Abstract
Enterocytozoon bieneusi is the most common microsporidian parasite recognized in human patients with AIDS. Recently, we identified a virtually identical organism causing a spontaneous infection associated with hepatobiliary and intestinal disease in simian immunodeficiency virus (SIV)-infected macaques. To examine the natural history of the infection, we examined captive rhesus macaques for E. bieneusi by PCR, in situ hybridization, and cytochemical techniques. PCR performed on fecal DNA detected enterocytozoon infection in 22 (16.7%) of 131 normal rhesus macaques (Macaca mulatta), compared to 18 (33.8%) of 53 rhesus macaques experimentally inoculated with SIV. In normal rhesus macaques, persistence of infection was demonstrated for up to 262 days and was usually not associated with clinical signs. In six of seven normal rhesus animals, E. bieneusi was detected by PCR in bile obtained through percutaneous cholecystocentesis but not by in situ hybridization performed on endoscopic biopsies of duodenum and proximal jejunum.
Enterocytozoon bieneusi is the most common microsporidian parasite to infect human patients with AIDS; it represents a significant cause of diarrhea, malabsorption, and wasting in the terminal stages of human immunodeficiency virus infection (4, 9, 13, 14, 18). Recently, E. bieneusi has been recognized as a cause of diarrhea in both healthy individuals and patients on immunosuppressive therapy, suggesting that infection of humans may be more widespread than previously thought (11, 12, 15, 17). Despite its relatively common occurrence in AIDS patients, basic aspects of E. bieneusi biology, epidemiology, and host immunity are poorly understood. The source, duration, and natural reservoir of E. bieneusi infection in humans is presently unknown. While E. bieneusi has been identified as a cause of acalculous cholecystitis and cholangitis in human patients with AIDS (1, 5, 8, 10), the distribution of E. bieneusi in tissue of immunologically normal individuals has not been investigated.
We recently described spontaneous enterocytozoon infection as a common cause of hepatobiliary and intestinal disease in simian immunodeficiency virus (SIV)-infected macaques at the New England Regional Primate Research Center (NERPRC) (6). The infecting organism is virtually indistinguishable from E. bieneusi of human origin at the light, ultrastructural, and genetic levels (3, 6). In this investigation, we utilized PCR, in situ hybridization, and cytochemical techniques to demonstrate asymptomatic and persistent infection of normal captive rhesus macaques. The epizootiology of spontaneous E. bieneusi in rhesus macaques may give insight to the mechanisms of enterocytozoon pathogenesis, transmission, and persistence in humans.
All rhesus macaques (Macaca mulatta) were housed at the NERPRC in accordance with the standards of the American Association for Accreditation of Laboratory Animal Care and Harvard Medical School’s Animal Care and Use Committee. Normal rhesus macaques were serologically negative for simian retrovirus type D and SIV. Animals were individually caged (n = 72) or housed in small breeding harems (n = 54) consisting of 1 adult male, 6 to 10 females, and a similar number of infants under 6 months old. Macaques experimentally inoculated with SIV (n = 51) were individually housed in biolevel 2 and 3 containment facilities, as previously described (Health and Human Services publication no. 93-8395) (7).
Fecal samples (n = 184) were collected over a 2-month period during physical examinations performed as a component of routine colony health management. Repeat fecal samples were obtained from selected animals over the next 30 to 269 days. Biopsy samples (n = 31) were obtained with an Olympus GIF XP10 pediatric endoscope under sedation induced with ketamine hydrochloride (10 mg/kg of body weight, administered intramuscularly). Percutaneous cholecystocentesis (n = 27) was performed with ultrasonography through the right cranioventral abdomen. A 22-g 1 1/2-in. needle was guided to the gall bladder through the hepatic parenchyma, and 2 to 3 ml of bile was aseptically aspirated. Serum chemistry was performed on 18 animals at the time of endoscopy and cholecystocentesis.
Extraction of total fecal DNA from formalin-fixed stool samples was performed with mechanical disruption and proteolytic digestion (2). Two hundred microliters of bile obtained by cholecystocentesis was mixed with 1,000 μl of 4% paraformaldehyde in 1× phosphate-buffered saline and centrifuged (Eppendorf model 5417C centrifuge) for 10 min at 14,000 rpm to pellet spores and cellular debris. The pellet was washed three times in distilled water, resuspended to a final volume of 20 μl, and boiled for 3 min. DNA was then extracted with 200 μl of Instagene matrix (Bio-Rad, Hercules, Calif.).
PCR was performed on fecal DNA with primers EBIEF1 and EBIER1, as previously described (2, 6). Specificity of the amplified target was verified by Southern transfer (Pharmacia Biotech, Uppsala, Sweden) and hybridization to an internal oligonucleotide probe (5′ TAC AGC GGT GTC TAA TCA CTT TCG ATA CTC) end labeled with digoxigenin by deoxyterminal transferase (Boehringer Mannheim, Indianapolis, Ind.). In situ hybridization was performed on formalin-fixed, paraffin-embedded tissues with a digoxigenin-labeled probe directed at the small subunit rRNA (6).
Fecal samples from 131 normal animals were analyzed for the presence of E. bieneusi by DNA isolation followed by PCR and Southern hybridization. Twenty-two (16.7%) animals were positive on the initial sample (Table 1). Of the seven animals which were positive on the initial sample and retested, five continued to be positive by PCR on subsequent tests. Two of the 22 animals had clinically apparent diarrhea at the time of initial examination. Of the 13 animals which were PCR negative on the initial sample and retested, two became PCR positive on subsequent examination. Fecal samples from 53 SIV-infected rhesus macaques were similarly tested. Eighteen (33.9%) were positive on initial examination. Six of 18 had clinically apparent diarrhea at the time of sample collection. The rate of E. bieneusi infection was significantly higher in SIV-infected macaques than in normal animals (chi-square test [P < 0.02]).
TABLE 1.
E. bieneusi infection in normal rhesus macaques
| Characteristica |
E. bieneusi
|
|
|---|---|---|
| Present (n = 22) | Absent (n = 109) | |
| Source∗ | ||
| NERPRC | 21 (95.4) | 73 (66.9) |
| Other | 1 (4.6) | 36 (33.1) |
| Mean age (yr)† | 5.6 | 5.9 |
| Housing∗ | ||
| Single | 8 (36.4) | 68 (62.4) |
| Group | 14 (63.6) | 41 (37.6) |
| Sex† | ||
| Male | 9 (40.9) | 32 (29.3) |
| Female | 13 (49.1) | 77 (70.7) |
Data are expressed as number (%) unless otherwise noted. ∗, statistically significant difference (chi-square test [P < 0.05]); †, no statistical difference.
The source, age, type of housing, and sex of immunologically normal animals infected with E. bieneusi on initial examination were compared to those of animals found to be negative. There was no statistically significant difference for age or sex. Animals positive for E. bieneusi were more likely to be in group housing (chi-square test [P < 0.02]) and to have been born at the NERPRC colony (chi-square test [P < 0.04]). Of the five normal animals with two or more positive tests for E. bieneusi, persistent infection was demonstrated for 30 to 269 days. All animals were in good health and asymptomatic for clinical signs of diarrhea and wasting on their final fecal examinations.
To attempt to localize the site of persistent E. bieneusi infection in normal rhesus macaques, 31 animals underwent endoscopic examination and biopsy of the duodenum and proximal jejunum. Twenty-seven of these animals underwent ultrasonographic examination of the hepatobiliary tree with cholecystocentesis. Endoscopic biopsy samples were examined by hematoxylin and eosin stain, Weber’s modified trichrome, and in situ hybridization. In no case was E. bieneusi found in the sampled sections of small intestine from immunologically normal rhesus macaques. E. bieneusi was identified by in situ hybridization in sections of small intestine obtained from three SIV-infected macaques identified prospectively.
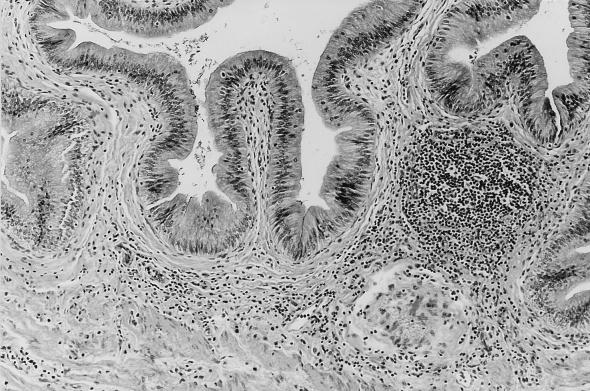
In contrast, PCR performed on DNA isolated from bile was positive in six of seven normal animals with E. bieneusi DNA detected in feces. Small numbers of E. bieneusi spores were visualized by Weber’s modified trichrome in the concentrated bile of three of these six animals. E. bieneusi DNA was detected in the bile of 3 of 20 animals which had been negative for enterocytozoon DNA in feces 7 to 28 days previously. Three normal macaques in which enterocytozoon DNA was detected in feces were euthanized for other reasons. In two of these animals, E. bieneusi had been identified prospectively in bile and a multifocal lymphoplasmacytic choledochitis and mild cholecystitis was present in tissue obtained at necropsy (Fig. 1).
FIG. 1.
Multifocal lymphoplasmacytic cholecystitis in a normal rhesus macaque (M. mulatta) infected with E. bieneusi.
Serum chemistry was performed on 18 immunocompetent animals while anesthetized for ultrasonography and endoscopic biopsy. Alkaline phosphatase values were significantly higher (P < 0.04) in animals with enterocytozoon DNA detected in bile (n = 5, mean = 526.3 IU) than in those animals negative for enterocytozoon DNA (n = 13; mean = 314.9 IU). There were no significant differences in γ-glutamyl transpeptidase, aspartate amino transferase, or alanine amino transferase levels between these groups.
Although there is extensive data on infection with E. bieneusi in immunocompromised patients, the incidence and tissue distribution of infection in immunocompetent patients have not been reported. Moreover, the source and natural reservoir for this organism are unknown. We have shown that E. bieneusi causes a common asymptomatic infection of immunologically normal rhesus macaques at the NERPRC. The higher rate of infection in colony-born animals and our ability to identify the organism in archival samples stored for over 6 years indicate that infection with E. bieneusi is enzootic at the NERPRC (6). We have previously shown extensive genetic and ultrastructural similarities between E. bieneusi organisms derived from rhesus macaques and from humans (3, 6). Furthermore, we have successfully transmitted E. bieneusi obtained from a human patient with AIDS to SIV-infected macaques, indicating that the host specificity of this organism may not be as strict as previously thought (16). Taken together, these findings indicate that the human- and macaque-derived enterocytozoons are essentially identical and suggest that the pathophysiology of parasite infection in these hosts will also reveal similarities.
Housing practices at the NERPRC would tend to promote the the fecal oral route of infection and could account for the relatively high rate of parasitism present in our colony. Virtually all rhesus macaques at the NERPRC are kept in group housing at some point during their lives. Due to the feeding habits of nonhuman primates in this type of housing, food is often placed on the floor, where it is easily contaminated by feces. This potential route of transmission, taken together with persistent shedding of E. bieneusi in the feces of some animals and the resistance of microsporidian spores to environmental influences, suggests that most animals housed in our colony are exposed to E. bieneusi. We suspect that the majority of these macaques acquire a self-limiting infection and that chronic or persistent parasitism develops in the minority of animals. Biochemical and morphologic evidence of hepatic dysfunction was mild in immunologically normal animals. Clinically significant disease may be restricted to acute natural infection and to the progressive phases of immunodeficiency. Nonetheless, persistently infected immunologically normal animals may play a critical role in perpertuation of the parasite within the colony.
E. bieneusi has been associated with chronic diarrhea, acalculous cholecystitis, and cholangitis in human patients with AIDS (4, 5, 10). While E. bieneusi has been linked to episodes of acute diarrhea in immunologically normal hosts, the tissue distribution and duration of infection in such individuals are unknown (15, 17). Our findings indicate that E. bieneusi is a potential cause of idiopathic cholecystitis and acalculous cholecystitis in immunologically normal macaques. Furthermore, our ability to detect enterocytozoon DNA in the bile of animals shedding E. bieneusi in feces suggests that the hepatobiliary tree may represent a reservoir of infection in the persistently infected host.
Acknowledgments
Financial support was provided by Public Health Service grants RR07000, RR00168, and DK50550. A. A. Lackner is the recipient of an Elizabeth Glaser Scientist Award.
REFERENCES
- 1.Beaugerie L, Teilhac M F, Deluol A, Fritsch J, Girard P, Rozenbaum W, Le Quintrec Y, Chatelet F. Cholangiopathy associated with Microsporidia infection of the common bile duct mucosa in a patient with HIV infection. Ann Intern Med. 1992;117:401–402. doi: 10.7326/0003-4819-117-5-401. [DOI] [PubMed] [Google Scholar]
- 2.Carville A, Mansfield K G, Widmer G, Lackner A, Kotler D, Weist P, Gumbo T, Sarbah S, Tzipori S. Development and application of genetic probes for detection of Enterocytozoon bieneusi in formalin-fixed stools and in intestinal biopsy specimens from infected patients. Clin Diagn Lab Immunol. 1997;4:1–3. doi: 10.1128/cdli.4.4.405-408.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chalifoux, L., J. MacKey, A. Carville, D. Shvetz, K. C. Lin, A. Lackner, and K. G. Mansfield. Ultrastructural morphology of Enterocytozoon bieneusi in biliary epithelium of rhesus macaques (Macaca mulatta). Vet. Pathol., in press. [DOI] [PubMed]
- 4.Desportes I, Le Charpentier Y, Galian A, Bernard F, Cochand-Priollet B, Lavergne A, Ravisse P, Modigliani R. Occurrence of a new microsporidian, Enterocytozoon bieneusi gn, nsp, in the enterocytes of a human patient with AIDS. J Protozool. 1985;32:250–254. doi: 10.1111/j.1550-7408.1985.tb03046.x. [DOI] [PubMed] [Google Scholar]
- 5.French A L, Beaudet L M, Benator D A, Levy C S, Kass M, Orenstein J M. Cholecystectomy in patients with AIDS: clinicopathologic correlations in 107 cases. Clin Infect Dis. 1995;21:852–858. doi: 10.1093/clinids/21.4.852. [DOI] [PubMed] [Google Scholar]
- 6.Mansfield K G, Carville A, Shvetz D, MacKey J, Tzipori S, Lackner A A. Identification of an Enterocytozoon bieneusi-like microsporidian parasite in simian immunodeficiency virus inoculated macaques with hepatobiliary disease. Am J Pathol. 1997;150:1395–1405. [PMC free article] [PubMed] [Google Scholar]
- 7.Mansfield K G, Pauley D, Young H L, Lackner A A. Mycobacterium avium complex in macaques with AIDS is associated with a specific strain of simian immunodeficiency virus and prolonged survival after primary infection. J Infect Dis. 1995;172:1149–1152. doi: 10.1093/infdis/172.4.1149. [DOI] [PubMed] [Google Scholar]
- 8.McWhinney P H M, Nathwani D, Green S T, Boyd J F, Forrest J A. Microsporidia detected in association with AIDS-related sclerosing cholangitis. AIDS. 1991;5:1394–1395. doi: 10.1097/00002030-199111000-00024. [DOI] [PubMed] [Google Scholar]
- 9.Orenstein J M. Intestinal microsporidiosis. Adv Anat Pathol. 1996;3:46–58. [Google Scholar]
- 10.Pol S, Romana C A, Richard S, Amouyal P, Desportes-Livage I, Carnot F, Pays J, Berthelot P. Microsporidia infection in patients with the human immunodeficiency virus and unexplained cholangitis. N Engl J Med. 1993;328:95–99. doi: 10.1056/NEJM199301143280204. [DOI] [PubMed] [Google Scholar]
- 11.Rabodonirina M, Bertocchi M, Desportes-Livage I, Cotte L, Levrey H, Piens M A, Monneret G, Celard M, Mornex J F, Mojon M. Enterocytozoon bieneusi as a cause of chronic diarrhea in a heart-lung transplant recipient who was seronegative for HIV. Clin Infect Dis. 1996;23:114–117. doi: 10.1093/clinids/23.1.114. [DOI] [PubMed] [Google Scholar]
- 12.Sax P E, Rich J D, Pieciak W S, Trnka Y M. Intestinal microsporidiosis in a liver transplant recipient. Transplantation. 1995;60:617–618. doi: 10.1097/00007890-199509270-00018. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz D A, Sobottka I, Leitch G J, Cali A, Visvesvara G S. Pathology of microsporidiosis: emerging parasitic infections in patients with acquired immunodeficiency syndrome. Arch Pathol Lab Med. 1996;120:173–188. [PubMed] [Google Scholar]
- 14.Shadduck J A, Orenstein J M. Comparative pathology of microsporidiosis. Arch Pathol Lab Med. 1993;117:1215–1219. [PubMed] [Google Scholar]
- 15.Sobottka I, Albrecht H, Schottelius J, Schmetz C, Bentfeld M, Laufs R, Schwartz D A. Self-limited traveller’s diarrhea due to a dual infection with Enterocytozoon bieneusi and Cryptosporidium parvum in an immunocompetent HIV-negative child. Eur J Clin Microbiol Infect Dis. 1995;14:919–920. doi: 10.1007/BF01691502. [DOI] [PubMed] [Google Scholar]
- 16.Tzipori S, Carville A, Widmer G, Kotler D, Mansfield K G, Lackner A. Transmission and establishment of a persistent infection of E. bieneusi derived from a human with AIDS in SIV-infected rhesus monkeys. J Infect Dis. 1997;175:1016–1020. doi: 10.1086/513962. [DOI] [PubMed] [Google Scholar]
- 17.Wanke C A, Degirolami P, Federman M. E. bieneusi infection and diarrheal disease in patients who were not infected with HIV: case report and review. Clin Infect Dis. 1996;23:816–818. doi: 10.1093/clinids/23.4.816. [DOI] [PubMed] [Google Scholar]
- 18.Weber R, Bryan R T, Schwartz D A, Owen R L. Human microsporidial infection. Clin Microbiol Rev. 1996;7:426–461. doi: 10.1128/cmr.7.4.426. [DOI] [PMC free article] [PubMed] [Google Scholar]