Abstract
Colony-stimulating factor 1 receptor (CSF1R) inhibition has been proposed as a specific method for microglia depletion. However, recent work revealed that in addition to microglia, CSF1R inhibition also affects other innate immune cells, such as peripheral monocytes and tissue-resident macrophages of the lung, liver, spleen, and peritoneum. Here, we show that this effect is not restricted to innate immune cells only but extends to the adaptive immune compartment. CSF1R inhibition alters the transcriptional profile of bone marrow cells that control T helper cell activation. In vivo or ex vivo inhibition of CSF1R profoundly changes the transcriptional profile of CD4+ cells and suppresses Th1 and Th2 differentiation in directionally stimulated and unstimulated cells and independently of microglia depletion. Given that T cells also contribute in CNS pathology, these effects may have practical implications in the interpretation of relevant experimental data.
Keywords: T-cells, microglia, CNS, differentiation, CSF1R
Introduction
We recently showed that colony stimulating factor 1 receptor (CSF1R) inhibition not only affects microglia but also alters the function of bone marrow-derived macrophages1–3; confirmed by others using CSF1R knock-out mice4,5 and in a recent article summarizing the effects of PLX5622 in innate immune cells6. However, it is still unknown if these off-target effects extend cells of the adaptive immune compartment. This is critical since recent studies have employed such inhibitors to investigate interactions between innate and adaptive immune cells and have demonstrated specific roles of T cells in CNS pathology, especially in models of autoimmune diseases and glaucoma7–9.
Here, we adopt in vivo and ex vivo models, along with targeted transcriptomics and Ingenuity Pathway Analysis (IPA), to investigate the role of CSF1R inhibition on the adaptive immune system. Here provides a brief report of the off-target effects of CSF1R inhibition, which extend to the adoptive immune system, and alert the community about the implications of these findings in interpreting relevant experimental data.
Results
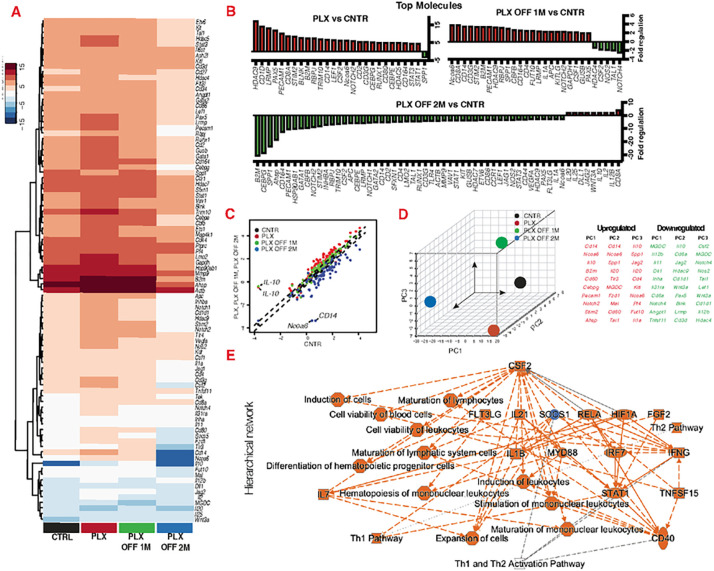
Using targeted transcriptomics, we show that CSF1R inhibition causes overexpression of Hdac9, Cd1d, Lrmp, Pax5, Pecam1, Cd8A, Stim2, Blnk, B2m, Rbpj and repression of Spp1 genes in bone marrow cells (Fig. 1A, B). Cessation of the inhibitor for 1 month does not restore these changes. Moreover, 1 month after cessation of the inhibitor, Nocoa6, Cd8a, Cd14, Cd3g, Stim2, B2m, Pecam1, Hdac9, Spp1 become over expressed and Hdac4, CSF2, Nos2, Tal1 and Notch4 repressed (Fig. 1A, B). We identified Spp1 as the single gene that switches from repression to overexpression after cessation of the inhibition (Fig. 1B), while interleukin 10 (Il-10), nuclear receptor co-activator 6 (Ncoa6) and CD14 (Fig. 1B) were identified as the most differentially expressed genes immediately, 1 and 2 months after cessation of the inhibitor. Principal Component Analysis (PCA) of the gene array confirms that CSF1R inhibition alters the transcriptional profile of hematopoiesis, and this change perdures long-term (Fig. 1D). Further analysis using ingenuity pathway predicts that CSF1R inhibition leads to upregulation of colony stimulating factor 2 (CSF2), which alters canonical pathways associated with Th1 and Th2 activation (Fig. 1E).
Figure 1.
CSF1R inhibition alters the transcriptional profile of bone marrow cells and that control Th1/Th2 activation. Gene expression analysis of bone marrow cells isolated from C57BL/6J mice using targeted transcriptomics for common hematopoietic genes immediately, 1 and 2 months after cessation of CSF1R inhibition. (A, B) CSF1R inhibition causes a wide range of transcriptional changes in hematopoietic gene expression, (C) especially through the deregulation of Il-10, Cd14and Ncoa6. (D) Principal component analysis suggests that CSF1R inhibition causes long-term transcriptional changes in bone marrow cells, even 2 months after cessation of the inhibitor. (E) Ingenuity pathway analysis of the gene array predicts that CSF1R inhibition upregulates CSF2, which alters the regulation of canonical pathways that control Th1 and Th2 activation. (A-D) n=5 per group. All entities have p-value ≤ 0.05, regulators and processes have |z-score| ≥ 2, and regulators are limited to genes only.
To assess if the model predictions are correct, we performed studies in CD4+ cells acquired from mice treated with PLX5622 (chow). CD4+ cells were analyzed with targeted transcriptomics to assess T helper cell differentiation pathway. Administration of CSF1R inhibitor for 3 weeks in mice profoundly affected T helper cell differentiation, as evident by transcriptional profile changes, notably through suppression of interleukin 12 receptor subunit beta 2 (Il12rb2) and over expression of CSF2 (Fig. 2A). Further analysis using ingenuity pathway predicted that these transcriptional changes would affect Th1 and Th2 cell activation (Fig. 2B). Indeed, this prediction was confirmed by showing that CSF1R inhibition causes a dose-dependent effect on CD4+ cell survival and led to suppression of Th1/Th2 differentiation ex vivo (Fig. 2C), independently of microglia depletion. Cessation of the inhibitor restored Th1/Th2 differentiation, while IL-12 or IL-4 stimulation of CD4+ cells enhanced Th1/Th2 differentiation, respectively, in mice treated with CSF1R inhibitor (Fig. 2D–K), though mice fed with PLX5622 had lower number of CD4+ cells (Fig. 2E).
Figure 2. CSF1R inhibition alters the transcriptional profile CD4+ cells and suppressed Th1 and Th2 differentiation.
(A) Targeted transcriptomics on CD4+ cells reveals that CSF1R inhibition causes significant changes in genes that control T helper cell differentiation. (B) Ingenuity pathway analysis predicts that these gene changes suppress Th1 and Th2 activation pathways. (C) CSF1R inhibition ex vivo causes dose dependent reduction in CD4+ cell number and suppression of Th1/Th2 differentiation. (D) Schematic representation of the experimental setup to assess Th1/Th2 differentiation after cessation of CSF1R inhibitor. (E) Percentage of CD4+ cells recovered from control and PLX treated mice. Histogram, mean fluorescent intensity and percentage of CD4+ cells that express (F-H) INFγ (Th1) or Il-4 (Th2). (F-K) Although mice treated with CSF1R inhibition have lower numbers of CD4+ cells, cessation of the inhibitor ex vivo restores their ability to differentiate, though directional Th1/Th2 stimulation ex vivo enhances the response.
Conclusion
Here, we adopt in vivo and ex vivo models, along with targeted transcriptomics and Ingenuity Pathway Analysis (IPA), to show that CSF1R inhibition not only affects innate immune cells, especially microglia and macrophages, but also bone marrow processes that control T-cell activation and differentiation. Previous studies have assumed that CSF1R inhibition may only affects microglia10–13, however, our recent studies demonstrated that CSF1R inhibitors profoundly affect peripheral monocytes and the function of macrophages1,2,4,5. To this end, additional off-target effects in other immune compartments became the target of this investigation. Here, we provide insights into the role of CSF1R inhibition on the adaptive immune cells and show that it causes changes in the regulation CD4+ and T helper cell differentiation. Our findings are important considering that T cells have recently implicated in CNS pathologies, especially in autoimmune conditions and glaucoma, and such studies have derived their conclusions by employed small-molecule CSF1R inhibitors to deplete microglia. Future experiments using CSF1R inhibitors need to account for these off-target effects in the interpretation of relevant experimental data.
Materials and Methods
Mouse model:
Animal experiments were performed in accordance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research, and the National Institutes of Health (NIH) Guidance for the Care and Use of Laboratory Animals. This study was approved by the Mass. Eye and Ear Animal Care Committee. Mice at 6–8 weeks old of both genders were used: C57BL/6J (Jackson Laboratory, Stock#: 000664). PLX5622 (PLexxikon, Inc) was formulated into AIN-76A chow (Research Diets, Inc) at the dose of 1200 ppm and given ad libitum for 3 weeks.
Ex vivo differentiation:
Spleen and lymph nodes from C57BL/6 mice were surgically removed and single cells were obtained mechanically using a 70μm cell strainer (BD Falcon). Red blood cells were lysed by ACK lysis buffer (Lonza) before CD4+ isolation, using the CD4+ T cell isolation Kit (Miltenyi). 0.5 ×106 CD4+ cells/mL were suspended in 10% FBS RPMI-1640 (ThermoFisher) supplemented with 5 ng/mL IL-2 (PeproTech) and 0.5 μg/mL anti-CD28 antibody (clone:37.51, BioLegend) and seeded into 2 μg/mL anti-CD3 antibody (clone: 145–2C11, BioLegend) pre-coated 24-well plates. Th1 differentiation was achieved using 1 μg/mL anti-IL-4 antibody (clone: 11B11, Biolegend), 5 ng/mL IL-2 (PeproTech), and 10 ng/mL IL-12 (PeproTech) in the culture media. Th2 differentiation was achieved using 1 μg/mL anti-IFN-γ antibodies (clone: XMG1.2, Biolegend), 5 ng/mL IL-2(PeproTech), and 10 ng/mL IL-4 (PeproTech) in the culture media. Cells were incubated for 96 hours in 5% CO2 at 37°C. Cell Activation Cocktail with Brefeldin A (Biolegend) was added to the culture media 5 hours prior to harvesting and staining the cells. For PLX5622 treatment, different concentrations of PLX5622 were added to the culture media during the stage of differentiation to Th1/Th2.
Flow cytometry:
Cultured CD4+ T cells and ex vivo splenocytes were processed for flow cytometry. Anti-murine CD16/32 (Clone: 2.4G2, eBiosciences) was used for blocking. Anti-CD3 (Clone: 17A2), anti-CD4 (Clone: GK1.5), anti-IL-4 (Clone: 11B11), anti-INF-γ (Clone: XMG1.2) were purchased from BioLegend. Data were acquired by LSRII cytometer (BD Biosciences) and analyzed by FLowJo V10 (Tree Star).
RT2 profiler and qPCR:
A total of 1 μg of RNA was used for each array analysis. cDNA was synthesized by RT2 First Strand Kit (Qiagen, Hilden, Germany) and then mixed with RT2 SYBR Green ROX qPCR Mastermix (Qiagen) before loading into the array well (Mouse hematopoiesis: PAMM-054ZA; Mouse T helper cell differentiation: PAMM-503ZA and Mouser Retinoic Acid Signaling: PAMM-180ZA). qPCR was performed by using QuantStudio 3 (ThermoFisher) according to manufacturer’s manual. Data analysis was performed by using Qiagen’s data analysis web portal (https://geneglobe.qiagen.com/us/analyze/) to generate a normalized expression profile. Further normalization was performed based on 2^ (- Delta Delta CT). Gene expression heat-map, 3D PCA plot, and scatter plot were generated using the normalized gene set in R-software version 1.14.4.
Network analysis:
Ingenuity Pathway Analysis (IPA, Redwood City, CA, USA) was employed to assess how gene changes effect canonical pathways, upstream regulators, and disease/functions associated14. A threshold of 2-fold change in gene expression and P < 0.05 was set for analysis. Data were ranked based on their z-score.
Statistical analysis:
Data were analyzed with GraphPad (Prism 2.8.1, San Diego, CA) using two-tailed unpaired t-test and ordinary one-way ANOVA with Dunnet’s correction for multiple comparisons. Statistical significance was set at P < 0.05.
Significance statement.
Here we show that CSF1R inhibition affects not only innate immune cells and microglia, but also the adaptive immune compartment by suppressing Th1/Th2 differentiation of CD4+ cells, independently of microglia depletion.
Acknowledgments:
This work was supported by the Boston Keratoprosthesis Research Fund, DoD 2019A016948, and core grant P30EY003790; PLX5622 was kindly provided by Plexxikon Inc. We would like to thank Dr. Thomas Dohlman for providing columns for T-cell isolation.
Funding:
Boston Keratoprosthesis Fund, Mass Eye and Ear, Core grant 5P30EY003790 “Core Grant for Vision Research
Footnotes
Competing interests: The authors declare no conflict of interest.
Ethical Approval: The animal study protocol was approved by the Institutional Review Board of the Mass Eye and Ear under the protocols 10–033A and 2021N000008.
Contributor Information
Fengyang Lei, Massachusetts Eye and Ear, Harvard Medical School.
Naiwen Cui, Harvard University.
Chengxin Zhou, Massachusetts Eye and Ear, Harvard Medical School.
James Chodosh, University of New Mexico.
Demetrios G. Vavvas, Massachusetts Eye and Ear, Harvard Medical School.
Eleftherios I. Paschalis, Massachusetts Eye and Ear, Harvard Medical School.
Data Availability:
All data necessary for study replication have been included in the submission. Materials are available commercially and are listed in Materials and Methods.
References
- 1.Lei F., Cui N., Zhou C., Chodosh J., Vavvas D. G. & Paschalis E. I. CSF1R inhibition by a small-molecule inhibitor is not microglia specific; affecting hematopoiesis and the function of macrophages. Proc Natl Acad Sci U S A (2020). 10.1073/pnas.1922788117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lei F., Cui N., Zhou C., Chodosh J., Vavvas D. G. & Paschalis E. I. Reply to Green and Hume: Nonmicroglia peripheral immune effects of short-term CSF1R inhibition with PLX5622. Proceedings of the National Academy of Sciences of the United States of America 118 (2021). 10.1073/pnas.2020660118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paschalis E. I. et al. Permanent neuroglial remodeling of the retina following infiltration of CSF1R inhibition-resistant peripheral monocytes. Proceedings of the National Academy of Sciences of the United States of America 115, E11359–E11368 (2018). 10.1073/pnas.1807123115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Delaney C. et al. Attenuated CSF-1R signalling drives cerebrovascular pathology. EMBO molecular medicine, e12889 (2020). 10.15252/emmm.202012889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Han J. et al. Underestimated Peripheral Effects Following Pharmacological and Conditional Genetic Microglial Depletion. Int J Mol Sci 21 (2020). 10.3390/ijms21228603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spiteri A. G. & King N. J. C. Putting PLX5622 into perspective: microglia in central nervous system viral infection. Neural Regen Res 18, 1269–1270 (2023). 10.4103/1673-5374.360170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen H. et al. Commensal microflora-induced T cell responses mediate progressive neurodegeneration in glaucoma. Nature communications 9, 262–213 (2018). 10.1038/s41467-018-05681-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaskow B. J. & Baecher-Allan C. Effector T Cells in Multiple Sclerosis. Cold Spring Harb Perspect Med 8 (2018). 10.1101/cshperspect.a029025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kunkl M., Frascolla S., Amormino C., Volpe E. & Tuosto L. T Helper Cells: The Modulators of Inflammation in Multiple Sclerosis. Cells 9 (2020). 10.3390/cells9020482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elmore M. R. P. et al. Colony-stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain. Neuron 82, 380–397 (2014). 10.1016/j.neuron.2014.02.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mok S. et al. Inhibition of CSF-1 Receptor Improves the Antitumor Efficacy of Adoptive Cell Transfer Immunotherapy. Cancer research 74, 153–161 (2014). 10.1158/0008-5472.CAN-13-1816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hilla A. M., Diekmann H. & Fischer D. Microglia Are Irrelevant for Neuronal Degeneration and Axon Regeneration after Acute Injury. Journal of Neuroscience 37, 6113–6124 (2017). 10.1523/JNEUROSCI.0584-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bellver-Landete V. et al. Microglia are an essential component of the neuroprotective scar that forms after spinal cord injury. Nature communications 10, 518 (2019). 10.1038/s41467-019-08446-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomas S. & Bonchev D. A survey of current software for network analysis in molecular biology. Hum Genomics 4, 353–360 (2010). 10.1186/1479-7364-4-5-353 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data necessary for study replication have been included in the submission. Materials are available commercially and are listed in Materials and Methods.