Abstract
Currently, clinical strategies for the treatment of wounds are limited, especially in terms of achieving rapid wound healing. In recent years, based on the technique of electrospinning (ES), cell electrospinning (C-ES) has been developed to better repair related tissues or organs (such as skin, fat and muscle) by encapsulating living cells in a microfiber or nanofiber environment and constructing 3D living fiber scaffolds. Therefore, C-ES has promising prospects for promoting wound healing. In this article, C-ES technology and its advantages, the differences between C-ES and traditional ES, the parameters suitable for maintaining cytoactivity, and material selection and design issues are summarized. In addition, we review the application of C-ES in the fields of biomaterials and cells. Finally, the limitations and improved methods of C-ES are discussed. In conclusion, the potential advantages, limitations and prospects of C-ES application in wound healing are presented.
Keywords: Cell electrospinning, Wound healing, Tissue regeneration, Wound repair, Biomaterial, Tissue engineering
Highlights.
The development of C-ES and the factors impacting its process are reviewed, and the differences between C-ES and traditional ES are discussed.
Parameter selection for C-ES and its application in the fields of biomaterials and cells are summarized. The challenges of maintaining cytoactivity while performing C-ES as well as the corresponding solutions are introduced.
The advantages, limitations and prospects of C-ES application in wound healing are discussed.
Background
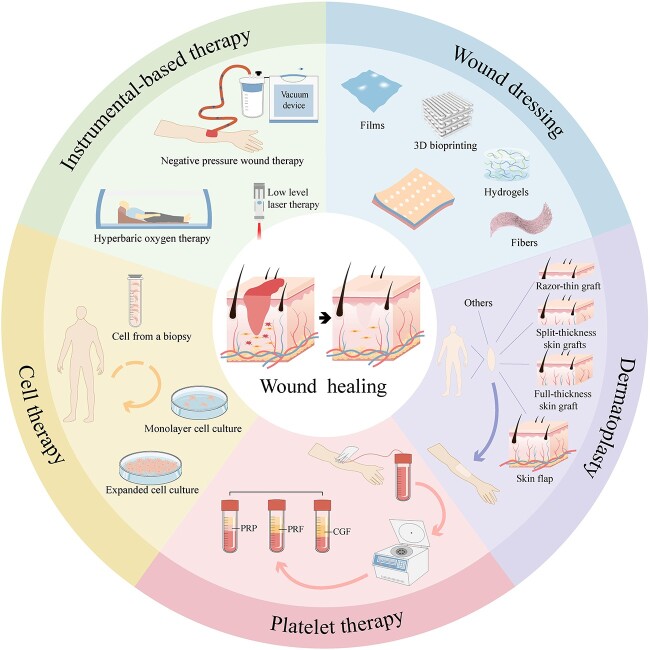
Skin injuries, tissue defects and wound infections impose a heavy burden on patients and healthcare systems both socially and economically [1]. The healing of skin wounds is a complex and highly coordinated process that is impacted by individual health conditions and other physiological factors [2]. The wound healing process can be divided into four stages: hemostasis, inflammation, proliferation and remodeling [3]. The process may stop at any stage due to changes in the wound microenvironment and the dysfunction of cells, which will cause the formation of chronic wounds, leading to the failure of wound healing, such as in the case of diabetic foot ulcers [4]. Small and simple wounds can usually heal with skin tissue repair, while large burns and mechanical injuries need to be treated with external wound dressings or other medical means [5,6]. Treatment methods vary based on the type of wound and the health workers treating the wound. Currently, common methods include skin/stem cell/cell transplantation, platelet therapy, instrumental therapy (such as negative-pressure wound therapy, low-level laser therapy, hyperbaric oxygen therapy, etc.) and wound dressing (Figure 1).
Figure 1.
Methodologies used for wound healing. Some common methods include skin/stem cell/cell transplantation, instrumental therapy (such as negative-pressure wound therapy, low-level laser therapy, hyperbaric oxygen therapy, etc.), wound dressing and platelet therapy. PRF platelet-rich fibrin, PRP platelet-rich plasma, CGF concentrate growth factors
Autogenic tissue implantation and allogenic tissue implantation have been the gold standards for the regeneration and repair of skin tissue when treating large areas of skin injury and refractory wounds [2]. However, the method of implantation has certain limitations, such as immune rejection by patients, trauma caused in another area, limited practicability and high cost [7]. Fortunately, the rise of tissue engineering offers an alternative to traditional wound repair. As the core of tissue engineering, nanofiber scaffolds can provide an ideal support structure that enables cells to survive, thus leading to the promotion of wound repair and healing. Electrospinning (ES) is a simple, efficient and economical technology compared to other methods of preparing nanofiber scaffolds. Polymer filaments with solid, hollow, core–shell or nanoscale structures can be prepared according to the requirements of the target products [8]. Fiber scaffolds made by ES have the advantages of high porosity, ideal mechanical properties and excellent biocompatibility. Such scaffolds are also conducive to cell respiration and water retention on the wound surface [9]. Therefore, it is worth researching the application of ES in wound dressings.
However, ES creates a native extracellular matrix-mimicking environment that lacks the key element (i.e. living cells) in the reconstruction of 3D living tissue function. In 2006, Townsend-Nicholson and Jayasinghe [10] successfully encapsulated astrocytoma (1321 N1) cells in fibers using ES technology for the first time, laying a foundation for the study of cell electrospinning (C-ES). By embedding living cells into fiber scaffolds based on ES technology, C-ES is able to achieve the regeneration and repair of specific tissues or organs. In recent years, many scholars have focused on how to evenly seed cells into the interior of fiber scaffolds to give full play to the function of loaded cells. However, various parameters concerning the preparation of C-ES fiber scaffolds may affect cell distribution and cytoactivity, which brings challenges to researchers [11]. The porosity and size of fiber scaffolds have significant effects on cell migration and growth [12]. Therefore, it is important to carefully analyze the preparation process of C-ES nanofibers. In addition, the maintenance of cytoactivity while performing C-ES and applying it in wound dressing cannot be achieved without the participation of various biomaterials or bioactive molecules. The selection and application of related biomaterials and bioactive molecules in the operation of C-ES have been summarized and analyzed, which will be of great value for the preparation of multifunctional wound dressing products in the future. Wound infection includes a series of dynamic pathophysiological processes, including microbial colonization, biofilm formation and invasive infection [13]. In recent years, various antibiotics and botanical drugs have been explored in the treatment of wound infection, some of which exhibit ideal therapeutic effects. The rapid development of pharmaceuticals, combined with C-ES technology, has led to the trend of designing drug-loaded nanofiber scaffolds.
In this review, we summarize the C-ES technique and compare the differences between C-ES and traditional ES. Aimed at the promotion of wound healing, the selection and application of cells and biomaterials while performing C-ES are discussed. As maintaining cytoactivity during the C-ES process is important, parameter regulation and improvement methods for C-ES are summarized. Furthermore, the difficulties and challenges researchers may encounter in the future while applying C-ES to wound healing are analyzed, and an effort has been made to propose solutions to these challenges. Finally, it is hoped that this review will inspire researchers to make breakthroughs in the treatment of wounds and extend the application of C-ES in the repair of burns and wounds.
Review
C-ES technology
C-ES is a technique that uses an electric field to create fibers with embedded living cells [10]. As shown in Figure 2, the process basically requires three components, namely, a nozzle tip connected to a high-voltage direct current or high-voltage alternating current, a flow controller and a capture device [14]. A large number of papers about the application of ES in wound healing have been published, which is largely attributed to ES’s simple process and its ability to manufacture multifunctional nanofiber products. However, the ES technique has some disadvantages, such as the use of toxic solvents, insufficient cell infiltration, uneven cell distribution, and inability to achieve encapsulation and culture of cells in vitro [11]. In contrast, by encapsulating different types of living cells, C-ES can achieve ideal cell infiltration and even cell distribution, thus increasing functional connections among cells and accelerating the regeneration and repair of specific tissues and organs [15]. Therefore, C-ES may bring new hope for the treatment of skin defects and refractory wounds with large areas. For instance, with the help of sodium alginate (SA)/polyethylene oxide (PEO) solution, Yeo and Kim [16] encapsulated human umbilical vein endothelial cells (HUVECs) in fibers based on C-ES technology, thereafter promoting the generation of microvessels. In addition, they found that the encapsulation of myoblasts (C2C12 cells) also promoted muscle formation.
Figure 2.
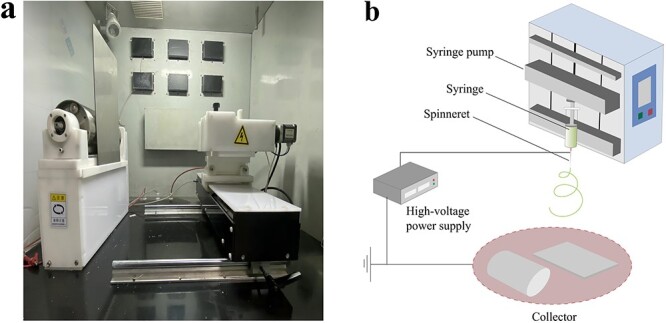
Basic schematic diagram of an electrospinning (ES) device. (a) Physical diagram of an ES system. (b) Schematic diagram of an ES system
Parameters related to C-ES
The preparation of fiber scaffolds/fiber membranes using C-ES is affected by various technological, solution and environmental parameters. The regulation of parameters is crucial for the preparation of target fibers, the maintenance of cytoactivity and the exercise of cell function [17]. For instance, the intensity of the electric field, the distance between the nozzle tip and the capture device, the concentration, viscosity and propulsion speed of the bioink, and environmental factors (temperature and humidity) need to be strictly controlled while preparing multifunctional fiber scaffolds with high porosity. As shown in Figure 3, we found in an experiment that nanofibers made in an environment of high humidity had bead- or droplet-like structures, making it difficult to prepare fiber scaffolds with even morphology.
Figure 3.
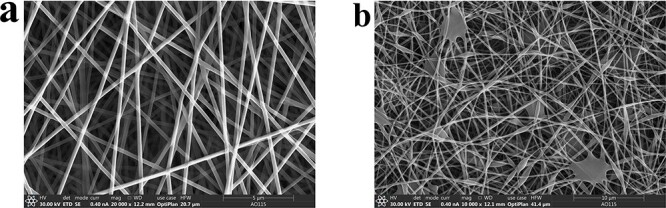
Electrospun nanofiber electron microscopy at different humidities. (a) Electrospun nanofiber morphology at normal humidity. (b) Electrospun nanofibers in high-humidity environments contain beads and droplets
In this section, we introduce the effects of technological, solution and environmental on fiber preparation and cytoactivity during the C-ES process and propose corresponding solutions, as shown in Table 1.
Table 1.
Effects of relevant parameters on cell viability and fiber morphology and methods for resolution
| Parameter | Effects on cytoactivity | Effect on fiber morphology | Methods for maintaining cytoactivity | Methods of maintaining fiber morphology | References |
|---|---|---|---|---|---|
| Molecular weight, molecular structure of the polymer (branched, linear, etc.) | Polymers with certain molecular weight are beneficial, while polymers with high molecular weight have negative effects on cytoactivity | High molecular weight produces fibers, low molecular weight produces beads | Select polymers with appropriate molecular-weight range | Select polymer materials with different molecular weights or add physical crosslinking | [8,18,19] |
| Polymer concentration | A certain concentration is beneficial, while high concentration has a negative impact on cytoactivity | Within a certain range, the fiber diameter is positively related to the polymer concentration, and low concentration produces electrospray | Properly reduce polymer concentration and surface tension | Adjust the ratio of polymer and solvent | [8,20–22] |
| Solution viscosity | Low viscosity is beneficial, while high viscosity has a negative effect on cytoactivity | Low viscosity causes bead formation, High viscosity increases fiber diameter, making it too high to eject solution | Properly reduce solution viscosity and surface tension | Adjust the ratio of polymer and solvent | [8,11,23] |
| Conductivity | Low conductivity is beneficial, while high conductivity has a negative effect on cytoactivity | Higher conductivity produces finer fibers | Select polymers with low conductivity as much as possible | Introduce ionic compounds, such as salts or inorganic acids | [8,11,21] |
| Surface tension | The lower the surface tension, the smaller the negative effect on cytoactivity | High surface tension leads to jet instability and low surface tension is conducive to the production of thinner fibers | Reduce surface tension | Add surfactants | [8,11,24,25] |
| Injection rate/flow rate | The smaller the flow rate, the smaller the negative effect on cytoactivity | Low flow-rate produces finer fibers, while high flow-rate produces beaded fibers or droplets | Keep the liquid inlet-rate as low as possible to reduce the shear force | Control the solution advancing speed | [11,23,24] |
| Size of electromotive force | The lower the electromotive force, the smaller the effect on cytoactivity | Higher electromotive force produces finer fibers | In the case of fiber preparation, electromotive force is reduced as far as possible | Regulate voltage | [8,11,26] |
| Distance between capillary tube and collecting screen | The smaller the distance, the less the exposure time of the cell electric field | A certain distance is conducive to the maintenance of fiber morphology, and bead fibers or droplets are produced at a small distance | Select the appropriate distance between the capillary tube and the collection screen | Adjust the distance between capillary tube and collecting screen | [8,11,27,28] |
| Motion pattern of the collection device | A movement trend with uniform embedding and small shear force is beneficial to maintain cytoactivity | It has nothing to do with fiber morphology, it has to do with fiber arrangement | By modifying the receiving device, the cell activity is maintained | Fix or move as required | [8,29] |
| Volatility of solvent | The volatilization and type of solvent have negative effects on cytoactivity | Too high a value will cause the jet to solidify and too low a value will cause the collected fiber to become wet | Select low cytotoxicity or avoid negative effects of solvent on cells | Modify the proportion and type of solvent | [8,30,31] |
| Temperature | Either a high or a low temperature has a negative effect on cytoactivity | High temperature is conducive to producing finer fibers, but too high a value is not conducive to extension | Select temperature conducive for cell survival | Control the working temperature to achieve the preparation of target fiber | [8,11,32,33] |
| Humidity | Too high or too low humidity has a negative effect on cytoactivity | Lower humidity forms drier and finer fibers, and higher humidity forms beaded fibers or liquid droplets | Select the proper humidity to maintain cytoactivity | Regulate working humidity | [8,11,34] |
Technological parameters
In the process of C-ES, the electric field is essential to spin the bioink into micro/nano filaments [35]. The electric-field intensity also affects cytoactivity and fiber morphology [16,26]. If the intensity is too strong, the cytoactivity will decline, and fibers with finer diameters will be produced. For instance, some scholars have investigated the cytoactivity of C2C12 myocytes in nanofibers generated by SA and PEO. When the field intensity was >0.1 kV/mm, cytoactivity showed a significant decrease. An intensity in the range 0.05–0.075 kV/mm corresponds to ideal cytoactivity [16]. Although the application of a low-intensity electric field (0.05 kV/mm) maintained cytoactivity, the prepared fibers did not meet the requirements of the target product. In another study, when encapsulated into a mixed nanofiber scaffold consisting of SA/polycaprolactone (PCL)/lecithin, MG63 osteoblast-like cells showed significantly decreased cytoactivity when the electric-field intensity was >0.16 kV/mm. When the intensity was in the range 0.1–0.13 kV/mm, no fiber was produced [26]. Therefore, aiming for ideal cytoactivity and fiber morphology during the C-ES process, it is important to adjust the range of electric-field intensity according to the type of cell and biomaterials. In addition, the distance between the nozzle tip and the electrode is an essential parameter. It not only affects the morphology of nanofibers but also impacts how long cells are exposed to the electric field [28]. In the C-ES process, the velocity of flow is related to the rate of pushing the syringe. The faster the flow velocity is, the greater the shear force will be. An increase in shear force will have a negative effect on cells and lead to the formation of fibers with beaded structures [11]. Although increasing the electric-field intensity or the distance between the nozzle tip and the electrode may help produce ideal fibers, cytoactivity cannot be guaranteed at the same time. Therefore, to achieve scaffolds with ideal morphology and the maintenance of cytoactivity, the velocity of flow needs to be regulated strictly.
Solution parameters
The solution parameters affecting the C-ES process include solution viscosity, surface tension, polymer concentration, molecular weight, and the type and proportion of solvent. The selection of solvent is essential for the maintenance of cytoactivity [36]. It is well known that most solvents, such as alcohols, dichloromethane, chloroform, dimethylformamide, tetrahydrofuran, acetone and dimethyl sulfoxide, are toxic to cells to some extent. Some researchers have demonstrated that chloroform, tetrahydrofuran and acetone cannot be used for C-ES, especially for instant ES using hand-held devices [36]. The volatility of the solvent is another element that should be noted. Volatility that is too high will cause the jet flow to solidify after being discharged. When the volatility is too low, the fibers become wet when deposited on the capture device [8]. Furthermore, the volatilization of solvents exerts negative effects on cells in the C-ES process. For example, Canbolat et al. [37] found that residual solvents in ES scaffolds reduced cytoactivity, while clean scaffolds could only increase cytoactivity by 5–10%. Therefore, many factors need to be taken into account when selecting solvents for C-ES. The appropriate surface tension and electrical conductivity of bioinks, which are also critical to cytoactivity, are impacted by the solvent ratio [8]. For instance, studies have shown that the ratio of dichloromethane to dimethylformamide determines the surface tension of PCL solution [31]. The appropriate ratio of solvent to polymer provides the required viscosity, surface tension and electrical conductivity [8]. In addition, from the perspective of 3D printing, the viscosity of the solution is closely related to the uniform encapsulation of cells and the formation of a good fiber morphology in the C-ES process [22,38]. Viscosity that is too high will not only affect the preparation of fiber scaffolds but also inhibit the even distribution of cells and reduce the efficiency of encapsulation. Moreover, high viscosity normally corresponds to a larger shear force generated by the solution, thus having an adverse effect on cytoactivity [39,40]. A solution with a viscosity that is too low is also not suitable for C-ES due to the difficulty in forming even fiber filaments in the electric field [30]. Therefore, to satisfy the preparation of target fibers, it is necessary to strictly adjust the viscosity of the solution to facilitate the even distribution and ideal encapsulation of cells in the C-ES process.
Environmental parameters
Environmental factors, such as temperature, humidity and aseptic conditions, also have a great influence on C-ES [11]. Temperature affects the rheology, viscosity and surface tension of the solution [32,33,41]. Cytoactivity is also influenced by temperature. As a result, temperature is strictly limited in the C-ES process so as not to cause damage to cells. Humidity is also an important element impacting the formation of C-ES fibers. As shown in Figure 3, we found experimentally that fiber scaffolds produced in different seasons showed different fiber morphologies under scanning electron microscopy, which may be due to different levels of humidity. Humidity that is too high normally causes elongation of the initial jet flow, leading to the formation of beaded fibers, which inhibits the even distribution of cells during the C-ES process [34]. Low humidity is also not conducive to the maintenance of cytoactivity. Therefore, C-ES experiments must be carried out under precisely appropriate conditions of indoor humidity [42].
In summary, changes in any parameter may directly or indirectly affect the formation of fibers and the maintenance of cytoactivity. It is of vital significance to strictly control these parameters.
C-ES and wound healing
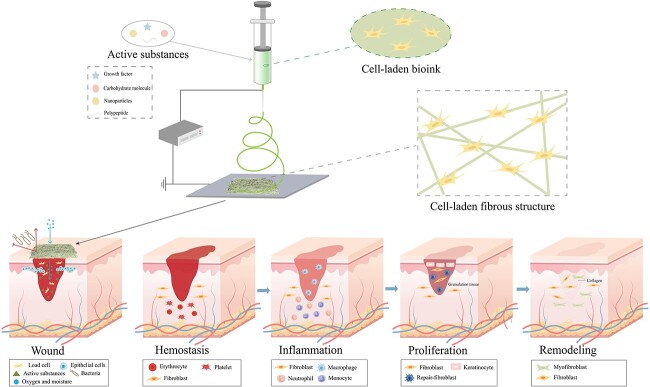
Skin is the first barrier for resisting harmful factors in the external environment and is thus maintains the stability of the internal environment, which means it is susceptible to numerous factors. Recently, researchers have shown an increased interest in the preparation of ES nanofibers and their application in wound healing [2]. The nanofiber scaffolds prepared by ES, acting as support structures, can promote the proliferation and differentiation of wound tissue cells through physiological and biochemical effects. However, ES does not involve the participation of exogenous cells, which leads to a lack of cells during the repair of areas with tissue defects. C-ES can address this issue by encapsulating different types of cells, thus accelerating wound healing and repair. Figure 4 shows the process of preparing a wound dressing using C-ES. This section summarizes the advantages of C-ES. Moreover, the application of cells and biomaterials in C-ES as well as their contribution to wound healing are reviewed. Finally, depending on the characteristics of wound healing, methods to achieve cell migration and growth, and drug loading in C-ES fiber scaffolds, are introduced.
Figure 4.
Cell electrospinning of cells and active substances for skin wound healing. A variety of active substances can be electrospun as multifunctional wound dressings to provide a suitable microenvironment for different stages of wound healing, including hemostasis, inflammation, proliferation and remodeling
Advantages of C-ES in wound healing
In recent years, some researchers have reported achievements while applying C-ES to wound healing. For instance, Xu et al. [36] prepared an 8% polyvinyl alcohol (PVA) solution, mixed stem cells with it and then made a fiber pad using a hand-held ES device. They proved that the fiber pad could promote the healing of full-thickness skin wounds in rats. In addition, some scholars have shown that C-ES can process substantial numbers of cells, achieving ideal infiltration and an even cell distribution [43]. As shown in Table 2, research on C-ES has achieved varying degrees of progress from 2006 to 2023. Based on principles of tissue regeneration and repair, loading cells and molecules crucial for wound healing through C-ES and constructing an environment similar to extracellular matrix (ECM) may be a potential method of wound treatment.
Table 2.
Evolution of cell electrospinning (C-ES)
| Year of publication | Article title (main content) | Reference |
|---|---|---|
| 2006 | Electrohydrodynamic jet processing: an advanced electric-field-driven jetting phenomenon for processing living cells | [44] |
| 2006 | Stable electric-field driven cone-jetting of concentrated biosuspensions | [109] |
| 2006 | Cell electrospinning: a unique biotechnique for encapsulating living organisms for generating active biological microthreads/scaffolds | [10] |
| 2007 | Cell electrospinning highly concentrated cellular suspensions containing primary living organisms into cell-bearing threads and scaffolds | [58] |
| 2008 | Pilot study to investigate the possibility of cytogenetic and physiological changes in bio-electrosprayed human lymphocyte cells | [45] |
| 2008 | Bio-protocols for directly forming active encapsulations containing living primary cells | [59] |
| 2010 | The differentiation and engraftment potential of mouse hematopoietic stem cells is maintained after bio-electrospray | [46] |
| 2011 | Bio-electrosprayed living composite matrix implanted into mouse models | [47] |
| 2011 | Bio-electrospraying and cell electrospinning: progress and opportunities for basic biology and clinical sciences | [48] |
| 2013 | Cell electrospinning: an in vitro and in vivo study | [110] |
| 2013 | Cell electrospinning: a novel tool for functionalising fibres, scaffolds and membranes with living cells and other advanced materials for regenerative biology and medicine | [29] |
| 2014 | Cell electrospinning cardiac patches for tissue engineering the heart | [43] |
| 2015 | Platform technologies for directly reconstructing 3D living biomaterials | [49] |
| 2015 | Fabrication of cell-laden electrospun hybrid scaffolds of alginate-based bioink and PCL microstructures for tissue regeneration | [26] |
| 2015 | A novel bioactive membrane by cell electrospinning | [15] |
| 2018 | Recent advances in cell electrospining of natural and synthetic nanofibers for regenerative medicine | [50] |
| 2019 | Modified cell-electrospinning for 3D myogenesis of C2C12s in aligned fibrin microfiber bundles | [61] |
| 2019 | Cell-electrospinning and its application for tissue engineering | [11] |
| 2020 | Electrospinning live cells using gelatin and pullulan | [51] |
| 2020 | Electrospun nanofibers as carriers of microorganisms, stem cells, proteins and nucleic acids in therapeutic and other applications | [52] |
| 2020 | Micro/nano-hierarchical scaffold fabricated using a cell electrospinning/3D printing process for co-culturing myoblasts and HUVECs to induce myoblast alignment and differentiation | [57] |
| 2022 | In situ cell electrospun using a portable handheld electrospinning apparatus for the repair of wound healing in rats | [36] |
| 2023 | Esophageal wound healing by aligned smooth muscle cell-laden nanofibrous patch | [53] |
PCL polycaprolactone, C2C12s mouse myoblasts, HUVECs human umbilical vein endothelial cells
Compared with other synthesis methods of nanofiber scaffolds (e.g. cell printing), C-ES produces nanoscale fiber scaffolds that offer higher resolution and better ECM-like structures [11]. Some researchers have found that stem-cell-derived smooth muscle cells (SMCs) undergoing the C-ES process showed higher levels of maturation than those undergoing cell printing [53]. In addition, C-ES nanofibers demonstrate the synergistic effect of nanoscale patterning by guiding cells to align along the fibers, rather than merely simulating the size of the structure; furthermore, the C-ES nanofiber scaffolds can exchange nutrients and oxygen efficiently, facilitating intercellular interactions [11]. By loading cells crucial for wound healing, C-ES can not only achieve the adhesion, migration and proliferation of tissue cells outside the scaffold but also compensate for the shortage of cells in the defect area by introducing cells. However, there also exist some limitations while performing C-ES. For instance, the mechanical strength of scaffolds is reduced to maintain cytoactivity, and the selection of biomaterials and solvents is relatively limited. The advantages and limitations of C-ES are listed in Table 3.
Table 3.
Advantages and disadvantages of C-ES and traditional ES
| Advantages/disadvantages | C-ES | Traditional ES |
|---|---|---|
| Advantages | Can better achieve cell-to-cell interactions Good for cell infiltration and migration Efficient and rapid exchange of gases and substances Uniform distribution of the cells in the scaffold Use of nontoxic/low-toxicity solvent (better biocompatibility) The structure of the fibrous scaffold is fine Many cells can be processed |
The process is simple and easy to operate Provides controllable micro/nanofiber structures Models the native ECM structure High surface area and porosity There is a wide variety of spinnable substances Spinning is cheap Products can be prepared with various properties |
| Disadvantages | The 3D structures are very difficult to implement The mechanical strength of the stent is low Difficult to balance maintenance of cellular activity with scaffold properties The cost is relatively high Selection of materials is relatively limited The parameter requirements are more stringent |
The use of toxic solvents The distribution of the cells is not uniform Insufficient infiltration and migration of cells Insufficient structural refinement |
ECM extracellular matrix, C-ES cell electrospinning, ES electrospinning
Polymers in wound-healing applications
As mentioned above, the key to the success of C-ES lies in the maintenance of cytoactivity. As shown in Table 4, in recent years researchers have used a variety of biomaterials to construct C-ES fiber scaffolds loaded with active cells. Table 5 lists common biomaterials used for ES in wound dressing studies. The selection of biomaterials and their application in C-ES are summarized, which may help with the preparation of C-ES products aimed at wound healing in the future. This section introduces related biomaterials, including natural polymers and synthetic polymers.
Table 4.
Description of materials used for C-ES classified into natural and synthetic polymers
| Polymer | Material | Research portfolio | Advantages | Disadvantages | References |
|---|---|---|---|---|---|
| Natural polymer | Collagen | Collagen/PEO solution | Highly biocompatible and biodegradable Relative nonimmunogenicity |
Poor mechanical strength Expensive |
[29,54,55] |
| GEL | Not mentioned | Biocompatible and biodegradable Relatively low cost |
Poor mechanical strength | [29,56] | |
| SA | SA/PEO/lecithin solution SA/PEO solution |
Biocompatible and biodegradable Sufficient cross-linking capacity Relatively low cost Non-toxic |
Poor mechanical strength without crosslinking Low biological characteristics |
[16,26,53,57] | |
| Synthetic polymer | PDMS | PDMS | Non-toxic Inertia Viscoelasticity is uniform and isotropic High solubility and biodegradability |
Lack of biological function clues | [10,58,59] |
| PVA | PVA | Relatively low cost Durable High temperature stability |
Lack of biological function clues | [15,36] | |
| PLLA | PLLA | Good biodegradability Good biocompatibility |
Lack of biological function clues | [60] | |
| PEO | Collagen/PEO solution SA/PEO/lecithin solution SA/PEO solution |
Good biodegradability Good biocompatibility |
Lack of biological function clues | [16,26,53,57,61] |
C-ES cell electrospinning, PEO polyethylene oxide, SA sodium alginate, PDMS polydimethylsiloxane, PVA polyvinyl alcohol, PLLA poly(L-lactic acid), GEL gelatin
Table 5.
Some common biomaterials for ES classified into natural and synthetic polymers in wound dressing studies
| Polymer | Material | Describe | Applications | References |
|---|---|---|---|---|
| Natural polymer | CS | Chitosan is a natural polysaccharide with good antibacterial property, high biocompatibility and controllable biodegradability | Wound dressing Biomedical materials Drug delivery |
[62,63] |
| HA | Hyaluronic acid is a kind of proteoglycan in ECM, which can be decomposed and absorbed in human body, non-toxic and low immune response | Medical implants Skin tissue engineering |
[64,65] | |
| SF | Silk fibroin is a natural macromolecular fibrin extracted from silk, with good biocompatibility and biodegradability | Biological materials Wound dressing |
[66,67] | |
| GEL | Collagen is a water-soluble elastin monomer with good biocompatibility and hydrophilicity | Tissue engineering scaffold Wound healing Wound dressing |
[68–70] | |
| Synthetic polymer | PEG | A water-soluble synthetic polymer with good wettability, commonly used in drug carriers and fiber products | Nonwoven tissue engineering scaffolds | [71,72] |
| PU | The composite membrane prepared from synthetic polymer has the characteristics of good elasticity, light weight, good oxygen permeability, high mechanical strength and good barrier performance, and is often used in biomedical applications | Non-woven fabric tissue template Wound healing |
[73,74] | |
| BG | A kind of material that can repair, replace and regenerate the body tissue, and can form a bond between the tissue and the material | Tissue engineering scaffold | [75,76] | |
| PLGA | It is formed by random polymerization of two monomers, lactic acid and glycolic acid, with good biocompatibility, non-toxicity, good encapsulation and biodegradability | Wound healing Biomedical applications |
[77,78] | |
| PLA | A non-toxic, degradable, biocompatible and good mechanical properties synthetic polymer | Tissue repair Wound healing Wound dressing |
[79,80] | |
| PCL | A synthetic polymer that can be used for human tissue engineering, with high mechanical strength, good processability and biodegradability | Bone tissue engineering Biomedical applications |
[81,82] |
ES electrospinning, CS chitosan, HA hyaluronic acid, SF silk fibroin, GEL gelatin, PEG polyethylene glycol, PU polyurethane, BG bioactive glass, PLGA polylactic acid-glycolic acid, PLA polylactic acid, PCL polycaprolactone, ECM extracellular matrix
Natural polymers
Natural polymers come from a variety of renewable sources and have inherent biocompatibility and biodegradability. For instance, chitosan (CS), hyaluronic acid (HA), SA and collagen and silk fibroin (SF) have been widely used in skin tissue engineering. SA, PEO, lecithin and gelatin (Gel) have attracted much attention for application in C-ES (Table 4).
CS, a biological polysaccharide polymer, possesses good biocompatibility, biodegradability and antimicrobial properties [83]. Interestingly, proteoglycan, the building block for connective tissue and cartilage, can be derived from glucosamine and monosaccharides produced during the hydrolysis of CS [84]. Therefore, CS shows great potential in the repair of tissue and invention of new wound dressings. Ma et al. [85] produced porous scaffolds by crosslinking CS and collagen with glutaraldehyde and then using a freeze-drying method. They found that the potential cytotoxicity of glutaraldehyde was reduced in the presence of CS. CS can improve the cross-linking efficiency in collagen-based scaffolds. In addition, CS can play a role in wound cleaning by accelerating the transfer of inflammatory cells and the secretion of type III collagen, thus promoting the formation of granulation tissue and epithelial tissue [86]. Santos et al. [87] demonstrated that CS accelerated wound healing by promoting the infiltration of polymorphonuclear cells at the wound site.
Collagen, an ECM protein produced mainly by fibroblasts and chondrocytes, has good elasticity and malleability [54]. No significant changes in cytoactivity or cell function were observed in primary muscle cells in collagen solution when applying collagen in C-ES, indicating that collagen is not cytotoxic. Rho et al. [88] developed a collagen fiber scaffold and investigated its effects on human keratinocytes. The results showed that the scaffold promoted the cell adhesion and proliferation of human keratinocytes. Moreover, in healing tests of open wounds, collagen scaffolds accelerated the removal of surface tissue debris and proliferation of fibroblasts and neonatal capillaries.
SA, a natural polysaccharide derived from brown algae or cell walls, also shows great prospects in skin repair. For instance, a mixture consisting of SA, PEO and lecithin can be used for the regeneration of soft tissue and bone [26]. The use of SA improved cytoactivity to a certain extent during the C-ES process. Some researchers prepared a bioink by mixing A2P3 solution (2% SA and 3% PEO) with mesenchymal stem cells (MSCs) and SMCs using C-ES technology, and a PCL fiber pad was used as the receiving platform to provide mechanical support. In this study, a 300 μm PCL scaffold was prepared by 3D printing PCL to provide mechanical support for C-ES. MSCs and SMCs were then printed onto the PCL pillar at a pneumatic pressure of 120 kPa and a nozzle movement speed of 10 mm/s. In this way, a patch with aligned SMC fibers was developed to promote esophageal healing. The results showed that the cytoactivity of MSCs and SMCs was >90% and was maintained for 21 days [53]. In the clinic, SA fibers can also be made into medical swabs and surgical absorbent dressings.
HA is a proteoglycan widely found in ECM. It can be decomposed and absorbed in the human body, but the mechanical properties of homogenous HA fiber scaffolds are not stable. Hence, to achieve mechanical stability and biological compatibility, some scholars used ES to prepare a double-layer polymer scaffold composed of CS, PCL and HA, which possessed ideal antibacterial properties due to the incorporation of CS [89]. Most noteworthy is that the scaffold also has good hydrophilicity and elasticity in both dry and humid environments, which can be taken advantage of to promote the adhesion and proliferation of renal epithelial cells, providing a new method for wound treatment.
SF is a protein containing 5263 amino acid residues whose β-folded structure gives it excellent mechanical properties [90]. Owing to its ability to promote skin collagen synthesis and epithelialization through the rapid proliferation of epithelial cells, SF has been widely used in various forms, such as hydrogels, sponges, films and microspheres [91]. Roh et al. [92] prepared a hybrid SF/SA scaffold and found that it accelerated the healing of full-thickness wounds in rats. In addition, Yoo et al. [93] prepared CS/SF biomimetic nanostructure scaffolds through ES and found that the combination of 25% chitin and 75% SF was promising for the adhesion and proliferation of normal human keratinocytes.
However, most of the naturally occurring polymers discussed above are hydrophilic, with weak molecular-chain entanglement and large repulsive forces between ions, which inevitably leads to defects in fiber structure and morphology in the process of performing C-ES [94]. To overcome these limitations, core–shell nozzles or mixtures containing multiple polymers can be used. For instance, the key factor that interferes with SA-based ES is the repulsive force between polyanions. Therefore, the addition of PEO to SA can reduce the repulsive force and decrease the degree of ionization of carboxylate, leading to an increase in molecular-chain entanglement [95]. In addition, these natural polymers can be chemically cross-linked or physically treated before the ES process, thus avoiding the structural and morphological shortcomings of ES.
Synthetic polymers
Synthetic polymers are widely used in the medical field because of their excellent physical and chemical properties, good biological characteristics, and easy processing and production [96]. For example, polyurethane (PU) film, which possesses high elasticity, light weight, good oxygen permeability, high mechanical strength and ideal barrier properties, is often used in biomedical fields [97]. Furthermore, PU membranes can be used as nanofiber materials to accelerate wound epithelialization and dermal tissue formation. Two types of synthetic polymers used earlier in C-ES were PVA and polydimethylsiloxane (PDMS). PVA, a safe macromolecular organic substance with good biocompatibility, can be used in ophthalmology and wound-dressing preparation [98,99]. One of the steps in the earliest version of C-ES was to encapsulate cells into PDMS fibers. Specifically, it was proven that after astrocytoma cells were successfully embedded into a fibrous scaffold using a 0.09 kV/mm electric field, they maintained a certain level of vitality (67.6 ± 1.9%) [10]. PCL, a kind of synthetic polymer, can be used in human tissue engineering. It has the advantages of strong mechanical properties, easy processing and good biodegradability [100]. Therefore, the use of PCL can make up for the defects of GEL fiber. During wound healing, fibroblasts play an important role in enhancing wound contraction and intercellular matrix recombination. Studies have shown that PCL/GEL nanofibers can provide a favorable substrate for the growth of dermal fibroblasts. Nanofiber scaffolds of PCL have high porosity, which can promote the adhesion and migration of fiber cells and offer space for their growth [101]. Therefore, the combination of PCL and GEL, which guarantees certain mechanical properties and biodegradability, has become a promising alternative to real leather [102]. In addition, the good biocompatibility and in vivo degradability of polylactic acid-glycolic acid (PLGA) has attracted the attention of researchers. The combination of PLGA and inorganic nanocomposites may promote tissue regeneration, accelerate wound healing and inhibit inflammatory responses [103]. Bioactive glass (BG) is a class of material with the ability to bond with both bone tissue and soft tissue [104]. The degradation products of BG can also promote the generation of growth factors and enhance the proliferation of cells [105]. These synthetic polymers have promising prospects for application in wound healing. Proper selection of these polymers, based on their physical, chemical and biological properties, combined with the use of other bioactive/nonbioactive substances and the accurate regulation of C-ES-related parameters, makes it possible to prepare ideal nanofiber scaffolds, which possess great application value in drug delivery and wound dressing. At present, most of these synthetic polymers are used in ES processes. A few of them, in the form of fiber pads, are applied in C-ES to increase mechanical strength.
C-ES nanofibers
In the C-ES process, multiple types of cells and various molecules can be fixed in the fiber simultaneously during the preparation of the scaffold. However, the wound healing process includes several successive phases of cellular and biochemical activity [2]. Moreover, different wound sites and injury conditions determine the selection of different treatment methods, which also increased the requirements in the preparation of tissue engineering scaffolds. It is important to regulate the specific parameters to produce nanofiber scaffolds with a specific function and morphology according to the characteristics of the wound surface. In addition, C-ES nanofibers are expected to fully simulate the biological and physicochemical properties of skin tissue. According to the site of the wound, the depth range of the wound and the healing condition of the wound, C-ES nanofibers should be adjusted in terms of several aspects, including the selection of materials, diameter, arrangement and fiber morphology. For example, the corneal stromais composed of orthogonally arranged collagen nanofiber layers, which are sensitive to light transparency. If the fiber structures made through ES simulate this orthogonal arrangement, the phenotype of corneal cells may be maintained, thus achieving corneal regeneration [106]. For muscle regeneration, the uniaxial arrangement of micropatterns is important for mimicking the structure of the native ECM [16]. In skin regeneration and repair, cross-linked nanofibers performed better in promoting the migration of fibroblasts and keratinocytes in vivo than axially arranged nanofibers [107,108]. Moreover, based on the treatment requirements of specific wound surfaces, nanofiber scaffolds of various forms, such as solid, porous, banded, core–shell, hollow, beaded and multichannel, can be obtained [8]. However, it is difficult to maintain cytoactivity while preparing nanofiber scaffolds with specific morphology and properties during the C-ES process. One recognized method to address this issue is coaxial ES, in which a cell suspension is placed in the inner needle and a biopolymer solution is placed in the outer needle. On one hand, the biopolymer solution in the outer layer shields the effect of high voltage on the inner cells. On the other hand, it encapsulates the cells and offers the matrix necessary for their survival. For example, some researchers placed a suspension containing 1321 N1 cells in the inner needle of the core–shell nozzle and placed PDMS solution in the outer needle for C-ES. The results showed an enhancement of the mechanical properties of the fibers, and the cytoactivity of the encapsulated cells was close to 70% of their original value [58].
C-ES and cells
In tissue engineering, cells need to proliferate and differentiate in a customized micro- or nano-environment to achieve tissue repair. The application of relevant cells in the C-ES process and the main cells involved in wound healing are reviewed in this section, which may help with the future application of C-ES in burn and wound repair.
Cells used for C-ES
Various types of cells, such as neuroblastoma cells, cardiomyocytes, osteosarcoma cells, osteoblasts, mesenchymal stem cells and adipose stem cells, have been used for C-ES since 2006. In these studies, different types of bioink solutions tailored to the corresponding cells were used for the repair and reconstruction of different tissues. Table 6 shows cells and solutions selected for use during the C-ES process.
Table 6.
Effect of cells, solutions and range of electric fields used in C-ES studies on cytoactivity
| Cell types | Solution | Solvent | Electrospinning method | Electric field | Cytoactivity | References |
|---|---|---|---|---|---|---|
| Osteoblast (MG63) cells | SA/PEO/lecithin | PBS | Blend electrospinning | 0.16 kV/mm | >80% | [26] |
| Primary cardiomyocytes | Matrigel-rich collagen biopolymer | Not mentioned | Blend electrospinning | 0.47–0.67 kV/mm | >80% | [43] |
| C2C12 myoblast cells | SA/PEO | Distilled water | Blend electrospinning | 0.075 kV/mm | >90% | [16] |
|
C2C12 myoblast cells |
Fibrin/PEO | Deionized water | Blend electrospinning | 0.056 kV/mm | Not mentioned | [61] |
| Primary porcine vascular SMCs and RASMCs | PDMS | Not mentioned | Coaxial electrospinning (inner cell suspension and outer polymer solution) | Not mentioned | Rabbit aorta smooth-muscle cells 88.44%–89.57% Primary porcine vascular cells 89.99%–95.14% |
[58,59] |
| PC-12 cells | PLLA | Not mentioned | Coaxial electrospinning (inner cell suspension and outer polymer solution) | Not mentioned | Not mentioned | [109] [60] |
| Human astrocytes (1321 N1) | PDMS | Not mentioned | Coaxial electrospinning (inner cell suspension and outer polymer solution) | 0.09 kV/mm | 65–75% | [10,109] |
| Neuroblastoma (N2A) cells | Matrigel with high concentration of laminin | Not mentioned | Coaxial electrospinning (inner cell suspension and outer polymer solution) | 0.05–0.25 kV/mm | >80% | [110] |
| ASCs | PVA | Distilled water | Blend electrospinning | 0.08 kV/mm | 133% | [15] |
| BMSCs in rats | PVA | PBS | Blend electrospinning | 0.075 kV/mm | >90% | [36] |
| SMCs | SA/PEO | Tridistilled water | Blend electrospinning | 0.075 kV/mm | >90% | [53] |
| HUVECs | SA/PEO | Tridistilled water | Blend electrospinning | 0.075 kV/mm | >90% | [57] |
| RASMCs | PDMS or PEO | Not mentioned | Coaxial electrospinning | Not mentioned | >70% | [58] |
PEO polyethylene oxide, SA sodium alginate, PDMS polydimethylsiloxane, PVA polyvinyl alcohol, PLLA poly(L-lactic acid), SMCs smooth muscle cells, ASCs adipose stem cells, BMSCs bone marrow mesenchymal stem cells, HUVECs human umbilical vein endothelial cells, RASMCs rabbit aorta smooth muscle cells, C2C12s mouse myoblasts
Cells involved in the wound-healing process
Certain cells are involved in different stages of wound healing, and these cells are highly coordinated in the regulation of the wound-healing process. At the initial stage of wound healing, platelets are activated and release platelet-derived growth factor and transforming growth factor-β. Platelet-derived growth factor attracts platelets, macrophages, endothelial cells, smooth muscle and other cells, which can promote hemostasis [111]. During the inflammatory stage, neutrophils and mononuclear cells play a major role. When monocytes are recruited to the wound, they differentiate into macrophages, which engulf necrotic tissue and cell debris [112,113]. In addition, bone marrow mesenchymal stem cells (BMSCs) migrate to the wound site to regulate the proliferation and migration of dermal mesenchymal and epithelial cells [114]. Studies have proven that scaffolds containing BMSCs have good skin affinity and are conducive to wound healing. As a result, they have become candidate cells for skin tissue engineering [115]. During the proliferative stage of the wound, fibroblasts secrete and synthesize various collagens and fibrins to promote wound healing and repair. Vascular endothelial cells, macrophages and epidermal cells secrete vascular endothelial growth factor and basic fibroblast growth factor, promoting the generation of microvessels [111]. Inflammatory cells, vascular endothelial cells, keratinocytes and secreted fibroblasts play an important role in the remodeling stage of the wound [116]. Stimulated by keratinocyte migration, the wound tends to possess the property of water retention, thus promoting rapid healing of the wound bed. In addition, neural stem cells have been shown to play an active role in the healing process by forming keratinocytes and fibroblasts and releasing angiopoietin-1 (Ang-1)or ECM proteins [117]. Stem cells are usually susceptible to external stimulation while performing C-ES, so construction of the ECM microenvironment is essential for using stem cells to regenerate tissue. Chen et al. [15] used PVA and adipose-derived stem cells (ASCs) to develop a bioactive membrane through C-ES and proved that the nanofiber membrane could guarantee the cytoactivity of ASCs, indicating that it is feasible to encapsulate stem cells and give full play to their functions using C-ES. Furthermore, making full use of cells at wound sites, such as platelets, fibroblasts and endothelial cells, is the key to promoting wound healing.
Migration and growth of cells in scaffolds
ECM, a noncellular component found in all tissues and organs, is made up of water, proteins and polysaccharides and is essential for the survival of cells [118]. ECM also plays an important role in structural support, signal transduction, etc. (Figure 5). Interestingly, C-ES, an effective method to simulate ECM structure in vitro, can facilitate the adhesion, proliferation and differentiation of cells [119,120]. Wound healing is inseparable from the migration and infiltration of cells. Fiber scaffolds prepared by C-ES should not only satisfy the migration and movement of cells inside the scaffolds but also facilitate the attachment and growth of cells on the tissue surface [121]. The morphology of nanofibers has a significant effect on the migration and growth of wound-healing-related cells, including keratinocytes and fibroblasts [122]. Likewise, the porosity, orientation and spatial size of C-ES nanofiber scaffolds have a great influence on cell migration and infiltration [12]. For instance, studies have shown that cross-linked nanofibers are more conducive to the migration of fibroblasts and keratinocytes in vivo than coaligned and randomly aligned nanofibers [108]. However, the cell types and characteristics of specific tissues should also be taken into consideration during comparisons [123]. For example, in the repair of nerve tissue, oriented nanofiber scaffolds are more conducive to the attachment and growth of nerve cells. When embedded in randomly aligned nanofibers, cells tend to migrate in all possible directions, resulting in short-distance translocations [8].
Figure 5.
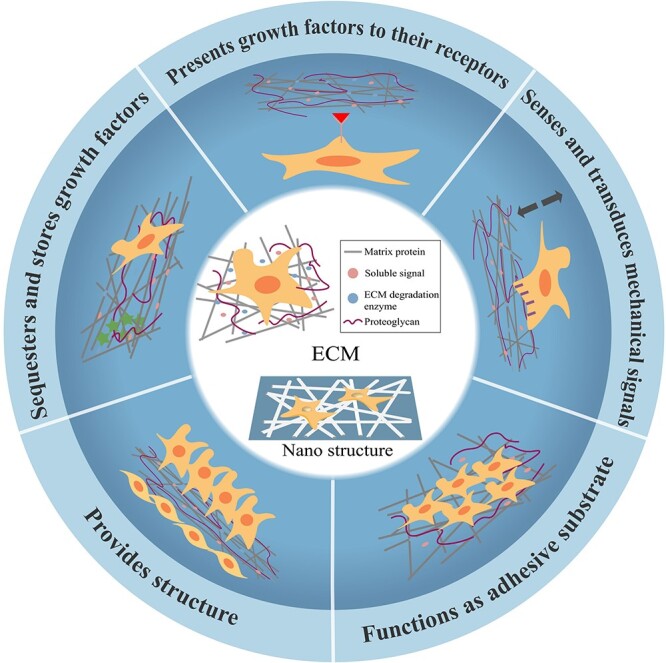
Schematic diagram of the structure and function of the ECM prepared by C-ES. The ECM functions as an adhesive substrate, provides structure, presents growth factors to their receptors, sequesters and stores growth factors, and senses and transduces mechanical signals. ECM extracellular matrix, C-ES cell electrospinning
Cell migration and growth take place in a 3D structure. Chen et al. [124] developed an interconnected 3D network scaffold that promotes effective infiltration and growth of fibroblasts and keratinocytes in vitro. In addition, the porosity and pore size of C-ES nanofiber scaffolds have a great influence on cell migration and infiltration. It has been reported that human dermal fibroblasts are 6–20 μm in diameter, whereas fibers with larger pore sizes (>20 μm) cause the cells to grow along them rather than being distributed inside the 3D structure [125]. Specifically, when the pores are small, cells will be distributed and grow on the surface of the fiber during the process of wound healing. Conversely, when the porosity and pore size are large, cells proliferate and deposit rapidly [126]. Therefore, it is of great significance to prepare C-ES nanofiber scaffolds conducive to cell migration and growth according to the characteristics of wound-healing-related cells and tissues.
Design of drug-loading in C-ES
Infection is a common cause of wound-healing failure. Common pathogens of traumatic wound infection are gram-positive Staphylococcus aureus and gram-negative Pseudomonas aeruginosa [127]. Various antibiotics, such as sulfonamides, penicillin, streptomycin, tetracycline and vancomycin, have been used clinically for infection control [128]. However, overuse of various antibiotics may create a crisis of drug resistance. Fortunately, the evenly arranged nanofiber scaffolds made by ES can effectively reduce drug absorption and deliver drugs to the site of infection at a constant rate, thus overcoming drug resistance to some extent [2,129]. Sustained release of drugs for anti-inflammatory and analgesic purposes, such as aspirin, ibuprofen, lidocaine and others, can be achieved by encapsulating the drugs in nanofibers. In addition, botanical drugs, such as metformin, cannabinoids and curcumin, have been introduced in research on nanofiber scaffolds for wound healing or tissue engineering [130,131]. In recent years, nanoenzymes have also attracted much attention in the field of wound anti-infection [132,133]. However, nanoenzymes mainly kill bacteria by producing reactive oxygen species, which means that their application in C-ES is bound to have adverse effects on cytoactivity.
A variety of drugs have been employed in the treatment of wound infection, but the application of relevant drugs in C-ES is still unexplored. This is largely attributed to the adverse effects of drugs on the maintenance of cytoactivity and the preparation of nanofibers. In addition, further research on the loading of drugs in the C-ES process remains to be conducted. Based on the basic process of C-ES, Figure 6 shows several possible drug-loading methods for reference, among which coaxial ES is the most promising. By placing the cells and drugs in the inner layer and the polymer solution in the outer shell, coaxial ES not only reduces the effects of the electric field on the cells but also achieves constant release of the inner drugs.
Figure 6.
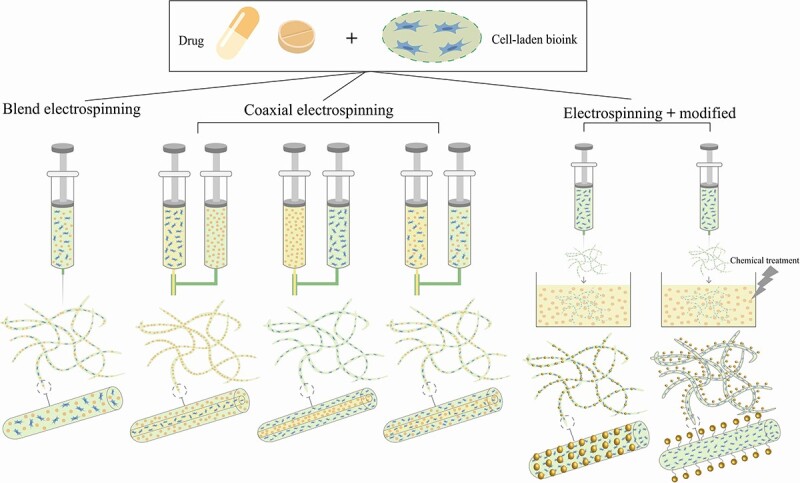
C-ES techniques for loading of drugs. Blending electrospinning: cells are electrospun with a polymer solution. Coaxial electrospinning: depending on demand, cells can be spun into the inner shell, the outer shell, or both. Electrospinning and modified: nanofiber scaffolds can be loaded with drugs either by physical adsorption or by chemical crosslinking. C-ES cell electrospinning
Present challenges and future prospects
Challenges and possible solutions
A few shortcomings may limit the application of C-ES-based nanofiber scaffolds in wound repair. First, maintenance of cytoactivity and cell extraction should be achieved, which requires assessment of the degree of damage to the patient’s skin tissue, the cell culture and the uniformity of encapsulation of cells into the nanofiber scaffold. To solve this problem, a long period of discussion and research is needed to form a professional treatment model, which limits the development and clinical application of C-ES in tissue repair and regeneration. Furthermore, platforms for coculture or multicellular culture should be implemented to simulate the physiological function of complex tissues, i.e. vascularized muscle structures or skin containing multiple cell types, such as keratinocytes and fibroblasts [11]. Second, most studies have been conducted in vitro (in mice, rats and rabbits), which means that their outcomes might differ in histology and pathology from those in humans [134]. Therefore, it is necessary to conduct similar studies in large animals, such as primates, to further validate the clinical effects of C-ES nanofiber scaffolds on tissue repair and regeneration. Third, the key to C-ES lies in the successful acquisition and encapsulation of cells, which makes it difficult to commercialize. A perfect clinical application system based in medical institutions needs to be developed to address this issue. However, with the development of stem-cell transplantation technology and the progress of tissue engineering, ranging from the selection of cells to the synthesis of multifunctional nanofiber scaffolds, the disadvantages of C-ES will be continuously improved and remedied [135]. Fourth, the mechanical strength of C-ES nanofiber scaffolds decreases with time while maintaining cytoactivity. This problem can be solved through other tissue-engineering techniques. For instance, by combining 3D printing and freeze-drying techniques, fiber pads with excellent mechanical properties can be prepared to act as receiving plates. In recent years, the rise of metal nanoparticles has provided a new option for the preparation of antibacterial nanofiber scaffolds. For example, Ag, Fe, Au and Pt ions have excellent antibacterial properties, while Ca, Ti, Mg and Zn ions have good anti-inflammatory and osteogenic effects. In addition, copper-based nanomaterials with enzyme-like activity have shown great potential in the development of novel broad-spectrum antibiotics [136]. By incorporating gold nanoparticles (AuNPs) coated with the small molecule 6-aminopenicillin into polymer ES fibers, some researchers successfully prepared products with significant antibacterial activity against multidrug-resistant bacteria [137]. Zhang et al. [138] prepared nickeliferous hydrogel nanorods with antioxidant and anti-inflammatory properties, which accelerated wound healing in mice. Considering the complexity of the internal physiological environment of the human body and the possibility of uncontrolled release of metal ions, issues of biological security should be considered [139]. In this regard, the design of nanofiber scaffolds needs to be perfected to allow ions to be released in a controlled manner within a safe and treatment-relevant range. It is hoped that these challenges can be addressed through multidisciplinary collaboration among clinicians, biologists and materials engineers.
Future prospects
C-ES shows potential application prospects in the biomedical field, and the trend of skin-tissue engineering is to develop multifunctional nanofiber scaffolds through C-ES in the future. The development of stem-cell research has brought hope for the treatment of chronic wounds [140]. For instance, simulated ECM prepared through C-ES can address the lack of functional ECM at diabetic wound sites. Moreover, cells loaded on nanofiber scaffolds can compensate for the shortage of cells in the defect area, helping to repair wounds. Anti-infection is the basis of normal wound healing. For wound healing in the future, with the help of C-ES technology, preparing fiber scaffolds with antibacterial properties by incorporating effective drugs and nanoparticles will be the trend. In the C-ES process, drugs can be delivered to the wound area at a constant rate by preparing a coaxial core–shell structure containing nanoparticles and drugs, thus effectively solving the problem of sudden drug release in simple blended ES [141] (Figure 7).
Figure 7.
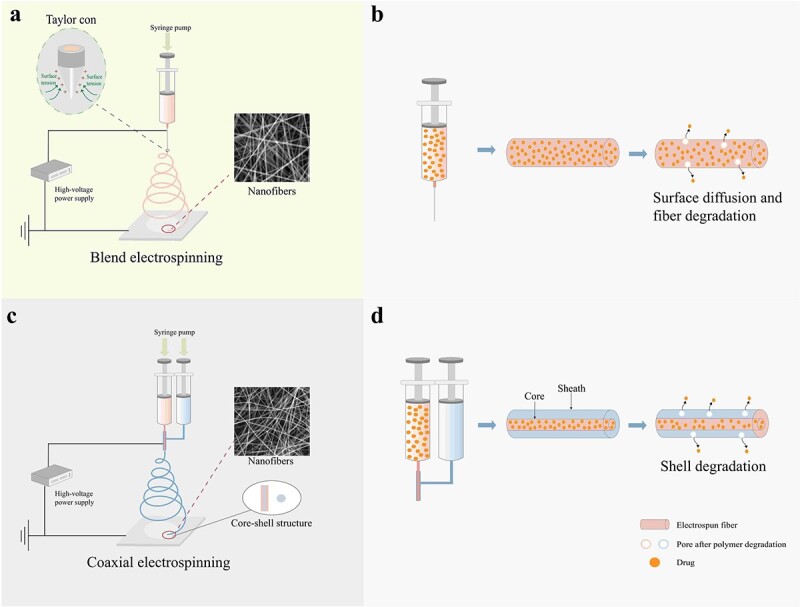
Schematic diagrams of blend electrospinning and coaxial electrospinning and illustration of the release of drugs from electrospun fibers. (a) Blend electrospinning.Taylor cone:when the electric fied is applied between the nozzle tip and the grounded collector, a microsphere is formed at the end of the nozzle.As the strength of the electric field increases, the microsphere at the tip elongates forming a conical shape called the taylor cone. (b) Drugs released through simple surface diffusion and pores caused by the degradation of the fiber. (c) Coaxial electrospinning. (d) Drugs in the core–shell structure drug-loaded fiber are released depending on the degradation of the shell layer
In addition, via modification with various bioactive materials, drugs, growth factors and technologies, nanofiber scaffolds can be endowed with multifunctional properties. For instance, chitosan modified with quaternary ammonium salt has significant anti-S. aureus and anti-Escherichia coli properties, thus improving the anti-infection ability of wound dressings [142]. Graphene is an ideal scaffold for wound healing due to its high strength, high elasticity, light weight, antibacterial properties and ability to support adipose stem cells [143,144]. GEL and collagen bioactive substances have hydrophilic properties and can effectively improve cytoactivity when combined with other biological materials in the process of C-ES. Platelet therapy is one of the potential methods for wound treatment. The prepared concentrate growth factors (CGF) is rich in growth factors and stem cells and has a dense grid structure. Compared with platelet-rich fibrin, it has greater tensile strength and viscosity, and its slow release of growth factors is closer to the natural process of tissue healing [145]. The application of CGF in C-ES may also be a bright spot in the future. In addition, various growth factors that are crucial for cell proliferation and differentiation are activated and play a role in different stages of wound healing. Some scholars collected the fibers produced by C-ES through an electriferous network, which was then periodically dipped into a culture medium containing various growth factors [29]. In this way, they found that cytoactivity during the C-ES process could be maintained well. The combination of a range of other technologies, including solvent casting, new membrane–liquid interface culture, salt immersion, spin coating, ES, vacuum freeze-drying and 3D printing, with C-ES can provide various options for the preparation of multifunctional fiber scaffolds. Among them, 3D printing is a technology that uses a variety of biological inks at the same time to quickly print new composite scaffolds with different gradient structures to achieve the spatiotemporal integration of bionic scaffolds in regeneration [124]. Yeo and Kim [26] combined 3D printing and C-ES, achieving high mechanical strength and ECM-like structures beneficial for bone regeneration. Therefore, this type of combination, which constructs a double-layer composite membrane, is a promising method for the preparation of nanofiber scaffolds (Figure 8).
Figure 8.
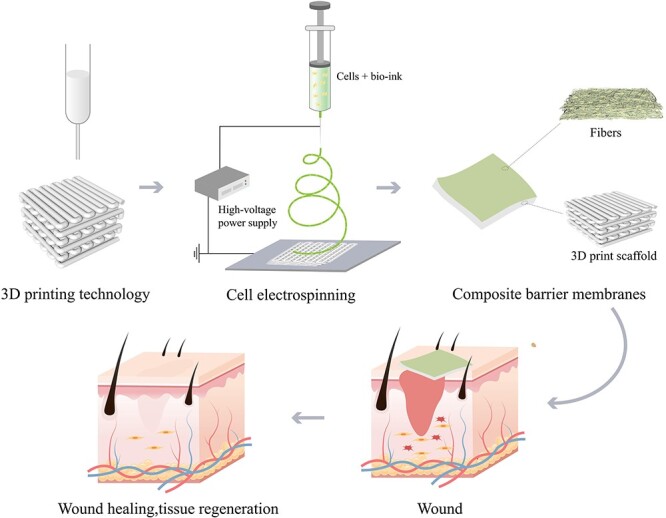
Schematic diagram of 3D printing and C-ES combined for wound healing. C-ES cell electrospinning
In terms of maintenance of cytoactivity, triboelectric nanogenerators may be a good choice as a power source for cell printing technology in the future [146]. Target products with different properties can be obtained by adjusting the parameters, the ratios among polymers and the reaction conditions. With the help of multiple nozzles, coaxial ES or blending ES, different fiber film dressings can be prepared. For example, Zhao et al. [147] successfully developed a novel and efficient coaxial electrospray technology using an alginate hydrogel shell and mouse embryonic stem cell aqueous solution (instead of hydrogel) as the core to prepare microcapsules. Encapsulated cells can survive and proliferate well. In addition, according to the characteristics of the process of wound healing, the preparation of smart dressings that can cover the wound during the whole healing process also has a bright future. For example, the healing process can be controlled by inducing healing-ion release in response to wound temperature or PH [148]. In conclusion, with the application of C-ES and the in-depth study of the wound healing process providing novel ideas and guiding principles, more practical alternative products for wound treatment may be discovered in the future.
Conclusions
Nanofiber scaffolds made by ES show promise in the promotion of skin wound healing and the regeneration and repair of blood vessels, muscle, skeleton and nerve tissue. However, there is still a long way to go before C-ES can achieve the maintenance of cytoactivity and be applied in the field of skin tissue engineering. Further in-depth research needs to be conducted. It is hoped that C-ES technology will advance and more breakthroughs will be made in the treatment of burns and wounds in the future.
Contributor Information
Zonghao Hu, Department of Implantology, School/Hospital of Stomatology, Lanzhou University, Lanzhou, 730000, China.
Zishun Qin, Department of Implantology, School/Hospital of Stomatology, Lanzhou University, Lanzhou, 730000, China; The First Clinical Medical College, Lanzhou University, Lanzhou, 730000, China.
Yue Qu, Department of Implantology, School/Hospital of Stomatology, Lanzhou University, Lanzhou, 730000, China.
Feng Wang, Department of Implantology, School/Hospital of Stomatology, Lanzhou University, Lanzhou, 730000, China.
Benheng Huang, Department of Implantology, School/Hospital of Stomatology, Lanzhou University, Lanzhou, 730000, China.
Gaigai Chen, Department of Implantology, School/Hospital of Stomatology, Lanzhou University, Lanzhou, 730000, China; The First Clinical Medical College, Lanzhou University, Lanzhou, 730000, China.
Xiaoyuan Liu, Department of Implantology, School/Hospital of Stomatology, Lanzhou University, Lanzhou, 730000, China; The First Clinical Medical College, Lanzhou University, Lanzhou, 730000, China.
Lihua Yin, Department of Implantology, School/Hospital of Stomatology, Lanzhou University, Lanzhou, 730000, China; The First Clinical Medical College, Lanzhou University, Lanzhou, 730000, China.
Abbreviations
ASCs: Adipose-derived stem cells; BG: Bioactive glass; BMSCs: Bone marrow mesenchymal stem cells; C2C12s: Mouse myoblasts; C-ES: Cell electrospinning; CGF: Concentrate growth factors; CS: Chitosan; ECM: Extracellular matrix; ES: Electrospinning; HA: Hyaluronic acid; HUVECs: Human umbilical vein endothelial cells; SA: Sodium alginate; SF: Silk fibroin; PCL: Polycaprolactone; PDMS: Polydimethylsiloxane; PEO: Polyethylene oxide; PLLA: Poly(L-lactic acid); PLGA: Polylactic acid-glycolic acid; PU: Polyurethane; PVA: Polyvinyl alcohol; SMC: smooth muscle cell.
Authors’ contributions
All authors wrote the manuscript and have read and approved the final manuscript.
Funding
This research was supported by the Competitive Projects for Science and Technology Innovation and Development of Gansu Province (2018ZX-10), Gansu Provincial Key Research and Development Project-International Science and Technology Cooperation (22YF7WA013), Gansu Provincial Department of Science and Technology, Gansu Provincial Science and Technology Program (Nature Science Foundation)-Excellent Doctoral Program (22JR5RA893), Lanzhou Talent Innovation and Venture Project (2017-RC-31), CSA West China Clinical Research Fund (CSA-W2022-11), Clinical Research Project of Hospital of Stomatology Lanzhou University (lzukqky-2022-t06).
Conflict of interest
None declare.
References
- 1. Farahani M, Shafiee A. Wound healing: from passive to smart dressings. Adv Healthc Mater 2021; 10: e2100477. 10.1002/adhm.202100477. [DOI] [PubMed] [Google Scholar]
- 2. Gao C, Zhang L, Wang J, Jin M, Tang Q, Chen Z, et al. Electrospun nanofibers promote wound healing: theories, techniques, and perspectives. J Mater Chem B. 2021;9:3106–30. [DOI] [PubMed] [Google Scholar]
- 3. Enoch S, Grey JE, Harding KG. Recent advances and emerging treatments. BMJ. 2006;332:962–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bandyk DF. The diabetic foot: pathophysiology, evaluation, and treatment. Semin Vasc Surg. 2018;31:43–8. [DOI] [PubMed] [Google Scholar]
- 5. Pazyar N, Yaghoobi R, Rafiee E, Mehrabian A, Feily A. Skin wound healing and phytomedicine: a review. Skin Pharmacol Physiol. 2014;27:303–10. [DOI] [PubMed] [Google Scholar]
- 6. Krishnan R, Rajeswari R, Venugopal J, Sundarrajan S, Sridhar R, Shayanti M, et al. Polysaccharide nanofibrous scaffolds as a model for in vitro skin tissue regeneration. J Mater Sci Mater Med. 2012;23:1511–9. [DOI] [PubMed] [Google Scholar]
- 7. Pereira RF, Barrias CC, Granja PL, Bartolo PJ. Advanced biofabrication strategies for skin regeneration and repair. Nanomedicine (Lond). 2013;8:603–21. [DOI] [PubMed] [Google Scholar]
- 8. Xue J, Wu T, Dai Y, Xia Y. Electrospinning and electrospun nanofibers: methods, materials, and applications. Chem Rev. 2019;119:5298–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang Y, Lim CT, Ramakrishna S, Huang ZM. Recent development of polymer nanofibers for biomedical and biotechnological applications. J Mater Sci Mater Med. 2005;16:933–46. [DOI] [PubMed] [Google Scholar]
- 10. Townsend-Nicholson A, Jayasinghe SN. Cell electrospinning: a unique biotechnique for encapsulating living organisms for generating active biological microthreads/scaffolds. Biomacromolecules. 2006;7:3364–9. [DOI] [PubMed] [Google Scholar]
- 11. Hong J, Yeo M, Yang GH, Kim G. Cell-electrospinning and its application for tissue engineering. Int J Mol Sci. 2019;20:6208. 10.3390/ijms20246208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Qin S, Clark RA, Rafailovich MH. Establishing correlations in the en-mass migration of dermal fibroblasts on oriented fibrillar scaffolds. Acta Biomater. 2015;25:230–9. [DOI] [PubMed] [Google Scholar]
- 13. Zhang P, Zou B, Liou YC, Huang C. The pathogenesis and diagnosis of sepsis post burn injury. Burns Trauma. 2021,9: tkaa047. 10.1093/burnst/tkaa047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pham QP, Sharma U, Mikos AG. Electrospinning of polymeric nanofibers for tissue engineering applications: a review. Tissue Eng. 2006;12:1197–211. [DOI] [PubMed] [Google Scholar]
- 15. Chen H, Liu Y, Hu Q. A novel bioactive membrane by cell electrospinning. Exp Cell Res. 2015;338:261–6. [DOI] [PubMed] [Google Scholar]
- 16. Yeo M, Kim GH. Anisotropically aligned cell-laden Nanofibrous bundle fabricated via cell electrospinning to regenerate skeletal muscle tissue. Small. 2018;14:e1803491. 10.1002/smll.201803491. [DOI] [PubMed] [Google Scholar]
- 17. Bhardwaj N, Kundu SC. Electrospinning: a fascinating fiber fabrication technique. Biotechnol Adv. 2010;28:325–47. [DOI] [PubMed] [Google Scholar]
- 18. Gupta, P, Elkins C, Long TE, Wilkes GL. Electrospinning of linear homopolymers of poly (methyl methacrylate): exploring relationships between fiber formation, viscosity, molecular weight and concentration in a good solvent. Polymer 2005; 46:4799–810. 10.1016/j.polymer.2005.04.021. [DOI] [Google Scholar]
- 19. Demir M, Yilgor I, Yilgor E, Erman B. Electrospinning of polyurethane fibers. Polymer. 2002;43:3303–9. [Google Scholar]
- 20. Son W, Youk J, Lee T, Park W. The effects of solution properties and polyelectrolyte on electrospinning of ultrafine poly (ethylene oxide) fibers. Polymer. 2004;45:2959–66. [Google Scholar]
- 21. Jun Z, Hou H, Schaper A, Wendorff J, Greiner A. Poly-L-lactide nanofibers by electrospinning — influence of solution viscosity and electrical conductivity on fiber diameter and fiber morphologye. Polymer. 2003;9:1–9. [Google Scholar]
- 22. Gopinathan J, Noh I. Recent trends in bioinks for 3D printing. Biomater Res. 2018;22:11. 10.1186/s40824-018-0122-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jiang H, Fang D, Hsiao B, Chu B, Chen W. Optimization and characterization of dextran membranes prepared by electrospinning. Biomacromolecules. 2004;5:326–33. [DOI] [PubMed] [Google Scholar]
- 24. Zuo W, Zhu M, Yang W, Yu H, Chen Y, Zhang Y. Experimental study on relationship between jet instability and formation of beaded fibers during electrospinning. Polym Eng Sci. 2005;45:704–9. [Google Scholar]
- 25. Mit-uppatham C, Nithitanakul M, Supaphol P. Ultrafine electrospun polyamide-6 fibers: effect of solution conditions on morphology and average fiber diameter. Macromol Chem Phys. 2004;205:2327–38. [Google Scholar]
- 26. Yeo M, Kim G. Fabrication of cell-laden electrospun hybrid scaffolds of alginate-based bioink and PCL microstructures for tissue regeneration. Chem Eng J. 2015;275:27–35. [Google Scholar]
- 27. Ki C, Baek D, Gang K, Lee K, Um L, Park Y. Characterization of gelatin nanofiber prepared from gelatin-formic acid solution. Polymer. 2005;46:5094–102. [Google Scholar]
- 28. Reneker D, Yarin A, Fong H, Koombhongse S. Bending instability of electrically charged liquid jets of polymer solutions in electrospinning. J Appl Phys. 2000;87:4531–47. [Google Scholar]
- 29. Jayasinghe SN. Cell electrospinning: a novel tool for functionalising fibres, scaffolds and membranes with living cells and other advanced materials for regenerative biology and medicine. Analyst. 2013;138:2215–23. [DOI] [PubMed] [Google Scholar]
- 30. Huang Z, Zhang Y, Kotaki M, Ramakrishna S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. ComposSciTechnol. 2003;63:2223–53. [Google Scholar]
- 31. Lee K, Kim H, Khil M, Ra Y, Lee D. Characterization of nano-structured poly (ε-caprolactone) nonwoven mats via electrospinning. Polymer. 2003;44:1287–94. [Google Scholar]
- 32. Zhao Y, Li Y, Mao S, Sun W, Yao R. The influence of printing parameters on cell survival rate and printability in microextrusion-based 3D cell printing technology. Biofabrication. 2015;7:045002. 10.1088/1758-5090/7/4/045002. https://doi. [DOI] [PubMed] [Google Scholar]
- 33. Ouyang L, Yao R, Zhao Y, Sun W. Effect of bioink properties on printability and cell viability for 3D bioplotting of embryonic stem cells. Biofabrication. 2016;8:035020. 10.1088/1758-5090/8/3/035020. [DOI] [PubMed] [Google Scholar]
- 34. Tripatanasuwan S, Zhong Z, Reneker D. Effect of evaporation and solidification of the charged jet in electrospinning of poly (ethylene oxide) aqueous solution. Polymer. 2007;48:5742–6. [Google Scholar]
- 35. Yarin A, Koombhongse S, Reneker D. Bending instability in electrospinning of nanofibers. J Appl Phys. 2001;89:3018–26. [Google Scholar]
- 36. Xu S, Lu T, Yang L, Luo S, Wang Z, Ye C. In situ cell electrospun using a portable handheld electrospinning apparatus for the repair of wound healing in rats. Int Wound J. 2022;19:1693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Canbolat MF, Tang C, Bernacki SH, Pourdeyhimi B, Khan S. Mammalian cell viability in electrospun composite nanofiber structures. Macromol Biosci. 2011;11:1346–56. [DOI] [PubMed] [Google Scholar]
- 38. Williams D, Thayer P, Martinez H, Gatenholm E, Khademhosseini A. A perspective on the physical, mechanical and biological specifications of bioinks and the development of functional tissues in 3D bioprinting. Bioprinting. 2018;9:19–36. [Google Scholar]
- 39. Kim Y, Lee H, Kim G. Strategy to achieve highly porous/biocompatible macroscale cell blocks, using a collagen/genipin-bioink and an optimal 3D printing process. ACS Appl Mater Interfaces. 2016;8:32230–40. [DOI] [PubMed] [Google Scholar]
- 40. Ding B, Kim H, Lee S, Lee D, Choi K. Preparation and characterization of nanoscaled poly (vinyl alcohol) fibers via electrospinning. Fiber Polym. 2002;3:73–9. [Google Scholar]
- 41. Yang G, Li H, Yang J, Wan J, Yu D. Influence of working temperature on the formation of electrospun polymer nanofibers. Nanoscale Res Lett. 2017;12:55. 10.1186/s11671-016-1824-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bonino C, Efimenko K, Jeong S, Krebs M, Alsberg E, Khan S. Three-dimensional electrospun alginate nanofiber mats via tailored charge repulsions. Small. 2012;8:1928–36. [DOI] [PubMed] [Google Scholar]
- 43. Ehler E, Jayasinghe SN. Cell electrospinning cardiac patches for tissue engineering the heart. Analyst. 2014;139:4449–52. [DOI] [PubMed] [Google Scholar]
- 44. Jayasinghe SN, Qureshi AN, Eagles PA. Electrohydrodynamic jet processing: an advanced electric-field-driven jetting phenomenon for processing living cells. Small. 2006;2:216–9. [DOI] [PubMed] [Google Scholar]
- 45. Kempski H, Austin N, Roe A, Chatters S, Jayasinghe SN. Pilot study to investigate the possibility of cytogenetic and physiological changes in bio-electrosprayed human lymphocyte cells. Regen Med. 2008;3:343–9. [DOI] [PubMed] [Google Scholar]
- 46. Bartolovic K, Mongkoldhumrongkul N, Waddington SN, Jayasinghe SN, Howe SJ. The differentiation and engraftment potential of mouse hematopoietic stem cells is maintained after bio-electrospray. Analyst. 2010;135:157–64. [DOI] [PubMed] [Google Scholar]
- 47. Jayasinghe SN, Warnes G, Scotton CJ. Bio-electrosprayed living composite matrix implanted into mouse models. Macromol Biosci. 2011;11:1364–9. [DOI] [PubMed] [Google Scholar]
- 48. Poncelet D, de Vos P, Suter N, Jayasinghe SN. Bio-electrospraying and cell electrospinning: progress and opportunities for basic biology and clinical sciences. Adv Healthc Mater. 2012;1:27–34. [DOI] [PubMed] [Google Scholar]
- 49. Jayasinghe SN, Auguste J, Scotton CJ. Platform technologies for directly reconstructing 3D living biomaterials. Adv Mater. 2015;27:7794–9. [DOI] [PubMed] [Google Scholar]
- 50. Zamani R, Aval SF, Pilehvar-Soltanahmadi Y, Nejati-Koshki K, Zarghami N. Recent dvances in cell Electrospining of natural and synthetic nanofibers for regenerative medicine. Drug Res (Stuttg). 2018;68:425–35. [DOI] [PubMed] [Google Scholar]
- 51. Nosoudi N, Oommen AJ, Stultz S, Jordan M, Aldabel S, Hohne C, et al. Electrospinning live cells using gelatin and pullulan. Bioengineering (Basel). 2020;7:21. 10.3390/bioengineering7010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Stojanov S, Berlec A. Electrospun nanofibers as carriers of microorganisms, stem cells, proteins, and nucleic acids in therapeutic and other applications. Front Bioeng Biotechnol. 2020;8:130. 10.3389/fbioe.2020.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yeo M, Yoon JW, Park GT, Shin SC, Song YC, Cheon YI, et al. Esophageal wound healing by aligned smooth muscle cell-laden nanofibrous patch. Mater Today Bio. 2023;19:100564. 10.1016/j.mtbio.2023.100564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ferreira A, Gentile P, Chiono V, Ciardelli G. Collagen for bone tissue regeneration. Acta Biomater. 2012;8:3191–200. [DOI] [PubMed] [Google Scholar]
- 55. Dong C, Lv Y. Application of collagen scaffold in tissue engineering: recent advances and new perspectives. Polymers (Basel). 2016;8:42. 10.3390/polym8020042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ross-Murphy S. Structure and rheology of gelatin gels: recent progress. Polym. 1992;33:2622–7. [Google Scholar]
- 57. Yeo M, Kim G. Micro/nano-hierarchical scaffold fabricated using a cell electrospinning/3D printing process for co-culturing myoblasts and HUVECs to induce myoblast alignment and differentiation. Acta Biomater. 2020;107:102–14. [DOI] [PubMed] [Google Scholar]
- 58. Jayasinghe SN, Irvine S, McEwan JR. Cell electrospinning highly concentrated cellular suspensions containing primary living organisms into cell-bearing threads and scaffolds. Nanomedicine (Lond). 2007;2:555–67. [DOI] [PubMed] [Google Scholar]
- 59. Patel P, Irvine S, McEwan JR, Jayasinghe SN. Bio-protocols for directly forming active encapsulations containing living primary cells. Soft Matter. 2008;4:1219–29. [DOI] [PubMed] [Google Scholar]
- 60. Shih Y, Yang J, Li S, Yang W, Chen C. Bio-electrospinning of poly (l-lactic acid) hollow fibrous membrane. Text Res J. 2012;82:602–12. [Google Scholar]
- 61. Guo Y, Gilbert-Honick J, Somers SM, Mao HQ, Grayson WL. Modified cell-electrospinning for 3D myogenesis of C2C12s in aligned fibrin microfiber bundles. Biochem Biophys Res Commun. 2019;516:558–64. [DOI] [PubMed] [Google Scholar]
- 62. Balagangadharan K, Dhivya S, Selvamurugan N. Chitosan based nanofibers in bone tissue engineering. Int J Biol Macromol. 2017;104:1372–82. [DOI] [PubMed] [Google Scholar]
- 63. Augustine R, Rehman SR, Ahmed R, Zahid A, Sharifi M, Falahati M, et al. Electrospun chitosan membranes containing bioactive and therapeutic agents for enhanced wound healing. Int J Biol Macromol. 2020;156:153–70. [DOI] [PubMed] [Google Scholar]
- 64. Litwiniuk M, Krejner A, Speyrer M, Gauto A, Grzela T. Hyaluronic acid in inflammation and tissue regeneration. Wounds. 2016;28:78–88. [PubMed] [Google Scholar]
- 65. Yang H, Song L, Zou Y, Sun D, Wang L, Yu Z, et al. Role of hyaluronic acids and potential as regenerative biomaterials in Wound healing. ACS Appl Bio Mater. 2021;4:311–24. [DOI] [PubMed] [Google Scholar]
- 66. Farokhi M, Mottaghitalab F, Fatahi Y, Khademhosseini A, Kaplan DL. Overview of silk fibroin use in Wound dressings. Trends Biotechnol. 2018;36:907–22. [DOI] [PubMed] [Google Scholar]
- 67. Kamalathevan P, Ooi P, Loo Y. Silk-based biomaterials in cutaneous Wound healing: a systematic review. Adv Skin Wound Care. 2018;31:565–73. [DOI] [PubMed] [Google Scholar]
- 68. Zhang Q, Qiao Y, Li C, Lin J, Han H, Li X, et al. Chitosan/gelatin-tannic acid decorated porous tape suture with multifunctionality for tendon healing. Carbohydr Polym. 2021;268:118246. 10.1016/j.carbpol.2021.118246. [DOI] [PubMed] [Google Scholar]
- 69. Zhou K, Zhang Z, Xue J, Shang J, Ding D, Zhang W, et al. Hybrid ag nanoparticles/polyoxometalate-polydopamine nano-flowers loaded chitosan/gelatin hydrogel scaffolds with synergistic photothermal/chemodynamic/ag anti-bacterial action for accelerated wound healing. Int J Biol Macromol. 2022;221:135–48. [DOI] [PubMed] [Google Scholar]
- 70. Sakthiguru N, Sithique M. Fabrication of bioinspired chitosan/gelatin/allantoin biocomposite film for wound dressing application. Int J Biol Macromol. 2020;152:873–83. [DOI] [PubMed] [Google Scholar]
- 71. Paskal M, Paskal W, Pietruski P, Wlodarski P. Polyethylene glycol: the future of posttraumatic nerve repair? Systemic review. Int J Mol Sci. 2019;20:1478. 10.3390/ijms20061478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Zhang X, Yang D, Nie J. Chitosan/polyethylene glycol diacrylate films as potential wound dressing material. Int J Biol Macromol. 2008;43:456–62. [DOI] [PubMed] [Google Scholar]
- 73. Heo DN, Yang DH, Lee JB, Bae MS, Kim JH, Moon SH, et al. Burn-wound healing effect of gelatin/polyurethane nanofiber scaffold containing silver-sulfadiazine. J Biomed Nanotechnol. 2013;9:511–5. [DOI] [PubMed] [Google Scholar]
- 74. Kim SE, Heo DN, Lee JB, Kim JR, Park SH, Jeon SH, et al. Electrospun gelatin/polyurethane blended nanofibers for wound healing. Biomed Mater. 2009;4:044106. 10.1088/1748-6041/4/4/044106. [DOI] [PubMed] [Google Scholar]
- 75. Zhang P, Jiang Y, Liu D, Liu Y, Ke Q, Xu H. A bioglass sustained-release scaffold with ECM-like structure for enhanced diabetic wound healing. Nanomedicine (Lond). 2020;15:2241–53. [DOI] [PubMed] [Google Scholar]
- 76. Rajzer I, Dziadek M, Kurowska A, Cholewa-Kowalska K, Ziąbka M, Menaszek E, et al. Electrospun polycaprolactone membranes with Zn-doped bioglass for nasal tissues treatment. J Mater Sci Mater Med. 2019;30:80. 10.1007/s10856-019-6280-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Zhao W, Li J, Jin K, Liu W, Qiu X, Li C. Fabrication of functional PLGA-based electrospun scaffolds and their applications in biomedical engineering. Mater Sci Eng C Mater Biol Appl. 2016;59:1181–94. [DOI] [PubMed] [Google Scholar]
- 78. Wu L, Li H, Li S, Li X, Yuan X, Li X, et al. Composite fibrous membranes of PLGA and chitosan prepared by coelectrospinning and coaxial electrospinning. J Biomed Mater Res A. 2010;92:563–74. [DOI] [PubMed] [Google Scholar]
- 79. Zhong G, Qiu M, Zhang J, Jiang F, Yue X, Huang C, et al. Fabrication and characterization of PVA@PLA electrospinning nanofibers embedded with Bletilla striata polysaccharide and Rosmarinic acid to promote wound healing. Int J Biol Macromol. 2023;234:123693. 10.1016/j.ijbiomac.2023.123693. [DOI] [PubMed] [Google Scholar]
- 80. Li L, Liu X, Niu Y, Ye J, Huang S, Liu C, et al. Synthesis and wound healing of alternating block polyurethanes based on poly(lactic acid) (PLA) and poly(ethylene glycol) (PEG). J Biomed Mater Res B Appl Biomater. 2017;105:1200–9. [DOI] [PubMed] [Google Scholar]
- 81. Azari A, Golchin A, Mahmoodinia Maymand M, Mansouri F, Ardeshirylajimi A. Electrospun Polycaprolactone nanofibers: current research and applications in biomedical application. Adv Pharm Bull. 2022;12:658–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Croisier F, Atanasova G, Poumay Y, Jérôme C. Polysaccharide-coated PCL nanofibers for wound dressing applications. Adv Healthc Mater. 2014;3:2032–9. [DOI] [PubMed] [Google Scholar]
- 83. Ahmed S, Ikram S. Chitosan based scaffolds and their applications in Wound healing. Achievements in the Life Sciences. 2016;10:27–37. [Google Scholar]
- 84. Singh B, Maharjan S, Cho KH, Cui L, Park IK, Choi YJ, et al. Chitosan-based particulate systems for the delivery of mucosal vaccines against infectious diseases. Int J Biol Macromol. 2018;110:54–64. [DOI] [PubMed] [Google Scholar]
- 85. Ma L, Gao C, Mao Z, Zhou J, Shen J, Hu X, et al. Collagen/chitosan porous scaffolds with improved biostability for skin tissue engineering. Biomaterials. 2003;24:4833–41. [DOI] [PubMed] [Google Scholar]
- 86. Islam MM, Shahruzzaman M, Biswas S, Nurus Sakib M, Rashid TU. Chitosan based bioactive materials in tissue engineering applications-a review. Bioact Mater. 2020;5:164–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Santos TC, Marques AP, Silva SS, Oliveira JM, Mano JF, Castro AG, et al. In vitro evaluation of the behaviour of human polymorphonuclear neutrophils in direct contact with chitosan-based membranes. J Biotechnol. 2007;132:218–26. [DOI] [PubMed] [Google Scholar]
- 88. Rho KS, Jeong L, Lee G, Seo BM, Park YJ, Hong SD, et al. Electrospinning of collagen nanofibers: effects on the behavior of normal human keratinocytes and early-stage wound healing. Biomaterials. 2006;27:1452–61. [DOI] [PubMed] [Google Scholar]
- 89. Chanda A, Adhikari J, Ghosh A, Chowdhury SR, Thomas S, Datta P, et al. Electrospun chitosan/polycaprolactone-hyaluronic acid bilayered scaffold for potential wound healing applications. Int J Biol Macromol. 2018;116:774–85. [DOI] [PubMed] [Google Scholar]
- 90. Sogawa H, Ifuku N, Numata K. 3,4-Dihydroxyphenylalanine (DOPA)-containing silk fibroin: its enzymatic synthesis and adhesion properties. ACS Biomater Sci Eng. 2019;5:5644–51. [DOI] [PubMed] [Google Scholar]
- 91. Kundu B, Rajkhowa R, Kundu SC, Wang X. Silk fibroin biomaterials for tissue regenerations. Adv Drug Deliv Rev. 2013;65:457–70. [DOI] [PubMed] [Google Scholar]
- 92. Roh DH, Kang SY, Kim JY, Kwon YB, Young Kweon H, Lee KG, et al. Wound healing effect of silk fibroin/alginate-blended sponge in full thickness skin defect of rat. J Mater Sci Mater Med. 2006;17:547–52. [DOI] [PubMed] [Google Scholar]
- 93. Yoo CR, Yeo IS, Park KE, Park JH, Lee SJ, Park WH, et al. Effect of chitin/silk fibroin nanofibrous bicomponent structures on interaction with human epidermal keratinocytes. Int J Biol Macromol. 2008;42:324–34. [DOI] [PubMed] [Google Scholar]
- 94. Khorshidi S, Solouk A, Mirzadeh H, Mazinani S, Lagaron JM, Sharifi S, et al. A review of key challenges of electrospun scaffolds for tissue-engineering applications. J Tissue Eng Regen Med. 2016;10:715–38. [DOI] [PubMed] [Google Scholar]
- 95. Saquing CD, Tang C, Monian B, Bonino CA, Manasco JL, Alsberg E, et al. Alginate–polyethylene oxide blend nanofibers and the role of the carrier polymer in electrospinning. Ind Eng Chem Res. 2013;52:8692–704. [Google Scholar]
- 96. Akbarinejad A, Ghoorchian A, Kamalabadi M, Alizadeh N. Electrospun soluble conductive polypyrrole nanoparticles for fabrication of highly selective n-butylamine gas sensor. Sens Actuators B Chem. 2016;236:99–108. [Google Scholar]
- 97. Joshi M, Adak B, Butola BS. Polyurethane nanocomposite based gas barrier films, membranes and coatings: a review on synthesis, characterization and potential applications. Prog Mater Sci. 2018;97:230–82. [Google Scholar]
- 98. Vijayasekaran S, Chirila TV, Hong Y, Tahija SG, Dalton PD, Constable IJ, et al. Poly(1-vinyl-2-pyrrolidinone) hydrogels as vitreous substitutes: histopathological evaluation in the animal eye. J Biomater Sci Polym Ed. 1996;7:685–96. [DOI] [PubMed] [Google Scholar]
- 99. Liu X, Lin T, Fang J, Yao G, Zhao H, Dodson M, et al. In vivo wound healing and antibacterial performances of electrospun nanofibre membranes. J Biomed Mater Res A. 2010;94:499–508. [DOI] [PubMed] [Google Scholar]
- 100. Hiep NT, Lee BT. Electro-spinning of PLGA/PCL blends for tissue engineering and their biocompatibility. J Mater Sci Mater Med. 2010;21:1969–78. [DOI] [PubMed] [Google Scholar]
- 101. Venugopal JR, Zhang Y, Ramakrishna S. In vitro culture of human dermal fibroblasts on electrospun polycaprolactone collagen nanofibrous membrane. Artif Organs. 2006;30:440–6. [DOI] [PubMed] [Google Scholar]
- 102. Cheng Z, Teoh SH. Surface modification of ultra thin poly (epsilon-caprolactone) films using acrylic acid and collagen. Biomaterials. 2004;25:1991–2001. [DOI] [PubMed] [Google Scholar]
- 103. Go EJ, Kang EY, Lee SK, Park S, Kim JH, Park W, et al. An osteoconductive PLGA scaffold with bioactive β-TCP and anti-inflammatory mg(OH)(2) to improve in vivo bone regeneration. Biomater Sci. 2020;8:937–48. [DOI] [PubMed] [Google Scholar]
- 104. El-Rashidy AA, Roether JA, Harhaus L, Kneser U, Boccaccini AR. Regenerating bone with bioactive glass scaffolds: a review of in vivo studies in bone defect models. Acta Biomater. 2017;62:1–28. [DOI] [PubMed] [Google Scholar]
- 105. Homaeigohar S, Li M, Boccaccini AR. Bioactive glass-based fibrous wound dressings. Burns Trauma. 2022;10:tkac038. 10.1093/burnst/tkac038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Kong B, Chen Y, Liu R, Liu X, Liu C, Shao Z, et al. Fiber reinforced GelMA hydrogel to induce the regeneration of corneal stroma. Nat Commun. 2020;11:1435. 10.1038/s41467-020-14887-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. El-Lakany SA, Abd-Elhamid AI, Kamoun EA, El-Fakharany EM, Samy WM, Elgindy NA. α-Bisabolol-loaded cross-linked Zein Nanofibrous 3D-scaffolds for accelerating Wound healing and tissue regeneration in rats. Int J Nanomedicine. 2019;14:8251–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Sun L, Gao W, Fu X, Shi M, Xie W, Zhang W, et al. Enhanced wound healing in diabetic rats by nanofibrous scaffolds mimicking the basketweave pattern of collagen fibrils in native skin. Biomater Sci. 2018;6:340–9. [DOI] [PubMed] [Google Scholar]
- 109. Jayasinghe SN, Townsend-Nicholson A. Stable electric-field driven cone-jetting of concentrated biosuspensions. Lab Chip. 2006;6:1086–90. [DOI] [PubMed] [Google Scholar]
- 110. Sampson SL, Saraiva L, Gustafsson K, Jayasinghe SN, Robertson BD. Cell electrospinning: an in vitro and in vivo study. Small. 2014;10:78–82. [DOI] [PubMed] [Google Scholar]
- 111. Diegelmann RF, Evans MC. Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci. 2004;9:283–9. [DOI] [PubMed] [Google Scholar]
- 112. Ellis S, Lin EJ, Tartar D. Immunology of Wound healing. Current Dermatology Reports. 2018;7:350–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Robson MC, Wound infection. A failure of wound healing caused by an imbalance of bacteria. Surg Clin North Am. 1997;77:637–50. [DOI] [PubMed] [Google Scholar]
- 114. Rajabian MH, Ghorabi GH, Geramizadeh B, Sameni S, Ayatollahi M. Evaluation of bone marrow derived mesenchymal stem cells for full-thickness wound healing in comparison to tissue engineered chitosan scaffold in rabbit. Tissue Cell. 2017;49:112–21. [DOI] [PubMed] [Google Scholar]
- 115. Cui B, Zhang C, Gan B, Liu W, Liang J, Fan Z, et al. Collagen-tussah silk fibroin hybrid scaffolds loaded with bone mesenchymal stem cells promote skin wound repair in rats. Mater Sci Eng C Mater Biol Appl. 2020;109:110611. 10.1016/j.msec.2019.110611. [DOI] [PubMed] [Google Scholar]
- 116. Demidova-Rice TN, Hamblin MR, Herman IM. Acute and impaired wound healing: pathophysiology and current methods for drug delivery, part 2: role of growth factors in normal and pathological wound healing: therapeutic potential and methods of delivery. Adv Skin Wound Care. 2012;25:349–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Zimolag E, Borowczyk-Michalowska J, Kedracka-Krok S, Skupien-Rabian B, Karnas E, Lasota S, et al. Electric field as a potential directional cue in homing of bone marrow-derived mesenchymal stem cells to cutaneous wounds. Biochim Biophys Acta Mol Cell Res. 2017;1864:267–79. [DOI] [PubMed] [Google Scholar]
- 118. Schlie-Wolter S, Ngezahayo A, Chichkov BN. The selective role of ECM components on cell adhesion, morphology, proliferation and communication in vitro. Exp Cell Res. 2013;319:1553–61. [DOI] [PubMed] [Google Scholar]
- 119. Barker TH. The role of ECM proteins and protein fragments in guiding cell behavior in regenerative medicine. Biomaterials. 2011;32:4211–4. [DOI] [PubMed] [Google Scholar]
- 120. Alenghat FJ, Ingber DE. Mechanotransduction: all signals point to cytoskeleton, matrix, and integrins. Sci STKE. 2002;119:pe6. 10.1126/stke.2002.119.pe6. [DOI] [PubMed] [Google Scholar]
- 121. Berthiaume F, Maguire TJ, Yarmush ML. Tissue engineering and regenerative medicine: history, progress, and challenges. Annu Rev Chem Biomol Eng. 2011;2:403–30. [DOI] [PubMed] [Google Scholar]
- 122. Sengupta S, Parent CA, Bear JE. The principles of directed cell migration. Nat Rev Mol Cell Biol. 2021;22:529–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Amores De Sousa MC, Rodrigues CAV, IAF F, Diogo MM, Linhardt RJ, Cabral JMS, et al. Functionalization of electrospun nanofibers and Fiber alignment enhance neural stem cell proliferation and neuronal differentiation. Front Bioeng. Biotechnol. 2020;8:580135. 10.3389/fbioe.2020.580135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Chen H, Peng Y, Wu S, Tan LP. Electrospun 3D fibrous scaffolds for chronic Wound repair. Materials (Basel). 2016;9:272. 10.3390/ma9040272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Lowery JL, Datta N, Rutledge GC. Effect of fiber diameter, pore size and seeding method on growth of human dermal fibroblasts in electrospun poly(epsilon-caprolactone) fibrous mats. Biomaterials. 2010;31:491–504. [DOI] [PubMed] [Google Scholar]
- 126. Torres-Sanchez C, Mclaughlin J, Bonallo R. Effect of pore size, morphology and orientation on the bulk stiffness of a porous Ti35Nb4Sn alloy. J Mater Eng Perform. 2018;27:2899–909. [Google Scholar]
- 127. Dai T, Kharkwal GB, Tanaka M, Huang YY, Bil De Arce VJ, Hamblin MR. Animal models of external traumatic wound infections. Virulence. 2011;2:296–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Ding X, Tang Q, Xu Z, Xu Y, Zhang H, Zheng D, et al. Challenges and innovations in treating chronic and acute wound infections: from basic science to clinical practice.. Burns Trauma. 2022;10:tkac014. 10.1093/burnst/tkac014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Pelgrift RY, Friedman AJ. Nanotechnology as a therapeutic tool to combat microbial resistance. Adv Drug Deliv Rev. 2013;65:1803–15. [DOI] [PubMed] [Google Scholar]
- 130. Kamali A, Oryan A, Hosseini S, Ghanian MH, Alizadeh M, Baghaban Eslaminejad M, et al. Cannabidiol-loaded microspheres incorporated into osteoconductive scaffold enhance mesenchymal stem cell recruitment and regeneration of critical-sized bone defects. Mater Sci Eng C Mater Biol Appl. 2019;101:64–75. [DOI] [PubMed] [Google Scholar]
- 131. Wang H, Liu Y, Cai K, Zhang B, Tang S, Zhang W, et al. Antibacterial polysaccharide-based hydrogel dressing containing plant essential oil for burn wound healing. Burns Trauma. 2021;9:tkab041. 10.1093/burnst/tkab041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Ma Y, Jiang K, Chen H, Shi Q, Liu H, Zhong X, et al. Liquid exfoliation of V(8)C(7) nanodots as peroxidase-like nanozymes for photothermal-catalytic synergistic antibacterial treatment. Acta Biomater. 2022;149:359–72. [DOI] [PubMed] [Google Scholar]
- 133. Jin X, Zhang W, Shan J, He J, Qian H, Chen X, et al. Thermosensitive hydrogel loaded with nickel-copper bimetallic hollow Nanospheres with SOD and CAT enzymatic-like activity promotes acute Wound healing. ACS Appl Mater Interfaces. 2022;14:50677–91. [DOI] [PubMed] [Google Scholar]
- 134. Grada A, Mervis J, Falanga V. Research techniques made simple: animal models of Wound healing. J Invest Dermatol. 2018;138:2095–2105.e2091. 10.1016/j.jid.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 135. Mahla RS. Stem cells applications in regenerative medicine and disease therapeutics. Int. J Cell Biol. 2016;2016:6940283. 10.1155/2016/6940283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Wu K, Zhu D, Dai X, Wang W, Zhong X, Fang Z, et al. Bimetallic oxide Cu1.5Mn1.5O4 cage-like frame nanospheres with triple enzyme-like activities for bacterial-infected wound therapy. Nano Today. 2022;43:101380. 10.1016/j.nantod.2022.101380. [DOI] [Google Scholar]
- 137. Yang X, Yang J, Wang L, Ran B, Jia Y, Zhang L, et al. Pharmaceutical intermediate-modified gold nanoparticles: against multidrug-resistant bacteria and Wound-healing application via an electrospun scaffold. ACS Nano. 2017;11:5737–45. [DOI] [PubMed] [Google Scholar]
- 138. Zhang W, Dai X, Jin X, Huang M, Shan J, Chen X, et al. Promotion of wound healing by a thermosensitive and sprayable hydrogel with nanozyme activity and anti-inflammatory properties. Smart Materials in Medicine. 2023;4:134–45. [Google Scholar]
- 139. Fang Y, Wang N, Shi L, Barker T, Zhang P. Pain relief during wound care for patients with a deep open wound in an orthopedic trauma department: a best practice implementation project. JBI Database System Rev Implement Rep. 2019;17:2559–69. [DOI] [PubMed] [Google Scholar]
- 140. Chen S, Wang H, Su Y, John JV, Mccarthy A, Wong SL, et al. Mesenchymal stem cell-laden, personalized 3D scaffolds with controlled structure and fiber alignment promote diabetic wound healing. Acta Biomater. 2020;108:153–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Elahi MF, Lu W. Core-shell Fibers for biomedical applications-a review. J Bioeng Biomed Sci. 2013;3:1–14. [Google Scholar]
- 142. Peng Z-X, Wang L, Du L, Guo S-R, Wang X-Q, Tang T-T. Adjustment of the antibacterial activity and biocompatibility of hydroxypropyltrimethyl ammonium chloride chitosan by varying the degree of substitution of quaternary ammonium. Carbohydr Polym. 2010;81:275–83. [Google Scholar]
- 143. Orsu P, Haider HY, Koyyada A. Bioengineering for curcumin loaded carboxymethyl guargum/reduced graphene oxide nanocomposites for chronic wound healing applications. Int J Pharm. 2021;606:120928. 10.1016/j.ijpharm.2021.120928. [DOI] [PubMed] [Google Scholar]
- 144. Liang Y, Li M, Yang Y, Qiao L, Xu H, Guo B. pH/glucose dual responsive metformin release hydrogel dressings with adhesion and self-healing via dual-dynamic bonding for athletic diabetic foot Wound healing. ACS Nano. 2022;16:3194–207. [DOI] [PubMed] [Google Scholar]
- 145. Kabilamurthi RS, Abhinav RP, Thiyaneswaran N, Subhashree R, Gajendran PL. Effectiveness of concentrated growth factor on surgical Wound healing: a pilot study. J Long-Term Eff Med Implants. 2021;31:27–32. [DOI] [PubMed] [Google Scholar]
- 146. Huo H, Liu F, Luo Y, Gu Q, Liu Y, Wang Z, et al. Triboelectric nanogenerators for electro-assisted cell printing. Nano Energy. 2020;67:104150. 10.1016/j.nanoen.2019.104150. [DOI] [Google Scholar]
- 147. Zhao S, Agarwal P, Rao W, Huang H, Zhang R, Liu Z, et al. Coaxial electrospray of liquid core-hydrogel shell microcapsules for encapsulation and miniaturized 3D culture of pluripotent stem cells. Integr Biol (Camb). 2014;6:874–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Chen Y, Abdalkarim SYH, Yu HY, Li Y, Xu J, Marek J, et al. Double stimuli-responsive cellulose nanocrystals reinforced electrospun PHBV composites membrane for intelligent drug release. Int J Biol Macromol. 2020;155:330–9. [DOI] [PubMed] [Google Scholar]