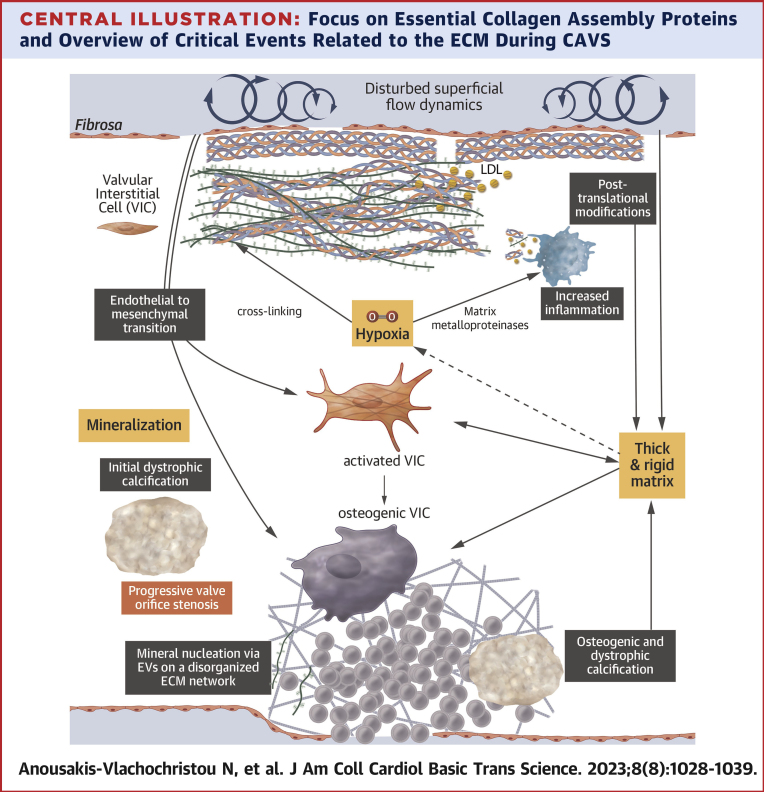
Central Illustration
Key Words: Biomineralization, calcific aortic valve stenosis, calcification, extracellular matrix proteins, mechanobiology
Highlights
-
•
CAVS remains without pharmaceutical therapy.
-
•
Sustained destruction and defective remodeling of the ECM, facilitates cellular activation and osteoblastic differentiation.
-
•
Altered mechanical function of the valve has a direct impact on ECM remodeling and osteoblastic differentiation.
-
•
Diverse and complex phases of calcium phosphate depositions are found in the lesion.
-
•
Calcification development is tightly related to native ECM protein alterations, where further research is needed.
Summary
Calcific aortic valve stenosis (CAVS) is a widespread valvular heart disease affecting people in aging societies, primarily characterized by fibrosis, inflammation, and progressive calcification, leading to valve orifice stenosis. Understanding the factors associated with CAVS onset and progression is crucial to develop effective future pharmaceutical therapies. In CAVS, native extracellular matrix proteins modifications, play a significant role in calcification in vitro and in vivo. This work aimed to review the evidence on the alterations of structural native extracellular matrix proteins involved in calcification development during CAVS and highlight its link to deregulated biomechanical function.
Calcific aortic valve stenosis (CAVS) affects mainly people ages >65 years, with a prevalence of 12.4% in people ages >75 years in Western countries. It develops silently over more than a decade, and 24.4% of patients remain asymptomatic at the final stage.1 When angina, syncope, or dyspnea develop and the valve is left unreplaced, it is characterized by death rates of ≤50% by year 2.2 In the congenital bicuspid valve, characterized either by 2 sinuses of Valsalva or 3 sinuses with cusp fusion,3 degeneration, and stenosis are seen as early as the fourth decade.4
The human aortic valve consists of 3 semilunar cusps, with a 3-layered tissue structure: the fibrosa at the aortic side comprised mainly of collagens, the ventricularis at the ventricular side consisted of elastin and collagen fibers, and the spongiosa as the medial layer, rich in proteoglycans5 (Figure 1, Table 1). Each layer of the valve confers different biomechanical properties: the fibrosa bears the high circumferential stress during aortic backflow, being stiffer in comparison with the ventricularis, which in turn is responsible for the flexibility, stretch, and elastic recoil.6,7 Collagen fibers constantly align their orientation depending on the mechanical loading.8 Although mostly avascular, on the cusp basis, where tissue is thicker and oxygen diffusion is expected to be inadequate to meet demands, a microcirculation system in the spongiosa has been described.5 The main resident cell population responsible for the maintenance of the extracellular matrix (ECM), are the valve interstitial cells (VICs), which are characterized by significant phenotypic heterogeneity; they are recognized as a specialized type of fibroblasts, although evidence exists on a small population of α-smooth muscle actin + VICs in the ventricularis.9, 10, 11
Figure 1.
Simplified Architecture of the Aortic Valve
The aortic valve consists of 3 layers, with different dominating extracellular matrix proteins. SMA = smooth muscle actin; VIC = valvular interstitial cell.
Table 1.
Native Structural ECM Protein Alterations in CAVS
| Reference | Source | |
|---|---|---|
| Proteins with specific mechanistic insights in CAVS | ||
| Physiological collagens | ||
| Collagens I, II, III, IV, V, VI, XVI, and XVIII | 24,27,31,32 | Protein, mRNA |
| New collagen isoforms in calcified valves | ||
| Collagens VIII, X, XI, and XII | 24,33, 34, 35, 36, 37 | Protein, mRNA |
| Proteins with increased synthesis | ||
| Collagens I, II, III, IV, V, and VI | 27,31,33, 34, 35,37, 38, 39 | Protein, mRNA |
| Laminins | 24,34,36 | Protein, mRNA |
| Fibronectin-1 | 24,32 | Protein, mRNA |
| Periostin | 31,38 | Protein, mRNA |
| Prolargin | 38 | Protein |
| Osteonectin | 24,35 | Protein, mRNA |
| Microfibrillar-associated protein 4,5 | 34,35,37 | Protein, mRNA |
| Fibulin-1, 6 | 35,37 | Protein, mRNA |
| Lumican | 31,32,35,38,40 | Protein, mRNA |
| Biglycan | 31,38 | Protein, mRNA |
| Decorin | 32,38 | Protein |
| Aggrecan | 24,34,35 | Protein, mRNA |
| Versican | 32 | Protein |
| Testicans | 24 | Protein, mRNA |
| Tenascin-C | 24,35 | Protein, mRNA |
| Annexin A2 | 32,37,38 | Protein, mRNA |
| Galectin-1 | 38 | Protein |
| Proteins with decreased synthesis | ||
| Tenascin-X | 24,25 | Protein, mRNA |
| Proteins without known specific mechanistic insights in CAVS | ||
| Multimerin-2 | 24,34,37 | Protein, mRNA |
| Galectin-3-binding protein | 24,37 | Protein, mRNA |
| SPARC-related modular calcium-binding protein | 24,37 | Protein, mRNA |
| Vitronectin | 24,32 | Protein, mRNA |
| Chondroadherin | 24 | Protein, mRNA |
| Myocilin | 37 | Protein, mRNA |
| Nephronectin | 35 | mRNA |
| Collagen triple helix repeat-containing protein 1 | 34 | mRNA |
| Hyaluronan and proteoglycan link protein 3 | 34 | mRNA |
| Keratin, type I cytoskeletal 18 | 34 | mRNA |
| Angiopoietin-related protein 4 | 34 | mRNA |
Significantly altered native structural ECM proteins in CAVS, as derived from proteomics and transcriptomics analyses.
CAVS = calcific aortic valve stenosis; ECM = extracellular matrix; mRNA.
The current understanding of CAVS pathobiology focuses on the osteoblastic metaplasia, where VICs are initially activated to a myofibroblast type, remodel the ECM leading to fibrosis, subsequently develop osteoblastic pathways such as RunX2, and then actively deposit calcium phosphate on the remodeled ECM. The exact causes are unknown, although lipid oxidation, apoptosis, excessive inflammation, mitochondrial dysfunction, endothelial-to-mesenchymal transition (EndMT), impaired autophagy, active participation of extracellular vesicles (EVs), and indirect evidence of DNA damage have been reported. The detailed description of CAVS pathogenesis in the cellular and molecular level has been discussed previously.12 In this review, we highlight findings on the pathogenetic role of the structural ECM proteins alterations per se, along with insights gained from relevant research from in-vitro experiments.
Calcification of the aortic valve is a special case of the broader ectopic soft tissue calcification entity, produced by diverse mechanisms.13 In arterial calcification, apart from the intima-bound pathology linked to atherosclerosis and aging, a distinct form located in the media is linked to end-stage renal disease, hypertension, or diabetes mellitus. The corresponding lesion to the end-stage renal disease–related clinical phenotype of CAVS has not been demonstrated yet, and the extent of overlap in mechanisms with degenerative CAVS is not well-understood yet.14
Mineral phase identification in the calcific aortic valves15 has revealed nanoscale to submicron-scale calcium phosphate crystals, mainly hydroxyapatite (HAP; Ca10[PO4]6[OH]2), the main inorganic phase of bone,16 along with plate-like octacalcium phosphate crystals (OCP; Ca8[HPO4]2[PO4]4·5H2O) and canonical geometric dicalcium phosphate dehydrate (CaHPO4·2H2O) crystals.17 X-ray diffraction and Fourier transform infrared spectroscopy techniques have shown that at an initial mineralization state, cardiovascular calcified deposits are poorly crystalline carbonated-substituted apatite (B-type, CO32– group substitute PO43– group).18 Raman microimaging analysis has shown that the calcification progresses from initial OCP-like compounds to tricalcium phosphate and finally into stable B-type HAP.19
Amorphous calcium phosphate, containing Na+, Mg2+, CO32–, and pyrophosphate (PPi), has also been identified in valve calcifications.20 In biological and prosthetic heart valves, OCP structurally consisting of apatitic layers (similar to HAP) separated by layers of water molecules has been suggested as a precursor phase of biological apatite.20 In heavily calcified valves, substantial incorporation of magnesium and silicon has been explored by energy-dispersive x-ray analysis of the calcium phosphate deposits.21 Magnesium, also detected in bioprosthetic valves that showed slight calcification, can substitute calcium within the crystal lattice of HAP and stabilize amorphous calcium phosphate within vesicles where the calcification initiates.21 Furthermore, silicon seems to be involved in the initial and later calcification stages.21 Therefore, the mineralogical and chemical analysis of the calcified deposits could provide evidence of the calcification mechanism.
Evidence on Structural ECM Protein Alterations in CAVS
The ECM provides structural support, harbors growth factors and cytokines, regulates cell-to-matrix communication22 and is an emerging drug target in cancer,23 making it a putative pharmaceutical target in CAVS, as well. Structural ECM proteins interact extensively directly or mediated by receptors or secreted molecules, comprising a highly regulated network.22 In CAVS, collagen fibers are broken down enzymatically,12,24,25 with proteinases colocalizing in vivo with macrophages.12 Unbiased tissue investigations reveal a wealth of synthesized proteins. Focusing on the native ECM structural proteins, we have included in Table 1 differentially expressed proteins derived either from mass spectrometry analyses or protein-coding RNA sequences and characterized as “extracellular matrix” proteins, using Uniprot database.26 New nonphysiological ECM proteins, growth factors, enzymes, and serum-derived proteins were excluded.
The majority of collagen synthesis happens in the spongiosa, with fibers found spatially disorganized.27 The importance of collagen synthesis is highlighted in the rare congenital pediatric CAVS, where stenosis is tightly related to increased depositions of structurally immature collagens and a decrease in the glycosaminoglycans.27 In adult CAVS, the most abundant forms are collagens I, II, III, IV, and XVIII, comprising the collagen triple helixes, and the basal membranes, collagens V and XI, regulators of fibril spacing and diameter; collagen XVII anchors cells on the basal membrane. The stability and strength of the collagen fibers is mediated by hydroxylated proline residues of the triple helix, a post-translational modification mediated by prolyl hydroxylases, along with the degree of collagen cross-linking, which is catalyzed by lysyl oxidase.27,28 Elastin fibrils are fragmented and disorganized and colocalize with calcification.29 Elastin fragmentation is also linked to neoangiogenesis in early CAVS, in the absence of inflammation.30
Critical regulating glycoproteins are increased (Table 1). Among them, the laminins are major basal membrane components. VICs cultured on a laminin-coated substrate, demonstrated extensive calcification, mainly of dicalcium phosphate dihydrate (instead of OCP), tricalcium phosphate, or HAP, which was statistically higher than in normal samples.41 Fibronectin is necessary for the ordinary collagen fibril packing and is closely associated with calcification.42 It harbors binding sites for proteoglycans and integrins bridging ECM components with cells.43 Periostin is essential for the normal embryonic development of the aortic valve.30 It regulates collagen fibrillogenesis, acting as a polymerized scaffold,44 and stabilization by activation of lysyl oxidase.28 A lack of periostin induced extensive calcium deposits in the aortic valve cusps.44 Periostin is required for the observed function of VICs during disease: its absence decreased aortic valve fibrosis and metalloproteinase-2,13 expression,30 osteonectin regulates collagen fibril formation and cross-linking with other ECM proteins in mineralized tissues, and is upregulated in pediatric and adult CAVS.27,45 Fibulin, along with microfibril-associated glycoprotein, interacts with elastin fibers, forming a fine network.
Calcification is associated with proteoglycan expansion toward the fibrosa layer, particularly at the hinge region.46 Lumican, which affects collagen fiber packing,47 interacts directly with HAP.47 It inhibits matrix metalloproteinases47 and its decrease in calcified areas impairs collagen architecture,48 leading to intracollagen calcification.29 Aggrecan affects calcium phosphate binding capacity.49 Decorin, biglycan, and versican, which also bind to collagen, are present around the calcified nodules, decreased in the nodular edge, and absent or considerably decreased in the actual nodules.50,51 Proteoglycans are also expected to interact with galectins and elastin fibers.52
Tenascins are expressed in the basal membrane, where they interact with fibronectin, proteoglycans, and cellular integrins. Tenascin-C substantially upregulates especially in the spongiosa, around, but not within, the calcified area24,35 and is associated with the progression of CAVS.53 It is expected to directly interact with osteopontin.54 In contrast, tenascin-X is decreased in the disease,24,25,55 suggesting an impaired synthesis or a consuming process. The family of annexins are released by implicated cells into the ECM, incorporated in the EV membrane, and mediate binding to the ECM and calcium accumulation inside the EVs.56 Galectin-3 is increased in CAVS and induced inflammation and osteogenic turnover in VICs.57
Protein–protein interaction networks suggest close coregulation of ECM regulating proteins with collagen II.38 Similarly increased production of collagens, proteoglycans, and regulating glycoproteins is observed during development of the aortic valve.27,55 Overall, the above constitute an embryonic shift in the ECM synthesis. This is consistent, along with the activation of respective pathways46 and the EndMT phenomenon.27,58
Impact of Biomechanical and ECM Alterations in CAVS Mechanisms
The biomechanical role of the ECM is highlighted by the fact that cells typically differentiate into phenotypes responding to the mechanical forces imposed on their environment, through a constant process mediated by specialized receptors or ion channels (cellular mechanosensation) and intracellular signaling pathways (mechanotransduction), leading to respective changes in gene expression.
In CAVS, among the recognized mechanotransduction pathways, evidence exists on the activation of the transforming growth factor (TGF)-β axis,7 the noncanonical Wnt axis,59 the RhoA axis,12 the PI3K/AKT, AMPK,60, 61, 62 the Hippo/YAP,63 and the Notch axis,64 leading to fibrosis or RunX2-mediated osteoblastic differentiation. Among mechanosensitive channels, TREK-1, Kir6.1, TRPV4, and TRPC6 are found in aortic VICs, with TRPV4 channel affecting the expression of collagen III in response to mechanical stretch65 while inhibiting the DDR2, which directly senses collagen fibers, produces fibrosis and RunX2-mediated osteogenic calcification of the valve.66
At the tissue level, a progressive valve stiffening impairs its mechanical function, activates VICs, and facilitates osteogenic differentiation via RhoA/ROCK pathway.7,12 The calcification pattern coincides with areas of high flexion stress and increased strain.7 The importance of valve stiffness becomes apparent as stretching of the valve alone can induce osteoblastic transformation and produce mineralized spheroids.12 In other tissues, mechanical stress-induced myofibroblast activation implicates fibronectin, in a reversible process directly linked to the imposed mechanical forces,67 which also directly affects matrix metalloproteinase–mediated proteolysis68 and directly shapes the collagen–fibronectin interactions.42 In contrast, myofibroblast activation of VICs, results in greater ECM stiffness, as they contract and add mechanical forces upon the remodeling tissue.69
The role of biomechanics becomes apparent in the case of the bicuspid valve, which is linked to mutations to several genes, especially of the Notch family.64 In the bicuspid valve, similar increases in collagen, proteoglycan, and elastin content are seen, with a possible overexpression of osteonectin and laminins, and less collagen XI and metalloproteinases as compared with tricuspid degenerative CAVS.70 There is a maldistribution of the mechanical load during diastole, and stenosis appears earlier in the cases of anatomical asymmetry among cusps.71 The fusion area is modelled to increase the stiffness of the valve,72 and the blood flow dynamics on the fibrosa are disturbed resulting in endothelial dysfunction.64
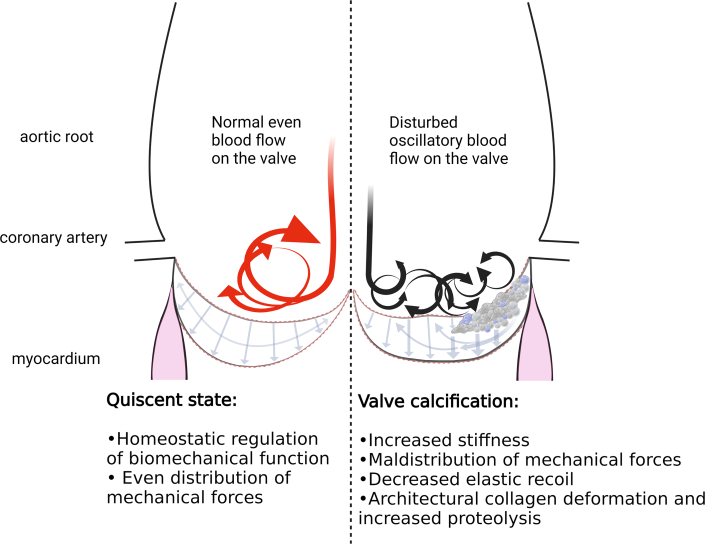
Superficial blood flow dynamics play an important role in CAVS. Increased oscillatory forces induce endothelial cell activation, leucocyte infiltration, and the overexpression of critical pathways such as TGF-β, nuclear factor-κβ, and Notch axes,12 Altered hemodynamics affect in a different way the fibrosa and the ventricularis, owing to inherent characteristics of the respective layer’s valvular endothelial cells (VECs), which are developed in different flow conditions. In a VEC–VIC co-culture, the ECM synthesis depends on the exposure to the physiological flow pattern on the fibrosa and the ventricularis, respectively.12 Similarly, changes in flow conditions alter the production of matrix degradation enzymes; thus, the total ECM content of the valve is influenced directly by blood flow conditions. Increased shear stress imposed on a VIC–VEC co-culture produced di- and octa-calcium phosphates (Figure 2).73
Figure 2.
Impact of Biomechanics on CAVS
Oscillatory superficial blood flow and increasing tissue stiffness, maldistribute mechanical forces upon aortic valvular endothelial cells and valvular interstitial cells, triggering extracellular matrix remodeling.
Moreover, the mechanical properties of the ECM are directly related to EndMT, a fundamental mechanism in CAVS, which precedes osteoblastic transformation.6 In particular, VECs exposed to a stiff matrix demonstrated increased EndMT under TGB-β1 stimulation, compared with a soft matrix.74 Cyclic strain imposed ex vivo in a different direction to the cells alignment resulted in severe EndMT, via TGF-β or Wnt pathways, depending on the external force imposed.75,76 Glycosaminoglycans facilitate EndMT in ex vivo models of calcific aortic valve disease, via TGF-β1 and ERK1/2 pathway (a member of MAPK family).6,74 Moreover, the mechanosensitive integrin-linked kinase, was downregulated in CAVS, and its silencing induced EndMT and RunX2-mediated osteoblastic differentiation in vitro,75 while a similar role is proposed for the midkine pathway, which can be activated by several mechanosensitive and ECM-related receptors.76
Glycosaminoglycan expansion facilitates lipid oxidation; they help more lipid particles to be trapped in the ECM, providing more substrate for oxidation reactions.77 Biglycan colocalized with phospholipid transfer protein and Toll-like receptor-2 receptor, suggesting an increased cellular absorption of the trapped lipid particles.78 Furthermore, ECM protein fragmentation products can serve as a source of immune cell activation via the Toll-like receptors and integrins. Local macrophages can migrate into areas of collagen deformation, driven by mechanical stretching forces alone.79 Fibronectin, biglycan, decorin, versican, and tenascin C activate Toll-like receptor-2/4,80 whereas endostatin, a collagen XVIII fragment, is significantly increased.81 Thus, ECM proteolysis can directly increase local inflammation. Moreover, a fibrotic and disorganized ECM can bind more EVs, through interaction with fibronectin, or by providing more positively charged sites on collagen and glycosaminoglycans.82 A thickened ECM, may also induce hypoxia in vivo.5 Hypoxia-induced factors 1 and 2 are overexpressed in calcified aortic valves and, in vitro, are correlated with collagen X, local metalloproteinase activity, neoangiogenesis, and the nuclear factor-κβ pathway.5,83,84 Matrix metalloproteinase 9 increased as O2 saturation decreased. Hypoxia enriches the EVs released to the ECM with lysyl oxidase,85 which might increase collagen cross-linking.
Post-Translational Modifications
There are >400 post-translational modifications identified in homeostasis and disease. Still, only a few are recognized to play a role in CAVS.
Oxidation
Protein oxidative reactions include the removal of electrons from peptides through several diverse nonenzymatic and enzymatic reactions, initiated by reactive oxygen species, reactive nitrogen species, or reactive sulfur species. They lead to a variety of unstable products that further react until a stable byproduct is formed. Excessive oxidation is well-documented regarding lipid oxidation.12 Nitric oxide, a reactive oxygen species scavenger, affects calcification of the aortic valve through Notch signaling, as shown in vivo and in vitro.86 Indirect evidence implicates reactive oxygen species–mediated DNA damage to the valve, with the levels of poly(ADP-ribose) polymerase having an inverse correlation to severity of stenosis.87 In contrast, isolated structural valve proteins oxidation and their consequences in calcification are less understood.
Enzymatic and nonenzymatic glycation of proteins
Glycation, the most frequent post-translational modification of proteins, is mainly controlled enzymatically and involves 10 monosaccharides and ≥12 pathways.88 The aortic valve contains mainly sialylated and core fucosylated N-glycans, which follow specific spatial distribution in the layers of the valve. In congenital CAVS, deregulated collagen glycosylation characterizes the thickened parts of the valve.55 Interestingly, lumican was found to have decreased glycosylation in calcified valves, suggesting a possible role in collagen disarray during calcification.
Furthermore, glycation of lysine and arginine residues of protein moieties is also produced nonenzymatically during the formation of advanced glycation end products (AGEs). Prolonged increased concentrations of sugars induce permanent glycation of proteins or lipids. Oxidation is chemically necessary; thus, AGEs are an indirect marker of oxidative stress. Both enzymatic and nonenzymatic glycation of proteins coexist in vivo, posing analytical challenges in interpreting research findings on protein glycation.
Well-controlled in vitro studies reveal that glycated collagen fibers demonstrate microstructural deformation, possibly owing to charging changes, hydrophobic interactions, and decreased physiological enzymatic cross-linking that stabilizes and protects collagen fibrils.89,90 The ex vivo glycation of collagenous tissues increases their mass and stiffness and decreases tensile strength, relaxation ability, elasticity, and intrafibril sliding.91 Patients with diabetes mellitus demonstrate increased calcification and aortic valve stenosis rates. AGEs induce the activation of nuclear factor-κβ,92 the AGE products receptor is upregulated in aortic valve stenosis,93 and antidiabetic medication reduced calcification. Moreover, AGE products receptor–deficient mice developed less aortic calcification.94,95 Nε-(Carboxymethyl)lysine and pentosidine, have been validated in CAVS.96
Calcification development
The ECM significantly impacts the formation of calcified aortic valves since matrix can be calcified even in the absence of cells.97 In CAVS, the presence of HAP—particles surrounding a dense, poorly crystalline apatite—has been found on cusp surfaces and embedded within lesions.98 The apatitic mineral deposits cause further stiffening and mechanical failure of the aortic valve cusps.99
Calcification deposits have been discriminated pathologically in the dystrophic (amorphous basophilic-stained material) and the osteogenic subtype (lamellar bone-like matrix in the presence of osteoblasts and osteoclasts).100 Dystrophic calcification has been observed in damaged tissues with extensive cellular necrosis. In contrast, osteogenic calcification is linked to active osteoblast-mediated biomineralization. Osteogenic calcification has been classically identified in CAVS, but it coexists with dystrophic calcification. Dystrophic calcification is found in 83% of diseased valves and is characterized by amorphous and crystalline deposits, whereas osteogenic calcification is present in 13% of valves having dystrophic calcification and is characterized by an osteoid matrix similar to active bone formation.101 Dystrophic and osteogenic calcification formation depend on mechanical stress and proinflammatory factors. Dystrophic calcification affects more often the aortic valves102 and possibly precedes the osteogenic form long before the rapid phase of valve stenosis. In vitro models of valvular calcification describe the formation of calcified nodules in VIC cultures with conditions mimicking the dystrophic and osteogenic calcification of aortic valve cusps.101 Dystrophic nodules, evident in dead cells, are formed on stiff substrates and lead themselves to the differentiation of VICs into activated myofibroblasts. In contrast, smaller than dystrophic, osteogenic nodules are formed on bone matrix secreted by VICs undergoing osteogenic differentiation.101 It is unclear whether these calcification processes are linked or independent.103 Osteopontin, a highly acidic and phosphorylated ECM protein not normally expressed in the aortic valve, binds strongly to HAP and has been found in vascular dystrophic calcification regions.104 This finding could be indirect evidence that these 2 processes are interconnected. Both calcified nodules contain calcium and phosphorus, with osteogenic nodules having abundant calcified spheres on the surface, in contrast with dystrophic nodules having negligible or no surface calcification.101 Furthermore, aggregations of elongated cells have been observed around the dystrophic nodules, whereas living cells occurred within osteogenic nodules.101
The calcification nucleation and growth processes significantly depend on the local chemical and biological microenvironments. The existence of calcified spherical nodules could denote a particle-based crystallization pathway. However, more evidence should be provided to prove this, along with the amorphous or crystalline state of the observed material.105 Calcium salt deposition and nucleation is facilitated through EVs, which actively promote mineralization, providing locally high calcium concentrations and enzymatically liberating free phosphate ions.82 Furthermore, calcification can simultaneously be promoted through passive deposition when constantly present natural inhibitors of calcification are reduced, such as PPi and matrix Gla protein. Degenerative CAVS patient-derived VICs demonstrated lowered levels of adenosine triphosphate, a PPi donor, owing to mitochondrial dysfunction,106 whereas the induction of adenosine triphosphate and PPi decreases calcification of porcine valves and VICs ex vivo.107,108 Matrix Gla protein inhibits HAP crystal growth and is reduced in the serum of patients with degenerative CAVS, correlating with stenosis severity.109 Moreover, The DNA damage response product, poly(ADP-ribose) colocalizes with calcification in both physiological (bone) and pathological ECM calcification (vascular and valvular), and mediates crystal nucleation.87,110 To date, damaged DNA fragments have not been associated causally with calcification deposits. Recently, DNA-based biomaterials have been used as biomimetics for growing hierarchically mineralized structures, meaning that DNA can act as a scaffold for mineralization.105,111 Under physiological conditions, collagen functions as a template, which directs the nucleation and growth of ∼2-nm-thick calcium phosphate crystals like the ones found in bone. During this process, charged macromolecules stabilizing transient amorphous calcium phosphate precursor phases facilitate its infiltration to the collagen.112 Interestingly, in Jurkat cells, oxidative stress, which is also present in CAVS, resulted in the exocytosis of EVs with DNA or DNA-binding proteins bound to their membrane, which mediated the EVs binding to fibronectin,113 suggesting a putative mechanism of active calcification propagation through ordinal EV buildup on the ECM template.
Future Research Directions
Collectively, the presented research data delineate the role of ECM alterations in critical aspects of the pathobiology recognized in CAVS, linking changes in the local mechanobiology with osteogenic differentiation and excessive calcification of the valve cusps (Figure 3). Data describe a vicious cycle between significant mechanobiological alterations and ECM remodeling with VIC activation and osteoblastic differentiation. The possibility that aging and comorbidities lead to the gradual mechanical degradation of the valve, with chronically persistent ECM remodeling as a maladaptive reparative mechanism, which in turn acts as a causal factor for osteoblastic metaplasia, needs to be evaluated.
Figure 3.
Future Research Directions
Future research should provide novel insights in the discrete histopathological phenotype of renal disease-associated CAVS, the role of the microvasculature, the possible diverging pathways expressed on α-smooth muscle actin + VICs, the mechanotransduction signaling, and the role of specific ECM proteins and their post-translational modifications on mineral nucleation and growth mechanisms. Abbreviations as in Figure 2.
Further investigation is required for a better understanding of disease progression and provide possible treatment options. The Central Illustration summarizes essential research areas that should be developed. Further investigation is required to identify ECM proteins that regulate collagen and ECM damage at early CAVS stages, and which post-translational modifications impact the stiffness alterations and calcification of the valves at a high level. Despite data on calcified spherical particles, there is no direct evidence about the calcification in multiple scales, which ECM proteins participate during this process, and what their specific role would be. Furthermore, there is no clear evidence of whether dystrophic and osteogenic calcification are part of the same process. The histopathology of CAVS at a final stage of renal disease has not been identified yet, in contrast with arterial calcification. More evidence should be provided on the native matrix proteins at the earlier stages of the CAVS, and their interactions during disease. This work could lead to pathophysiological hypothesis generation and ultimately the design of new therapeutics for decreasing the rate of disease progression.
Central Illustration.
Focus on Essential Collagen Assembly Proteins and Overview of Critical Events Related to the ECM During CAVS
Funding Support and Author Disclosures
Drs Anousakis-Vlachochristou, Athanasiadou, and Toutouzas acknowledge the European Union (E.U.), and Greek funds through the Operational Program “Human Resources Development, Education and Lifelong Learning” (NSRF 2014-2020), under the call “Supporting Researchers with an Emphasis on Young Researchers – Cycle B” (MIS: 5047949), Athens, Greece, for funding. Dr Carneiro has reported that she has no relationships relevant to the contents of this paper to disclose.
Acknowledgments
The authors thank Antigoni Miliou, PhD, for comments and reading of the final manuscript. Figures were created using BioRender.com. Ethical approval was not obtained, as per review paper.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Contributor Information
Dimitra Athanasiadou, Email: dimath@chalmers.se.
Konstantinos Toutouzas, Email: ktoutouz@gmail.com.
References
- 1.Yang C., Xu H., Jia R., Jin Z., Zhang C., Yuan J. Global burden and improvement gap of non-rheumatic calcific aortic valve disease: 1990–2019 findings from Global Burden of Disease Study 2019. J Clin Med. 2022;11:6733. doi: 10.3390/jcm11226733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eveborn G.W., Schirmer H., Heggelund G., Lunde P., Rasmussen K. The evolving epidemiology of valvular aortic stenosis. The Tromsø study. Heart Br Card Soc. 2013;99:396–400. doi: 10.1136/heartjnl-2012-302265. [DOI] [PubMed] [Google Scholar]
- 3.Sievers H.-H., Schmidtke C. A classification system for the bicuspid aortic valve from 304 surgical specimens. J Thorac Cardiovasc Surg. 2007;133:1226–1233. doi: 10.1016/j.jtcvs.2007.01.039. [DOI] [PubMed] [Google Scholar]
- 4.Shen M., Tastet L., Capoulade R., et al. Effect of bicuspid aortic valve phenotype on progression of aortic stenosis. Eur Heart J Cardiovasc Imaging. 2020;21:727–734. doi: 10.1093/ehjci/jeaa068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chester A.H., Grande-Allen K.J. Which biological properties of heart valves are relevant to tissue engineering? Front Cardiovasc Med. 2020;7:63. doi: 10.3389/fcvm.2020.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Islam S., Boström K.I., Di Carlo D., et al. The mechanobiology of endothelial-to-mesenchymal transition in cardiovascular disease. Front Physiol. 2021;12 doi: 10.3389/fphys.2021.734215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kodigepalli K.M., Thatcher K., West T., et al. Biology and biomechanics of the heart valve extracellular matrix. J Cardiovasc Dev Dis. 2020;7:57. doi: 10.3390/jcdd7040057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hudson L.T., Laurence D.W., Lau H.M., Mullins B.T., Doan D.D., Lee C.-H. Linking collagen fiber architecture to tissue-level biaxial mechanical behaviors of porcine semilunar heart valve cusps. J Mech Behav Biomed Mater. 2022;125 doi: 10.1016/j.jmbbm.2021.104907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bairati A., DeBiasi S. Presence of a smooth muscle system in aortic valve leaflets. Anat Embryol (Berl) 1981;161:329–340. doi: 10.1007/BF00301830. [DOI] [PubMed] [Google Scholar]
- 10.Cimini M., Rogers K.A., Boughner D.R. Smoothelin-positive cells in human and porcine semilunar valves. Histochem Cell Biol. 2003;120:307–317. doi: 10.1007/s00418-003-0570-z. [DOI] [PubMed] [Google Scholar]
- 11.Rabkin-Aikawa E., Farber M., Aikawa M., Schoen F.J. Dynamic and reversible changes of interstitial cell phenotype during remodeling of cardiac valves. J Heart Valve Dis. 2004;13:841–847. [PubMed] [Google Scholar]
- 12.Driscoll K., Cruz A.D., Butcher J.T. Inflammatory and biomechanical drivers of endothelial-interstitial interactions in calcific aortic valve disease. Circ Res. 2021;128:1344–1370. doi: 10.1161/CIRCRESAHA.121.318011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kempf H., Komarova S., Murshed M. Editorial: ectopic mineralization of tissues: mechanisms, risk factors, diseases, and prevention. Front Cell Dev Biol. 2021;9 doi: 10.3389/fcell.2021.759702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shroff G.R., Bangalore S., Bhave N.M., et al. Evaluation and management of aortic stenosis in chronic kidney disease: a scientific statement from the American Heart Association. Circulation. 2021;143:e1088–e1114. doi: 10.1161/CIR.0000000000000979. [DOI] [PubMed] [Google Scholar]
- 15.Radvar E., Griffanti G., Tsolaki E., et al. Engineered in vitro models for pathological calcification: routes toward mechanistic understanding. Adv NanoBiomed Res. 2021;1 [Google Scholar]
- 16.Pasteris J.D. A mineralogical view of apatitic biomaterials. Am Mineral. 2016;101:2594–2610. [Google Scholar]
- 17.Krings M., Kanellopoulou D., Koutsoukos P.G., Mavrilas D., Glasmacher B. Development of a new combined test setup for accelerated dynamic pH-controlled in vitro calcification of porcine heart valves. Int J Artif Organs. 2009;32:794–801. doi: 10.1177/039139880903201105. [DOI] [PubMed] [Google Scholar]
- 18.Danilchenko S.N., Kalinkevich A.N., Moskalenko R.A., et al. Structural and crystal-chemical characteristics of the apatite deposits from human aortic walls. Interv Med Appl Sci. 2018;10:110–119. doi: 10.1556/1646.10.2018.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Czamara K., Natorska J., Kapusta P., Baranska M., Kaczor A. Raman microspectroscopy of human aortic valves: investigation of the local and global biochemical changes associated with calcification in aortic stenosis. Analyst. 2015;140:2164–2170. doi: 10.1039/c4an01856g. [DOI] [PubMed] [Google Scholar]
- 20.Dorozhkin S.V. Calcium orthophosphates. J Mater Sci. 2007;42:1061–1095. [Google Scholar]
- 21.Delogne C., Lawford P.V., Habesch S.M., Carolan V.A. Characterization of the calcification of cardiac valve bioprostheses by environmental scanning electron microscopy and vibrational spectroscopy. J Microsc. 2007;228:62–77. doi: 10.1111/j.1365-2818.2007.01824.x. [DOI] [PubMed] [Google Scholar]
- 22.Sainio A., Järveläinen H. Extracellular matrix-cell interactions: focus on therapeutic applications. Cell Signal. 2020;66 doi: 10.1016/j.cellsig.2019.109487. [DOI] [PubMed] [Google Scholar]
- 23.Huang J., Zhang L., Wan D., et al. Extracellular matrix and its therapeutic potential for cancer treatment. Signal Transduct Target Ther. 2021;6:1–24. doi: 10.1038/s41392-021-00544-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schlotter F., Halu A., Goto S., et al. Spatiotemporal multi-omics mapping generates a molecular atlas of the aortic valve and reveals networks driving disease. Circulation. 2018;138:377–393. doi: 10.1161/CIRCULATIONAHA.117.032291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alvarez-Llamas G., Martín-Rojas T., de la Cuesta F., et al. Modification of the secretion pattern of proteases, inflammatory mediators, and extracellular matrix proteins by human aortic valve is key in severe aortic stenosis. Mol Cell Proteomics MCP. 2013;12:2426–2439. doi: 10.1074/mcp.M113.027425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.The UniProt Consortium UniProt: the universal protein knowledgebase in 2021. Nucleic Acids Res. 2021;49:D480–D489. doi: 10.1093/nar/gkaa1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clift C.L., Su Y.R., Bichell D., et al. Collagen fiber regulation in human pediatric aortic valve development and disease. Sci Rep. 2021;11:9751. doi: 10.1038/s41598-021-89164-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maruhashi T., Kii I., Saito M., Kudo A. Interaction between periostin and BMP-1 promotes proteolytic activation of lysyl oxidase. J Biol Chem. 2010;285:13294–13303. doi: 10.1074/jbc.M109.088864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perrotta I., Davoli M. Collagen mineralization in human aortic valve stenosis: a field emission scanning electron microscopy and energy dispersive spectroscopy analysis. Ultrastruct Pathol. 2014;38:281–284. doi: 10.3109/01913123.2014.901468. [DOI] [PubMed] [Google Scholar]
- 30.Hinton R.B., Juraszek A.L., Opoka A.M., et al. Early aberrant angiogenesis due to elastic fiber fragmentation in aortic valve disease. J Cardiovasc Dev Dis. 2021;8:75. doi: 10.3390/jcdd8070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu K., Xie S., Huang Y., et al. Cell-type transcriptome atlas of human aortic valves reveal cell heterogeneity and endothelial to mesenchymal transition involved in calcific aortic valve disease. Arterioscler Thromb Vasc Biol. 2020;40:2910–2921. doi: 10.1161/ATVBAHA.120.314789. [DOI] [PubMed] [Google Scholar]
- 32.Bouchareb R., Guauque-Olarte S., Snider J., et al. Proteomic architecture of valvular extracellular matrix: FNDC1 and MXRA5 are new biomarkers of aortic stenosis. J Am Coll Cardiol Basic Trans Science. 2021;6:25–39. doi: 10.1016/j.jacbts.2020.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Greene C.L., Jaatinen K.J., Wang H., Koyano T.K., Bilbao M.S., Woo Y.J. Transcriptional profiling of normal, stenotic, and regurgitant human aortic valves. Genes. 2020;11:E789. doi: 10.3390/genes11070789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guauque-Olarte S., Droit A., Tremblay-Marchand J., et al. RNA expression profile of calcified bicuspid, tricuspid, and normal human aortic valves by RNA sequencing. Physiol Genomics. 2016;48:749–761. doi: 10.1152/physiolgenomics.00041.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kossar A.P., Anselmo W., Grau J.B., et al. Circulating and tissue matricellular RNA and protein expression in calcific aortic valve disease. Physiol Genomics. 2020;52:191–199. doi: 10.1152/physiolgenomics.00104.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bossé Y., Miqdad A., Fournier D., Pépin A., Pibarot P., Mathieu P. Refining molecular pathways leading to calcific aortic valve stenosis by studying gene expression profile of normal and calcified stenotic human aortic valves. Circ Cardiovasc Genet. 2009;2:489–498. doi: 10.1161/CIRCGENETICS.108.820795. [DOI] [PubMed] [Google Scholar]
- 37.Heuschkel M.A., Skenteris N.T., Hutcheson J.D., et al. Integrative multi-omics analysis in calcific aortic valve disease reveals a link to the formation of amyloid-like deposits. Cells. 2020;9:2164. doi: 10.3390/cells9102164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martin-Rojas T., Mourino-Alvarez L., Alonso-Orgaz S., et al. iTRAQ proteomic analysis of extracellular matrix remodeling in aortic valve disease. Sci Rep. 2015;5 doi: 10.1038/srep17290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mourino-Alvarez L., Iloro I., de la Cuesta F., et al. MALDI-imaging mass spectrometry: a step forward in the anatomopathological characterization of stenotic aortic valve tissue. Sci Rep. 2016;6 doi: 10.1038/srep27106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martín-Rojas T., Gil-Dones F., Lopez-Almodovar L.F., Padial L.R., Vivanco F., Barderas M.G. Proteomic profile of human aortic stenosis: insights into the degenerative process. J Proteome Res. 2012;11:1537–1550. doi: 10.1021/pr2005692. [DOI] [PubMed] [Google Scholar]
- 41.Rodriguez K.J., Masters K.S. Regulation of valvular interstitial cell calcification by components of the extracellular matrix. J Biomed Mater Res A. 2009;90A:1043–1053. doi: 10.1002/jbm.a.32187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nashchekina Y., Nikonov P., Prasolov N., Sulatsky M., Chabina A., Nashchekin A. The structural interactions of molecular and fibrillar collagen type I with fibronectin and its role in the regulation of mesenchymal stem cell morphology and functional activity. Int J Mol Sci. 2022;23 doi: 10.3390/ijms232012577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yamada K.M., Doyle A.D., Lu J. Cell-3D matrix interactions: recent advances and opportunities. Trends Cell Biol. 2022;32:883–895. doi: 10.1016/j.tcb.2022.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.del Monte-Nieto G., Fischer J.W., Gorski D.J., Harvey R.P., Kovacic J.C. Basic biology of extracellular matrix in the cardiovascular system, part 1/4: JACC focus seminar. J Am Coll Cardiol. 2020;75:2169–2188. doi: 10.1016/j.jacc.2020.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Karamanos N.K., Theocharis A.D., Piperigkou Z., et al. A guide to the composition and functions of the extracellular matrix. FEBS J. 2021;288:6850–6912. doi: 10.1111/febs.15776. [DOI] [PubMed] [Google Scholar]
- 46.Gomez-Stallons M.V., Tretter J.T., Hassel K., et al. Calcification and extracellular matrix dysregulation in human postmortem and surgical aortic valves. Heart. 2019;105:1616–1621. doi: 10.1136/heartjnl-2019-314879. [DOI] [PubMed] [Google Scholar]
- 47.Matsushima N., Miyashita H., Kretsinger R.H. Sequence features, structure, ligand interaction, and diseases in small leucine rich repeat proteoglycans. J Cell Commun Signal. 2021;15:519–531. doi: 10.1007/s12079-021-00616-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Suzuki H., Chikada M., Yokoyama M.K., et al. Aberrant glycosylation of lumican in aortic valve stenosis revealed by proteomic analysis. Int Heart J. 2016;57:104–111. doi: 10.1536/ihj.15-252. [DOI] [PubMed] [Google Scholar]
- 49.Eanes E.D., Hailer A.W. Effect of ultrafilterable fragments from chondroitinase and protease-treated aggrecan on calcium-phosphate precipitation in liposomal suspensions. Calcif Tissue Int. 1994;55:176–179. doi: 10.1007/BF00425872. [DOI] [PubMed] [Google Scholar]
- 50.Stephens E.H., Saltarrelli J.G., Baggett L.S., et al. Differential proteoglycan and hyaluronan distribution in calcified aortic valves. Cardiovasc Pathol. 2011;20:334–342. doi: 10.1016/j.carpath.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen D., Smith L.R., Khandekar G., et al. Distinct effects of different matrix proteoglycans on collagen fibrillogenesis and cell-mediated collagen reorganization. Sci Rep. 2020;10 doi: 10.1038/s41598-020-76107-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Itoh A., Nonaka Y., Ogawa T., Nakamura T., Nishi N. Small leucine-rich repeat proteoglycans associated with mature insoluble elastin serve as binding sites for galectins. Biosci Biotechnol Biochem. 2017;81:2098–2104. doi: 10.1080/09168451.2017.1374828. [DOI] [PubMed] [Google Scholar]
- 53.Satta J., Melkko J., Pöllänen R., et al. Progression of human aortic valve stenosis is associated with tenascin-C expression. J Am Coll Cardiol. 2002;39:96–101. doi: 10.1016/s0735-1097(01)01705-3. [DOI] [PubMed] [Google Scholar]
- 54.Sun J.-Y., Hua Y., Shen H., et al. Identification of key genes in calcific aortic valve disease via weighted gene co-expression network analysis. BMC Med Genomics. 2021;14:135. doi: 10.1186/s12920-021-00989-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Angel P.M., Drake R.R., Park Y., et al. Spatial N-glycomics of the human aortic valve in development and pediatric endstage congenital aortic valve stenosis. J Mol Cell Cardiol. 2021;154:6–20. doi: 10.1016/j.yjmcc.2021.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Krohn J.B., Aikawa E., Aikawa M., Hutcheson J.D., Sahoo S., Fish J.E. Editorial: extracellular vesicles in cardiovascular inflammation and calcification. Front Cardiovasc Med. 2022;9 doi: 10.3389/fcvm.2022.1077124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Luo J., Wang S., Liu X., et al. Galectin-3 promotes calcification of human aortic valve interstitial cells via the NF-kappa B signaling pathway. Cardiovasc Deign Ther. 2022;12:196–207. doi: 10.21037/cdt-21-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aikawa E., Hutcheson J.D. The developmental origin of calcific aortic stenosis. N Engl J Med. 2022;386:1372–1374. doi: 10.1056/NEJMcibr2200439. [DOI] [PubMed] [Google Scholar]
- 59.Akoumianakis I., Polkinghorne M., Antoniades C. Non-canonical WNT signalling in cardiovascular disease: mechanisms and therapeutic implications. Nat Rev Cardiol. 2022;19:783–797. doi: 10.1038/s41569-022-00718-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.En Q., Zeping H., Yuetang W., Xu W., Wei W. Metformin alleviates the calcification of aortic valve interstitial cells through activating the PI3K/AKT pathway in an AMPK dependent way. Mol Med. 2021;27:156. doi: 10.1186/s10020-021-00416-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Qiao E., Huang Z., Wang W. Exploring potential genes and pathways related to calcific aortic valve disease. Gene. 2022;808 doi: 10.1016/j.gene.2021.145987. [DOI] [PubMed] [Google Scholar]
- 62.Huang K., Wu L., Gao Y., et al. Transcriptome sequencing data reveal lncRNA-miRNA-mRNA regulatory network in calcified aortic valve disease. Front Cardiovasc Med. 2022;9 doi: 10.3389/fcvm.2022.886995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gao C., Hu W., Liu F., et al. Aldo-keto reductase family 1 member B induces aortic valve calcification by activating hippo signaling in valvular interstitial cells. J Mol Cell Cardiol. 2021;150:54–64. doi: 10.1016/j.yjmcc.2020.10.002. [DOI] [PubMed] [Google Scholar]
- 64.Wang Y., Fang Y., Lu P., Wu B., Zhou B. NOTCH signaling in aortic valve development and calcific aortic valve disease. Front Cardiovasc Med. 2021 Jun 22;8 doi: 10.3389/fcvm.2021.682298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Al-Shammari H., Latif N., Sarathchandra P., et al. Expression and function of mechanosensitive ion channels in human valve interstitial cells. PLoS One. 2020;15 doi: 10.1371/journal.pone.0240532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Carracedo M., Pawelzik S.-C., Artiach G., et al. The tyrosine kinase inhibitor nilotinib targets the discoidin domain receptor DDR2 in calcific aortic valve stenosis. Br J Pharmacol. 2022;179:4709–4721. doi: 10.1111/bph.15911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hinz B., McCulloch C.A., Coelho N.M. Mechanical regulation of myofibroblast phenoconversion and collagen contraction. Exp Cell Res. 2019;379:119–128. doi: 10.1016/j.yexcr.2019.03.027. [DOI] [PubMed] [Google Scholar]
- 68.Saini K., Cho S., Dooling L.J., Discher D.E. Tension in fibrils suppresses their enzymatic degradation—a molecular mechanism for ‘use it or lose it. ’ Matrix Biol. 2020;85-86:34–46. doi: 10.1016/j.matbio.2019.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Büttner P., Feistner L., Lurz P., Thiele H., Hutcheson J.D., Schlotter F. Dissecting calcific aortic valve disease—the role, etiology, and drivers of valvular fibrosis. Front Cardiovasc Med. 2021;8 doi: 10.3389/fcvm.2021.660797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Padang R., Bagnall R.D., Tsoutsman T., Bannon P.G., Semsarian C. Comparative transcriptome profiling in human bicuspid aortic valve disease using RNA sequencing. Physiol Genomics. 2015;47:75–87. doi: 10.1152/physiolgenomics.00115.2014. [DOI] [PubMed] [Google Scholar]
- 71.Beppu S., Suzuki S., Matsuda H., Ohmori F., Nagata S., Miyatake K. Rapidity of progression of aortic stenosis in patients with congenital bicuspid aortic valves. Am J Cardiol. 1993;71:322–327. doi: 10.1016/0002-9149(93)90799-i. [DOI] [PubMed] [Google Scholar]
- 72.Qin T., Caballero A., Mao W., et al. The role of stress concentration in calcified bicuspid aortic valve. J R Soc Interface. 2020;17 doi: 10.1098/rsif.2019.0893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mendoza M., Chen M.-H., Huang P., Mahler G.J. Shear and endothelial induced late-stage calcific aortic valve disease-on-a-chip develops calcium phosphate mineralizations. Lab Chip. 2022;22:1374–1385. doi: 10.1039/d1lc00931a. [DOI] [PubMed] [Google Scholar]
- 74.Bramsen J.A., Alber B.R., Mendoza M., et al. Glycosaminoglycans affect endothelial to mesenchymal transformation, proliferation, and calcification in a 3D model of aortic valve disease. Front Cardiovasc Med. 2022 Sep 29;9 doi: 10.3389/fcvm.2022.975732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sánchez-Esteban S., Castro-Pinto M., Cook-Calvete A., et al. Integrin-linked kinase expression in human valve endothelial cells plays a protective role in calcific aortic valve disease. Antioxidants. 2022;11:1736. doi: 10.3390/antiox11091736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhou Q., Cao H., Hang X., et al. Midkine prevents calcification of aortic valve interstitial cells via intercellular crosstalk. Front Cell Dev Biol. 2021;9 doi: 10.3389/fcell.2021.794058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Grande-Allen K.J., Osman N., Ballinger M.L., Dadlani H., Marasco S., Little P.J. Glycosaminoglycan synthesis and structure as targets for the prevention of calcific aortic valve disease. Cardiovasc Res. 2007;76:19–28. doi: 10.1016/j.cardiores.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 78.Nsaibia M.J., Devendran A., Goubaa E., Bouitbir J., Capoulade R., Bouchareb R. Implication of lipids in calcified aortic valve pathogenesis: why did statins fail? J Clin Med. 2022;11:3331. doi: 10.3390/jcm11123331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Poulis N., Martin M., Hoerstrup S.P., Emmert M.Y., Fioretta E.S. Macrophage-extracellular matrix interactions: perspectives for tissue engineered heart valve remodeling. Front Cardiovasc Med. 2022;9 doi: 10.3389/fcvm.2022.952178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Blokland K.E.C., Pouwels S.D., Schuliga M., Knight D.A., Burgess J.K. Regulation of cellular senescence by extracellular matrix during chronic fibrotic diseases. Clin Sci. 2020;134:2681–2706. doi: 10.1042/CS20190893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chalajour F., Treede H., Ebrahimnejad A., Lauke H., Reichenspurner H., Ergun S. Angiogenic activation of valvular endothelial cells in aortic valve stenosis. Exp Cell Res. 2004;298:455–464. doi: 10.1016/j.yexcr.2004.04.034. [DOI] [PubMed] [Google Scholar]
- 82.Bakhshian Nik A., Hutcheson J.D., Aikawa E. Extracellular vesicles as mediators of cardiovascular calcification. Front Cardiovasc Med. 2017;4:78. doi: 10.3389/fcvm.2017.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ferrari S., Pesce M. The complex interplay of inflammation, metabolism, epigenetics, and sex in calcific disease of the aortic valve. Front Cardiovasc Med. 2022;8 doi: 10.3389/fcvm.2021.791646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Salim M.T., Villa-Roel N., Vogel B., Jo H., Yoganathan A.P. HIF1A inhibitor PX-478 reduces pathological stretch-induced calcification and collagen turnover in aortic valve. Front Cardiovasc Med. 2022;9 doi: 10.3389/fcvm.2022.1002067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.de Jong O.G., van Balkom B.W.M., Gremmels H., Verhaar M.C. Exosomes from hypoxic endothelial cells have increased collagen crosslinking activity through up-regulation of lysyl oxidase-like 2. J Cell Mol Med. 2016;20:342–350. doi: 10.1111/jcmm.12730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Majumdar U., Manivannan S., Basu M., et al. Nitric oxide prevents aortic valve calcification by S-nitrosylation of USP9X to activate NOTCH signaling. Sci Adv. 2021;7 doi: 10.1126/sciadv.abe3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bäck M., Michel J.-B. From organic and inorganic phosphates to valvular and vascular calcifications. Cardiovasc Res. 2021;117:2016–2029. doi: 10.1093/cvr/cvab038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schjoldager K.T., Narimatsu Y., Joshi H.J., Clausen H. Global view of human protein glycosylation pathways and functions. Nat Rev Mol Cell Biol. 2020;21:729–749. doi: 10.1038/s41580-020-00294-x. [DOI] [PubMed] [Google Scholar]
- 89.Bansode S., Bashtanova U., Li R., et al. Glycation changes molecular organization and charge distribution in type I collagen fibrils. Sci Rep. 2020;10:3397. doi: 10.1038/s41598-020-60250-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li R., Rajan R., Wong W.C.V., et al. In situ characterization of advanced glycation end products (AGEs) in collagen and model extracellular matrix by solid state NMR. Chem Commun. 2017;53:13316–13319. doi: 10.1039/c7cc06624d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Werbner B., Lee M., Lee A., et al. Non-enzymatic glycation of annulus fibrosus alters tissue-level failure mechanics in tension. J Mech Behar Biomed Mater. 2022;126 doi: 10.1016/j.jmbbm.2021.104992. [DOI] [PubMed] [Google Scholar]
- 92.Kopek M., Mazur P., Ząbczyk M., Undas A., Natorska J. Diabetes concomitant to aortic stenosis is associated with increased expression of NF-κB and more pronounced valve calcification. Diabetologia. 2021;64:2562–2574. doi: 10.1007/s00125-021-05545-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Saku K., Tahara N., Takaseya T., et al. Pathological role of receptor for advanced glycation end products in calcified aortic valve stenosis. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.119.015261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lin C.-P., Huang P.-H., Chen C.-Y., et al. Sitagliptin attenuates arterial calcification by downregulating oxidative stress-induced receptor for advanced glycation end products in LDLR knockout mice. Sci Rep. 2021;11 doi: 10.1038/s41598-021-97361-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang B., Cai Z., Liu B., et al. RAGE deficiency alleviates aortic valve calcification in ApoE−/− mice via the inhibition of endoplasmic reticulum stress. Biochim Biophys Acta BBA Mol Basis Dis. 2017;1863:781–792. doi: 10.1016/j.bbadis.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 96.Baidoshvili A., Niessen H.W.M., Stooker W., et al. Nε-(Carboxymethyl)lysine depositions in human aortic heart valves: similarities with atherosclerotic blood vessels. Atherosclerosis. 2004;174:287–292. doi: 10.1016/j.atherosclerosis.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 97.Schoen F.J. Aortic valve structure-function correlations: role of elastic fibers no longer a stretch of the imagination. J Heart Valve Dis. 1997;6:1–6. [PubMed] [Google Scholar]
- 98.Bertazzo S., Gentleman E., Cloyd K.L., Chester A.H., Yacoub M.H., Stevens M.M. Nano-analytical electron microscopy reveals fundamental insights into human cardiovascular tissue calcification. Nat Mater. 2013;12:576–583. doi: 10.1038/nmat3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Giachelli C.M. Ectopic Calcification. Am J Pathol. 1999;154:671–675. doi: 10.1016/S0002-9440(10)65313-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mohler E.R., Gannon F., Reynolds C., Zimmerman R., Keane M.G., Kaplan F.S. Bone formation and inflammation in cardiac valves. Circulation. 2001;103:1522–1528. doi: 10.1161/01.cir.103.11.1522. [DOI] [PubMed] [Google Scholar]
- 101.Chen J., Peacock J.R., Branch J., David Merryman W. Biophysical analysis of dystrophic and osteogenic models of valvular calcification. J Biomech Eng. 2015;137:0209031–0209036. doi: 10.1115/1.4029115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Karwowski W., Naumnik B., Szczepański M., Myśliwiec M. The mechanism of vascular calcification—a systematic review. Med Sci Monit. 2011;18:RA1–RA11. doi: 10.12659/MSM.882181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Merryman W.D., Schoen F.J. Mechanisms of calcification in aortic valve disease: role of mechanokinetics and mechanodynamics. Curr Cardiol Rep. 2013;15:355. doi: 10.1007/s11886-013-0355-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wada T., McKee M.D., Steitz S., Giachelli C.M. Calcification of vascular smooth muscle cell cultures inhibition by osteopontin. Circ Res. 1999;84:166–178. doi: 10.1161/01.res.84.2.166. [DOI] [PubMed] [Google Scholar]
- 105.Athanasiadou D., Carneiro K.M.M. DNA nanostructures as templates for biomineralization. Nat Rev Chem. 2021;5:93–108. doi: 10.1038/s41570-020-00242-5. [DOI] [PubMed] [Google Scholar]
- 106.Morciano G., Patergnani S., Pedriali G., et al. Impairment of mitophagy and autophagy accompanies calcific aortic valve stenosis favouring cell death and the severity of disease. Cardiovasc Res. 2022;118:2548–2559. doi: 10.1093/cvr/cvab267. [DOI] [PubMed] [Google Scholar]
- 107.Rathan S., Yoganathan A.P., O’Neill W.C. The role of inorganic pyrophosphate in aortic valve calcification. J Heart Valve Dis. 2014;23:387–394. [PMC free article] [PubMed] [Google Scholar]
- 108.Rattazzi M., Bertacco E., Iop L., et al. Extracellular pyrophosphate is reduced in aortic interstitial valve cells acquiring a calcifying profile: implications for aortic valve calcification. Atherosclerosis. 2014;237:568–576. doi: 10.1016/j.atherosclerosis.2014.10.027. [DOI] [PubMed] [Google Scholar]
- 109.Liu C., Liu H., Xie T. Impact of Fetuin-A, Lp(a), matrix gla protein and macrophage density on calcific aortic valve disease: a clinical study. Lipids Health Dis. 2022;21:14. doi: 10.1186/s12944-022-01625-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Müller K.H., Hayward R., Rajan R., et al. Poly(ADP-Ribose) links the DNA damage response and biomineralization. Cell Rep. 2019;27:3124–3138.e13. doi: 10.1016/j.celrep.2019.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Danesi A.L., Athanasiadou D., Aderinto A.O., Rasie P., Chou L.Y.T., Carneiro K.M.M. Peptide-decorated DNA nanostructures promote site-specific hydroxyapatite growth. ACS Appl Mater Interface. 2022;14:1692–1698. doi: 10.1021/acsami.1c19271. [DOI] [PubMed] [Google Scholar]
- 112.Patterson J.P., Xu Y., Moradi M.-A., Sommerdijk N.A.J.M., Friedrich H. CryoTEM as an advanced analytical tool for materials chemists. Acc Chem Res. 2017;50:1495–1501. doi: 10.1021/acs.accounts.7b00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Németh A., Orgovan N., Sódar B.W., et al. Antibiotic-induced release of small extracellular vesicles (exosomes) with surface-associated DNA. Sci Rep. 2017;7:8202. doi: 10.1038/s41598-017-08392-1. [DOI] [PMC free article] [PubMed] [Google Scholar]