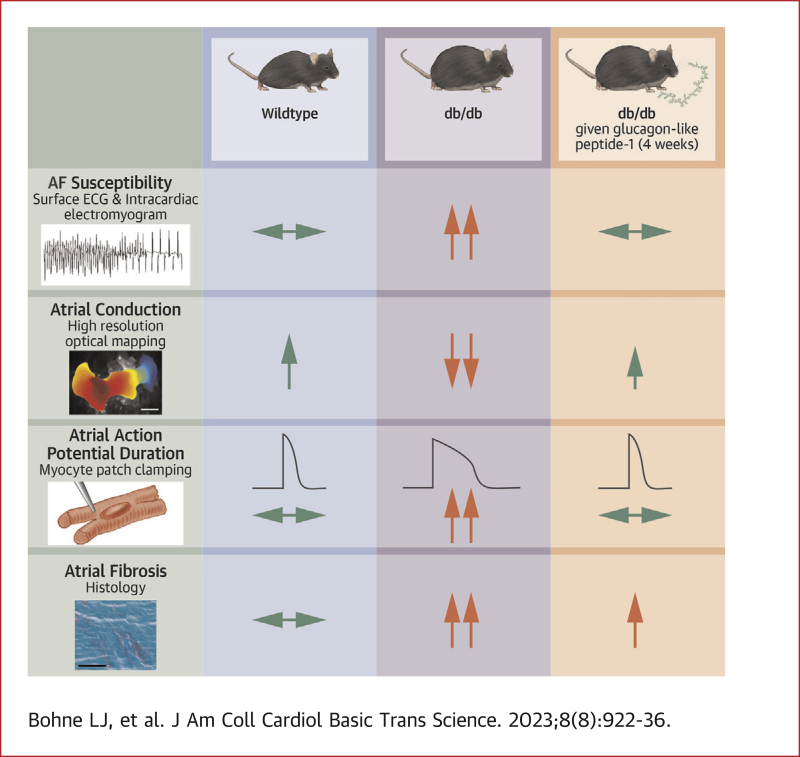
Visual Abstract
Key Words: arrhythmia, electrophysiology, fibrosis. incretin, ion channels
Highlights
-
•
AF is highly prevalent in type 2 diabetes mellitus where it increases morbidity and mortality.
-
•
GLP-1R agonists are used to treat T2DM, but their effects on AF are unclear.
-
•
T2DM db/db mice are highly susceptible to AF in association with atrial electrical and structural remodeling.
-
•
GLP-1 and liraglutide (GLP-1R agonist) prevented AF as well as atrial remodeling in db/db mice.
-
•
GLP-1R agonists may represent a therapeutic option to prevent AF in patients with T2DM.
Summary
Atrial fibrillation (AF) is highly prevalent in type 2 diabetes where it increases morbidity and mortality. Glucagon-like peptide (GLP)-1 receptor agonists are used in the treatment of type 2 diabetes (T2DM), but their effects on AF in T2DM are poorly understood. The present study demonstrates type 2 diabetic db/db mice are highly susceptible to AF in association with atrial electrical and structural remodeling. GLP-1, as well as the long-acting GLP-1 analogue liraglutide, reduced AF and prevented atrial remodeling in db/db mice. These data suggest that GLP-1 and related analogues could protect against AF in patients with T2DM.
Atrial fibrillation (AF) is highly prevalent in patients with type 2 diabetes mellitus (T2DM), which accounts for ∼90% of the diabetic population.1, 2, 3, 4 With obesity occurring at epidemic proportions, T2DM and AF occurrence are expected to continue to rise. AF is associated with increased risk of stroke and death, as well as severe impairments in quality of life.5 In addition, patients with T2DM that develop AF experience substantial increases in total mortality, cardiovascular death, and stroke compared to patients who are diabetic without AF.4,6 Current therapeutic approaches for AF, including in T2DM, are limited.
AF can develop in association with electrical and structural remodeling of the atria.7, 8, 9 Atrial action potential (AP) morphology is a critical determinant of susceptibility to AF and can be affected by electrical remodeling. Specifically, the AP upstroke is determined by the Na+ current (INa) (carried by NaV1.5 channels), which importantly affects atrial conduction velocity.7,10 Changes in conduction velocity can affect susceptibility to re-entry and AF occurrence. Action potential duration (APD) is determined in part by several repolarizing K+ currents including the transient outward K+ current (Ito) (carried by KV4.2 and KV4.3), the ultrarapid delayed rectifier K+ current (IKur) (carried by KV1.5 channels), and the inward rectifier K+ current (IK1) (carried by Kir2 channels).10 Alterations in these currents can lead to changes in APD that can also favor re-entry or triggered activity in AF.7,9
Changes in atrial electrical conduction can also result from structural remodeling caused by fibrosis following enhanced collagen production and deposition.7,8 This inappropriate collagen deposition can disrupt connectivity between myocytes leading to slow or irregular conduction, which can further promote re-entry and favor AF development. Recent studies have demonstrated that the db/db mouse model of T2DM is characterized by increased susceptibility to AF caused by impaired atrial conduction in association with electrical and structural remodeling in the atria.11
Glucagon-like peptide (GLP)-1 is an incretin hormone produced in endocrine cells in the gut that mediates effects via the glucagon-like peptide 1 receptor (GLP-1R).12,13 GLP-1 can contribute to blood glucose regulation via insulinotropic effects; however, GLP-1R agonists also have a number of additional beneficial actions including in the cardiovascular system.14, 15, 16 GLP-1R is highly expressed in the atria of the heart,13,17 suggesting that GLP-1 may be important in regulating atrial function. A number of GLP-1R agonists have been developed for therapeutic use in patients with T2DM, including liraglutide, which is a long-acting form of GLP-1 with a half-life of 13 hours compared to ∼2 minutes for native GLP-1.13,18
Clinical studies have shown that GLP-1R agonists such as liraglutide do not increase the incidence of AF in T2DM,4 and some studies have suggested that GLP-1R agonists could reduce the risk of AF in diabetes.16,19 Liraglutide has also been shown to protect against AF in a nondiabetic canine model of AF.20 Overall, however, whether GLP-1 or GLP-1R agonists are protective against AF in T2DM, and the mechanisms involved, are poorly understood. Accordingly, the purpose of this study was to investigate the effects of chronic GLP-1 treatment on AF susceptibility, as well as atrial remodeling, in type 2 diabetic db/db mice.
Methods
An expanded Methods section can be found in the Supplemental Appendix.
Mice
This study used male and female littermate wild-type and db/db mice21 aged 16-20 weeks. All experimental procedures were approved by the University of Calgary Animal Care and Use Committee and conformed to the guidelines of the Canadian Council on Animal Care as described in the Supplemental Appendix.
For chronic GLP-1 studies, db/db mice were treated with GLP-1 (7-36 amide; 15 μg/kg/d), or saline as a control, via osmotic minipumps, for 4 weeks beginning at 12-16 weeks of age. This dose of GLP-1 was chosen based on studies showing that similar GLP-1 doses and treatment durations have protective effects in mouse models of diabetes.22 For chronic liraglutide studies, db/db mice were given subcutaneous injections of liraglutide (0.25 mg/kg/d), or vehicle control, once per day for 28 to 30 days beginning at 12 to 16 weeks of age. This dose of liraglutide was calculated to be equivalent to the dose used in obese humans.16,23 Additional details are available in the Supplemental Appendix.
Blood glucose levels were assessed by oral glucose tolerance tests. Plasma insulin levels were measured using commercially available assays. Blood pressure was monitored by tail-cuff plethysmography. Cardiac structure and function were assessed by echocardiography. Additional details are available in the Supplemental Appendix.
In vivo electrophysiology
Surface electrocardiograms (ECGs) were measured in anesthetized mice using subdermal needle electrodes. In conjunction, a 1.2-F octapolar electrophysiology catheter was inserted into the right heart via an incision in the jugular vein and used to measure AF susceptibility, atrial effective refractory period (AERP), atrioventricular node effective refractory period (AVERP), and corrected sinoatrial node (SAN) recovery time (cSNRT) as described previously.24, 25, 26 AF was defined as a rapid and irregular atrial rhythm (fibrillatory baseline in the ECG) with irregular RR intervals lasting at least 1 second. Additional details are provided in the Supplemental Appendix.
High-resolution optical mapping
Activation patterns, atrial conduction velocities, and atrial optical APD were measured in isolated atrial preparations using high-resolution optical mapping as described previously24,27 and in the Supplemental Appendix.
Patch-clamping of isolated atrial myocytes
Isolated left and right atrial myocytes were used to record stimulated APs and ionic currents including INa, total IK, Ito, and IKur using the whole-cell patch-clamp technique as described previously24,25 and in the Supplemental Appendix.
Quantitative polymerase chain reaction
Expression of messenger RNA for Kcnd2, Kcnd3, Kcnip2, Kcna5, Col1a, Col3a, and Hprt1 (reference gene) was measured in left atrial tissue samples using approaches previously described.24,25 Primer sequences and experimental protocols are described in the Supplemental Appendix.
Western blotting
Western blotting for Kv4.2, Kv4.3, KChIP2, and Kv1.5 was performed in left atrial tissue samples as described previously.11 Additional details are provided in the Supplemental Appendix.
Histology
Interstitial collagen levels were measured using picrosirius red (collagen) and fast green (myocardium) staining of paraffin embedded sections (5 μm) through the left and right atria. Fibrosis was quantified using ImageJ software (National Institutes of Health).
Statistical analysis
Data are presented as mean ± SEM. Categorical data were compared using Fisher exact test and continuous data were compared using one-way or two-way analysis of variance with Holm-Sidak post hoc test for multiple pairwise comparisons as indicated in each figure legend. Data were assessed for normality using Shapiro-Wilks test. Statistical differences are reported as P < 0.05, P < 0.01, or P < 0.001.
Results
Effects of chronic GLP-1 treatment on AF susceptibility and atrial electrophysiology in db/db mice
Compared to wild-type mice, db/db mice infused with saline for 4 weeks (db/dbsaline) exhibited increased blood glucose levels (Supplemental Figures 1A to 1C). Four weeks of GLP-1 (15 μg/kg/d) treatment in db/db mice (db/dbGLP-1) resulted in intermediate blood glucose levels that were between those of wild-type and db/dbsaline mice (Supplemental Figures 1A to 1C). Body mass and plasma insulin concentrations were similarly increased in db/dbsaline and db/dbGLP-1 mice compared with in wild-type mice (Supplemental Figures 1D and 1E). There were no differences in blood pressure between treatment groups at baseline or after 4 weeks of GLP-1 treatment (Supplemental Figure 2). Echocardiography demonstrated an increase in maximum left atrial area in db/dbsaline mice; however, there were no other differences in atrial area in db/dbsaline mice compared with in wild-type mice. There were also no effects of GLP-1 on atrial area compared with that of db/dbsaline mice (Supplemental Table 1). Furthermore, there were no differences between groups in measures of ventricular structure or function (Supplemental Table 1).
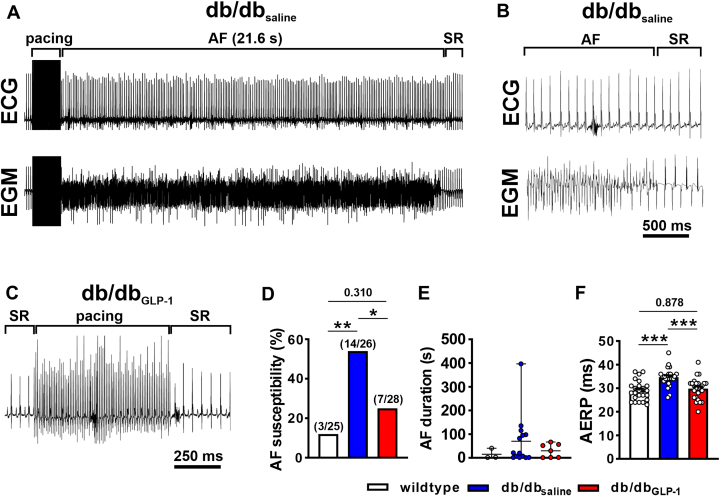
Atrial burst pacing in anesthetized mice (Figures 1A to 1C) demonstrates that AF susceptibility was increased in db/dbsaline mice compared with wild-type mice and that this elevated susceptibility to AF was prevented in db/dbGLP-1 mice (Figure 1D). When AF was induced, it was longer on average in db/dbsaline mice, whereas wild-type and db/dbGLP-1 mice exhibited shorter AF durations on average (Figure 1E). Programmed electrical stimulation studies demonstrate that AERP was increased in db/dbsaline mice compared with wild-type mice and GLP-1 treatment prevented this increase in AERP in db/db mice (Figure 1F). cSNRT was also prolonged in db/dbsaline mice and not significantly affected by GLP-1 treatment (Supplemental Table 2).
Figure 1.
Effects of chronic GLP-1 Treatment on AF and AERP in db/db Mice
(A) Representative surface electrocardiogram (ECG) (top) and atrial intracardiac electrogram (EGM) (lower) showing the induction of atrial fibrillation (AF) following burst pacing in an anesthetized db/db mouse after 4 weeks of saline infusion. AF lasted for 21.6 seconds and then reverted to sinus rhythm (SR). (B) Magnified view of the recording shown in A illustrating AF converting to SR. (C) Representative surface ECG showing the absence of AF induction after burst pacing in a db/db mouse infused with glucagon-like peptide-1 (GLP-1) for 4 weeks. (D) Inducibility of AF in wild-type mice, db/db mice infused with saline (db/dbsaline), and db/db mice infused with GLP-1 (db/dbGLP-1) for 4 weeks. Numbers in parentheses indicate the number of mice induced into AF. Data analyzed by Fisher exact test. (E) Duration of AF in the wild-type, db/dbsaline, and db/dbGLP-1 mice that were induced into AF. (F) Atrial effective refractory period (AERP) in wild-type (n = 25), db/dbsaline (n = 26), and db/dbGLP-1 (n = 28) mice. Data analyzed by one-way analysis of variance with Holm-Sidak post hoc test. ∗P< 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001.
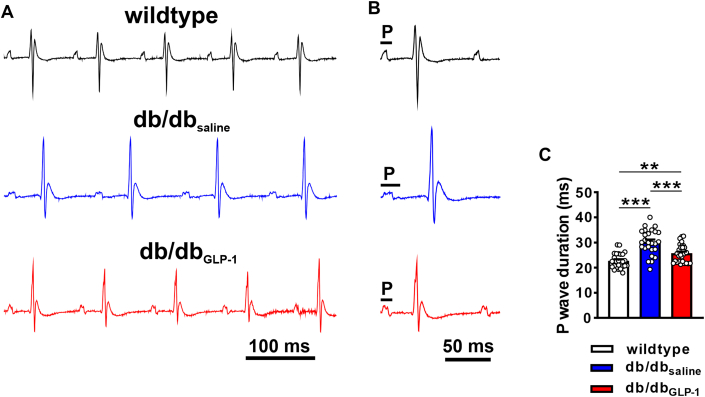
Surface ECG analysis (Figures 2A and 2B, Supplemental Table 2) demonstrates that P-wave duration as well as PR interval were increased in male and female db/dbsaline mice (Figure 2C). These increases were largely prevented in db/dbGLP-1 although P-wave duration was still longer in db/dbGLP-1 mice compared with in wild-type mice (Figure 2C). In contrast, there were no effects of GLP-1 treatment on QRS duration, QT interval, or AVERP in db/db mice (Supplemental Table 1). These effects of GLP-1 on ECG parameters were comparable between male and female mice (Supplemental Tables 3 and 4); therefore, male and female data were combined in all studies.
Figure 2.
Effects of Chronic GLP-1 Treatment on P-Wave Duration in db/db Mice
(A) Representative surface ECGs in anesthetized wild-type, db/dbsaline, and db/dbGLP-1 mice. (B) Magnified ECGs in wild-type, db/dbsaline, and db/dbGLP-1 mice illustrating differences in P-wave duration. (C) Summary of P-wave duration in wild-type (n = 25), db/dbsaline (n = 26), and db/dbGLP-1 (n = 28) mice. Data analyzed by one-way analysis of variance with Holm-Sidak post hoc test. Refer to Supplemental Tables 2 to 4 for additional ECG analyses. ∗∗P < 0.01, ∗∗∗P < 0.001. Abbreviations as in Figure 1.
Effects of chronic liraglutide treatment on AF susceptibility and atrial electrophysiology in db/db mice
To test the impact of targeting the GLP-1 pathway in a more clinically relevant fashion, db/db mice were treated with the long-acting GLP-1 analogue liraglutide by once daily subcutaneous injection (0.25 mg/kg/d). Vehicle-injected db/db mice (db/dbveh) were hyperglycemic compared to wild-type mice, whereas db/db mice treated with liraglutide (db/dblira) showed reduced blood glucose levels, although not fully to wild-type levels (Supplemental Figure 3A to 3C). Body mass was similarly elevated in db/db mice injected with vehicle or liraglutide at baseline; however, after 4 weeks of treatment, body mass was lower in db/dblira mice compared with in db/dbveh mice, although still increased compared to wild-type mice (Supplemental Figure 3D). Plasma insulin levels were similarly elevated in db/dbveh and db/dblira mice (Supplemental Figure 3E).
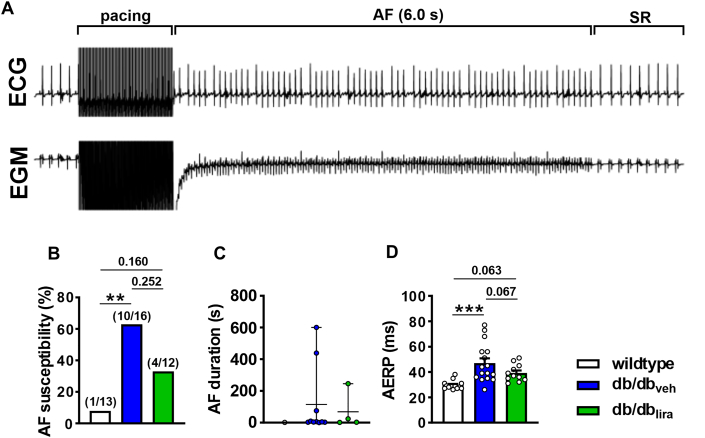
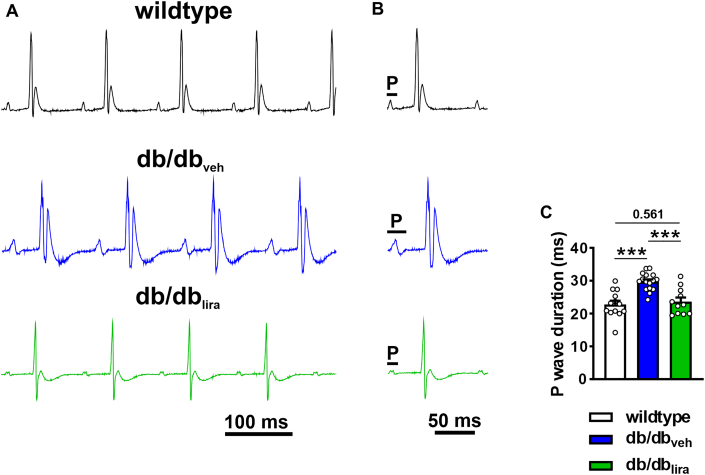
Vehicle-injected db/db mice exhibited an increased susceptibility to burst pacing induced AF that was longer lasting than for wild-type mice (Figures 3A to 3C). In contrast, db/dblira mice had an intermediate susceptibility to AF between wild-type and db/dbveh mice that on average was shorter in duration than for db/dbveh mice (Figures 3B and 3C). AERP was increased in db/dbveh mice compared with wild-type mice, and this was prevented in db/dblira mice (Figure 3D). P-wave duration was also increased in db/dbveh mice compared with in wild-type and normalized in db/dblira mice (Figures 4A to 4C). Similar to GLP-1, liraglutide had no effects on ventricular ECG intervals, cSNRT, or AVERP in db/db mice (Supplemental Table 5).
Figure 3.
Effects of Chronic Liraglutide Treatment on AF and AERP in db/db Mice
(A) Representative surface ECG (top) and atrial intracardiac EGM (bottom) showing induction of AF and conversion to SR in a vehicle-injected db/db mouse. (B) Inducibility of AF in wild-type mice, db/db mice given daily vehicle injections (db/dbveh), and db/db mice given daily injections of liraglutide for 4 weeks (db/dblira). Numbers in parentheses indicate the number of mice induced into AF. Data analyzed by Fisher exact test. (C) Duration of AF in wild-type, db/dbveh, and db/dblira mice that were induced into AF. (D) AERP in wild-type (n = 13), db/dbveh (n = 16), and db/dblira (n = 11) mice. Data analyzed by one-way analysis of variance with Holm-Sidak post hoc test. ∗∗P < 0.01, ∗∗∗P < 0.001. Abbreviations as in Figure 1.
Figure 4.
Effects of Chronic Liraglutide Treatment on P-Wave Duration in db/db Mice
(A) Representative surface ECGs in anesthetized wild-type, db/dbveh, and db/dblira mice. (B) Magnified ECGs in wild-type, db/dbveh, and db/dblira mice illustrating differences in P-wave duration. (C) Summary of P-wave duration in wild-type (n = 13), db/dbveh (n = 16), and db/dblira (n = 11) mice. Data analyzed by one-way analysis of variance with Holm-Sidak post hoc test. Refer to Supplemental Table 5 for additional ECG analysis. ∗∗∗P < 0.001. Abbreviations as in Figures 1 and 3.
Effects of chronic GLP-1 on atrial conduction and atrial optical AP morphology
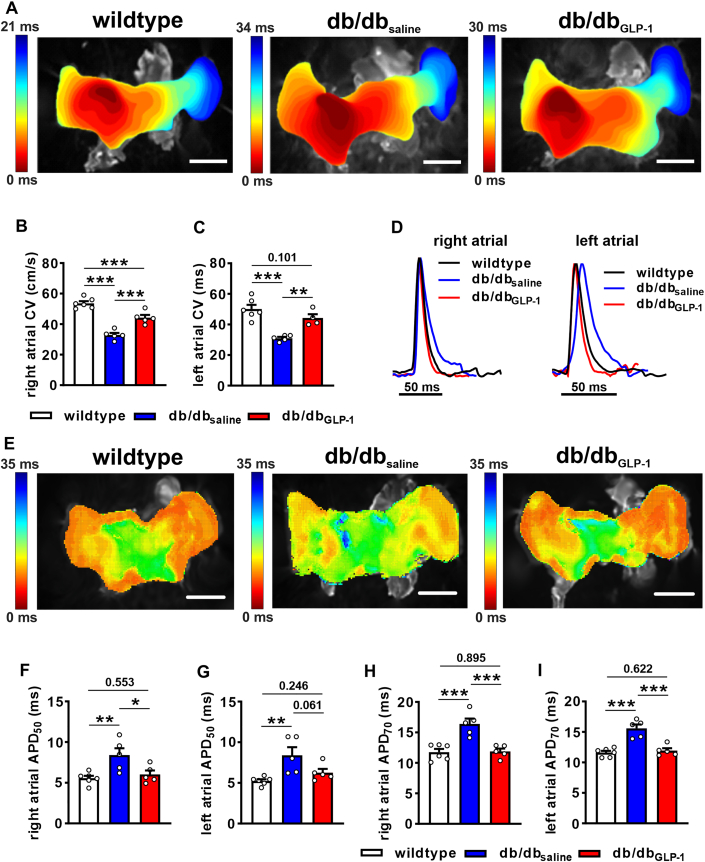
Changes in P-wave duration are indicative of altered atrial conduction; therefore, this was directly assessed in isolated atrial preparations paced at 8 Hz using optical mapping. Activation maps show that atrial conduction time was longer in db/dbsaline mice than in wild-type mice and that this was shortened in db/dbGLP-1 mice (Figure 5A). Right and left atrial conduction velocities were reduced in db/dbsaline mice compared with wild-type mice, but conduction velocities were substantially improved in db/dbGLP-1 mice (Figures 5B and 5C). Representative optical APs (Figure 5D) and APD maps (Figure 5E) demonstrate changes in APD in db/db mice treated with GLP-1. Specifically, right and left atrial APD50 (Figures 5F and 5G) and APD70 (Figures 5H and 5I) were increased in db/dbsaline mice compared with wild-type mice and normalized in db/dbGLP-1 mice. Similar changes in atrial conduction and APD were observed in optical mapping experiments in atrial preparations in sinus rhythm (ie, no pacing) (Supplemental Figure 4).
Figure 5.
Effects of Chronic GLP-1 on Atrial Conduction and Atrial Optical AP Morphology in db/db Hearts
(A) Representative activation maps in isolated atrial preparations from wild-type, db/dbsaline, and db/dbGLP-1 mice paced at 8 Hz. Right atrial appendage is on the left side of the image. Color scale indicates total conduction time across the preparation. Bars = 2 mm. (B, C) Summary of right atrial (B) and left atrial (C) conduction velocity (CV) in wild-type, db/dbsaline, and db/dbGLP-1 mice. (D) Representative right atrial and left atrial optical action potentials (APs) in isolated atrial preparations from wild-type, db/dbsaline, and db/dbGLP-1 mice. (E) Representative action potential duration maps at 50% repolarization time (APD50) in atrial preparations from wild-type, db/dbsaline, and db/dbGLP-1 mice. Bars = 2 mm. (F, G) Summary of right (F) and left (G) atrial APD50 in atrial preparations from wild-type, db/dbsaline, and db/dbGLP-1 mice. (H, I) Summary of right (H) and left (I) atrial APD70 in atrial preparations from wild-type, db/dbsaline, and db/dbGLP-1 mice. Summary data analyzed by one-way analysis of variance with Holm-Sidak post hoc test; n = 5-6 atrial preparations per group. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001. Abbreviations as in Figure 1.
Effects of chronic GLP-1 on atrial myocyte electrophysiology in db/db mice
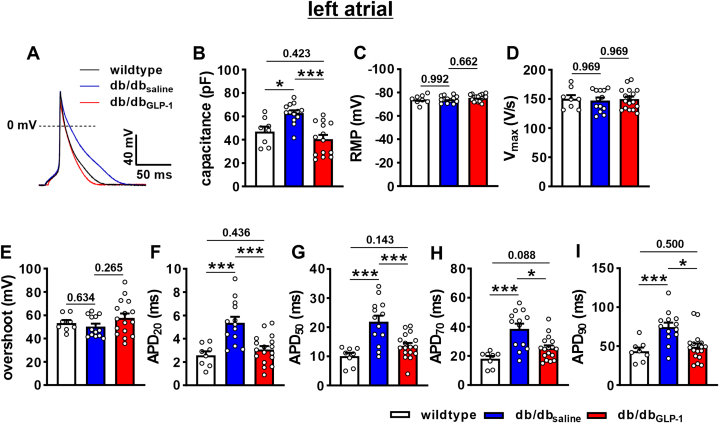
AP recordings in isolated left atrial myocytes (Figure 6A) revealed increases in cell capacitance in db/dbsaline mice that were normalized in db/dbGLP-1 mice (Figure 6B). There were no differences in left atrial resting membrane potential, AP upstroke velocity (Vmax), or AP overshoot between treatment groups (Figures 6C to 6E). Consistent with the data in intact atrial preparations, left atrial APD was prolonged throughout repolarization in db/dbsaline mice and normalized in db/dbGLP-1 mice (Figure 6F to 6I). Very similar effects of GLP-1 on AP morphology were observed in isolated right atrial myocytes (Supplemental Figure 5).
Figure 6.
Effects of Chronic GLP-1 on AP Morphology in Isolated Left Atrial Myocytes in db/db Mice
(A) Representative stimulated APs in left atrial myocytes isolated from wild-type, db/dbsaline, and db/dbGLP-1 mice. (B to I) Summary data for cell capacitance (B), resting membrane potential (RMP) (C), AP upstroke velocity (Vmax) (D), overshoot (E), APD20(F), APD50(G), APD70(H), and APD90(I) in isolated left atrial myocytes from wild-type, db/dbsaline, and db/dbGLP-1 mice. Data analyzed by one-way analysis of variance with Holm-Sidak post hoc test; n = 8 myocytes from 2 mice for wild type, 13 myocytes from 5 mice for db/dbsaline, and 17 cells from 9 mice for db/dbGLP-1. ∗P < 0.05, ∗∗∗P < 0.001. Abbreviations as in Figures 1 and 5.
In addition, variability in APD was assessed using Poincaré plot analysis (Supplemental Figures 6A and 6B). Quantification of SDs (SD1 and SD2) of Poincaré plots demonstrate that APD heterogeneity was increased in db/dbsaline mice throughout repolarization compared to wild-type mice and largely normalized in db/dbGLP-1 mice (Supplemental Figures 6C to 6J).
Consistent with the absence of differences in AP upstroke velocity or Vmax, there were no differences in peak INa density (Supplemental Figures 7A and 7B) or INa steady-state activation kinetics (Supplemental Figure 7C, Supplemental Table 6) between treatment groups. However, the INa steady-state inactivation curve was shifted to more positive membrane potentials in db/dbsaline right atrial myocytes compared to wild-type mice and normalized in db/dbGLP-1 myocytes (Supplemental Figure 7D, Supplemental Table 6). This resulted in a larger INa window current in db/dbsaline atrial myocytes, which was normalized in db/dbGLP-1 atrial myocytes (Supplemental Figures 7E and 7F).
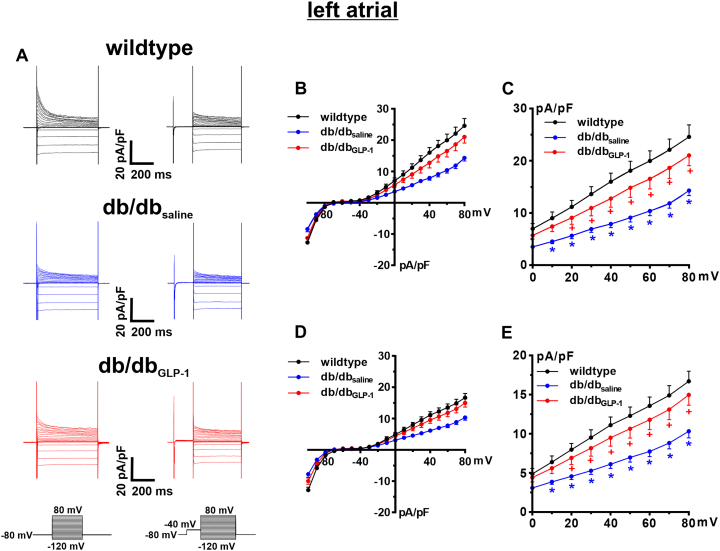
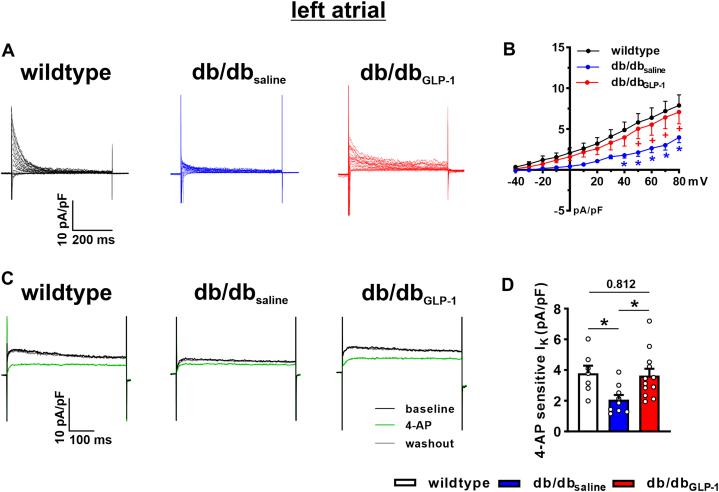
Total K+ current (IK) was measured with and without a prepulse to −40 mV to inactivate Ito in left atrial myocytes from wild-type, db/dbsaline, and db/dbGLP-1 mice (Figure 7A). IK current-voltage relationships measuring peak outward IK demonstrate that outward IK was reduced in db/dbsaline left atrial myocytes and significantly improved in, db/dbGLP-1 left atrial myocytes before (Figures 7B and 7C) and after (Figures 7D and 7E) the inactivating prepulse. The difference current between the IK recordings with and without the prepulse is a measure of Ito (Figure 8A). Ito was reduced in left atrial myocytes from db/dbsaline mice compared with wild-type mice and normalized in db/dbGLP-1 left atrial myocytes (Figure 8B). In addition, IKur was measured as the 4-aminopyridine (4-AP) (100 μmol/L)-sensitive component of IK in left atrial myocytes from wild-type, db/dbsaline, and db/dbGLP-1 mice (Figure 8C). The 4-AP–sensitive IKur was reduced in left atrial db/dbsaline myocytes compared with wild-type myocytes and normalized in left atrial db/dbGLP-1 myocytes (Figure 8D). Similar changes in repolarizing K+ currents were observed in right atrial myocytes from GLP-1–treated db/db mice (Supplemental Figure 8). Specifically, right atrial Ito was reduced in db/dbsaline mice compared with wild-type mice and significantly improved in db/dbGLP-1 mice (Supplemental Figures 9A and 9B). Right atrial 4-AP–sensitive IKur was also reduced in db/dbsaline mice compared with in wild-type mice and normalized in db/dbGLP-1 mice (Supplemental Figures 9C and 9D).
Figure 7.
Effects of Chronic GLP-1 on K+ Currents in Isolated Left Atrial Myocytes in db/db Mice
(A) Representative K+ current (IK) recordings for total IK(left) and IK after an inactivating prepulse to −40 mV (right) in left atrial myocytes isolated from wild-type, db/dbsaline, and db/dbGLP-1 mice. Voltage clamp protocols shown below recordings. (B) IK current-voltage (IV) curves measured at the peak of the total IK recordings. (C) IK IV curves (same data as B) showing repolarizing IK at membrane potentials positive to 0 mV. (D) IK IV curves measured at the peak of the IK recordings after an inactivating prepulse. (E) IK IV curves after an inactivating prepulse (same data as D) showing IK at membrane potentials positive to 0 mV. ∗P < 0.05 vs wild type, +P < 0.05 vs db/dbsaline by mixed-effects analysis of variance with Holm-Sidak post hoc test; n = 7 myocytes from 2 mice for wild type, 11 myocytes from 3 mice for db/dbsaline, and 15 myocytes from 5 mice for db/dbGLP-1. Statistical symbols shown only on C and E for clarity. Abbreviations as in Figure 1.
Figure 8.
Effects of Chronic GLP-1 on Ito and 4-AP–sensitive IKur in Isolated Left Atrial Myocytes in db/db Mice
(A) Representative transient outward K+ current (Ito) recordings in isolated left atrial myocytes from wild-type, db/dbsaline, and db/dbGLP-1 mice generated by digitally subtracting the IK recordings with and without a prepulse as seen in Figure 7. (B) Ito IV curves measured as the difference current between IK recordings with and without a prepulse as shown in Figure 7. ∗P < 0.05 vs wild-type mice; +P < 0.05 vs db/dbGLP-1 mice by mixed effects analysis of variance with Holm-Sidak post hoc test; n = 7 myocytes from 2 mice for wild type, 11 myocytes from 3 mice for db/dbsaline, and 15 myocytes from 5 mice for db/dbGLP-1. (C) Representative IK recordings at +30 mV showing the effects of 4-aminopyridine (4-AP) (100 μmol/L), which inhibits KV1.5-mediated IKur, in isolated left atrial myocytes from wild-type, db/dbsaline, and db/dbGLP-1 mice. (D) Summary of the amplitude of 4-AP–sensitive IK in isolated left atrial myocytes from wild-type, db/dbsaline, and db/dbGLP-1 mice. Data analyzed by one-way analysis of variance with Holm-Sidak post hoc test; n = 7 myocytes from 2 mice for wild type, 9 myocytes from 3 mice for db/dbsaline, and 12 myocytes from 5 mice for db/dbGLP-1. IKur = ultra-rapid delayed rectifier K+ current; other abbreviations as in Figures 1 and 7.
Expression of Kcnd2 (encodes KV4.2), Kcnd3 (encodes KV4.3), and Kcnip2 (encodes KChIP2) were each reduced in the left atrium of db/dbsaline mice compared with wild-type mice and remained reduced compared to wild-type in db/dbGLP-1 left atria (Supplemental Figures 10A to 10C). Expression of Kcna5 (encodes KV1.5) was not different between treatment groups (Supplemental Figure 10D). Protein expression of Kv4.2 showed a trend (P = 0.055) toward reduction in the left atrium of db/dbsaline mice and was not affected by GLP-1 treatment (Supplemental Figure 10E). Protein expression of Kv4.3, KChIP2, and Kv1.5 was not different among groups (Supplemental Figures 10F to 10H).
Effects of acute GLP-1 on atrial myocyte action potential morphology in db/db mice
The effects of acute GLP-1 (100 nmol/L) application on isolated atrial myocytes from untreated db/db mice were also measured to determine whether this would mimic the effects of chronic GLP-1 treatment described herein. In these experiments, GLP-1 was superfused on isolated atrial myocytes for 5-10 minutes. Acute GLP-1 application had no effects on atrial AP morphology in isolated db/db atrial myocytes (Supplemental Figure 11).
Effects of chronic GLP-1 on atrial fibrosis in db/db mice
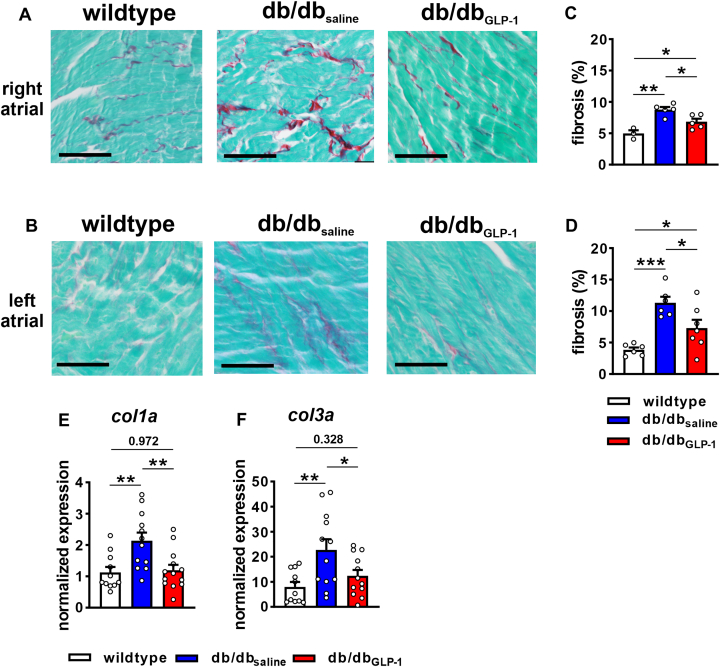
Interstitial fibrosis at study endpoints was increased in the right and left atria of db/dbsaline mice and this was significantly attenuated in db/dbGLP-1 mice (Figures 9A to 9D). The progression of fibrosis was also assessed in the left atrium in db/db mice (Supplemental Figure 12). These data demonstrate that left atrial fibrosis was also increased at 12-14 weeks of age (ie, the time point at which GLP-1 or saline infusions began) in db/db mice and tended to be further increased by the study endpoint. Expression of Col1a (encodes collagen type I) and Col3a (encodes collagen type III) was increased in the left atrium of db/dbsaline mice compared to wild-type mice and normalized in db/dbGLP-1 left atria (Figures 9E and 9F).
Figure 9.
Effects of Chronic GLP-1 on Atrial Fibrosis in db/db Mice
(A, B) Representative images showing patterns of interstitial fibrosis (red staining) in right (A) and left (B) atria of wild-type, db/dbsaline, and db/dbGLP-1 mice. Bars = 50 μm. (C,D) Summary of right (C) and left (D) atrial fibrosis. Data analyzed by one-way analysis of variance with Holm-Sidak post hoc test. For right atrium, n = 3 wild-type, 5 db/dbsaline, and 5 db/dbGLP-1 mice. For left atrium, n = 6 wild-type, 6 db/dbsaline, and 7 db/dbGLP-1 mice. (E-F) Messenger RNA expression of Col1a(E) and Col3a(F) in the left atrium in wild-type, db/dbsaline, and db/dbGLP-1 mice. Data analyzed by one-way analysis of variance with Holm-Sidak post hoc test, n = 11 wild-type, 12, db/dbsaline, and 13 db/dbGLP-1 mice. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001. Abbreviations as in Figure 1.
Discussion
AF is highly prevalent in patients with T2DM where it substantially worsens morbidity and mortality.2,4,5 Consistent with this, the present study, as well as previous studies,11 show that the db/db mouse model of T2DM is highly susceptible to pacing-induced AF caused by impaired atrial electrical conduction in association with electrical and structural remodeling of the atria. Although some clinical studies have suggested that antidiabetic drugs such as GLP-1R agonists may protect against AF in T2DM,19 this is very poorly understood. Accordingly, this was investigated in the present study. Strikingly, chronic treatment of db/db mice with GLP-1 (continuous infusion by osmotic pump) reduced susceptibility to AF. Furthermore, AF durations were shorter on average in GLP-1–treated db/db mice, indicating a less severe AF burden. Protection against AF in GLP-1–treated db/db mice occurred in association with improvements in atrial conduction as indicated by shorter P-wave durations in vivo and faster atrial conduction velocities in isolated atrial preparations compared to saline-treated db/db mice. Importantly, treatment of db/db mice with the long-acting GLP-1R analogue liraglutide, using a clinically relevant dose and treatment approach (daily subcutaneous injections), also reduced AF burden. Specifically, liraglutide-treated db/db mice exhibited intermediate levels of AF susceptibility and on average had shorter AF durations. Liraglutide also reduced AERP and shortened P-wave duration to wild-type levels, indicating a normalization of atrial conduction in db/db mice. The intermediate effect of liraglutide compared to GLP-1 on AF susceptibility may be related to dosing and route of delivery as GLP-1 was delivered continuously by osmotic pump whereas liraglutide was delivered by once-daily subcutaneous injection. Overall, the present study demonstrates that GLP-1 (and related GLP-1R agonists) can potently protect against AF and preserve atrial conduction in db/db mice.
db/db mice also exhibited evidence of SAN dysfunction (ie, reduced heart rate, increased cSNRT), which is common in association with AF.28,29 Previous studies have also demonstrated SAN dysfunction in db/db mice.30 Overall, GLP-1 did not prevent these changes in SAN function. We did not observe substantial changes in atrial or ventricular size or ventricular function in db/db mice by echocardiography, which is consistent with our previous study.11 The present study shows that echocardiography measures were also not significantly affected by GLP-1. On the other hand, patch-clamp studies demonstrated increased cell capacitance in db/dbsaline mice that were normalized in db/dbGLP-1 mice. This suggests that db/db mice may exhibit atrial myocyte hypertrophy, which was prevented by GLP-1.
Whereas atrial electrophysiology was substantially altered in db/db mice and potently affected by GLP-1, ventricular ECG intervals were not markedly altered in db/db mice and were not significantly affected by GLP-1 or liraglutide. GLP-1R is highly expressed in the atria, possibly at levels higher than in the ventricles,13 suggesting that GLP-1 may act, at least in part, via direct effects in the atria. Consistent with this, blood pressure was not different in db/db mice and not affected by GLP-1. There were also no effects of GLP-1 or liraglutide on plasma insulin levels, which were similarly elevated in saline- and drug-treated db/db mice. On the other hand, GLP-1 and liraglutide caused some improvement in blood glucose levels in db/db mice, although not to wild-type levels. This is consistent with previous studies showing that liraglutide partially improves glucose tolerance in db/db mice.31,32 Thus, it is possible that a partial reduction in the level of hyperglycemia could contribute to the improvements in atrial electrophysiology elicited by GLP-1R agonists via indirect effects. It is important to note, however, that the relationship between AF prevalence and glycemic control in patients with diabetes remains unresolved with some studies suggesting worsening AF with poor glycemic control and others finding no relationship.2,4,6,33 Further studies will be needed to delineate direct and indirect effects of GLP-1R agonists on atrial electrophysiology.
Several factors can affect atrial conduction velocity including AP upstroke velocity caused by INa, gap junctions, and fibrosis.7 We observed no differences in atrial AP upstroke velocity or atrial peak INa in db/db mice and neither of these were altered by GLP-1 treatment. We have also previously shown that messenger RNA expression of major atrial connexins (Cx40 and Cx43) is not altered in db/db mice.11 In contrast, db/db mice exhibited fibrosis in the right and left atria in association with increases in expression of collagen type I and collagen type III. Atrial fibrosis was significantly lower in db/db atria after GLP-1 treatment. Atrial fibrosis was also increased at 12-14 weeks of age in db/db mice (which corresponds to the time point at which GLP-1 treatment began) and tended to increase further at time points corresponding to study endpoints when functional studies were conducted. This suggests that GLP-1 acts to prevent further fibrosis from developing but may also partially reverse fibrosis. Consistent with this, GLP-1 also normalized atrial collagen gene expression in db/db mice. These data indicate that changes in fibrosis are critically involved in the impairments in atrial conduction velocity in db/db mice and their prevention following treatment with GLP-1. GLP-1 could directly affect fibroblast function; however, it is also possible that GLP-1 could affect inflammatory signaling, which could then have an impact on fibroblast activation and profibrotic signaling. Inflammation has been linked to fibrosis and atrial arrhythmias in prior studies.34 These possibilities will be explored in future studies.
Impaired conduction caused by atrial fibrosis can create a substrate for re-entry, which favors the occurrence and maintenance of AF.7,35 Consistent with the findings in our study, previous studies have shown that GLP-1R agonists or dipeptidyl peptidase 4 inhibitors (which prevent the hydrolysis of GLP-1) can have antifibrotic effects in the heart in nondiabetic mouse models of disease.36, 37, 38
In addition, we observed substantial heterogeneity in APD during repolarization in db/db mice, which was prevented by GLP-1 treatment. Increased heterogeneity in repolarization can also lead to re-entry;39 therefore, the ability of GLP-1 to protect against this heterogeneity in APD may also contribute to the protective effects of GLP-1 against AF in db/db mice.
Consistent with previous studies,11 we also observed increases in atrial APD in db/db mice. Increased APD was evident from optical mapping studies in intact atrial preparations and from patch-clamp studies in isolated atrial myocytes and coincided with increases in AERP in vivo. Increased atrial APD occurred because of reductions in repolarizing Ito and IKur. Although peak INa was not altered in db/db atrial myocytes, the INa steady-state inactivation curve was shifted to more positive membrane potentials resulting in a larger INa window current that could also contribute to AP prolongation. Chronic GLP-1 treatment normalized APD, prevented the reductions in Ito and IKur, and normalized INa steady-state inactivation in db/db atrial myocytes. In addition to fibrosis, prolongation of APD could also contribute to the slowing of atrial conduction, particularly at high heart rates, while also increasing the potential for conduction block.40,41 Each of these could further contribute to a substrate for re-entry and AF in db/db mice. In addition, AP prolongation could increase the likelihood of early afterdepolarizations, which could trigger AF occurrence. Thus, the prevention of increases in APD by GLP-1 is likely centrally involved in the improvements in atrial conduction and protection against AF in db/db mice treated with GLP-1.
In agreement with previous studies,11 Ito was reduced in db/db atria in association with reduced expression of Kcnd2, Kcnd3, and Kcnip2 and lower protein expression of Kv4.2. Interestingly, these changes were not prevented by GLP-1 treatment. GLP-1 treatment also had no effects on the protein expression of Kv4.3 or KChIP2. Furthermore, there were no changes in expression of Kcna5 or KV1.5 protein in db/db mice before or after GLP-1 treatment. Thus, GLP-1 was able to increase Ito and IKur current density independently of changes in ion channel gene or protein expression. As noted, cell capacitance was increased in db/db atrial myocytes (indicating atrial hypertrophy) and this was prevented by GLP-1 treatment; therefore, the improvements in Ito and IKur density in db/dbGLP-1 mice may be associated with these differences in cell capacitance and the prevention of atrial myocyte hypertrophy. The mechanisms by which GLP-1 improves atrial K+ currents in db/db mice will be studied further in future investigations.
Acute application of GLP-1 had no effects on AP morphology in isolated atrial myocytes from db/db mice. It is also reasonable to infer that an acute exposure to GLP-1 treatment would have minimal affects, if any, on atrial fibrosis. Together, these data indicate that GLP-1 must be delivered chronically to achieve protection against atrial electrical remodeling (ie, changes in AP morphology and associated ionic currents) and structural remodeling (ie, fibrosis) in db/db mice.
Study limitations
The present study was conducted in mice, which exhibit some differences in repolarizing K+ currents compared to humans.42 Specifically, the rapid and slow delayed rectifier K+ currents (IKr and IKs) do not play a major functional role in mouse atria. However, Ito and IKur, which are centrally involved in the changes in AP morphology in db/db mice before and after GLP-1 treatment, are critical in the atria of mice and humans, suggesting the effects identified in the present study will be relevant to humans. Further studies to determine optimal doses and duration of treatment with specific GLP-1R agonists to optimize effects on atrial electrophysiology while also ensuring appropriate glycemic control are warranted.
Conclusions
The present study demonstrates that chronic treatment of type 2 diabetic db/db mice with GLP-1 protects against AF by preventing impairments in atrial conduction, atrial electrical remodeling, and atrial fibrosis. These findings suggest that GLP-1R agonists could protect against AF in human patients with T2DM. Given the wide use of GLP-1R agonists for the treatment of T2DM, the effects of these compounds on AF in these patients should be further studied.
Funding Support and Author Disclosures
Dr Bohne has received a University of Calgary Graduate Scholarship. Dr Jansen has received a Killam Postdoctoral Fellowship. Dr Dorey has received a Canadian Institutes of Health Research Doctoral Research Award. Dr Jamieson has received a Libin Cardiovascular Institute Postdoctoral Fellowship. Dr Rose has received support from the Canadian Institutes of Health Research (grants PJT180474 and PJT166105). All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Atrial fibrillation is highly prevalent in patients with T2DM. Furthermore, patients with T2DM that develop AF experience worse outcomes including increased mortality. GLP-1R agonists are used to treat patients with T2DM; however, their effects on atrial remodeling and AF are unclear. We demonstrate that GLP-1 and the GLP-1 analogue liraglutide protect against AF in type 2 diabetic mice by preventing electrical and structural remodeling in the atria.
TRANSLATIONAL OUTLOOK: This study identifies GLP-1 and the related GLP-1 analogue liraglutide as a potential approach for treating or preventing AF in a mouse model of T2DM. Further studies will be required to translate these finding to human patients with T2DM to reduce the influences of AF in this patient population.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For a supplemental Methods section, and supplemental figures, and tables, please see the online version of this paper.
Appendix
References
- 1.Staerk L., Sherer J.A., Ko D., Benjamin E.J., Helm R.H. Atrial fibrillation: epidemiology, pathophysiology, and clinical outcomes. Circ Res. 2017;120(9):1501–1517. doi: 10.1161/CIRCRESAHA.117.309732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bohne L.J., Johnson D., Rose R.A., Wilton S.B., Gillis A.M. The association between diabetes mellitus and atrial fibrillation: clinical and mechanistic insights. Front Physiol. 2019;10:135. doi: 10.3389/fphys.2019.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kahn S.E., Cooper M.E., Del Prato S. Pathophysiology and treatment of type 2 diabetes: perspectives on the past, present, and future. Lancet. 2014;383(9922):1068–1083. doi: 10.1016/S0140-6736(13)62154-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bell D.S.H., Goncalves E. Atrial fibrillation and type 2 diabetes: prevalence, etiology, pathophysiology and effect of anti-diabetic therapies. Diabetes Obes Metab. 2019;21(2):210–217. doi: 10.1111/dom.13512. [DOI] [PubMed] [Google Scholar]
- 5.Kornej J., Borschel C.S., Benjamin E.J., Schnabel R.B. Epidemiology of atrial fibrillation in the 21st century: novel methods and new insights. Circ Res. 2020;127(1):4–20. doi: 10.1161/CIRCRESAHA.120.316340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andrade J., Khairy P., Dobrev D., Nattel S. The clinical profile and pathophysiology of atrial fibrillation: relationships among clinical features, epidemiology, and mechanisms. Circ Res. 2014;114(9):1453–1468. doi: 10.1161/CIRCRESAHA.114.303211. [DOI] [PubMed] [Google Scholar]
- 7.Jansen H.J., Bohne L.J., Gillis A.M., Rose R.A. Atrial remodeling and atrial fibrillation in acquired forms of cardiovascular disease. Heart Rhythm O2. 2020;1(2):147–159. doi: 10.1016/j.hroo.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aguilar M., Rose R.A., Takawale A., Nattel S., Reilly S. New aspects of endocrine control of atrial fibrillation and possibilities for clinical translation. Cardiovasc Res. 2021;117(7):1645–1661. doi: 10.1093/cvr/cvab080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heijman J., Voigt N., Nattel S., Dobrev D. Cellular and molecular electrophysiology of atrial fibrillation initiation, maintenance, and progression. Circ Res. 2014;114(9):1483–1499. doi: 10.1161/CIRCRESAHA.114.302226. [DOI] [PubMed] [Google Scholar]
- 10.Bartos D.C., Grandi E., Ripplinger C.M. Ion channels in the heart. Compr Physiol. 2015;5(3):1423–1464. doi: 10.1002/cphy.c140069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bohne L.J., Jansen H.J., Daniel I., et al. Electrical and structural remodeling contribute to atrial fibrillation in type 2 diabetic db/db mice. Heart Rhythm. 2021;18(1):118–129. doi: 10.1016/j.hrthm.2020.08.019. [DOI] [PubMed] [Google Scholar]
- 12.Ussher J.R., Drucker D.J. Cardiovascular biology of the incretin system. Endocr Rev. 2012;33(2):187–215. doi: 10.1210/er.2011-1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ussher J.R., Drucker D.J. Cardiovascular actions of incretin-based therapies. Circ Res. 2014;114(11):1788–1803. doi: 10.1161/CIRCRESAHA.114.301958. [DOI] [PubMed] [Google Scholar]
- 14.Ussher J.R., Greenwell A.A., Nguyen M.A., Mulvihill E.E. Cardiovascular effects of incretin-based therapies: integrating mechanisms with cardiovascular outcome trials. Diabetes. 2022;71(2):173–183. doi: 10.2337/dbi20-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nauck M.A., Meier J.J., Cavender M.A., Abd El Aziz M., Drucker D.J. Cardiovascular actions and clinical outcomes with glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors. Circulation. 2017;136(9):849–870. doi: 10.1161/CIRCULATIONAHA.117.028136. [DOI] [PubMed] [Google Scholar]
- 16.Marso S.P., Daniels G.H., Brown-Frandsen K., et al. LEADER Steering Committee and Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311–322. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baggio L.L., Yusta B., Mulvihill E.E., et al. GLP-1 receptor expression within the human heart. Endocrinology. 2018;159(4):1570–1584. doi: 10.1210/en.2018-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drucker D.J., Dritselis A., Kirkpatrick P. Liraglutide. Nat Rev Drug Discov. 2010;9(4):267–268. doi: 10.1038/nrd3148. [DOI] [PubMed] [Google Scholar]
- 19.Shi W., Zhang W., Zhang D., et al. Comparison of the effect of glucose-lowering agents on the risk of atrial fibrillation: a network meta-analysis. Heart Rhythm. 2021;18(7):1090–1096. doi: 10.1016/j.hrthm.2021.03.007. [DOI] [PubMed] [Google Scholar]
- 20.Nakamura H., Niwano S., Niwano H., et al. Liraglutide suppresses atrial electrophysiological changes. Heart Vessels. 2019;34(8):1389–1393. doi: 10.1007/s00380-018-01327-4. [DOI] [PubMed] [Google Scholar]
- 21.Hsueh W., Abel E.D., Breslow J.L., et al. Recipes for creating animal models of diabetic cardiovascular disease. Circ Res. 2007;100(10):1415–1427. doi: 10.1161/01.RES.0000266449.37396.1f. [DOI] [PubMed] [Google Scholar]
- 22.Zhang J., Tokui Y., Yamagata K., et al. Continuous stimulation of human glucagon-like peptide-1 (7-36) amide in a mouse model (NOD) delays onset of autoimmune type 1 diabetes. Diabetologia. 2007;50(9):1900–1909. doi: 10.1007/s00125-007-0737-6. [DOI] [PubMed] [Google Scholar]
- 23.Nair A.B., Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016;7(2):27–31. doi: 10.4103/0976-0105.177703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Polina I., Jansen H.J., Li T., et al. Loss of insulin signaling may contribute to atrial fibrillation and atrial electrical remodeling in type 1 diabetes. Proc Natl Acad Sci U S A. 2020;117(14):7990–8000. doi: 10.1073/pnas.1914853117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jansen H.J., Mackasey M., Moghtadaei M., et al. Distinct patterns of atrial electrical and structural remodeling in angiotensin II mediated atrial fibrillation. J Mol Cell Cardiol. 2018;124:12–25. doi: 10.1016/j.yjmcc.2018.09.011. [DOI] [PubMed] [Google Scholar]
- 26.Ripplinger C.M., Glukhov A.V., Kay M.W., et al. Guidelines for assessment of cardiac electrophysiology and arrhythmias in small animals. Am J Physiol Heart Circ Physiol. 2022;323(6):H1137–H1166. doi: 10.1152/ajpheart.00439.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jansen H.J., Mackasey M., Moghtadaei M., et al. NPR-C (natriuretic peptide receptor-C) modulates the progression of angiotensin ii-mediated atrial fibrillation and atrial remodeling in mice. Circ Arrhythm Electrophysiol. 2019;12(1) doi: 10.1161/CIRCEP.118.006863. [DOI] [PubMed] [Google Scholar]
- 28.Bukari A., Wali E., Deshmukh A., et al. Prevalence and predictors of atrial arrhythmias in patients with sinus node dysfunction and atrial pacing. J Interv Card Electrophysiol. 2018;53(3):365–371. doi: 10.1007/s10840-018-0463-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang H.Y., Lin Y.J., Lo L.W., et al. Sinus node dysfunction in atrial fibrillation patients: the evidence of regional atrial substrate remodelling. Europace. 2013;15(2):205–211. doi: 10.1093/europace/eus219. [DOI] [PubMed] [Google Scholar]
- 30.Liu Y., Jansen H.J., Krishnaswamy P.S., Bogachev O., Rose R.A. Impaired regulation of heart rate and sinoatrial node function by the parasympathetic nervous system in type 2 diabetic mice. Sci Rep. 2021;11(1) doi: 10.1038/s41598-021-91937-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Y., Zheng J., Shen Y., et al. Comparative study of liraglutide and insulin glargine on glycemic control and pancreatic beta-cell function in db/db mice. Med Sci Monit. 2018;24:3293–3300. doi: 10.12659/MSM.907227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koike M., Saito H., Kohno G., Takubo M., Watanabe K., Ishihara H. Effects of GLP-1RA and SGLT2i, alone or in combination, on mouse models of type 2 diabetes representing different disease stages. Int J Mol Sci. 2021;22(21) doi: 10.3390/ijms222111463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benjamin E.J., Levy D., Vaziri S.M., D'Agostino R.B., Belanger A.J., Wolf P.A. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA. 1994;271(11):840–844. [PubMed] [Google Scholar]
- 34.Scott L., Jr., Fender A.C., Saljic A., et al. NLRP3 inflammasome is a key driver of obesity-induced atrial arrhythmias. Cardiovasc Res. 2021;117(7):1746–1759. doi: 10.1093/cvr/cvab024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nattel S. Molecular and cellular mechanisms of atrial fibrosis in atrial fibrillation. J Am Coll Cardiol EP. 2017;3(5):425–435. [Google Scholar]
- 36.Wang J., Guo R., Ma X., et al. Liraglutide inhibits AngII-induced cardiac fibroblast proliferation and ECM deposition through regulating miR-21/PTEN/PI3K pathway. Cell Tissue Bank. 2023;24(1):125–137. doi: 10.1007/s10561-022-10021-9. [DOI] [PubMed] [Google Scholar]
- 37.Withaar C., Meems L.M.G., Markousis-Mavrogenis G., et al. The effects of liraglutide and dapagliflozin on cardiac function and structure in a multi-hit mouse model of heart failure with preserved ejection fraction. Cardiovasc Res. 2021;117(9):2108–2124. doi: 10.1093/cvr/cvaa256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Banks T.E., Rajapaksha M., Zhang L.H., Bai F., Wang N.P., Zhao Z.Q. Suppression of angiotensin II-activated NOX4/NADPH oxidase and mitochondrial dysfunction by preserving glucagon-like peptide-1 attenuates myocardial fibrosis and hypertension. Eur J Pharmacol. 2022;927 doi: 10.1016/j.ejphar.2022.175048. [DOI] [PubMed] [Google Scholar]
- 39.Avula U.M.R., Abrams J., Katchman A., et al. Heterogeneity of the action potential duration is required for sustained atrial fibrillation. JCI Insight. 2019;5(11) doi: 10.1172/jci.insight.128765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carmeliet E. Conduction in cardiac tissue. Historical reflections. Physiol Rep. 2019;7(1) doi: 10.14814/phy2.13860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burton F.L., Cobbe S.M. Dispersion of ventricular repolarization and refractory period. Cardiovasc Res. 2001;50(1):10–23. doi: 10.1016/s0008-6363(01)00197-3. [DOI] [PubMed] [Google Scholar]
- 42.Nerbonne J.M., Kass R.S. Molecular physiology of cardiac repolarization. Physiol Rev. 2005;85(4):1205–1253. doi: 10.1152/physrev.00002.2005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.