Abstract
Purpose
Evaluate which factors are involved in the increased rate of mosaicism in embryos.
Methods
A systematic review and meta-analysis was performed. After an exhaustive search of the literature, a total of seven papers were included in the analysis. In addition, data collected from IVF cycles performed in our fertility clinic were also analysed. Day of biopsy, embryo quality, maternal and paternal age and seminal quality were the chosen factors to be studied.
Results
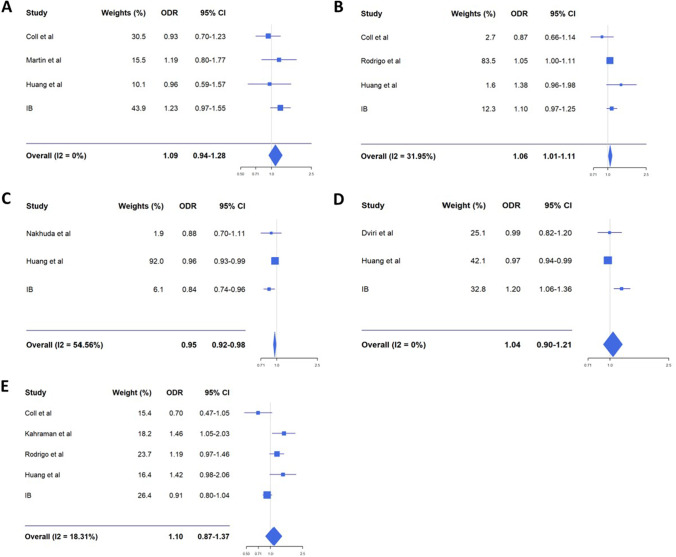
The results of the meta-analysis show that neither embryo quality nor seminal quality were related to mosaic embryo rate (OR: 1.09; 95% CI: 0.94–1.28 and OR: 1.10; 95% CI: 0.87–1.37, respectively). A positive association was observed for the variable “biopsy day” with embryos biopsied at day 6 or 7 having the highest rate of mosaicism (OR: 1.06; 95% CI: 1.01–1.11). In opposite to what happens with aneuploidy rate, which increases with maternal age, embryo mosaicism is higher in younger women (<34 years) rather than in older ones (≥34 years) (OR: 0.95; 95% CI: 0.92–0.98). However, for the “paternal age” factor, no association with mosaicism was found (OR: 1.04; 95% CI: 0.90–1.21).
Conclusions
With the present study, we can conclude that the factors related to the presence of mosaicism in embryos are the embryo biopsy day and maternal age. The rest of the studied factors showed no significant relationship with mosaicism. These results are of great importance as knowing the possible causes leading to mosaicism helps to improve the clinical results of reproductive treatments.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10815-023-02914-9.
Keywords: Meta-analysis, Embryo mosaicism, Next-generation sequencing (NGS), Preimplantation genetic testing for aneuploidies (PGT-A)
Introduction
The development of preimplantation genetic testing for aneuploidy (PGT-A) has allowed to reduce the impact of aneuploid embryos in in vitro fertilization (IVF) success. Being able to identify aneuploid embryos may prevent adverse clinical outcomes and improve pregnancy and live birth rates per embryo transfer [1].
Over the last few years, some sensitive genetic techniques such as next-generation sequencing (NGS) have been introduced into clinical practice to help us identify the chromosomal status of embryos generated in the laboratory. When we analyse trophectoderm (TE) embryo biopsies with these techniques, we can detect not only euploid and aneuploid embryos but also mosaic embryos. Mosaicism is a phenomenon characterized by the simultaneous existence of two or more chromosomally distinct cell lines. In contrast to full aneuploidies which are mainly meiotic and the result of a faulty chromosome segregation in the gamete development [2], mosaicism is primarily generated during post-zygotic mitosis by chromosomal malsegregation via non-disjunction or by aberrations in the centrosome and mitotic spindle or defects in chromatid cohesion [3–7]. With NGS, we can also identify small chromosome deletions or duplications (about 10 Mb) (segmental mosaicism) [8, 9]. It exist technical and biological limitations in interpreting PGT-A results. Genetic testing of preimplantation embryos is performed on a limited number of biopsied embryonic cells (3 to 10 cells), from a single TE biopsy, rather than the cell lineage programmed to form the proper embryo (i.e. inner cell mass (ICM)) [10]. This is an important limitation when it comes to give an accurate diagnosis, especially when it concerns mosaicism. Technical factors influencing the diagnosis of mosaicism also include the whole-genome amplification protocol, which may lead to over- or under-representation of certain parts of the genome [5]. For this reason, diagnostics based on intermediate copy number values can only suggest that the embryo may be at risk of being mosaic but does not ensure that the embryo is mosaic [11, 12].
The factors that may give rise to mosaicism are unclear and generate controversy in the scientific community. Some investigations have suggested that ovarian stimulation, culture media or laboratory conditions may cause an increase in mosaicism rate [9, 13, 14]. However, these statements are not quite evident.
With the increasing use of NGS techniques to perform PGT-A, it becomes more and more frequent to find mosaic embryos. The reported incidence of mosaic embryos is highly variable between genetic laboratories, ranging from as low as 2% to as high as 40% [7, 8]. This is probably due to differences in sensitivity and specificity of the analysis platform used and the cut-off points applied for data interpretation, especially in the classification of low-grade mosaic embryos [15, 16]. It could also be due to other factors related to biopsy technique or the number of cells biopsied [16].
Although there are guidelines on how to deal with an embryo classified as mosaic in clinical practice, such as ESHRE’s Guidelines [17], there are still many doubts and uncertainty about its management among specialists. The effect of mosaicism on implantation and the development potential of these embryos are still unknown [1]. Some studies, such as that of Capalbo et al. [10] or Lledó et al. [12], show that embryos can result in pregnancy and birth of healthy babies, especially when we transfer low-level mosaic embryos. However, other studies affirm that mosaic embryos are associated with reduced implantation and higher miscarriage rates [18–20], which complicates the embryo transfer decision.
Identifying the mosaicism origin has become a challenge for clinicians. Since there is speculation that some extrinsic and intrinsic factors could increase the incidence of mosaicism in embryos [7, 15], we undertook a systematic review of the literature and meta-analysis of the results published to evaluate if some specific factors could be related with mosaicism rate.
Materials and methods
The present meta-analysis was performed following the preferred reporting item for systematic reviews and meta-analysis (PRISMA) statement [21].
Search strategy
To carry out the bibliographic search, the MEDLINE database (via PubMed) and Google Scholar were used.
Searches were limited to English or Spanish using database supplied limits. Only papers published between January 2016 and November 2022 were considered. To perform the bibliographic search, we used some specific keywords, such as “Mosaicism”, “Semen quality”, “Blastocyst” and “Preimplantation Diagnosis”. These words should have been either in the title or in the abstract of searched papers. The full detailed search strategies for the databases are available in the supplemental Appendix.
Study selection criteria and data abstraction
Studies addressing factors associated with embryo mosaicism were comprehensively analysed. As inclusion criteria, mosaicism should have been detected from a blastocyst TE biopsy on day 5, 6 or 7, and analysed by NGS. Views, reviews, letters, opinions, surveys, case reports, editorials, congress abstracts and committee opinions were excluded. Search, screen and data extraction were performed by two reviewers independently based on inclusion criteria. Characteristics of the study population, analysed embryos and IVF cycles have been described in Supplemental table 1. Embryo quality, maternal and paternal age, day of biopsy and seminal quality were the outcome variables extracted and included for analysis.
Embryo quality was assessed according to the criteria established by Istanbul Consensus [22] or to guidelines from the Spanish Association for the Study of Biology of Reproduction (ASEBIR) [23], depending on the centre. To study the effect of embryo quality, we formed two study groups: embryos considered of excellent/good quality as defined by Istanbul Consensus (A+B, according to ASEBIR’s guideline) versus those of fair/poor quality as indicated in Istanbul Consensus (C+D, according to ASEBIR’s guideline). Regarding the effect of maternal and paternal age, we also carried out two study groups: <34 years versus ≥34 years (maternal age) and <40 years versus ≥40 years (paternal age). When studying the effect of biopsy day on mosaicism rate, we also formed two study groups: those embryos that were biopsied at day 5 against those biopsied at day 6 or 7 of embryonic development. Finally, we have defined two groups for sperm quality: normal and altered. Normal semen quality has been considered for all those samples that do not have any altered semen parameters, according to the latest guidelines published by the World Health Organization in 2022 [24].
An embryo was considered mosaic when it had an intermediate copy number for one or more chromosomes. Most of the papers included in the systematic review and meta-analysis consider global mosaicism rate (diploid-aneuploid and aneuploid-aneuploid). Only two of them considered exclusively the rate of diploid-aneuploid mosaics (Supplemental table 1).
Statistical methods
Summary estimates were calculated to determine the factors associated with embryonic mosaicism for this systematic review and meta-analysis. Results for dichotomous variables are expressed as odds ratios (ODR) and 95% confidence interval (CI). We used fixed-effect models to calculate the summary estimates and their 95% CIs. Studies were weighted using the inverse variance method. As for the potential factor total, we report the χ2-test statistic for heterogeneity across studies with its p-value. When the different studies to be combined did not show heterogeneity (p>0.1), the fixed-effect method was applied; otherwise, the random-effects method was used.
In addition, the I2 statistic measuring the degree of inconsistency of the results was calculated. I2 values above 50% suggested considerable heterogeneity, between 25 and 50% moderate heterogeneity, and values below 25% indicated low heterogeneity.
Statistical analysis and meta-analysis were performed using Statistical Product and Service Solutions, version 23.0 (SPSS, Chicago, IL, USA) and R statistical software (v. 4.2.0). Specifically, meta-analysis was performed using the !MAR macro [25] for SPSS Statistics and forest plots with the R forest plot library.
Results
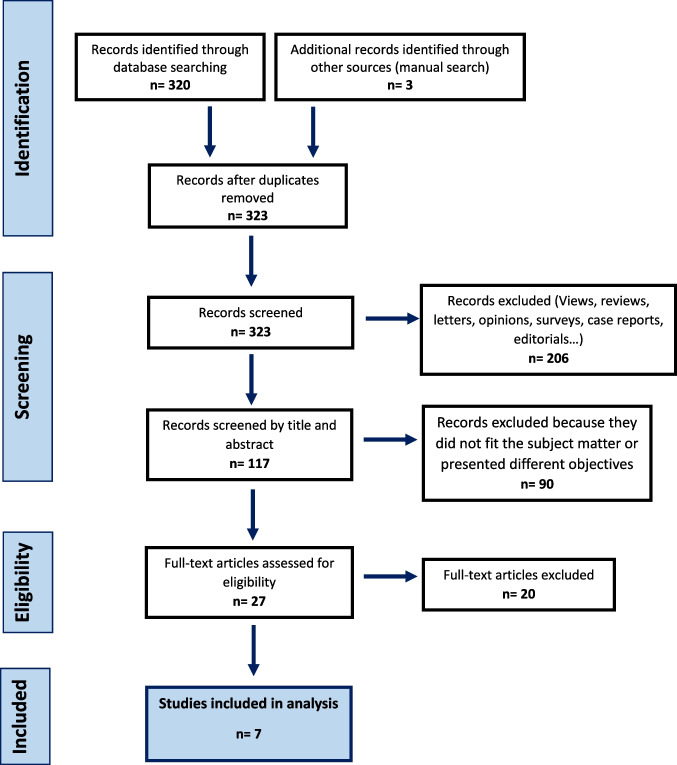
By searching the available literature on PubMed and Google Scholar using the above criteria, 619 results returned. After applying the corresponding filters and considering the articles published in the last 6 years, we obtained a total of 320 papers. After screening title and abstract, some papers were discarded because they did not meet certain requirements: studies not carried out on humans, studies using analysis techniques different from our objectives, articles with a very small number of cases or not corresponding to our specific study topic. Finally, twenty-seven potentially eligible articles were retrieved for full-text screening (Fig. 1).
Fig. 1.
Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) flow chart of literature search by the application of inclusion and exclusion criteria
Then, we critically and thoroughly read the selected papers, and 7 of them were included in this systematic review and meta-analysis. Data obtained from PGT-A cycles conducted in our reproductive clinic (Instituto Bernabeu, Spain) were also enclosed in the analysis (2513 cycles, 7242 embryos). The characteristics of the 7 selected studies are listed in Table 1.
Table 1.
Qualitative analysis of included studies
| Authors | Year | Location | Study design | Embryos analysed (n) | Results (association with mosaicism) |
|---|---|---|---|---|---|
| Coll et al. | 2020 | Spain | Retrospective cohort study | 1708 | Positive association between mosaicism prevalence and paternal age |
| Dviri et al. | 2020 | Canada | Retrospective cohort study | 3118 | No significant association |
| Huang et al. | 2022 | China | Retrospective study | 1910 | Semen quality, fertilization method and detection system are independent factors associated with embryonic mosaicism |
| Kahraman et al. | 2020 | Turkey | Retrospective study | 1570 | When testicular sperm is used, the rate of mosaicism increases |
| Martin et al. | 2021 | Spain | Retrospective cohort study | 1511 | No significant association |
| Nakhuda et al. | 2018 | Canada | Retrospective cross-sectional study | 1743 | |
| Rodrigo et al. | 2020 | Spain | Retrospective observational study | 111,860 | Older female and male patients showed higher rates of high-mosaic degree. Severe oligozoospermic patients had higher rates of mosaicism |
Seminal quality was analysed to study its association with embryonic mosaicism based on whether semen parameters were normal or not. The results of the meta-analysis did not show association between seminal quality and mosaicism rate: OR: 1.10; 95% CI: 0.87–1.37. Embryo quality was studied according to whether the embryos were of good or poor quality (A+B vs. C+D) according to the classification described in the “Materials and methods” section, but no association with mosaicism was shown (OR: 1.09; 95% CI: 0.94–1.28). In contrast, a positive association with embryo mosaicism was observed for the variable “biopsy day”: embryos biopsied at day 6 or 7 of embryo development show a slight increase in the mosaicism rate in comparison with those biopsied in day 5 (OR: 1.06; 95% CI: 1.01–1.11). All these results are shown in Fig. 2.
Fig. 2.
Forest plots comparing embryo mosaicism rate with different factors (A embryo quality; B embryo biopsy day; C maternal age; D paternal age; E seminal quality). ODR, odds ratio; CI, confidence interval
On the other hand, the association with maternal and paternal age was also analysed in the study. To study the effect of paternal age on embryonic mosaicism, two groups were established, one for younger men (<40 years) and one for older men (≥40 years), but no statistically significant relationship was observed between paternal age and embryonic mosaicism rate (OR: 1.04; 95% CI: 0.90–1.21). In contrast, when studying the effect of maternal age, a statistically significant relationship between this factor and the embryonic mosaicism rate was observed: mosaicism rate is higher in the younger women group (<34 years) compared with the older women group (≥34 years) (Fig. 2).
Discussion
Because the mosaicism prevalence is relatively high and variable between centres [1, 7], it is important to understand the origins, mechanism and incidences of mosaicism through embryo development. Mosaic embryos are a challenge for clinicians in IVF cycles, especially when there are no euploid embryos to be transferred. Mosaic embryo transfer is a controversial topic as the current literature is inconclusive on the clinical outcomes of these embryos. Some studies show that implantation rates are lower and miscarriage rates increase compared to the transfer of euploid embryos [18–20]. It is important to know that these results should be interpreted with caution, as all of them are retrospective studies, so there could be an increased risk of bias in terms of patient selection, incomplete information or variability in data quality. Nonetheless, the prospective non-selection study performed by Capalbo et al. [10] reports no differences in reproductive outcomes following the transfer of euploid and low-level mosaic embryos (<50%). Further prospective and well-designed studies are needed to confirm these findings.
On the other hand, identification of mosaicism at the blastocyst stage is not an easy task as the embryo genetic analysis is based on the premise that aneuploid cells are distributed equally in the biopsied fraction compared with the remaining embryo. Furthermore, it is unknown whether there is a real association between trophectoderm cells and inner mass cells, so analysing only a small portion of the TE is no guarantee of an accurate diagnosis. For this reason, using the term “mosaic embryo” causes some controversy in the scientific community, so it would be more correct to speak about the risk of an embryo being a mosaic.
Current literature has reported a significant association between blastocyst morphology (embryo quality) and chromosomal content [11, 26, 27]. Martin et al. [11] affirm in their study that the blastocyst morphological quality increases gradually from aneuploid embryos (lowest quality) to mosaic (intermediate) and euploid embryos, which have the highest quality. Although several studies show that low-quality embryos are often associated with high rates of aneuploidy [11, 26, 28], this association between quality and mosaicism rate is not so evident [5, 7, 29]. The results obtained in this meta-analysis showed that embryo quality is not associated to mosaicism rates: mosaic blastocysts have the same quality than non-mosaic blastocysts. Nevertheless, it is true that as only blastocysts were assessed in the analysis, an association between mosaicism and compromised embryo development at early stages cannot be excluded. The day on which the embryo biopsy is performed is closely related to embryo development, which in turn is associated with the quality of the embryo. Embryos that take longer to develop and are thus biopsied at days 6 or 7 of development are usually associated with poorer embryo quality and, therefore, as discussed above, are more likely to have aneuploid cells. After meta-analysis, we observed that the frequency of mosaicism in embryos is slightly higher when they are biopsied at day 6 or 7 of embryonic development. This could be explained by the fact that the aneuploid cell line of the mosaic embryos may slow embryo development; however, the methodological limitations could be interfering as well.
On the other hand, maternal age is one of the most important factors that will determine the success of assisted reproduction treatments. This is mainly due to the decrease in the number of available oocytes as well as their quality [30]. Different studies have shown that advanced maternal age is the determining factor in the appearance of meiotic errors, which give rise to aneuploid embryos [31–34]. Mosaicism, by contrast, mainly results from post-fertilization mitotic errors [5, 35]. This would lead us to believe that there is no correlation between the incidence of mosaicism and maternal age. However, after the meta-analysis, we observed an inverse correlation between maternal age and the incidence of mosaicism in embryos. Unhealthy lifestyle habits, exposure to toxic substances and oxygen free radicals or radiation could be the explanation for this, as they cause mitochondrial damage and telomere shortening, leading to mitotic errors [36, 37]. Telomeres are repetitive sequences and associated proteins, which cap and protect chromosome ends [38]. Telomeres shorten both during DNA replication and from the response to oxidative DNA damage [37]. When telomeres become critically short, genomic instability occurs, making cell division failure more likely. Furthermore, the telomeres of human oocytes are particularly short compared to other species [36, 37], which makes these cells much more sensitive to processes or toxic substances that increase telomere decay. Since the interval between foetal oogenesis and ovulation is exceptionally prolonged in women, older women have a higher risk of cumulative exposure to free radicals, so the telomeres of their oocytes would be expected to be shorter due to inefficient DNA repair of oxidative damage [36, 37]. This damage affects both younger and older women; however, because the telomeres of young females are longer, cell damage is lower, resulting in fewer complete aneuploidies, but rather small mitotic defects that could be generating mosaicism. Older women, in addition, have reduced centromere cohesion in oocytes and less control of spindle assembly, so misseparation of sister chromatids in early meiosis is more likely to occur, leading to complete aneuploidies [29].
To date, the relationship between paternal age and chromosomal alterations in embryos remains unclear. In the current literature, there is no obvious correlation between paternal age and the rate of embryo aneuploidy: some works showed no association [39, 40] whereas others found a positive association [7]. Some studies associate the increase in paternal age with other factors that could be increasing in turn chromosomal alterations in the embryos. Pino et al. [41] have observed a positive relationship between paternal age and sperm DNA fragmentation, being 4.58 times higher in males older than 50 than in males younger than 30. On the other hand, regarding embryonic mosaicism, with the in-depth review and analysis carried out during the present study, we have also found no association between paternal age and the presence of mosaicism in the embryos. This reinforces the idea that paternal age is not closely related to the occurrence of chromosomal alterations in embryos. Regarding seminal quality, although some works such as that of Tarozzi et al. [42] observed a positive relationship with the appearance of mosaicism in embryos when seminal quality decreases, other works, such as Coll et al., Rodrigo et al. and Kahraman et al. [7, 16, 43], did not find this association. This difference may be due to the fact that in these papers no distinction is made regarding to what an altered male factor implies (whether it is due just to sperm concentration, motility, morphology or a mix of them; or even a distinction between mild or moderate male factor). It would be interesting to carry out future analyses by studying individually each seminal parameter to find out if any of them are associated with the appearance of chromosomal alterations of mitotic origin.
There are other factors related to the handling of the embryos in the laboratory that are hypothesized to be interfering in the correct embryo development and promoting the appearance of an aneuploid cell line, increasing mosaicism rate. These factors have not been included in the meta-analysis, mainly because these data are rarely published and also due to the great variability between centres since, although each laboratory has its own standardized conditions, these do not necessarily have to be the same as those of another laboratory, which makes it difficult to make a proper comparison. The culture media used in IVF laboratories have different compositions depending on the manufacturer [13]. This composition could be interfering with embryo development that could also induce spindle disassembly and thus lead the appearance of mosaicism in the embryos. Studies, such as Coll et al. [7], evaluated the possible effect of culture medium on the mosaicism rate comparing two different single-step media with no differences observed in mosaicism prevalence. Other factors related to embryo biopsy could also be interfering with the mosaicism rate by producing damage to the embryo: number of laser shots, assisted hatching or biopsy technique used (pulling or flicking). Also, poorer biopsy samples may result in higher rates of artefactual mosaicism. Future studies comparing data from different centres are needed to obtain more accurate results about mosaicism origin.
One of the main strengths of this study is that we have also included in the meta-analysis the results obtained in our clinic. This is an important number of cycles, which makes the conclusions of the analysis even more relevant. The main limitation of this study is the low number of papers analysed in the meta-analysis. Limitations also include the retrospective design, heterogeneity of studies, selection bias or incomplete outcome data. On the other hand, there is no worldwide consensus on the criteria for the diagnosis of mosaicism from a trophectoderm biopsy. Each laboratory sets its own aneuploidy rate threshold for mosaic diagnosis, and this makes it difficult to carry out homogeneous and well-designed studies.
To the best of our knowledge, this is the first meta-analysis carried out about factors affecting embryo mosaicism rate. Our result, combined with the body of medical evidence available, suggest that embryo mosaicism rate is influenced by TE biopsy day and maternal age. This information will add in the knowledge for elucidating the uncertainties surrounding the factors by which mosaicism is generated in embryos.
Supplementary information
(DOCX 18 kb)
Appendix. Systematic review search strategies
PubMed—(“semen quality”[Title/Abstract] OR “embryo”[Title/Abstract] OR “preimplantation diagnosis”[Title/Abstract] OR “blastocyst”[Title/Abstract] OR “blastocysts”[Title/Abstract]) AND (“Mosaicism”[Title/Abstract] OR “embryo mosaicism”[Title/Abstract]) Filters: from 2016 to 2022.
Data Availability
The authors confirm that the data supporting this article are available under request.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Greco E, Minasi MG, Fiorentino F. Healthy babies after intrauterine transfer of mosaic aneuploid blastocysts. N Engl J Med. 2015;373(21):2089–2090. doi: 10.1056/NEJMc1500421. [DOI] [PubMed] [Google Scholar]
- 2.Webster A, Schuh M. Mechanisms of aneuploidy in human eggs. Trends Cell Biol. 2017;27:55–68. doi: 10.1016/j.tcb.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Taylor TH, Gitlin SA, Patrick JL, Crain JL, Wilson JM, Griffin DK. The origin, mechanisms, incidence and clinical consequences of chromosomal mosaicism in humans. Hum Reprod Update. 2014;20(4):571–581. doi: 10.1093/humupd/dmu016. [DOI] [PubMed] [Google Scholar]
- 4.Alksere B, Grinfelde I, Kornejeva L, Dzalbs A, Vedmedovska N, Kovalova I, Conka U, Andersone S, Krasucka S, Blumberga A, Berzina D, Fodina V. The outcomes after transfers of embryos with chromosomal mosaicism: a single reproductive medicine center experience at IVF Riga clinic. Gynecol Endocrinol. 2020;36(sup1):53–57. doi: 10.1080/09513590.2020.1816719. [DOI] [PubMed] [Google Scholar]
- 5.Popovic M, Dhaenens L, Boel A, Menten B, Heindryckx B. Chromosomal mosaicism in human blastocysts: the ultimate diagnostic dilemma. Hum Reprod Update. 2020;26:313–334. doi: 10.1093/humupd/dmz050. [DOI] [PubMed] [Google Scholar]
- 6.Zhang YX, Chen JJ, Nabu S, Yeung QSY, Li Y, Tan JH, Suksalak W, Chanchamroen S, Quangkananurug W, Wong PS, Chung JPW, Choy KW. The pregnancy outcome of mosaic embryo transfer: a prospective multicenter study and meta-analysis. Genes. 2020;11(9):973. doi: 10.3390/genes11090973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coll L, Parriego M, Mateo S, García-Monclús S, Rodríguez I, Boada M, Coroleu B, Polyzos NP, Vidal F, Veiga A. Prevalence, types and possible factors influencing mosaicism in IVF blastocysts: results from a single setting. Reprod Biomed Online. 2021;42(1):55–65. doi: 10.1016/j.rbmo.2020.09.025. [DOI] [PubMed] [Google Scholar]
- 8.Cram DS, Leigh D, Handyside A, Rechitsky L, Xu K, Harton G, Grifo J, Rubio C, Fragouli E, Kahraman S, Forman E, Katz-Jaffe M, Tempest H, Thornhill A, Strom C, Escudero T, Qiao J, Munne S, Simpson JL, Kuliev A. PGDIS position statement on the transfer of Mosaic Embryos 2019. Reprod Biomed Online. 2019;39(Suppl 1):e1–e4. doi: 10.1016/j.rbmo.2019.06.012. [DOI] [PubMed] [Google Scholar]
- 9.Swain JE. Controversies in ART: can the IVF laboratory influence preimplantation embryo aneuploidy? Reprod Biomed Online. 2019;39:599–607. doi: 10.1016/j.rbmo.2019.06.009. [DOI] [PubMed] [Google Scholar]
- 10.Capalbo A, Poli M, Rienzi L, Girardi L, Patassini C, Fabiani M, Cimadomo D, Benini F, Farcomeni A, Cuzzi J, Rubio C, Albani E, Sacchi L, Vaiarelli A, Figliuzzi M, Findikli N, Coban O, Boynukalin FK, Vogel I, Hoffmann E, Livi C, Levi-Setti PE, Ubaldi FM, Simón C. Mosaic human preimplantation embryos and their developmental potential in a prospective, non-selection clinical trial. Am J Hum Genet. 2021;108(12):2238–2247. doi: 10.1016/j.ajhg.2021.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martín Á, Rodrigo L, Beltrán D, Meseguer M, Rubio C, Mercader A, de Los Santos MJ. The morphokinetic signature of mosaic embryos: evidence in support of their own genetic identity. Fertil Steril. 2021;116(1):165–173. doi: 10.1016/j.fertnstert.2020.12.031. [DOI] [PubMed] [Google Scholar]
- 12.Lledó B, Morales R, Ortiz JA, Blanca H, Ten J, Llácer J, Bernabeu R. Implantation potential of mosaic embryos. Syst Biol Reprod Med. 2017;63(3):206–208. doi: 10.1080/19396368.2017.1296045. [DOI] [PubMed] [Google Scholar]
- 13.Morbeck DE, Baumann NA, Oglesbee D. Composition of single-step media used for human embryo culture. Fertil Steril. 2017:107. 10.1016/j.fertnstert.2017.01.007. [DOI] [PubMed]
- 14.Katz-Jaffe M, Parks J, McReynolds S, Henry L, Schoolcraft WB. Chromosomal mosaicism is impacted by compromised embryo culture conditions. Fertil Steril. 2018;110:e431. doi: 10.1016/j.fertnstert.2018.08.037. [DOI] [Google Scholar]
- 15.Monahan D, Harton G, Griffin D, Angle M, Smikle C. Clinical comparison of two PGT-A platforms utilizing different thresholds to determine ploidy status. Reprod Biomed Online. 2019;39:e27–e28.2. doi: 10.1016/j.rbmo.2019.04.055. [DOI] [Google Scholar]
- 16.Rodrigo L, Clemente-Císcar M, Campos-Galindo I, Peinado V, Simón C, Rubio C. Characteristics of the IVF cycle that contribute to the incidence of mosaicism. Genes. 2020;11(10):1151. doi: 10.3390/genes11101151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.ESHRE Working Group on Chromosomal Mosaicism and others. ESHRE survey results and good practice recommendations on managing chromosomal mosaicism. Human Reproduction Open. 2022(4):hoac044. 10.1093/hropen/hoac044. [DOI] [PMC free article] [PubMed]
- 18.Victor AR, Tyndall JC, Brake AJ, Lepkowsky LT, Murphy AE, Griffin DK, McCoy RC, Barnes FL, Zouves CG, Viotti M. One hundred mosaic embryos transferred prospectively in a single clinic: exploring when and why they result in healthy pregnancies. Fertil Steril. 2019;111(2):280–293. doi: 10.1016/j.fertnstert.2018.10.019. [DOI] [PubMed] [Google Scholar]
- 19.Zore T, Kroener LL, Wang CM, Liu L, Buyalos R, Hubert G, Shamonki M. Transfer of embryos with segmental mosaicism is associated with a significant reduction in live-birth rate. Fertil Steril. 2019;111:69–76. doi: 10.1016/j.fertnstert.2018.08.057. [DOI] [PubMed] [Google Scholar]
- 20.Zhang L, Wei D, Zhu Y, Gao Y, Yan J, Chen ZJ. Rates of live birth after mosaic embryo transfer compared with euploid embryo transfer. J Assist Reprod Genet. 2019;36(1):165–172. doi: 10.1007/s10815-018-1322-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 22.Alpha Scientists in Reproductive Medicine and ESHRE Special Interest Group of Embryology The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Hum Reprod. 2011;26:1270–1283. doi: 10.1093/humrep/der037. [DOI] [PubMed] [Google Scholar]
- 23.Ardoy M, Caderón G, Cuadros J, Figueroa MJ, Herrer R, Moreno JM, et al. Criterios ASEBIR de valoración morfológica de oocitos, embriones tempranos y blastocistos humanos. Cuadernos de Embriología Clínica. 2008;II:1–59. [Google Scholar]
- 24.Björndahl L, Kirkman Brown J. The sixth edition of the WHO Laboratory Manual for the Examination and Processing of Human Semen: ensuring quality and standardization in a basic examination of human ejaculates. Fertil Steril. 2022;117(2):246–251. doi: 10.1016/j.fertnstert.2021.12.012. [DOI] [PubMed] [Google Scholar]
- 25.Domenech JM. Macro !MAR for SPSS Statistics. Meta-Analysis OR, RR, RD, IR, ID, B & MD Combined [computer program].V2012.03.23. Bellaterra: Universitat Autònoma de Barcelona; 2012. [Google Scholar]
- 26.Minasi MG, Colasante A, Riccio T, Ruberti A, Casciani V, Scarselli F, Spinella F, Fiorentino F, Varricchio MT, Greco E. Correlation between aneuploidy, standard morphology evaluation and morphokinetic development in 1730 biopsied blastocysts: a consecutive case series study. Hum Reprod. 2016;31(10):2245–2254. doi: 10.1093/humrep/dew183. [DOI] [PubMed] [Google Scholar]
- 27.Capalbo A, Rienzi L, Cimadomo D, Maggiulli R, Elliott T, Wright G, Nagy ZP, Ubaldi FM. Correlation between standard blastocyst morphology, euploidy and implantation: an observational study in two centers involving 956 screened blastocysts. Hum Reprod. 2014;29(6):1173–1181. doi: 10.1093/humrep/deu033. [DOI] [PubMed] [Google Scholar]
- 28.Ortiz JA, Morales R, Lledó B, Vicente JA, González J, García-Hernández EM, Cascales A, Ten J, Bernabeu A, Bernabeu R. Application of machine learning to predict aneuploidy and mosaicism in embryos from in vitro fertilization cycles. AJOG Glob Rep. 2022;2(4):100103. doi: 10.1016/j.xagr.2022.100103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang QX, Wang ZH, Huang WJ, Mao LH, Lin CL, Chen GY, Wang CX, Chen ZB, Lin YL, He LY, Liu Y. Factors influencing mosaicism: a retrospective analysis. Reprod Biomed Online. 2022;45(3):491–500. doi: 10.1016/j.rbmo.2022.04.020. [DOI] [PubMed] [Google Scholar]
- 30.ESHRE Capri Workshop Group Fertility and ageing. Hum Reprod Update. 2005;11:261–276. doi: 10.1093/humupd/dmi006. [DOI] [PubMed] [Google Scholar]
- 31.Babariya D, Fragouli E, Alfarawati S, Spath K, Wells D. The incidence and origin of segmental aneuploidy in human oocytes and preimplantation embryos. Hum Reprod. 2017;32(12):2549–2560. doi: 10.1093/humrep/dex324. [DOI] [PubMed] [Google Scholar]
- 32.Barash OO, Hinckley MD, Rosenbluth EM, Ivani KA, Weckstein LN. High gonadotropin dosage does not affect euploidy and pregnancy rates in IVF PGS cycles with single embryo transfer. Hum Reprod. 2017;32(11):2209–2217. doi: 10.1093/humrep/dex299. [DOI] [PubMed] [Google Scholar]
- 33.Munné S, Cohen J. Advanced maternal age patients benefit from preimplantation genetic diagnosis of aneuploidy. Fertil Steril. 2017;107(5):1145–1146. doi: 10.1016/j.fertnstert.2017.03.015. [DOI] [PubMed] [Google Scholar]
- 34.Nakhuda G, Jing C, Butler R, Guimond C, Hitkari J, Taylor E, Tallon N, Yuzpe A. Frequencies of chromosome-specific mosaicisms in trophoectoderm biopsies detected by next-generation sequencing. Fertil Steril. 2018;109(5):857–865. doi: 10.1016/j.fertnstert.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 35.Munné S, Grifo J, Wells D. Mosaicism: “survival of the fittest” versus “no embryo left behind”. Fertil Steril. 2016;105(5):1146–1149. doi: 10.1016/j.fertnstert.2016.01.016. [DOI] [PubMed] [Google Scholar]
- 36.Keefe DL. Telomeres and genomic instability during early development. Eur J Med Genet. 2020;63(2):103638. doi: 10.1016/j.ejmg.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 37.Keefe DL, Liu L. Telomeres and reproductive aging. Reprod Fertil Dev. 2009;21(1):10–14. doi: 10.1071/rd08229. [DOI] [PubMed] [Google Scholar]
- 38.Blackburn EH. Structure and function of telomeres. Nature. 1991;350(6319):569–573. doi: 10.1038/350569a0. [DOI] [PubMed] [Google Scholar]
- 39.Carrasquillo RJ, Kohn TP, Cinnioglu C, Rubio C, Simon C, Ramasamy R, Al-Asmar N. Advanced paternal age does not affect embryo aneuploidy following blastocyst biopsy in egg donor cycles. J Assist Reprod Genet. 2019;36(10):2039–2045. doi: 10.1007/s10815-019-01549-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dviri M, Madjunkova S, Koziarz A, Antes R, Abramov R, Mashiach J, Moskovtsev S, Kuznyetsova I, Librach C. Is there a correlation between paternal age and aneuploidy rate? An analysis of 3,118 embryos derived from young egg donors. Fertil Steril. 2020;114(2):293–300. doi: 10.1016/j.fertnstert.2020.03.034. [DOI] [PubMed] [Google Scholar]
- 41.Pino V, Sanz A, Valdés N, Crosby J, Mackenna A. The effects of aging on semen parameters and sperm DNA fragmentation. JBRA Assist Reprod. 2020;24(1):82–86. doi: 10.5935/1518-0557.20190058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tarozzi N, Nadalini M, Lagalla C, Coticchio G, Zacà C, Borini A. Male factor infertility impacts the rate of mosaic blastocysts in cycles of preimplantation genetic testing for aneuploidy. J Assist Reprod Genet. 2019;36(10):2047–2055. doi: 10.1007/s10815-019-01584-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kahraman S, Sahin Y, Yelke H, Kumtepe Y, Tufekci MA, Yapan CC, Yesil M, Cetinkaya M. High rates of aneuploidy, mosaicism and abnormal morphokinetic development in cases with low sperm concentration. J Assist Reprod Genet. 2020;37(3):629–640. doi: 10.1007/s10815-019-01673-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 18 kb)
Data Availability Statement
The authors confirm that the data supporting this article are available under request.