Abstract
Typhoid fever is a contagious disease that is generally caused by bacteria known as Salmonella typhi. This disease spreads through manure contamination of food or water and infects unprotected people. In this work, our focus is to numerically examine the dynamical behavior of a typhoid fever nonlinear mathematical model. To achieve our objective, we utilize a conditionally stable Runge–Kutta scheme of order 4 (RK-4) and an unconditionally stable non-standard finite difference (NSFD) scheme to better understand the dynamical behavior of the continuous model. The primary advantage of using the NSFD scheme to solve differential equations is its capacity to discretize the continuous model while upholding crucial dynamical properties like the solutions convergence to equilibria and its positivity for all finite step sizes. Additionally, the NSFD scheme does not only address the deficiencies of the RK-4 scheme, but also provides results that are consistent with the continuous system's solutions. Our numerical results demonstrate that RK-4 scheme is dynamically reliable only for lower step size and, consequently cannot exactly retain the important features of the original continuous model. The NSFD scheme, on the other hand, is a strong and efficient method that presents an accurate portrayal of the original model. The purpose of developing the NSFD scheme for differential equations is to make sure that it is dynamically consistent, which means to discretize the continuous model while keeping significant dynamical properties including the convergence of equilibria and positivity of solutions for all step sizes. The numerical simulation also indicates that all the dynamical characteristics of the continuous model are conserved by discrete NSFD scheme. The theoretical and numerical results in the current work can be engaged as a useful tool for tracking the occurrence of typhoid fever disease.
Subject terms: Applied mathematics, Bacterial infection
Introduction
Infectious diseases can be transmitted among people either directly or indirectly. These diseases take place when germs enter into the body, enhance in quantity, and then cause a reaction of the body. Typhoid fever is a severe infection disease that can spread in the whole body, influencing numerous organs. If not treated on time, it can cause genuine difficulties and can be life threatening. It is caused by bacteria named Salmonella Typhi1. This bacteria is found in constipated food or water, and it causes illness when it enters the body through drinking or eating. Although, the sanitation of water coverage is improved but the spread of typhoid disease is still a noteworthy public health issue in several emergent countries2. The most common typhoid symptoms are migraine, stomach ache, knee pain, spine, muscular pain, lack of appetite, spew, dysentery, spots, and fever3.
Typhoid fever can become more dangerous if not treated instantly. It can damage the internal flow of blood and cause infection in the tissue that lies in the stomach4. Typhoid fever can be diagnosed by using some simple blood or stool tests. These tests identify the existence of Salmonella typhi in blood or stool samples. Typhoid fever preventive and control strategies include antibiotic treatments, stool standard precautions, vaccination, environmental sanitation and clean water5. Typhoid fever maybe treated with medicines and the symptoms improve within four weeks. The symptoms may return if treatment is not completed6. Over 110 years ago, the first typhoid vaccine was developed. Typhoid fever vaccines are available in two forms: oral and injectable. Vi polysaccharide vaccine is a licensed injectable vaccine which is secure and 65% defensive while Ty21a is a licensed liquid oral vaccine that can be used for children of 2 years and older. The oral vaccine is more costly than the Vi polysaccharide vaccine7. Many studies have been carried out over the last few decades, and different studies have resulted into different mathematical models4–7.
Numerous physical phenomena are being explained by researchers using fractional and integer order mathematical models8–15. The implementation of mathematical modelling enables us to focus on the process by which an infectious disease spreads throughout a region. To comprehend various infectious diseases and their dynamical properties, experts from all around the world developed several mathematical models16–18. Mathematical models can be used to relate the evolving dynamics of infection and biological mechanisms of transmission at the population level19,20. By using optimal control techniques, Getachew et al.21 built a deterministic mathematical model of typhoid disease to investigate its effects. The author also utilized some control strategies with cost-effective approaches. Musa et al.22 inspected the spread dynamics of the typhoid fever epidemic. The model evaluates how public health education initiatives affect the pathogenesis of typhoid fever, which can lead to significant outbreaks, especially in areas with limited resources. To analyze the dynamics of typhoid fever sickness while considering infection resistance, a mathematical model was formed by Nthiiri et al.23 using a set of ordinary differential equations. Adeboye et al.24 constructed and investigated a mathematical model of typhoid and malaria co-infection that tackles the control of the spread of malaria and typhoid simultaneously. Pitzer et al.25 considered a parsimonious age-structured mathematical model of typhoid fever to estimate the initial and indirect impact of vaccination. Cook et al.26 examined a mathematical model that discusses both direct and indirect vaccine safety as well as the benefits of a widespread vaccination programme.
Recently, Karunditu et al.27 investigated a nonlinear epidemic model for typhoid disease transmission. The stability of the disease-free and endemic equilibria was thoroughly examined for the continuous model. The main intention of this work is to use two finite difference schemes, i.e. RK-4 and NSFD schemes to evaluate various properties of the continuous model to exhibit its sustainability and biological viability. The RK-4 scheme cannot exactly maintain the basic dynamical aspects of the continuous model, resulting in numerical solutions that change from the solutions of the original system. On the other hand, our findings demonstrate that the NSFD method is not only suitable for the continuous model but also yields incredibly accurate and efficient outcomes. Mickens28 actually came up with the concept for this. He proposed the name NSFD scheme to differentiate the new numerical system from the previous SFD scheme. In order to compensate for the shortcomings of the RK-4 scheme, the NSFD scheme was developed.
The present paper is structured as follows: The mathematical model is given in Sect. 2. In Sect. 3, the equilibria and basic reproduction number are provided for the model. In Sect. 4, we utilized the RK-4 scheme for the nonlinear mathematical model. We consider the advanced NSFD scheme in Sect. 5 to solve a variety of problems. Using the Schur-Cohn criterion, the local stability of disease-free equilibrium point is examined. However, the Routh-Hurwitz condition is engaged to validate the local stability of the endemic equilibrium point for the NSFD scheme. The global stability of endemic and disease-free equilibria is examined employing the properties of the Lyapunov function. In order to explain our analytical conclusions, numerical simulations are provided in each section. To summarize the whole paper, the conclusions are given in the final section.
Mathematical model and parameters explanation
The present paper discusses and evaluates a deterministic model for the dynamics of typhoid disease. We presume that the whole population is separated into four sections: Susceptible (), Exposed (), Infected (), and Recovered (), i.e. =. The model employs the subsequent procedure as: . A fractional map for SEIR model of typhoid disease transmission among unprotected people compartments is shown in Fig. 1.
Figure1.
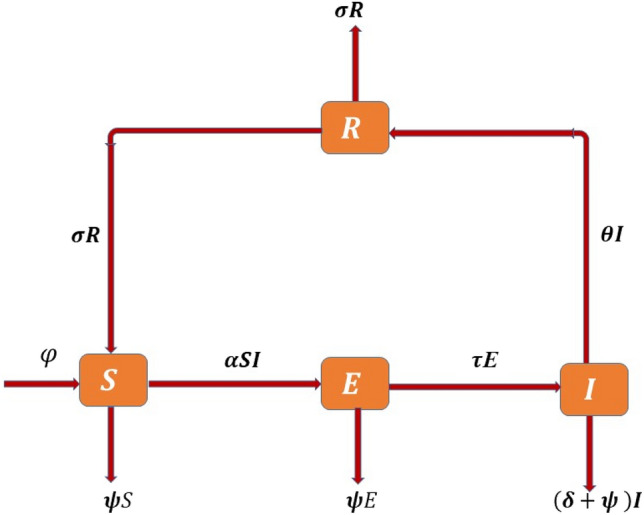
The detailed description of epidemic model for typhoid fever disease transmission.
From Fig. 1, we can describe the following SEIR disease system27 of nonlinear ordinary differential equations as:
| 1 |
Parameters
The following are descriptions of the parameters used in model (1).
The parameters are all positive constants. As we know that.
Consequently, by combining all dynamical equations in system (1), we get.
| 2 |
From Eq. (2), we can write
and.
Therefore, the feasible area for the continuous system (1) becomes.
| 3 |
Equilibrium points and basic reproductive number
In this section, we establish the equilibrium points of the model (1) and basic reproductive number.
Equilibrium points
For system (1), there exist the following two nonnegative equilibria.
Disease-free equilibrium (DFE) point
Disease endemic equilibrium (DEE) point = (,
where
Basic reproductive number
The fundamental reproductive number ), which measures the average rate of latest cases in a residents that is perfectly susceptible, is a critical threshold value in epidemiology. To find the reproductive number, we utilize the idea of next generation matrix provided by the authors in29. For typhoid fever disease model (1), we can easily get
In the following two sections, a comparison between NSFD scheme and RK-4 is provided. As our main concern is NSFD scheme, therefore the advantages and uses of NSFD are discussed in detail. Specifically, traditional issues concerned to the behavior of these schemes, i.e. equilibrium points, positivity and stability are discussed with respect to increment of the time step.
The RK-4 scheme
The RK-4 scheme30 is widely used approach to solve a system of ordinary differential equations. In numerous situations, we frequently employ the RK-4 scheme, unless stated otherwise. Let for , then system (1) may be signified using the RK-4 scheme as given below.
Stage -1
Stage-2
Stage-3
Stage-4
Thus general form is
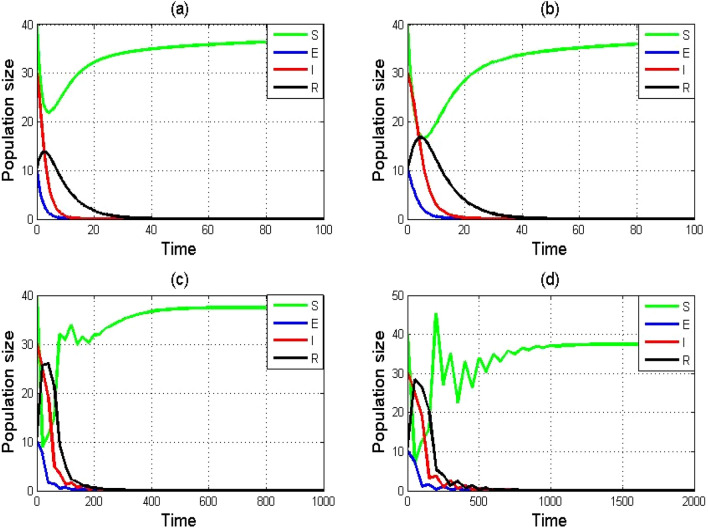
The RK-4 approach is graphically depicted in Fig. 2a–d with various step sizes. The RK-4 approach clearly yields stable and positive solutions for small step sizes, as seen in Fig. 2a,b. As the step size is increased, the equilibrium point stability is shattered for model (1), as illustrated in Fig. 2c,d. Hence, we conclude that the RK-4 technique cannot be employed for large step sizes.
Figure 2.
Numerical simulation of SEIR model by using RK-4 scheme with (a) =0.01, (b) =0.1, (c) =0.9, and (d) =1. Remaining parameters are set as
The NSFD scheme
In this section, our main objective is to discuss the dynamics of NSFD scheme for model (1). The NSFD scheme concept was presented by Mickens in 199428. The NSFD scheme is an iterative method in which we move closer to a solution through iterations31. The NSFD scheme is a valuable technique used to solve problems in epidemiology32–36, ecology37,38, and meta-population modeling39. The following will show that, despite the step size , the discrete NSFD scheme sustains the dynamical properties of the corresponding continuous model (1).
Construction of NSFD scheme
For model (1), we use the notation , and to indicate the numerical estimates of and at time step , where is nonnegative time step size and is a nonnegative integer40.
| 4 |
It is assumed that the starting quantities of NSFD SEIR model (4) are also nonnegative. From (4), we get
| 5 |
In the same way like continuous model (1), we can determine a feasible region for the discrete scheme (5). If we indicate , then by combining all the four equations in system (4) we obtain
and.
By employing the discrete Gronwall inequality41–43, if then.
Since , so we get as . Therefore, the feasible region for NSFD scheme (5) becomes.
| 6 |
In the following, we provide the stability conditions of DFE and DEE points for the discrete NSFD scheme (5). We first describe the local stability of both equilibrium points in order to achieve this goal.
Local stability for NSFD Scheme
To prove that DFE and DEE points are locally asymptotically stable (LAS), we consider
| 7 |
To demonstrate that DFE and DEE points are LAS, as stated in Lemma 1, we shall utilize the Schur-Cohn condition44,45.
Lemma 1
The roots of equation guarantee , =1,2 if and only if the requirements listed below are met.
-
i.
-
ii.
-
iii.
where and stand for the Jacobian matrix trace and determinant, respectively.
Theorem 1
For all , the DFE point of the NSFD model (5) is LAS whenever .
Proof
Let us consider the Jacobian matrix.
| 8 |
In the following, we first find all the derivatives used in (8).
By substituting the values of all the derivatives in Eq. (8), we get.
The aforementioned matrix becomes at DFE point
To determine the eigenvalues, we take into consideration.
The above equation can be rewritten as.
| 9 |
The Eq. (9) gives . To discuss the other two eigenvalues, we consider.
| 10 |
From Eq. (10), we obtain.
| 11 |
Comparing (11) with , we get and . If , i.e. then all the three conditions of Lemma 1 are satisfied, i.e.
All of the Schur–Cohn requirements described in Lemma 1 are consequently satisfied whenever . As a result, the DFE point of discrete NSFD scheme (5) is LAS, provided that .
When a disease is prevalent in a population, it will continue to exist in that community. In the following theorem, we will use Routh-Hurwitz criterion46,47 to examine that DEE point is LAS.
Theorem 2
For all , the DEE point of the NSFD model (5) is LAS whenever .
Proof
The Jacobian matrix is obtained according to Theorem 1 as.
By putting DEE point , we obtain.
To determine the eigenvalues, we take into consideration.
The characteristic equation for above equation becomes as.
| 12 |
where
From above informations, it is clear that if , then
According to the Routh-Hurwitz criteria, all of the solutions to the Eq. (12) must have negative real parts. As a result, the DEE point of the discrete NSFD scheme (5) is LAS whenever .
Global stability for NSFD Scheme
In the following, we now show that is a critical value for the global stability. If , then the DFE point is globally asymptotically stable (GAS) and when , then DEE point becomes GAS. To discuss the global stability of equilibria, we employ the same criterion used by Vaz et al.48.
Theorem 3
For all , the DFE point of the NSFD model (5) is GAS whenever .
Proof
From the feasible region (6) discussed for NSFD scheme (5), it is clear that . If , then . Therefore, if we take , then there exists an integer such that for any . Consider the sequence such that.
where and . For , we have
By applying the NSFD scheme (5), we obtain
Let , then the above expression becomes
If we put , the above expression becomes
We can choose a very small positive number such that
Therefore, we get
Let , then we get
Since is a very small number and is imprecise Therefore, if then we reach the conclusion that for any , and . The sequence is a monotonically decreasing and . Therefore, the DFE point is GAS whenever .
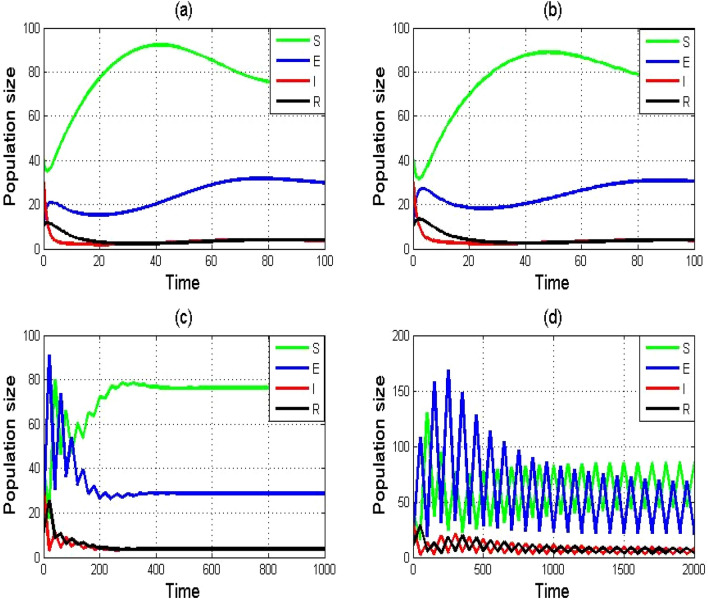
The numerical simulations shown in Figs. 3a–d and 4a–d for and , respectively also exhibit that the solutions of NSFD scheme (5) converges to the DFE point independent of the step size. By combining the above two conditions, we conclude that if then the DFE point is GAS for NSFD scheme (5). The NSFD scheme is hence convergent for model (1) for all finite step sizes.
Figure 3.
Numerical simulation of SEIR model by using NSFD scheme with . Remaining parameters are set as .
Figure 4.
Numerical simulation of SEIR model by using NSFD scheme with . Remaining parameters are set as .
Theorem 4
For all , the DEE point of the NSFD model (5) is GAS whenever .
Proof
Let us construct a sequence such that.
Where such that . It is evident that and if , the equality holds. From above, we can write
In the same way
Let , then
and
and
The difference of satisfies
Hence, the sequence for any is monotonic decreasing. Since and , we conclude that , , and . This completes the proof.
The numerical illustration in Fig. 5a–d additionally illustrates that, for any step size, the NSFD scheme (5) solutions converge to the DEE point if . This reveals the NSFD scheme's unconditional convergence.
Figure 5.
Numerical simulation of SEIR model by using NSFD scheme with . Remaining parameters are set as
Conclusions
In the present paper, a nonlinear epidemic model of a typhoid fever disease is numerically studied using two finite difference schemes. It was shown that the spread of the disease is mostly determined by the rate of contact with sick individuals within a community. The RK-4 and NSFD schemes are employed to discuss the dynamical characteristics of DFE and DEE points, including their local and global stabilities. The findings demonstrate that the NSFD method provides precise numerical solutions while eliminating drawback of RK-4 scheme. The convergence is demonstrated that presents that the NSFD scheme retains their stability and positivity characteristics. The key benefits of NSFD are demonstrated theoretically as well as numerically which reveal that this scheme have good dynamical behavior even for large time step size. At the same time, the RK-4 scheme cannot exactly sustain the fundamental properties of the original continues model and consequently, it can produce numerical solutions which are not quite the same as the solutions of the original model. The NSFD scheme is an easy approach that exhibits how discrete and continuous models act appropriately and produce results that are mathematically accurate. Figures 3, 4, and 5 depicts that the NSFD scheme (5) remains stable for each step size. This demonstrates that for each step size, the NSFD scheme is positive forever and unconditionally convergent. The RK-4 scheme, however, shows convergence only for lower step sizes, as shown in Fig. 3a–d. We can effectively monitor the spread of typhoid fever disease by utilizing the NSFD system. The results provided in this study are advantageous to humanity as well as in the sector of healthcare. Numerical simulations are included in each section to support our theoretical conclusions.
Author contributions
I.U.K.: Conceptualization, Methodology, Software. S.M.: Writing, paper Original draft setting, Software A.S.: Reviewing and Editing, visualization S.L.: Reviewing and Editing, original draft preparation A.A.: Reviewing and Editing, visualization A.B.: Reviewing and Editing, visualization.
Data availability
The data used to support the findings of this study are included within the article.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mushayabasa S, Bhunu CP, Ngarakana-Gwasira ET. Mathematical analysis of a typhoid model with carriers, direct and indirect disease transmission. Int. J. Math. Sci. Eng. Appl. 2013;7(1):79–90. [Google Scholar]
- 2.Ivanoff B, Levine MM, Lambert P. Vaccination against typhoid fever: Present status. Bull. World Health Organ. 1994;72(6):957. [PMC free article] [PubMed] [Google Scholar]
- 3.Nsutebu EF, Martins P, Adiogo D. Prevalence of typhoid fever in febrile patients with symptoms clinically compatible with typhoid fever in Cameroon. Trop. Med. Int. Health. 2003;8(6):575–578. doi: 10.1046/j.1365-3156.2003.01012.x. [DOI] [PubMed] [Google Scholar]
- 4.Schemmer, A. K. Heterogeneity of inflammation and host metabolism in a typhoid fever model (Doctoral dissertation, University_of_Basel) (2012).
- 5.Bhan MK, Bahl R, Bhatnagar Typhoid and paratyphoid fever. Lancet. 2005;366(9487):749–762. doi: 10.1016/S0140-6736(05)67181-4. [DOI] [PubMed] [Google Scholar]
- 6.Butler T. Treatment of typhoid fever in the 21st century: promises and shortcomings. Clin. Microbiol. Infect. 2011;17(7):959–963. doi: 10.1111/j.1469-0691.2011.03552.x. [DOI] [PubMed] [Google Scholar]
- 7.Cook J, Jeuland M, Whittington D, et al. The cost-effectiveness of typhoid Vi vaccination programs: Calculations for four urban sites in four Asian countries. Vaccine. 2008;26(50):6305–6316. doi: 10.1016/j.vaccine.2008.09.040. [DOI] [PubMed] [Google Scholar]
- 8.Gao XY, Guo YJ, Shan WR. Similarity reductions for a (3+ 1)-dimensional generalized Kadomtsev–Petviashvili equation in nonlinear optics, fluid mechanics and plasma physics. Appl. Comput. Math. 2021;20(3):421–429. [Google Scholar]
- 9.Pankov, P. S., Zheentaeva, Z. K., & Shirinov, T. Asymptotic reduction of solution space dimension for dynamical systems. TWMS J. Pure Appl. Math. 12(2) (2021)
- 10.Musaev, H. K. (2021). The Cauchy problem for degenerate parabolic convolution equation. TWMS J. Pure Appl. Math. 12(2) (2021)
- 11.Sunday J, Shokri A, Kwanamu JA, Nonlaopon K. Numerical integration of stiff differential systems using non-fixed step-size strategy. Symmetry. 2022;14(8):1575. doi: 10.3390/sym14081575. [DOI] [Google Scholar]
- 12.Juraev DA, Shokri A, Marian D. Regularized solution of the Cauchy problem in an unbounded domain. Symmetry. 2022;14(8):1682. doi: 10.3390/sym14081682. [DOI] [Google Scholar]
- 13.Rafiq M, Ahmad W, Abbas M, Baleanu D. A reliable and competitive mathematical analysis of Ebola epidemic model. Adv. Diff. Equ. 2020;2020:1–24. doi: 10.1186/s13662-020-02994-2. [DOI] [Google Scholar]
- 14.Ahmad W, Rafiq M, Abbas M. Mathematical analysis to control the spread of Ebola virus epidemic through voluntary vaccination. Eur. Phys. J. Plus. 2020;135(10):775. doi: 10.1140/epjp/s13360-020-00683-3. [DOI] [Google Scholar]
- 15.Ahmad W, Abbas M, Rafiq M, Baleanu D. Mathematical analysis for the effect of voluntary vaccination on the propagation of Corona virus pandemic. Results Phys. 2021;31:104917. doi: 10.1016/j.rinp.2021.104917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Butt AIK, Shah NA, Ahmad W, Botmart T, Ahmad N. On implementation of a semi-analytic strategy to develop an analytical solution of a steady-state isothermal tube drawing model. Sci. Rep. 2022;12(1):7636. doi: 10.1038/s41598-022-11708-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanif, A., Kashif Butt, A. I., & Ahmad, W. Numerical approach to solve Caputo‐Fabrizio‐fractional model of corona pandemic with optimal control design and analysis. Math. Methods Appl. Sci. (2023).
- 18.Butt AIK, Rafiq M, Ahmad W, Ahmad N. Implementation of computationally efficient numerical approach to analyze a Covid-19 pandemic model. Alex. Eng. J. 2023;69:341–362. doi: 10.1016/j.aej.2023.01.052. [DOI] [Google Scholar]
- 19.Kumar P, Erturk VS, Abboubakar H, Nisar KS. Prediction studies of the epidemic peak of coronavirus disease in Brazil via new generalised Caputo type fractional derivatives. Alex. Eng. J. 2021;60:3189–3204. doi: 10.1016/j.aej.2021.01.032. [DOI] [Google Scholar]
- 20.Nazir A, Ahmed N, Khan U, Mohyud-Din ST, Nisar KS, Khan I. An advanced version of a conformable mathematical model of Ebola virus disease in Africa. Alex. Eng. J. 2020;59:3261–3268. doi: 10.1016/j.aej.2020.08.050. [DOI] [Google Scholar]
- 21.Getachew, T. T., Makinde, O. D. & Malonza, D. Modelling and optimal control of typhoid disease with cost-effective strategies. Comput. Math. Methods Med., Article ID 2324518 (2017). [DOI] [PMC free article] [PubMed]
- 22.Musa SS, Zhao S, Hussaini N, Usaini S, He D. Dynamics analysis of typhoid fever with public health education programs and final epidemic size relation. Results Appl. Math. 2021;10:100153. doi: 10.1016/j.rinam.2021.100153. [DOI] [Google Scholar]
- 23.Nthiiri JK, Lawi GO, Akinyi CO, Oganga DO, Muriuki WC, Musyoka MJ, Otieno PO, Koech L. Mathematical modelling of typhoid fever disease incorporating protection against infection. Br. J. Math. Comput. Sci. 2016;14(1):1–10. doi: 10.9734/BJMCS/2016/23325. [DOI] [Google Scholar]
- 24.Adeboye KR, Haruna M. A Mathematical model for the transmission and control of malaria typhoid co-infection using sirs approach. Niger. Res. J. Math. 2015;2(2):1–24. [Google Scholar]
- 25.Pitzer VE, Bowles CC, Baker S, Kang G, Balaji V, Farrar JJ, Grenfell BT. Predicting the impact of vaccination on the transmission dynamics of typhoid in South Asia: A mathematical modeling study. PLoS Negl. Trop. Dis. 2014;8(1):e2642. doi: 10.1371/journal.pntd.0002642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cook, J. H. Are cholera and typhoid vaccines a good investment for a slum in Kolkata, India? Ph.D. Dissertation. Chapel Hill, NC: Department of Environmental Sciences and Engineering, The University of North Carolina at Chapel Hill (2007).
- 27.Karunditu JW, Kimathi G, Osman S. Mathematical modeling of typhoid fever disease incorporating unprotected humans in the spread dynamics. J. Adv. Math. Comput. Sci. 2019;32(3):1–11. doi: 10.9734/jamcs/2019/v32i330144. [DOI] [Google Scholar]
- 28.Mickens, R. E. Nonstandard finite difference models of differentials equations. World Sci. (1994).
- 29.Van den Driessche P, Watmough J. Reproduction numbers and sub-threshold endemic equilibria for compartmental models of disease transmission. Math. Biosci. 2002;180(1–2):29–48. doi: 10.1016/S0025-5564(02)00108-6. [DOI] [PubMed] [Google Scholar]
- 30.Chamuchi MN, Sigey JK, Okello JA, Okwoyo JM. SI IC R Model and Simulation of the effects of carriers on the transmission dynamics of typhoid fever in Kisii town, Kenya. SIJ Trans. Comput. Sci. Eng. Appl. (CSEA) 2014;2(3):109–116. [Google Scholar]
- 31.Gentile I, Borgia G. Vertical transmission of hepatitis B virus: Challenges and solutions. Int. J. Womens Health. 2014;6:605–611. doi: 10.2147/IJWH.S51138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Edward S, Raymond KE, Gabriel KT, Nestory F, Godfrey MG, Arbogast MP. Global stability in a mathematical modeling of tuberculosis. Appl. Comput. Math. 2015;4(6):396–408. doi: 10.11648/j.acm.20150406.12. [DOI] [Google Scholar]
- 33.Mickens RE. Nonstandard finite difference schemes for differential equations. J. Differ. Equ. Appl. 2002;8(9):823–847. doi: 10.1080/1023619021000000807. [DOI] [Google Scholar]
- 34.Cui Q, Xu J, Zhang Q, Wang K. An NSFD scheme for SIR epidemic models of childhood diseases with constant vaccination strategy. Adv. Diff. Equ. 2014;2014(1):1–15. doi: 10.1186/1687-1847-2014-172. [DOI] [Google Scholar]
- 35.Hattaf K, Lashari AA, Boukari BE, Yousfi N. Effect of discretization on dynamical behavior in an epidemiological model. Diff. Equ. Dyn. Syst. 2015;23(4):403–413. doi: 10.1007/s12591-014-0221-y. [DOI] [Google Scholar]
- 36.Rao PRS, Ratnam KV, Murthy MSR. Stability preserving non-standard finite difference schemes for certain biological models. Int. J. Dyn. Control. 2018;6(4):1496–1504. doi: 10.1007/s40435-018-0410-6. [DOI] [Google Scholar]
- 37.Darti, I., Suryanto, A. & Hartono, M. Global stability of a discrete SIR epidemic model with saturated incidence rate and death induced by the disease. Commun. Math. Biol. Neurosci. (2020).
- 38.Darti I, Suryanto A. Dynamics of a SIR epidemic model of childhood diseases with a saturated incidence rate: Continuous model and its nonstandard finite difference discretization. Mathematics. 2020;8(9):1459. doi: 10.3390/math8091459. [DOI] [Google Scholar]
- 39.Shabbir MS, Din Q, Safeer M, Khan MA, Ahmad K. A dynamically consistent nonstandard finite difference scheme for a predator-prey model. Adv. Differ. Equ. 2019;2019(1):1–17. doi: 10.1186/s13662-019-2319-6. [DOI] [Google Scholar]
- 40.Dang QA, Hoang MT. Complete global stability of a meta population model and its dynamically consistent discrete models. Qual. Theory Dyn. Syst. 2019;18(2):461–475. doi: 10.1007/s12346-018-0295-y. [DOI] [Google Scholar]
- 41.Pachpatte BG. On the discrete generalizations of Gronwall’s inequality. J. Indian Math. Soc. 1973;37:147–156. [Google Scholar]
- 42.Clark DS. Short proof of a discrete Gronwall inequality. Discret. Appl. Math. 1987;16(3):279–281. doi: 10.1016/0166-218X(87)90064-3. [DOI] [Google Scholar]
- 43.Dixon J, McKee S. Weakly singular discrete Gronwall inequalities. Z. Angew. Math. Mech. 1986;66(11):535–544. doi: 10.1002/zamm.19860661107. [DOI] [Google Scholar]
- 44.Brauer F, Castillo-Chavez C. Mathematical models in population biology and epidemiology. New York: Springer; 2012. p. 508. [Google Scholar]
- 45.Elaydi S. An international to difference equation. 3. Springer- Verlag; 2005. [Google Scholar]
- 46.Jia J, Li P. Global analysis of an SVEIR epidemic model with partial immunity. Math. Terna. 2011;8(8):547–561. [Google Scholar]
- 47.Khatun Z, Islam MS, Ghosh U. Mathematical modeling of hepatitis B virus infection incorporating immune responses. Sens. Int. 2020;1:100017. doi: 10.1016/j.sintl.2020.100017. [DOI] [Google Scholar]
- 48.Vaz S, Torres DFM. A dynamically-consistent nonstandard finite difference scheme for the SICA model. Math. Biosci. Eng. 2021;18(4):4552–4571. doi: 10.3934/mbe.2021231. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are included within the article.