Abstract
Indium tin oxide (ITO) is one of the most widely used semiconductor among transparent conducting oxides (TCOs) due to their electrical conductivity and optical transparency properties. Since the development of low temperature deposition methods, coating of ITO on polymer substrates especially for use in flexible electronics has been a popular topic. The existence of adequate adhesion strength between ITO and polymer is critical in producing a successful film. Nowadays, polycarbonate (PC), poly(methyl methacrylate) (PMMA) and polyethyleneterephtalate (PET) are frequently used as substrates for such coatings. However, there may be other polymeric alternatives that have a potential to be used for this purpose in the future. To evaluate these alternatives, work of adhesion (Wa) knowledge between ITO and polymers is necessary, and it has not been handled systematically previously. In this study, the interphase interaction parameters and Wa values between ITO and various polymers were calculated based on the Dupré, Fowkes and Girifalco-Good equations. PC, PMMA, PET, polystyrene (PS), polyphenylene sulfide (PPS), Nylon 66, polypropylene (PP), polyvinylchloride (PVC), styrene-butadiene rubber (SBR), high density polyethylene (HDPE), low density polyethylene (LDPE), polyvinyl acetate (PVAc), polyvinyl fluoride (PVF), polyvinylidene fluoride (PVDF), polytetrafluoroethylene (PTFE), polytrifluoroethylene (PTrFE) and polyperfluoroalkylethyl acrylate (PPFA) were considered as substrate material. Surface free energy (SFE) components calculated by acid-base, geometric mean and harmonic mean approaches for polymeric substrates were used during the calculations. In the present study, the polymers that can be used as substrates were evaluated in terms of adhesion ability to ITO, the significance of calculation methods on Wa values were also investigated simultaneously. It was determined that the Wa between ITO and polymer substrates was directly related with the total SFE value of the polymers.
Keywords: Indium tin oxide, work of adhesion, surface free energy, contact angle, polymer substrate
1. Introduction
Sn doped In2O3, also known as indium tin oxide (ITO), is one of the most widely used n-type semiconductor among transparent conducting oxides (TCOs) due to their electrical conductivity and optical transparency. It is typically comprised from the solution of 90% In2O3 and 10% SnO2 by weight, and used in many technological systems such as liquid crystal displays (LCDs), organic light-emitting diodes (OLEDs), photovoltaics and biosensors [1,2]. The demand to produce ITO films by adjusting the electrical conductivity and optical transparency has led to the development of many deposition methods [3]. DC magnetron sputtering [4], RF magnetron sputtering [5], ion beam sputtering [6], electron beam evaporation [7], chemical vapor deposition [8] and chemical solution deposition [9] are the examples of deposition methods used to coat ITO on a suitable substrate. The use of glass as substrate is quite common in the field due to its ability to withstand high temperatures, since a significant part of the coatings made with the aforementioned methods was carried out by heating the substrate to elevated temperatures of over 200 °C [10].
However, the use of polymeric materials as substrates to produce ITO films is critical for many applications such as plastic LCD devices, electromagnetic interference shielding materials and flexible electronics [11–14]. Since the development of low temperature deposition methods [3,10,15–19], preparation of ITO films by using appropriate polymer substrates has been a popular topic. Polycarbonate (PC) [3,14,20,21], polyethyleneterephtalate (PET) [17,20,22–26] and poly(methyl methacrylate) (PMMA) [5,26] are the most commonly used polymer substrates for this purpose. Although there are various advantages of using polymers as substrate, insufficient adhesion of ITO to the polymeric substrates is still a big problem. In order to overcome this, many modification methods have been applied to the polymer substrate or ITO. Air plasma, argon plasma and O2 plasma are some of the plasma treatment methods used for this purpose. During these processes, surface free energy (SFE) properties of ITO and substrate have been put forward as an important parameter many times [4,21], because a detailed SFE knowledge for solid surfaces is critical in evaluating of many interface phenomena such as adhesion, adsorption, wettability, and lubrication behaviour. For example, Vunnam et al. improved the ITO surface by changing the wettability properties of ITO for direct writing of silver nanoparticulate ink micropatterns by using air plasma treatment [27]. Lee et al. increased the SFE of the ITO by using argon atmospheric pressure plasma, and reported that the optoelectronic properties of the ITO can be optimized by this way [28]. You and Dong treated the surface of ITO by O2 plasma to improve the ITO/polymer interface for use in organic light emitting diodes [29]. Recently, we adjusted SFE properties of the PC substrate by O2 plasma treatment to prepare ITO-based transparent and conducting multilayer thin films that could be potentially used in optoelectronic industry [21]. In addition to plasma treatment methods, chemical treatments were also applied in order to modify SFE properties of TCOs. For example, Arazna et al. reported that the treatment of ITO surface in ultrasonic bath using organic solvents such as acetone, ethyl alcohol and isopropyl alcohol is effective for increasing the SFE of the ITO [30]. Davenas et al. immersed the ITO substrate in a solution of 2-chloroethylphosphonic acid in order to functionalize it with a molecular layer, and increased the SFE value of the ITO for improvement of charge injection in organic light emitting diodes [31]. Similarly, Besbes et al. increased the SFE value of ITO with 2-chloroethanephosphonic acid and developed an ITO/polymer interface that is more suitable for use in organic light emitting devices by this way [32]. Apart from these studies, silane based chemicals are effective for improving the adhesion ability of ITO’s contact surface due to the Si-O bonds that can be react with the hydroxyl groups of ITO [2,33–35]. In this context, Chiang and Hsieh used five different types of organo-functional silanes containing vinyl, epoxy, amino, methacrylic and acrylic groups in the cationic polymerization of epoxide resin to enhance adhesion between epoxide resin and ITO [36]. Maksimenko et al. used 3-methacryloxypropyltrimethoxysilane to improve the attachment between ITO and polyvinylpyrrolidone for the synthesis of ITO/polymer nanocomposites [37]. Similarly, Ginzburg-Turgeman et al. reported that the use of 3-(trimethoxysilyl)propyl methacrylate as a silane-based molecular adhesive was very effective in establishing covalent attachment between PMMA and ITO, and thus improved the adhesion [38]. All of these studies clearly indicate that the interfacial tension forces, and hence work of adhesion (Wa), between ITO and organic substrates have an important role in order to design a desired structure.
Besides, there are many unknown information in this field about the adhesion relationships between ITO and polymer-based substrates. As known, there are numerous methods in the literature to calculate SFE from contact angle results. Which of these might be better associated with adhesion of ITO to polymer substrates? On the other hand, although PC, PMMA and PET are most commonly used substrates for ITO-based coatings, what other industrial polymers would be a good candidate as substrates for such coatings? In order to answer these questions, the adhesion between ITO and various polymer substrates must be known. In this context, calculation of thermodynamic work of adhesion between interlayers by using SFE values stands out as a good alternative [4].
The main aim of this study is to estimate the thermodynamic Wa values between ITO and various common polymeric substrates as accurately as possible by using previously reported contact angle and SFE values of ITO and polymeric substrates. To do this, SFE components of ITO and polymeric substrates have been listed by using acid-base [39], geometric mean [40] and harmonic mean [41] approaches. Common and industrial polymers such as polystyrene (PS), poly(methyl methacrylate) (PMMA), polycarbonate (PC), polyethyleneterephtalate (PET), Nylon 66, polypropylene (PP), polyvinylchloride (PVC), styrene-butadiene rubber (SBR), polyphenylene sulfide (PPS), high density polyethylene (HDPE), low density polyethylene (LDPE), polyvinyl acetate (PVAc), polyvinyl fluoride (PVF), polyvinylidene fluoride (PVDF), polytetrafluoroethylene (PTFE), polytrifluoroethylene (PTrFE) and polyperfluoroalkylethyl acrylate (PPFA) were selected as substrate material. Following this, the interaction parameter of interphase (ϕ) and Wa values between ITO and selected polymeric substrates were estimated using the SFE values based on the Dupré [42], Fowkes [43–45] and Girifalco-Good equations [46]. Finally, alternative polymers to be used as substrates for ITO coatings have been proposed considering calculated Wa values.
2. Theoretical background and estimation of the work of adhesion between ITO and polymer substrates
Thermodynamic work of adhesion ( ) between two material surfaces can be defined as the reversible work required to separate two phases at the interface to infinite distance [47,48]. According to Dupré equation, work of adhesion between two phases depending on the interfacial tensions can be written as [42]
| (1) |
Wa can also be described by the sum of intermolecular interactions as proposed by Fowkes [43–45],
| (2) |
where superscript d denotes London-dispersion forces, h denotes hydrogen-bonding, p denotes dipole-dipole (polar) interactions, i denotes dipole-induced dipole interactions, π denotes π-bonds, da denotes donor-acceptor bonds, and e denotes electrostatic interactions. Fowkes suggests that London-dispersion forces are always present, and component is usually dominant when compared to the other components [44].
According to Berthelot’s approach, attractive constants between like (Aaa and Abb) and unlike (Aab) molecules can be expressed as [46,49]
| (3) |
Girifalco and Good modified Equation (3) by using the free energies of adhesion and cohesion for two phases. Their basic principle is to equate ratio to the interphase interaction parameter (ϕ) [46]. According to Girifalco and Good, the interphase interaction parameter can be defined as,
| (4) |
where denotes the free energy of adhesion for the interface between a and b phases, and denote free energy of cohesion for a and b phases, respectively. This equation can also be defined as [50],
| (5) |
where Wc denotes work of cohesion, and it can be expressed using surface tension terms as.
| (6) |
By combining Equations (1), (5), and (6), interfacial tension between two material surfaces can be written as.
| (7) |
By combining Equations (1) and (7), the main equation of Girifalco-Good for Wa between two material surfaces can be expressed as.
| (8) |
Equation (8) can be written for polymer/ITO systems as
| (9) |
where γP is SFE of the polymer substrate, and γITO is SFE of the ITO. The ϕ value of the interphase between polymer and ITO can be written by geometric mean approach as [4,40,50],
| (10) |
where denotes dispersive component fraction of the SFE, denotes polar component fraction of the SFE, subscript j represents the polymer or ITO, and .
The ϕ value of the interphase between polymer and ITO can be written by harmonic mean approach as [4,41,50].
| (11) |
The parameters in Equations (10) and (11) are calculated as
| (12) |
| (13) |
| (14) |
Apart from ϕ values, the SFE values of the polymer and ITO must also be known in order to calculate Wa by using Equation (9). There are three main approaches that can be used to calculate the SFE. One of them is the van Oss-Chaudhury-Good method, which is based on the acid-base (AB) approach [39]. The main equation of the van Oss-Chaudhury-Good method can be written as,
| (15) |
where subscript S denotes solid; L liquid; V vapor, γLW is the Lifshitz-van der Waals SFE term, and θ denotes contact angle. Other equations used in this method are
| (16) |
| (17) |
where subscript i denotes liquid or solid, denotes Lewis acid parameter, denotes Lewis base parameter and comprises all the electron acceptor-donor interactions. The Owens and Wendt’s method based on the geometric mean (GM) approach [40], and Wu’s method based on the harmonic mean (HM) approach [41] are the other commonly used methods for calculation of SFE and its parameters. The equations used for the determination of SFE based on the geometric mean approach and the harmonic mean approach can be written as follows, respectively:
| (18) |
| (19) |
where γd denotes dispersive component of the surface tension, and γp denotes polar component of the surface tension. The surface tension components of the test liquids used in the equations can be easily retrieved from the literature [4,21,47,51]. In brief, after determination of the ϕ values, SFE values calculated with AB, GM and HM approaches are used in equation (9) to determine Wa values of polymer/ITO interfaces.
3. Results and discussion
The SFE components of ITO coated on different substrates taken from the literature and are presented in Table 1. Although used substrates have quite different physical and chemical properties, the SFE values of ITO were distributed in a narrow range. For example, the values of ITO coated on PET and glass substrates are reported as 29.09 and 29.30 mJ/m2, respectively, by using acid-base approach [23,31]. Similarly, the value of ITO coated on O2 plasma treated PC is reported as 32.08 mJ/m2 by using acid-base approach, and this value is close to value (33.31 mJ/m2) of ITO deposited on a gold interlayer [21]. While the parameters were reported as zero or very close to zero under all deposition conditions, the mean value for was calculated as 4.38 mJ/m2, signifying that all of the ITO surfaces have a monopolar basic character (Table 1). As known, acid-base approach sometimes gives negative values in the square roots of and , causing to be calculated as zero. The values close to zero originating from the negative values of the square roots of caused values to be calculated as zero for ITO surfaces. For this reason, determination of the polar interactions for ITO surfaces by acid-base approach is very difficult [4,21,23].
Table 1.
Surface free energy components (mJ/m2) of ITO calculated by acid-base (AB), geometric mean (GM) and harmonic mean (HM) approaches.
| Method | Substrate | Reference | |||||
|---|---|---|---|---|---|---|---|
| AB | PC | 31.95 | 0.00 | 5.90 | 0.13 | 32.08 | [21] |
| PET | 30.07 | 0.00 | 5.45 | 0.00 | 30.07 | [23] | |
| PET | 29.09 | 0.00 | 5.15 | 0.00 | 29.09 | [23] | |
| Glass | 26.60 | 0.30 | 7.30 | 2.80 | 29.30 | [31] | |
| Glass | 26.88 | 0.00 | 1.69 | 0.02 | 26.90 | [4] | |
| ITO | 27.66 | 0.00 | 2.11 | 0.01 | 27.68 | [4] | |
| Gold | 33.26 | 0.00 | 6.41 | 0.05 | 33.31 | [21] | |
| Silver | 25.84 | 0.00 | 1.05 | 0.00 | 25.84 | [4] | |
| Mean | 28.92 | 0.04 | 4.38 | 0.38 | 29.28 | ||
| Deviation | ±2.17 | NA | ±2.07 | NA | ±1.91 | ||
| GM | PC | 31.95 | - | - | 0.64 | 32.59 | [21] |
| PET | 27.82 | - | - | 2.41 | 30.23 | [23] | |
| PET | 27.08 | - | - | 2.12 | 29.20 | [23] | |
| Glass | 26.88 | - | - | 0.42 | 27.30 | [4] | |
| ITO | 27.66 | - | - | 0.36 | 28.03 | [4] | |
| Gold | 33.26 | - | - | 0.85 | 34.11 | [21] | |
| Silver | 25.84 | - | - | 0.21 | 26.05 | [4] | |
| Mean | 28.64 | - | - | 1.00 | 29.64 | ||
| Deviation | ±2.26 | - | - | ±0.72 | ±2.28 | ||
| HM | PC | 33.04 | - | - | 1.82 | 34.86 | [21] |
| Glass | 28.66 | - | - | 2.12 | 30.78 | [4] | |
| ITO | 29.33 | - | - | 1.98 | 31.32 | [4] | |
| Gold | 34.19 | - | - | 2.36 | 36.56 | [21] | |
| Silver | 27.77 | - | - | 1.31 | 29.08 | [4] | |
| Mean | 30.60 | - | - | 1.92 | 32.52 | ||
| Deviation | ±2.41 | - | - | ±0.28 | ±2.55 |
NA: not applicable due to very close zero values.
The dispersive ( ) and polar ( ) components of the SFE for ITO calculated with geometric and harmonic mean approaches are listed in Table 1. The mean values for , and were calculated as 28.64, 1.00, and 29.64 mJ/m2, respectively, by using geometric mean approach. These values are close to those obtained from acid-base approach. However, when using harmonic mean approach, the mean values for , and were calculated as 30.60, 1.92, and 32.52 mJ/m2. These results show that SFE components calculated by harmonic mean approach are higher than the geometric mean and acid-base approaches as seen in Wu’s previous determinations [41,50]. In summary, while SFE values of ITO are distributed in a narrow range, polymers show a wide range of SFE distribution (around 7–45 mJ/m2) depending on their molecular structure [51]. Accordingly, the adhesion strength of ITO to polymer base substrates varies predominantly according to the SFE properties of used polymer.
SFE components of polymer to be used for substrate material must also be known in order to determine Wa between ITO and the polymer. In this context, contact angle measurements, which quantifies liquid/solid interactions, are one of the most popular techniques used to determine the SFE of a surface. However, SFE components of a surface can vary depending on the calculation methods and type of liquid used. For this reason, the SFE values obtained by using the same liquids in the same calculation method can only be compared with each other. In this work, we have retrieved contact angle results of water (W), formamide (FA) and diiodomethane (DM) liquids on common and industrial polymers from previous literature reports [21,51–60], and presented in Table 2. We then listed the SFE components calculated for polymer surfaces with acid-base, geometric mean and harmonic mean approaches using W, FA and DM contact angle results (Table 3). The results show that surface wettability properties of the polymers are very different from each other. For instance, W contact angle results of the listed polymers ranged between 56° and 125°. Similar wide range distributions are also seen for FA and DM contact angle results. The wide distributions observed in contact angle results of the polymer surfaces naturally resulted in wide distributions of the SFE results. As can be seen from Table 3, values of the listed polymers change between 7.45 mJ/m2 and 64.73 mJ/m2 depending on the chemical structure of the polymer and calculation method. In means of and values, most of the polymers (except PPS and PPFA) evaluated in this work have larger values compared with the components, indicating that most of the common polymers are on the monopolar basic character. For example, while the values for PMMA, PET and PC are 15.58, 6.42 and 5.70, respectively, it is observed that values for these polymers are 0 mJ/m2. Also, SFE values calculated by acid-base approach are close to those obtained from geometric mean approach. However, when using harmonic mean approach, SFE parameters of the polymers especially for and are found to be higher than that of acid-base and geometric mean approaches. Although all of these results help to understand the adhesion behaviour of polymers, thermodynamic Wa values of ITO/polymer interlayers should be determined in order to comment on how these changes of SFE components affect the adhesion strength between ITO and polymers properly.
Table 2.
Water (W), formamide (FA) and diiodomethane (DM) contact angle results of various polymer surfaces.
| Polymer | θW (°) | θFA (°) | θDM (°) | Reference |
|---|---|---|---|---|
| PCa | 56 | 32 | 25 | [21] |
| PVAc | 60 | 43 | 41 | [52] |
| PC | 82 | 61 | 32 | [21] |
| Nylon 66 | 70 | 50 | 41 | [53] |
| PPS | 96 | 54 | 34 | [51] |
| PS | 91 | 74 | 35 | [53] |
| PVC | 87 | 66 | 36 | [54] |
| PET | 81 | 61 | 38 | [53] |
| PMMA | 71 | 59 | 40 | [55] |
| PVF | 80 | 54 | 49 | [54] |
| HDPE | 102 | 85 | 53 | [52] |
| PVDF | 82 | 59 | 63 | [54] |
| LDPE | 102 | 82 | 55 | [56] |
| PP | 95 | 77 | 56 | [57] |
| SBR | 98 | 78 | 63 | [58] |
| PTrFE | 92 | 76 | 71 | [54] |
| PTFE | 108 | 92 | 88 | [59] |
| PPFA | 125 | 109 | 104 | [60] |
shows the contact angle results of O2 treated PC.
Table 3.
Comparison of surface free energy components of various polymer surfaces calculated by acid-base, geometric mean, and harmonic mean approaches.
| Polymer | Acid-base approacha | GM approachb | HM approachb | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PCc | 46.15d | 0.59d | 17.54d | 6.45d | 52.60d | 46.15d | 12.29d | 58.44d | 46.25d | 18.47d | 64.73d |
| PVAc | 39.10e | 0.55e | 18.37e | 6.38e | 45.49e | 39.10e | 12.65e | 51.76e | 39.70f | 17.86f | 57.56f |
| PC | 43.37d | 0.00d | 5.70d | 0.00d | 43.37d | 43.37d | 2.25d | 45.63d | 43.63d | 7.05d | 50.68d |
| Nylon 66 | 39.10e | 0.32e | 11.05e | 3.77e | 42.87e | 39.10e | 7.57e | 46.68e | 39.70f | 12.99f | 52.69f |
| PPS | 42.49e | 0.96e | 0.00e | 0.00e | 42.49e | 42.49e | 0.09e | 42.58e | 42.80e | 1.92e | 44.72e |
| PS | 42.03e | 0.00e | 4.67e | 0.00e | 42.03e | 42.03e | 0.59e | 42.62e | 42.37f | 3.74f | 46.12f |
| PVC | 41.56e | 0.00e | 4.10e | 0.00e | 41.56e | 41.56e | 1.32e | 42.88e | 41.94f | 5.31f | 47.25f |
| PET | 40.60e | 0.00e | 6.42e | 0.00e | 40.60e | 40.60e | 2.99e | 43.59e | 41.06f | 7.86f | 48.92f |
| PMMA | 39.61e | 0.00e | 15.58e | 0.00e | 39.61e | 39.61e | 6.98e | 46.59e | 40.16f | 12.43f | 52.59f |
| PVF | 34.83e | 0.95e | 4.12e | 3.95e | 38.78e | 34.83e | 4.51e | 39.34e | 35.89f | 9.21f | 45.10f |
| HDPE | 32.59e | 0.00e | 2.04e | 0.00e | 32.59e | 32.59e | 0.09e | 32.68e | 33.93f | 1.17f | 35.10f |
| PVDF | 26.85e | 1.80e | 4.33e | 5.58e | 32.43e | 26.85e | 5.85e | 32.70e | 29.01f | 9.89f | 38.90f |
| LDPE | 31.45e | 0.00e | 1.22e | 0.00e | 31.45e | 31.45e | 0.14e | 31.58e | 32.94f | 1.33f | 34.28f |
| PP | 30.87e | 0.00e | 2.98e | 0.00e | 30.87e | 30.87e | 1.04e | 31.92e | 32.45f | 3.84f | 36.29f |
| SBR | 26.85e | 0.01e | 1.70e | 0.29e | 27.14e | 26.85e | 1.00e | 27.85e | 29.01f | 3.44f | 32.45f |
| PTrFE | 22.32e | 0.30e | 4.18e | 2.23e | 24.54e | 22.32e | 3.35e | 25.67e | 25.18f | 6.65f | 31.83f |
| PTFE | 13.60e | 0.29e | 1.06e | 1.11e | 14.72e | 13.60e | 1.23e | 14.84e | 17.73f | 2.96f | 20.69f |
| PPFA | 7.30e | 0.14e | 0.04e | 0.15e | 7.45e | 7.30e | 0.17e | 7.46e | 11.88f | 0.07f | 11.95f |
The interphase interaction parameter (ϕ), also known as Girifalco-Good interaction parameter, is a good indicator to determine the degree of interaction between two phases, and thus work of adhesion [46,61]. The ϕ values of ITO/polymer systems were calculated from SFE components of the polymeric substrates and ITO by applying geometric and harmonic mean approaches as explained in the theoretical background section. As seen in Table 4, all of the ϕ values between polymeric surfaces and ITO were close to 1 in most cases, although they varied somewhat depending on the calculation methods. This indicates that Wa values are directly related to the total SFE value of the polymeric surfaces due to the nature of the Girifalco-Good calculation approach. These results also indicate that the polymers considered in this study may be good substrate candidates for ITO coatings, given that the ϕ values can range between 0.5 and 1.15 [46,61]. However, Wa knowledge between ITO and polymers is also necessary in order to predict the extent of adhesion of ITO/polymer interfaces.
Table 4.
Interphase interaction parameters (ϕ) and work of adhesion (Wa) values (mJ/m2) between various polymers and ITO.
| Substrate | AB methoda | GM methodb | HM methodc | |||
|---|---|---|---|---|---|---|
| ϕ | W a | ϕ | W a | ϕ | W a | |
| PCd | 0.971 | 76.20 | 0.958 | 79.72 | 0.879 | 80.62 |
| PVAc | 0.964 | 70.37 | 0.945 | 74.04 | 0.879 | 76.06 |
| PC | 0.994 | 70.83 | 0.999 | 73.49 | 0.960 | 77.98 |
| Nylon 66 | 0.983 | 69.65 | 0.974 | 72.43 | 0.916 | 75.81 |
| PPS | 0.994 | 70.11 | 0.990 | 70.37 | 0.986 | 75.21 |
| PS | 0.994 | 69.73 | 0.998 | 70.93 | 0.983 | 76.15 |
| PVC | 0.994 | 69.34 | 1.000 | 71.30 | 0.975 | 76.41 |
| PET | 0.994 | 68.53 | 0.997 | 71.66 | 0.957 | 76.31 |
| PMMA | 0.994 | 67.69 | 0.977 | 72.65 | 0.920 | 76.12 |
| PVF | 0.978 | 65.93 | 0.987 | 67.41 | 0.946 | 72.43 |
| HDPE | 0.994 | 61.40 | 0.991 | 61.70 | 0.995 | 67.27 |
| PVDF | 0.952 | 58.64 | 0.968 | 60.30 | 0.928 | 66.00 |
| LDPE | 0.994 | 60.32 | 0.993 | 60.77 | 0.997 | 66.60 |
| PP | 0.994 | 59.76 | 1.000 | 61.51 | 0.991 | 68.12 |
| SBR | 1.000 | 56.40 | 1.000 | 57.46 | 0.993 | 64.50 |
| PTrFE | 0.982 | 52.65 | 0.983 | 54.23 | 0.951 | 61.21 |
| PTFE | 0.987 | 40.96 | 0.994 | 41.69 | 0.955 | 49.56 |
| PPFA | 1.000 | 29.54 | 1.000 | 29.74 | 0.875 | 34.51 |
SFE components calculated by acid-base approach were used to determine ϕ and Wa values.
SFE components calculated by geometric mean approach were used to determine ϕ and Wa values.
SFE components calculated by harmonic mean approach were used to determine ϕ and Wa values.
shows the ϕ and Wa values between O2 treated PC and ITO.
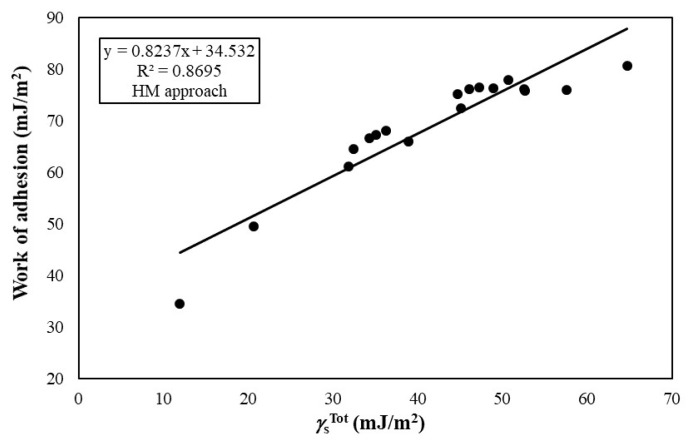
The Wa values between ITO and polymeric substrates are given in Table 4. The Wa values of the ITO films to the polymeric substrates varied from 29.54 to 80.62 mJ/m2 depending on the type of polymer and calculation model. The Wa values increased from 29.54 to 76.20 mJ/m2 with the increase of the of polymeric substrates from 7.45 to 52.60 mJ/m2, which calculated by acid-base approach as seen in Figure 1. The regression coefficient value of the line of this graph was found to be 0.97, and at first glance, it can be thought that the use of values determined by the acid-base approach is directly related to the calculated Wa values. However, the and values of many surfaces reported in this work had to be considered zero due to the negative values of and [4,21,51]. This assumption caused to be calculated as zero, and contribution to the was mainly originated from the . Thus, the relationship between and Wa for ITO/polymer systems should also be evaluated by other SFE calculation approaches to verify mentioned strong correlation. According to the values calculated from geometric mean approach, the Wa values increased from 29.74 to 79.72 mJ/m2 with the increase of the of polymeric substrates from 7.46 to 58.44 mJ/m2 as seen in Figure 2. Similar to acid-base and geometric mean approaches, the Wa values calculated by harmonic mean approach were also increased sharply from 34.51 to 80.62 mJ/m2 with the increase of the of polymeric substrates as seen in Figure 3. All of these results clearly show that the Wa between ITO and polymeric substrates is highly correlated with the value of the used polymeric material.
Figure 1.
Change of work of adhesion between ITO and polymer substrates with the change of SFE values of polymer substrates calculated by acid-base approach.
Figure 2.
Change of work of adhesion between ITO and polymer substrates with the change of SFE values of polymer substrates calculated by geometric mean approach.
Figure 3.
Change of work of adhesion between ITO and polymer substrates with the change of SFE values of polymer substrates calculated by harmonic mean approach.
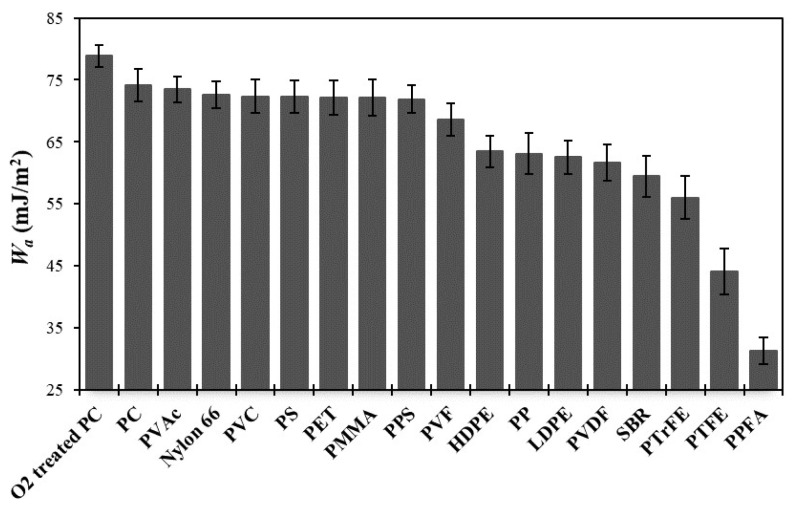
In order to make a more precise comparison, the Wa values between ITO and polymeric substrates were interpreted by averaging the results obtained from acid-base, geometric mean and harmonic mean approaches (Figure 4). According to the results obtained, the Wa value of ITO film to O2 treated PC was 78.85 ± 1.8 mJ/m2, whereas the Wa for untreated PC was calculated as 74.10 ± 2.6 mJ/m2. This is due to the differences in the SFE components of both surfaces. For example, the values of O2 treated PC surface were 52.60, 58.44, and 64.73 mJ/m2 by acid-base, geometric mean and harmonic mean methods, respectively. Where as, the values of untreated PC surface were 43.37, 45.63, and 50.68 mJ/m2 by acid-base, geometric mean and harmonic mean methods, respectively [21]. On the other hand, electron donor functional component ( ) of the SFE shows the hydrogen bonding ability of the carbonyl groups present on the surface [55]. The value of the PC surface increased from 5.70 to 17.54 mJ/m2 with O2 plasma treatment, and it shows that the intensity of polar oxygenated groups on the surface increases and carbonyl groups ready to form hydrogen bonds cover most of the PC surface [21]. This change may have caused higher Wa at the O2 treated PC/ITO interface comparing to the untreated PC/ITO.
Figure 4.
Comparison of work of adhesion between ITO and various polymer substrates by averaging the results obtained from acid-base, geometric mean and harmonic mean approaches.
PET is another important substrate that often used for ITO coatings, and the Wa value of PET/ITO interface was calculated as 72.17 ± 2.8 mJ/m2. This value is very close to those obtained from PS (72.27 ± 2.6 mJ/m2), PMMA (72.15 ± 3.0 mJ/m2), and PPS (71.90 ± 2.2 mJ/m2). Considering the very close Wa values of PET and PMMA, it can be better understood why these two polymers seem to be good alternatives to each other. On the other hand, close Wa values of PC, PS, PET and PPS are due to the close values of resulting from the presence of benzene rings in their structures [21,51,53]. Comparing PC, PMMA and PET, the Wa values between polymer and ITO decreased in the following order: PC (74.10 ± 2.6 mJ/m2) > PET (72.17 ± 2.8 mJ/m2) > PMMA (72.15 ± 3.0 mJ/m2). Apart from these, PVAc (73.49 ± 2.1 mJ/m2), Nylon 66 (72.63 ± 2.1 mJ/m2), and PVC (72.35 ± 2.7 mJ/m2) have also high Wa values at the polymer/ITO interface. This is an expected situation, because it is a known fact that PVAc is used in the adhesive industry due to its high surface free energy [52,56], and this feature naturally caused the Wa value of the PVAc/ITO interface to be high. As for Nylon 66, the Wa value of this polymer seems to be higher than that of the many other polymers that have listed in this work. The reason for this is the high surface polarity (χs = 16.22%) due to the presence of amide groups instead of aliphatic hydrocarbons in the structure [51,53]. The Wa between PVC and ITO (72.35 ± 2.7 mJ/m2) was also found to be higher than that of the many other polymers such as HDPE (63.46 ± 2.5 mJ/m2), LDPE (62.56 ± 2.7 mJ/m2), PP (63.13 ± 3.3 mJ/m2) and SBR (59.45 ± 3.4 mJ/m2). Because, replacement of covalent hydrogen atoms by chlorine atoms causing higher adhesional energies and thus higher Wa values, as in the previous determinations of Zisman and coworkers [54,62].
Fluoropolymers are generally known for their nonadhesive behaviours, and they are widely used in many sectors such as aerospace, automotive, electronic, chemical processing, medical devices, and pharmaceutical because of their favourable physical and chemical properties [63]. For this reason, the Wa values between ITO and most common fluoropolymers were also determined in this study. It was observed that significant portion of the fluorinated polymers has lower Wa than that of nonfluorinated polymers due to their low SFE values originating from high electronegativity of fluorine atoms and low polarizability of C-F bonds [55,58,64]. Thus, the Wa values of fluorine containing polymers decreased with the increased of the number of fluorine atoms in the macromolecular structure in the following order: PVF (68.59 ± 2.6 mJ/m2) > PVDF (61.65 ± 2.9 mJ/m2) > PTrFE (56.03 ± 3.5 mJ/m2) > PTFE (44.07 ± 3.7 mJ/m2). Also, the Wa value of PPFA (31.26 ± 2.2 mJ/m2) was very low when compared to both fluorinated and nonfluorinated polymers. This is because long side chains having CF2 units and CF3 end groups in perfluorinated acrylate polymers tend to migrate to the outermost part of the polymer surface than being in the bulk [55,58,60,64,65]. Among the fluorinated polymers only PVF shows Wa value that close to commonly used polymers in ITO coatings such as PC, PET, PMMA, while the others are quite far. These results indicate that fluorinated polymers (except PVF) are not suitable for use as substrates in ITO coatings without improving their adhesion properties with appropriate treatments. To sum up, in addition to widely used usual polymers (PC, PET, PMMA), in terms of adhesion strength values PVAc, Nylon 66, PVC, PS, PPS and PVF polymers are promising alternatives that can be used as substrate for ITO coating processes in the future. However, apart from adhesion values, transparency of the aforementioned polymers should also be considered for a successful application.
4. Conclusion
The thermodynamic Wa knowledge between coating and substrate is a beneficial parameter that can be used to predict the compatibility of the interlayers in the product to be obtained. In the present study, the compatibility of ITO, which is widely used in optoelectronic industry, with different kind of polymeric substrates was evaluated by utilizing the Wa values of ITO/polymer interfaces. For this purpose, the Wa values between ITO and various polymers were determined by applying the proposals of Dupré, Fowkes and Girifalco-Good to the SFE components obtained by acid-base, geometric mean and harmonic mean approaches. As well as the Wa, the interphase interaction parameters were also determined to comment on the degree of interaction between ITO and polymers. It was found that the ϕ values between polymeric surfaces and ITO were close to 1, indicating that all polymers considered in this study have adequate interaction with ITO. According to the calculations made for the determination of Wa values, it has been observed that the magnitude of Wa was related with the total SFE value of the polymeric substrates, and Wa values gradually increased with the increase of the total SFE of the polymer. Calculations performed with acid-base, geometric mean and harmonic mean methods showed similar tendencies in terms of SFE/Wa relationships. Among the considered polymers, PVAC, Nylon 66, PVC, PS, and PPS seems to be a good alternative to PC, PET, and PMMA in terms of Wa values. Among the fluorinated polymers, only PVF has been showed a partially high Wa value with ITO. Overall, there are various alternative polymers that can be considered for use as substrate in ITO coating processes, as well as PC, PET and PMMA, and the interactions of these alternatives with ITO could be controlled by knowing the SFE properties of the polymeric substrate.
References
- 1. Stadler A. Transparent Conducting Oxides—An Up-To-Date Overview. Materials. 2012;5(4):661–683. doi: 10.3390/ma5040661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aydın EB, Sezgintürk MK. Indium tin oxide (ITO): A promising material in biosensing technology. TrAC Trends in Analytical Chemistry. 2017;97:309–315. doi: 10.1016/j.trac.2017.09.021. [DOI] [Google Scholar]
- 3. Wu WF, Chiou BS. Deposition of indium tin oxide films on polycarbonate substrates by radio-frequency magnetron sputtering. Thin Solid Films. 1997;298(1–2):221–227. doi: 10.1016/S0040-6090(96)09311-X. [DOI] [Google Scholar]
- 4. Ozbay S, Erdogan N, Erden F, Ekmekcioglu M, Rakop B, et al. Surface free energy and wettability properties of transparent conducting oxide-based films with Ag interlayer. Applied Surface Science. 2021;567:150901. doi: 10.1016/j.apsusc.2021.150901. [DOI] [Google Scholar]
- 5. Kim DH, Park MR, Lee GH. Preparation of high quality ITO films on a plastic substrate using RF magnetron sputtering. Surface and Coatings Technology. 2006;201(3–4):927–931. doi: 10.1016/j.surfcoat.2006.01.004. [DOI] [Google Scholar]
- 6. Kim D, Han Y, Cho JS, Koh SK. Low temperature deposition of ITO thin films by ion beam sputtering. Thin Solid Films. 2000;377–378:81–86. doi: 10.1016/S0040-6090(00)01388-2. [DOI] [Google Scholar]
- 7. Liu C, Mihara T, Matsutani T, Asanuma T, Kiuchi M. Preparation and characterization of indium tin oxide films formed by oxygen ion beam assisted deposition. Solid State Communications. 2003;126(9):509–513. doi: 10.1016/S0038-1098(03)00237-0. [DOI] [Google Scholar]
- 8. Maruyama T, Tabata K. Indium-tin oxide thin films prepared by chemical vapor deposition from metal acetates. Japanase Journal of Applied Physics. 1990;29(2A):L355–L357. doi: 10.1143/JJAP.29.L355. [DOI] [Google Scholar]
- 9. Khondoker MAH, Yang SY, Mun SC, Kim J. Flexible and conductive ITO electrode made on cellulose film by spin-coating. Synthetic Metals. 2012;162(21–22):1972–1976. doi: 10.1016/j.synthmet.2012.09.005. [DOI] [Google Scholar]
- 10. Zhang K, Zhu F, Huan CHA, Wee ATS. Indium tin oxide films prepared by radio frequency magnetron sputtering method at a low processing temperature. Thin Solid Films. 2000;376(1–2):255–263. doi: 10.1016/S0040-6090(00)01418-8. [DOI] [Google Scholar]
- 11. Chiou B-S, Hsieh S-T. R.f. magnetron-sputtered indium tin oxide film on a reactively ion-etched acrylic substrate. Thin Solid Films. 1993;229(2):146–155. doi: 10.1016/0040-6090(93)90357-U. [DOI] [Google Scholar]
- 12. Park SK, Han JI, Kim WK, Kwak MG. Deposition of indium-tin-oxide films on polymer substrates for application in plastic-based flat panel displays. Thin Solid Films. 2001;397(1–2):49–55. doi: 10.1016/S0040-6090(01)01489-4. [DOI] [Google Scholar]
- 13. Königer T, Münsted H. Coatings of indium tin oxide nanoparticles on various flexible polymer substrates: Influence of surface topography and oscillatory bending on electrical properties. Journal of the Society for Information Display. 2008;16(4):559–568. doi: 10.1889/1.2905043. [DOI] [Google Scholar]
- 14. Erdogan N, Erden F, Astarlioglu AT, Ozdemir M, Ozbay S, et al. ITO/Au/ITO multilayer thin films on transparent polycarbonate with enhanced EMI shielding properties. Current Applied Physics. 2020;20(4):489–497. doi: 10.1016/j.cap.2020.01.012. [DOI] [Google Scholar]
- 15. Davis L. Properties of transparent conducting oxides deposited at room temperature. Thin Solid Films. 1993;236(1–2):1–5. doi: 10.1016/0040-6090(93)90632-Y. [DOI] [Google Scholar]
- 16. Karasawa T, Miyata Y. Electrical and optical properties of indium tin oxide thin films deposited on unheated substrates by d.c. reactive sputtering. Thin Solid Films. 1993;223(1):135–139. doi: 10.1016/0040-6090(93)90737-A. [DOI] [Google Scholar]
- 17. Ma J, Li S-Y, Zhao J, Ma H-L. Preparation and properties of indium tin oxide films deposited on polyester substrates by reactive evaporation. Thin Solid Films. 1997;307(1–2):200–202. doi: 10.1016/S0040-6090(97)00203-4. [DOI] [Google Scholar]
- 18. Laux S, Kaiser N, Zöller A, Götzelmann R, Lauth H, et al. Room-temperature deposition of indium tin oxide thin films with plasma ion-assisted evaporation. Thin Solid Films. 1998;335(1–2):1–5. doi: 10.1016/S0040-6090(98)00861-X. [DOI] [Google Scholar]
- 19. Wu Y, Marée CHM, Haglund RF, Hamilton JD, Morales Paliza MA, et al. Resistivity and oxygen content of indium tin oxide films deposited at room temperature by pulsed-laser ablation. Journal of Applied Physics. 1999;86(2):991–994. doi: 10.1063/1.370864. [DOI] [Google Scholar]
- 20. Kulkarni AK, Schulz KH, Lim T-S, Khan M. Electrical, optical and structural characteristics of indium-tin-oxide thin films deposited on glass and polymer substrates. Thin Solid Films. 1997;308–309:1–7. doi: 10.1016/S0040-6090(97)00526-9. [DOI] [Google Scholar]
- 21. Ozbay S, Erdogan N, Erden F, Ekmekcioglu M, Ozdemir M, et al. Surface free energy analysis of ITO/Au/ITO multilayer thin films on polycarbonate substrate by apparent contact angle measurements. Applied Surface Science. 2020;529:147111. doi: 10.1016/j.apsusc.2020.147111. [DOI] [Google Scholar]
- 22. Henry BM, Erlat AG, McGuigan A, Grovenor CRM, Briggs GAD, et al. Characterization of transparent aluminium oxide and indium tin oxide layers on polymer substrates. Thin Solid Films. 2001;382(1–2):194–201. doi: 10.1016/S0040-6090(00)01769-7. [DOI] [Google Scholar]
- 23. Zhong Z, Yin S, Liu C, Zhong Y, Zhang W, et al. Surface energy for electroluminescent polymers and indium-tin-oxide. Applied Surface Science. 2003;207(1–4):183–189. doi: 10.1016/S0169-4332(02)01328-4. [DOI] [Google Scholar]
- 24. Wuu D-S, Lien S-Y, Mao H-Y, Wang J-H, Wu B-R, et al. Improvement of indium-tin oxide films on polyethylene terephthalate substrates using hot-wire surface treatment. Thin Solid Films. 2006;501(1–2):346–349. doi: 10.1016/j.tsf.2005.07.147. [DOI] [Google Scholar]
- 25. Lee J, Jung H, Lee J, Lim D, Yang K, et al. Growth and characterization of indium tin oxide thin films deposited on PET substrates. Thin Solid Films. 2008;516(7):1634–1639. doi: 10.1016/j.tsf.2007.05.028. [DOI] [Google Scholar]
- 26. Yin X, Tang W, Weng X, Deng L. Surface morphology modelling for the resistivity analysis of low temperature sputtered indium tin oxide thin films on polymer substrates. Journal of Physics D: Applied Physics. 2009;42(22):225304. doi: 10.1088/0022-3727/42/22/225304. [DOI] [Google Scholar]
- 27. Vunnam S, Ankireddy K, Kellar J, Cross W. Surface modification of indium tin oxide for direct writing of silver nanoparticulate ink micropatterns. Thin Solid Films. 2013;531:294–301. doi: 10.1016/j.tsf.2013.01.047. [DOI] [Google Scholar]
- 28. Lee G, Park E, Nguyen V-T, Heo S, Nguyen N-A, et al. Plasma-assisted ITO sol coating for optimizing the optoelectronic properties of ITO glass. Applied Surface Science. 2021;551:149414. doi: 10.1016/j.apsusc.2021.149414. [DOI] [Google Scholar]
- 29. You ZZ, Dong JY. Surface modifications of ITO electrodes for polymer light-emitting devices. Applied Surface Science. 2006;253(4):2102–2107. doi: 10.1016/j.apsusc.2006.04.009. [DOI] [Google Scholar]
- 30. Arazna A, Koziol G, Janeczek K, Futera K, Stęplewski W. Investigation of surface properties of treated ITO substrates for organic light-emitting devices. Journal of Materials Science: Materials in Electronics. 2013;24:267–271. doi: 10.1007/s10854-012-0731-8. [DOI] [Google Scholar]
- 31. Davenas J, Besbes S, Abderrahmen A, Jaffrezic N, BenOuada H. Surface characterisation and functionalisation of indium tin oxide anodes for improvement of charge injection in organic light emitting diodes. Thin Solid Films. 2008;516(7):1341–1344. doi: 10.1016/j.tsf.2007.03.163. [DOI] [Google Scholar]
- 32. Besbes S, BenOuada H, Davenas J, Ponsonnet L, Jaffrezic N, et al. Effect of surface treatment and functionalization on the ITO properties for OLEDs. Materials Science and Engineering C. 2006;26(2–3):505–510. doi: 10.1016/j.msec.2005.10.078. [DOI] [Google Scholar]
- 33. Wu D, Liu J, Wang Y. Enhancing indium tin oxide (ITO) thin film adhesiveness using the coupling agent silane. Applied Surface Science. 2010;256(9):2934–2938. doi: 10.1016/j.apsusc.2009.11.053. [DOI] [Google Scholar]
- 34. Armstrong NR, Carter C, Donley C, Simmonds A, Lee P, et al. Interface modification of ITO thin films: organic photovoltaic cells. Thin Solid Films. 2003;445(2):342–352. doi: 10.1016/j.tsf.2003.08.067. [DOI] [Google Scholar]
- 35. Arya SK, Prusty AK, Singh SP, Solanki PR, Pandey MK, et al. Cholesterol biosensor based on N-(2-aminoethyl)-3-aminopropyl-trimethoxysilane self-assembled monolayer. Analytical Biochemistry. 2007;363(2):210–218. doi: 10.1016/j.ab.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 36. Chiang TH, Hsieh TE. The effect of organo-functional silanes on the adhesion of epoxide resins to ITO glass. Journal of Adhesion Science and Technology. 2005;19(1):1–18. doi: 10.1163/1568561053066927. [DOI] [Google Scholar]
- 37. Maksimenko I, Gross M, Königer T, Münstedt H, Wellmann PJ. Conductivity and adhesion enhancement in low-temperature processed indium tin oxide/polymer nanocomposites. Thin Solid Films. 2010;518(10):2910–2915. doi: 10.1016/j.tsf.2009.10.151. [DOI] [Google Scholar]
- 38. Ginzburg-Turgeman R, Guion JB, Mandler D. Improving the adhesion of polymethacrylate thin films onto indium tin oxide electrodes using a silane-based “Molecular Adhesive”. Journal of Solid State Electrochemistry. 2011;15:2401–2407. doi: 10.1007/s10008-011-1454-0. [DOI] [Google Scholar]
- 39. van Oss CJ, Chaudhury MK, Good RJ. Interfacial Lifshitz-van der Waals and polar interactions in macroscopic systems. Chemical Reviews. 1988;88(6):927–941. doi: 10.1021/cr00088a006. [DOI] [Google Scholar]
- 40. Owens DK, Wendt RC. Estimation of the surface free energy of polymers. Journal of Applied Polymer Science. 1969;13(8):1741–1747. doi: 10.1002/app.1969.070130815. [DOI] [Google Scholar]
- 41. Wu S. Calculation of interfacial tension in polymer systems. Journal of Polymer Science Part C: Polymer Symposia. 1971;34(1):19–30. doi: 10.1002/polc.5070340105. [DOI] [Google Scholar]
- 42.Dupré A, Dupré P. Théorie méchanique de la chaleur. Gauthier-Villars; Paris: 1869. [Google Scholar]
- 43. Fowkes FM. Attractive Forces at Interfaces. Industrial & Engineering Chemistry. 1964;56(12):40–52. doi: 10.1021/ie50660a008. [DOI] [Google Scholar]
- 44. Fowkes FM. Calculation of work of adhesion by pair potential suummation. Journal of Colloid and Interface Science. 1968;28(3–4):493–505. doi: 10.1016/0021-9797(68)90082-9. [DOI] [Google Scholar]
- 45. Fowkes FM. Determination of interfacial tensions, contact angles, and dispersion forces in surfaces by assuming additivity of intermolecular interactions in surfaces. The Journal of Physical Chemistry. 1962;66(2):382–382. doi: 10.1021/j100808a524. [DOI] [Google Scholar]
- 46. Girifalco LA, Good RJ. A theory for the estimation of surface and interfacial energies. I. Derivation and application to interfacial tension. The Journal of Physical Chemistry. 1957;61(7):904–909. doi: 10.1021/j150553a013. [DOI] [Google Scholar]
- 47.Erbil HY. Surface Chemistry of Solid and Liquid Interfaces. Blackwell Publishing; Oxford: 2006. [Google Scholar]
- 48. Erbil HY. The debate on the dependence of apparent contact angles on drop contact area or three-phase contact line: A review. Surface Science Reports. 2014;69(4):325–365. doi: 10.1016/j.surfrep.2014.09.001. [DOI] [Google Scholar]
- 49. Berthelot D. Sur le mélange des gaz. Comptes rendus hebdomadaires des séances de l’Académie des Sciences. 1898;126:1703–1706. [Google Scholar]
- 50.Wu S. Polymer Interface. Adhesion Marcel Dekker; New York: 1982. [Google Scholar]
- 51. Ozbay S. Evaluation of polyphenylene sulfide by surface thermodynamics approaches: Comparison with common polymers. Journal of Applied Polymer Science. 2022;139(18):e52082. doi: 10.1002/app.52082. [DOI] [Google Scholar]
- 52. Ucar IO, Doganci MD, Cansoy CE, Erbil HY, Avramova I, et al. Combined XPS and contact angle studies of ethylene vinyl acetate and polyvinyl acetate blends. Applied Surface Science. 2011;257(22):9587–9594. doi: 10.1016/j.apsusc.2011.06.070. [DOI] [Google Scholar]
- 53. Ellison AH, Zisman WA. Wettability studies of nylon, polyethylene terephthalate and polystyrene. The Journal of Physical Chemistry. 1954;58(6):503–506. doi: 10.1021/j150516a013. [DOI] [Google Scholar]
- 54. Ellison AH, Zisman WA. Wettability of halogenated organic solid surfaces. The Journal of Physical Chemistry. 1954;58(3):260–265. doi: 10.1021/j150513a020. [DOI] [Google Scholar]
- 55. Ozbay S, Erbil HY. Solution copolymerization of perfluoroalkyl ethyl methacrylate with methyl methacrylate and butyl acrylate: Synthesis and surface properties. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2014;452:9–17. doi: 10.1016/j.colsurfa.2014.03.054. [DOI] [Google Scholar]
- 56. Doganci MD, Cansoy CE, Ucar IO, Erbil HY, Mielczarski E, et al. Combined XPS and contact angle studies of flat and rough ethylene-vinyl acetate copolymer films. Journal of Applied Polymer Science. 2012;124(3):2100–2109. doi: 10.1002/app.35189. [DOI] [Google Scholar]
- 57. Ozbay S, Erbil HY. Ice accretion by spraying supercooled droplets is not dependent on wettability and surface free energy of substrates. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2016;504:210–218. doi: 10.1016/j.colsurfa.2016.05.065. [DOI] [Google Scholar]
- 58. Ozbay S, Erbil HY. Superhydrophobic and oleophobic surfaces obtained by graft copolymerization of perfluoroalkyl ethyl acrylate onto SBR rubber. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2015;481:537–546. doi: 10.1016/j.colsurfa.2015.05.049. [DOI] [Google Scholar]
- 59. Fox HW, Zisman WA. The spreading of liquids on low energy surfaces. I. polytetrafluoroethylene. Journal of Colloid Science. 1950;5(6):514–531. doi: 10.1016/0095-8522(50)90044-4. [DOI] [Google Scholar]
- 60. Cengiz U, Erbil HY. The lifetime of floating liquid marbles: The influence of particle size and effective surface tension. Soft Matter. 2013;9(37):8980–8991. doi: 10.1039/C3SM51304A. [DOI] [Google Scholar]
- 61. Remya VPR, Jose Varghese R, Parani S, Sakho EHM, Oluwafemi OS, et al. Compatibilization of epoxidized triblock copolymer on the generation of self-assembled nanostructured epoxies and their surface wettability. Journal of Applied Polymer Science. 2021;138(10):e49985. doi: 10.1002/app.49985. [DOI] [Google Scholar]
- 62. Bowers RC, Clinton WC, Zisman WA. Effect of halogenation on frictional properties of plastics. Journal of Applied Physics. 1953;24(8):1066–1067. doi: 10.1063/1.1721443. [DOI] [Google Scholar]
- 63.Ebnesajjad S. Introduction to fluoropolymers: Materials, technology, and applications. William Andrew Elsevier; 2020. [Google Scholar]
- 64. Lee S, Park J-S, Lee TR. The wettability of fluoropolymer surfaces: Influence of surface dipoles. Langmuir. 2008;24(9):4817–4826. doi: 10.1021/la700902h. [DOI] [PubMed] [Google Scholar]
- 65. Nishino T, Meguro M, Nakamae K, Matsushita M, Ueda Y. The lowest surface free energy based on -CF3 alignment. Langmuir. 1999;15(13):4321–4323. doi: 10.1021/la981727s. [DOI] [Google Scholar]