Abstract
In this study, 7 new isoxazole compounds (8–14) were synthesized from the cyclization of chalcone compounds (1–7) containing different functional groups with hydroxylamine hydrochloride in alkaline medium. Tyrosinase and antioxidant properties of 8–14 were investigated. IC50 values for the tyrosinase enzyme inhibition of compounds 8, 11, 12, and 13 were varied between 61.47 ± 3.46 and 188.52 ± 5.85, while compounds 9, 10 and 14 did not show any inhibition effect. The antioxidant properties of 8–14 were investigated by DPPH and CUPRAC methods. Compound 12 showed the best DPPH radical scavenging activity (SC50: 40.21 ± 2.71), while 12 and 13 had shown the greatest Cupric ion reducing effect as 1.233 ± 0.015 and 1.245 ± 0.019 mg TEAC/mg, respectively. As a result, the change of functional groups in the synthesized compounds caused a significant difference in the biological properties of 8–14.
Keywords: Isoxazole, tyrosinase inhibition, antioxidant activity
1. Introduction
The isoxazole and its derivatives are an important intermediate for the synthesis of new chemicals in medicinal chemistry, which have been increased in last decades. Isoxazole nucleus had shown a wide spectrum of biological activity. Easy synthesis of isoxazole ring has been as an object of interest for the scientist from research group all over the world. The synthesis of isoxazole compounds have resulted in multiple corresponding antibacterial drugs in the market (Sulfisoxazole, Flucloxacillin, Dicloxacillin, Leflunomide, Risperidone, Oxacillin, Sulfamethoxazole, and Cloxacillin) and there are many patent related with isoxazole [1]. Leflunomide has been approved for the management of the signs and symptoms of active rheumatoid arthritis (RA) to improve physical function. Sulfisoxazole has been approved for the treatment of severe, repeated, or long-lasting urinary tract infections by inhibiting the enzyme dihydropteroate synthetase. Risperidone has been approved for the treatment of schizophrenia in adults [1]. 1,3-Dipolar cycloaddition of α, β-unsaturated ketone or 1,3-diketone is a widely used method for the synthesis of isoxazole [2]. One of the substituted isoxazole synthesis described using oxime of a ketone [3]. Although there are many methods were reported for the synthesis or modification of the isoxazole ring [1–9]. Isoxazoles play a fundamental role in the synthesis of numerous biologically active drugs such as antimicrobial, antiviral, anticancer, immunomodulatory, antiinflammatory, antiplatelet, antithrombotic, antitriglyceride, antidiabetic, analgesic, anticonvulsant, and anti-Alzheimer agents [2], pesticides and insecticides [10–11]. Thus, the synthesis of novel isoxazole derivatives remains a main focus of medicinal research. In this work, the synthesis and biological activities of a new series of isoxazole analogues with a substituted two aromatic rings still needed and are reported here.
2. Experimental
2.1. Material and methods
Solvents (n-hexane, ethyl alcohol, and diethyl ether), aldehydes and ketone compounds (2/3/4-methoxyacetophenone, 3/4-hydroxyacetophenone, 2,4-dimethoxy-benzaldehyde, 2,5-dimethoxybenzaldehyde, 2,3,4-trimethoxybenzaldehyde, benzaldehyde, pyridine-2-carbaldeyde, and pyridine-4-carbaldeyde), 1,1-diphenyl-2-picrylhydrazil (DPPH), kojic acid and any used reagent were purchased from by Sigma-Aldrich, Fluka or Merck unless otherwise stated.
1H and 13C NMR spectra were obtained on a Bruker 400 MHz NMR spectrometer (400 MHz for 1H, 100 MHz for 13C), using TMS as an internal standard. CDCl3 and DMSO-d6 were used as NMR solvents. 13C and APT spectra were adjusted according to deutero solvent peaks. Chemical shifts were expressed in δ (ppm), and coupling constants (J) were reported in hertz (Hz). ACD NMR program was used for the interpretation of spectra. FT-IR spectra were taken using the Perkin-Elmer 1600 (ATR) (4000–400 cm−1) spectrophotometer. Melting points were determined using the Thermo-var apparatus fitted with a microscope. Normal phase silica gel (230–400 mesh) was used in vacuum column chromatography (VLC). TLC was carried out on Silica gel 60 F254, and the spots were visualized by UV lamp (254 nm and 366 nm) or spraying with 20% H2SO4 and heating.
2.1. General procedure for the synthesis of compounds 1–14
The synthesis of known chalcone compounds (1–7) was carried out using the Claisen-Schmidt condensation [12,13]. A mixture of chalcone (1–7) (10 mmol) and hydroxylamine hydrochloride (15 mmol) in ethyl alcohol (30 mL) was refluxed for 12 h in presence of 40% KOH (5 mL) [1–9]. The reaction mixture was controlled by TLC then cooled, poured into crushed ice and extracted with diethyl ether (30 mL × 3). Solvent was evaporated to give crude products which were chromatographed by column chromatography using increased polarity of n-hexane then ether mixture to give compounds 8–14 in the range of 45%–63% yield, respectively.
Compound 8 (3-(2-methoxyphenyl)-5-(2,3,4-trimethoxyphenyl)isoxazole): Yield: 63%, m.p. = 102–103 oC, FT-IR (cm−1):1589 (C=C, aromatic ring),1606 (C=N), 2940 (=CH), 1H-NMR (δ, ppm, DMSO-d6): 3.97, 3.98 (s, 12 H, −OCH3), ar-H:[6.80–6.87 (m,1H), 7.04–7.15 (m, 2H), 7.42–7.50 (m,1H), 7.69–7.75 (m,1H), 7.91–7.97 (m,1H)], 7.25 (s,1H, isoxazole-H4), APT-NMR (100 MHz, DMSO-d6): 61.04, 60.67, 56.11, 55.61 (4× −OCH3), 103.35 (isoxazole-C4), ar-C [107.78 (CH), 115.25 (C),115.51 (CH), 118.54 (C), 120.93 (CH), 122.53 (CH), 129.65 (CH), 131.04 (CH), 142.65 (C), 151.27(C), 155.10 (C), 157.32 (C), 160.93 (isoxazole-C3), 165.35 (isoxazole-C5), ]. LC-TOF-MS: (m/z, %) [M+Na]+: 364.1159 (100), calc. 364,1187. Supplementary data are in Figures S1–S4.
Compound 9 (3-(4-methoxyphenyl)-5-(2,4-dimethoxyphenyl)isoxazole): Yield: 45%, m.p. = 93–94 oC, FT-IR (cm−1):1594 (C=C, aromatic ring),1613 (C=N), 3058, 3006, 2987 (=CH), 1H-NMR (δ, ppm, DMSO-d6): 3.90 (s, 6H, −OCH3), 4.00 (s, 3H, −OCH3), ar-H:[ 6.60–6.66 (m, 2H), 6.94–6.98 (m, 2H), 7.82–7.98 (m, 3H)], 7.03 (s,1H, isoxazole-H4), APT-NMR (100 MHz, DMSO-d6): 55.39, 55.56, 55,64 (3 × −OCH3), 98.92 (isoxazole-C4), ar-C [99.66 (CH), 106.12 (CH), 109.95(C), 114.21 (2CH), 122.26 (C), 128.24 (2CH), 128.84 (CH), 157.61(C), 160.80 (C), 162.26 (C)], 162.58 (isoxazole-C3), 166.26 (isoxazole-C5),. LC-TOF-MS: (m/z, %) [M+H]+: 312.1235 (100), calc. 312.1222, [M+Na]+: 334.1059 (70), calc. 334.1049. Supplementary data are in Figures S5–S8.
Compound 10 (3-(3-hydroxyphenyl)-5-(phenyl)isoxazole): Yield: 62%, m.p. = 230–231 oC, FT-IR (cm−1):1605 (C=N), 2962, 2836 (=CH), 3193, 3484 (−OH), 1H-NMR (δ, ppm, DMSO-d6): ar-H: [6.94 (d, 1H, J=6.8 Hz), 7.35–7.38 (m, 2H), 7.54 (d, 4H, J=8.0 Hz), 7.93 (d, 2H, J=6.4 Hz )], 7.31(s,1H, isoxazole-H4), 9.21 (phenolic,−OH) (s, 1H), APT-NMR (100 MHz, DMSO-d6): 99.00 (isoxazole-C4), ar-C [112.50 (CH), 116.92 (CH), 118.07 (CH), 127.06 (2 CH), 128.42 (C), 129,61 (C), 129.61 (2 CH), 130.76 (CH), 130.96 (CH), 158.40 (C)], 163.03 (isoxazole-C3), 170.29 (isoxazole-C5). LC-TOF-MS: (m/z, %) [M+H]+: 238.0856 (100), calc. 238.0844. Supplementary data are in Figures S9–S12.
Compound 11 (3-(3-hydroxyphenyl)-5-(2,4-dimethoxyphenyl)isoxazole): Yield: 60%, m.p. = 168–169 oC, FT-IR (cm−1): 1572(C=C, aromatic ring), 1608 (C=N), 2940, 3010, 3007 (=CH), 3256 (−OH), 1H-NMR (δ, ppm, DMSO-d6): 3,85 (s, 3H, −OCH3), 3.98 (s, 3H, −OCH3) ar-H:[ 6.71 (d, 1H, J=8.8 Hz), 6.90 (d, 1H, J=7.2 Hz), 7.14 (s,1H), 7.30–7.36 (m,3H), 7.81 (d, 1H, J=8.8 Hz)], 6.75 (s,1H, isoxazole-H4), 9.76 (phenolic,−OH) (s, 1H), APT-NMR (100 MHz, DMSO): 56.33, 55.99 (2 × −OCH3), 99.24 (isoxazole-C4), ar-C [100.22 (CH), 106.48 (CH), 108.99 (C), 113.49 (CH), 117.59 (CH), 118.00 (CH), 128.56 (CH), 130.49 (C),130.63 (CH), 157.99 (C), 158.27(C), 162.79 (C)], 162.82 (isoxazole-C3), 166.56 (isoxazole-C5). LLC-TOF-MS: (m/z, %) [M+H]+: 298.1074, calc. 298.1080. Supplementary data are in Figures S13–S16.
Compound 12 (3-(4-hydroxyphenyl)-5-(2,5-dimethoxyphenyl)isoxazole): Yield: 65%, m.p. = 176–178 oC,, FT-IR (cm−1):1575(C=C, aromatic ring), 1603 (C=N), 3061 (=CH), 3479 (−OH), 1H-NMR (δ, ppm, DMSO-d6): 3.76 (s, 3H,−OCH3), 3.84 (s, 3H,−OCH3), ar-H:[ 6.95 (d, 2H, J=8.8 Hz), 7.04–7.07 (m, 1H),7.33 (d, 1H, J=3.2 Hz), 7.16 (s, 1H), 7.76 (d, 2H, J=8.4 Hz)], 10.1 (phenolic,−OH) (s, 1H), 7.11 (m,1H, isoxazole −H4), APT-NMR (100 MHz, DMSO-d6): 55.94 (−OCH3), 56.60 (−OCH3), 99.86 (isoxazole-C4), ar-C [114.00 (CH), 114.06 (CH), 116.43 (2CH), 117.10 (CH), 127.85 (2CH),118.39 (C), 118.66 (C), 151.69 (C), 153.61(C), 159.84 (C)], 160.45 (isoxazole-C3), 169.67 (isoxazole-C5), LC-TOF-MS: (m/z, %) [M+H]+: 298.1065 (100), calc. 298.1080. Supplementary data are in Figures S17–S20.
Compound 13 (3-(3-methoxyphenyl)-5-(2-pyridinyl)isoxazole): Yield: 58%, m.p. = 98 oC, FT-IR (cm−1): 1568 (C=C, aromatic ring), 1582 (C=N), 2962, 3049, 3084 (C=C), 1H-NMR (δ, ppm, DMSO-d6): 3.35 (s, 3H,−OCH3), ar-H:[ 7.10 (d, 1H, J=7.6 Hz), 7.45–7.56 (m, 4H), 8.0 (d, 1H, J=7.6 Hz), 8.07 (d, 1H, J=7.6 Hz), 8.76 (s,1H)], 7.65 (s,1H,isoxazole-H4), APT-NMR (100 MHz, DMSO-d6): 55.89 (−OCH3), 99.89 (isoxazole-C4), ar-C [111.22 (CH), 117.13 (CH), 118.40 (CH), 121.86 (CH), 125.66 (CH), 128.39 (C), 130.98 (CH), 138.04 (CH), 148.06 (C), 150.40 (CH), 160.23(C)], 164.06 (isoxazole-C3), 170.36 (isoxazole-C5), LC-TOF-MS: (m/z, %) [M+H]+: 253.0990 (100), calc. 253.0890. Supplementary data are in Figures S21–S24.
Compound 14 (3-(4-methoxyphenyl)-5-(4-pyridinyl)isoxazole): Yield: 55%, m.p. = 141–143 oC, FT-IR (cm−1): 1573 (C=C, aromatic ring), 1610 (C=N), 2967, 3019, 3112 (=CH), 1H-NMR (δ, ppm, DMSO-d6): 3.83(s, 3H,−OCH3), ar-H:[7.09–7.11 (d, 2H, J=7.6 Hz), 7.78 (d, 2H, J=7.6 Hz), 7.82 (d, 2H, J=6.4 Hz), 8.78 (d, 2H, J=6.4 Hz)], 7.80 (s,1H, isoxazole-H4), APT-NMR (100 MHz, DMSO-d6): 55 (−OCH3), 102 (isoxazole-C4), ar-C [116 (2CH), 119 (2CH), 121 (C), 128 (2CH), 134 (C), 152 (2CH), 162 (C)], 164 (isoxazole-C3), 168 (isoxazole-C5). LC-TOF-MS: (m/z, %) [M+H]+: 253.0977 (100), calc., 253.0890, [M+Na]+: 275.0805 (60), calc. 275.0825. Supplementary data are in Figures S25–S28.
2.3. Biological activities
2.3.1. Antioxidant activity
2.3.1.1. Cupric ion reducing (CUPRAC) method
The capacity of 8–14 compounds to reduce cupric ions was established as it was described before [14]. A reaction mixture was prepared by mixing a volume of CuCl2 (0.250 mL, 10 mM), neocuproine (0.250 mL, 7.5 mM in ethanol) and NH4Ac buffer (0.250 mL, 1 M, pH 7.0) with standard/compound solutions at different concentrations. SA blank was prepared by adding sample solution to premixed reaction mixture CuCl2. The absorbances of the blank and sample were read at 450 nm after a 30-min incubation at room temperature. The absorbance of the blank was subtracted from that of the sample. CUPRAC activity was expressed as equivalents of trolox (mg TEAC/mg compound).
2.3.1.2. DPPH free radical scavenging method
The DPPH free radical scavenging activities of 8–14 compounds were determined using the method described before [15]. Alcoholic solution of DPPH (1 mL, 0.1 M in methanol) was mixed with 8–14 compound solutions prepared in DMSO. The absorbance was noted at 517 nm after incubation in the dark for 50 min. DPPH gives a purple color in methanol. This color is decayed by antioxidant. So reduction in the absorbance indicates DPPH• scavenging activity. Trolox was used as standard radical scavenger and values were expressed as SC50 (μg compound per mL), the concentration of the samples that causes 50% scavenging of DPPH radical.
2.3.2. Tyrosinase inhibition
The mushroom tyrosinase inhibition assay was carried out according previously reported method. A total of 100 μL of 100 mM phosphate buffer (pH 6.8), 20 μL of 250 U/mL mushroom and compounds 8–14 (5 μL) were prepared in 96 well plate [16,17]. After 10 min of a preincubation period at room temperature, the enzymatic reaction was initiated with the addition of l-DOPA (3 mM, 20 μL) as substrate. The absorbance was read at 475 nm. The compound concentration giving 50% inhibition of tyrosinase activity was determined by interpolation of concentration-response curves. Kojic acid was used as a positive control. The extract concentration giving 50% (IC50) of the original tyrosinase activity was determined.
3. Results
3.1. Synthesis
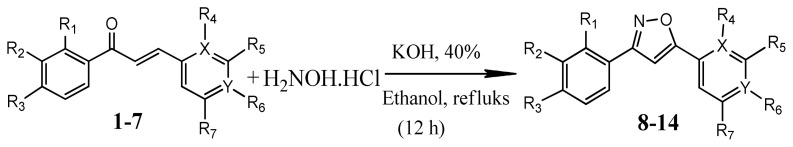
The synthetic procedure of the target compounds was shown in Figure. Commercially available different aromatic ketone with aldehydes using NaOH were yielded known chalcones (1–7) [18–24] through the Claisen–Schmidt condensation [12,13] which then were condensed with hydroxylamine hydrochloride in alkaline medium to give their corresponding isoxazole by intermolecular cycloaddition reaction (Figure) [25]. All new compounds (8–14) were characterized by 1H NMR, 13C/APT NMR, FT-IR, and LC-TOF/ MS (Supplementary data are in Figures S1–S28). Then these compounds were screened for their tyrosinase enzyme inhibition and antioxidant properties.
Figure.
Synthetic scheme for the isoxazole derivatives (8–14).
Isoxazole is an azole with an oxygen atom next to the nitrogen. Isoxazole rings are found in some natural products and a number of drugs such as ibotenic acid, COX-2 inhibitor valdecoxib, furoxan, cloxacillin, dicloxacillin, and flucloxacillin. The synthetic androgenic steroid danazol also has an isoxazole ring. The synthesis of substituted isoxazoles are well developed in literature to possess significant biological activities. In the literature, Kang et al. [26] described the synthesis of a series of new isoxazole derivatives showed potent antibacterial properties against different gram-positive and gram-negative organisms. Lamani et al. [27] reported the synthesis of a new series of benzisoxazolyl imidazothiadiazoles and evaluated their antibacterial potential. Yamuna et al. [28] also described a synthesis and biological properties of isoxazolo pyrimidocyclohepta[b]indoles, the best activity against Mycobacterium tuberculosis strain H37Rv were reported for an isoxazole derivative with MIC value 3.12 mg/mL. Another group of isoxazole derivatives is a series of (1,4-phenylene)bis(arylsulfonylisoxazoles) synthesized by Lavanya et al. [29] by 1,3-dipolar cycloaddition. Sharp et al. [30] described the properties of another isoxazole Hsp90 inhibitor. Kamal et al. [31] also reported the synthesis of 3,5-diaryl isoxazole linked with quinazolinone and assayed for their anticancer activity. There are many manuscript related with the synthesis and biological evaluation of isoxazoles [1–9].
3.2. Biological activities
3.2.1. Tyrosinase activity of compounds 8–14
The activities on tyrosinase of synthesized compounds 8–14 were performed according to a modified method with Kojic acid as positive control [17, 32]. The activity effects of 8–14 were summarized in Table 1. As shown in Table 1, among the synthesis compounds 8, and 11–13 showed good to moderate activating effect on tyrosinase. The potencies of compound 8 (with the value of IC50 = 66.74 ± 3.96 μg/mL), 11 (IC50 = 61.47 ± 3.46 μg/mL), 12 (IC50 = 91.41 ± 4.15 μg/mL) and 13 (IC50 = 188.52 ± 5.85 μg/mL) were lower to the positive control kojic acid, of which IC50 was 3.2 ± 0.29 μg/mL. The position, number and nature of the substituent on benzene and pyridine rings were varied in order to identify the most appropriate group. The compounds with 3-hydroxy and 2,4-dimethoxy (11) showed higher activity compared to with 2-methoxy and 2,3,4-trimethoxy (8), 4-hydroxy and 2,5-dimethoxy (12), and 3-methoxy and 4-pyridinyl (13), which suggested that the strong electron donating group and the number of methoxy may be favorable to enhance the activity. However, the activity dropped rapidly when the less methoxy substituted and the benzene on isoxazole was replaced by pyridine, which suggested that benzene was fundamental for the activity [25].
Table 1.
Tyrosinase inhibition of compounds 8–14.
| No | Tyrosinase (IC50 μg/mL) |
|---|---|
| 8 | 66.74 ± 3.96 |
| 9 | No inhibition |
| 10 | No inhibition |
| 11 | 61.47 ± 3.46 |
| 12 | 91.41 ± 4.15 |
| 13 | 188.52 ± 5.85 |
| 14 | No inhibition |
| Kojic acid | 3.2 ± 0.29 |
Synthetic and natural tyrosinase inhibitors are promising therapeutic agents for skincare and cosmetic products. Tyrosinase is the key enzyme in the melanogenesis pathway, responsible for the formation of melanin pigment. The most common target for inhibiting the melanogenesis pathway is the direct inhibition of the catalytic activity of tyrosinase, and the most widely used hypopigmented agents are tyrosinase inhibitors [17]. Tyrosinase inhibitors, which can inhibit melanin biosynthesis, are currently used in various hyperpigmentation and cosmetic products to control freckle formation [32]. Kojic acid is a fungal metabolite that exhibits inhibitory activity against tyrosinase. Kojic acid is often used as a positive control to discover new tyrosinase inhibitors [33,34].
In the literature, a series of novel isoxazole chalcone derivatives have been synthesized, and evaluated for tyrosinase activities. Among them, 1-(4-((3-phenylisoxazol-5-yl) methoxy) phenyl) -3-phenylprop-2-en-1-one was reported as a potent tyrosinase activator [35]. In another study, isoxazole chalcone was reported that they inhibit the monopenolase activity of fungal tyrosinase [25]. In another work, novel 2- (3, 5-disubstituted isoxazolyl) -1, 5-benzodiazepines has been reported and those hybrid compounds exhibited moderate to significant antityrosinase activities [36]. The tyrosinase inhibitory activity of the natural curcuminoid analogs on both L-tyrosine and DOPA substrates were reported. The isoxazole analog showed IC50 values of 8.3, μM, and were 20.9-fold more active than kojic acid [37]. Thus, they showed awareness of tyrosinase activities according to the substitution difference of isoxazole compounds.
3.2.2. Antioxidant activity of compounds 8–14
Antioxidant properties of synthesized compounds 8–14 were made according to CUPRAC and DPPH methods [14, 38–39] as seen in Table 2. The highest CUPRAC values of isoxazole compounds (8–14) were 1.245 ± 0.019, 1.233.33 ± 0.015, and 1.189.33 ± 0.021 (μg/mL trolox/gram DW) in compounds 13, 12, and 14, and the lowest DPPH values for compounds 11 and 10 were found to be 0.584 ± 0.011 mg/mL and 0.789 ± 0.009 mg/mL, respectively. When the activities of all compounds according to CUPRAC and DPPH were examined, it was seen that compound 13 for CUPRAC, and compound 11 for DPPH were the most effective antioxidant compounds. When looking at the substitution positions of these compounds, it has been found that they are generally more effective when they are substituted at the 3-position phenyl ring which is substituted at the C-3 position of isoxazole. Isoxazole having 3-hydroxy and 3-methoxy at the phenyl group which are attached at the C-3 position of isoxazoles (11 and 13) showed the greatest activity due to the electron donation and formation of phenoxy radical.
Table 2.
Antioxidant (CUPRAC and DPPH) activities of compounds 8–14.
| No | DPPH, SC50 (μg/mL) | CUPRAC (mg TEAC/mg) |
|---|---|---|
| 8 | 61.42 ± 1.41 | 1.156 ± 0.026 |
| 9 | 71.96 ± 2.36 | 0.856 ± 0.013 |
| 10 | 65.65 ± 1.69 | 0.789 ± 0.009 |
| 11 | 81.52 ± 3.66 | 0.584 ± 0.011 |
| 12 | 40.21 ± 2.71 | 1.233 ± 0.015 |
| 13 | 57.32 ± 2.12 | 1.245 ± 0.019 |
| 14 | 54.41 ± 2.87 | 1.189 ± 0.021 |
| Trolox | 5.56 ± 0.85 | - |
Cancer is one of the leading causes of mortality in the world. There has been a lot of effort to discover new anticarcinogenic agents that allow treatment with fewer side effects. Isoxazole is the anticancer agent. A series of isoxazole-carboxamide derivatives were reported and evaluated for their antioxidant activity in DPPH assay. One of the compound was reported the most active as antioxidant agent (IC50 = 7.8 ± 1.21 μg/mL) compared with Trolox (IC50 2.75 μg/mL) [40]. Antioxidant activity of isoxazole-based chalcones were reported [41]. A novel series of indolyl isoxazole derivatives were synthesized and their antioxidant activity were evaluated [42]. The substituted isoxazole derivatives displayed antioxidant activity [43]. The disubstituted and trisubstituted isoxazoles have been reported to exhibit antioxidant activity [44]. Antioxidant activity of novel functionalized isoxazole derivatives were reported and among them 5-amino-3(pyridine-4-yl) isoxazole-4-carbonitrile was highly significant [45]. As seen in the literature, compounds having isoxazole ring with different substituents had shown very different biological activities. In this study, tyrosinase and antioxidant activities of 7 different isoxazole compounds, which are not found in the literature, were reported.
4. Conclusion
In summary, this article is based on synthesized new isoxazole derivatives which display wide spectrum of biological potential such as anticancer, antiinflammatory, antimicrobial, antiviral, antiplatelet, immunomodulatory, antithrombotic, antitriglyceride, antidiabetic, analgesic, anticonvulsant, anti-Alzheimer agents, pesticides and insecticides. The heterocyclic moiety being so versatile in nature offers the medicinal chemist to explore more about the data mentioned in this article, which will be a great help to prospective researches working in this area for further studies. Thus, the synthesis of new isoxazole is still needed. Compounds 11 and 8 could be used as new potential tyrosinase inhibitor and 13 as antioxidant agent. It may be concluded that isoxazole moiety is an important heterocyclic compound as an essential constituent of large number of marketed drugs. Isoxazole compounds have a great potential to be explored for the development of new chemical compounds to treat a variety of clinically important diseases.
Supplementary Material
| Table of Contents | Page |
|---|---|
| Figure S1. 1H-NMR spectrum of compound 8 (CDCl3, 400 MHz) ........................................... | 2 |
| Figure S2. APT-NMR spectrum of compound 8 (CDCl3,100 MHz) ......................................... | 2 |
| Figure S3. LC-Q-TOF/MS spectrum of compound 8 ................................................................ | 3 |
| Figure S4. ATR (FT-IR) spektrum of compounds 8 .................................................................. | 3 |
| Figure S5. 1H-NMR spectrum of compound 9 (CDCl3, 400 MHz) ........................................... | 4 |
| Figure S6. APT-NMR spectrum of compound 9 (CDCl3,100 MHz) ......................................... | 4 |
| Figure S7. LC-Q-TOF/MS spectrum of compound 9 ................................................................ | 5 |
| Figure S8. ATR (FT-IR) spektrum of compounds 9 .................................................................. | 5 |
| Figure S9. 1H-NMR spectrum of compound 10 (DMSO, 400 MHz) ....................................... | 6 |
| Figure S10. APT-NMR spectrum of compound 10 (DMSO,100 MHz) .................................... | 6 |
| Figure S11. LC-Q-TOF/MS spectrum of compound 10 ............................................................ | 7 |
| Figure S12. ATR (FT-IR) spektrum of compound 10 ............................................................... | 7 |
| Figure S13. 1H-NMR spectrum of compound 11 (DMSO, 400 MHz) ...................................... | 8 |
| Figure S14. APT-NMR spectrum of compound 11 (DMSO,100 MHz) .................................... | 8 |
| Figure S15. LC-Q-TOF/MS spectrum of compound 11 ............................................................ | 9 |
| Figure S16. ATR (FT-IR) spektrum of compound 11 ............................................................... | 9 |
| Figure S17. 1H-NMR spectrum of compound 12 (DMSO, 400 MHz) .................................... | 10 |
| Figure S18. APT-NMR spectrum of compound 12 (DMSO,100 MHz) .................................. | 10 |
| Figure S19. LC-Q-TOF/MS spectrum of compound 12 .......................................................... | 11 |
| Figure S20. ATR (FT-IR) spektrum of compound 12 ............................................................. | 11 |
| Figure S21. 1H-NMR spectrum of compound 13 (DMSO, 400 MHz) .................................... | 12 |
| Figure S22. 13C-NMR spectrum of compound 13 (DMSO, 100 MHz) ................................... | 12 |
| Figure S23. LC-Q-TOF/MS spectrum of compound 13 .......................................................... | 13 |
| Figure S24. ATR (FT-IR) spektrum of compound 13 ............................................................. | 13 |
| Figure S25. 1H-NMR spectrum of compound 14 (DMSO, 400 MHz) .................................... | 14 |
| Figure S26. APT-NMR spectrum of compound 14 (DMSO, 100 MHz) ................................ | 14 |
| Figure S27. LC-Q-TOF/MS spectrum of compound 14 ......................................................... | 15 |
| Figure S28. ATR (FT-IR) spektrum of compound 14 ............................................................. | 15 |
1H-NMR spectrum of compound 8 (CDCl3, 400 MHz)
APT-NMR spectrum of compound 8 (CDCl3, 100 MHz)
LC-Q-TOF/MS spectrum of compound 8
ATR (FT-IR) spectrum of compound 8
1H-NMR spectrum of compound 9 (CDCl3, 400 MHz)
APT-NMR spectrum of compound 9 (CDCl3, 100 MHz)
LC-Q-TOF/MS spectrum of compound 9
ATR (FT-IR) spektrum of compound 9
1H-NMR spectrum of compound 10 (DMSO-d6, 400 MHz)
APT-NMR spectrum of compound 10 (DMSO-d6, 100 MHz)
LC-Q-TOF/MS spectrum of compound 10
ATR (FT-IR) spektrum of compound 10
1H-NMR spectrum of compound 11 (DMSO-d6, 400 MHz)
APT-NMR spectrum of compound 11 (DMSO-d6, 100 MHz)
LC-Q-TOF/MS spectrum of compound 11
ATR (FT-IR) spectrum of compound 11
1H-NMR spectrum of compound 12 (DMSO-d6, 400 MHz)
APT-NMR spectrum of compound 12 (DMSO-d6, 100 MHz)
LC-Q-TOF/MS spectrum of compound 12
ATR (FT-IR) spectrum of compound 12
1H-NMR spectrum of compound 13 (DMSO-d6, 400 MHz)
13C-NMR spectrum of compound 13 (DMSO-d6, 100 MHz)
LC-Q-TOF/MS spectrum of compound 13
ATR (FT-IR) spectrum of compounds 13
1H-NMR spectrum of compound 14 (DMSO-d6, 400 MHz)
APT-NMR spectrum of compound 14 (DMSO-d6, 100 MHz)
LC-Q-TOF/MS spectrum of compound 14
ATR (FT-IR) spectrum of compound 14
Acknowledgment
The author is thankful to Dr. Özlem FAİZ (Recep Tayyip Erdoğan University, Faculty of Science, and Department of Chemistry) for the help of biological activities. I also thank to Karadeniz Technical University for using the research facilities.
References
- 1. Zhu J, Mo J, Lin HZ, Chen Y, Sun HP. The recent progress of isoxazole in medicinal chemistry. Bioorganic and Medicinal Chemistry. 2018;26(12):3065–3075. doi: 10.1016/j.bmc.2018.05.013. [DOI] [PubMed] [Google Scholar]
- 2. Sysak A, Obminska-Mrukowicz B. Isoxazole ring as a useful scaffold in a search for new therapeutic agents. European Journal of Medicinal Chemistry. 2017;137:292–309. doi: 10.1016/j.ejmech.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 3. Beam CF, Dyer MCD, Schwarz RA, Hauser CR. A new synthesis of isoxazoles from 1,4-dianions of oximes having an alpha hydrogen - mass spectrometry. Journal of Organic Chemistry. 1970;35(6):1806–1810. doi: 10.1021/jo00831a020. [DOI] [Google Scholar]
- 4. Parul S, Ruchi B, Renu S. Various synthetic pathways for the synthesis of 3, 4-disubstituted isoxazole by one pot multicomponent reaction. Orbital: The Electronic Journal of Chemistry. 2020;12(4):267–275. doi: 10.17807/orbital.v12i4.1549. [DOI] [Google Scholar]
- 5. Rizvana KK, Hareeshbabu E. Synthesis and antimicrobial screening of modified isoxazoles: a short review. International Journal of Pharmacy and Pharmaceutical Research. 2019;15(3):136–148. [Google Scholar]
- 6. Chikkula KV, Raja S. Isoxazole-a potent pharmacophore. International Journal of Pharmacy and Pharmaceutical Sciences. 2017;9(7):13–24. doi: 10.22159/ijpps.2017.v9i7.1-9097. [DOI] [Google Scholar]
- 7. Ruthu M, Pradeepkumar Y, Madhusudhana CC, Prasanthi G, Redd VJS. Pharmacological activities of isoxazole derivatives. Journal of Global Trends in Pharmaceutical Sciences. 2011;2(1):55–62. [Google Scholar]
- 8. Koufaki M, Fotopoulou T, Kapetanou M, Heropoulos GA, Gonos ES, et al. Microwave-assisted synthesis of 3, 5-disubstituted isoxazoles and evaluation of their anti-ageing activity. European Journal of Medicinal Chemistry. 2014;83:508–515. doi: 10.1016/j.ejmech.2014.06.046. [DOI] [PubMed] [Google Scholar]
- 9. Kuchana M. Synthesis and in vitro antioxidant activity of substituted α-cyano-N-(5-methylisoxazol-3-yl) cinnamides. World Journal of Pharmacy and Pharmaceutical Sciences. 2014;3(6):1800–1808. [Google Scholar]
- 10. Prashanthi Y, Kiranmai K, Shivaraj Subhashini NJP. Synthesis, potentiometric and antimicrobial studies on metal complexes of isoxazole Schiff bases. Spectrochimica Acta, Part A: Molecular and Biomolecular Spectroscopy. 2008;70(1):30–35. doi: 10.1016/j.saa.2007.07.028. [DOI] [PubMed] [Google Scholar]
- 11. Ahrens H, Lange G, Muller T, Rosinger C, Willms L, et al. 4-Hydroxyphenylpyruvate dioxygenase inhibitors in combination with safeners: solutions for modern and sustainable agriculture. Angewandte Chemie International Edition. 2013;52(36):9388–9398. doi: 10.1002/anie.201302365. [DOI] [PubMed] [Google Scholar]
- 12. Fandaklı S, Kahriman N, Yücel TB, Karaoğlu SA, Yaylı N. Biological evaluation and synthesis of new pyrimidine-2(1H)-ol/-thiol derivatives derived from chalcones using the solid phase microwave method. Turkish Journal of Chemistry. 2018;42:520–535. doi: 10.3906/kim-1711-9. [DOI] [Google Scholar]
- 13. Kahriman N, Serdaroğlu V, Peker K, Aydın A, Usta A, et al. Synthesis and biological evaluation of new 2,4,6-trisubstituted pyrimidines and their N-alkyl derivatives. Bioorganic Chemistry. 2019;83:580–594. doi: 10.1016/j.bioorg.2018.10.068. [DOI] [PubMed] [Google Scholar]
- 14. Apak R, Güçlü K, Özyürek M, Karademir SE. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC Method. Journal of Agricultural and Food Chemistry. 2004;52(26):7970–7981. doi: 10.1021/jf048741x. [DOI] [PubMed] [Google Scholar]
- 15. Brand-Williams W, Cuvelier ME, Berset C. Use of a Free-Radical Method to Evaluate Antioxidant Activity. LWT - Food Science and Technology. 1995;28(1):25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- 16. Fu R, Zhang Y, Guo Y, Chen F. Antioxidant and tyrosinase inhibition activities of the ethanol-insoluble fraction of water extract of Sapium sebiferum (L.) Roxb. leaves. South African Journal of Botany. 2014;93:98–104. [Google Scholar]
- 17. Chang TS. Natural melanogenesis inhibitors acting through the down regulation of tyrosinase activity. Materials. 2012;5(9):1661–1685. doi: 10.3390/ma5091661. [DOI] [Google Scholar]
- 18. Fandaklı S, Doğan İS, Sellitepe HE, Yaşar A, Yaylı N. Synthyesis, theoretical calculation and a-glucodsdes inhibition of new chalcone oximes. Organic Communications. 2018;11(1):23–34. doi: 10.25135/acg.oc.38.18.02.067. [DOI] [Google Scholar]
- 19. Küçükoqlu K, Fatih Oral, Aydın T, Yamalı C, Algül O, et al. Synthesis, cytotoxicity and carbonic anhydrase inhibitory activities of new pyrazolines. Journal of Enzyme Inhibition and Medicinal Chemistry. 2016;31(4):20–24. doi: 10.1080/14756366.2016.1217852. [DOI] [PubMed] [Google Scholar]
- 20. Sultan A, Shajahan S, Ahamad T, Alshehri SM, Sajjad N, et al. Silica-supported heterogeneous catalysts-mediated synthesis of chalcones as potent urease inhibitors: in vitro and molecular docking studies. Monatshefte fuer Chemie. 2020;151(1):123–133. doi: 10.1007/s00706-019-02534-z. [DOI] [Google Scholar]
- 21. Mopur VBR, Yi H, Ping-Chung H, Guan-Jhong H, Yu-Yi C, et al. 4-Synthesis and biological evaluation of chalcone, dihydrochalcone, and 1, 3-diaryl propane analogs as anti-inflammatory agents. Bioorganic & Medicinal Chemistry Letters. 2017;27(7):1547–1550. doi: 10.1016/j.bmcl.2017.02.038. [DOI] [PubMed] [Google Scholar]
- 22. Shubhalaxmi, Patlak L, Ananda K, Subrahmanya BK. Synthesis of focused library of novel aryloxyacids and pyrazoline derivatives: Molecular docking studies and antimicrobial investigation. Cogent Chemistry. 2016;2(1):1–18. doi: 10.1080/23312009.2016.1141388. [DOI] [Google Scholar]
- 23. Albay C, Kahriman N, Yılmaz Iskender N, Alpay Karaoglu S, Yaylı N. Synthesis and antimicrobial activity of methoxy azachalcones and N-alkyl substituted methoxy azachalconium bromides. Turkish Journal of Chemistry. 2011;35(3):441–454. doi: 10.3906/kim-1007-790. [DOI] [Google Scholar]
- 24. Singh JV, Sharma S, Rahar S. Synthesis and spermicidal activity of substituted (E)-3- (aryl/heteroaryl)-1-phenylprop-2-en-1-ones. Pharma Chemica. 2015;7(11):93–103. [Google Scholar]
- 25. Niu C, Yin L, Nie LF, Dou J, Zhao JY, et al. Synthesis and bioactivity of novel isoxazole chalcone derivatives on tyrosinase and melanin synthesis in murine B16 cells for the treatment of vitiligo. Bioorganic & Medicinal Chemistry. 2016;24(21):5440–5448. doi: 10.1016/j.bmc.2016.08.066. [DOI] [PubMed] [Google Scholar]
- 26. Kang YK, Shin KJ, Yoo KH, Seo KJ, Hong CY, et al. Synthesis and antibacterial activity of new carbapenems containing isoxazole moiety. Bioorganic & Medicinal Chemistry Letters. 2000;10(2):95–99. doi: 10.1016/S0960-894X(99)00646-0. [DOI] [PubMed] [Google Scholar]
- 27. Lamani RS, Shetty NS, Kamble RR, Khazi IAM. Synthesis and antimicrobial studies of novel methylene bridged benzisoxazolyl imidazo 2,1-b 1,3,4 thiadiazole derivatives. European Journal of Medicinal Chemistry. 2009;44(7):2828–2833. doi: 10.1016/j.ejmech.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 28. Yamuna E, Kumar RA, Zeller M, Prasad KJR. Synthesis, antimicrobial, antimycobacterial and structure-activity relationship of substituted pyrazolo-, isoxazolo-, pyrimido- and mercaptopyrimidocyclohepta b indoles. European Journal of Medicinal Chemistry. 2012;47(1):228–238. doi: 10.1016/j.ejmech.2011.10.046. [DOI] [PubMed] [Google Scholar]
- 29. Lavanya G, Reddy LM, Padmavathi V, Padmaja A. Synthesis and antimicrobial activity of (1,4-phenylene) bis (arylsulfonylpyrazoles and isoxazoles. European Journal of Medicinal Chemistry. 2014;73:187–194. doi: 10.1016/j.ejmech.2013.11.041. [DOI] [PubMed] [Google Scholar]
- 30. Sharp SY, Prodromou C, Boxall K, Powers MV, Holmes JL, et al. Inhibition of the heat shock protein 90 molecular chaperone in vitro and in vivo by novel, synthetic, potent resorcinylic pyrazole/isoxazole amide analogues. Molecular Cancer Therapeutics. 2007;6(9):1198–1211. doi: 10.1158/1535-7163.MCT-07-0149. [DOI] [PubMed] [Google Scholar]
- 31. Kamal A, Bharathi EV, Reddy JS, Ramaiah MJ, Dastagiri D, et al. Synthesis and biological evaluation of 3,5-diaryl isoxazoline/isoxazole linked 2, 3- dihydroquinazolinone hybrids as anticancer agents. European Journal of Medicinal Chemistry. 2011;46(2):691–703. doi: 10.1016/j.ejmech.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 32. Saqib F, Janbaz KH, Sherwani MK. In vitro inhibitory potential of methanolic extract of Celosia argentea var. Cristata on tyrosinase, acetylcholinesterase and butyrylcholinesterase enzymes. Bangladesh Journal of Pharmacology. 2015;10(2):449–454. doi: 10.3329/bjp.v10i2.22880. [DOI] [Google Scholar]
- 33. Burnett CL, Bergfeld WF, Belsito DV, Hill AR, Klaassen CD, et al. Final report of the safety assessment of kojic acid as used in cosmetics. International Journal of Toxicology. 2010;29(4):244S–273S. doi: 10.1177/1091581810385956. [DOI] [PubMed] [Google Scholar]
- 34. Kanwal R, Khan AU, Saeed A, Nadeem H, Khalid W. Synthesis, docking, antioxidant, antihyperlipidemic, antiplatelet, anticoagulant, and vasodilatory activities of isoxazole newly synthesized derivative. Latin American Journal of Pharmacy. 2020;39(5):969–979. [Google Scholar]
- 35. Yin L, Niu C, Liao LX, Dou J, Habasi M, et al. An isoxazole chalcone derivative enhances melanogenesis in B16 melanoma cells via the Akt/GSK3β /β-catenin signaling pathways. Molecules. 2017;22(12):1–12. doi: 10.3390/molecules22122077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wejdane A, Mejda DA, Mansour Z, Hichem BJ, Rafik G. Design and synthesis of (3, 5-disubstituted isoxazole)-linked [1, 5]-benzodiazepine conjugates: evaluation of their antimicrobial and anti-tyrosinase activities. Journal of Chemical Research. 2017;41(1):12–17. doi: 10.3184/174751917X14815427219121. [DOI] [Google Scholar]
- 37. Athipornchai A, Niyomtham N, Pabuprapap W, Ajavakom V, Duca M, et al. Potent tyrosinase inhibitory activity of curcuminoid analogues and inhibition kinetics studies. Cosmetics. 2021;8(2):35. doi: 10.3390/cosmetics8020035. [DOI] [Google Scholar]
- 38. Deng J, Cheng W, Yang G. A novel antioxidant activity index (AAU) for natural products using the DPPH assay. Food Chemistry. 2011;125(4):1430–1435. doi: 10.1016/j.foodchem.2010.10.031. [DOI] [Google Scholar]
- 39. Sharma OP, Bhat TK. DPPH antioxidant assay revisited. Food Chemistry. 2009;113(4):1202– 1205. doi: 10.1016/j.foodchem.2008.08.008. [DOI] [Google Scholar]
- 40. Eid AM, Hawash M, Amer J, Jarrar A, Qadri S, et al. Synthesis and biological evaluation of novel isoxazole-amide analogues as anticancer and antioxidant agents. BioMed Research International. 2021:9. doi: 10.1155/2021/6633297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shaik A, Bhandare RR, Palleapati K, Nissankararao S, Kancharlapalli V, et al. Antimicrobial, antioxidant, and anticancer activities of some novel isoxazole ring containing chalcone and dihydropyrazole derivatives. Molecules. 2020;25(5):1047. doi: 10.3390/molecules25051047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Matilda JJ, Reji TFAF. Synthesis, antioxidant activity and DFT study of some novel N-methylated indole incorporating isoxazole moieties. Asian Journal of Chemistry. 2020;32(2):244–248. doi: 10.14233/ajchem.2020.22288. [DOI] [Google Scholar]
- 43. Rizvana KK, Hareeshbabu E. Synthesis and antimicrobial screening of modified isoxazoles: a short review. International Journal of Pharmacy and Pharmaceutical Research. 2019;15(3):136–148. [Google Scholar]
- 44. Voskienė A, Mickevičius V. Cyclization of chalcones to isoxazole and pyrazole derivatives. Chemistry of Heterocyelic Compounds. 2009;45(12):1485–1488. doi: 10.1007/s10593-010-0455-8. [DOI] [Google Scholar]
- 45. Beyzaei H, Deljoo MK, Aryan R, Ghasemi B, Zahedi MM, et al. Green multicomponent synthesis, antimicrobial and antioxidant evaluation of novel 5-amino-isoxazole-4-carbonitriles. Chemistry Central Journal. 2018;12:114. doi: 10.1186/s13065-018-0488-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
1H-NMR spectrum of compound 8 (CDCl3, 400 MHz)
APT-NMR spectrum of compound 8 (CDCl3, 100 MHz)
LC-Q-TOF/MS spectrum of compound 8
ATR (FT-IR) spectrum of compound 8
1H-NMR spectrum of compound 9 (CDCl3, 400 MHz)
APT-NMR spectrum of compound 9 (CDCl3, 100 MHz)
LC-Q-TOF/MS spectrum of compound 9
ATR (FT-IR) spektrum of compound 9
1H-NMR spectrum of compound 10 (DMSO-d6, 400 MHz)
APT-NMR spectrum of compound 10 (DMSO-d6, 100 MHz)
LC-Q-TOF/MS spectrum of compound 10
ATR (FT-IR) spektrum of compound 10
1H-NMR spectrum of compound 11 (DMSO-d6, 400 MHz)
APT-NMR spectrum of compound 11 (DMSO-d6, 100 MHz)
LC-Q-TOF/MS spectrum of compound 11
ATR (FT-IR) spectrum of compound 11
1H-NMR spectrum of compound 12 (DMSO-d6, 400 MHz)
APT-NMR spectrum of compound 12 (DMSO-d6, 100 MHz)
LC-Q-TOF/MS spectrum of compound 12
ATR (FT-IR) spectrum of compound 12
1H-NMR spectrum of compound 13 (DMSO-d6, 400 MHz)
13C-NMR spectrum of compound 13 (DMSO-d6, 100 MHz)
LC-Q-TOF/MS spectrum of compound 13
ATR (FT-IR) spectrum of compounds 13
1H-NMR spectrum of compound 14 (DMSO-d6, 400 MHz)
APT-NMR spectrum of compound 14 (DMSO-d6, 100 MHz)
LC-Q-TOF/MS spectrum of compound 14
ATR (FT-IR) spectrum of compound 14