Abstract
Purpose
To identify new mutations in DNAH17 that cause male infertility and analyze intracytoplasmic sperm injection (ICSI) outcomes in patients with DNAH17 mutations.
Methods
A total of five cases of new DNAH17 mutations exhibiting the multiple morphological abnormalities of the sperm flagella (MMAF) phenotype were identified through semen analysis and genetic testing. They were recruited at our reproductive medicine center from September 2018 to July 2022. Information on DNAH17 genetic mutations and ICSI outcomes was systematically explored following a literature review.
Results
Three novel compound mutations in DNAH17 were identified in patients with male infertility caused by MMAF. This study and previous publications included 21 patients with DNAH17 mutations. DNAH17 has been associated with asthenozoospermia and male infertility, but different types of DNAH17 variants appear to be involved in different sperm phenotypes. In 11 couples of infertile patients with DNAH17 mutations, there were 17 ICSI cycles and 13 embryo transplantation cycles. Only three men with DNAH17 variants ultimately achieved clinical pregnancy with their partners through ICSI combined with assisted oocyte activation (AOA).
Conclusions
Loss-of-function mutations in DNAH17 can lead to severe sperm flagellum defects and male infertility. Patients with MMAF-harboring DNAH17 mutations generally have worse pregnancy outcomes following ICSI. ICSI combined with AOA may improve the outcome of assisted reproductive techniques (ARTs) for men with DNAH17 variants.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10815-023-02897-7.
Keywords: DNAH17, Whole exome sequencing, Intracytoplasmic sperm injection, MMAF
Introduction
Multiple morphological abnormalities of the sperm flagella (MMAF) is a special phenotype of asthenoteratospermia, mainly manifested by various abnormally different combinations of sperm flagella (including absent, coiled, short, and irregular-caliber flagella) and severely impaired sperm mobility [1]. In humans, motile cilia and sperm flagella have similar ultrastructures, which are evolutionarily conserved. The ultrastructure of motile cilia and sperm flagella is represented as the typical “9 + 2” axis structure, comprising nine peripheral microtubule doublets (MTDs) plus two central pairs, a radiation axis, an inner and outer dynamic protein arm (IDA and ODA, respectively), and several attached structures. Abnormalities in the ultrastructure can lead to primary ciliary dyskinesia (PCD) and MMAF [2].
Genetic factors are the major causes of MMAF. With the popularity of whole exome sequencing (WES) and the decreased cost of this test, numerous pathogenic genes associated with MMAF have been identified by several research teams. Currently, more than 30 MMAF pathogenic genes have been identified, including DNAH family genes, CFAP family genes, DRC family genes, etc. [3]. Intracytoplasmic sperm injection (ICSI) is an important method for patients with MMAF to obtain biological offspring, but studies have suggested an increased rate of aneuploidy of the embryo, adverse pregnancy outcomes, and high miscarriage rates in the teratozoospermia group [4]. Currently, the earliest reported MMAF-related gene is DNAH1, which explains approximately 30% of the causes, and patients with DNAH1 gene mutations have a good ICSI prognosis [5].
Although motile cilia and sperm flagella have axonemal structures that are very similar, those organelles may have certain structural and/or protein content changes that could explain the existence of sperm flagella axonemal abnormalities. Several detrimental mutations in additional dynein axonemal heavy chain genes have been documented to cause male infertility due to asthenozoospermia, but not PCD. DNAH17 is located on chromosome 17 and contains 81 exons encoding an outer dynein arm protein, which is associated with ODA composition in sperm flagella [6]. Our previous study and several other groups have shown that DNAH17 mutations can lead to asthenoteratospermia and male infertility [7–9]. These studies suggest that DNAH17 plays a significant role in sperm flagellar assembly. Previous studies have found that patients harboring DNAH17 mutations generally have an unsuccessful pregnancy outcome following ICSI. Our previous study reported two cases with DNAH17 mutations that achieved satisfactory pregnancy outcomes after ICSI combined with assisted oocyte activation (AOA) [7]. This study aimed to explore new homozygous or compound heterozygous DNAH17 mutations with MMAF by WES and to determine their possible influences on assisted reproductive technique (ART) outcomes.
Materials and methods
Patient samples
This was a retrospective cohort study. We analyzed the clinical data, semen parameters, and genetic information of patients presenting with a typical MMAF and found five men diagnosed with DNAH17 mutations. All patients had a normal karyotype (46, XY) and no large-scale deletions in the Y chromosomes. All patients were enrolled from the First Affiliated Hospital of Anhui Medical University in China between September 2018 and July 2022. This study was approved by the institutional review board of the First Affiliated Hospital of the Anhui Medical University, and written informed consent was obtained from each patient.
Semen parameter analysis and sperm morphological analysis
Conventional semen parameters, including semen volume, sperm concentration, and progressive motility rate (PR), were determined using computer-assisted sperm analysis (CASA). All semen samples were collected by masturbation into sterile containers after 2–7 days of sexual abstinence and processed according to the WHO Processing Laboratory Manual (Fifth Edition). For sperm morphology analysis, semen smears were stained with Papanicolaou, and the percentage of abnormal sperm was determined by analyzing at least 200 sperm in each sample. Abnormal morphological features of sperm flagella include absent, short, coiled, or bent flagella and irregular caliber. All patients answered questions regarding the clinical manifestations of primary ciliary dyskinesia (PCD), and none of them had any other PCD-related symptoms besides infertility.
WES and Sanger sequencing
WES and bioinformatics analyses were performed according to our previously described protocols [7]. The novel variants were identified according to absence or rare reports in genetic variation databases (OMIM, 1000 Genomes Project, Exome Aggregation Consortium, and GnomAD). Missense, nonsense, frameshift, splice-site, and compound heterozygous variants were retained for subsequent analyses. Functional annotation of the genetic variants was conducted using ANNOVAR and other bioinformatics tools, such as SIFT, PolyPhen-2, and MutationTaster. The variants and their parental origins were subsequently validated using Sanger sequencing.
Assisted reproductive procedures
The female partners were also under fertility evaluation with normal ovary response and were treated with standard control ovarian stimulation of gonadotropin administration by a long protocol or antagonist protocol. Sperm were selected for ICSI after density graduation. After oocyte collection, intracytoplasmic sperm injection (ICSI) was performed as previously described. If AOA was used, 30 min after ICSI, the injected oocytes were chemically activated by exposure to 10 mM calcium ionophore (A23187, Sigma-Aldrich, St. Louis, MO, USA) for 5 min. Fertilization rate was assessed 18–19 h after ICSI. The embryos were cultured in cleavage medium and then blastocyst medium (Cook Medical, Bloomington, IN, USA) and incubated for 5 or 6 days. One to two viable embryos were transferred into the uterus of the female partner or those that were subjected to freeze–thaw cycles. Clinical pregnancy was confirmed by detection of a fetal heartbeat 5 to 6 weeks after embryo transfer.
Results
Mutation sites of DNAH17 reported in previous studies and this study
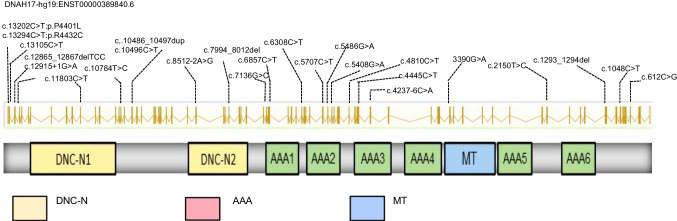
Three novel compound heterozygous variants in DNAH17 were reported in patients with MMAF after filtering variants through bioinformatic analyses and subsequent Sanger sequencing (Table 1, Sup Fig. 1). These variants are absent or very rare in human populations according to data from the 1000 Genomes Project, ExAC, and GnomAD databases. Furthermore, these variants were predicted to be disease causing by the MutationTaster, SIFT, and PolyPhen-2 tools. Nine previous studies included 18 patients with DNAH17 mutations, all of whom showed the MMAF phenotype or asthenoteratospermia (Table 1). A total of 21 cases of male infertility were confirmed with DNAH17 mutation, and the information of DNAH17 variants is shown in Fig. 1.
Table 1.
DNAH17 variants identified in previous studies and this study
| Individuals | Zygosity | Mutation | Phenotype | Ethnic (origin) |
References | |
|---|---|---|---|---|---|---|
| P1 | Het | c.1293_1294del; p.Tyr431* | c.7994_8012del; p.Gly2665Glufs*4 | MMAF | France/Italy | Whitfifield (2019) |
| P2 | Hom | c.5486G > A; p.Cys1829Tyr | MMAF | Algeria | Whitfifield (2019) | |
| P3 | Hom | c.5486G > A; p.Cys1829Tyr | MMAF | Algeria | Whitfifield (2019) | |
| P4 | Hom | c.10496C > T; p.Pro3499Leu | MMAF | Morocco | Whitfifield (2019) | |
| P5 | Hom | c.10486_10497dup; p.Val3496_Pro3499dup | MMAF | France | Whitfifield (2019) | |
| P6 | Het | c.C4445T; p.A1482V | c.C6857T; p.S2286L | MMAF | China | Sha (2019) |
| P7*3 | Hom | c.G5408A; p.C1803Y | Asthenozoospermia | Pakistan | Zhang (2020) | |
| P8 | Het | c.12915 + 1G > A | c.13202C > T;p. Pro4401Leu | MMAF | China | Song (2020) |
| P9 | Het | c.8512-2A > G | c.13294C > T; p. R4432C | MMAF | China | Song (2020) |
| P10*2 | Hom | c.6308C > T;p.Ala2103Val | c.11803C > T; p.Gln3935* | MMAF | Pakistan | Zhang (2021) |
| P11 | Hom | c.5707C > T;p.Arg1903Cys) | MMAF | China | Zhang (2021) | |
| P12 | Hom | c. 4810C > T; p.R1604C | MMAF | China | Zheng (2021) | |
| P13 | Het | c.1048 C > T; p.Arg350* | c.3390G > A;p.Met1130Ile | Asthenozoospermia | China | Jia (2021) |
| P14 | Hom | c.4810C > T; p.R1604C | MMAF | China | Li (2022) | |
| P15 | Hom | p.Trp1489* | MMAF | India | Sudhakar (2022) | |
| P16 | Het | c.12865_12867delTCC; p.Ser4289del | c.13105C > T; p.Arg4369* | MMAF | China | This study |
| P17 | Het | c.612C > G; p.Ile204Met | c.4237-6C > A; | MMAF | China | This study |
| P18 | Het | c.T2150C; p.L717P | c.G7136C; p.W2379S | MMAF | China | This study |
Het, heterozygous; Hom, homogeneous; MMAF, multiple morphological abnormalities of the sperm flagella
Fig. 1.
Identification of variants in DNAH17 in patients with asthenoteratospermia in previous studies and this study. DNAH17 protein contains two dynein heavy chain domains (DHC N1 and N2), six ATPase domains (ATPases associated with diverse cellular activities; AAAs), and a microtubule-binding region (MT). The positions of the identified variants in previous studies and this study are indicated
Analysis of semen parameters in patients with MMAF with DNAH17 mutations
In the analysis of the sperm parameters of patients with MMAF with DNAH17 mutations, our team found a total of five cases in patients with novel compound heterozygous variants in DNAH17; two cases (P8 and P9) were reported in our previous study, and three new cases (P16, P17, and P18) of DNAH17 mutation were identified in this study. The spermatozoa of P16, P17, and P18 presented very low motility (7.56%, 11.9%, and 27.3%, respectively), and progressive motility (6.48%, 0.8%, and 0.4%, respectively). Under the SEM and TEM scans of all patients with mutations, a large number of sperm had abnormal flagella, including shortness, irregular shape, coiling, or complete absence. In combination with other studies, all patients with DNAH17 mutations showed severe decreases in sperm vitality and most of them displayed abnormal sperm morphology with defects in sperm flagella (Table 2).
Table 2.
Semen characteristics and sperm flagellar morphology in the DNAH17-mutated men
| Semen characteristics | Flagellar abnormalities | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Individuals | Volume (ml) |
sperm concentration (106/ml) |
Total Motility (%) |
Progressive Motility (%) |
Absent (%) |
Short (%) |
Irregular Caliber (%) |
Coiled (%) |
Multiple (%) |
Angulation (%) |
Typical Forms (%) |
| P1 | 2.5 | 20 | 10 | 1 | 5 | 2 | 36 | 27 | 1 | 11 | |
| P2 | 3.5 | 119 | 1 | 0 | 12 | 34 | 16 | 38 | 2 | 2 | |
| P3 | 3.8 | 27.7 | 1 | 0 | 16 | 34 | 14 | 38 | 0 | 4 | |
| P4 | 4.5 | 88.6 | 25 | 5 | 2 | 1 | 6 | 18 | 2 | 22 | |
| P5 | 6 | 198 | 20 | 2 | 2 | 2 | 12 | 22 | 0 | 12 | |
| P6 | 2.2 | 12.6 | 6.8 | 0 | 32 | 40 | 35 | 13 | 8 | 0.5 | |
| P7-1 | 3.3 | 18 | 11.5 | 5.5 | |||||||
| P7-2 | 2.0 | 30 | 25 | 17.5 | |||||||
| P7-3 | 3.5 | 78.4 | 15.1 | 9.4 | |||||||
| P8 | 2.7 | 30.7 | 21.6 | 12.8 | 6.9 | 24.1 | 0.6 | 41.3 | 0 | 27.1 | |
| P9 | 2.2 | 80.2 | 0.7 | 0.2 | 8.3 | 25.2 | 5.8 | 48.0 | 7.8 | 4.9 | |
| P10-1 | 2.7 | 27.3 | 2.8 | 0 | 7.2 | 14.9 | 7.6 | 32.3 | 22.6 | ||
| P10-2 | 3.0 | 32.5 | 4.4 | 0 | 9.3 | 14.9 | 8.8 | 35.3 | 18.4 | ||
| P11 | 2.0 | 35.3 | 4.0 | 2 | 8.8 | 35.3 | 12.2 | 23.3 | 2.0 | ||
| P12 | 4.9 | 10 | 5 | 38 | 18 | 13 | 13 | 17.5 | 0.5 | ||
| P16 | 3.6 | 47.85 | 7.56 | 6.48 | 7.5 | 35.1 | 4.6 | 47.3 | 8.0 | 3.5 | |
| P17 | 2.7 | 10.6 | 11.9 | 0.8 | 9.2 | 26.8 | 5.8 | 48.0 | 9.2 | 1.8 | |
| P18 | 3.5 | 44.7 | 27.3 | 0.4 | 10.2 | 33.5 | 10.2 | 43.3 | 1.5 | 1.3 | |
Assisted reproduction outcome of patients with MMAF with DNAH17 mutations
In combination with previous study, a total of 11 patients with DNAH17 mutant MMAF received assisted reproduction treatment (Table 3). Patients P1–P4 and P12 received 1–3 rounds of ICSI, but did not get pregnant. Patients P13 with IVF/ICSI/ICSI + AOA failed to fertilize. Finally, they successfully became pregnant after IVF with donor sperm. Patients P8, P9, and P16 received ICSI + AOA treatment in our center and obtained successful clinical pregnancies. Patient P17 with ICSI joint AOA did not succeed received five high-quality embryos with ICSI with donor sperm. Patients P18 received many ICSI/ICSI + AOA attempts with no clinical pregnancies, and the remaining two high-quality embryos (ICSI + AOA cycle) were not transplanted. In general, three couples ultimately got pregnant ICSI combined with AOA and one couple achieved clinical pregnancy with donor sperm.
Table 3.
Outcomes of ART in the couples with the DNAH17-mutated men
| Individuals | Male age (years) | Female age (years) | Oocytes collected | Oocytes | Source of sperm | Fertilization | Insemination | Blastocyst | No. of transferred embryos | Clinical pregnancy |
|---|---|---|---|---|---|---|---|---|---|---|
| P1 | 36 | Husband | ICSI | No | ||||||
| P2 | 35 | Husband | ICSI | No | ||||||
| P3 | 37 | Husband | ICSI | No | ||||||
| P4 | 27 | Husband | ICSI | No | ||||||
| P8 | 32 | 33 | 14 | 14 | Husband | 12 | ICSI + AOA | 6 | 2 | Yes |
| P9 | 34 | 29 | 16 | 16 | Husband | 11 | ICSI + AOA | 8 | 2 | Yes |
| P12 | 32 | 31 | 13 | 11 | Husband | 8 | ICSI | 4 | 3 | No |
| P13 | 31 | 20 | 13 | Husband | 0 | IVF + R-ICSI | No | |||
| 15 | 10 | Husband | 0 | ICSI/ICSI + AOA | No | |||||
| 11 | Donor | 8 | IVF | Yes | ||||||
| P16 | 28 | 23 | Husband | ICSI | ||||||
| 28 | 23 | 14 | 8 | Husband | 6 | ICSI + AOA | 2 | 2 | Yes | |
| P17 | 32 | 30 | 8 | 8 | Husband | 4 | ICSI | 2 | 2 | No |
| 34 | 32 | 10 | 9 | Husband | 3/4 | ICSI + AOA | 2 | 2 | No | |
| Donor | 5/5 | ICSI | 5 | N | Not transplanted | |||||
| P18 | 33 | 32 | 5 | 3 | Husband | 1 | ICSI | 0 | 0 | No |
| 33 | 32 | 8 | 4 | Husband | 2 | ICSI + AOA | 2 | N | Not transplanted | |
| 34 | 32 | 9 | 4 | Husband | 1 | ICSI + AOA | 0 | 0 | No |
ART, assisted reproductive technique; ICSI, intracytoplasmic sperm injection; AOA, assisted oocyte activation; N, embryo not yet transplanted
Discussion
MMAF is a severe deformity of sperm due to multiple deformities of the sperm tail, which causes severe decreases in sperm activity and fertility ability. In tail movement, the ODA and IDA of the outer tubes play an important role. Essentially, they are ATP-enzymes, which provide energy for the movement of the spermatic tail. The destruction of the dynamical protein arm can also lead to a decrease in the stability of the axis structure of the sperm tail, which leads to insufficient sperm vitality and abnormal sperm forms.
Currently, 13 dynein axonemal heavy chains (DNAHs) have been identified in humans: DNAH1–3, DNAH5–12, DNAH14, and DNAH17 [8, 10, 11]. Several DNAH-related gene mutations can lead to MMAF. DNAH1 (MIM:603,332) was the first human MMAF pathogenic gene to be discovered. Patients with DNAH1 mutations displayed impaired sperm motility and morphological and ultrastructural disruptions of sperm flagella, thereby leading to male infertility associated with MMAF [11]. DNAH2 [12], DNAH6 [13], and DNAH10 [14] have also been associated with sperm flagella function. Loss of function of DNAH8, which encodes an ODA component, can also induce PCD and MMAF [10]. DNAH17 is a heavy chain protein located on the axonemal dynein arm that can hydrolyze ATP, thus providing energy for sperm movement. Animal studies have found a deficiency of 13 bp in the 55th intron of DNAH17 in pigs. This affects the normal expression of DNAH17, which produces multiple abnormal morphological and ultrastructural sperm flagella sperm, leading to pig infertility [15].
Whitfield et al. first discovered five DNAH17 genetic mutations in four families leading to MMAF and severe asthenozoospermia, but without PCD symptoms. The research team carried out ICSI treatment on the four families, but all were unsuccessful [8]. Subsequently, scholars found three infertile siblings in a Pakistani family with consanguineous union and asthenozoospermia. Transmission electron microscopy (TEM) revealed that the cross sections of the principal piece and end piece of sperm flagella showed a high proportion of MTD(s) 4–7 missing with a concomitant loss of the associated ODF(s) [9]. The group also found three novel loss-of-function variants of DNAH17 in men diagnosed with MMAF from two consanguineous families of Pakistani and Chinese origins. Their findings indicate that loss-of-function variants in DNAH17 disrupt sperm head shaping and flagellar development based on both patient and mouse models [16]. Other groups have subsequently reported multiple cases of DNAH17 mutations leading to severe asthenozoospermia and the MMAF phenotype. These studies showed a significant decrease in DNAH17 expression and a lack of ODA in mutant patients, indicating the special role played by DNAH17 in sperm vitality and sperm flagellar formation [17, 18]. It appears that different types of DNAH17 variants may also lead to different phenotypes. Jia et al. found one case of asthenozoospermia with DNAH17 mutation, with one ICSI and one ICSI + AOA showing complete fertilization failure, and eventually achieved successful pregnancy through IVF with donor sperm. They speculated that this full-fertilization dysfunction may be associated with DNAH17 mutation [19]. Yu et al. found that two patients with left–right asymmetry disorders carried a compound heterozygous mutation in DNAH17. The two patients were identified as one 50-year-old woman with no symptoms of PCD and one 5-year-old boy with severe congenital cardiomyopathy, who did not undergo ultrastructural analyses of the cilia and flagella [20].
Patients with MMAF may have different assisted reproductive outcomes based on their genetic background. Mutations such as DNAH1, DNAH8, TTC29, CFAP43, and CFAP44 typically lead to good ICSI outcomes, whereas other genes, such as CEP135 and FSIP2, can lead to unsuccessful pregnancies [21–23]. Similarly, DNAH17 mutations are associated with equally worse ICSI pregnancies. However, most studies on assisted pregnancies in infertile male patients with DNAH17 mutations failed to obtain satisfactory results. DNAH17 plays an important role not only in flagellar development, but also in sperm head shaping, according to Dnah17–/– mouse models [16]. Mice lacking DNAH17 displayed a misshaped spermatid heads with inappropriately enlarged acrosome, a distorted nucleus shape, and an ectopically positioned manchette [16]. This could explain the reduced ICSI fertilization rate and poor ART result in patients with the DNAH17 mutation. Taking all of this into consideration, we used AOA to ameliorate the situation. Our research team found that two patients with MMAF carrying DNAH17 variants acquired several high-quality embryos and ultimately achieved clinical pregnancy with their partners through ICSI combined with oocyte activation. One of the three newly discovered patients with the DNAH17 mutation had already received clinical pregnancy through the same protocol, and another couple acquired two available blastocyst embryos. Analyses of spermiation abnormalities and nuclear normalcy in patients with DNAH17 mutations would provide important clues regarding the fertilization and subsequent embryo development issues in these patients.
Studies have shown differences in clinical results after ICSI in patients with DNAH17 gene mutations, suggesting that the prognosis of ICSI treatment may be dose dependent with the expression of DNAH17, and that ICSI combined with AOA may improve the ART outcome in some patients. A study suggested that shorter periods of abstinence may produce sperm with a high proportion of normal sperm flagella, and that morphologically normal sperm may exist in the testis and epididymis [9]. For some patients with DNAH17 mutations, sperm selection for ICSI with secondary masturbation or testicular sperm can be considered. It is also recommended that donor sperm be considered after multiple cycles of ICSI failures. The clinical outcome of ICSI in patients with MMAF may have some correlation with the phenotypic and genetic background and should be further explored in the future on the basis of large, multi-centered samples.
In conclusion, our findings and systematic review of recent studies provide a deeper understanding of the complexities of MMAF etiology and pathogenesis, giving a theoretical foundation for genetic diagnosis and counseling in patients with MMAF.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (82101682) and Key research and development plan of Anhui Province (202004j07020032).
Data availability
Data are available upon reasonable request.
Declarations
Ethics approval and consent to participate
This study was approved by the Ethical Review Board of The First Affiliated Hospital of Anhui Medical University and was conducted according to the Declaration of Helsinki principles. Written informed consents were obtained from all enrolled patients.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Bing Song, Tianjin Yang, Qunshan Shen and Yiyuan Liu contributed equally to this work.
Contributor Information
Bing Song, Email: songbing060907@126.com.
Yunxia Cao, Email: caoyunxia6@126.com.
Xiaojin He, Email: hxj0117@126.com.
References
- 1.Coutton C, Escoffier J, Martinez G, Arnoult C, Ray PF. Teratozoospermia: spotlight on the main genetic actors in the human. Hum Reprod Update. 2015;21:455–485. doi: 10.1093/humupd/dmv020. [DOI] [PubMed] [Google Scholar]
- 2.Touré A, Martinez G, Kherraf ZE, Cazin C, Beurois J, Arnoult C, et al. The genetic architecture of morphological abnormalities of the sperm tail. Hum Genet. 2021;140:21–42. doi: 10.1007/s00439-020-02113-x. [DOI] [PubMed] [Google Scholar]
- 3.Jiao SY, Yang YH, Chen SR. Molecular genetics of infertility: loss-of-function mutations in humans and corresponding knockout/mutated mice. Hum Reprod Update. 2021;27:154–189. doi: 10.1093/humupd/dmaa034. [DOI] [PubMed] [Google Scholar]
- 4.Yang H, Liu Y, Niu W, Yang Z, Wang Y, Jin H, et al. Correlation study of male semen parameters and embryo aneuploidy in preimplantation genetic testing for aneuploidy. Front Endocrinol. 2022;13:1072176. doi: 10.3389/fendo.2022.1072176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wambergue C, Zouari R, Fourati Ben Mustapha S, Martinez G, Devillard F, Hennebicq S, et al. Patients with multiple morphological abnormalities of the sperm flagella due to DNAH1 mutations have a good prognosis following intracytoplasmic sperm injection. Hum Reprod. 2016;31:1164–72. doi: 10.1093/humrep/dew083. [DOI] [PubMed] [Google Scholar]
- 6.Chen L, Ouyang J, Li X, Xiao X, Sun W, Li S, et al. DNAH17 is essential for rat spermatogenesis and fertility. J Genet. 2021;100:14. 10.1007/s12041-021-01264-8 [PubMed]
- 7.Song B, Liu C, Gao Y, Marley JL, Li W, Ni X, et al. Novel compound heterozygous variants in dynein axonemal heavy chain 17 cause asthenoteratospermia with sperm flagellar defects. J Genet Genomics. 2020;47:713–717. doi: 10.1016/j.jgg.2020.07.004. [DOI] [PubMed] [Google Scholar]
- 8.Whitfield M, Thomas L, Bequignon E, Schmitt A, Stouvenel L, Montantin G, et al. Mutations in DNAH17, encoding a sperm-specific axonemal outer dynein arm heavy chain, cause isolated male infertility due to asthenozoospermia. Am J Hum Genet. 2019;105:198–212. doi: 10.1016/j.ajhg.2019.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang B, Ma H, Khan T, Ma A, Li T, Zhang H, et al. A DNAH17 missense variant causes flagella destabilization and asthenozoospermia. J Exp Med. 2020;217(2):e20182365. 10.1084/jem.20182365 [DOI] [PMC free article] [PubMed]
- 10.Liu C, Miyata H, Gao Y, Sha Y, Tang S, Xu Z, et al. Bi-allelic DNAH8 variants lead to multiple morphological abnormalities of the sperm flagella and primary male infertility. Am J Hum Genet. 2020;107:330–341. doi: 10.1016/j.ajhg.2020.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ben Khelifa M, Coutton C, Zouari R, Karaouzène T, Rendu J, Bidart M, et al. Mutations in DNAH1, which encodes an inner arm heavy chain dynein, lead to male infertility from multiple morphological abnormalities of the sperm flagella. Am J Hum Genet. 2014;94:95–104. doi: 10.1016/j.ajhg.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Y, Sha Y, Wang X, Ding L, Liu W, Ji Z, et al. DNAH2 is a novel candidate gene associated with multiple morphological abnormalities of the sperm flagella. Clin Genet. 2019;95:590–600. doi: 10.1111/cge.13525. [DOI] [PubMed] [Google Scholar]
- 13.Tu C, Nie H, Meng L, Yuan S, He W, Luo A, et al. Identification of DNAH6 mutations in infertile men with multiple morphological abnormalities of the sperm flagella. Sci Rep. 2019;9:15864. doi: 10.1038/s41598-019-52436-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tu C, Cong J, Zhang Q, He X, Zheng R, Yang X, et al. Bi-allelic mutations of DNAH10 cause primary male infertility with asthenoteratozoospermia in humans and mice. Am J Hum Genet. 2021;108:1466–1477. doi: 10.1016/j.ajhg.2021.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nosková A, Hiltpold M, Janett F, Echtermann T, Fang ZH, Sidler X, et al. Infertility due to defective sperm flagella caused by an intronic deletion in DNAH17 that perturbs splicing. Genetics. 2021;217(2):iyaa033. 10.1093/genetics/iyaa033 [DOI] [PMC free article] [PubMed]
- 16.Zhang B, Khan I, Liu C, Ma A, Khan A, Zhang Y, et al. Novel loss-of-function variants in DNAH17 cause multiple morphological abnormalities of the sperm flagella in humans and mice. Clin Genet. 2021;99:176–186. doi: 10.1111/cge.13866. [DOI] [PubMed] [Google Scholar]
- 17.Liu Z, Wang C, Ni F, Yang F, Wei H, Li T, et al. Novel compound heterozygous variants of DNAH17 in a Chinese infertile man with multiple morphological abnormalities of sperm flagella. Andrologia. 2022;54:e14553. doi: 10.1111/and.14553. [DOI] [PubMed] [Google Scholar]
- 18.Sha Y, Wei X, Ding L, Mei L, Huang X, Lin S, et al. DNAH17 is associated with asthenozoospermia and multiple morphological abnormalities of sperm flagella. Ann Hum Genet. 2020;84:271–279. doi: 10.1111/ahg.12369. [DOI] [PubMed] [Google Scholar]
- 19.Jia M, Shi R, Xue X. Novel DNAH17 mutations associated with fertilization failures after ICSI. Gynecol Endocrinol. 2021;37:769–771. doi: 10.1080/09513590.2021.1937979. [DOI] [PubMed] [Google Scholar]
- 20.Yu X, Yuan L, Deng S, Xia H, Tu X, Deng X, et al. Identification of DNAH17 variants in Han-Chinese patients with left-right asymmetry disorders. Front Genet. 2022;13:862292. doi: 10.3389/fgene.2022.862292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martinez G, Kherraf ZE, Zouari R, Fourati Ben Mustapha S, Saut A, Pernet-Gallay K, et al. Whole-exome sequencing identifies mutations in FSIP2 as a recurrent cause of multiple morphological abnormalities of the sperm flagella. Hum Reprod. 2018;33:1973–84. doi: 10.1093/humrep/dey264. [DOI] [PubMed] [Google Scholar]
- 22.Sha YW, Xu X, Mei LB, Li P, Su ZY, He XQ, et al. A homozygous CEP135 mutation is associated with multiple morphological abnormalities of the sperm flagella (MMAF) Gene. 2017;633:48–53. doi: 10.1016/j.gene.2017.08.033. [DOI] [PubMed] [Google Scholar]
- 23.Ferreux L, Bourdon M, Chargui A, Schmitt A, Stouvenel L, Lorès P, et al. Genetic diagnosis, sperm phenotype and ICSI outcome in case of severe asthenozoospermia with multiple morphological abnormalities of the flagellum. Hum Reprod. 2021;36:2848–2860. doi: 10.1093/humrep/deab200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request.