Coronary artery disease is the most common manifestation of thoracic radiation therapy (RT)–induced heart dysfunction, and ischemic events can occur within 2 years after RT. However, how cardiac RT influences ischemic injury responses is unknown. In this study, we evaluated cardiac susceptibility to injury and ability to recover following ex vivo ischemic reperfusion (IR) injury in rats that received targeted cardiac RT.
Echocardiograms with M-mode readings were performed on 10- to 12-week-old female Dahl salt-sensitive/Mcw (SS) rats (n = 9/group) 10 weeks after 24 Gy of localized, computed tomography–guided cardiac RT or sham.1 Animal care use and approval were received at the Medical College of Wisconsin (AUA4200), and all national care/use requirements were followed. Rats were randomly assigned to groups and fed a low-salt diet.1 Mean ± SD and unpaired Student’s t-test analyses are reported. Hearts were isolated and perfused ex vivo using the Langendorff method by researchers blinded to groups. Hearts either underwent 25 minutes of ischemia followed by 120 minutes of reperfusion (IR; n = 6 each sham/RT) or perfusion without IR for the same duration (no IR; n = 3 each sham/RT). Cardiac function, mitochondrial redox state (reduced nicotinamide adenine dinucleotide [NADH]/flavin adenine dinucleotide [FAD]), vascular reactivity, and infarct size were assessed as previously described.2 Two-way repeated-measures analyses of variance with Tukey post hoc test for multiple comparisons were conducted using Prism GraphPad 9.3.1.
The RT group showed increased interventricular septal thickness (1.3 ± 0.11 cm/kg vs 0.8 ± 0.1 cm/kg; P < 0.001) and left ventricular (LV) posterior wall thickness (1.03 ± 0.1 cm/kg vs 0.8 ± 0.1 cm/kg; P < 0.001) vs sham. The RT group had decreased end-diastolic (1.8 ± 0.5 mL/kg vs 2.4 ± 0.3 mL/kg; P = 0.036) and end-systolic (0.1 ± 0.05 mL/kg vs 0.2 ± 0.1 mL/kg; P = 0.019) volume. Ejection fraction (94.5% ± 2.1% vs 90.3% ± 3.9%; P = 0.08) and stroke volume (1.7 ± 0.4 mL/kg vs 2.2 ± 0.3 mL/kg; P = 0.08) did not significantly vary post-RT. Thus, 10 weeks following targeted cardiac RT, rats exhibit ventricular remodeling and thickening, with LV function preserved.
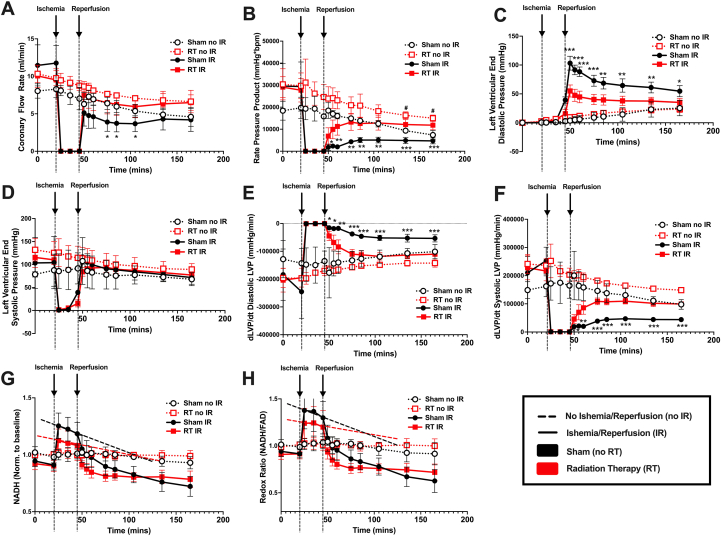
In the no IR sham vs RT groups, coronary flow rate (CFR) did not differ. Rate pressure product was lower in sham vs RT, suggesting that RT sustained myocardial work overtime in ex vivo hearts. Following ischemia, the RT group showed enhanced recovery of CFR and rate pressure product vs sham (Figures 1A and 1B). There were no differences in mean heart rate in the no IR or IR groups (data not shown). Given the enhanced recovery of CFR in the irradiated group, we postulated that RT improved vascular reactivity. No significant differences in endothelium-dependent (percent change in CFR with bradykinin with no IR: 26.8% ± 15.4%; no IR RT: 17.8% ± 8.8%; sham IR: 41.0% ± 44.2% vs RT IR: 14.5% ± 10.8%; P = 0.42) or independent (percent change in CFR with sodium nitroprusside with no IR sham: 23.4% ± 15.2%; no IR RT: 15.0% ± 9.6%; sham IR: 19.7% ± 31.7% vs RT IR: 19.6% ± 18.8%; P = 0.98) vascular reactivity were observed.
Figure 1.
Response to Ex Vivo Ischemia Reperfusion Injury in Irradiated Versus Sham Treated Rat Hearts
(A) Coronary flow rate (mL/min). (B) Rate pressure product (mm Hg × beats/min). (C) Left ventricular end-diastolic pressure (mm Hg). (D) Left ventricular end-systolic pressure (mm Hg). (E) Diastolic and (F) systolic developed left ventricular pressure over time (dLVP/dt; mm Hg/min). (G) NADH fluorescence normalized to baseline levels. (H) Redox state (NADH/FAD fluorescence) normalized to baseline levels. All graphs show mean ± SD. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001: sham vs RT exposed to IR injury. #P < 0.05: sham vs RT not exposed to IR injury. Two-way repeated-measures analyses of variance with Tukey post hoc test for multiple comparisons. FAD = flavin adenine dinucleotide; IR = ischemia reperfusion; LVP = left ventricular pressure; mins = minutes; NADH = reduced nicotinamide adenine dinucleotide; no IR = no ischemia reperfusion; Norm. = normalized; RT = radiation therapy.
Following reperfusion, the RT group showed greater recovery of LV end-diastolic pressure compared to sham (Figure 1C). No significant differences were seen in LV end-systolic pressure (Figure 1D); however, following reperfusion, the RT group showed more significant recovery of diastolic (Figure 1E) and systolic (Figure 1F) developed LV pressure over time (dLVP/dt) vs sham, suggesting improved contractility and relaxation during reperfusion in the RT group. No differences were seen between the no IR sham and RT groups. Hence, cardiac RT enhanced recovery from IR but did not significantly affect cardiovascular function at baseline. Infarct sizes did not vary between the sham and RT groups following IR (sham IR: 34.9% ± 17.9% vs RT IR 42.9% ± 12.7%; P = 0.45).
Although RT is classically associated with cell death and organ dysfunction, kidney3 and brain4 RT can up-regulate protective stress-response mechanisms that minimize subsequent IR damage. Here, we show, for the first time to our knowledge, that a single dose of cardiac RT decreases susceptibility to IR injury and enhances recovery following IR in ex vivo SS rat hearts, although the mechanism for this protection remains unclear. Mitochondrial reactive oxygen species production with radiation exposure may up-regulate protective pathways against IR injury, similar to preconditioning. In preconditioned hearts, this results in the stabilization of mitochondrial redox states, which presents as a slow decline in NADH and redox ratio (NADH/FAD) during ischemia. In the RT group, a slower decline in NADH levels and redox ratio was seen vs sham hearts (Figures 1G and 1H). As such, future work will elucidate whether RT mimics preconditioning by activating reactive oxygen species–mediated signaling to confer cardioprotection.
For this study, we selected female SS rats based on prior work characterizing their high susceptibility for RT-induced heart dysfunction.1,5 Further work evaluating sex- and strain- differences using in vivo IR models is necessary. Our prior work also showed that fractioned (9 Gy × 5) and 1-time 24-Gy (easier to administer, limited anesthesia-related complications) cardiac radiation results in similar phenotypes.1 Future studies comparing the effects of 1-time high-dose (clinically representative of RT for ventricular tachycardia) vs fractionated low-dose (eg, thoracic cancer) cardiac RT, with a focus on elucidating the timeline and mechanism of RT-mediated cardioprotection vs detrimental outcomes, can greatly inform clinical care.
Footnotes
This work was supported by the National Institutes of Health R01HL147884 (Dr Bergom). Additional support was provided by the Medical College of Wisconsin Cancer Center (Dr Bergom); the Michael H. Keelan, Jr., MD, Research Foundation Grant (Dr Bergom); and the Cardiovascular Center at the Medical College of Wisconsin (Dr Bergom). Dr Javaheri has received research support from AstraZeneca and Bitterroot, is a member of the Scientific Advisory Board of Mobius Scientific, and holds patents for the use of apolipoproteins to treat heart failure and eye diseases. Dr Lavine serves as a consultant for Implicit Biosciences and Medtronic; is the recipient of sponsored research agreements with Amgen and Novartis; and has a pending patent entitled “Methods for Detecting CCR2 Receptors.” All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Contributor Information
Amadou K.S. Camara, Email: aksc@mcw.edu.
Carmen Bergom, Email: cbergom@wustl.edu.
References
- 1.Schlaak R.A., Frei A., Schottstaedt A.M., et al. Mapping genetic modifiers of radiation-induced cardiotoxicity to rat chromosome 3. Am J Physiol Heart Circ Physiol. 2019;316:H1267–H1280. doi: 10.1152/ajpheart.00482.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.la Cour M.F., Mehrvar S., Heisner J.S., et al. Optical metabolic imaging of irradiated rat heart exposed to ischemia-reperfusion injury. J Biomed Opt. 2018;23:1–9. doi: 10.1117/1.JBO.23.1.016011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim J., Park J.W., Park K.M. Increased superoxide formation induced by irradiation preconditioning triggers kidney resistance to ischemia-reperfusion injury in mice. Am J Physiol Renal Physiol. 2009;296:F1202–F1211. doi: 10.1152/ajprenal.90592.2008. [DOI] [PubMed] [Google Scholar]
- 4.Kokosova N., Danielisova V., Smajda B., Burda J. Ionizing radiation as preconditioning against transient cerebral ischemia in rats. Gen Physiol Biophys. 2014;33:403–410. doi: 10.4149/gpb_2014021. [DOI] [PubMed] [Google Scholar]
- 5.Andruska N., Schlaak R.A., Frei A., et al. Differences in radiation-induced heart dysfunction in male versus female rats. Int J Radiat Biol. 2023:1–31. doi: 10.1080/09553002.2023.2194404. [DOI] [PMC free article] [PubMed] [Google Scholar]