Abstract
Preimplantation genetic testing for aneuploidy (PGT-A) is a common add-on to IVF cycles. As it is presently performed, PGT-A relies on whole genome amplification of small amounts of DNA from cells removed from the trophectoderm (TE) of a blastocyst for determination of gain or loss of chromosomal material by next-generation sequencing. Whole genome amplification may introduce artifacts such as allele dropout and loss of heterozygosity in up to 25% of cases. In addition, the high prevalence of mosaicism in human embryos is a complicating factor in interpreting the results of PGT-A screening. In the presence of mosaicism, biopsy of TE cells cannot provide accurate results regarding the chromosomal make-up of the inner cell mass. The available clinical data suggest that PGT-A is probably harmful when IVF outcomes are analyzed by intention to treat or by live birth rate per cycle started rather than per embryo transfer, especially in women with three or fewer blastocysts. In addition, hypothesized advantages of reduced spontaneous abortion rate and reduced time to conception may be modest at best.
Keywords: In vitro fertilization, IVF, Preimplantation genetic testing for aneuploidy, PGT-A, Mosaicism, Trophectoderm biopsy, False positive, Clonal expansion, Clonal depletion, Self-correction
Introduction
Preimplantation genetic screening (PGS) or preimplantation genetic testing for aneuploidy (PGT-A) is a common add-on for in vitro fertilization. It has been estimated that in 2017, more than 20% of IVF clinics in the USA offered PGT-A to over 50% of their patients [1]. The theoretical advantages of PGT-A are: (1) to increase the live birth rate with single embryo transfer, (2) to reduce the time to pregnancy, and (3) to lower the chance of a miscarriage. Although the initial concept of testing the chromosomal make-up of the embryo to achieve these advantages appeared to be beyond reasonable doubt, in fact the situation is complicated by several factors including the presence of mosaicism in human embryos.
Meiotic errors can occur in the oocyte and less commonly in the spermatozoan. Meiotic errors are overwhelmingly attributed to nondisjunction and premature separation of sister chromatids as a result of defective chromosome-spindle attachments and/or weakening of cohesion [2] which occurs in the oocyte at the time of the first polar body extrusion during oocyte maturation or at the time of the second polar body extrusion with fertilization. Meiotic errors increase with maternal age [2, 3] and affect all the chromosomes of the embryo uniformly. Fertilization errors such as dispermy will also affect all the cells of the embryo. In contrast, mosaicism may result from an error in cytokinesis during mitosis as early as the first cleavage division after fertilization [4, 5]. The first mitotic division in the zygote appears to have a prolonged prometaphase/metaphase duration allowing nondisjunction with aberrant chromosome segregations and lagging chromosomes that may be visible as micronuclei [5]. As a result, embryos may contain a mixture of cells with different chromosomal ploidy. Mitotic errors do not seem to be affected by increasing age [5]. An important concept to understand is that mitotic activity in the cells of a human embryo results in clonal proliferation resulting in a cluster of contiguous cells with the same chromosomal make-up. In other words, if a cell undergoes a mitotic error to become aneuploid, it will divide into two complementary daughter cells, one with a gain of chromosomes and the other with a loss of chromosomes. Both cells will be aneuploid and continue to divide to form two clusters of contiguous aneuploid cells.
A less common cause of mosaicism is rescue of some cells in an aneuploid embryo. The embryo may undergo nondisjunction in meiosis to become aneuploid and then one cell could undergo trisomy or monosomy rescue. Most common is trisomy rescue of a single chromosome aneuploidy with the formation of a mosaic embryo with diploid/aneuploid cells [6, 7]; however, in this case, the diploid cell line may have uniparental disomy (UPD) of the rescued chromosome. UPD, the inheritance of two homologous chromosomes from the same parent, may lead to unmasking of recessive gene mutations or an error in imprinted genes from abnormal methylation patterns. The incidence of this type of mosaicism is unknown but is thought to be rare [7].
Mosaicism and problems with blastomere biopsies of embryos
Mosaicism happens very early after fertilization and is surprisingly frequent in early embryos [4, 5]. A 2011 review [8] looked at 815 spare human embryos that were donated for research. Each of the embryos was taken apart and each cell was tested for ploidy using fluorescence in situ hybridization (FISH) in 88% of embryos and array comparative genomic hybridization (aCGH) or other technique for the remaining 12%. The authors found that 60–70% of the embryos were aneuploid/diploid mosaics on day three and this percentage increased to 86% in 267 blastocysts [8].
This observation leads to the first problem with PGT-A. The first widespread commercial application of PGS involved biopsying one or two cells from an eight-cell day 3 embryo for determination of embryo ploidy. This test was initiated in the late 1990s [9] and was still being used by 2015, although the majority of cycles by then were undergoing blastocyst biopsy with comprehensive chromosome screening [10].
The technique of PGS in early embryos initially looked promising to increase the pregnancy rate and reduce the miscarriage rate, especially in patients with balanced translocations and chromosomally unbalanced segregation products of the rearrangement. However, a randomized controlled study published in the New England Journal of Medicine in 2007 by Mastenbroek and his colleagues [11] sent shock waves through the industry with the observation that PGS, rather than improving pregnancy rates, was detrimental with a reduction in live birth rate and no improvement in miscarriage rate.
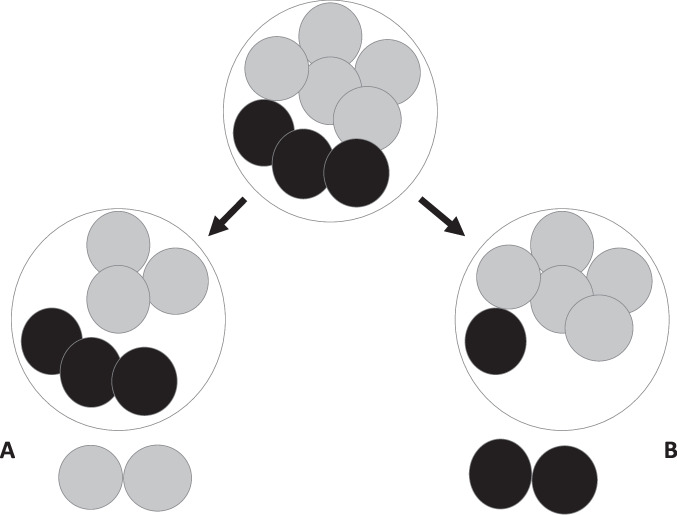
The authors believed a possible explanation for the findings was that biopsy of a blastomere on day 3 of embryonic development physically hampered the potential of an embryo to successfully implant. The second explanation was the FISH technique used to analyze the chromosome complement at the time. FISH used DNA probes attached to a fluorochrome and the probes bound to specific sites on a particular chromosome. This technique could only analyze a small number of chromosomes because there was a limited number of colored fluorochromes detectable using a fluorescence microscope. PGS might lead to the transfer of embryos labeled as normal but were aneuploid for chromosomes not detected by FISH. Suggestions were to abandon day 3 blastomere biopsy and move to trophectoderm biopsy at the blastocyst stage and to use a comprehensive assay of chromosomal number such as aCGH or other method to test all the chromosomes [11]. A less obvious problem at the time, but with potentially more impact on the ability of PGS to assess pregnancy outcome, was the presence of mosaicism and is illustrated in Fig. 1.
Fig. 1.
Illustration of effect of biopsying 2 blastomeres from an eight-cell mosaic embryo containing 5 euploid cells (grey) and three aneuploid cells (black). In example A, the diagnosis from the biopsy is euploid and the embryo is transferred but the biopsy has converted the embryo to a high-level mosaic. In example B, the diagnosis from the biopsy is aneuploid and the embryo would be discarded when in fact, the biopsy has converted the embryo to predominantly euploid cells
For example, if there is an 8-cell mosaic embryo with 3 aneuploid and 5 diploid cells, and the embryologist, by chance, selected two of the diploid cells for biopsy, the diagnosis would return euploid. The embryo would be transferred but unfortunately has been converted to 50% aneuploid cells by the biopsy (Fig. 1A). In contrast, if the embryologist, by chance, biopsies 2 of the aneuploid cells, the diagnosis of aneuploidy will be made. However, what has really happened is that the biopsy almost self-corrected the embryo which now contains >80% diploid cells, but nevertheless is discarded (Fig. 1B). In either case, nothing good has come from the biopsy and there is now less chance for the embryo to implant and become a live birth.
Mitotic errors occur early in development
It seems apparent that mitotic errors occur very early on in embryo development if 60–70% of embryos are already mosaic by day 3. Although trophectoderm (TE) biopsy was suggested to be more accurate with less impact on embryo implantation, the situation in terms of embryo testing becomes more complicated at the blastocyst stage especially if the mitotic errors happen even earlier than day 3.
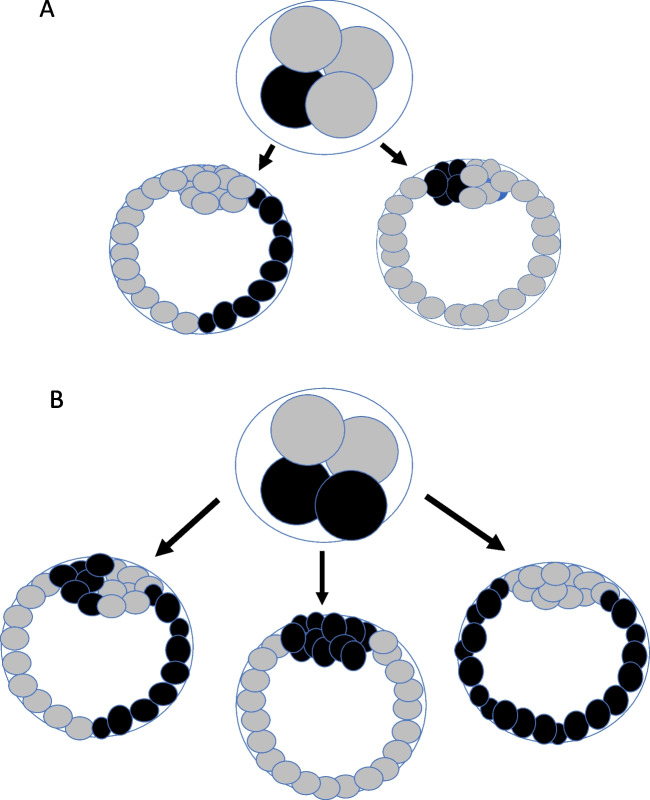
Some very simplistic scenarios are shown in Fig. 2 illustrating potential outcomes of TE biopsy if mitotic errors occur in 1 or 2 cells of a 4-cell embryo. Actual outcomes could be more complicated since further mitotic errors could occur at subsequent embryo developmental stages but the figures are illustrative of the concept of early mosaicism. Keeping in mind clonal proliferation, there are two possible results if one of the 4 cells is aneuploid depending on whether the aneuploid cells become incorporated into the inner cell mass (ICM) or the TE (Fig. 2A). If the aneuploid cells are in the TE, there is a 50% chance of a false-positive diagnosis of aneuploidy, if the biopsy happens in the group of contiguous cells that are aneuploid. If the aneuploid cells are incorporated into the ICM, the TE biopsy will always be diploid and the pregnancy rate will depend on whether the mosaic ICM self-corrects or not. If 2 of the 4 cells are aneuploid, there are three possible blastocyst outcomes (Fig. 2B). If both aneuploid cell lines are selected to form the ICM, the TE biopsy will be diploid, but the embryo will not implant or miscarry in most cases (false-negative error). If the 2 aneuploid cell lines are directed to form the TE, the biopsy result will be aneuploid, but the ICM is diploid and a normal baby could be born (false-positive error). Thirdly, if one aneuploid and one diploid cell line form both the ICM and the TE, the embryo will be uniformly mosaic and the biopsy result, by chance, could be euploid, aneuploid, or mosaic and the ICM may or may not self-correct.
Fig. 2.
Potential outcomes at the blastocyst stage if mitotic errors occur in one cell of a 4-cell embryo (A) or 2 cells of a 4-cell embryo (B) keeping in mind the concept of clonal proliferation
Any number of mitotic errors could happen further along in embryo development and could develop into multiple contiguous clumps of aneuploid cells throughout the embryo similar to the soccer ball analogy used previously to describe mosaic embryos [12].
Embryo self-correction
Another factor complicating the potential accuracy of PGT-A is the potential of the embryo, specifically the ICM, to self-correct. There were several mechanisms proposed for removal of aneuploid cells from an embryo including increased death or reduced proliferation of aneuploid cells, extrusion of an extra chromosome or duplication of a missing chromosome in aneuploid cells (trisomy or monosomy rescue), or preferential contribution of euploid cells to the inner cell mass [13]. However, there is convincing evidence from animal experiments that the most likely explanation is clonal depletion [14]. Clonal depletion refers to lineage specific loss of aneuploid cells at the blastocyst stage onwards. In the inner cell mass, aneuploid cells seem to be lost predominantly through apoptosis, a relatively quick process. In contrast, aneuploid cells in the trophectoderm are depleted more slowly through differential proliferation, i.e., increased cycle length and development of senescence [14]. In general, murine mosaic embryos undergo depletion of aneuploid cells from the blastocyst stage onwards and can develop fully into healthy pups as long as the starting number of euploid cells is adequate (likely in the range of 33% or more) [14]. Recently, a study of human embryos in extended culture demonstrated self-correction by their ability to eliminate abnormal blastomeres as cell debris or fragments [15].
Problems with whole genome amplification and other technical issues
PGT-A requires biopsy of a small number of trophectoderm cells which initially were thought to be representative of the entire blastocyst. Because of the small quantity of DNA extracted from these cells, whole genome amplification of the DNA is required in order to obtain enough DNA for sequencing. Whole genome amplification of a very low quantity of DNA can introduce artifacts, specifically allelic dropouts, that can lead to loss of heterozygosity. In addition, technical problems can arise and may vary with different embryology labs. Highly skilled embryologists are needed for precise blastomere biopsy and to prevent pipetting and loading errors in transferring the biopsied cells for transport to the genetic testing lab. Specifically, the biopsied TE cells should be washed several times to remove any contamination with overlay oil or culture medium. The washed cells should be loaded in the smallest possible volume of lysis buffer, ideally 2 μl (Dr Chaim Jalas, personal communication). Poor preparation and loading of samples or low amounts of DNA can lead to failure of amplification or excessive background “noise” leading to a no-result or inaccurate diagnosis. A recent study compared whole genome amplification errors in both human blastomeres and human embryonic stem cell lines [16]. The authors showed that 26.6% of human blastomeres with normal heterozygous loci on their alleles appeared to have loss of heterozygosity after whole genome amplification indicative of allelic dropouts. Loss of heterozygosity was more frequent in blastomeres than in embryonic stem cell lines [16]. This finding suggests that allelic dropouts are common with whole genome amplification of small amounts of DNA and may be a limiting step in the accuracy of NGS in human preimplantation embryos, leading to artifactual false-positive results.
Problems with data analysis
Almost all papers published regarding PGT-A give the results and analyze the data as pregnancy or live birth rate per embryo transfer. This type of analysis is misleading and obscures the potential inaccuracy of the technique as recently outlined nicely by Wilkinson [17]. The relevant statistic is number of live births per cycle started or live births based on an intention to treat (ITT) analysis. Below is a description of 4 relevant PGT-A studies selected to illustrate the importance of ITT analysis.
The first study is the STAR trial [18], thought to be a definitive study of the use of PGT-A. This was a multicenter study including 34 IVF centers and 9 testing labs all utilizing the Illumina platform for NGS. A total of 661 subjects between age 25 and 40 years was randomized to a single frozen embryo transfer (FET) with or without PGT-A. These were good prognosis patients with a mean of 7 blastocysts per subject in each group. In the study group, all blastocysts were biopsied for PGT-A and vitrified with the best morphological quality euploid embryo subsequently transferred. In the control group, the best morphological quality embryo was selected and was vitrified for subsequent FET and the remaining embryos were tested by PGT-A.
The results of the first embryo transfer showed that the live birth rate per embryo transfer and spontaneous abortion rate were not different in the study or control groups under age 35 years. A benefit of PGT-A was seen as an improved LBR/embryo transfer in the group over age 35. The authors concluded that there is a benefit to performing PGT-A in women over the age of 35 years [18]. However, when examining the data more closely, 42 subjects in the study group had no euploid embryos for transfer and 4 subjects in the control group had embryos that did not survive warming. Using ITT analysis in this study, there was no significant difference in LBR in the study group compared to the control group regardless of age (all subjects, PGT-A 33.8% LBR and control 35.8% LBR, P = non-significant and for subjects over age 35, PGT-A 31.6% LBR vs 28% LBR in controls, P = non-significant). Of interest, there was also no difference in the spontaneous abortion rate between the PGT-A and control groups [19].
The second study published in the New England Journal of Medicine in 2021 [20] was designed as an ITT analysis, again in good prognosis patients. A total of 1212 women with 3 or more blastocysts were enrolled and randomized to a study group (PGT-A) or a control group. The study group included 606 women in whom PGT-A was performed on the 3 best quality blastocysts. The control group had three good quality blastocysts selected by morphology alone. Cumulative live birth rate after all transfers was the primary outcome measure. There were 468 women with a live birth in the PGT-A group (77.2%) and 496 live births in the control group (81.8%). The difference between the two group just reached significance, therefore, suggesting a possible detrimental effect of PGT-A on LBR in good prognosis patients [20].
The third selected study was performed in relatively poor prognosis patients with 3 or fewer blastocysts [21]. The study group included 130 women who decided to do PGT-A on their blastocysts and the control group included 130 women matched for age, BMI, number of embryos, and embryo morphologic grade who did not select to do PGT-A. There were 263 blastocysts in each group but only 90 euploid embryos (34% of the total) available for transfer in the PGT-A group. Live birth rates and spontaneous abortion rates are shown in Fig. 3. The LBR was more than double in the control group compared to the study group but the spontaneous abortion rate was identical in each group.
Fig. 3.
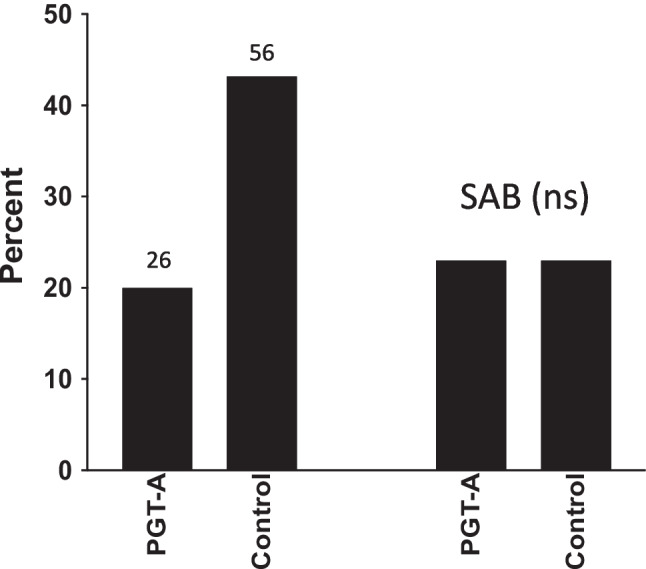
Live birth rate (LBR) and spontaneous abortion rate (SAB) in 130 subjects with 3 or fewer blastocysts who underwent trophectoderm biopsy for PGT-A compared to 130 matched controls with 3 or fewer blastocysts who did not do PGT-A. ns, non-significant (adapted from ref 21)
This study also explored the time to pregnancy which was shorter in the control group since about a third of the women who conceived did so with their fresh embryo transfer whereas women in the PGT-A group had their first embryo transfer delayed by at least a month while waiting for the PGT-A result. The cumulative live birth rate over time was always greater in the control group [21]. In summary, this study in poor prognosis patients showed more than double the LBR in the control group vs the PGT-A group with no difference in spontaneous abortion rates. The control group also had another 85 embryos stored frozen awaiting transfer compared to only 7 in the PGT-A group, suggesting that the overall difference in LBR would be even greater.
The final selected study might also be considered definitive based on its large size and the transfer of all suitable embryos [22]. This study included 2064 cycles in women with diminished ovarian reserve in whom only one good quality blastocyst was available after IVF. The study group included 1126 women who decided to do PGT-A while the control group included 938 women who had an embryo transfer cycle without PGT-A. The authors analyzed the data by live birth rate per embryo transfer and concluded that PGT-A should be offered to women with one blastocyst since the LBR was significantly higher [22]. However, there were only 225 diagnosed euploid embryos available for transfer in the 1126 women undergoing PGT-A (20% of cycles). That means that 80% of women in the PGT-A group had no embryos for transfer whereas in the control group, all 938 women had an embryo transfer. There were 115 live births in the 225 women with PGT-A for a live birth rate of 50% per embryo transfer. In the 938 non-PGT cycles, there were 278 live births for a live birth rate of 30%. The authors conclusion of benefit from PGT-A was based on this difference in pregnancies per embryo transfer.
The actual LBR per cycle is shown in Fig. 4 and illustrates a 3-fold higher LBR in the control group. In addition, there was no difference in the SAB rate. If PGT-A had not been performed in the 1126 cycles, it is possible that another 223 babies could have been born based on the LBR of 30% in the control group. This large study, therefore, demonstrated that PGT-A resulted in irreparable harm in women who only have one blastocyst for transfer and only one chance to conceive from their IVF cycle.
Fig. 4.
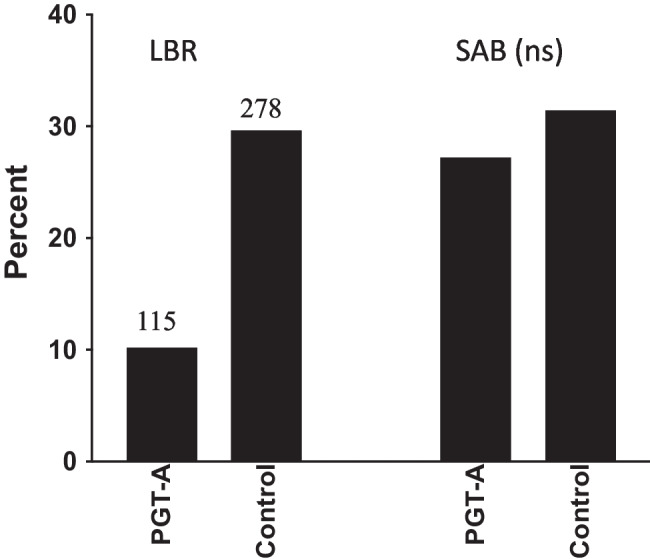
Comparison of live birth rate (LBR) and spontaneous abortion rate (SAB) in 1126 women with one good quality blastocyst who had TE biopsy for PGT-A and 938 control women with one good quality blastocyst who transferred their embryos without PGT-A. ns, non-significant (adapted from ref 22)
Spontaneous abortion rate and PGT-A
It should be a foregone conclusion that the transfer of euploid embryos would reduce the spontaneous loss rate in IVF embryo transfers. However, only one study [20] showed minimal benefit while none of the other studies described here [18, 21, 22] showed any effect of PGT-A to reduce the spontaneous abortion rate compared to untested embryos. Many of the studies were likely underpowered and it is possible that much larger studies would show a difference. A meta-analysis published in 2020 [19] examined 13 trials involving 2794 women and showed no difference in SAB between PGT-A and non-PGT-A cycles but various technologies were used for PGT-A. A large study in women with recurrent pregnancy loss [23] compared 4288 cycles with PGT-A and 4116 cycles without PGT-A in couples with RPL. They found an increased LBR per embryo transfer in the PGT-A group (no report of number of cycles without embryo transfer) and a modest (10.8% vs 12.6%, P = 0.02) reduction in SAB rate overall.
The issue of SAB rate is complicated since many factors other than aneuploidy may be important for pregnancy loss. It appears that about half of pregnancy losses in the general population are euploid [24–26]. Even women with recurrent pregnancy loss have been shown to have about a 50% rate of euploid products of conception [27]. There are several possible causes for euploid embryo losses. One factor may be poor overall blastocyst quality including grading of reduced trophectoderm quality or poor inner cell mass development or overall developmental delay [28]. Endometrial factors such as dysbiosis may be associated with loss of a euploid fetus [29, 30]. Autoimmune or thrombophilia issues have been linked to euploid pregnancy loss [31] as has elevated BMI [28, 32]. Finally, while PGT-A can identify chromosomal aneuploidies, it cannot determine ploidy level nor the presence of pathogenic microdeletions responsible for genomic disorders that could lead to SAB [33]. All of these factors may obscure the benefit of PGT-A in preventing pregnancy loss.
Cost-effectiveness of PGT vs no-PGT
In terms of cost-effectiveness, there are financial and emotional costs to both PGT-A cycles and non-PGT-A cycles. Assuming PGT-A is accurate, one would financially compare the cost of the embryo biopsy and PGT-A assay with the possibility and cost of multiple FET cycles to obtain a pregnancy in non-PGT-A patients. Emotionally, one would have to balance the possibility of having no embryos for transfer in a PGT-A cycle vs failed FET cycles or increased risk of spontaneous abortion or a chromosomal syndromic baby. On the other hand, if PGT-A is not accurate, cost-effectiveness shifts in favor of non-PGT-A. False-positive PGT-A diagnoses lead to increased risk of no embryos for transfer, especially in women with few embryos, and the requirement of another IVF cycle with both financial and emotional costs. In addition, most studies showed no reduction in spontaneous abortion rates with PGT-A (whether due to false negatives or other issues) leading to equal emotional costs from pregnancy loss for both PGT-A and non-PGT-A cycles. Nevertheless, there are some indications for PGT-A in its present state. Patients with a previous pregnancy termination for trisomy 21 or other syndromic chromosomal pregnancy will likely request PGT-A in an effort to prevent another highly emotional poor pregnancy outcome. Older women with a large number of blastocysts may also benefit from PGT-A to avoid multiple embryo transfers with negative outcome and to shorten the time to pregnancy. Finally, PGT-A with transfer of a euploid embryo is likely indicated with the use of a gestational carrier to reduce the number of embryo transfers and potentially avoid an adverse outcome.
Conclusion
PGT-A, as it is presently performed, relies on whole genome amplification of small amounts of DNA from the trophectoderm for determination of loss or gain of chromosomal material by next-generation sequencing. Whole genome amplification may introduce artifacts such as allele dropout and loss of heterozygosity in up to 25% of cases. In addition, there are technical issues which may affect DNA amplification and these can vary from lab to lab. As long as biopsy of TE cells is the only option, PGT-A cannot provide accurate results regarding the chromosomal make-up of the inner cell mass because of the high prevalence of mosaicism, which begins in the early stages of embryo development. The available data suggest that PGT-A is probably harmful when the results are analyzed by intention to treat or by live birth rate per cycle started rather than per embryo transfer, especially in women with three or fewer blastocysts. In addition, the hypothesized advantages of reduced spontaneous abortion rate and reduced time to conception are likely to be modest at best. It appears that the only way to improve the diagnostic accuracy of PGT-A is by finding a method (preferably non-invasive) to assess the inner cell mass at later stages of embryo development so that self-correction has had a chance to occur in the face of mosaicism. There are already some interesting preliminary results to support analysis of morphokinetic data using artificial intelligence algorithms as a potential way to rank embryos most likely to be diploid [34, 35]. At the present time, however, none of these technologies has been validated nor is ready for general clinical use.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Roche K, Racowsky C, Harper J. Utilization of preimplantation genetic testing in the USA. J Assist Reprod Genet. 2021;38:1045–1053. doi: 10.1007/s10815-021-02078-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patel J, Tan SL, Hartshorne GM, McAinsh AD. Unique geometry of sister kinetochores in human oocytes during meiosis I may explain maternal age-associated increases in chromosomal abnormalities. Biol. Open. 2016;5:178–184. doi: 10.1242/bio.016394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herbert M, Kalleas D, Cooney D, Lamb M, Lister L. Meiosis and maternal aging: insights from aneuploid oocytes and trisomy births. Cold Spring Harb. Perspect. Biol. 2015;7:a017970. doi: 10.1101/cshperspect.a017970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCoy RC. Mosaicism in preimplantation human embryos: when chromosomal abnormalities are the norm. Trends Genet. 2017;33:448–463. doi: 10.1016/j.tig.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Currie CE, Ford E, Benham Whyte L, Taylor DM, Mihalas BP, Erent M, Marston AL, Hartshorne GM, McAinsh AD. The first mitotic division of human embryos is highly error prone. Nat Commun. 2022;13:6755. doi: 10.1038/s41467-022-34294-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Polonis K, Lopes JL, Cabral H, Babcock HE, Kline L, Ruiz KM, Schwartz S, Hasadsri L, Rowsey RA, Hoppman NL. Uniparental disomy of multiple chromosomes in two cases with a complex phenotype. Am J Med Genet A. 2023;191:1978–1983. doi: 10.1002/ajmg.a.63224. [DOI] [PubMed] [Google Scholar]
- 7.Kotzot D. Complex and segmental uniparental disomy (UPD): review and lessons from rare chromosomal complements. J Med Genet. 2001;38:497–507. doi: 10.1136/jmg.38.8.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Echten-Arends J, Mastenbroek S, Sikkema-Raddatz B, Korevaar JC, Heineman MJ, van der Veen F, Repping S. Chromosomal mosaicism in human preimplantation embryos: a systematic review. Hum Reprod Update. 2011;17:620–627. doi: 10.1093/humupd/dmr014. [DOI] [PubMed] [Google Scholar]
- 9.Munné S, Magli C, Cohen J, Morton P, Sadowy S, Gianaroli L, et al. Positive outcome after preimplantation diagnosis of aneuploidy in human embryos. Hum Reprod. 1999;14:2191–2199. doi: 10.1093/humrep/14.9.2191. [DOI] [PubMed] [Google Scholar]
- 10.Coonen E, van Montfoort A, Carvalho F, Kokkali G, Moutou C, Rubio C, et al. ESHRE PGT Consortium data collection XVI–XVIII: cycles from 2013 to 2015. Human Reprod Open. 2020;4:hoaa043. doi: 10.1093/hropen/hoaa043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mastenbroek S, Twisk M, van Echten-Arends J, Sikkema-Raddatz B, Korevaar JC, Verhoeve HR, et al. In vitro fertilization with preimplantation genetic screening. N Engl J Med. 2007;357:9–17. doi: 10.1056/NEJMoa067744. [DOI] [PubMed] [Google Scholar]
- 12.Paulson RJ. https://uscfertility.org/video-preimplantation-genetic-screening/.
- 13.Bazrgar M, Gourabi H, Valojerdi MR, Yazdi PE, Baharvand H. Self-correction of chromosomal abnormalities in human preimplantation embryos and embryonic stem cells. Stem Cells Dev. 2013;22:2449–2456. doi: 10.1089/scd.2013.0053. [DOI] [PubMed] [Google Scholar]
- 14.Bolton H, Graham SJL, Van der Aa N, Kumar P, Theunis K, Fernandez Gallardo E, Voet T, Zernicka-Goetz M. Mouse model of chromosome mosaicism reveals lineage-specific depletion of aneuploid cells and normal developmental potential. Nat Commun. 2016;7:11165. doi: 10.1038/ncomms11165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orvieto R, Shimon C, Rienstein S, Jonish-Grossman A, Shani H, Aizer A. Do human embryos have the ability of self-correction? Reprod Biol Endocrinol. 2020;18:98. doi: 10.1186/s12958-020-00650-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liang D, Mikhalchenko A, Ma H, Marti Gutierrez N, Chen T, Lee Y, Park SW, et al. Limitations of gene editing assessments in human preimplantation embryos. Nat Commun. 2023;14:1219. doi: 10.1038/s41467-023-36820-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilkinson J. Neither relevant nor randomized: the use of “per embryo transfer” in the analysis of preimplantation genetic testing for aneuploidy trials. Fertil Steril. 2023;119:910–912. doi: 10.1016/j.fertnstert.2023.03.020. [DOI] [PubMed] [Google Scholar]
- 18.Munné S, Kaplan B, Frattarelli JL, Child T, Nakhuda G, Shamma FN, et al. STAR Study Group. Preimplantation genetic testing for aneuploidy versus morphology as selection criteria for single frozen-thawed embryo transfer in good-prognosis patients: a multicenter randomized clinical trial. Fertil Steril. 2019;112:1071–1079.e7. doi: 10.1016/j.fertnstert.2019.07.1346. [DOI] [PubMed] [Google Scholar]
- 19.Cornelisse S, Zagers M, Kostova E, Fleischer K, van Wely M, Mastenbroek S. Preimplantation genetic testing for aneuploidies (abnormal number of chromosomes) in in vitro fertilisation. Cochrane Database Syst Rev. 2020;9(9):CD005291. doi: 10.1002/14651858.CD005291.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yan J, Qin Y, Zhao H, Sun Y, Gong F, Li R, et al. Live birth with or without preimplantation genetic testing for aneuploidy. N Engl J Med. 2021;385:2047–2058. doi: 10.1056/NEJMoa2103613. [DOI] [PubMed] [Google Scholar]
- 21.Mahesan AM, Chang PT, Ronn R, Paul ABM, Meriano J, Casper RF. Preimplantation genetic testing for aneuploidy in patients with low embryo numbers: benefit or harm? J Assist Reprod Genet. 2022;39:2027–2033. doi: 10.1007/s10815-022-02588-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kahraman S, Duzguner INB, Sahin Y, Irez T. What to advise to patients with only one good quality blastocyst, PGT-A or not? Outcomes of 2064 cycles. J Assist Reprod Genet. 2022;39:2555–2562. doi: 10.1007/s10815-022-02617-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhatt SJ, Marchetto NM, Roy J, Morelli SS, McGovern PG. Pregnancy outcomes following in vitro fertilization frozen embryo transfer (IVF-FET) with or without preimplantation genetic testing for aneuploidy (PGT-A) in women with recurrent pregnancy loss (RPL): a SART-CORS study. Hum Reprod. 2021;36:2339–2344. doi: 10.1093/humrep/deab117. [DOI] [PubMed] [Google Scholar]
- 24.Gu C, Li K, Li R, Li L, Li X, Dai X, He Y. Chromosomal aneuploidy associated with clinical characteristics of pregnancy loss. Front Genet. 2021;12:667697. doi: 10.3389/fgene.2021.667697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao W, Chen P, Liu X, Li Y, Liang X, Li J. Comparison of aneuploidy rate in spontaneous abortion chorionic villus between D6 and D5 thawed-frozen blastocyst transfer. BMC Pregnancy Childbirth. 2023;23:130. doi: 10.1186/s12884-023-05452-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Menasha J, Levy B, Hirschhorn K, Kardon NB. Incidence and spectrum of chromosome abnormalities in spontaneous abortions: new insights from a 12-year study. Genet Med. 2005;7:251–263. doi: 10.1097/01.GIM.0000160075.96707.04. [DOI] [PubMed] [Google Scholar]
- 27.Zhu D, Wei X, Zhou XY, Deng LB, Xiong SY, Chen JP, Chen GQ, Zou G, Sun LM. Chromosomal abnormalities in recurrent pregnancy loss and its association with clinical characteristics. J Assist Reprod Genet. 2023;40:1713–1720. doi: 10.1007/s10815-023-02816-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cimadomo D, Rienzi L, Conforti A, Forman E, Canosa S, Innocenti F, et al. Opening the black box: why do euploid blastocysts fail to implant? A systematic review and meta-analysis. Hum Reprod Update. 2023;16:dmad010. doi: 10.1093/humupd/dmad010. [DOI] [PubMed] [Google Scholar]
- 29.Grewal K, Lee YS, Smith A, Brosens JJ, Bourne T, Al-Memar M, Kundu S, MacIntyre DA, Bennett PR. Chromosomally normal miscarriage is associated with vaginal dysbiosis and local inflammation. BMC Med. 2022;20:38. doi: 10.1186/s12916-021-02227-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mori R, Hayakawa T, Hirayama M, Ozawa F, Yoshihara H, Goto S, Kitaori T, Ozaki Y, Sugiura-Ogasawara M. Cervicovaginal microbiome in patients with recurrent pregnancy loss. J Reprod Immunol. 2023;157:103944. doi: 10.1016/j.jri.2023.103944. [DOI] [PubMed] [Google Scholar]
- 31.Vandevelde A, Gris JC, Moore GW, Musiał J, Zuily S, Wahl D, Devreese KMJ. Added value of antiphosphatidylserine/prothrombin antibodies in the workup of obstetric antiphospholipid syndrome: communication from the ISTH SSC Subcommittee on Lupus Anticoagulant/Antiphospholipid Antibodies. J Thromb Haemost. 2023;21:1981–1994. doi: 10.1016/j.jtha.2023.04.001. [DOI] [PubMed] [Google Scholar]
- 32.Melado Vidales L, Lawrenz B, Vitorino RL, Patel R, Ruiz FJ, Marques LM, Bayram A, Elkhatib I, Fatemi H. Clinical and laboratory parameters associated with cycle outcomes in patients undergoing euploid frozen blastocyst transfer. Reprod Biomed Online. 2023;46:917–925. doi: 10.1016/j.rbmo.2023.02.014. [DOI] [PubMed] [Google Scholar]
- 33.Caroselli S, Figliuzzi M, Picchetta L, Cogo F, Zambon P, Pergher I, et al. Improved clinical utility of preimplantation genetic testing through the integration of ploidy and common pathogenic microdeletions analyses. Hum Reprod. 2023;38:762–775. doi: 10.1093/humrep/dead033. [DOI] [PubMed] [Google Scholar]
- 34.Yuan Z, Yuan M, Song X, Huang X, Yan W. Development of an artificial intelligence based model for predicting the euploidy of blastocysts in PGT-A treatments. Sci Rep. 2023;13:2322. doi: 10.1038/s41598-023-29319-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Diakiw SM, Hall JMM, VerMilyea MD, Amin J, Aizpurua J, Giardini L, et al. Development of an artificial intelligence model for predicting the likelihood of human embryo euploidy based on blastocyst images from multiple imaging systems during IVF. Hum Reprod. 2022;37:1746–1759. doi: 10.1093/humrep/deac131. [DOI] [PMC free article] [PubMed] [Google Scholar]