Abstract
Purpose
We aimed to investigate the additional value of preoperative PET/CT and reveal relationships between metabolic parameters, pericolic fat stranding finding, postoperative histopathology, and overall survival in colorectal cancer (CRC).
Methods
CRC patients who underwent preoperative PET/CT between January 2017-December 2021 were analyzed. Lymph nodes, organ metastases, and metabolic parameters were evaluated from PET/CT. The pericolic fat stranding was evaluated from CT component. Relationships between these factors and postoperative histopathological findings were statistically analyzed. Survival analyses were performed.
Results
Ninety-one patients (59 males, 32 females) were included in the study. All tumors showed high FDG uptake (mean SUVmax 19.5 ± 9.9). SUVmax of the tumor differed significantly at T3 and T4 stages (p = 0.041). A significant correlation was found between MTV, TLG values and the differentiation degree (p = 0.005, 0.003, respectively). PET/CT predicted the N stage with a high accuracy rate (80%). PET/CT found additional metastases that changed treatment decisions in one-third of patients. A relationship was found between tumor length, surgical margin, lymphovascular invasion and pericolic fat stranding. In multivariate analysis, differentiation degree (HR = 26.1, 95%CI 1.672–408.467), MTV (HR = 0.3, 95%CI 0.071–0.841), TLG (HR = 3.5, 95%CI 1.065–11.193), and lymphovascular invasion (HR = 0.2, 95%CI 0.026–0.853, p = 0.033) were independent factors affecting overall survival.
Conclusion
Preoperative PET/CT contributes to CRC management by detecting additional metastases as well as predicting prognosis and postoperative findings such as T stage, N stage and tumor differentiation. The SUVmax may differentiate between T3 and T4 tumor. Reporting of pericolic fat stranding may contribute to the estimation of lymphatic invasion and positive surgical margin.
Keywords: F-18-FDG PET, PET/CT, FDG, Colorectal cancer, Colon cancer, Pericolic fat stranding
Introduction
Colorectal carcinoma (CRC) is the third most common cancer and the second leading cause of death worldwide [1]. The main treatment is surgery, which may be combined with chemotherapy, and radiotherapy. Preoperative imaging plays a substantial role as clinical outcome and treatment strategies depend on tumor stage [2]. Guidelines recommend pan-colonoscopy in clinical suspicion of CRC and contrast-enhanced CT of the thorax, abdomen, and pelvis for pre-treatment staging after diagnosis [3, 4]. However, published data show that CT has insufficient accuracy in the evaluation of TNM staging [5, 6].
Fluorodeoxyglucose (FDG) positron emission tomography (PET) is widely used in the diagnosis, staging, restaging, evaluation of treatment response, and prognosis prediction of various malignancies [7]. PET/CT allows both visual and quantitative evaluation of lesions in the whole body. The most common metabolic parameter used to show the metabolic activity of the lesions is the standardized uptake value (SUV), which is obtained by dividing the mean activity in the area of interest by the injected dose. In addition to SUV, volumetric parameters such as metabolic tumor volume (MTV), which represents the volume of interest (VOI) of the lesion segmented by a threshold method, and total lesion glycolysis (TLG), calculated by multiplying the MTV by the SUV mean, are also being investigated to predict outcome in patients with malignancy [8–10].
PET-CT is used in CRC to detect distant metastases in advanced disease, to evaluate treatment response, and to detect recurrence in case of increased tumor markers [11]. Preoperative use of PET/CT is currently limited to the detection of other distant metastases in the presence of a surgically treatable metastasis. Recently, studies investigating the contribution of preoperative PET/CT to primary staging and prognosis in CRC have been increasingly reported [12, 13].
Pericolic fat stranding is the appearance of linear or nodular opacities on CT images in the adipose tissue around the colon wall. There are some studies on whether the pericolic fat stranding can be used in the staging by predicting extension beyond the muscle tissue in CRC [14, 15]. The compatibility of the pericolic fat stranding with postoperative pathology in CRC was investigated in a study [16]. However, its prognostic significance is still unclear.
In this study, we aimed to investigate the contribution of preoperative PET/CT metabolic parameters in predicting postoperative findings and prognosis in the CRC, as well as the complementary role of pericolic fat stranding finding to be reported in the CT component.
Methods
Patients
Data from 763 CRC patients, who underwent PET/CT between January 2017- December 2021 were retrospectively evaluated. Patients with preoperative PET/CT available clinical and radiological data were included in the study. Exclusion criteria were: age < 18 years, previous CRC treatment, the presence of other malignancies, abdominal inflammatory diseases, and lost to follow-up (Fig. 1). In a subgroup, whose detailed postoperative histopathology reports could be accessed, PET/CT parameters were analyzed in terms of predicting postoperative findings.
Fig. 1.

Flowchart of patient enrollment
Gender, age at the time of diagnosis, preoperative carcinoembryonic antigen (CEA) level, and postoperative histopathological data including tumor segmental length, depth, differentiation, surgical margin, lymphatic/blood vessel, perineural invasion were recorded.
This study was performed in accordance with the ethical concepts of the Helsinki Declaration in October 2013 and approved by the institutional ethical review board (No: TUTF-BAEK 2022/390).
Imaging and Image Analysis
The patients fasted for at least 4 h and oral contrast was administered 2 h prior to the examination. Patients with blood glucose < 200 mg/dl, received an intravenous injection of approximately 3.7 MBq/kg of FDG. All PET/CT scans were performed using a combined PET/CT system (Discovery STE; GE Medical Systems, Milwaukee, WI) after one hour of injection. CT images were taken from the skull to the mid-thigh by adjusting the CT parameters to 120 kV, 200 mAs with 16 slice CT. After that, PET images were recorded from the skull to the mid-thigh in 3D mode, 4 min per bed position, and reconstructed by an iterative method. Axial, coronal, and sagittal reformatted images and PET-CT fusion images were created.
PET/CT images were interpreted blindly by two nuclear medicine physicians. By visual evaluation, organs and lymph nodes showing more FDG uptake than normal organs or surrounding tissue in PET/CT images were considered metastatic lesions. A consensus was obtained to classify lesions as metastatic by examining follow-up PET, CT, MR images, histopathological sampling, and treatment response. The SUVmax, MTV, and TLG values for primary tumor and metastatic lesions were automatically calculated by PET VCAR application (Advanced Workstation 4.4; GE Medical Systems). The SUVmax value was the highest SUV in the lesion by plotting a VOI on PET images. The MTV was the total tumor volume greater than 41% of SUVmax [7]. TLG was calculated automatically as the product of MTV and SUVmean. The pericolic fat stranding was assessed visually from the CT component.
Statistical Analysis
Statistical analyses were performed using SPSS version 25 (SPSS, Inc., Chicago, IL). Descriptive data were presented as numbers, mean and ± SD for quantitative data. Percentage values were given for qualitative data. Normality test was performed to determine whether the sample data were normally distributed. The relationship between categorical variables was evaluated by Chi-square and Fisher’s exact test. Student’s t-tests were used for the analysis of paired independent samples. Differences between groups were assessed with one-way ANOVA or Kruskal–Wallis test followed by Tukey post-hoc tests for continuous variables. All p values with P < 0.05 was considered statistically significant. Univariate analyzes were conducted to analyze the effect of age, gender, TNM staging, histopathological features, PET findings, and pericolic fat stranding on overall survival (OS). OS curves were created using Kaplan–Meier estimates. Using the Cox proportional hazard model, multivariate analyzes were performed to identify the best independent factors. Only variables that predicted OS by univariate analyses were included in the multivariate analysis. Since TLG was calculated by multiplying MTV and SUVmean, separate models were created for MTV and TLG due to the correlation between them.
Results
Patient and Tumor Characteristics
Ninety-one patients (32 females, 59 males) with preoperative PET/CT were included in the study. The mean age at the time of diagnosis was 66 ± 11 (Minimum: 33, maximum: 91) years. The mean preoperative CEA value was 73.4 ± 75.2 ng/mL (Minimum: 0.6 ng/mL, maximum: 1048 ng/mL). The mean tumor length was 6.0 ± 2.4 (Minimum: 1.0, maximum: 11.8) cm. The details of patient and tumor characteristics are given in Table 1.
Table 1.
Clinicopathological data of 91 patients with CRC (n)
| n (%) | |
|---|---|
| All patients | 91 |
| Age | |
| < 60 years | 20 (22.0) |
| ≥ 60 years | 71(78.0) |
| Gender | |
| Female | 32 (35.2) |
| Male | 59 (64.8) |
| Tumor lenght | |
| < 3 cm | 8 (8.8) |
| ≥ 3 cm | 83 (91.2) |
| Localization | |
| Ascending | 12 (13.2) |
| Transvers | 5 (5.5) |
| Descending | 9 (9.9) |
| Sigmoid | 30 (32.9) |
| Rectum | 35 (38.5) |
| Pathological type | |
| Adenocarcinoma | 85 (93.4) |
| Mucinous adenocarcinoma | 6 (6.6) |
| Tumor site | |
| Right | 16 (17.6) |
| Left | 75 (82.4) |
PET/CT Findings
High FDG affinity was detected in the primary tumors of all patients who underwent PET/CT imaging. The mean SUVmax of the primary tumor was 19.5 ± 9.9. There was a positive correlation between SUVmax of the primary tumor and tumor length (r = 0.345, p = 0.001). SUVmax was significantly higher in tumors ≥ 3 cm long (20.3 ± 9.9 vs 11.6 ± 5.2, p = 0.017). The mean tumor SUVmax was significantly higher in right colon tumors compared to left (24.0 ± 10.1 vs 18.6 ± 9.6, p = 0.004).
Twenty-four (26.4%) patients had PET-positive abdominal LNs, with an average SUVmax of 5.8 ± 3.9. A significant relationship was found between PET positive peri-intestinal, abdominal lymph nodes and higher T stages (p = 0.026, 0.010, respectively). Twenty-six (28.6%) patients had PET-positive liver metastasis, with an average SUVmax of 9.9 ± 3.9. Liver metastases were significantly higher in left-sided tumors and in patients over 60 years of age (p = 0.033, 0.037, respectively). Twelve (13.2%) patients had PET-positive lung metastasis, with an average SUVmax of 6.5 ± 4.3. Twelve (13.3%) patients had PET-positive omental metastasis, with an average SUVmax of 9.9 ± 8.6. Nine (9.9%) patients had bone metastasis, and one (1.1%) patient had brain metastasis. When compared with conventional imaging methods, additional findings such as synchronous colonic lesions (Fig. 2), undetectable distant metastases (Fig. 3), and distant LNs were observed in a total of 30 patients, which changed the treatment decision. Forty-six (50.5%) patients of 91 patients had PET-positive peri-intestinal LNs, with an average SUVmax of 3.3 ± 2.2. Peri-intestinal lymph node metastasis was significantly higher in tumors with segment lengths longer than 3 cm (p = 0.030). SUVmax of the primary tumor showed a correlation with SUVmax of metastatic pelvic LNs (r = 0.307, p = 0.036).
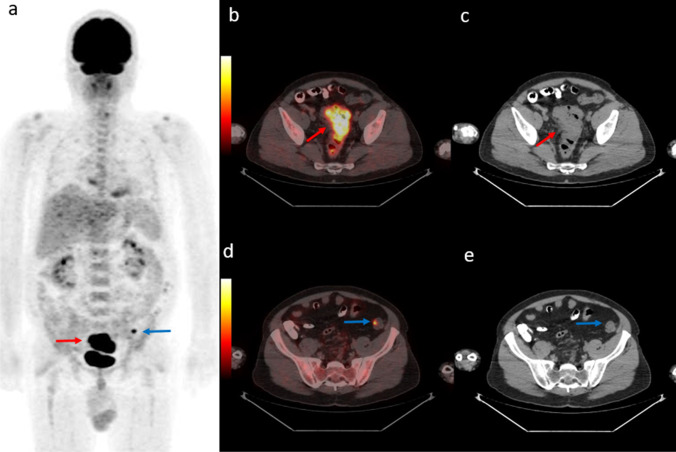
Fig. 2.
54-year-old man diagnosed with rectosigmoid adenocancer. PET maximum intensity projection (MIP) images (a) shows primary lesion (red arrow) and another focal uptake in the left pelvic region (blue arrow). Axial fused PET/CT and CT images demonstrate rectosigmoid lesion with SUVmax 21.9 (b, c, red arrow) and a synchronous descending colon lesion with SUVmax 5.5 undetectable on CT (d, e, blue arrow)
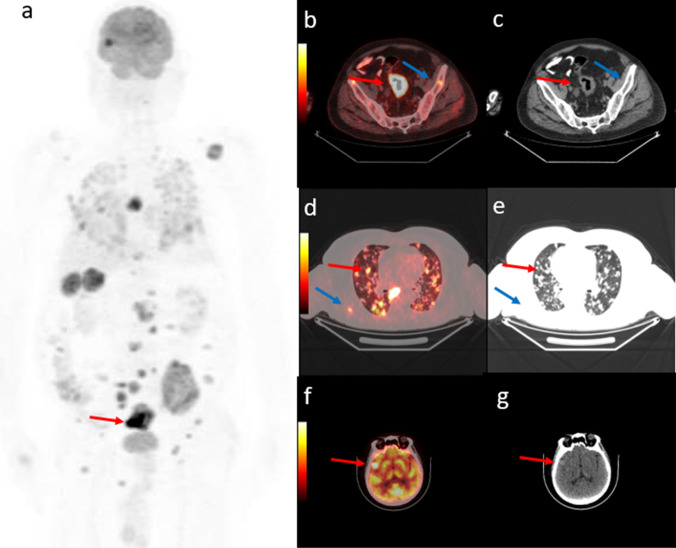
Fig. 3.
67-year-old man diagnosed with rectosigmoid adenocancer. PET maximum intensity projection (MIP) images (a) shows primary lesion (red arrow), and multiple lesions that have disseminated throughout the body. Axial fused PET/CT and CT images demonstrate rectosigmoid lesion with SUVmax 35.2 (b, c, red arrow), bilateral multiple lung metastasis (d, e, red arrow), bone metastases (b-e, blue arrow) and a right temporal lobe metastasis (f, g, red arrow) which cannot dedectable on standard thoracoabdominapelvic CT procedure
In a subgroup of fifty patients, whose detailed postoperative histopathology reports could be accessed, PET/CT parameters were analyzed in terms of predicting postoperative findings. PET/CT detected 16 true positive and 24 true negative peri-intestinal LN metastases in 50 patients. False positive results were found in 7 patients and false negative results in 3 patients. Sensitivity, specificity, PPD, NPD, and accuracy rates were calculated as 0.84, 0.77, 0.57, 0.59, and 0.80, respectively.
The mean SUVmax, MTV, and TLG increased as the T stage increased. The mean SUVmax of the primary tumor differs significantly in T3 and T4 stages (p = 0.041). Analyzes revealed a SUVmax cutoff of 19.9 to separate the T3 and T4 stages (AUC = 0.684, SE = 0.086; 95%CI = 0.515–0.853; sensitivity 74%, specificity 73%). A statistically significant correlation was found between MTV, TLG values and the degree of differentiation (p = 0.005, 0.003, respectively). There was no statistically significant relationship between histopathological parameters such as lymphovascular invasion, positive surgical margins, perineural invasion, and PET/CT metabolic parameters. Postoperative histopathology and PET/CT metabolic findings of the patients are evaluated together in Table 2.
Table 2.
Relationship between postoperative histopathology and PET/CT metabolic parameters
| n | Mean tumor SUVmax | p | Mean tumor MTV, cm3 | p | Mean tumor TLG, g | p | |
|---|---|---|---|---|---|---|---|
| All patients | 50 | ||||||
| Tumor differentiation | NS | 0.007* | 0.002* | ||||
| Well | 11 | 20.1 ± 10.4 | 19.3 ± 11.6 | 191.2 ± 83.6 | |||
| Moderate | 35 | 20.0 ± 9.8 | 30.0 ± 24.1 | 402.8 ± 445.0 | |||
| Poorly | 4 | 28.3 ± 9.6 | 77.5 ± 86.1 | 1668.0 ± 2274.8 | |||
| Lymphovascular invasion | NS | NS | NS | ||||
| negative | 28 | 20.0 ± 8.5 | 32.4 ± 26.8 | 415.3 ± 478.2 | |||
| positive | 22 | 21.6 ± 11.7 | 30.1 ± 40.3 | 511.1 ± 1041.6 | |||
| Vascular invasion | NS | NS | NS | ||||
| negative | 45 | 20.7 ± 9.8 | 29.3 ± 22.6 | 385.1 ± 409.3 | |||
| positive | 5 | 21.0 ± 13.1 | 50.9 ± 85.2 | 1108.3 ± 2199.4 | |||
| Perineural invasion | NS | NS | NS | ||||
| negative | 40 | 21.4 ± 9.8 | 33.3 ± 35.1 | 509.6 ± 846.1 | |||
| positive | 10 | 18.1 ± 10.9 | 23.9 ± 23.1 | 249.0 ± 260.3 | |||
| Surgical margin | NS | NS | NS | ||||
| negative | 31 | 19.4 ± 10.1 | 24.9 ± 23.7 | 334.3 ± 463.8 | |||
| positive | 19 | 23.9.7 | 42.1 ± 43.0 | 658.3 ± 1089.3 | |||
| T stage | 0.005* | NS | NS | ||||
| 1 | 1 | 42.8 | 8.2 | 212.4 | |||
| 2 | 8 | 16.1 ± 3.5 | 23.0 ± 18.6 | 244.6 ± 201.6 | |||
| 3 | 22 | 17.5 ± 8.4 | 27.6 ± 24.2 | 359.3 ± 514.0 | |||
| 4 | 19 | 24.4 ± 10.7 | 45.0 ± 43.9 | 719.7 ± 1083.3 | |||
| N stage | NS | NS | NS | ||||
| 0 | 30 | 18.8 ± 8.1 | 32.5 ± 26.4 | 404.5 ± 466.4 | |||
| 1 | 14 | 20.3 ± 9.8 | 34.8 ± 49.6 | 595.5 ± 1296.9 | |||
| 2 | 6 | 28.3 ± 15.3 | 32.1 ± 19.9 | 519.7 ± 290.4 |
SUVmax maximum standardized uptake value, MTV metabolic tumour volume, TLG total lesion glycolysis, NS not significant, *Statistically significant
Pericolic Fat Stranding
The pericolic fat stranding was observed in 66 (72.5%) of 91 patients. A relationship was observed between pericolic fat stranding and tumor length. The rate of pericolic fat stranding was significantly higher in tumors larger than 3 cm compared to tumors smaller than 3 cm (72.5% vs 25.0%, p = 0.005). When patients with and without pericolic fat stranding findings were compared, the mean SUVmax (20.8 ± 10.2 vs 16.5 ± 8.2), MTV (34.9 ± 32.1 cm3 vs 18.3 ± 18.2 cm3), and TLG (468 ± 689 g vs 165 ± 149 g) values of the primary tumor were higher in the presence of pericolic fat stranding, but a statistical significance was found only with MTV (p = 0.017) and TLG (p = 0.001). The analysis revealed a cut-off value of 19.8 cm3 for MTV to predict PFS (AUC = 0.726, SE = 0.063; 95%CI = 0.604–0.849; sensitivity 68.2%, specificity 72%).
A significant correlation was found between postoperative findings such as lymphovascular invasion, positive surgical margin, T stage and the pericolic fat stranding detected in preoperative PET/CT (p = 0.029, 0.015, 0.041, respectively) (Fig. 4). Postoperative histopathology findings and pericolic fat stranding are evaluated together in Table 3.
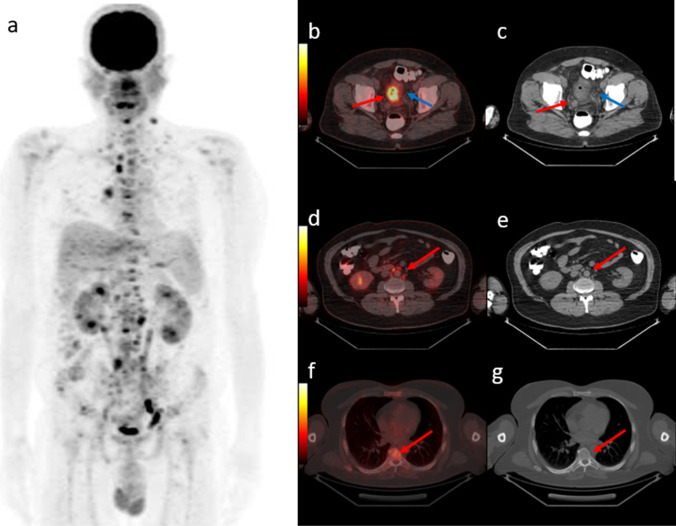
Fig. 4.
43-year-old man diagnosed with rectum cancer. PET maximum intensity projection (MIP) images (a) shows multiple lymph node and bone metastasis. Axial fused PET/CT and CT images demonstrate primary lesion with SUVmax 16,1 (b, c, red arrow), and additional pericolic fat stranding into the surrounding mesentery (blue arrow). Also multiple millimetric lymph nodes (d, e, red arrow) and bone lesions without CT changes (f, g, red arrow) were observed. Histopathology of the resected specimen showed a T4 tumor with 85 mm lenght, positive lymphovascular invasion and positive surgical margin
Table 3.
Univariate analysis between postoperative histopathology and pericolic fat stranding
| n | Pericolic fat stranding - | Pericolic fat stranding + | p | |
|---|---|---|---|---|
| All patients | 50 | 13 | 37 | |
| Tumor differentiation | NS | |||
| Well | 11 | 3 | 8 | |
| Moderate | 35 | 8 | 27 | |
| Poorly | 4 | 1 | 3 | |
| Lymphovascular invasion | 0.029* | |||
| negative | 28 | 10 | 18 | |
| positive | 22 | 2 | 20 | |
| Vascular invasion | NS | |||
| negative | 45 | 11 | 34 | |
| positive | 5 | 1 | 4 | |
| Perineural invasion | NS | |||
| negative | 40 | 11 | 29 | |
| positive | 10 | 1 | 9 | |
| Surgical margin | 0.015* | |||
| negative | 31 | 11 | 20 | |
| positive | 19 | 1 | 18 | |
| T stage | 0.041* | |||
| 1 | 1 | 0 | 1 | |
| 2 | 8 | 5 | 3 | |
| 3 | 22 | 6 | 16 | |
| 4 | 19 | 2 | 17 | |
| N stage | NS | |||
| 0 | 30 | 11 | 19 | |
| 1 | 14 | 1 | 13 | |
| 2 | 6 | 1 | 5 |
NS not significant, *Statistically significant
Overall Survival
The median follow‑up time was 18.9 months (Minimum:1, maximum:86). Twenty-eight patients died during follow-up. The mean age at death was 69 ± 10 years old.
The 2-year OS rate was 0.78, and the 5-year OS rate was 0.60. When analyzed separately, the 5-year OS rate was 0.51 in patients with PET-positive metastases and 0.70 in patients without PET-positive metastases.
In univariate analysis, OS was related to tumor differentiation degree (p = 0.017), lymphovascular invasion (p = 0.012), T stage (p = 0.045), N stage (p = 0.048), MTV (p = 0.039), and TLG (p = 0.006) (Fig. 5). The analysis revealed a cut-off of 25.0 cm3 for MTV (AUC = 0.708, SE = 0.080; 95%CI = 0.550–0.865; sensitivity 73.3%, specificity 62.9%), and a cut-off of 274.5 g for TLG to predict OS (AUC = 0.731, SE = 0.086; 95%CI = 0.563–0.900; sensitivity 73.3%, specificity 71.4%).
Fig. 5.
Kaplan Meier curves for overall survival based on (a) T stage, (b) tumor differentiation, (c) N stage, (d) lymphovascular invasion, (e) MTV, and (f) TLG values
The following variables were included in the multivariate analysis: tumor differentiation, lymphovascular invasion, T stage, N stage, MTV and TLG. The presence of lymphovascular invasion (HR = 0.2, 95%CI 0.026–0.853, p = 0.033), tumor differentiation (HR = 26.1, 95%CI 1.672–408.467, p = 0.020), MTV (HR = 0.3, 95%CI 0.071–0.841, p = 0.025) and TLG (HR = 3.5, 95%CI 1.065–11.193, p = 0.039) were the independent significant predictors.
Discussion
CRC treatment consists of surgery, neoadjuvant radiotherapy, adjuvant chemotherapy, and targeted therapies [2]. However, approximately 20–30% of CRC patients are diagnosed at an advanced stage, and the 5-year OS rate in these patients is quite low despite all these treatments [17]. Imaging is crucial for the accurate estimation of tumor extension, distant metastases, and prognosis. Preoperative PET/CT is currently used to detect further metastases in case of detection of a surgically treatable metastasis with conventional methods. The main finding of this study is that preoperative PET/CT may also predict prognosis and the postoperative findings such as T stage, N stage and tumor differentiation degree, thanks to metabolic data. The SUVmax value of the primary tumor may differentiate between T3 and T4 disease. In addition, reporting of the pericolic fat stranding in the CT component may contribute to the prediction of lymphatic invasion, and positive surgical margin.
F-18 FDG PET/CT is an effective whole-body imaging method that allows the detection of morphological changes with the low-dose CT component and also the metabolic changes with the PET component that may precede structural findings. In addition, quantitative parameters obtained from PET images, such as SUVmax, MTV, and TLG are used to predict prognosis [9, 10]. In CRC, PET-CT is used to detect distant metastases in advanced disease, evaluate treatment response, and detect recurrence in case of increased tumor markers [11, 18]. In the current guideline, preoperative PET/CT in CRC is recommended only in selected cases where metastatic lesions are suspected by a prior conventional imaging method [4]. However, recently, studies investigating the contribution of preoperative PET/CT to primary staging and prognosis in CRC have been increasingly reported [12, 13]. Studies have shown that PET/CT is superior to contrast-enhanced CT in tumor T staging with higher sensitivity and specificity [19]. A recent study reported that SUVmax value was significantly associated with tumor length, clinical stage, pathological type, and differentiation [20]. They found a SUVmax cutoff of 18.26 to predict prognosis. In our study, all primary tumors showed high FDG uptake. Consistent with previous studies, we found a positive correlation between SUVmax and tumor length. SUVmax values were significantly higher in tumors ≥ 3 cm. Although it is generally accepted that mucinous adenocarcinomas may show low FDG uptake [21], we also found high SUVmax values for mucinous adenocarcinoma, which is consistent with another study [22]. The mean tumor SUVmax was found to be significantly higher in the right colon tumors, which were considered to have a worse prognosis, compared to the left [23]. The degree of tumor differentiation significantly increased as MTV and TLG values increased. In addition, we found that the mean SUVmax of the primary tumor differed significantly at T3 and T4 stages. To our best knowledge, this is the first study to address this finding.
Interpretation of LN metastases on CT is based on size. However, sometimes LNs below 1 cm may contain metastases, whereas large LNs may be reactive. Although there are studies reporting very low sensitivity [24, 25] and low specificity [26, 27] values for N staging with PET/CT for CRC in the literature, in a meta-analysis the pooled estimated sensitivity and specificity for PET/CT were calculated as 0.70 and 0.63, respectively. This meta-analysis revealed that PET-CT had better diagnostic accuracy than CT in determining N staging of CRC, especially in distant LNs [19]. In a study supporting this, it was also reported that evaluating LNs according to SUVmax value is a better criterion than size, in determining malignant LNs [28]. In our study, PET/CT was able to predict the postoperative N stage with a high accuracy. We found that SUVmax of the primary tumor showed a correlation with SUVmax of metastatic pelvic LNs.
It is known that the value of PET/CT for M staging in CRC is quite high [19]. Knowing all metastases sites at the beginning of treatment will improve the prognosis of patients by optimizing the treatment plan, as well as reducing the risks of blind surgery [28]. In a study investigating the use of whole-body MRI in CRC staging and its comparison with PET/CT, it was reported that whole-body MRI can be used as a method for CRC staging, but it cannot replace PET-CT [29]. Ye et al. reported in their meta-analysis that with the addition of PET/CT in TNM staging of CRC, there was a 15.8% rate of change that would affect the therapeutic strategy [19]. In a meta-analysis compiling the effect of PET in the management of liver metastases in CRC, an average change of 24% was reported with the contribution of PET [30]. In our study, additional findings such as synchronous colonic lesions, undetectable distant metastases, and distant LNs were found in one-third of patients with PET/CT, which changed the treatment decision, consistent with the literature [31]. Also, in accordance with the literature, liver metastases were found to be significantly higher in left-sided tumors [32].
Besides the metabolic contribution of PET, another aim of this study was to investigate the contribution of additional CT findings around the lesion to be reported. Increased linear or nodular opacities observed in the fatty tissue around the colon wall on CT images are defined as pericolic fat stranding. Although pericolic fat stranding can be seen in inflammatory processes such as colitis and diverticulitis on CT, studies have investigated whether this finding is an indicator of extra-intestinal spread in the presence of known CRC [33]. In a study examining preoperative pericolic fat stranding and histopathological findings in CRC, it was noted that despite high sensitivity and PPV values (79% and 91%, respectively), very low specificity and NPV values were found (33% and 15%, respectively) [16]. In another study, pericolic fat stranding on CT was shown to be significantly associated with the circumferential ratio and length of the tumor [34]. Studies have similarly reported that pericolic fat stranding on CT may show a T3 or T4 stage tumor but cannot differentiate between stage T3 and T4 stages [14, 16, 34]. In our study, pericolic fat stranding was observed in the majority of patients (72%). A relationship was found between tumor length, positive surgical margin, lymphovascular invasion and the pericolic fat stranding. Supporting the literature, although pericolic fat stranding was associated with the T stage and showed a significant increase in T3-T4 stage compared to the T1-T2 stage, it could not differentiate between T3 and T4 stage. A statistical significance relationship was found between MTV, TLG values, and the pericolic fat stranding. The analysis revealed a cut-off value of 19.8 cm3 for MTV to predict PFS. Therefore, our results revealed that metabolic data such as SUVmax may be more predictive than structural findings for distinguishing between T3 and T4 stages. However, since the pericolic fat stranding may predict positive surgical margins and lymphovascular invasion, reporting it on PET/CT may contribute additionally when noticed. To our best knowledge, this is the first study to investigate the pericolic fat stranding and the metabolic data obtained from PET/CT together.
The extent of tumoral invasion into the intestinal wall, the number of regional lymph node metastases, and distant organ metastases were accepted as the most important prognostic determinants in CRC and formed the basis of staging systems. In addition to these factors, histopathological findings such as lymphovascular invasion, perineural invasion, tumor budding, and surgical margins have also been identified as prognostic factors [35, 36]. However, since these factors can be evaluated after the operation, alternative methods that can provide more information in the preoperative period continue to be investigated. Some previous studies have investigated the role of preoperative PET/CT parameters in predicting prognosis and neoadjuvant chemoradiotherapy response in CRC, but conflicting results have been reported [37–41]. We found a 2-year OS rate of 0.78, and a 5-year OS rate of 0.60, consistent with previous publications [42, 43]. When analyzed separately, the 5-year OS rate was 0.51 in patients with PET-positive metastases and 0.70 in patients without PET-positive metastases. In univariate analysis, OS was related to tumor differentiation degree, lymphovascular invasion, T stage, N stage, MTV, and TLG. The analysis revealed a cut-off of 25.0 for MTV, and a cut-off of 274.5 for TLG to predict overall survival. The SUVmax of the primary tumor was not a strong predictor of overall survival, in agreement with previous studies [44]. There was no prognostically significant difference between male and female genders, which differs in studies [42, 45]. Serum CEA is also used as a prognostic biomarker in CRC, but mostly in follow-up. Early postoperative CEA level has been reported to be a better predictor of prognosis than preoperative CEA level [46]. In the present study, preoperative CEA level was not a significant prognostic factor for OS. In the multivariate analysis, the presence of lymphovascular invasion, tumor differentiation, MTV and TLG were the independent significant predictors of overall survival.
Recently, it has been reported that the time from first diagnosis to treatment is also an effective factor in the prognosis of CRC. In a study that analyzed 2,279 CRC patients, a significantly better OS was reported in patients whose treatment was started within 60 days [42]. Another study reported that time from the initial diagnosis to surgery is an independent prognostic factor for OS in patients with non-metastatic CRC [47]. So, in addition to being a prognostic predictor by MTV and TLG values, PET/CT may also shorten the decision-making process and initiation time of treatments with its contributions.
As a limitation of the study, since our study was conducted as a retrospective study, we included patients referred to PET/CT, which resulted in a selection bias. Second, the histopathological correlation could not be made for all lesions considered metastatic. However, additional imaging methods, repeated PET scans, and sufficient follow-up periods have helped the characterization of the lesions. Third, numbers were low for statistics in some groups. Nevertheless, the short time between surgery and PET/CT imaging and the standard interpretation of all pathologies in our university hospital are strengths of the study. Further prospective studies are warranted to confirm our findings and determine the cutoff points for an accurate prediction.
Conclusion
In addition to its success in detecting distant metastases, preoperative PET/CT may contribute CRC management by predicting prognosis and the postoperative findings such as T stage, N stage and tumor differentiation degree, thanks to metabolic parameters. The SUVmax value of the primary tumor may differentiate between T3 and T4 disease. Likewise, reporting of the pericolic fat stranding in the CT component may contribute to the prediction of lymphatic invasion, and positive surgical margin. Thus, PET/CT may speed up the decision-making process and shorten the time to start surgery and treatments in CRC.
Acknowledgements
The authors thank Dr.Burak Günay for his contribution to the evaluation of the computed tomography data.
Authors’ Contributions
The study was designed by Selin Soyluoglu. Material preparation and data collection were performed by Busra Ozdemir. The data analysis was performed by Selin Soyluoglu and Busra Ozdemir. The first draft of the manuscript was written by Selin Soyluoglu and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Data Availability
Raw data were generated at Trakya University Hospital. Derived data supporting the findings of this study are available from the corresponding author Dr. Selin Soyluoglu on request.
Declarations
Competing Interests
The authors state that there are no conflicts of interest.
Ethics Approval and Consent to Participate
This study was performed in accordance with the ethical concepts of the Helsinki Declaration in October 2013 and approved by the institutional ethical review board (No: TUTF-BAEK 2022/390).
Consent for Publication
The authors give consent for the publication to be published in the Journal.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Selin Soyluoglu, Email: dr.selina@gmail.com, Email: fselinsoyluoglu@trakya.edu.tr.
Busra Ozdemir Gunay, Email: busraozdemir39@gmail.com.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Weitz J, Koch M, Debus J, Höhler T, Galle PR, Büchler MW. Colorectal Cancer. Lancet. 2005;365:153–165. doi: 10.1016/S0140-6736(05)17706-X. [DOI] [PubMed] [Google Scholar]
- 3.Benson AB, Venook AP, Al-Hawary MM, Arain MA, Chen YJ, Ciombor KK et al. Colon Cancer, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021;19:329–59. [DOI] [PubMed]
- 4.Benson AB, Venook AP, Al-Hawary MM, Azad N, Chen YJ, Ciombor KK et al. Rectal Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2022;20:1139–67. [DOI] [PubMed]
- 5.Thoeni RF, Rogalla P. CT for the Evaluation of Carcinomas in the Colon and Rectum. Semin Ultrasound CT MR. 1995;16:112–126. doi: 10.1016/0887-2171(95)90004-7. [DOI] [PubMed] [Google Scholar]
- 6.Isbister WH, al-Sanea O. The Utility of Pre-Operative Abdominal Computerized Tomography Scanning in Colorectal Surgery. J R Coll Surg Edinb. 1996;41:232–4. [PubMed]
- 7.Boellaard R, Delgado-Bolton R, Oyen WJ, Giammarile F, Tatsch K, Eschner W et al. FDG PET/CT: EANM Procedure Guidelines for Tumour Imaging: Version 2.0. Eur J Nucl Med Mol Imaging. 2015;42:328–54. [DOI] [PMC free article] [PubMed]
- 8.Wen W, Piao Y, Xu D, Li X. Prognostic Value of MTV and TLG of (18)F-FDG PET in Patients with Stage I and II Non-Small-Cell Lung Cancer: a Meta-Analysis. Contrast Media Mol Imaging. 2021;2021:7528971. doi: 10.1155/2021/7528971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee JW, Kang CM, Choi HJ, Lee WJ, Song SY, Lee JH, et al. Prognostic Value of Metabolic Tumor Volume and Total Lesion Glycolysis on Preoperative 18F-FDG PET/CT in Patients with Pancreatic Cancer. J Nucl Med. 2014;55:898–904. doi: 10.2967/jnumed.113.131847. [DOI] [PubMed] [Google Scholar]
- 10.Pak K, Cheon GJ, Nam HY, Kim SJ, Kang KW, Chung JK, et al. Prognostic Value of Metabolic Tumor Volume and Total Lesion Glycolysis in Head and Neck Cancer: A Systematic Review and Meta-Analysis. J Nucl Med. 2014;55:884–890. doi: 10.2967/jnumed.113.133801. [DOI] [PubMed] [Google Scholar]
- 11.Choi EK, Yoo Ie R, Park HL, Choi HS, Han EJ, Kim SH, et al. Value of Surveillance (18)F-FDG PET/CT in Colorectal Cancer: Comparison with Conventional Imaging Studies. Nucl Med Mol Imaging. 2012;46:189–195. doi: 10.1007/s13139-012-0145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He J, Wang Q, Zhang Y, Wu H, Zhou Y, Zhao S. Preoperative Prediction of Regional Lymph Node Metastasis of Colorectal Cancer Based on (18)F-FDG PET/CT and Machine Learning. Ann Nucl Med. 2021;35:617–627. doi: 10.1007/s12149-021-01605-8. [DOI] [PubMed] [Google Scholar]
- 13.Liu X, Xiang K, Geng GY, Wang SC, Ni M, Zhang YF, et al. Prognostic Value of Intratumor Metabolic Heterogeneity Parameters on (18)F-FDG PET/CT for Patients with Colorectal Cancer. Contrast Media Mol Imaging. 2022;2022:2586245. doi: 10.1155/2022/2586245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zeina AR, Mahamid A, Walid S, Nachtigal A, Shapira-Rootman M. The Diagnostic Accuracy of Pericolonic Fat Extension and Attenuation for Colorectal Tumors. Eur J Radiol. 2015;84:1724–1728. doi: 10.1016/j.ejrad.2015.05.036. [DOI] [PubMed] [Google Scholar]
- 15.Dighe S, Swift I, Brown G. CT Staging of Colon Cancer. Clin Radiol. 2008;63:1372–1379. doi: 10.1016/j.crad.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 16.Ng CS, Doyle TC, Dixon AK, Miller R, Arends MJ. Histopathological Correlates of Abnormal Pericolic Fat on CT in the Assessment of Colorectal Carcinoma. Br J Radiol. 2002;75:31–37. doi: 10.1259/bjr.75.889.750031. [DOI] [PubMed] [Google Scholar]
- 17.Lykke J, Roikjaer O, Jess P. The Relation Between Lymph Node Status and Survival in Stage I-III Colon Cancer: Results from a Prospective Nationwide Cohort Study. Colorectal Dis. 2013;15:559–565. doi: 10.1111/codi.12059. [DOI] [PubMed] [Google Scholar]
- 18.Bang JI, Lim Y, Paeng JC, Han SW, Park S, Lee JM, et al. Comparison of Quantitative Methods on FDG PET/CT for Treatment Response Evaluation of Metastatic Colorectal Cancer. Nucl Med Mol Imaging. 2017;51:147–153. doi: 10.1007/s13139-016-0449-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ye Y, Liu T, Lu L, Wang G, Wang M, Li J, et al. Pre-Operative TNM Staging of Primary Colorectal Cancer by (18)F-FDG PET-CT or PET: A Meta-Analysis Including 2283 Patients. Int J Clin Exp Med. 2015;8:21773–21785. [PMC free article] [PubMed] [Google Scholar]
- 20.Li D, Wang Y, Liu W, Chen Q, Cai L, Xing X, et al. The Correlation between (18)F-FDG PET/CT Imaging SUVmax of Preoperative Colon Cancer Primary Lesions and Clinicopathological Factors. J Oncol. 2021;2021:4312296. doi: 10.1155/2021/4312296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berger KL, Nicholson SA, Dehdashti F, Siegel BA. FDG PET Evaluation of Mucinous Neoplasms: Correlation of Fdg Uptake with Histopathologic Features. AJR Am J Roentgenol. 2000;174:1005–1008. doi: 10.2214/ajr.174.4.1741005. [DOI] [PubMed] [Google Scholar]
- 22.Barbaro B, Leccisotti L, Vecchio FM, Di Matteo M, Serra T, Salsano M, et al. The Potential Predictive Value of MRI and PET-CT in Mucinous And Nonmucinous Rectal Cancer to Identify Patients at High Risk of Metastatic Disease. Br J Radiol. 2017;90:20150836. doi: 10.1259/bjr.20150836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yahagi M, Okabayashi K, Hasegawa H, Tsuruta M, Kitagawa Y. The Worse Prognosis of Right-Sided Compared with Left-Sided Colon Cancers: a Systematic Review and Meta-analysis. J Gastrointest Surg. 2016;20:648–655. doi: 10.1007/s11605-015-3026-6. [DOI] [PubMed] [Google Scholar]
- 24.Llamas-Elvira JM, Rodríguez-Fernández A, Gutiérrez-Sáinz J, Gomez-Rio M, Bellon-Guardia M, Ramos-Font C, et al. Fluorine-18 Fluorodeoxyglucose PET in the Preoperative Staging of Colorectal Cancer. Eur J Nucl Med Mol Imaging. 2007;34:859–867. doi: 10.1007/s00259-006-0274-4. [DOI] [PubMed] [Google Scholar]
- 25.Kantorová I, Lipská L, Bêlohlávek O, Visokai V, Trubaĉ M, Schneiderová M. Routine (18)F-FDG PET Preoperative Staging of Colorectal Cancer: Comparison With Conventional Staging and its Impact on Treatment Decision Making. J Nucl Med. 2003;44:1784–1788. [PubMed] [Google Scholar]
- 26.Tateishi U, Maeda T, Morimoto T, Miyake M, Arai Y, Kim EE. Non-Enhanced CT Versus Contrast-Enhanced CT in Integrated PET/CT Studies for Nodal Staging of Rectal Cancer. Eur J Nucl Med Mol Imaging. 2007;34:1627–1634. doi: 10.1007/s00259-007-0455-9. [DOI] [PubMed] [Google Scholar]
- 27.Lee JH, Lee MR. Positron Emission Tomography/Computed Tomography in the Staging of Colon Cancer. Ann Coloproctol. 2014;30:23–27. doi: 10.3393/ac.2014.30.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsunoda Y, Ito M, Fujii H, Kuwano H, Saito N. Preoperative Diagnosis of Lymph Node Metastases of Colorectal Cancer by FDG-PET/CT. Jpn J Clin Oncol. 2008;38:347–353. doi: 10.1093/jjco/hyn032. [DOI] [PubMed] [Google Scholar]
- 29.Squillaci E, Manenti G, Mancino S, Cicciò C, Calabria F, Danieli R et al. Staging of Colon Cancer: Whole-Body MRI vs. Whole-Body PET-CT--Initial Clinical Experience. Abdom Imaging. 2008;33:676–88. [DOI] [PubMed]
- 30.Maffione AM, Lopci E, Bluemel C, Giammarile F, Herrmann K, Rubello D. Diagnostic Accuracy and Impact on Management of (18)F-FDG PET and PET/CT in Colorectal Liver Metastasis: A Meta-Analysis and Systematic Review. Eur J Nucl Med Mol Imaging. 2015;42:152–163. doi: 10.1007/s00259-014-2930-4. [DOI] [PubMed] [Google Scholar]
- 31.Heriot AG, Hicks RJ, Drummond EG, Keck J, Mackay J, Chen F, et al. Does Positron Emission Tomography Change Management in Primary Rectal Cancer? A Prospective Assessment. Dis Colon Rectum. 2004;47:451–458. doi: 10.1007/s10350-003-0089-3. [DOI] [PubMed] [Google Scholar]
- 32.Engstrand J, Nilsson H, Strömberg C, Jonas E, Freedman J. Colorectal Cancer Liver Metastases - A Population-Based Study on Incidence, Management and Survival. BMC Cancer. 2018;18:78. doi: 10.1186/s12885-017-3925-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dixon AK, Nightingale RC. Abnormal Fat: A Useful Marker of Intra-Abdominal Disease at Computed Tomography. Clin Radiol. 1984;35:469–473. doi: 10.1016/S0009-9260(84)80056-2. [DOI] [PubMed] [Google Scholar]
- 34.Morimoto T, Yamada T, Miyakawa K, Nakajima Y. Factors Associated with Pericolic Fat Stranding of Colon Cancer on Computed Tomography Colonography. Acta Radiol Open. 2018;7:2058460118757578. doi: 10.1177/2058460118757578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fujita S, Shimoda T, Yoshimura K, Yamamoto S, Akasu T, Moriya Y. Prospective Evaluation of Prognostic Factors in Patients with Colorectal Cancer Undergoing Curative Resection. J Surg Oncol. 2003;84:127–131. doi: 10.1002/jso.10308. [DOI] [PubMed] [Google Scholar]
- 36.Compton CC, Fielding LP, Burgart LJ, Conley B, Cooper HS, Hamilton SR et al. Prognostic Factors in Colorectal Cancer. College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med. 2000;124:979–94. [DOI] [PubMed]
- 37.Choi BW, Kang S, Bae SU, Jeong WK, Baek SK, Song BI, et al. Prognostic Value of Metabolic Parameters On (18)F-Fluorodeoxyglucose Positron Tomography/Computed Tomography in Classical Rectal Adenocarcinoma. Sci Rep. 2021;11:12947. doi: 10.1038/s41598-021-92118-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jo HJ, Kim SJ, Lee HY, Kim IJ. Prediction of Survival and Cancer Recurrence using Metabolic Volumetric Parameters Measured by 18F-FDG PET/CT in Patients with Surgically Resected Rectal Cancer. Clin Nucl Med. 2014;39:493–497. doi: 10.1097/RLU.0000000000000438. [DOI] [PubMed] [Google Scholar]
- 39.Shi D, Cai G, Peng J, Li D, Li X, Xu Y, et al. The Preoperative SUVmax for (18)F-FDG Uptake Predicts Survival in Patients with colorectal cancer. BMC Cancer. 2015;15:991. doi: 10.1186/s12885-015-1991-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ogawa S, Itabashi M, Kondo C, Momose M, Sakai S, Kameoka S. Prognostic Value of Total Lesion Glycolysis Measured by 18F-FDG-PET/CT in Patients with Colorectal Cancer. Anticancer Res. 2015;35:3495–3500. [PubMed] [Google Scholar]
- 41.Sakin A, Sahin S, Karyagar SS, Karyagar S, Atci M, Akboru MH, et al. The Predictive Value of Baseline Volumetric PET/CT Parameters on Treatment Response and Prognosis in Locally Advanced Rectal Cancer Treated with Neoadjuvant Chemoradiotherapy. J Gastrointest Cancer. 2022;53:341–347. doi: 10.1007/s12029-021-00608-y. [DOI] [PubMed] [Google Scholar]
- 42.Aguiar Junior S, Oliveira MM, Silva D, Mello CAL, Calsavara VF, Curado MP. Survival of Patients with Colorectal Cancer in a Cancer Center. Arq Gastroenterol. 2020;57:172–177. doi: 10.1590/s0004-2803.202000000-32. [DOI] [PubMed] [Google Scholar]
- 43.Roder D, Karapetis CS, Wattchow D, Moore J, Singhal N, Joshi R, et al. Colorectal Cancer Treatment and Survival: The Experience of Major Public Hospitals in South Australia Over Three Decades. Asian Pac J Cancer Prev. 2015;16:2431–2440. doi: 10.7314/APJCP.2015.16.6.2431. [DOI] [PubMed] [Google Scholar]
- 44.Moulton CA, Gu CS, Law CH, Tandan VR, Hart R, Quan D, et al. Effect of PET before Liver Resection on Surgical Management for Colorectal Adenocarcinoma Metastases: A Randomized Clinical Trial. JAMA. 2014;311:1863–1869. doi: 10.1001/jama.2014.3740. [DOI] [PubMed] [Google Scholar]
- 45.Weiss JM, Pfau PR, O'Connor ES, King J, LoConte N, Kennedy G, et al. Mortality by Stage for Right- Versus Left-Sided Colon Cancer: Analysis of Surveillance, Epidemiology, and End Results–Medicare Data. J Clin Oncol. 2011;29:4401–4409. doi: 10.1200/JCO.2011.36.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lin JK, Lin CC, Yang SH, Wang HS, Jiang JK, Lan YT, et al. Early Postoperative CEA Level is a Better Prognostic Indicator than is Preoperative Cea Level in Predicting Prognosis of Patients with Curable Colorectal Cancer. Int J Colorectal Dis. 2011;26:1135–1141. doi: 10.1007/s00384-011-1209-5. [DOI] [PubMed] [Google Scholar]
- 47.Kaltenmeier C, Shen C, Medich DS, Geller DA, Bartlett DL, Tsung A, et al. Time to Surgery and Colon Cancer Survival in the United States. Ann Surg. 2021;274:1025–1031. doi: 10.1097/SLA.0000000000003745. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Raw data were generated at Trakya University Hospital. Derived data supporting the findings of this study are available from the corresponding author Dr. Selin Soyluoglu on request.