Abstract
This is the first report on the isolation of Klebsiella planticola from neonates. Over a period of 1 year, oropharyngeal and rectal swab specimens from 131 newborns on a neonatal ward were monitored for Klebsiella spp. Thirteen strains of K. planticola could be isolated, and these represented 9% of all Klebsiella spp. found. Capsule type K27 predominated, followed by capsule type K68. Our findings suggest a significant rate of colonization by K. planticola among neonates.
Klebsiellae belong to the eight most common bacterial pathogens causing nosocomial infections (4). Immunocompromised patients, especially elderly people and infants, comprise the population most at risk. In pediatrics, too, nosocomial Klebsiella infections are remarkably troublesome, particularly in premature infants and intensive care units (3). Pediatric patients are easily colonized by Klebsiella spp. (5). Intestinal and oropharyngeal colonization acts as the main reservoir for nosocomial outbreaks (3, 13).
In 1981, a new Klebsiella species, Klebsiella planticola, was described (2). Originally thought to occur solely in aquatic, botanic, and soil environments, this species was also recently isolated from human clinical specimens. These investigations found a surprisingly high frequency of K. planticola among clinical Klebsiella isolates. However, to date only three studies have examined the occurrence of K. planticola in human clinical material (6, 8, 9). So far nothing is known about the expression of virulence factors by K. planticola, and no data on its ability to colonize the human host exist.
To our knowledge, the present study is the first to investigate whether and to what extent neonates are colonized by K. planticola. To evaluate the carriage rate, the intestinal and oropharyngeal flora of 131 infants from a neonatal after-care ward were examined.
Patient specimens.
The occupants of a ward for premature infants (after-care ward; 18 incubators) at the Pediatric Clinic, University of Heidelberg, Heidelberg, Germany, were monitored for a period of 12 months (August 1993 to August 1994). At 14-day intervals, rectal and oropharyngeal swab specimens were taken from a total of 131 infants and were investigated with respect to the isolation of Klebsiella species.
Identification of bacteria.
The swabs were streaked onto blood agar and onto MacConkey agar plates. Colonies suspected of being klebsiellae (mucoid and/or lactose-positive colonies) were isolated and were subjected to identification in the API 20E system. Klebsiella isolates were further differentiated by the tests recommended by Bagley et al. (2). Especially important for the identification of K. planticola are the fecal coliform test, growth at 10°C, pectate degradation, l-sorbose fermentation, melezitose fermentation, and utilization of m-hydroxybenzoate and hydroxy-l-proline.
Antimicrobial susceptibility tests.
The MICs of ampicillin, piperacillin, amoxicillin-clavulanic acid, cefuroxime, cefotaxime, imipenem, tobramycin, co-trimoxazole, and ciprofloxacin were determined by the agar dilution method.
Capsule typing.
The isolates were serotyped by the capsular swelling reaction. Polyvalent immune sera were used for screening, and monospecific sera were used for typing. Immune sera were produced in rabbits as described by Ullmann (14). Isolates were grown on Worfel-Ferguson agar (1) for 24 h at 37°C and at room temperature for a further 24 h. Polyvalent sera were used for screening, and monovalent sera were used for typing.
From the multiple specimens obtained from a total of 131 children investigated, 149 Klebsiella isolates could be isolated. Klebsiella carriage was demonstrated in 51 of these children (39%). This finding suggests that more than one-third of premature infants become colonized with klebsiellae at some time during their hospital stays. Two-thirds of the isolates were from rectal swab specimens (68%) and one-third (32%) were from oropharyngeal swab specimens. This high proportion of isolates from feces is in agreement with the current opinion that intestinal colonization is the main reservoir for Klebsiella infections (10).
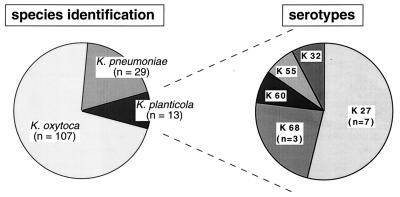
Species identification revealed that the great majority of the isolates were K. oxytoca (72%) (Fig. 1). Apart from K. oxytoca and K. pneumoniae, 13 isolates (8.7%) could be identified as K. planticola. An isolate was considered to be K. planticola if it was positive for l-sorbose fermentation, hydroxy-l-proline utilization, and growth at 10°C and negative by the pectate degradation test and if it failed to ferment melezitose, utilize m-hydroxybenzoate, or produce gas from lactose at 44.5°C (fecal coliform test).
FIG. 1.
Incidence and serotype distribution of K. planticola strains isolated from 131 premature children on a neonatal ward. A total of 149 Klebsiella isolates were recovered from neonatal rectal and oropharyngeal swab specimens.
Previous studies on the isolation of K. planticola from human clinical specimens have reported rates of isolation of K. planticola of from 3.5% (9) to 19% (8) among the Klebsiella strains isolated. Moreover, among the Klebsiella isolates from the feces of healthy adults (food handlers) isolated by our group, 9% were K. planticola (9). Thus, the proportion of K. planticola strains observed in this investigation (8.7%) confirms the previous findings that this new species is present at a surprisingly high frequency in human specimens.
Ten of the 13 K. planticola strains (77%) were shown to be indole positive. These findings correspond with our previous observation of a high incidence (72%) of indole-positive clinical K. planticola isolates (9). In Japan, however, only a minority (28%) of clinical K. planticola isolates were indole positive (8). It is as yet unclear whether geographical differences in the distribution of the indole-positive and the indole-negative biotypes exist.
Antimicrobial susceptibility testing revealed that all K. planticola strains were susceptible to piperacillin, amoxicillin-clavulanic acid, cefuroxime, cefotaxime, imipenem, tobramycin, co-trimoxazole, and ciprofloxacin. Characteristic of Klebsiella spp., most isolates (12 of 13 strains) were resistant to ampicillin.
Interestingly, in only one of the nine children colonized by K. planticola was this species isolated from both the oropharyngeal and the rectal sites. In most children (n = 7) K. planticola could be detected in rectal specimens only. This finding of a predominantly rectal colonization of neonates is in contrast to previous observations that the site of isolation of K. planticola was mainly the respiratory tract (8, 9).
Serotyping of the K. planticola isolates revealed a predominance of capsule type K27 (54%; 7 strains), followed by capsule type K68 (23%; 3 strains). After adjustment for multiple isolates, however, it was noted that both K27 and K68 strains had been isolated from three children each, suggesting a similar incidence of both K types. One strain each was found to express serotype K32, K55, or K60 (Fig. 1). In both of the previous studies mentioned above (8, 9), K27 or K68 was not a frequent capsular type of K. planticola. Thus, colonization of the neonates by particular clonal groups appears to be likely. This assumption accords with a number of observations on clonal outbreaks of K. oxytoca serotype K55 in neonatal wards (7, 11, 12). Moreover, our finding that K55 was one of the five K types detected confirms previous observations that this serotype is common among neonatal Klebsiella isolates. In one of the previous studies (9), Klebsiella serotypes K1, K2, and K5, which have been associated with enhanced virulence, were observed among the clinical K. planticola isolates. In the present study none of these K types was found.
The means of acquisition of this microorganism still remains unclear. During the period of this investigation, the hands of the staff and the inanimate ward environment were monitored by culture for K. planticola. K. planticola could not be detected among 130 hand specimens and 1,020 samples from the environment such as medical and nursing equipment, tubs, and washbasins. To clarify the question of acquisition of K. planticola, follow-up studies which include determination of whether the mothers are colonized are necessary.
Taken together, the present results and previously published data on clinical K. planticola strains demonstrate that this newly discovered Klebsiella species is much more common in human specimens than hitherto suspected. The present study is the first to isolate K. planticola from neonates. Its findings demonstrate a significant rate of neonatal colonization by K. planticola. Because the endogenous flora is thought to be the main reservoir for neonatal Klebsiella infections, further investigations on whether and to what extent neonatal Klebsiella infections are caused by K. planticola are warranted.
Acknowledgments
We thank Andrea Hölzgen for expert technical assistance.
REFERENCES
- 1.Atlas R M. In: Handbook of microbiological media. Parks L C, editor. Boca Raton, Fla: CRC Press, Inc.; 1993. [Google Scholar]
- 2.Bagley S, Seidler R J, Brenner D J. Klebsiella planticola sp. nov.: a new species of Enterobacteriaceae found primarily in nonclinical environments. Curr Microbiol. 1981;6:105–109. [Google Scholar]
- 3.Hart C A. Klebsiellae and neonates. J Hosp Infect. 1993;23:83–86. doi: 10.1016/0195-6701(93)90013-p. [DOI] [PubMed] [Google Scholar]
- 4.Horan T, Culver D, Jarvis W, Emori G, Banerjee S, Martone W, Thornsberry C. Pathogens causing nosocomial infections. Antimicrob Newsl. 1988;5:65–67. [Google Scholar]
- 5.Kühn I, Ayling-Smith B, Tullus K, Burman L G. The use of colonization rate and epidemic index as tools to illustrate the epidemiology of faecal Enterobacteriaceae strains in Swedish neonatal wards. J Hosp Infect. 1993;23:287–297. doi: 10.1016/0195-6701(93)90146-q. [DOI] [PubMed] [Google Scholar]
- 6.Monnet D, Freney J, Brun Y, Boeufgras J M, Fleurette J. Difficulties in identifying Klebsiella strains of clinical origin. Zentralbl Bakteriol Parasitenkd Infektionskr Hyg Abt 1 Orig. 1991;274:456–464. doi: 10.1016/s0934-8840(11)80081-2. [DOI] [PubMed] [Google Scholar]
- 7.Morgan M E, Hart C A, Cooke R W. Klebsiella infection in a neonatal intensive care unit: role of bacteriological surveillance. J Hosp Infect. 1984;5:377–385. doi: 10.1016/0195-6701(84)90005-7. [DOI] [PubMed] [Google Scholar]
- 8.Mori M, Ohta M, Agata N, Kido N, Arakawa Y, Ito H, Komatsu T, Kato N. Identification of species and capsular types of Klebsiella clinical isolates, with special reference to Klebsiella planticola. Microbiol Immunol. 1989;33:887–895. doi: 10.1111/j.1348-0421.1989.tb00976.x. [DOI] [PubMed] [Google Scholar]
- 9.Podschun R, Ullmann U. Incidence of Klebsiella planticola among clinical Klebsiella isolates. Med Microbiol Lett. 1994;3:90–95. [Google Scholar]
- 10.Selden R, Lee S, Wang W L, Bennett J V, Eickhoff T C. Nosocomial Klebsiella infections: intestinal colonization as a reservoir. Ann Intern Med. 1971;74:657–664. doi: 10.7326/0003-4819-74-5-657. [DOI] [PubMed] [Google Scholar]
- 11.Smith M J, Hart C A, Cooke R W I. Gentamicin-resistant Klebsiella oxytoca on a special care baby unit. Lancet. 1984;ii:586–587. doi: 10.1016/s0140-6736(84)90812-2. [DOI] [PubMed] [Google Scholar]
- 12.Tullus K, Aylingsmith B, Kuhn I, Rabsch W, Reissbrodt R, Burman L G. Nationwide spread of Klebsiella oxytoca K55 in Swedish neonatal special care wards. APMIS. 1992;100:1008–1014. [PubMed] [Google Scholar]
- 13.Tullus K, Berglund B, Fryklund B, Kühn I, Burman L G. Epidemiology of fecal strains of the family Enterobacteriaceae in 22 neonatal wards and influence of antibiotic policy. J Clin Microbiol. 1988;26:1166–1170. doi: 10.1128/jcm.26.6.1166-1170.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ullmann U. The distribution of Klebsiella pneumoniae serotypes from different sources and their sensitivity to cephalosporins. Infection. 1983;11:S28–S31. doi: 10.1007/BF01641102. [DOI] [PubMed] [Google Scholar]