differentiation58, suggesting shared disruption of TGF-b signaling in both human and mouse model systems.
DEGs shared between mouse and human model systems are consistent with conserved TIDS molecular pathology (Figure 6G). More Thoc6fs/fs DEGs overlapped with THOC6W100*/W100* (23 genes) than THOC6E188K/E188K (9 genes) samples, and include genes involved in neurogenesis, hypoxic response, and synapse regulation. Validation of Ier3, Islr2, Wnt7a, Kcnt2, Anax2, and Vegfa DEGs shared across affected models were confirmed by qRT-PCR in three additional E9.5 forebrain biological replicates for Thoc6+/+, Thoc6fs/+, and Thoc6fs/fs samples (Figures 6E and S6H). Overlapping affected human and mouse molecular mechanisms suggest shared pathology. However, the extent of upregulation of genes in response to increased apoptosis is exacerbated in mouse, highlighting species-specific phenotypic differences due to loss of THOC6.
Delayed differentiation and elevated apoptosis in THOC6-affected forebrain organoids
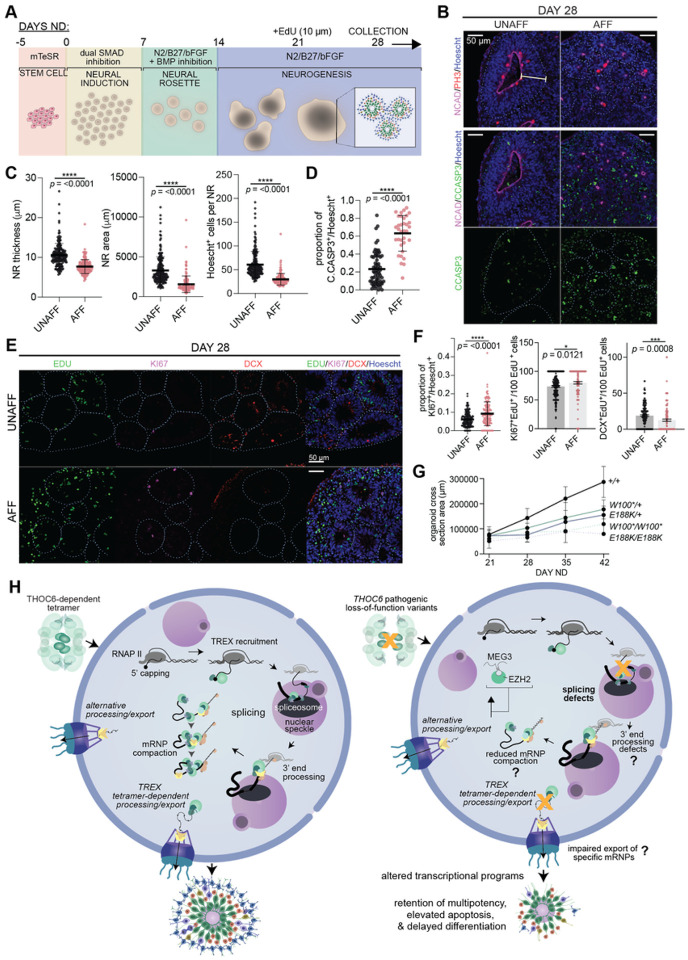
THOC6 pathogenesis in human cortical development was investigated using dorsal forebrain-fated organoids, neural differentiated from iPSC lines (Figure 7A). Forebrain organoids recapitulate the cellular heterogeneity and developmental dynamics of early corticogenesis69. Within each organoid, several neural rosette (NR) structures develop stochastically to recapitulate features of in vivo ventricular zone development, including hNPC proliferation and differentiation to cortical neuron fates (Figure 7A). NR morphology was evaluated in cortical organoids at 28 days of neural differentiation (ND) from three independent differentiations per genotype. To minimize the effect of inter-cell line NR variability, the following analyses focus on heterozygous unaffected and homozygous affected comparisons. To characterize the NR proliferative niche, the maximum thickness of the NR neuroepithelial center was measured as defined by N-cadherin immunostaining and pseudostratified NR cytoarchitecture by Hoescht staining (Figures 7B, 7C, S7A, and S7B). THOC6-affected organoids show significantly thinner pseudostratified neuroepithelium (p = <0.0001, two-tailed t test), concordant with reduced NR size composed of fewer cells (p = <0.0001, two-tailed t test) (Figures 7B, 7C, S7A, and S7B). Given the stark upregulation of apoptosis observed in Thoc6fs/fs E9.5 mouse forebrain, we investigated the contribution of apoptosis as a mechanism of reduced NR size in the THOC6-affected organoids (Figures 7B, 7D, S7A, and S7B). A significantly higher proportion of affected NR cells expressed the apoptotic marker C.CASP3, providing evidence for shared mechanism of altered corticogenesis (p = <0.0001, two-tailed t test) (Figure 7D).
Figure 7. Modeling of THOC6 variant pathogenesis in human cerebral organoids.
(A) Cerebral organoid differentiation protocol. ND, neural differentiation. (B) Immunostaining of PH3, N-Cadherin, apoptosis marker cleaved caspase3 (C.CASP3), and Hoescht in day 28 human cerebral organoids differentiated from unaffected and affected iPSCs, highlighting differences in neural rosette morphology. 40x magnification; Scale bar: 50 μm. UNAFF, unaffected; AFF, affected. (C-D) Quantifications of area, thickness, Hoescht+ cells, and fraction of C.CASP3+ cells per NR for THOC6W100*/+ and THOC6E188K/+ controls and THOC6W100*/W100* and THOC6E188K/E188K affected organoids. NR (organoid) number analyzed across one differentiation replicate per genotype: unaffected, n = 67 (15); affected, n = 34 (10). (E) Immunostaining of EDU, KI67, DCX to assess timing of differentiation in day 28 organoids with quantifications (F). NR (organoid) number analyzed across three differentiation replicates per genotype: unaffected, n = 187 (87); affected, n = 157 (67). (G) Growth rate of organoids across genotypes measured by cross section area (μm) from days 21–42. (C-D, F-G) Data shown as mean ±SEM. Significance, two-tailed unpaired t test. Representative image for unaffected and affected are THOC6E188K/+ and THOC6E188K/E188K, respectively. (H) Schematic of proposed model of THOC6 pathogenesis.
To assess alterations in the timing of differentiation in affected NRs, we performed EdU-pulse labeling at day 21ND for 24 hours to label mitotically active cells, followed by organoid immunohistochemistry analysis at day 28ND (Figures 7E, 7F, S7C, and S7D). To assess the balance of multipotency and differentiation EdU, KI67, and DCX co-labeled cells per NR were quantified. A significant increase in cells co-stained with the proliferation marker KI67 and EdU per affected NR were detected at day 28ND (p = <0.0001, two-tailed t test), indicating affected NPCs remain mitotically active longer than control NPCs (Figure 7F). This finding paired with elevated mRNA and protein expression of OCT4 in affected hNPCs (data not shown) supports retention of multipotency model. Consistent with this finding, we observed a significant reduction in the fraction of EdU cells co-labeled with the migrating neuron marker doublecortin (DCX) in affected NRs (p = <0.0001, two-tailed t test) (Figure 7F). Paired with the prolonged proliferation dynamics, this suggests a disruption to the differentiation timeline in affected organoids.
To investigate effects of reduced NR growth on organoid size, we measured whole organoid cross section areas weekly from day 21 to day 42. Compared to the steady size increase of THOC6+/+ organoids, affected organoids showed a slower growth rate (E188K/E188K: p = 0.0122; W100*/W100*: p = 0.0362) (Figure 7G). Together, our findings implicate a pathogenic mechanism of delayed differentiation due to reduced NPC proliferative capacity and elevated apoptosis with subsequent cortical growth impairment in affected organoids.
DISCUSSION
There is a growing cohort of individuals with TIDS due to variant discovery resulting from exome based genetic testing6,8–18,41,42. However, our understanding of the molecular functions of THOC6 are limited, despite robust research on the TREX complex25,34,36,46,70–82. Information implicating important species differences in TREX composition and function that are THOC6-dependent serve to highlight this gap in knowledge83–86. Our findings support a novel THOC6 LOF model of TIDS pathogenesis whereby pathogenic variants impair the formation of the THO/TREX tetramer complex that facilitates multivalent protein-mRNA interactions to support coordination of mRNP processing and export functions (Figure 7H). The resulting molecular impact preferentially disrupts processing of long mRNAs with a high incidence of intron retention. The neurological features of TIDS and the enrichment of long genes expressed in the brain further implicate a critical role for the THOC6-dependent TREX tetramer in providing a platform for enhanced coalescence of mRNP processing cofactors and maintenance of mRNA structural integrity during splicing of long mRNA transcripts important for brain development. Cell-type and organism specific requirements for splicing and gene length are predicted to inform the variation in tolerance that underlies distinct human phenotypic presentations and interspecific differences.
Our analysis of the THOC6 alleles, carrying either nonsense and missense variants, indicates pathogenic mRNA transcripts do not undergo NMD. Stable THOC6 nonsense mRNAs correlate with a notable reduction in full length THOC6 expression, with no evidence for expression of a truncated product. Very low THOC6 expression in homozygous THOC6 nonsense cells is consistent with the expression of a minimal readthrough product, by a nonsense suppression mechanism observed in both human and mouse cells. THOC6 missense variants do not trigger NMD or affect protein abundance. The shared clinical phenotypes between biallelic THOC6 nonsense and missense variants indicates that THOC6 variants generally function through a LOF genetic mechanism, with both low protein abundance and normal expression of variant protein disrupting TREX tetramer complex formation. Given the conserved functions of TREX in mammals, the phenotypic discrepancy between Thoc6fs/fs mouse embryonic lethality and human biallelic THOC6 TIDS features is notable and suggests species-specific aspects of THOC6 pathogenic mechanisms. Superficially, this finding suggests that humans are more tolerant to THOC6 variants. Alternatively, this may reflect divergence in the downstream genetic mechanisms of the human and mouse variants investigated. Human pathogenic THOC6 variants reside in the WD40 repeat domains whereas the mouse Thoc6fs variant is located upstream of the first WD40 repeat domain. Nonsense THOC6 variant transcripts are stable and readthrough permits limited THOC6 expression. Conversely, Thoc6fs transcripts are subject to NMD, impairing THOC6 expression, and readthrough causes low expression of full-length THOC6 protein at restricted developmental timepoints. This suggests that minimal THOC6 expression may be sufficient for human embryogenesis.
TREX has a prominent role in nuclear RNA export46,76. As such, a significant reduction in global polyA+ RNA nuclear export was predicted to result from loss of THOC6-mediated TREX function. However, the absence of global export defects suggests that THO dimers in THOC6-affected hNPCs maintain their conserved function for RNA export. Given this finding, alternative THOC6 pathogenic mechanisms may represent tetramer functions. The significant splicing changes implicate a pathogenic mechanism where THOC6-dependent disruption of TREX tetramer formation indirectly disrupts coordination of multiple steps of mRNP processing, including splicing, upstream of polyA+ mRNA packaging and export. This interpretation is supported by the diversity of mRNP processing functions attributed to tetramer-associated cofactors. UAP56 and ALYREF play important roles in mediating pre-mRNA splicing decisions46,87. This finding does not rule out the possibility that THOC6 plays a direct role in pre-mRNA splicing outside of mediating TREX core tetrameric assembly on the EJC. That THO member THOC5 can interact with unspliced transcripts34, and WD40-repeat domains facilitate splicing factor interactions with pre-mRNA48, are evidence in support of this possibility.
The crystal structure of human THO-UAP56 was recently solved, implicating several putative consequences of THOC6-dependent TREX tetramer disruption (Figure 7H)25. Defects in TREX tetramer assembly are not predicted to disrupt formation of stable functional dimers, allowing THOC6-depleted models to discriminate between dimer and tetramer functions. The tetramer affords greater surface area for mRNP processing and permits enhanced co-adaptor loading of known and potentially species-specific TREX cofactors in species with a substantial splicing burden. Certainly, we see evidence for reduced association of ALYREF with THO complexes by THOC6 co-immunoprecipitation in THOC6-affected iPSCs, providing indirect evidence for altered TREX tetramer formation with impaired binding of tetramer-associated mRNP processing cofactors (Figure 2F). Tetramer formation juxtaposes two UAP56 helicases on each end of dimers88 to support a greater number of cofactors with broad mRNP processing capabilities (e.g., CHTOP in 3’ end processing and ALYREF in splicing)46 known to compete for the limited number of UAP56 binding sites36,89,90. The tetramer also affords a greater surface area to maintain the structural integrity of long mRNA transcripts during mRNP processing and export. Through this process, TREX serves as a mRNA chaperone to prevent formation of DNA-RNA hybrid or R-loop structures that can promote genome instability38,39. Thus, the TREX tetramer helps ensure mRNP quality control and evasion of degradation by the nuclear exosome91. The tetramer may also enable multiple TREX complexes to simultaneously bind several mRNP regions25 to facilitate compaction and/or protection of longer transcripts with elevated splicing.
Enhanced mRNA processing efficiency provided by UAP56, ALYREF, CHTOP, and other export adaptors associated with the TREX tetramer is susceptible to THOC6 pathogenic variants. Likewise, the diversity of expressed isoforms increases phylogenetically (yeast versus mammals), as well as during mammalian differentiation29,30,93, especially in the brain, highlighting the vulnerability of neural development to THOC6 loss40,94. Neuronal expression of long genes is imperative for proper neuronal differentiation and synaptogenesis95. ALYREF is predicted to interact with other UAP56/TREX-bound adaptors associated to the same mRNP25, suggesting that the tetramer facilitates increased mRNP compaction required for proper export, especially of longer mRNAs. It is possible that enhanced selectivity of mRNP processing and export co-evolved with TREX conformation, contributing to organismal complexity and distinguishing mammalian and yeast cells.
Our findings implicating THOC6-dependent TREX tetramers as indirect facilitators of splicing by coordinating the mechanics of mRNP processing is also supported by enrichment of aberrant splicing events at weaker splice sites. Weak splice sites are most often utilized by transcripts during alternative splicing, and genes with elevated isoform diversity from alternative splicing are more susceptible to disruption of the overall integrity of mRNP processing in THOC6-affected hNPCs. Long genes that are highly expressed in the brain particularly rely on such infrastructure to ensure pro-neural gene expression. This may also be supported by the observed enrichment of retained introns in THOC6-deficient cells. Indeed, retained introns in unaffected tissues typically have weaker 5’ and 3’ splice sites compared to other splice junctions96. In addition, intron retention increases during mammalian differentiation97, again suggesting differentiated cells may be more susceptible to loss of THOC6-mediated TREX tetramer functions by miscoordination affecting splicing outcomes.
Differences in splicing requirements may also account for interspecific phenotypic differences. While SE and RI events were the predominant splicing defects in both model systems, AS events affect more protein coding transcripts and lncRNAs in THOC6-affected hNPCs. Despite less AS events in Thoc6fs/fs cells, lethality in the mouse could be attributed to aberrant alternative splicing of specific transcripts important for mouse embryogenesis. Additionally, previous findings indicate that RI events account for a substantial portion of splicing variation in the primate prefrontal cortex, a trend that is most pronounced in humans98. Although intron retention is a known mechanism of mouse neuronal gene regulation by initiating RNA exosome-mediated degradation99, it is possible that human cells are more tolerant than mouse cells to elevated intron retention. Further investigation of these interspecific differences is important for generating translationally relevant discoveries.
The number of ID genes that are mis-spliced in THOC6 affected hNPCs relative to controls implicate shared underlying developmental mechanisms of ID pathology. However, developmental impact of individual defects on TIDS neuropathology is complicated by the compounding effects of constitutive THOC6 LOF models. In addition to trends shared with the mouse model, we show that biallelic THOC6 LOF is responsible for disruption of key TGF-β and Wnt signaling pathways via a mechanism that involves dysregulation of signaling components and lncRNAs resulting in delayed hNPC differentiation, prolonged retention of multipotency, and enhanced apoptosis. This is exemplified by intron retention and upregulation of MEG3 in affected hNPCs. MEG3 is linked to the regulation of TGF-b signaling and other EZH2 common target genes66. Our findings suggest that RI events alter MEG3 subcellular localization, expression, and downstream WNT signaling that increases multipotency and disrupts the balance of proliferation and differentiation in affected hNPCs. A shift towards cytoplasmic localization of lncRNAs has evolved in human cells, which is important for the maintenance of stem cell pluripotency (e.g., cytoplasmic FAST binds E3 ubiquitin ligase b-TrCP to block its interaction with b-catenin and enable activation of Wnt signaling)100,101. Given the increased diversity of lncRNA functions in human developmental biology, mouse cells may be less tolerant to lncRNA dysregulation than human cells. In addition, MEG3 is also upregulated by CREB102 whose target genes are affected in Thoc5 conditional knockout mouse cortical neurons82, potentially reflecting a shared mechanism of THO dysregulation in neural cells. While our analyses from mouse and human organoid models of Thoc6 and THOC6 disruption provide insight into the molecular pathology of early neural development, later analysis of synaptic physiology will be important to elucidate mechanisms of neuronal dysfunction in TIDS.
Altogether, our findings expand the TIDS clinical population and provide novel functional insight into the pathogenic mechanisms of biallelic LOF variants in THOC6 using comparative mammalian model systems. Functional studies with THOC6 enable us to assess TREX tetramer function while retaining THO subcomplex formation, and our findings provide novel insight into TREX splicing functions separate from export. Future work is needed to dissect the direct and indirect effects of THOC6 loss and confirm endogenous tetramer disruption under native protein conditions. In addition, the well-known role of several TREX members in determination of polyadenylation site choice necessitates research focused on characterizing global aberrant alternative polyadenylation changes that could be contributing to dysregulation. Follow-up investigation at later-stage cortical organoids, and with use of unbiased single-cell RNAseq profiling, will allow for more detailed assessment of the developmental consequences of observed defects for cortical lamination and cell type composition. Lastly, it will be important to determine if alterations in mRNA processing/export also underlie synaptic defects—a morphological basis of ID, and a prominent clinical feature shared between THOC2 and THOC6-associated neurodevelopmental disorders.
METHODS
Human subjects
All subjects or parents/guardians provided informed consent and were enrolled in institutional review board-approved research studies. In all cases, the procedures followed were in accordance with the ethical standards of the respective institution’s committee on human research and were in keeping with international standards. Probands 1–3 and 5 were identified through GeneMatcher103. Details for all subjects are provided in Table S1.
Animal models
All mice were maintained according with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and were approved by the Case Western Reserve Institutional Animal Care and Use Committee. CRISPR genome editing was performed in the University of California, San Diego Transgenic and Knockout Mouse Core. C57BL/6JN hybrid mice (Jackson Laboratory, 005304) were used for CRISPR editing of the Thoc6 locus. Founder mice with the Thoc6fs/+ allele were intercrossed with C57BL/6JN mice (Jackson Laboratory, 005304) for line maintenance. All ex vivo analyses were performed on tissue collected from mice of both sexes at embryonic day (E) 8.5–10.5. Sex-dependent differences were not assessed.
Litters were genotyped by allele-specific polymerase chain reaction (AS-PCR). Genomic DNA was prepared from mouse tissue samples as previously described104. AS-PCR for each allele was assembled using the standard GoTaq DNA polymerase (Promega) protocol. Reaction conditions were executed as recommended by the manufacturer. Primers and sgRNA sequences are provided in Table S3.
Human ESC/iPSC culture
Human ESC and iPSC lines were cultured using feeder free conditions on Matrigel (Corning) with mTeSR-1 (STEMCELL Technologies). Lines used in this manuscript include: H9 (THOC6+/+, 46XX, ESCs, WA09, WiCell), AS0035 (THOC6+/+, 46XX, iPSCs, New York Stem Cell Foundation (NYSCF) Diabetes iPSC Panel), AS0041 (THOC6+/+, 46XY, iPSCs, NYSCF Diabetes iPSC Panel), KMC6002 (THOC6E188K/+, 46XY, iPSCs), KMC6003 (THOC6E188K/E188K, 46XY, iPSCs), KMC7001 (THOC6W100*/+, 46XY, iPSCs), KMC7002 (THOC6W100*/W100*, 46XY, iPSCs). Passaging was performed using mTeSR-1 supplemented 1 nM ROCK inhibitor (BD Biosciences 562822) to prevent differentiation. Both manual and chemical dissociation with Versene (Gibco 15040066) were performed for splitting. Sanger sequencing validation of genotypes (Figure S1B and S1C) (primers listed in Table S3) and CNV microarray analysis (Illumina Bead Array, analysis with Genome Studio v2.0) were performed on all lines to ensure no pathogenic changes were acquired during culturing. No further validation of iPSCs lines was performed in our lab.
Whole exome sequencing and analysis
Exome libraries from genomic DNA of all BBIS-affected probands were prepared and captured with the Agilent SureSelectXT Human All Exon 50Mb Kit for Probands 1 & 4–7 and Individual 5, the Agilent SureSelectXT Clinical Research Exome kit for Proband 3, and the TrueSeq Rapid Exome Kit for Proband 2. Further, exome libraries were sequenced on an Illumina HiSeq or NextSeq instrument as described previously105.
Reads were aligned to the human reference genome NCBI builds 37 (GRCh37) and 38 (GRCh38) and 38 using Burrows-Wheeler Aligner (BWA)106. Variant calling of single nucleotide variants (SNVs) and copy number variants (CNVs) was performed using GATK107, VEP, and CoNIFER108. Average depth of coverage was calculated across all targeted regions. The data were filtered and annotated from the canonical THOC6 transcript (ENST00000326266.8 and ENSP00000326531.8) using in-house bioinformatics software109. Variants were also filtered against public databases including the 1000 Genomes Project phase 311, Exome Aggregation Consortium (ExAC) v.0.3.1, Genome Aggregate Database (gnomAD), National Heart, Lung, and Blood Institute Exome Sequencing Project Exome Variant Server (ESP6500SI-V2). Those with a minor allele frequency >3.3% were excluded. Additionally, variants flagged as low quality or putative false positives (Phred quality score 14; 15 < 20, low quality by depth <20) were excluded from the analysis. Variants in genes known to be associated with ID were selected and prioritized based on predicted pathogenicity.
Sanger sequencing
All variants discovered by WES were confirmed with Sanger sequencing of THOC6 for each individual and respective family members who submitted samples except Proband 1 where high-coverage WES of THOC6 in the proband and parents was deemed sufficient to report without Sanger confirmation. Chromatograms were analyzed using NextGENe, Sequencer, and Geneious Prime Software (v.2022.1.1).
Cerebral organoid generation
Telencephalic cerebral organoids were generated based on previously published protocols110, with few modifications to start with low cell density in order to generate smaller and more consistent embryoid bodies (EBs). Briefly, human and chimpanzee iPSCs were passaged into 96-well V-shaped bottom ultra-low attachment cell culture plates (PrimeSurface® 3D culture, MS-9096VZ) to achieve a starting cell density of 600–1,000 cells per well in 30 μl of mTesR™1 with 1 nM ROCK inhibitor. After 36 hours, 150 μl of N-2/SMAD inhibition media (cocktail of 1X N-2 supplement (Invitrogen 17502048), 2 μM A-83–01 inhibitor (Tocris Bioscience 2939), and 1 mM dorsomorphin (Tocris Bioscience 309350) in DMEM-F12 (Gibco 11330032)) was added for neural induction. On day 7, EBs were transferred to Matrigel-coated plates to enrich for neural rosettes at a density of 20–30 EBs per well of a 6-well plate, and media was changed to neural differentiation media (0.5X N-2 supplement, 0.5X B-27 supplement (Invitrogen 17504044) with 20 pg/ml bFGF and 1mM dorsomorphin inhibitor in DMEM/F-12). For organoid differentiation EBs were outlined on day 14 using a pipet tip and uplifted carefully with a cell scraper to minimize organoid fusion and tissue ripping. Media was changed once more to N-2/B-27 with bFGF only and plates with uplifted organoids were placed on a shaker in the incubator set at a rotation speed of 90. On day 14, media was changed once more to N-2/B-27 with bFGF only. Prior to day 14, media changes were performed every 48 hours. After day 14, daily media changes were performed until collection. For monolayer NPC differentiation, neural rosettes were scored and uplifted on day 14, dissociated in Accutase (Gibco A1110501), and re-plated on poly-L-ornithine (PLO)/Laminin-coated plates for NPC expansion, selection, and passaging. 15 μg/mL PLO (Sigma-Aldrich P4957) diluted in DPBS (Gibco 14040–133); 10 μg/mL laminin (Sigma-Aldrich L2020) diluted in DMEM/F-12.
Western blot analysis and immunoprecipitation
ESCs, iPSCs, and NPCs used for western blot analysis were pelleted and lysed in RIPA buffer supplemented with 1:50 protease inhibitor cocktail (Sigma-Aldrich P8340) and 1:100 phosphatase inhibitor cocktail 3 (Sigma-Aldrich P0044) using mortar and pestle coupled with end-over-end rotation for 30 minutes to 1 hr at 4°C. Protein concentration was quantified by BCA (Thermo Scientific Pierce A53227). Lysis samples were then incubated at a 1:3 ratio with 4x Laemmli sample buffer (Bio-Rad) supplemented with 10% BME and incubated at 95°C on a heat block for 5 minutes for denaturation. For co-immunoprecipitation, primary antibodies anti-THOC5 and anti-THOC6 (1:50 dilution in 1x PBS with Tween-20) were incubated overnight at 4°C with Dynabeads Protein G (Invitrogen, 10003D). Beads were washed and cell lysis (35 μg of protein) was added for incubation overnight at 4°C with rotation. IP samples were prepared according to manufacturer’s instructions with elution in Laemmli sample buffer with 10% BME. For promotion of readthrough of premature termination codons, ataluren (eMolecules NC1485023) was dissolved in DMSO added to ESC/iPSC media at a final concentration of 30 mM for 48 hours as previously described111. Protein was then extracted as described above.
Samples were loaded into 4–20% SDS-polyacrylamide gels (Bio-Rad) and proteins were separated by electrophoresis at 30V for ~4 hours room temperature. Separated proteins were then transferred to PVDF membranes (Millipore) overnight using a wet transfer system (Bio-Rad) at 4°C. For immunoblotting, membranes were incubated in 5% milk blocking buffer (1x TBS-T) followed by primary antibody incubation overnight at 4°C with rotation. Membranes were washed 3 times for 5 minutes in 1x TBS-T and then incubated with secondary antibodies for 1–2 hours at room temperature. Membranes underwent final washes before developing using West Femto Substrate (ThermoFisher 34095) with film exposure. Primary antibodies used: mouse anti-THOC6 (1:1000, Abnova H00079228-A01), rabbit anti-THOC1 (1:200, Bethyl Laboratories A302–839A), rabbit anti-THOC2 (1:200, Bethyl Laboratories A303–630A), rabbit anti-THOC5 (1:200, Bethyl Laboratories A302–120A), mouse anti-ALYREF (1:200, Sigma Aldrich A9979), rabbit anti-CHTOP (1:200, Invitrogen PA5–55929), mouse anti-b-actin (1:250, Abcam ab6276). Secondary antibodies used: donkey anti-rabbit HRP-conjugated (1:5000, Cytiva NA9340V) and goat anti-mouse HRP-conjugated (1:1000, Invitrogen 32430).
Immunofluorescence and Single-molecule Fluorescence in situ Hybridization
Human NPCs were fixed in 4% paraformaldehyde (PFA) for 20 minutes. Human cortical organoids and mouse embryos were fixed in 4% PFA for 24 hours at 4°C, cryoprotected in 15% and 30% sucrose in 1x DPBS for 24 hours at 4°C, then embedded in OCT with quick freezing in −50°C 2-methylbutane, followed by cryosectioning for immunostaining. Mouse embryos were sectioned at 13 μm and organoids at 16 μm. Samples for immunostaining were incubated for 1 hour with blocking buffer (5% NDS (Jackson ImmunoResearch) 0.1% Triton X-100, 5% BSA) at room temperature, then overnight with primary antibodies diluted in blocking buffer at 4°C, and for 1–2 hours in secondary dilution at room temperature. Washes performed in PBS. For nuclear staining, samples were incubated at room temperature for 10 minutes in Hoescht or DAPI (1:1000 dilution in PBS) prior to final washes. For EdU labeling detection, the Click-IT EdU imaging kit (Invitrogen C10337) was used according to the manufacturer’s instructions. After incubation with the Click-IT reaction cocktail, sections were blocked and immunostained as described above. Some antibodies required antigen retrieval via incubation in heated 10 mM sodium citrate solution (95–100°C) for 20 minutes prior to immunostaining. Primary antibodies used: mouse anti-PAX6 (1:250, Abcam MA-109), rabbit anti-KI67 (1:200, Abcam ab16667), rat anti-PH3 (1:250, Abcam ab10543), rabbit anti-cleaved caspase3 (1:100–1:400, Cell Signaling 9661), mouse anti-N-Cadherin (BD Biosciences 610920), goat anti-DCX (1:400, Santa Cruz Biotechnology A1313), rat anti-CTIP2 (1:500, Abcam ab18465), rabbit anti-PAX6 (1:100, BioLegend PRB-278P) and goat anti-SOX1 (1:100, R&D Biosystems AF3369). AlexaFluor-conjugated secondaries used: donkey anti-mouse 647 (1:400, Invitrogen A31571), donkey anti-rat 555 (1:400, Invitrogen A48270), and donkey anti-rabbit 488 (1:400, Invitrogen A21206).
Embryos and organoids used for RNA Fluorescence in situ Hybridization (FISH) were fixed and cryoprotected as indicated above using RNAse-free PBS. RNAse-zap treatment of sectioning equipment was performed prior to cryosectioning. NPCs for RNA FISH were fixed in RNAse-free 4% PFA then permeabilized in PBS-TritonX (0.1%) for 15 minutes. Hybridizations were then performed overnight at 37°C with a final concentration of 2 ng/μl of Cy3-conjugated oligo-dT(30-mer) probe, MALAT1 (Quasar-670, Stellaris VSMF-2211–5), and/or MEG3 (Quasar-570, Stellaris VSMF-20346–5). Saline-sodium citrate washes were performed before and after hybridization, followed by nuclear staining with RNAse-free Hoescht-PBS wash (1:1000 dilution) and final wash in RNAse-free PBS.
Glass covers were mounted onto all slides with Prolong Gold (Molecular Probes S36972) and incubated for 24 hours at room temperature prior to imaging. Imaging was performed with a Nikon A1ss inverted confocal microscope using NIS-Elements Advanced Research software. Image analysis was performed using Fiji (ImageJ) software112. For oligo-dT FISH, Z-series images were taken every 0.2 mm across entire width of cells for each genotype using same laser intensity settings and collapsed by max intensity using Z project tool in Fiji for quantification of nuclear and cytoplasmic fractions of polyA intensity by automated quantitation with CellProfiler (v4.2.1). Hoechst signal was used to segment nuclei and the oligo-dT signal to segment cell body. Three differentiation replicates per genotype. 3D surface plots were made in Fiji.
WGA inhibition of nuclear export
Confluent NPCs were incubated with digitonin at 30 mg/mL diluted in DMSO and WGA conjugated to Alexa Fluor 488 (Invitrogen, W11261) at 5 mg/mL diluted in DPBS for 5 minutes, as previously described45. Cells were washed to remove digitonin and WGA only was added to media at 5 mg/mL for 1 hour. Control NPCs were only treated with digitonin. Cells were fixed and prepped for oligo-dT FISH as described above.
RNA sequencing and bioinformatics analysis
Total RNA was extracted from cultured hNPCs (two biological replicates per genotype) using TRIzol Reagent (Invitrogen 15596026) followed by DNAse column treatment using PureLink RNA extraction kit (Invitrogen 12183018A). Total RNA from dissected E9.5 mouse forebrain tissue (three biological replicates per genotype) was extracted using Picopure RNA isolation kit (Applied Biosystems KIT0204) according to manufacturer’s recommendations. hNPC and E9.5 mouse forebrain RNA samples were ribo-depleted followed by 151 bp paired-end sequencing on the Illumina NovaSeq 300 cycle, ~20–30 million reads per sample. Library preparation and sequencing was conducted by the Advanced Genomics Core (AGC) at the University of Michigan. ERCC spike-ins (Invitrogen 4456740) were added for sequencing controls at starting concentrations according to the manufacturer’s instructions. FASTQ files were trimmed with Cutadapt v4.1 using default parameters113. Read quality was assessed by FASTQC v0.11.9114. MultiQC v1.7115 was used to visualize FASTQC outputs and compare samples. ERCC spike-in FASTA and GTF annotation files were merged with human GRCh38.p13 reference genome FASTA with GTF release 39 or mouse GRCm39 reference genome FASTA with GTF release M28. FASTQ reads were then mapped to merged files using STAR alignment with parameter ‘--outSAMtype BAM SortedByCoordinate’116. Count analysis was performed on sorted BAM files using RSEM with paired-end alignment specified117. Differential expression analysis was carried out using DESeq2 v1.34.0118 in R v4.1.2119. ERCC spike-in counts were used to estimate size factors for each sample for DESEq2 analysis. Genes were considered dysregulated if FDR < 0.05 and fold-change > 2 or < −2. Volcano and PCA plots were made using ggplot2 and pcaExplorer packages in R.
Alternative splicing analysis was performed on sorted BAM files using rMATS v4.1.2120 with the following parameters: ‘-t paired --readLength 150 --variable-read-length --nthread 4’47. AS events were called if FDR < 0.05 and ΔPSI > 10%. Events with less than 5 average reads were filtered out using the MASER package in R121. To calculate splice site strength at 5’ and 3’ splice sites in AS transcripts identified by rMATS, maximum entropy modeling was carried out using MaxEntScan54. The required input is a 9-mer sequence at 5’ splice sites (3 bases in exon and 6 bases in downstream intron) and a 23-mer at 3’ splice site (20 bases of intron and 3 bases of downstream exon). Scores were plotted in GraphPad Prism (v9.3.1).
DAVID (david.ncifcrf.gov/tools)122 and Metascape (metascape.org)123 analyses were performed to identify enriched biological pathways based on Benjamini-Hochberg multiple hypothesis corrections of the p-values. To identify potential transcription factors responsible for expression differences, Gene Set Enrichment Analysis (GSEA v4.2.3) against the MSigDB transcription factor motif gene set (c4.tftv7.5.1.symbols.gmt) and ChIP-X Enrichment Analysis v3 (ChEA3) were performed124. Ensembl BioMart tool (http://useast.ensembl.org/biomart) was used to obtain coding sequence length, transcript number per gene, gene type, and sequences for AS events. The GeneOverlap v1.32 R package was used to identify overlapping DE and AS hits between affected genotypes. Primary and candidate syndromic ID genes were obtained from the SysID database (https://www.sysid.dbmr.unibe.ch).
qRT-PCR and mRNA half-live analysis
Reverse transcription for cDNA synthesis was performed using 1 μg of total RNA with Superscript III first-strand synthesis kit (Invitrogen 18080051) according to manufacturer’s instructions. For validation of AS events, standard PCR was performed as described above. Abundances of THOC6, FOS, GAPDH, MEG3, MEG8, ESRG, and NEAT1 mRNA was determined by quantitative real-time PCR (qPCR) using the Applied Biosystems 7500 system with 7500 Software v2.3 and Radiant Green 2X qPCR Mix Lo-ROX 2X qPCR Mix (Alkali Scientific inc., QS1020) according to manufacturer’s instructions. Cycler parameters used: cDNA activation (1 cycle at 95°C for 2 minutes), denaturation (40 cycles 95°C for 5 seconds) and annealing/extension (40 cycles at 60°C for 30 seconds). The ΔΔCt method was used to analyze data with GAPDH as a reference gene. ΔΔCt values obtained by subtracting mean THOC6+/+ ΔCt values for each sample. Data shown represent mean values of three qPCR technical replicates per sample for three biological replicates per genotype (independent differentiations for NPCs). Melt curve analysis was performed on all primers to ensure temperature peaks at ~80–90°C. GAPDH and FOS primer sequences were obtained from43. NEAT1 was obtained from65 and MEG3 was obtained from66. All others were designed using NCBI primer blast. Primer sequences provided in Table S3.
mRNA decay analysis was performed using transcription inhibition by Actinomycin D (ActD) based on43. Human ESCs/iPSCs were first passaged into five 12-well plates. Each plate had the following lines: THOC6+/+ (H9 ESCs), THOC6+/+ (AS0041 iPSCs), THOC6W100*/W100*, THOC6W100*/+, THOC6E188K/E188K, THOC6E188K/+. Once confluent, ActD was added to media of all four plates at 10 mg/mL (Sigma-Aldrich A9415). After 30 minutes, media was removed from one plate and 1 mL of TRIzol Reagent was added directly to each well (t = 0). Cells were uplifted in TRIzol by pipetting and transferred to a fresh tube. Tubes were immediately frozen in TRIzol at ‒80°C. This was repeated every 30 minutes to obtain the following time points 30 minutes post-ActD treatment: t = 0.5, 1, 1.5, and 2 hrs. Extractions were performed in batches per time point based on protocol described above. Standard curve analysis was performed to validate primers (Figure S1C). This experiment was repeated to capture longer decay window using the following time points: t = 0, 2, 4, 8 (Figure S1D). ΔΔCt values obtained by subtracting mean t = 0 ΔCt for each genotype. Abundances of THOC6, GAPDH, and FOS (positive control for rapid decay) mRNA was determined.
Quantification and statistical analyses
Statistical significance of all quantifications from microscopy images, western blot images, gel images, and qRT-PCR abundances was tested using a student’s two-tailed t-test and data was plotted using GraphPad Prism (v9.3.1) as mean ±SEM or mean ±SD, as specified in figure legends. Simple linear regression was performed in qRT-PCR standard curve analysis, organoid growth curves, and intron retention analysis. Statistical significance of gene overlaps were tested using Fisher’s exact test via GeneOverlap package function testGeneOverlap() in R. Benjamini-Hochberg multiple hypothesis corrections were performed in pathway enrichment analyses.
ACKNOWLEDGEMENTS
The authors would like to especially thank all the family members who participated in this study and gave permission to use genetic and clinical information. We thank Stephanie Moon for guidance on mRNA stability assay, Sumin Kim for reagents and protocols for oligo-dT FISH, and the University of Michigan Advanced Genomics, Transgenic Animal Model, and Microscopy Cores for sequencing and equipment. This research was supported by NSF Doctoral Dissertation Research Improvement Grant 1919671: F055138, Wenner-Gren Foundation for Anthropological Research N02820: 9967, Leakey Foundation Dissertation Fieldwork Grant, University of Michigan Rackham Candidate Research Grant, the Joan B. Kessler Award, and the University of Michigan Pandemic Research Recovery Award to E.A.W, as well as the National Institutes of Health award numbers R01AWD010411 to S.L.B, A.S., and K.M.G, R00HD099403 to A.E.S, F31NS122207 and T32NS077888 to G.R.L, and T32GM008056 to K.J.
Footnotes
COMPETING INTERESTS
The authors declare no competing interests.
Supplementary Files
Contributor Information
Elizabeth Werren, University of Michigan–Ann Arbor.
Geneva LaForce, Case Western Reserve University.
Anshika Srivastava, Sanjay Gandhi Postgraduate Institute of Medical Sciences.
Delia Perillo, University of Michigan.
Katherine Johnson, Case Western Reserve University.
Brandon Berger, Case Western Reserve University.
Samantha Regan, University of Michigan.
Christian Pfennig, University of Michigan.
Safa Baris, Marmara University Hospital.
Sonja de Munnik, Radboud University Medical Centre Nijmegen.
Rolph Pfundt, Radboud University Medical Centre.
Malavika Hebbar, University of Washington.
Raul Jimenez Heredia, CeMM Research Center for Molecular Medicine.
Elif Karakoc-Aydiner, Marmara University Hospital.
Ahmet Ozen, Marmara University Hospital.
Jasmin Dmytrus, CeMM Research Centre for Molecular Medicine of the Austrian Academy of Sciences.
Ana Krolo, Ludwig Boltzmann Institute for Rare and Undiagnosed Diseases.
Ken Corning, Greenwood Genetic Center.
Eloise Prijoles, Greenwood Genetic Center.
Raymond Louie, Greenwood Genetic Center.
Robert Lebel, SUNY Upstate University Hospital.
Thuy-Linh Le, Imagine Institute, INSERM U1163, Paris Descartes University.
Jeanne Amiel, Necker.
Christopher Gordon, Institut National de la Santé et de la Recherche Médicale (INSERM) UMR 1163, Institut Imagine.
Kaan Boztug, Ludwig Boltzmann Institute for Rare and Undiagnosed Diseases.
Katta Girisha, Kasturba Medical College, Manipal, Manipal Academy of Higher Education.
Anju Shukla, Kasturba Medical College.
Stephanie Bielas, University of Michigan–Ann Arbor.
Ashleigh Schaffer, Case Western Reserve University.
DATA AVAILABILITY
Data generated in this study are provided in Supplemental Information. This study did not generate new unique reagents. This paper does not report original code. RNAseq data have been deposited on GEO and are publicly available as of the date of publication. Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Stephanie L. Bielas (sbielas@umich.edu).
REFERENCES
- 1.Schalock R. L., Luckasson R. & Tassé M. J. An Overview of Intellectual Disability: Definition, Diagnosis, Classification, and Systems of Supports (12th ed.). Am J Intellect Dev Disabil 126, 439–442 (2021). 10.1352/1944-7558-126.6.439 [DOI] [PubMed] [Google Scholar]
- 2.Yang Y. et al. Molecular findings among patients referred for clinical whole-exome sequencing. JAMA 312, 1870–1879 (2014). 10.1001/jama.2014.14601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Retterer K. et al. Clinical application of whole-exome sequencing across clinical indications. Genet Med 18, 696–704 (2016). 10.1038/gim.2015.148 [DOI] [PubMed] [Google Scholar]
- 4.Vasudevan P. & Suri M. A clinical approach to developmental delay and intellectual disability. Clin Med (Lond) 17, 558–561 (2017). 10.7861/clinmedicine.17-6-558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gieldon L. et al. Diagnostic value of partial exome sequencing in developmental disorders. PLoS One 13, e0201041 (2018). 10.1371/journal.pone.0201041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anazi S. et al. Clinical genomics expands the morbid genome of intellectual disability and offers a high diagnostic yield. Mol Psychiatry 22, 615–624 (2017). 10.1038/mp.2016.113 [DOI] [PubMed] [Google Scholar]
- 7.Kochinke K. et al. Systematic Phenomics Analysis Deconvolutes Genes Mutated in Intellectual Disability into Biologically Coherent Modules. Am J Hum Genet 98, 149–164 (2016). 10.1016/j.ajhg.2015.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amos J. S. et al. Autosomal recessive mutations in THOC6 cause intellectual disability: syndrome delineation requiring forward and reverse phenotyping. Clin Genet 91, 92–99 (2017). 10.1111/cge.12793 [DOI] [PubMed] [Google Scholar]
- 9.Casey J. et al. Beaulieu-Boycott-Innes syndrome: an intellectual disability syndrome with characteristic facies. Clin Dysmorphol 25, 146–151 (2016). 10.1097/MCD.0000000000000134 [DOI] [PubMed] [Google Scholar]
- 10.Anazi S. et al. Confirming the candidacy of THOC6 in the etiology of intellectual disability. Am J Med Genet A 170A, 1367–1369 (2016). 10.1002/ajmg.a.37549 [DOI] [PubMed] [Google Scholar]
- 11.Boycott K. M. et al. A novel autosomal recessive malformation syndrome associated with developmental delay and distinctive facies maps to 16ptel in the Hutterite population. Am J Med Genet A 152A, 1349–1356 (2010). 10.1002/ajmg.a.33379 [DOI] [PubMed] [Google Scholar]
- 12.Accogli A. et al. Novel CNS malformations and skeletal anomalies in a patient with Beaulieu-boycott-Innes syndrome. Am J Med Genet A 176, 2835–2840 (2018). 10.1002/ajmg.a.40534 [DOI] [PubMed] [Google Scholar]
- 13.Gupta N. et al. First report of THOC6 related intellectual disability (Beaulieu Boycott Innes syndrome) in two siblings from India. Eur J Med Genet 63, 103742 (2020). 10.1016/j.ejmg.2019.103742 [DOI] [PubMed] [Google Scholar]
- 14.Hassanvand Amouzadeh M., Akhavan Sepahi M. & Abasi E. Proteinuria in Two Sisters with Beaulieu-Boycott-Innes Syndrome, A Case Report. Iran J Kidney Dis 14, 312–314 (2020). [PubMed] [Google Scholar]
- 15.Kiraz A., Tubaş F. & Seber T. A truncating variant in the THOC6 gene with new findings in a patient with Beaulieu-Boycott-Innes syndrome. Am J Med Genet A 188, 1568–1571 (2022). 10.1002/ajmg.a.62667 [DOI] [PubMed] [Google Scholar]
- 16.Mattioli F. et al. Clinical and functional characterization of recurrent missense variants implicated in THOC6-related intellectual disability. Hum Mol Genet (2018). 10.1093/hmg/ddy391 [DOI] [PubMed] [Google Scholar]
- 17.Ruaud L. et al. Biallelic THOC6 pathogenic variants: Prenatal phenotype and review of the literature. Birth Defects Res 114, 499–504 (2022). 10.1002/bdr2.2011 [DOI] [PubMed] [Google Scholar]
- 18.Zhang Q., Chen S., Qin Z., Zheng H. & Fan X. The first reported case of Beaulieu-Boycott-Innes syndrome caused by two novel mutations in THOC6 gene in a Chinese infant. Medicine (Baltimore) 99, e19751 (2020). 10.1097/MD.0000000000019751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lemire G., Innes A. M. & Boycott K. M. THOC6 intellectual disability syndrome. GeneReviews®[Internet] (2020). [PubMed] [Google Scholar]
- 20.Jimeno S. & Aguilera A. The THO complex as a key mRNP biogenesis factor in development and cell differentiation. J Biol 9, 6 (2010). 10.1186/jbiol217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Strässer K. & Hurt E. Splicing factor Sub2p is required for nuclear mRNA export through its interaction with Yra1p. Nature 413, 648–652 (2001). 10.1038/35098113 [DOI] [PubMed] [Google Scholar]
- 22.Köhler A. & Hurt E. Exporting RNA from the nucleus to the cytoplasm. Nat Rev Mol Cell Biol 8, 761–773 (2007). 10.1038/nrm2255 [DOI] [PubMed] [Google Scholar]
- 23.Hautbergue G. M., Hung M. L., Golovanov A. P., Lian L. Y. & Wilson S. A. Mutually exclusive interactions drive handover of mRNA from export adaptors to TAP. Proc Natl Acad Sci U S A 105, 5154–5159 (2008). 10.1073/pnas.0709167105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taniguchi I. & Ohno M. ATP-dependent recruitment of export factor Aly/REF onto intronless mRNAs by RNA helicase UAP56. Mol Cell Biol 28, 601–608 (2008). 10.1128/MCB.01341-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pühringer T. et al. Structure of the human core transcription-export complex reveals a hub for multivalent interactions. Elife 9 (2020). 10.7554/eLife.61503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh G., Pratt G., Yeo G. W. & Moore M. J. The Clothes Make the mRNA: Past and Present Trends in mRNP Fashion. Annu Rev Biochem 84, 325–354 (2015). 10.1146/annurev-biochem-080111-092106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Venter J. C. et al. The sequence of the human genome. Science 291, 1304–1351 (2001). 10.1126/science.1058040 [DOI] [PubMed] [Google Scholar]
- 28.Lander E. S. et al. Initial sequencing and analysis of the human genome. Nature 409, 860–921 (2001). 10.1038/35057062 [DOI] [PubMed] [Google Scholar]
- 29.Nagasaki H., Arita M., Nishizawa T., Suwa M. & Gotoh O. Species-specific variation of alternative splicing and transcriptional initiation in six eukaryotes. Gene 364, 53–62 (2005). 10.1016/j.gene.2005.07.027 [DOI] [PubMed] [Google Scholar]
- 30.Chen F. C., Chen C. J., Ho J. Y. & Chuang T. J. Identification and evolutionary analysis of novel exons and alternative splicing events using cross-species EST-to-genome comparisons in human, mouse and rat. BMC Bioinformatics 7, 136 (2006). 10.1186/1471-2105-7-136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chervitz S. A. et al. Using the Saccharomyces Genome Database (SGD) for analysis of protein similarities and structure. Nucleic Acids Res 27, 74–78 (1999). 10.1093/nar/27.1.74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Juneau K., Miranda M., Hillenmeyer M. E., Nislow C. & Davis R. W. Introns regulate RNA and protein abundance in yeast. Genetics 174, 511–518 (2006). 10.1534/genetics.106.058560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kumar R. et al. THOC2 Mutations Implicate mRNA-Export Pathway in X-Linked Intellectual Disability. Am J Hum Genet 97, 302–310 (2015). 10.1016/j.ajhg.2015.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chi B. et al. Aly and THO are required for assembly of the human TREX complex and association of TREX components with the spliced mRNA. Nucleic Acids Res 41, 1294–1306 (2013). 10.1093/nar/gks1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xie Y. & Ren Y. Mechanisms of nuclear mRNA export: A structural perspective. Traffic 20, 829–840 (2019). 10.1111/tra.12691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heath C. G., Viphakone N. & Wilson S. A. The role of TREX in gene expression and disease. The Biochemical journal 473, 2911–2935 (2016). 10.1042/BCJ20160010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dufu K. et al. ATP is required for interactions between UAP56 and two conserved mRNA export proteins, Aly and CIP29, to assemble the TREX complex. Genes Dev 24, 2043–2053 (2010). 10.1101/gad.1898610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luna R., Rondón A. G., Pérez-Calero C., Salas-Armenteros I. & Aguilera A. The THO Complex as a Paradigm for the Prevention of Cotranscriptional R-Loops. Cold Spring Harb Symp Quant Biol 84, 105–114 (2019). 10.1101/sqb.2019.84.039594 [DOI] [PubMed] [Google Scholar]
- 39.Pérez-Calero C. et al. UAP56/DDX39B is a major cotranscriptional RNA-DNA helicase that unwinds harmful R loops genome-wide. Genes Dev 34, 898–912 (2020). 10.1101/gad.336024.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mauger O., Lemoine F. & Scheiffele P. Targeted Intron Retention and Excision for Rapid Gene Regulation in Response to Neuronal Activity. Neuron 92, 1266–1278 (2016). 10.1016/j.neuron.2016.11.032 [DOI] [PubMed] [Google Scholar]
- 41.Beaulieu C. L. et al. Intellectual disability associated with a homozygous missense mutation in THOC6. Orphanet J Rare Dis 8, 62 (2013). 10.1186/1750-1172-8-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mattioli F. et al. Clinical and functional characterization of recurrent missense variants implicated in THOC6-related intellectual disability. Hum Mol Genet 28, 952–960 (2019). 10.1093/hmg/ddy391 [DOI] [PubMed] [Google Scholar]
- 43.Moon S. L. et al. A noncoding RNA produced by arthropod-borne flaviviruses inhibits the cellular exoribonuclease XRN1 and alters host mRNA stability. RNA 18, 2029–2040 (2012). 10.1261/rna.034330.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bahar Halpern K. et al. Nuclear Retention of mRNA in Mammalian Tissues. Cell Rep 13, 2653–2662 (2015). 10.1016/j.celrep.2015.11.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mor A. et al. Dynamics of single mRNP nucleocytoplasmic transport and export through the nuclear pore in living cells. Nat Cell Biol 12, 543–552 (2010). 10.1038/ncb2056 [DOI] [PubMed] [Google Scholar]
- 46.Viphakone N. et al. Co-transcriptional Loading of RNA Export Factors Shapes the Human Transcriptome. Mol Cell 75, 310–323.e318 (2019). 10.1016/j.molcel.2019.04.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shen S. et al. rMATS: robust and flexible detection of differential alternative splicing from replicate RNA-Seq data. Proc Natl Acad Sci U S A 111, E5593–5601 (2014). 10.1073/pnas.1419161111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jin L. et al. STRAP regulates alternative splicing fidelity during lineage commitment of mouse embryonic stem cells. Nat Commun 11, 5941 (2020). 10.1038/s41467-020-19698-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chai G. et al. Mutations in Spliceosomal Genes PPIL1 and PRP17 Cause Neurodegenerative Pontocerebellar Hypoplasia with Microcephaly. Neuron 109, 241–256.e249 (2021). 10.1016/j.neuron.2020.10.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ellis J. D. et al. Tissue-specific alternative splicing remodels protein-protein interaction networks. Mol Cell 46, 884–892 (2012). 10.1016/j.molcel.2012.05.037 [DOI] [PubMed] [Google Scholar]
- 51.Weyn-Vanhentenryck S. M. et al. Precise temporal regulation of alternative splicing during neural development. Nat Commun 9, 2189 (2018). 10.1038/s41467-018-04559-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Licatalosi D. D. et al. HITS-CLIP yields genome-wide insights into brain alternative RNA processing. Nature 456, 464–469 (2008). 10.1038/nature07488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Llorian M. et al. Position-dependent alternative splicing activity revealed by global profiling of alternative splicing events regulated by PTB. Nat Struct Mol Biol 17, 1114–1123 (2010). 10.1038/nsmb.1881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yeo G. & Burge C. B. Maximum entropy modeling of short sequence motifs with applications to RNA splicing signals. J Comput Biol 11, 377–394 (2004). 10.1089/1066527041410418 [DOI] [PubMed] [Google Scholar]
- 55.Wang Y. et al. Mechanism of alternative splicing and its regulation. Biomed Rep 3, 152–158 (2015). 10.3892/br.2014.407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li Y. et al. Induction of Expansion and Folding in Human Cerebral Organoids. Cell Stem Cell 20, 385–396.e383 (2017). 10.1016/j.stem.2016.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Andrews M. G., Subramanian L. & Kriegstein A. R. mTOR signaling regulates the morphology and migration of outer radial glia in developing human cortex. Elife 9 (2020). 10.7554/eLife.58737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vogel T., Ahrens S., Büttner N. & Krieglstein K. Transforming growth factor beta promotes neuronal cell fate of mouse cortical and hippocampal progenitors in vitro and in vivo: identification of Nedd9 as an essential signaling component. Cereb Cortex 20, 661–671 (2010). 10.1093/cercor/bhp134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Meyers E. A. & Kessler J. A. TGF-β Family Signaling in Neural and Neuronal Differentiation, Development, and Function. Cold Spring Harb Perspect Biol 9 (2017). 10.1101/cshperspect.a022244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Qu Q. et al. Wnt7a regulates multiple steps of neurogenesis. Mol Cell Biol 33, 2551–2559 (2013). 10.1128/MCB.00325-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Harrison-Uy S. J. & Pleasure S. J. Wnt signaling and forebrain development. Cold Spring Harb Perspect Biol 4, a008094 (2012). 10.1101/cshperspect.a008094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jalali A. et al. HeyL promotes neuronal differentiation of neural progenitor cells. J Neurosci Res 89, 299–309 (2011). 10.1002/jnr.22562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tsui D., Vessey J. P., Tomita H., Kaplan D. R. & Miller F. D. FoxP2 regulates neurogenesis during embryonic cortical development. J Neurosci 33, 244–258 (2013). 10.1523/JNEUROSCI.1665-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang H., Ge G., Uchida Y., Luu B. & Ahn S. Gli3 is required for maintenance and fate specification of cortical progenitors. J Neurosci 31, 6440–6448 (2011). 10.1523/JNEUROSCI.4892-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cui Y. et al. LncRNA Neat1 mediates miR-124-induced activation of Wnt/β-catenin signaling in spinal cord neural progenitor cells. Stem Cell Res Ther 10, 400 (2019). 10.1186/s13287-019-1487-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mondal T. et al. MEG3 long noncoding RNA regulates the TGF-β pathway genes through formation of RNA-DNA triplex structures. Nat Commun 6, 7743 (2015). 10.1038/ncomms8743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Greijer A. E. & van der Wall E. The role of hypoxia inducible factor 1 (HIF-1) in hypoxia induced apoptosis. J Clin Pathol 57, 1009–1014 (2004). 10.1136/jcp.2003.015032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhou S. et al. IL-6/STAT3 Induced Neuron Apoptosis in Hypoxia by Downregulating ATF6 Expression. Front Physiol 12, 729925 (2021). 10.3389/fphys.2021.729925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Qian X. et al. Brain-Region-Specific Organoids Using Mini-bioreactors for Modeling ZIKV Exposure. Cell 165, 1238–1254 (2016). 10.1016/j.cell.2016.04.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dias A. P., Dufu K., Lei H. & Reed R. A role for TREX components in the release of spliced mRNA from nuclear speckle domains. Nat Commun 1, 97 (2010). 10.1038/ncomms1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Guria A. et al. Identification of mRNAs that are spliced but not exported to the cytoplasm in the absence of THOC5 in mouse embryo fibroblasts. RNA 17, 1048–1056 (2011). 10.1261/rna.2607011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Katahira J., Inoue H., Hurt E. & Yoneda Y. Adaptor Aly and co-adaptor Thoc5 function in the Tap-p15-mediated nuclear export of HSP70 mRNA. EMBO J 28, 556–567 (2009). 10.1038/emboj.2009.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Katahira J. et al. Human TREX component Thoc5 affects alternative polyadenylation site choice by recruiting mammalian cleavage factor I. Nucleic Acids Res 41, 7060–7072 (2013). 10.1093/nar/gkt414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Luna R., Rondón A. G. & Aguilera A. New clues to understand the role of THO and other functionally related factors in mRNP biogenesis. Biochim Biophys Acta 1819, 514–520 (2012). 10.1016/j.bbagrm.2011.11.012 [DOI] [PubMed] [Google Scholar]
- 75.Mancini A. et al. THOC5/FMIP, an mRNA export TREX complex protein, is essential for hematopoietic primitive cell survival in vivo. BMC Biol 8, 1 (2010). 10.1186/1741-7007-8-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Masuda S. et al. Recruitment of the human TREX complex to mRNA during splicing. Genes Dev 19, 1512–1517 (2005). 10.1101/gad.1302205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Peña A. et al. Architecture and nucleic acids recognition mechanism of the THO complex, an mRNP assembly factor. EMBO J 31, 1605–1616 (2012). 10.1038/emboj.2012.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rondón A. G., Jimeno S. & Aguilera A. The interface between transcription and mRNP export: from THO to THSC/TREX-2. Biochim Biophys Acta 1799, 533–538 (2010). 10.1016/j.bbagrm.2010.06.002 [DOI] [PubMed] [Google Scholar]
- 79.Tran D. D. et al. THOC5 controls 3’end-processing of immediate early genes via interaction with polyadenylation specific factor 100 (CPSF100). Nucleic Acids Res 42, 12249–12260 (2014). 10.1093/nar/gku911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wickramasinghe V. O. & Laskey R. A. Control of mammalian gene expression by selective mRNA export. Nat Rev Mol Cell Biol 16, 431–442 (2015). 10.1038/nrm4010 [DOI] [PubMed] [Google Scholar]
- 81.Zuckerman B., Ron M., Mikl M., Segal E. & Ulitsky I. Gene Architecture and Sequence Composition Underpin Selective Dependency of Nuclear Export of Long RNAs on NXF1 and the TREX Complex. Mol Cell 79, 251–267.e256 (2020). 10.1016/j.molcel.2020.05.013 [DOI] [PubMed] [Google Scholar]
- 82.Maeder C. I. et al. The THO Complex Coordinates Transcripts for Synapse Development and Dopamine Neuron Survival. Cell 174, 1436–1449.e1420 (2018). 10.1016/j.cell.2018.07.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jimeno S., Rondón A. G., Luna R. & Aguilera A. The yeast THO complex and mRNA export factors link RNA metabolism with transcription and genome instability. EMBO J 21, 3526–3535 (2002). 10.1093/emboj/cdf335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chávez S. et al. A protein complex containing Tho2, Hpr1, Mft1 and a novel protein, Thp2, connects transcription elongation with mitotic recombination in Saccharomyces cerevisiae. EMBO J 19, 5824–5834 (2000). 10.1093/emboj/19.21.5824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Strässer K. et al. TREX is a conserved complex coupling transcription with messenger RNA export. Nature 417, 304–308 (2002). 10.1038/nature746 [DOI] [PubMed] [Google Scholar]
- 86.Meinel D. M. et al. Recruitment of TREX to the transcription machinery by its direct binding to the phospho-CTD of RNA polymerase II. PLoS Genet 9, e1003914 (2013). 10.1371/journal.pgen.1003914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shen J., Zhang L. & Zhao R. Biochemical characterization of the ATPase and helicase activity of UAP56, an essential pre-mRNA splicing and mRNA export factor. J Biol Chem 282, 22544–22550 (2007). 10.1074/jbc.M702304200 [DOI] [PubMed] [Google Scholar]
- 88.Chang C. T. et al. Chtop is a component of the dynamic TREX mRNA export complex. EMBO J 32, 473–486 (2013). 10.1038/emboj.2012.342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hautbergue G. M. et al. UIF, a New mRNA export adaptor that works together with REF/ALY, requires FACT for recruitment to mRNA. Curr Biol 19, 1918–1924 (2009). 10.1016/j.cub.2009.09.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Izumikawa K., Ishikawa H., Simpson R. J. & Takahashi N. Modulating the expression of Chtop, a versatile regulator of gene-specific transcription and mRNA export. RNA Biol 15, 849–855 (2018). 10.1080/15476286.2018.1465795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fan J. et al. Exosome cofactor hMTR4 competes with export adaptor ALYREF to ensure balanced nuclear RNA pools for degradation and export. EMBO J 36, 2870–2886 (2017). 10.15252/embj.201696139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang L. et al. The THO complex regulates pluripotency gene mRNA export and controls embryonic stem cell self-renewal and somatic cell reprogramming. Cell Stem Cell 13, 676–690 (2013). 10.1016/j.stem.2013.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mazin P. V., Khaitovich P., Cardoso-Moreira M. & Kaessmann H. Alternative splicing during mammalian organ development. Nat Genet 53, 925–934 (2021). 10.1038/s41588-021-00851-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang E. T. et al. Alternative isoform regulation in human tissue transcriptomes. Nature 456, 470–476 (2008). 10.1038/nature07509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zylka M. J., Simon J. M. & Philpot B. D. Gene length matters in neurons. Neuron 86, 353–355 (2015). 10.1016/j.neuron.2015.03.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Monteuuis G., Wong J. J. L., Bailey C. G., Schmitz U. & Rasko J. E. J. The changing paradigm of intron retention: regulation, ramifications and recipes. Nucleic Acids Res 47, 11497–11513 (2019). 10.1093/nar/gkz1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Braunschweig U. et al. Widespread intron retention in mammals functionally tunes transcriptomes. Genome Res 24, 1774–1786 (2014). 10.1101/gr.177790.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mazin P. V. et al. Conservation, evolution, and regulation of splicing during prefrontal cortex development in humans, chimpanzees, and macaques. RNA 24, 585–596 (2018). 10.1261/rna.064931.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yap K., Lim Z. Q., Khandelia P., Friedman B. & Makeyev E. V. Coordinated regulation of neuronal mRNA steady-state levels through developmentally controlled intron retention. Genes Dev 26, 1209–1223 (2012). 10.1101/gad.188037.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Guo C. J. et al. Distinct Processing of lncRNAs Contributes to Non-conserved Functions in Stem Cells. Cell 181, 621–636.e622 (2020). 10.1016/j.cell.2020.03.006 [DOI] [PubMed] [Google Scholar]
- 101.Azam S. et al. Nuclear retention element recruits U1 snRNP components to restrain spliced lncRNAs in the nucleus. RNA Biol 16, 1001–1009 (2019). 10.1080/15476286.2019.1620061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhao J., Zhang X., Zhou Y., Ansell P. J. & Klibanski A. Cyclic AMP stimulates MEG3 gene expression in cells through a cAMP-response element (CRE) in the MEG3 proximal promoter region. Int J Biochem Cell Biol 38, 1808–1820 (2006). 10.1016/j.biocel.2006.05.004 [DOI] [PubMed] [Google Scholar]
- 103.Sobreira N., Schiettecatte F., Valle D. & Hamosh A. GeneMatcher: a matching tool for connecting investigators with an interest in the same gene. Hum Mutat 36, 928–930 (2015). 10.1002/humu.22844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Truett G. E. et al. Preparation of PCR-quality mouse genomic DNA with hot sodium hydroxide and tris (HotSHOT). Biotechniques 29, 52, 54 (2000). 10.2144/00291bm09 [DOI] [PubMed] [Google Scholar]
- 105.Srivastava A. et al. Genetic diversity of NDUFV1-dependent mitochondrial complex I deficiency. Eur J Hum Genet 26, 1582–1587 (2018). 10.1038/s41431-018-0209-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Li H. & Durbin R. Fast and accurate short read alignment with Burrows–Wheeler transform. bioinformatics 25, 1754–1760 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Van der Auwera G. A. et al. From FastQ data to high confidence variant calls: the Genome Analysis Toolkit best practices pipeline. Curr Protoc Bioinformatics 11, 11.10.11–11.10.33 (2013). 10.1002/0471250953.bi1110s43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Krumm N. et al. Copy number variation detection and genotyping from exome sequence data. Genome Res 22, 1525–1532 (2012). 10.1101/gr.138115.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Müller H. et al. VCF.Filter: interactive prioritization of disease-linked genetic variants from sequencing data. Nucleic Acids Res 45, W567–W572 (2017). 10.1093/nar/gkx425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lancaster M. A. et al. Cerebral organoids model human brain development and microcephaly. Nature 501, 373–379 (2013). 10.1038/nature12517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Roy B. et al. Ataluren stimulates ribosomal selection of near-cognate tRNAs to promote nonsense suppression. Proc Natl Acad Sci U S A 113, 12508–12513 (2016). 10.1073/pnas.1605336113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Schindelin J. et al. Fiji: an open-source platform for biological-image analysis. Nat Methods 9, 676–682 (2012). 10.1038/nmeth.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. journal 17, 10–12 (2011). [Google Scholar]
- 114.Wingett S. W. & Andrews S. FastQ Screen: A tool for multi-genome mapping and quality control. F1000Res 7, 1338 (2018). 10.12688/f1000research.15931.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ewels P., Magnusson M., Lundin S. & Käller M. MultiQC: summarize analysis results for multiple tools and samples in a single report. Bioinformatics 32, 3047–3048 (2016). 10.1093/bioinformatics/btw354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dobin A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013). 10.1093/bioinformatics/bts635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li B. & Dewey C. N. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics 12, 323 (2011). 10.1186/1471-2105-12-323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Love M. I., Huber W. & Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15, 550 (2014). 10.1186/s13059-014-0550-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Team, R. C. R: A language and environment for statistical computing, <https://www.R-project.org/> (2018).
- 120.Park J. W., Tokheim C., Shen S. & Xing Y. Identifying differential alternative splicing events from RNA sequencing data using RNASeq-MATS. Methods Mol Biol 1038, 171–179 (2013). 10.1007/978-1-62703-514-9_10 [DOI] [PubMed] [Google Scholar]
- 121.Veiga D. F. T. maser: Mapping Alternative Splicing Events to pRoteins . 2022).
- 122.Subramanian A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A 102, 15545–15550 (2005). 10.1073/pnas.0506580102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhou Y. et al. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat Commun 10, 1523 (2019). 10.1038/s41467-019-09234-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Keenan A. B. et al. ChEA3: transcription factor enrichment analysis by orthogonal omics integration. Nucleic Acids Res 47, W212–W224 (2019). 10.1093/nar/gkz446 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data generated in this study are provided in Supplemental Information. This study did not generate new unique reagents. This paper does not report original code. RNAseq data have been deposited on GEO and are publicly available as of the date of publication. Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Stephanie L. Bielas (sbielas@umich.edu).