Abstract
The cornerstone of structural biology is the unique relationship between protein sequence and the 3D structure at equilibrium. Although intrinsically disordered proteins (IDPs) do not fold into a specific 3D structure, breaking this paradigm, some IDPs exhibit large-scale organization, such as liquid-liquid phase separation. In such cases, the structural plasticity has the potential to form numerous self-assembled structures out of thermal equilibrium. Here, we report that high-temperature incubation time is a defining parameter for micro and nanoscale self-assembly of resilin-like IDPs. Interestingly, high-resolution scanning electron microscopy micrographs reveal that an extended incubation time leads to the formation of micron-size rods and ellipsoids that depend on the amino acid sequence. More surprisingly, a prolonged incubation time also induces amino acid composition-dependent formation of short-range nanoscale order, such as periodic lamellar nanostructures. We can correlate the lamellar structures to β-sheet formation and demonstrate similarities between the observed nanoscopic structural arrangement and spider silk. We, therefore, suggest that regulating the period of high-temperature incubation, in the one-phase regime, can serve as a unique method of controlling the hierarchical self-assembly mechanism of structurally disordered proteins.
Introduction
The organization of macromolecules into compartments allows cells tight regulation of physiological functions. In addition to classical membrane-bound organelles, eukaryotic cells also form dynamic, liquid-like membrane-less organelles (MLOs) to orchestrate biochemical reactions and cellular stress effectively.1–3
Intrinsically disordered proteins (IDPs) have significant stretches in their primary amino acid sequence-termed intrinsically disordered regions (IDRs) that lack a singular, stable 3D structure.4–7 Such IDPs have attracted significant attention as hubs of protein-interaction networks that regulate transcription, translation, cell cycle8–10, and modulate intracellular signaling.11–13 In addition, liquid-liquid phase separation (LLPS) of IDPs has been shown to play an important role in the formation of MLOs,14–16 and dysregulation of LLPS due to irreversible aggregation of IDPs can lead to a variety of pathological diseases.17–19
The LLPS of IDPs has also enabled diverse applications ranging from protein purification20, drug delivery21–23, self-assembly of artificial MLOs into nano-meso structures24–26, to injectable biomaterials and hydrogels.27–29 Importantly, beyond the sequence, other external stimuli, such as pH, protein concentration, and temperature, have also been shown to affect LLPS.30
Like synthetic polymers, IDPs can also exhibit thermoresponsive phase behavior, controlled by the spatial orientation of amino acid side chains and peptide-water interactions.31–33 IDPs with an upper critical solution temperature (UCST) undergo LLPS below their transition temperature (Tt), also called the cloud point temperature. At high temperatures (T > Tt), the system is a homogenous solution, while at lower temperatures, it exists as a two-phase system consisting of an IDP-rich coacervate phase (immiscible in water) and a dilute phase of IDPs dissolved in the solvent. The UCST phase -separation is reversible, and above the Tt, the coacervates become soluble again.20,34 In contrast, other IDP sequences with a lower critical solution temperature (LCST) exhibit phase separation above their cloud point temperature.34 Examples of UCST and LCST IDP-based thermoresponsive polymers are resilin-like polypeptides (RLPs)33 and elastin-like polypeptides (ELPs) 34, derived from the disordered sequences of the natural proteins resilin35 and tropoelastin36, respectively.
Designing new strategies to drive the self-assembly of resilin-like IDPs is important in light of their possible applications in tissue engineering, bioelectronics, bioimaging and biosensors.37 Here, we investigate the thermoresponsive behavior and self-assembly of RLPs, a class of synthetic intrinsically disordered proteins (SynIDPs) derived from resilin.35 We focus on their self-assembly into nano- and micro-scale structures modulated by changes in incubation time at temperatures above the LLPS transition temperature. In addition, we show that it is possible to fine-tune the resulting self-assembled structures by alterations in the sequence and the number of repeats of the RLPs.
Results
This study investigated the hierarchical self-assembly of SynIDPs derived from a 8 amino acid repeats (GRGDSPYS) inspired by the Drosophila Melanogaster Rec-1 resilin protein, which exhibits UCST phase behavior.20 Here, we focused on three RLPs, the octapeptide parent sequence is known as Wild type (WT), while the notation for the RLPs are [WT]XX, where XX represents the number of octapeptide repeats in the SynIDPs, for example, [WT]40 and [WT]80. Further, the terminology of mutant versions of the RLPs is according to the position and type of amino acids that is substituted for the amino acid into the WT octapeptide repeat. For example, the mutant [V7]40 has V substituted for the Y at the 7th position in the octapeptide WT repeat. Specifically, we examined the effect of incubation time at 80° C (t80), varying the number of octapeptide repeats and changing the sequence of the SynIDPs. All studied RLPs sequences are presented in Table 1.
Table 1.
Characteristics of the A-IDP sequences
| Protein name | Sequence | No. of amino acids | Molecular weight (Da) |
|---|---|---|---|
| [WT]20 | SKGP-(GRGDSPYS)20-GY | 166 | 17,004 |
| [WT]40 | SKGP-(GRGDSPYS)40-GY | 326 | 33,400 |
| [WT]60 | SKGP-(GRGDSPYS)60-GY | 486 | 49,797 |
| [WT]80 | SKGP-(GRGDSPYS)80-GY | 646 | 66,193 |
| [3Y7V7]40 | SKGP-[(GRGDSPYS)3 GRGDSPVS]10-GY | 326 | 32,760 |
| [Y7V7]40 | SKGP-[GRGDSPYSGRGDSPVS]20-GY | 326 | 32,119 |
| [V7]40 | SKGP-(GRGDSPVS)40-GY | 326 | 30,839 |
Microscale self-assembly
Previous reports suggest that RLPs [WT]40, and [WT]80, show reversible LLPS below the cloud point, Tt = 40°C, when measured in 150 mM phosphate buffer of pH 7.4.20 Both [WT]40 and [WT]80 RLPs are insoluble when prepared in buffer at 25°C. However, these samples do show LLPS after incubation at 80°C for t80 = 1 h and cooling back to 25°C. In contrast, the mutant [V7]40 did not show such reversible UCST phase behavior and remained insoluble after 1 h of incubation at 80°C.
We investigated the effect of incubation time on the microscale self-assembly of [WT]40, [WT]80, and [V7]40 by incubating 100 μM solutions of the RLPs in 150 mM phosphate buffer at 80°C for 1, 24, and 48 h. After incubation, the solutions were cooled back to 25°C for 10 min. For convenience, we term a sample with XX repeats incubated at 80°C for t80 hours and measured at 25°C as [sample]]xx-t80.
High-resolution scanning electron microscopic (HRSEM) was used to characterize the morphology of the microstructures after different incubation times (Figs. 1–3). Before hydration and self-assembly, the HRSEM images of [WT]40 powder samples are amorphous (Fig. 1a). However, after hydration and 1 h incubation at high temperature, images show that [WT]40 are predominantly composed of 400 nm wide and ~600 nm long micro-ellipsoids with a few ~ 500 nm diameter rods that are several μm in length (Fig. 1b). A more prolonged incubation (t80 = 24 h), increased the number of rods in this system (Fig. 1c). Longer incubation (t80 = 48 h) produced an interconnected system of rods ~ 500 nm wide, but with length in microns (Fig. 1c). These rods appear to be formed due to the systematic arrangement of ellipsoidal particles through inter-connecting nanofibers (arrows in Fig. 1b & Supplementary Fig. S1a).
Figure 1.
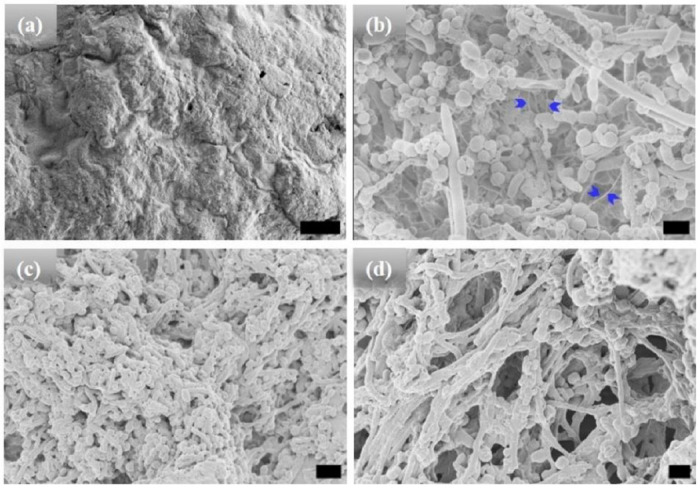
HRSEM micrographs of [WT]40 RLP (a) amorphous powder samples. (b)-(d) [WT]40 in phosphate buffer solutions after incubation at 80°C for 1, 24, and 48 h, respectively. Incubation for 1 h results in the formation of micro-ellipsoids of 500 nm width and ~1 μm in length and a few rods of ~ 500 nm in diameter and several μm in length (b). A longer incubation of 24 h leads to an increase in the micro-rod population (c). A 48 h incubation produced microstructures with interconnected rods (d). Arrows in (b) mark interconnecting nanofibers, which leads to self-assembly of micro-ellipsoids to form micro-rods. Scale bar:1μm.
Figure 3.

HRSEM images of [V7]40 RLP (a) powder samples, (b)-(d) [V7]40 RLP solutions after incubation at 80°C for 1, 24, and 48 h, respectively. The powder samples and solutions incubated for 1 and 24 h have an amorphous structure, but micro-ellipsoids accompanied by few nanorods are observed after 48 h incubation at 80°C. Scale bar:1μm.
The [WT]80 powder samples also exhibit an amorphous structure (Fig. 2a). However, imaging revealed that a hydrated [WT]80 solution undergoes LLPS after incubation at 80°C for 1 h, with the formation of condensates of several μm in length (Fig. 2b). Micro-rods and micro-ellipsoids were only seen after incubating the [WT]80 peptide in buffer solution for 24 h at 80°C (Fig. 2c). Similar to [WT]40 RLP incubated for 48 h, [WT]80-24 h sample also exhibits interconnecting nanofibers between the micro-ellipsoids which connect together to form micron size rods (Supplementary Fig. S1b). Thus, in both [WT]40 and [WT]80 systems, 48 h of incubation at 80°C induced the formation of long rods accompanied by a few micro-ellipsoids (Figs. 1d, 2d).
Figure 2.

HRSEM images of [WT]80 RLP (a) amorphous powder samples. Micrographs of RLP solutions in buffer after incubation at 80°C. (b) A 1h incubation results in the formation of several μm long condensates, (c) 24 h incubation produces a mixture of micro-rods and micro-ellipsoids, and (d) 48 h incubation results in several μm long micro-rods accompanied by some micro-ellipsoids. Scale bar:1μm.
Unlike the [WT] RLPs, a powder sample of [V7]40 does not undergo LLPS and exhibits an amorphous structure (Fig. 3a). The sample remained amorphous after 1 and 24h incubation at 80°C (Fig. 3b & c respectively) and only after extended incubation at high temperature (i.e., t80 = 48 h), the sample become turbid and exhibit ellipsoidal particles that were ~500nm in width and ~650 nm in length as observed by HRSEM with a few several μm long rods (Fig. 3d). Unlike [WT]40, the primary morphology observed for [V7]40 after 48 h incubation is micro-ellipsoidal rather than elongated rods.
Nanoscopic Self-Assembly
Motivated by the microscale self-assembly of SynIDPs and the correlation with incubation time, we used small- and wide-angle X-ray scattering (SAXS, WAXS) techniques to investigate the nanoscopic structures.38,39 RLP samples and excess supernatant were sealed in polycarbonate capillaries and placed into a measurement heating stage. This allowed temperature dependent investigation of the samples with the desired high temperature incubation. For phase separated samples the X-ray scattering measurements is of the pellet positioned at the bottom of the capillary. The lack of correlation peaks in the scattering profiles of [WT]40, [WT]80, and [V7]40 solutions after 1 h incubation (Fig. 4a) indicate that the structure is amorphous with no long- or short-range repeated order. Notably, there was no order in lyophilized powders of these A-IDPs (Supplementary Fig. S2a).
Figure 4.

The scattering profiles of the [WT]40, [WT]80, and [V7]40 RLPs after incubation at 80°C (t80) for the duration of (a) 1 h, (b) 24 h, and (c) 48 h. There were no correlation peaks after 1 h but [WT]80-24 h and [V7]40-48 h RLPs exhibit two intense correlation peaks qa, qb and q1, q’ respectively, indicative of nanoscopic long- or short-range ordering (d) Extended scattering profile of the [V7]40-48 h RLP exhibiting lamellar peaks (q1, q2, q4), and a singular correlation peak (q′) with no additional reflections, and peaks corresponding to the secondary structure of proteins (qβ1 and qβ2). The peak at q~3.4 nm−1 (shown by a dotted line) corresponds to a residual diffraction peak of the kapton window originating from the experimental setup.40
To our surprise, incubation of [WT]80 peptide solutions for t80 = 24 h resulted in the appearance of two sharp correlation peaks (i.e., qa and qb) in the scattering profile (Fig. 4b). Such scattering peaks indicate the presence of repeated nanoscopic structures. Increasing the incubation time to 48 h produced intense correlation peaks only in the [V7]40-48 h system (Figs. 4c & 4d). Interestingly, the scattering profiles of [3Y7V7] and [Y7V7], where 25% and 50% of Y are replaced by V, respectively, do not exhibit correlation peaks after 48 h incubation (Supplementary Fig. S2b). Therefore, we can conclude that the both the sequence and incubation time ultimately dictate the nanoscopic long- or short-range self-assembly in RLPs considered for our studies.Specifically, the scattering profile of the [V7]40-48 h system exhibits six correlation peaks (q1, q2, q4, q′, qβ1, and qβ2, Fig. 4d) where the positions of peaks q1, q2 and q4 are in the ratio q1:2q1:4q1 and q1 = 1.082 nm−1. This profile indicates the presence of an ordered lamellar morphology corresponding to an interplanar distance of 5.8 nm. In addition, there was a strong singular correlation peak, q′ = 1.646 nm−1, without any additional harmonics (Fig. 4d). The spacing of the correlation peaks at qβ1 = 15.09 nm−1 and qβ2 = 15.4 nm−1, corresponding to real space distances of 0.416 nm (dβ1) and 0.406 nm (dβ2), which is typical of protein secondary structure (Fig. 4d).
Previous measurements described the H-bonding distance between antiparallel strands of the β-sheets of some nylons to be 0.474 nm.41,42 In line with that, we presume that in our [V7]40-48 h system, the correlation peak qβ2 arises due to repetitive arrangement of β-strands through backbone H-bonds with inter-strand spacing of qβ2 (0.406 nm). Further, the reported inter-sheet distances between stacked β-sheets ranges from a minimum of 0.34 nm for poly(Gly)43 to a maximum of 0.79 nm for Nephila Senegalese silk fibroin, a silk that contains a high percentage of amino acid residues with bulky side chains.44 Therefore, the dβ1 = 0.416 nm spacing we observed corresponds to an average inter-sheet distance between stacked β-sheets.
To validate the presence of secondary structure, we performed circular dichroism (CD) studies for [V7]40 systems after various incubation times at high temperatures. We acknowledge that for IDPs, the analysis of CD spectra is limited, since current models were derived based on folded proteins.45,46 Nonetheless, after one hour of incubation the [V7]40-1h RLP exhibits a peak at 197nm and rather flat spectrum through rest of wavelength considered. This is because [V7]40-1h sample is insoluble in phosphate buffer. Thus, it’s not possible to predict secondary structures from the CD spectra of [V7]40-1h sample with certainty (Fig. 5a).
Figure 5.
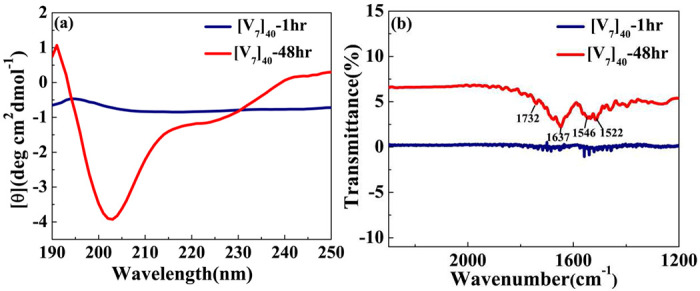
(a) CD spectra for [V7]40 RLP (a) After 1 and 48 hr incubation. The [V7]40-48hrsampie exhibits profile with vallies at the wavelengths of ~203 nm and ~225nm and a peak at 190nm characteristic to β-sheets. (b) FTIR spectra of [V7]40-1hr and [V7]40-48hr sample. The presence of infrared bands at 1522, 1637 and 1732 cm−1 in [V7]40-48hr sample confirms the presence of antiparallel β-sheets.
Following 48 h incubation, the [V7]40 RLP did develop the characteristic valleys at ~ 203 and 225 nm and a peak at 190 nm corresponding to β-type structures (Fig. 5a) in correlation with the scattering profiles demonstrating antiparallel β-strands through backbone H-bonds (Fig. 4d). Similar CD spectra is previously reported for the β-sheets of Bombyx mori silk solution in hexafluoroisopropyl alcohol.47
Acknowledging its limitation for IDPs, we used the BestSel algorithm to quantify the different types of secondary structures in the[V7]40 −48 h RLP.46 The quantification confirmed that the [V7]40 RLP after 48hrs of incubation exhibit a mixture of secondary structures including α-helix, parallel and antiparallel β-sheets, turns, and disordered coils (Supplementary Fig. S3), while the predominant structures are antiparallel β-sheets (34%, Supplementary Fig. S3). The CD data agree with the WAXS data in finding β-sheets correlation peaks for the [V7]40-48 h system (Fig. 4d).
Fourier transform infrared (FTIR) measurements for [V7]40 −1h and [V7]40 −48h further supported the secondary structure determination. Indeed for the 48 h incubation we find the amide I band at 1637cm−1 (corresponding to C=O stretch of peptide linkages) and amide II band at 1522cm−1 (mainly derived from in-plane NH bending) corresponds to β-sheets (Fig. 5b).48 The higher wavenumber band at 1732 cm−1 corresponds to antiparallel β-sheets in the system in support with our CD and X-ray data. No such bands are observed in FTIR spectra of [V7]40 −1hr sample (Fig. 5b), which confirms that incubation of 48hrs at 80°C lead to stabilization of antiparallel β-sheets in [V7]40 system.
In support of our experimental results, alphafold249 predicts completely disordered structure for [WT]40 system (Supplementary Figs. S4a) which is in fact is the characteristic of IDP. On the contrary, alphafold2 for [V7]40 shows more than 90% probability of presence of β-sheets (Supplementary Fig. S4b). Next, we used X-ray scattering to investigate the temperature-dependence phase behavior of the lamellar structure in the [V7]40-48 h samples during the heating and cooling cycle. The experiment involves incubating at 80°C for t80 hours and then cooling back to 25°C. The temperature ramped up to 80°C with a ramp rate of 1°C min−1 during the heating cycle. After every 5°C increment, the system was held at the given temperature for 15 min for equilibration before collecting the scattering profiles (Supplementary Fig. S5). Scattering profiles were collected every 5°C during the cooling cycle from 80°C to 20°C (Supplementary Fig. S6).
The results indicate that while heating the [V7]40-48 h sample from 25°C-45°C, the lamellar correlation peaks systematically shift towards higher q-values. However, at temperatures above 45°C, the higher-order lamellar harmonics (q2H, q4H) and β-sheet peaks disappear (qβ1H, qβ2H) while the principal lamellar peak ((q1H), persists (Fig. 6a & Supplementary Fig. S5). Further heating the system to 50°C, causes q1H to split into two peaks, where we designate the daughter peak as . Surprisingly, on further heating peak q1H disappears at 55°C, while persists. From 60°C to 80°C (the maximum temperature reached in our experiment), completely disappears (Fig. 6a). Above 60°C, the last ordered structure signature is the correlation peak of that also remains in the one-phase regime (Fig. 6a & Supplementary Fig. S5). Further details of will be discussed in the subsequent section.
Figure 6.

SAXS profiles of the [V7]40-48 h RLP at different temperatures during temperature cycle (a) heating and (b) cooling cycle. With an increase in temperature, the principal lamellar peak (q1H) systematically moves toward higher q-values and splits at 50°C and both correlation peaks disappear at 60°C. During the cooling cycle, a correlation peak reappears at 50°C as , which denotes a thermal hysteresis of 15°C in the self-assembly. The peak spilts to a daughter peak starting as a small hump at 35°C and becoming prominent at 30°C & 25°C. Variation in the interplanar distance (d in nm) during the heating-cooling cycle corresponding to correlation peaks (c) , and (d) qβ1 and qβ2 (β-sheet peaks) obtained by the relation d = 2 π/q. Arrows in (c) indicate the temperatures at which the system transit to the two-nanoscopic phase during the heating and cooling cycle.
There were no signs of lamellar or β-sheet correlation peaks when the temperature of the [V7]40-48 h sample was decreased from 80 to 50°C during the cooling cycle (Fig. 6b & Supplementary Fig. S6). However, once the temperature reaches 45°C, we observe a single reflection, denoted as , in the SAXS region (Fig. 6b & Supplementary Fig. S6).
Also, at 45°C, all the correlation peaks corresponding to a lamellar nanostructure (, where C in the superscript signifies the cool cycle) reappear, as well as the β-sheets ( and , Supplementary Fig. S6). During further cooling, the lamellar peaks systematically shift to lower q-values (Fig. 6b & Supplementary Fig. S6). In addition, starting at 35°C, and seen very clearly at 25°C, the peak splits into two reflections (1.07 nm−1) and (1.19 nm−1), similar to those observed in the heat cycle at 50°C.
Furthermore, lowering the temperature to 20° C diminishes the peak at while peak persists (Fig. 6b). The lamellar interplanar distance (d = 2π/q) hysteresis loop during the heating and cooling cycle is presented in Fig. 6c. We could detect only a weak anti-correlation between the temperature and the interplanar distance but could observe the coexistence of two nanoscopic phases at 50° C and 30° C for the heating and cooling cycles, respectively. Noticeably, the β-sheet spacing positively correlates with temperature between 25 to 45° C and disappears above this temperature (Fig. 6d).
The correlation peak designated as q′ remains present throughout the complete heating-cooling cycle (Fig. 7a, b). Interestingly, the intensity of the q′ peak is positively correlated with the temperature during both cycles, with the peak position (and hence the inter-planer distance) independent of the cycle direction (Supplementary Fig. S7). Both these findings differentiate this from the other nanoscopic structures and suggest that a discrete equilibrated state is present in the system.
Figure 7.

Scattering profiles of the [V7]40-48 h RLP exhibiting a variation in correlation peak q’as a function of temperature. Profiles during the (a) heating cycle and (b) cooling cycle . The q′ peak persists through all the temperature range examined, and the peak position is essentially independent of the heating and cooling cycle. The intensity of q’ increases with an increase in temperature for both cycles.
We also performed temperature-dependent CD studies for [V7]40-48 h system with same experimental conditions as for SAXS measurements. Upon ramping the temperature from 20 to 80° C at a ramp rate of 5° C, we observed that the valleys at ~ 203 and 225 nm corresponding to β-sheets persist upto 60° C during heat cycle. These valleys almost vanish at 70 and 80° C (Supplementary Fig. S8a: for clarity we have shown results at interval of 10° C). During cool cycle the valleys reappeared at 60° C and persist upto 20° C (Supplementary Fig. S8b).
The scattering profiles of the [WT]n RLPs with a variable number of octapeptide repeats after 24 h incubation at 80°C differ from that of [V7]40 RLP. We could not detect any correlation peaks in the scattering profiles where n = 20, 40, and 60, (Supplementary Fig. S9), which indicates the absence of short-or-long range-order in these RLPs. However, the SAXS/WAXS profiles of the [WT]80-24 h sample do contain two correlation peaks (Fig. 4b & Supplementary Fig. S10 (a) & (b)). These correlation peaks do not show corresponding higher-order reflections during both the heating and cooling cycle (Supplementary Fig. S10 (a) & (b)), thus preventing a description of the long-range symmetry. Noticeably the correlation peaks are rather sharp which suggests a homogeneous short-range correlation.
During the heating cycle of the [WT]80-24 h sample, qaH shifts systematically toward higher q-values and split into a daughter peak at 50°C (Fig. 8a) before both disappear at 55°C and 60°C respectively. In contrast, during the cooling cycle, the correlation peak reappears at 40°C (Fig. 8b). Upon further cooling below 30°C, the correlation peak diminishes and a new sharp correlation peak, nm−1 appears and becomes more prominent as the system cools down to 20°C (Fig. 8b). Thus, we can conclude that the disappearance of at 60°C during the heating cycle and the appearance of at 40°C in the cooling cycle are interrelated.
Figure 8.
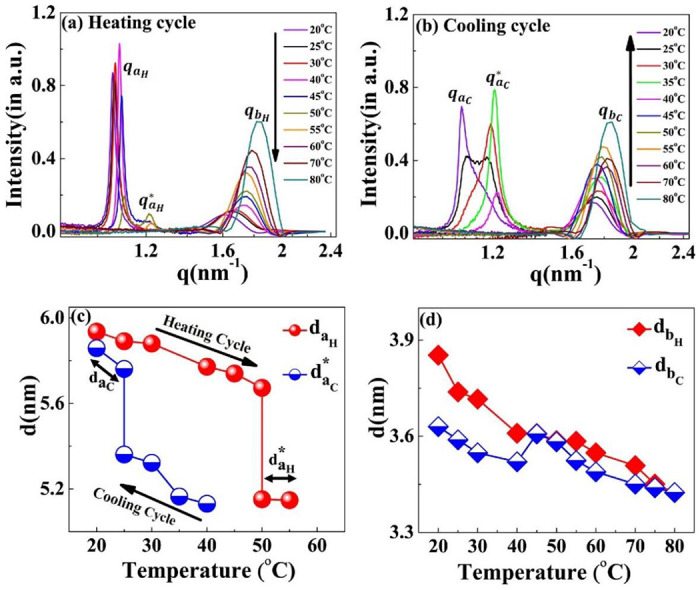
Scattering profiles of the [WT]80-24 h sample at different temperatures. During the (a) heating and (b) cooling cycle. The correlation peaks qa and qb indicates short-range nanoscopic organization. Upon ramping the temperature from 20°C-45°C, the peak qaH shifts towards higher q-values, splits into a daughter peak at 50°C and both peaks disappear at 60°C. Upon cooling, the correlation peak reappears first at 40°C as . This exhbits a thermal hysteresis of 20°C in the self-assembly. Below 30°C, the diminishes and a new sharp correlation peak, qac appears at 20°C. Variations in the interplanar distance (d in nm) (c) da (d) db during the heating and cooling cycle. The system exhibits the presence of two-nanoscopic phases characterized by two-interplanar distances, daH and at 50°C and 55°C during the heating cycle and by qaC and at 25°C during the cooling cycle. The correlation peak qb remains prominent throughout the heating-cooling cycle, with minimal changes in interplanar distance.
Similar to the results seen with [V7]40, the [WT]80-24 h system exhibits thermal hysteresis of the nanoscopic structure (Fig. 8c). However, the exact short-range nanoscopic organization differs. While a previous report suggested that UCST SynIDPs like [WT]80 show phase separation thermal hysteresis20, our results demonstrate that nanoscale organization also exhibits such hysteretic behavior. In addition, the correlation peak qb remains prominent with little alteration and minimal hysteresis throughout the entire heating and cooling cycle, as shown by minimal changes in the interplanar distance (Fig. 8d).
Discussion
The results presented here demonstrate modulation of the micro and nano self-assembly of resilin-like SynIDPs by periods of high-temperature incubation at 80°C and subsequent cooling. For the [WT]40 and [WT]80 RLPs, varying the incubation time correlates with a structural transition from micro-ellipsoids to rods structures. In contrast, the [V7]40 RLP, that has a V instead of Y amino acid at the 7th position of the GRGDSPYS repeat unit of WT RLP, requires a longer incubation time and only transitions from an amorphous coacervate to an ellipsoidal structure after 48 h at 80°C. Interestingly, upon cooling back to 25°C, prolonged incubation of both the [WT]80, and [V7]40 RLPs at 80°C leads to the formation of hierarchical self-assembled nanostructures, an observation that was confirmed by the sharp X-ray scattering correlation peaks.
Based on their absorbance measurements, Quiroz et al.34 reported that RLPs undergo loss of LLPS during multiple heating and cooling cycles around the UCST. They attributed such behavior to the formation of irreversible aggregates. A similar effect has also been observed for synthetic polymers exhibiting UCST phase behavior.50 The loss of LLPS and the formation of irreversible pathological aggregates have also been observed in the low complexity disordered domain of RNA-binding protein FUS.51 IDPs of Aβ, tau and α-synuclein proteins in aqueous solution also form such irreversible aggregates.52 In line with these findings, we expect long incubations at 80°C to lead to a progressive build-up of irreversible aggregates in the [WT]40 and [WT]80 RLPs. Indeed, on the microscopic scale, we did observe various ellipsoidal aggregates in the HRSEM images after long incubation times at 80°C (Figs. 1c, d & 2c, d). Moreover, these ellipsoidal aggregates systematically self-assemble to form rods in the [WT]40 and [WT]80 RLPs.
The driving force for this self-assembly could be the abundance of H-bond’s forming amino acids aspartic acid (D), serine (S) and tyrosine (Y) that can create metastable interchain interactions. Thus, we suggest that a long incubation time promotes additional mixing in the liquid state that can overcomes meta-stable states and accelerate the formation of self-assembled micro-scale structures via the hydrogen-bond’s forming amino acids. These interactions could also explain the formation of interconnected nanofibers observed in these systems (Supplementary Fig S1).
Upon prolonged incubation at 80°C, the micro-ellipsoids aggregate throughout the nanofibers’ connection to form rods or fiber-like structures. In contrast and supporting our hypothesis that intermolecular H-bonding is an important driving force for the hierarchical self-assembly in RLPs at higher temperatures is the lack of formation of interconnected fibers in [V7]40 RLP incubated for 48 h at 80°C. The [V7]40 RLP, has a non-H-bonding residue (V) instead of a H-bonding amino acid (Y). Further support is the reduction in the number of rods after prolonged 80°C incubation.
The incubation time also promotes order on the nanoscopic length scale in a sequence-dependent manner. The sequence of [V7]40 consists of a zwitterionic hydrophilic domain (i.e., GRGD), with positively charged arginine (R) and negatively charged aspartic acid (D). Each hydrophilic repeat is conjugated to a neutral-charged domain (i.e., SPVS) with a hydrophobic valine (V) residue. Thus, we presume that our sequence exhibits properties similar to amphiphilic polypeptides that possess alternating repeats of single hydrophilic and hydrophobic amino acids instead of alternating domains, each four amino acids long.53,54 Alternating amphiphilic peptides are known to form highly stable β-sheet structures in aqueous solution due to the interaction between their hydrophobic faces,53 while random polymers of the same composition lead to α-helical structures.55,56 Indeed, our X-ray scattering and CD data (Figs. 4d & 5d) provide evidence of β-sheets in the [V7]40 RLP.
The [WT]40 RLP also contains an alternating amphiphilic sequence (GRGDSPYS) with Y as a hydrophobic residue. Nonetheless, the CD spectra of the [WT]40 system after 48 h incubation at high temperature still exhibits an α-helical structure. According to Chou-Fasman’s potential scale, the valine has the highest propensity to form β-sheets57, which can explain the presence of β-sheets in [V7]40 RLP. In agreement with our findings, molecular dynamic simulations of β-sheet formation in self-assembled peptide amphiphile fibers exhibits that fibers with a higher valine/alanine ratio have a higher percentage of β-sheet.58 The molecular reason for the presence of α-helical structures in the WT system is however, unclear at this time.
Notably, the alternating amphiphilic sequence with valine is insufficient to induce β-sheet formation, which requires a prolonged incubation time (i.e., 48 h) at a high temperature (80°C). We attributed this finding to a rough but shallow energy landscape at high temperatures with alternative transient meta-stable minima of random coils and α-helix. A prolonged incubation allows the system to equilibrate to the lowest β-sheet energy state.59 Thus, the major driving force for β-sheets is the self-interaction between the hydrophobic valine side chains on neighboring peptides, which minimizes unfavorable contact with water molecules. The self-complementary ion pair interactions between the alternate positively charged arginine (R) and negatively charged aspartic acid (D) motifs may also assist in stabilizing β-sheet formation. Apparently, incubation up to 48 h is insufficient to induce β-sheets in the [WT]40 RLP, which shares identical ionic pairing.
In Fig. 9a we show schematic representation of resilin-like octapeptide unit GRGDSPVS having hydrophobic and hydrophilic domains due to the presence of arginine (R) and aspartic acid (D), and valine (V) amino acids amino acids, respectively. In [V7]40 RLP, the adjacent polypeptide chains (β-strands) in the β-sheets are knitted in an antiparallel fashion through a backbone stabilized via hydrogen bonds separated by a distance of dβ2 = 0.406 nm. Due to the amphiphilic nature of our system, the valine side chains of two neighboring β-strands can interact with each other to form a hydrophobic face on the side of the sheets away from the water molecules. On the other side of the sheet, electrostatic interactions between complementary ionic amino acid pairs stabilize a three-strand β-sheet structure (Fig. 9b). Such an arrangement has previously been reported for the alternating amphiphilic oligopeptide (EAK)16, which possesses a complementary ion-pair in the sequence.60 As another example, the peptide (VK)4VPPT(KV)4 exhibits the formation of self-assembled fibrils due to the presence polar β-sheet with distinctive hydrophobic (V) and hydrophilic (K) domains. β-sheets of this peptide collapse into fibrils due to collapse of hydrophobic domains. Further, molecular dynamics simulation of the amphiphilic peptides I4K2 and KI4K indicated that the non-bonded energies of the three-strand β-sheets are three times lower than that of individual monomers.61
Figure 9.
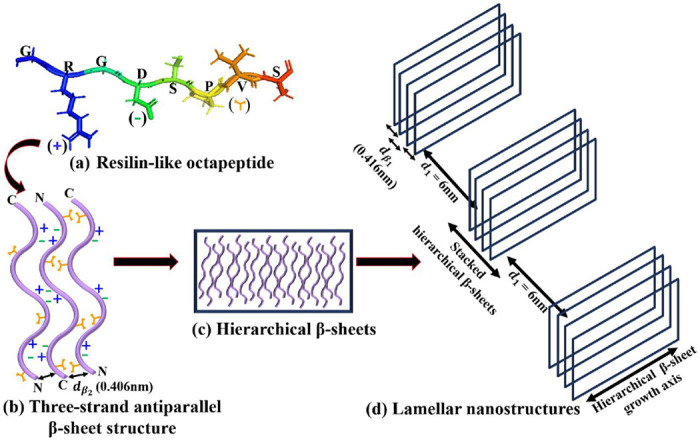
Schematic representation of self-assembly of hierarchical lamellar nanostructures in the [V7]40− 48h RLP (a) PyMOL structure of a single RLP unit GRGDSPVS having hydrophobic and hydrophilic domain due to the presence of arginine (R) and aspartic acid (D), and valine (V) amino acids respectively. The (+) and (−) represent the charges on R and D and used to denote these amino acids in (b). At the same time, the orange Y-shape symbol describes the hydrophobic valine (b) a three-strand antiparallel β-sheet structure formed due to the alternating arrangement of the ionic (R and D) and hydrophobic (V) amino side chains on each β-strand of the octapeptide repeat. The distance dβ2 = 0.406 nm represents the backbone H-bonding distance between antiparallel β-strands. (c) Hierarchical β-sheets formed by lateral stacking of three-strand antiparallel β-sheet structure. (d) Lamellar nanostructures having interplanar distance (d1) of 6 nm formed by lateral arrangement of stacked hierarchical β-sheets. Each hierarchical β-sheet in a stack is separated by a distance of dβ1 = 0.416 from the other. d1, dβ1 and dβ2 are obtained from X-ray correlation peaks.
At high peptide concentration, the three-stranded structure can laterally self-assemble via Van der Walls interactions to form correlated hierarchical β-sheets (Fig. 9c). Indeed, at low temperatures, the [V7]40 RLP shows a well-ordered lamellar morphology after 48 h of incubation, as evidenced by sharp correlation peaks in its X-ray scattering profiles, as well as anti-parallel β-sheet signatures. Spider silk fibers, whose sequence is also amphiphilic with highly conserved poly-(Ala-Gly) and poly-Ala repeats, similarly contain crosslinked nanoscale crystals of antiparallel β-sheets structures.62–64 These nanocrystals are further embedded in a semi-amorphous phase of less orderly structures, helices, and β-turns. Therefore, we conclude that the formation of lamellar structures in the [V7]40 RLP is due to the alternate arrangement of antiparallel β-sheets at a distance of ~ 6 nm. This arrangement is similar to silk’s, but in our [V7]40 RLP, the β-sheets self-assemble to form a lamellar nanostructure instead of fibers (Fig. 9d).
Of note, we also observed that high temperature diminishes the self-assembled nanoscopic structures in the [V7]40 and [WT]80 RLPs in a hysteretic manner. Previous reports suggest that IDPs exhibit temperature-dependent collapse, quantified as a decrease in their radius of gyration.65–67 This temperature-induced collapse in IDPs implies the presence of temperature-dependent interactions such as hydrophobic effects68,69 or changes in solvation-free energy.30 Interestingly, Wuttke et al.70 reported that the most hydrophilic and charged IDP sequences exhibit a significant temperature-induced collapse. They also suggested that along with the classical hydrophobic effects, the temperature-dependent solvation-free energies of the hydrophilic amino acids play a vital role in determining the collapse. Zerge et al.71 simulated the solvent-accessible surface area (SASA) and showed that this was decreased by hydrophobic amino acids up to a critical temperature and then subsequently increased. For negatively charged amino acids (Asp;D and Glu; E), the SASA decreases with increasing temperature, which avoids water contact, while the cationic amino acid arginine(R) exhibits the opposite tendency.70,71 Therefore, the temperature-dependent self-assembly in our [V7]40 and [WT]80 RLPs can be attributed to the collapse of the valine (V) or tyrosine (Y) domains due to the enhanced hydrophobic effect caused by an increase in temperature. Since the RLPs have R and D residues, which exhibit opposing SASA temperature dependencies, we might expect the combined temperature dependency to be more complex and require further investigation.
Summary
We have demonstrated that it is possible to modulate the hierarchical self-assembly in RLPs by simply adjusting the time of incubation at high temperatures. We also witnessed a temperature-dependent hysteresis in the self-assembled nanostructure of these SynIDPs that is determined by their sequence, and suggest that the lamellar nanostructures formed in this system are associated with the presence of β-sheets. A variation in incubation time also induced structural transitions from amorphous to rod/ellipsoids during the LLPS of these SynIDPs. In conclusion, our results introduce a new approach to access new forms of self-assembled structures by the interplay between the incubation time and hysteresis of these SynIDPs. The micro-phase separated nanoscale lamellar or short-range ordered structure observed in our RLPs can find application in heterogeneous catalysis, drug delivery and scaffolds to guide tissue regeneration, similar to block copolypeptides.
Materials and Methods
Protein Purification
The RLPs were recombinantly expressed from plasmid-borne genes in E.Coli, and were purified as reported previously.20 Briefly, E. coli (BL21) cells were grown overnight in Terrific Broth (TB) culture medium, followed by induction with 500 μM IPTG. The bacteria were pelleted at 3500 RCF, resuspended in PBS, then lysed by sonication and centrifuged at 4°C to separate the supernatant and pellet containing the RLP of interest. The pellets were suspended in an equal volume of 8M urea in 150 mM PBS buffer and heated for 10 min in a water bath maintained at 37°C, followed by centrifugation at 20,000 RCF for 20 min. The collected supernatants were dialyzed against milli-Q water at 4°C for 48 h with two changes of dialysis water. The suspension obtained after dialysis was centrifuged at 3500 RCF for 10 min at 4°C to pellet the RLP The protein pellets were then lyophilized for three days to obtain dry RLP and stored at −80°C as powder. The molecular weight and purity of each protein was confirmed by matrix-assisted laser desorption/ionization time of flight mass spectrometry (MALDI-TOF-MS). The experimental m/z obtained for [WT]40, [V7]40 and [WT]80 samples are 33379, 30839 and 66193 respectively (Supplementary Fig. S11(a)-(c)).
Sample preparation
For small and wide-angle X-ray studies (SAXS and WAXS), buffers for background subtraction were prepared for each RLP by using slide-A-lyzer MINI dialysis devices (3.5K MWCO). A 100 μM solution of each RLP, prepared in 2mL phosphate buffer (150 mM, at pH 7), was incubated at 60°C for 15 min. The hot solution was transferred to the MINI dialysis tubes and dialyzed against 50 mL of phosphate buffer for 4-5 h at 60°C on a shaker. Maintaining a temperature above the Tt (i.e., 40°C) was necessary to prevent phase separation. We secured the mouth of the dialysis tube with a cellophane sheet to prevent evaporation of the buffer, and the tube was further closed with the cap. The dialyzed buffers were stored at 4°C and used for SAXS measurements as required.
For the RLP measurements, we used 100 μM dialyzed RLP solutions of [WT]40, [WT]80, and [V7]40 in triplicate. We heated the RLP solutions at 80°C on a heating bath for an incubation time of 1, 24, or 48 h. We defined the samples based on RLP name and incubation time. For example, samples of [WT]40 RLP heated at 80°C for 1, 24, and 48 h are referred to as [WT]40−1 h, [WT]40-24 h, and [WT]40-48 h, respectively. Detailed material and methods are given in supporting information.
Acknowledgments
The synchrotron SAXS/WAXS data were collected on a beamline I22 at Diamond Light Source, Oxford, United Kingdom. We would like to thank beamline scientists Dr. Nick Terrill and Dr. Andy Smith for their assistance in using the beamlines. This work was supported by the National Science Foundation under Grant No. 1715627, the United States-Israel Bi-national Science Foundation under Grant No. 2020787, and the Israel Science Foundation under Grants No. 1454/20 to R.B, and by the NIH through Grant No. R35GM127042 and the AFOSR though Grant No. FA9550-20-1-0241 (SUB0000436) to A.C. D.C.-G. acknowledges the Marian Gertner Institute for Medical Nano systems at Tel Aviv University. D.C.-G. gratefully acknowledges the support of the Colton Foundation. The authors acknowledge the Chaoul Center for Nanoscale Systems at Tel Aviv University and the ADAMA Center for Novel Delivery Systems in Crop Protections for the use of instruments and staff assistance.
Footnotes
Competing Interest
The authors declare no competing interests.
Data Availability
All data generated or analyzed during this study are included in this published article.
References
- 1.Gomes E. & Shorter J. The molecular language of membraneless organelles. J. Biol. Chem. 294, 7115–7127 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Uversky V. N. Intrinsically disordered proteins in overcrowded milieu: Membrane-less organelles, phase separation, and intrinsic disorder. Curr. Opin. Struct. Biol. 44, 18–30 (2017). [DOI] [PubMed] [Google Scholar]
- 3.Marnik E. A. & Updike D. L. Membraneless organelles: P granules in Caenorhabditis elegans. Traffic 20, 373–379 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Habchi J., Tompa P., Longhi S. & Uversky V. N. Introducing protein intrinsic disorder. Chem. Rev. 114, 6561–6588 (2014). [DOI] [PubMed] [Google Scholar]
- 5.Ehm T. et al. Intrinsically disordered proteins at the nano-scale. Nano Futur. 5, 022501 (2021). [Google Scholar]
- 6.Uversky V. N. & Kulkarni P. Intrinsically disordered proteins: Chronology of a discovery. Biophys. Chem. 279, 106694 (2021). [DOI] [PubMed] [Google Scholar]
- 7.Chakrabarti P. & Chakravarty D. Intrinsically disordered proteins/regions and insight into their biomolecular interactions. Biophys. Chem. 283, 106769 (2022). [DOI] [PubMed] [Google Scholar]
- 8.Galea C. A., Wang Y., Sivakolundu S. G. & Kriwacki R. W. Regulation of cell division by intrinsically unstructured proteins: Intrinsic flexibility, modularity, and signaling conduits. Biochemistry 47, 7598–7609 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Roey K., Gibson T. J. & Davey N. E. Motif switches: decision-making in cell regulation. Curr. Opin. Struct. Biol. 22, 378–385 (2012). [DOI] [PubMed] [Google Scholar]
- 10.Gsponer J., Futschik M. E., Teichmann S. A. & Babu M. M. Tight regulation of unstructured proteins: From transcript synthesis to protein degradation. Science (80-.). 322, 1365–1368 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunker A. K., Cortese M. S., Romero P., Iakoucheva L. M. & Uversky V. N. Flexible nets. The roles of intrinsic disorder in protein interaction networks. FEBS J. 272, 5129–5148 (2005). [DOI] [PubMed] [Google Scholar]
- 12.Kim P. M., Sboner A., Xia Y. & Gerstein M. The role of disorder in interaction networks: A structural analysis. Mol. Syst. Biol. 4, 179 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iakoucheva L. M., Brown C. J., Lawson J. D., Obradović Z. & Dunker A. K. Intrinsic Disorder in Cell-signaling and Cancer-associated Proteins. J. Mol. Biol. 323, 573–584 (2002). [DOI] [PubMed] [Google Scholar]
- 14.Chong P. A. & Forman-Kay J. D. Liquid–liquid phase separation in cellular signaling systems. Curr. Opin. Struct. Biol. 41, 180–186 (2016). [DOI] [PubMed] [Google Scholar]
- 15.Cuevas-Velazquez C. L. & Dinneny J. R. Organization out of disorder: liquid–liquid phase separation in plants. Curr. Opin. Plant Biol. 45, 68–74 (2018). [DOI] [PubMed] [Google Scholar]
- 16.Abyzov A., Blackledge M. & Zweckstetter M. Conformational Dynamics of Intrinsically Disordered Proteins Regulate Biomolecular Condensate Chemistry. Chem. Rev. 122, 6719–6748 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Babinchak W. M. & Surewicz W. K. Liquid–Liquid Phase Separation and Its Mechanistic Role in Pathological Protein Aggregation. J. Mol. Biol. 432, 1910–1925 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alberti S. & Dormann D. Liquid-Liquid Phase Separation in Disease. Annu. Rev. Genet. 53, 171–194 (2019). [DOI] [PubMed] [Google Scholar]
- 19.Ray S. et al. α-Synuclein aggregation nucleates through liquid–liquid phase separation. Nat. Chem. 2020 128 12, 705–716 (2020). [DOI] [PubMed] [Google Scholar]
- 20.Dzuricky M., Rogers B. A., Shahid A., Cremer P. S. & Chilkoti A. De novo engineering of intracellular condensates using artificial disordered proteins. Nat. Chem. 12, 814–825 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Indulkar A. S., Box K. J., Taylor R., Ruiz R. & Taylor L. S. pH-dependent liquid–liquid phase separation of highly supersaturated solutions of weakly basic drugs. ACS Publ. 12, 2365–2377 (2015). [DOI] [PubMed] [Google Scholar]
- 22.Indulkar A. S., Gao Y., Raina S. A., Zhang G. G. Z. & Taylor L. S. Exploiting the Phenomenon of Liquid-Liquid Phase Separation for Enhanced and Sustained Membrane Transport of a Poorly Water-Soluble Drug. Mol. Pharm. 13, 2059–2069 (2016). [DOI] [PubMed] [Google Scholar]
- 23.Sun M., Shichao D., Tang W., Jia L. & Gong J. Design of Spherical Crystallization for Drugs Based on Thermal-Induced Liquid-Liquid Phase Separation: Case Studies of Water-Insoluble Drugs. Ind. Eng. Chem. Res. (2019). [Google Scholar]
- 24.Liu J., Zhorabek F., Dai X., Huang J. & Chau Y. Minimalist Design of an Intrinsically Disordered Protein-Mimicking Scaffold for an Artificial Membraneless Organelle. ACS Cent. Sci. 8, 493–500 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mu W. et al. Membrane-confined liquid-liquid phase separation toward artificial organelles. Sci. Adv. 7, 9000–9028 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simmel F. C. Synthetic organelles. Emerg. Top. Life Sci. 3, 587–595 (2019). [DOI] [PubMed] [Google Scholar]
- 27.Costa S. A. et al. Photo-Crosslinkable Unnatural Amino Acids Enable Facile Synthesis of Thermoresponsive Nano-to Microgels of Intrinsically Disordered Polypeptides. Adv. Mater. 30, 1704878 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ibáñez-Fonseca A., Flora T., Acosta S. & Rodríguez-Cabello J. C. Trends in the design and use of elastin-like recombinamers as biomaterials. Matrix Biol. 84, 111–126 (2019). [DOI] [PubMed] [Google Scholar]
- 29.Tikhonova T. N. et al. Tunable Self-Assembled Peptide Hydrogel Sensor for Pharma Cold Supply Chain. ACS Appl. Mater. Interfaces 14, 55392–55401 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dignon G. L., Best R. B. & Mittal J. Biomolecular phase separation: From molecular driving forces to macroscopic properties. Annu. Rev. Phys. Chem. 71, 53–75 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seuring J. & Agarwal S. Polymers with upper critical solution temperature in aqueous solution: Unexpected properties from known building blocks. ACS Macro Lett. 2, 597–600 (2013). [DOI] [PubMed] [Google Scholar]
- 32.Holehouse A. S. & Pappu R. V. Collapse Transitions of Proteins and the Interplay among Backbone, Sidechain, and Solvent Interactions. Annu. Rev. Biophys. 47, 19–39 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moses D. et al. Revealing the Hidden Sensitivity of Intrinsically Disordered Proteins to their Chemical Environment. J. Phys. Chem. Lett. 11, 10131–10136 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quiroz F. G. et al. Intrinsically disordered proteins access a range of hysteretic phase separation behaviors. Sci. Adv. 5, 5177–5195 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qin G., Hu X., Cebe P. & Kaplan D. L. Mechanism of resilin elasticity. Nat. Commun. 2012 31 3, 1–9 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wise S. G. et al. Tropoelastin - a versatile, bioactive assembly module. Acta Biomater. 10, 1532 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Balu R., Dutta N. K., Dutta A. K. & Choudhury N. R. Resilin-mimetics as a smart biomaterial platform for biomedical applications. Nat. Commun. 2021 121 12, 1–15 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kornreich M., Avinery R. & Beck R. Modern X-ray scattering studies of complex biological systems. Curr. Opin. Biotechnol. 24, 716–723 (2013). [DOI] [PubMed] [Google Scholar]
- 39.Li T., Senesi A. J. & Lee B. Small Angle X-ray Scattering for Nanoparticle Research. Chem. Rev. 116, 11128–11180 (2016). [DOI] [PubMed] [Google Scholar]
- 40.Masunaga H., Sakurai K., Akiba I., Ito K. & Takata M. Accurate measurements of intrinsic scattering from window materials by use of a vacuum camera. J. Appl. Cryst. 46, 577–579 (2013). [Google Scholar]
- 41.Atkins E. D. T., Keller A. & Sadler D. M. Structure analysis of chain-folded lamellar polyamide crystals from X-ray diffraction. J. Polym. Sci. Part A-2 Polym. Phys. 10, 863–875 (1972). [Google Scholar]
- 42.Magill J. H., Girolamo M. & Keller A. Crystallization and morphology of nylon-6,6 crystals: 1. Solution crystallization and solution annealing behaviour. Polymer (Guildf). 22, 43–55 (1981). [Google Scholar]
- 43.Lotz B. Rippled Sheets: The Early Polyglycine Days and Recent Developments in Nylons. ChemBioChem 23, e202100658 (2022). [DOI] [PubMed] [Google Scholar]
- 44.Riekel C. & Vollrath F. Spider silk fibre extrusion: combined wide- and small-angle X-ray microdiffraction experiments. Int. J. Biol. Macromol. 29, 203–210 (2001). [DOI] [PubMed] [Google Scholar]
- 45.Nagy G., Igaev M., Jones N. C., Hoffmann S. V. & Grubmüller H. SESCA: Predicting Circular Dichroism Spectra from Protein Molecular Structures. J. Chem. Theory Comput. 15, 5087–5102 (2019). [DOI] [PubMed] [Google Scholar]
- 46.Micsonai A., Bulyáki É. & Kardos J. BeStSel: From Secondary Structure Analysis to Protein Fold Prediction by Circular Dichroism Spectroscopy. Methods Mol. Biol. 2199, 175–189 (2021). [DOI] [PubMed] [Google Scholar]
- 47.Drummy L. F., Phillips D. M., Stone M. O., Farmer B. L. & Naik R. J. Thermally induced α-helix to β-sheet transition in renegated silk fibers and films. Biomacromolecules 6, 3328–3333 (2005). [DOI] [PubMed] [Google Scholar]
- 48.Kong J. & Yu S. Fourier Transform Infrared Spectroscopic Analysis of Protein Secondary Structures. Acta Biochim. Biophys. Sin. (Shanghai). 39, 549–559 (2007). [DOI] [PubMed] [Google Scholar]
- 49.Jumper J. et al. Highly accurate protein structure prediction with AlphaFold. Nat. 2021 5967873 596, 583–589 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Seuring J. & Agarwal S. First example of a universal and cost-effective approach: Polymers with tunable upper critical solution temperature in water and electrolyte solution. Macromolecules 45, 3910–3918 (2012). [Google Scholar]
- 51.Shin Y. et al. Spatiotemporal Control of Intracellular Phase Transitions Using Light-Activated optoDroplets. Cell 168, 159–171 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nguyen P. H. & Derreumaux P. Structures of the intrinsically disordered Aβ, tau and α-synuclein proteins in aqueous solution from computer simulations. Biophys. Chem. 264, 106421- (2020). [DOI] [PubMed] [Google Scholar]
- 53.Fukushima Y. Synthesis and Highly Stable β-Sheet Formation of Sequential Alternating Amphiphilic Polypeptides. Polym. J. 28, 113–120 (1996). [Google Scholar]
- 54.Seipke G., Arfmann H.-A & Wagner K. G. Synthesis and properties of alternating poly(Lys-Phe) and comparison with the random copolymer poly(Lys51, Phe49). Biopolymers 13, 1621–1633 (1974). [DOI] [PubMed] [Google Scholar]
- 55.Brack A. & Spach G. Multiconformational Synthetic Polypeptides. J. Am. Chem. Soc. 103, 6319–6323 (1981). [Google Scholar]
- 56.Brack A. & Caille A. SYNTHESIS AND β-CONFORMATION OF COPOLYPEPTIDES WITH ALTERNATING HYDROPHILIC AND HYDROPHOBIC RESIDUES. Int. J. Pept. Protein Res. 11, 128–139 (1978). [DOI] [PubMed] [Google Scholar]
- 57.Prevelige P. & Fasman G. D. Chou-Fasman Prediction of the Secondary Structure of Proteins. Predict. Protein Struct. Princ. Protein Conform. 391–416 (1989). [Google Scholar]
- 58.Lee O. S., Liu Y. & Schatz G. C. Molecular dynamics simulation of β-sheet formation in self-assembled peptide amphiphile fibers. J. Nanoparticle Res. 14, (2012). [Google Scholar]
- 59.Bryngelson J. D., Onuchic J. N., Socci N. D. & Wolynes P. G. Funnels, pathways, and the energy landscape of protein folding: A synthesis. Proteins Struct. Funct. Bioinforma. 21, 167–195 (1995). [DOI] [PubMed] [Google Scholar]
- 60.Zhang S., Lockshin C., Cook R. & Rich A. Unusually stable β-sheet formation in an ionic self-complementary oligopeptide. Biopolymers 34, 663–672 (1994). [DOI] [PubMed] [Google Scholar]
- 61.Zhao Y. et al. Tuning the self-assembly of short peptides via sequence variations. ACS Publ. 29, 13457–13464 (2013). [DOI] [PubMed] [Google Scholar]
- 62.Van Beek J. D., Hesst S., Vollrath F. & Meier B. H. The molecular structure of spider dragline silk: Folding and orientation of the protein backbone. Proc. Natl. Acad. Sci. U. S. A. 99, 10266–10271 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lefèvre T., Rousseau M. E. & Pézolet M. Protein Secondary Structure and Orientation in Silk as Revealed by Raman Spectromicroscopy. Biophys. J. 92, 2885–2895 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nova A., Keten S., Pugno N. M., Redaelli A. & Buehler M. J. Molecular and nanostructural mechanisms of deformation, strength and toughness of spider silk fibrils. Nano Lett. 10, 2626–2634 (2010). [DOI] [PubMed] [Google Scholar]
- 65.Makhatadze G. I. & Privalov P. L. Protein interactions with urea and guanidinium chloride: A calorimetric study. J. Mol. Biol. 226, 491–505 (1992). [DOI] [PubMed] [Google Scholar]
- 66.Nettels D. et al. Single-molecule spectroscopy of the temperature-induced collapse of unfolded proteins. Proc. Natl. Acad. Sci. U. S. A. 106, 20740–20745 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Langridge T. D., Tarver M. J. & Whitten S. T. Temperature effects on the hydrodynamic radius of the intrinsically disordered N-terminal region of the p53 protein. Proteins Struct. Funct. Bioinforma. 82, 668–678 (2014). [DOI] [PubMed] [Google Scholar]
- 68.Hummer G., Garde S., García A. E., Pohorille A. & Pratt L. R. An information theory model of hydrophobic interactions. Proc. Natl. Acad. Sci. 93, 8951–8955 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chandler D. Interfaces and the driving force of hydrophobic assembly. Nat. 2005 4377059 437, 640–647 (2005). [DOI] [PubMed] [Google Scholar]
- 70.Wuttke R. et al. Temperature-dependent solvation modulates the dimensions of disordered proteins. Proc. Natl. Acad. Sci. U. S. A. 111, 5213–5218 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zerze G. H., Best R. B. & Mittal J. Sequence- and Temperature-Dependent Properties of Unfolded and Disordered Proteins from Atomistic Simulations. J. Phys. Chem. B 119, 14622–14630 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.


