Abstract
Purpose:
In 2011 we conducted a survey regarding continuous EEG (CEEG) utilization in critically ill children. In the interim decade, the literature has expanded, and guidelines and consensus statements have addressed CEEG utilization. Thus, we aimed to characterize current practice related to CEEG utilization in critically ill children.
Methods:
We conducted an online survey of pediatric neurologists from 50 United States (US) and 12 Canadian institutions in 2022.
Results:
We assessed responses from 48 of 62 (77%) surveyed institutions. Reported CEEG indications were consistent with consensus statement recommendations and included altered mental status after a seizure or status epilepticus, altered mental status of unknown etiology, or altered mental status with an acute primary neurological condition. Since the prior survey, there was a 3–4-fold increase in the number of patients undergoing CEEG per month and greater use of written pathways for ICU CEEG. However, variability in resources and workflow remained, particularly regarding technologist availability, frequency of CEEG screening, communication approaches, and electrographic seizure management approaches.
Conclusions:
Among the surveyed institutions, which included primarily large academic centers, CEEG use in pediatric intensive care units has increased with some practice standardization, but variability in resources and workflow remained.
Keywords: Critical Care, EEG, Pediatric, Survey, EEG monitoring, neuromonitoring
Introduction
In 2011, we conducted a survey of child neurologists in the United States (US) and Canada regarding resources and practices related to continuous electroencephalographic monitoring (CEEG) in the pediatric intensive care unit (PICU).1 CEEG use was increasing, and there was substantial variability in workflow and resource availability. Over the last decade, expanding literature has established that electrographic seizures (ES) occur in 10–40% of critically ill children with acute encephalopathy who undergo CEEG, most ES lack a clinical correlate, and about one-third of patients with ES experience electroencephalographic status epilepticus.2–17 Further, there is increasing evidence that high ES exposure is associated with unfavorable outcomes.6, 7, 10, 15, 16 Thus, guidelines and consensus statements recommend that many critically ill children with acute encephalopathy undergo CEEG for ES detection and management.18–20 Given our enhanced understanding of the epidemiology, incidence, and impact of ES in critically ill children, we aimed to assess current resources and practices regarding CEEG in critically ill children in the US and Canada at an institutional level. Data regarding contemporary practice could contribute to the development of clinical pathways, detect gaps in resource availability, and identify areas of practice variability. We hypothesized that there would be greater CEEG use but persistent resource and practice variability.
Methods
We updated the web-based survey used in August 2011.1 The investigators performed two rounds of pilot testing to optimize clarity and focus the survey to enable completion in 10–15 minutes.
We distributed the new survey in July-August 2022 using REDCap,21 and participants completed the survey anonymously. We surveyed a limited cohort of institutions representing mostly larger academic medical centers, including (1) US centers ranked number 1–50 in Pediatric Neurology/Neurosurgery by the 2021–2022 US News and World Report22 and (2) 12 tertiary care institutions in Canada. We obtained one response per institution. We targeted physicians focused on ICU EEG trained in electroencephalography.
The survey consisted of 75 questions (Addendum Material) subdivided into seven sections: (1) institutional characteristics and CEEG volume, (2) physician and technologist characteristics, (3) CEEG indications, (4) workflow, (5) communication and reporting, (6) ES management, and (7) other issues, including affiliated institutions, quality improvement (QI), and quantitative EEG (QEEG). The survey defined CEEG as lasting at least three hours. Categorical responses were calibrated with percentage ranges (e.g., almost never 0%; rarely 1–20%; sometimes 25–50%; mostly 51–90%; almost always 91–100%). Neonatal ICU monitoring was excluded from the survey questions.
Analyses were conducted in Stata 15.1 (College Station, TX). We report descriptive statistics for all institutions and subdivided by country. The study was deemed exempt from review by the Institutional Review Board of the Children’s Hospital of Philadelphia. We applied standards from a consensus-based checklist for reporting survey studies.23
Results
Responses and Institution Characteristics
We surveyed 62 institutions (50 US; 12 Canada), received responses from 53 institutions, and excluded 5 institutions with mostly incomplete responses. Thus, we assessed responses from 48 institutions (77%) which had ≤5 incomplete questions, including 74% (37/50) of US and 92% (11/12) of Canadian institutions. Table 1 provides the full data. Figure 1 summarizes key institutional and workflow characteristics.
Table 1.
Full Data
| QUESTION | RESPONSES | UNITED STATES (N=37) |
CANADA (N=11) |
TOTAL (N=48) |
|---|---|---|---|---|
| Institutional Characteristics, Pathways, and Limitations | ||||
| Institution Characteristics | Academic / University | 33 (92) | 11 (100) | 44 (94) |
| Non-Academic / Community | 3 (8) | 0 (0) | 3 (6) | |
| Pediatric ICU Beds | 11–25 Beds | 12 (32) | 10 (91) | 22 (46) |
| 25–50 Beds | 17 (46) | 0 (0) | 17 (35) | |
| 51–75 Beds | 2 (5) | 1 (9) | 3 (6) | |
| ≥76 beds | 6 (16) | 0 (0) | 6 (13) | |
| Cardiac ICU | No Cardiac ICU | 5 (14) | 7 (64) | 12 (25) |
| Cardiac ICU Separate from Pediatric ICU | 32 (86) | 4 (36) | 36 (75) | |
| National Association of Epilepsy Centers | Level 4 | 33 (89) | ||
| Neurologists Specialized in NeuroICU Care | Most Not Specialized | 24 (65) | 9 (82) | 33 (69) |
| Most Specialized | 10 (27) | 2 (18) | 12 (25) | |
| All Specialized | 3 (8) | 0 (0) | 3 (6) | |
| Written Pathway for ICU CEEG | None | 4 (11) | 1 (10) | 5 (11) |
| Formal Written Pathway for Many Conditions | 12 (32) | 3 (30) | 15 (32) | |
| Formal Written Pathway for Few Conditions | 14 (38) | 3 (30) | 17 (36) | |
| Informal Approach for Many Conditions | 6 (16) | 1 (10) | 7 (15) | |
| Informal Approach for Few Conditions | 1 (3) | 1 (10) | 2 (4) | |
| Limitations to Performing More CEEG | None | 5 (14) | 1 (9) | 6 (13) |
| Insufficient Technologists | 23 (62) | 9 (82) | 32 (67) | |
| Insufficient Equipment | 20 (54) | 5 (45) | 25 (52) | |
| Insufficient Electroencephalographers | 4 (11) | 5 (45) | 9 (19) | |
| Insufficient Motivation by Intensivists | 5 (14) | 0 (0) | 5 (10) | |
| Insufficient Motivation by Neurologists | 2 (5) | 2 (18) | 4 (8) | |
| Physician Characteristics | ||||
| Physician Training | Certified EEG (ABPN or ABCN) | 30 (81) | 11 (100) | 41 (85) |
| Certified Epilepsy (ABPN) and Specific Training in EEG | 30 (81) | 5 (45) | 35 (73) | |
| Not Certified but Specific EEG Training | 1 (3) | 1 (9) | 2 (4) | |
| No Specific EEG Training | 0 (0) | 1 (9) | 1 (2) | |
| Physician Coverage | Same Covers EMU and ICU EEG Day and Night | 16 (43) | 7 (64) | 23 (48) |
| Separate Cover EMU and ICU EEG Day and Night | 12 (32) | 4 (36) | 16 (33) | |
| Separate Cover EMU and ICU EEG Day But Combine Night | 9 (24) | 0 (0) | 9 (19) | |
| Physician Staffing Nights in a Row | 1–2 Nights | 7 (10) | 2 (18) | 9 (19) |
| 3–4 Nights | 6 (16) | 0 (0) | 6 (13) | |
| 5–7 Nights | 21 (57) | 7 (64) | 28 (58) | |
| 8–14 Nights | 1 (3) | 2 (18) | 3 (6) | |
| Never (0 Nights) | 2 (5) | 0 (0) | 2 (4) | |
| Physician Overnight Calls | Almost Never | 3 (8) | 4 (36) | 7 (15) |
| 1–2 Nights / Week | 9 (24) | 5 (45) | 14 (29) | |
| 3–4 Nights / Week | 11 (30) | 0 (0) | 11 (23) | |
| 5–7 Nights / Week | 4 (11) | 0 (0) | 4 (8) | |
| Almost Every Night | 5 (14) | 2 (18) | 7 (15) | |
| Multiple times almost every night | 5 (14) | 0 (0) | 5 (10) | |
| Physician Time if Called Overnight | <5 Minutes | 0 (0) | 2 (18) | 2 (4) |
| 5–15 Minutes | 16 (43) | 5 (45) | 21 (44) | |
| 16–30 Minutes | 16 (43) | 4 (36) | 20 (42) | |
| 31–59 Minutes | 5 (14) | 0 (0) | 5 (10) | |
| ≥60 Minutes | 0 (0) | 0 (0) | 0 (0) | |
| Trainees Involved in EEG Interpretation | None | 5 (14%) | 6 (55%) | 11 (23%) |
| Child Neurology Residents | ||||
| Weekdays | 12 (32%) | 2 (18%) | 14 (29%) | |
| Weeknights | 15 (41%) | 0 (0%) | 15 (31%) | |
| Weekend Days | 11 (30%) | 0 (0%) | 11 (23%) | |
| Weekend Nights | 13 (35%) | 0 (0%) | 13 (27%) | |
| Adult Neurology Residents | ||||
| Weekdays | 6 (16%) | 2 (18%) | 8 (17%) | |
| Weeknights | 6 (16%) | 1 (9%) | 7 (15%) | |
| Weekend Days | 4 (10%) | 1 (9%) | 5 (10%) | |
| Weekend Nights | 4 (10%) | 1 (9%) | 5 (10%) | |
| EEG/Epilepsy/Neurophysiology Fellows | ||||
| Weekdays | 25 (68%) | 5 (45%) | 30 (63%) | |
| Weeknights | 15 (41%) | 3 (27%) | 18 (38%) | |
| Weekend Days | 18 (49%) | 3 (27%) | 21 (44%) | |
| Weekend Nights | 16 (43%) | 3 (27%) | 19 (40%) | |
| Neurocritical Care Fellows | ||||
| Weekdays | 1 (3%) | 0 (0) | 1 (2%) | |
| Weeknights | 1 (3%) | 0 (0) | 1 (2%) | |
| Weekend Days | 1 (3%) | 0 (0) | 1 (2%) | |
| Weekend Nights | 1 (3%) | 0 (0) | 1 (2%) | |
| EEG Technologist Characteristics | ||||
| Certified - ABRET | None Certified | 1 (3) | 1 (9) | 2 (4) |
| Some Certified | 7 (19) | 0 (0) | 7 (15) | |
| Most or All Certified | 27 (73) | 10 (91) | 37 (77) | |
| Unknown | 2 (5) | 0 (0) | 2 (4) | |
| Certified - Continuous Long-Term Monitoring Certified | None Certified | 3 (8) | ||
| Some Certified | 20 (56) | |||
| Most or All Certified | 6 (17) | |||
| Unknown | 7 (19) | |||
| Certified - Neuroanalyst | None Certified | 14 (38) | ||
| Some Certified | 10 (27) | |||
| Most or All Certified | 3 (8) | |||
| Unknown | 10 (27) | |||
| Technologist Continuing Education | Weekly Conference | 13 (35) | 3 (27) | 16 (33) |
| Monthly Conference | 9 (24) | 3 (27) | 12 (25) | |
| Daily Record Review | 10 (27) | 2 (18) | 12 (25) | |
| Specific Approach Targeting Weekend and Night Technologists | 10 (27) | 2 (18) | 12 (25) | |
| Technologist Availability | Not Available 24/7 | 2 (5) | 9 (82) | 11 (23) |
| Available 24/7 - Always In Hospital | 21 (57) | 0 (0) | 21 (44) | |
| Available 24/7 - Combination In Hospital and On Call | 6 (16) | 0 (0) | 6 (13) | |
| Available 24/7 - Always On Call | 8 (22) | 2 (18) | 10 (21) | |
| Technologist Availability | Weekdays In Hospital | 37 (100) | 11 (100) | 48 (100) |
| Weekdays On Call | 1 (3) | 1 (9) | 2 (4) | |
| Weekdays Not Available | 0 (0) | 0 (0) | 0 (0) | |
| Weeknights In Hospital | 32 (86) | 1 (9) | 33 (69) | |
| Weeknights On Call | 6 (16) | 2 (18) | 8 (17) | |
| Weeknights Not Available | 1 (3) | 5 (45) | 6 (13) | |
| Weekend Days In Hospital | 36 (97) | 2 (18) | 38 (79) | |
| Weekend Days On Call | 4 (11) | 8 (73) | 12 (25) | |
| Weekend Days Not Available | 0 (0) | 0 (0) | 0 (0) | |
| Weekend Nights In Hospital | 31 (84) | 1 (9) | 32 (67) | |
| Weekend Nights On Call | 8 (22) | 3 (27) | 11 (23) | |
| Weekend Nights Not Available | 1 (3) | 4 (36) | 5 (10) | |
| Technologist Work | Technical Work | 37 (100) | 11 (100) | 48 (100) |
| Screen CEEG | 26 (70) | 6 (55) | 32 (67) | |
| Communicate Urgent Findings to EEG Clinician | 27 (73) | 7 (64) | 34 (71) | |
| Communicate Urgent Findings to ICU Clinicians | 3 (8) | 0 (0) | 3 (6) | |
| Collect Clinical Data | 15 (41) | 6 (55) | 21 (44) | |
| Generate Preliminary Reports | 1 (3) | 0 (0) | 1 (2) | |
| ICU EEG Workflow | ||||
| Education Regarding CEEG Indications | Clinician Knowledge | 28 &76) | 10 (91) | 38 (79) |
| Lectures for Neurologists | 23 (62) | 5 (45) | 28 (58) | |
| Lectures for ICU Clinicians | 21 (57) | 7 (64) | 28 (58) | |
| Lectures for ICU Nurses | 7 (19) | 3 (27) | 10 (21) | |
| Daily Census / List Review | 1 (3) | 2 (18) | 3 (6) | |
| CEEG Embedded in Ordersets in Electronic Medical Record | 10 (27) | 3 (27) | 13 (27) | |
| Best Practice Alerts in Electronic Medical Record | 2 (5) | 0 (0) | 2 (4) | |
| Approval to Initiate CEEG | ICU Clinicians Never Need Approval | 13 (35) | 0 (0) | 13 (27) |
| ICU Clinicians Sometimes Need Approval | 10 (27) | 2 (18) | 12 (25) | |
| ICU Clinicians Always Need Approval | 14 (38) | 9 (82) | 23 (48) | |
| CEEG Initiation Availability | Sometimes Available | 0 (0) | 2 (18) | 2 (4) |
| Mostly Available | 5 (14) | 7 (64) | 12 (25) | |
| Almost Always Available | 32 (86) | 2 (18) | 34 (71) | |
| Electrodes Applied | Technologists | 37 (100) | 11 (100) | 48 (100) |
| Nurses | 0 (0) | 0 (0) | 0 (0) | |
| Neurology or ICU Clinicians | 0 (0) | 0 (0) | 0 (0) | |
| Limited Array Montages | Almost No Patients | 23 (62) | 8 (73) | 31 (65) |
| Some Patients Depending on Scenario | 14 (38) | 3 (27) | 17 (35) | |
| Almost All Patients | 0 (0) | 0 (0) | 0 (0) | |
| MRI Compatible Electrodes | Almost No Patients | 15 (41) | 7 (64) | 22 (46) |
| Some Patients Depending on Scenario | 13 (35) | 2 (18) | 15 (31) | |
| Almost All Patients | 9 (24) | 2 (18) | 11 (23) | |
| Electrode Caps | Some Patients Depending on Scenario | 1 (3) | 0 (0) | 1 (2) |
| Technologist Reviews Initial Portion Quickly (Within 1 Hour) | Almost Never | 5 (14) | 2 (18) | 7 (15) |
| Sometimes | 3 (8) | 2 (18) | 5 (11) | |
| Mostly | 3 (8) | 4 (36) | 7 (15) | |
| Almost Always | 25 (69) | 3 (27) | 28 (60) | |
| Physician Reviews Initial Portion Quickly (Within 1 Hour) | Sometimes | 6 (16) | 2 (18) | 8 (17) |
| Mostly | 7 (19) | 4 (36) | 11 (23) | |
| Almost Always | 24 (65) | 5 (45) | 29 (60) | |
| Physician Performing Initial Review | Attending - Electroencephalographer | 24 (65) | 4 (36) | 28 (58) |
| Attending - Neurologist | 3 (8) | 3 (27) | 6 (13) | |
| Fellow - Electroencephalography | 8 (22) | 4 (36) | 12 (25) | |
| Resident - Neurology | 2 (5) | 0 (0) | 2 (4) | |
| EEG Pages Reviewed by Technologist (among technologists involved in screening) | 1–25% of Pages | 0 (0) | 2 (25) | 2 (6) |
| 26–50% of Pages | 1 (4) | 0 (0) | 1 (3) | |
| 51–75% of Pages | 1 (4) | 0 (0) | 1 (3) | |
| 76–99% of Pages | 7 (27) | 2 (25) | 9 (26) | |
| All Pages | 17 (65) | 4 (50) | 21 (62) | |
| EEG Pages Reviewed by Physician | 1–25% of Pages | 3 (8) | 0 (0) | 3 (6) |
| 26–50% of Pages | 2 (5) | 0 (0) | 2 (4) | |
| 51–75% of Pages | 1 (3) | 0 (0) | 1 (2) | |
| 76–99% of Pages | 7 (19) | 2 (18) | 9 (19) | |
| All Pages | 24 (65) | 9 (82) | 33 (69) | |
| Technologist Review Frequency (among technologists involved in screening) | 1 Per Day | 1 (3) | 2 (29) | 3 (8) |
| 2 Per Day | 1 (3) | 4 (57) | 5 (14) | |
| 3 Per Day | 1 (3) | 0 (0) | 1 (3) | |
| 4 Per Day | 2 (7) | 0 (0) | 2 (6) | |
| 6 Per Day | 3 (10) | 0 (0) | 3 (8) | |
| 12 Per Day | 4 (14) | 0 (0) | 4 (11) | |
| About Hourly | 4 (14) | 0 (0) | 4 (11) | |
| Nearly Continuously | 13 (45) | 1 (14) | 14 (39) | |
| Physician Review Frequency | 1 Per Day | 1 (3) | 0 (0) | 1 (2) |
| 2 Per Day | 8 (22) | 3 (27) | 11 (23) | |
| 3 Per Day | 9 (24) | 3 (27) | 12 (25) | |
| 4 Per Day | 7 (19) | 2 (18) | 9 (19) | |
| 6 Per Day | 8 (22) | 3 (17) | 11 (23) | |
| 12 Per Day | 0 (0) | 0 (0) | 0 (0) | |
| About Hourly | 2 (5) | 0 (0) | 2 (4) | |
| Nearly Continuously | 2 (5) | 0 (0) | 2 (4) | |
| Review Frequency Approach | Same Basic Approach for All Patients | 21 (57) | 5 (45) | 26 (54) |
| Individualized Based on Clinical and EEG Variables | 16 (43) | 6 (55) | 22 (46) | |
| Patients Concurrently Screened Per Technologist During Day (among technologists involved in screening during the day) | 1–2 Patients | 1 (4) | 3 (60) | 4 (13) |
| 3–4 Patients | 17 (63) | 1 (20) | 18 (56) | |
| 5–8 Patients | 7 (26) | 1 (20) | 8 (25) | |
| 9–12 Patients | 2 (7) | 0 (0) | 2 (6) | |
| ≥13 Patients | 0 (0) | 0 (0) | 0 (0) | |
| Patients Concurrently Screened Per Technologist During Night (among technologists involved in screening during the night) | 1–2 Patients | 0 (0) | 0 (0) | 0 (0) |
| 3–4 Patients | 10 (38) | 0 (0) | 10 (37) | |
| 5–8 Patients | 10 (38) | 0 (0) | 10 (37) | |
| 9–12 Patients | 5 (19) | 0 (0) | 5 (19) | |
| ≥13 Patients | 1 (4) | 1 (100) | 2 (7) | |
| CEEG Discontinuation Decisions Approach | No Specific Criteria | 19 (51) | 2 (18) | 21 (44) |
| Protocol / Pathway / Algorithm | 7 (19) | 6 (55) | 13 (27) | |
| Individualized Approach | 11 (30) | 3 (27) | 14 (29) | |
| Decision To End CEEG | Protocol / Pathway / Algorithm | 9 (24) | 3 (27) | 12 (25) |
| Critical Care Team | 15 (41) | 1 (9) | 16 (33) | |
| NeuroICU Consult Team | 37 (100) | 11 (100) | 48 (100) | |
| EEG Team | 10 (27) | 0 (0) | 10 (21) | |
| CEEG Remote Availability | Almost No Patients | 2 (5) | 0 (0) | 2 (4) |
| Most Patients | 1 (3) | 1 (9) | 2 (4) | |
| Almost All Patients | 34 (92) | 10 (91) | 44 (92) | |
| Video Availability | Some Patients | 0 (0) | 1 (9) | 1 (2) |
| Most Patients | 0 (0) | 1 (9) | 1 (2) | |
| Almost All Patients | 37 (100) | 9 (82) | 46 (96) | |
| Reactivity Assessment Standards | None | 17 (46) | 7 (64) | 23 (50) |
| Frequency of Assessment | 10 (27) | 1 (9) | 11 (23) | |
| Stimulus for Assessment | 16 (43) | 4 (36) | 20 (42) | |
| EEG Changes Required for Determination | 8 (22) | 1 (9) | 9 (19) | |
| Reactivity Assessment Procedure | Not Performed Routinely | 6 (16) | 6 (55) | 12 (25) |
| Specific Procedure by Technologists | 22 (59) | 4 (36) | 26 (54) | |
| Specific Procedure by ICU or Neurology Clinicians | 2 (5) | 0 (0) | 2 (4) | |
| No Specific Procedure and Assessed During Care | 7 (19) | 1 (9) | 8 (17) | |
| Reports and Communication | ||||
| Report Frequency | Daily | 33 (94) | 7 (64) | 40 (87) |
| More Than 1 Per Day | 2 (6) | 0 (0) | 2 (4) | |
| Less Than Daily | 0 (0) | 4 (36) | 4 (9) | |
| Templated Report Use | Almost Never | 3 (9) | 4 (36) | 7 (15) |
| Rarely | 2 (6) | 0 (0) | 2 (4) | |
| Sometimes | 3 (9) | 4 (36) | 7 (15) | |
| Mostly | 9 (26) | 0 (0) | 9 (20) | |
| Almost Always | 18 (51) | 3 (27) | 21 (46) | |
| ACNS Terminology in Templated Reports | Almost All ACNS Terminology | 15 (48) | 4 (57) | 19 (50) |
| Some ACNS Terminology | 16 (52) | 2 (29) | 18 (47) | |
| Almost No ACNS Terminology | 0 (0) | 0 (0) | 0 (0) | |
| Policy to Convey CEEG Findings in Real Time | Yes | 22 (63) | 4 (36) | 26 (57) |
| Technologist Communication for Urgent Findings to Whom | Technologists Do Not Screen EEG | 6 (17) | 3 (27) | 9 (19) |
| EEG Clinician | 19 (53) | 6 (55) | 25 (53) | |
| Neuro Consultation Team | 11 (31) | 2 (18) | 13 (28) | |
| ICU Team | 0 (0) | 0 (0) | 0 (0) | |
| Technologist Communication for Which Urgent Findings | Technologists Do Not Screen EEG | 7 (19) | 4 (36) | 11 (23) |
| Technologist Discretion | 8 (22) | 3 (27) | 11 (23) | |
| Seizures | 20 (54) | 5 (45) | 25 (52) | |
| Status Epilepticus | 20 (54) | 5 (45) | 25 (52) | |
| Background Change | 20 (54) | 3 (27) | 23 (48) | |
| Individualized Criteria for Each Patient | 18 (49) | 1 (9) | 19 (40) | |
| EEG Clinician Communication for Urgent Findings | EEG Clinician Calls Critical Care Team | 3 (8) | 0 (0) | 3 (6) |
| EEG Clinician Calls NeuroICU Consult Team | 27 (75) | 10 (91) | 37 (79) | |
| EEG Clinician is Also NeuroICU Consult Team | 6 (17) | 1 (9) | 7 (15) | |
| Meeting / Huddle / Rounds to Discuss CEEG | Never | 9 (25) | 8 (73) | 17 (36) |
| Once Per Day | 16 (44) | 0 (0) | 16 (34) | |
| Twice Per Day | 3 (8) | 1 (9) | 4 (9) | |
| Weekly | 7 (19) | 0 (0) | 7 (15) | |
| Huddle Attendees | Multiple Technologists Covering Shift | 9 (19) | 1 (9) | 8 (17) |
| Lead Technologist Covering Shift | 5 (14) | 0 (0) | 5 (10) | |
| EEG Attending | 23 (62) | 3 (27) | 26 (54) | |
| EEG Fellow | 20 (54) | 2 (18) | 22 (46) | |
| ICU Clinicians | 3 (8) | 1 (9) | 4 (8) | |
| NeuroICU Consult Clinicians | 20 (54) | 2 (18) | 22 (46) | |
| Seizure Management | ||||
| Anti-Seizure Medication Planning | Selected and Ordered if Seizures Occur | 17 (46) | 7 (64) | 25 (50) |
| Selected but Not Ordered Unless Seizures Occur | 7 (19) | 3 (27) | 10 (21) | |
| Selected and Ordered in Case Seizures Occur | 12 (32) | 1 (9) | 13 (27) | |
| Anti-Seizure Medication Selection | Clinicians Make Plan if Seizures Occur | 21 (57) | 7 (64) | 28 (58) |
| Guided by Pathway / Guideline / Algorithm | 19 (51) | 6 (55) | 25 (52) | |
| Individualized Seizure Action Plan | 17 (46) | 2 (18) | 19 (40) | |
| First Anti-Seizure Medication | Diazepam | 1 (3) | 0 (0) | 1 (2) |
| Fosphenytoin | 1 (3) | 1 (9) | 2 (4) | |
| Levetiracetam | 14 (38) | 6 (55) | 20 (42) | |
| Lorazepam | 20 (54) | 3 (27) | 23 (48) | |
| Midazolam | 0 (0) | 1 (9) | 1 (2) | |
| Second Anti-Seizure Medication | Fosphenytoin | 12 (32) | 2 (18) | 14 (29) |
| Lacosamide | 4 (11) | 0 (0) | 4 (8) | |
| Levetiracetam | 12 (32 | 4 (36) | 16 (33) | |
| Midazolam | 2 (5) | 0 (0) | 2 (4) | |
| Phenobarbital | 1 (3) | 0 (0) | 1 (2) | |
| Phenytoin | 3 (8) | 5 (45) | 8 (17) | |
| Valproate | 1 (3) | 0 (0) | 1 (2) | |
| Treatment Goal | No Treatment for Non-Convulsive Seizures | 1 (3) | 0 (0) | 1 (2) |
| Tolerate Some Brief Non-Convulsive Seizures | 15 (41) | 1 (9) | 16 (33) | |
| Aim to Terminate All Non-Convulsive Seizures | 21 (57) | 10 (91) | 31 (65) | |
| Quantitative EEG | ||||
| Quantitative EEG Utilization | Not Used | 11 (30) | 2 (18) | 13 (27) |
| Used by EEG Reader for Some Patients | 14 (38) | 5 (45) | 19 (40) | |
| Used by EEG Reader for Most Patients | 12 (32) | 2 (18) | 14 (29) | |
| Used by Bedside Clinician for Some Patients | 3 (8) | 3 (27) | 6 (13) | |
| Used by Bedside Clinician for Most Patients | 3 (8) | 1 (9) | 4 (8) | |
| Quantitative EEG Use by EEG Reader | Seizure Identification | 25 (68) | 5 (45) | 30 (63) |
| Ischemia Identification | 11 (30) | 1 (9) | 12 (25) | |
| Targeting Burst-Suppression | 15 (41) | 3 (27) | 18 (38) | |
| Prognosis | 3 (8) | 0 (0) | 3 (6) | |
| Quantitative EEG Use by Bedside Clinician | Seizure Identification | 6 (16) | 3 (27) | 9 (19) |
| Ischemia Identification | 2 (5) | 0 (0) | 2 (4) | |
| Targeting Burst-Suppression | 5 (14) | 0 (0) | 5 (10) | |
| Prognosis | 1 (3) | 0 (0) | 1 (2) | |
| Quality Improvement | ||||
| Ongoing Quality Improvement | None | 23 (62) | 10 (91) | 33 (69) |
| Timing from Order to CEEG Initiation | 11 (30) | 0 (0) | 11 (23) | |
| Timing from Seizure Onset to Identification | 8 (22) | 0 (0) | 8 (17) | |
| Timing of Team Notification Regarding Seizures | 8 (22) | 0 (0) | 8 (17) | |
| Timing of Anti-Seizure Medication Administration | 9 (24) | 0 (0) | 9 (19) | |
| Proportion of Appropriate Patients Undergoing CEEG | 4 (11) | 0 (0) | 4 (8) | |
| Affiliated Institution Monitoring and Companies | ||||
| Affiliated Institution Monitoring | None | 16 (43) | 8 (73) | 24 (50) |
| PICU at 1 Affiliate | 2 (5) | 1 (9) | 3 (6) | |
| PICU at ≥2 Affiliates | 10 (27) | 0 (0) | 10 (21) | |
| NICU at 1 Affiliate | 7 (19) | 2 (18) | 9 (19) | |
| NICU at ≥2 Affiliates | 13 (35) | 0 (0) | 13 (27) | |
| CICU at 1 Affiliate | 1 (3) | 0 (0) | 1 (2) | |
| CICU at ≥2 Affiliates | 2 (5) | 0 (0) | 2 (4) | |
| Company for CEEG | No | 33 (89) | 11 (100) | 44 (92) |
| Rare Patients | 2 (5) | 0 (0) | 2 (4) | |
| Some Patients | 2 (5) | 0 (0) | 2 (4) | |
Some questions allowed multiple responses. Some questions were missing responses from a small number of respondents.
Figure 1.

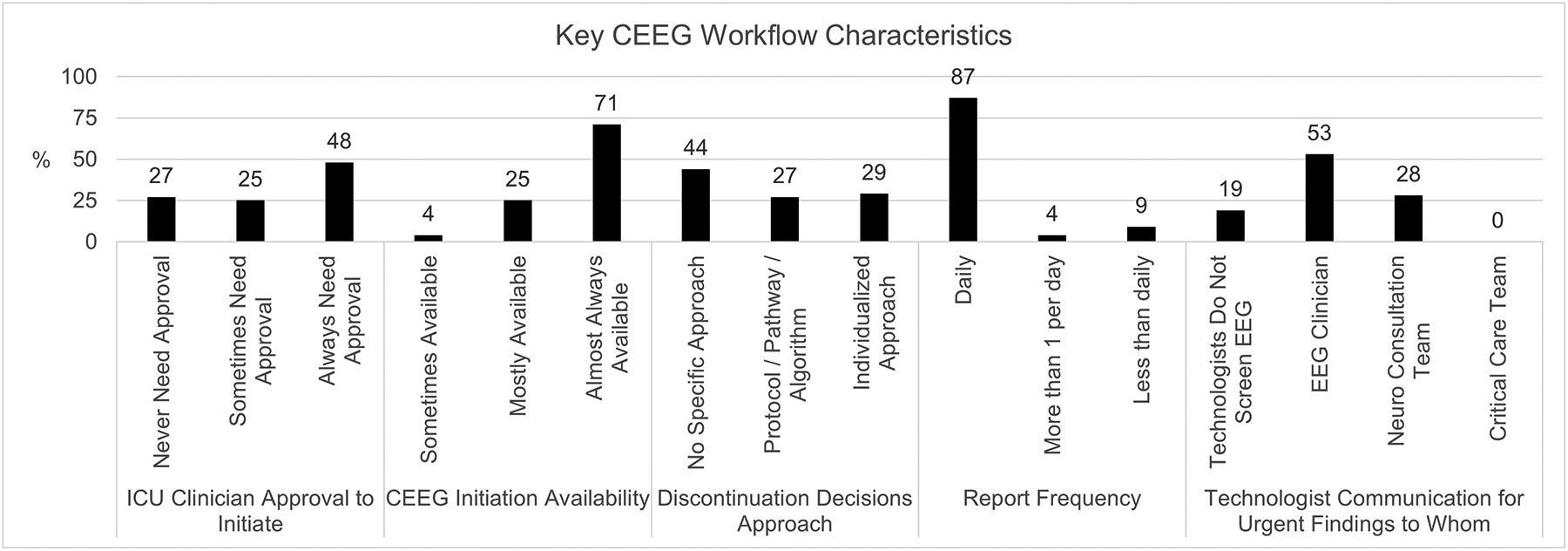
Key institutional (1A) and and workflow (1B) findings.
The institutions were primarily academic medical centers (94%) with 11–50 PICU beds (81%) and separate Pediatric and Cardiac ICUs (75%). All institutions had neurologic consultations available in the PICU. Consulting neurologists were generally not specialized in Neuro-ICU care (69%). Written pathways regarding CEEG were available at 68% of institutions, divided between institutions with pathways for many conditions (32%) or a few conditions (36%).
CEEG Availability, Volume and Key Limitations
CEEG was almost always available at 86% of US institutions and 18% of Canadian institutions. A median of 40 (interquartile range 25–70) and 10 (interquartile range 5–15) patients underwent CEEG per month at institutions in the US and Canada, respectively. The main limitations to performing additional CEEG were insufficient technologists (67%), equipment (52%), and electroencephalographers (19%).
Physician Characteristics and Coverage
Often one attending physician simultaneously covered the Epilepsy Monitoring Unit and ICU EEG services both day and night (48%). Some institutions had separate coverage during the day and night (33%). Attendings covered 5–7 nights (58%), 1–2 nights (19%), or 3–4 nights (13%) in a row. There was variability in the number of times attendings estimated being awoken each week: 29% awoken 1–2 nights/week, 23% awoken 3–4 nights/week, and 15% awoken nightly. When called overnight, respondents estimated the work required 5–15 minutes (44%) or 16–30 minutes (42%). Epilepsy fellows were often involved in EEG interpretation on weekdays (63%) but less often weeknights and weekends (38–44%). Child neurology residents were less involved in EEG interpretation across all times (23–31%).
Technologist Characteristics and Coverage
In the US, 95% of institutions had technologists available 24/7, including in-hospital 24/7 at 57% of institutions and through a combination of in-hospital and on-call coverage at 38% of institutions, while only 5% had times without technologist coverage. US institutions had in-hospital technologist coverage on weekdays (100%), weeknights (86%), weekend days (97%), and weekend nights (84%), and the remaining times were covered by on-call technologists. In Canada, 82% of institutions had periods without technologist availability, and no institutions had 24/7 in-hospital availability. Technologists in Canada were available in-hospital on weekdays (100%) and on-call on weekend days (73%), but there was more limited availability weeknights (45%) and weekend nights (36%).
Technologists applied electrodes at all institutions, and technologists often screened CEEG (67%) and collected clinical data (44%). Specific approaches to technologist education were rare, including weekly conferences (33%), monthly conferences (25%), or daily record reviews with attendings (25%).
CEEG Indications
Clinicians reported CEEG indications that generally aligned with the main American Clinical Neurophysiology Society indications19 (84–100%), except for the identification of cerebral ischemia (42%) (Table 2). Identification of patients who should undergo CEEG generally relied on individual clinician knowledge (79%), but some institutions had educational lectures addressing ICU CEEG indications for intensivists (58%), neurologists (58%), and ICU nurses (21%). CEEG was rarely embedded into specific order sets (27%) or best practice alerts (4%). Only 6% of institutions identified patients requiring CEEG by daily census review.
Table 2.
Indications for Continuous EEG Monitoring.
| Indications | % |
|---|---|
| American Clinical Neurophysiology Society Indications | |
| Diagnosis of Nonconvulsive Seizures and Status Epilepticus | 100% |
| Diagnosis of Paroxysmal Events | 94% |
| Assessment of Efficacy of Therapy for Seizures and Status Epilepticus | 92% |
| Identification of Cerebral Ischemia | 42% |
| Monitoring of Sedation and High-Dose Suppressive Therapy | 84% |
| Assessment of Severity of Encephalopathy and Prognostication | 86% |
| Other Indications | |
| Persistently Abnormal MS Following Convulsive Status Epilepticus | 100% |
| Persistently Abnormal Mental Status Following Clinically Evident Seizures (not Constituting Status Epilepticus) | 98% |
| Acute Supratentorial Brain Injury with Altered Mental Status | 88% |
| Fluctuating or Unexplained Alteration of Mental Status without Known Acute Brain Injury | 96% |
| Generalized, Lateralized, or Bilateral Independent Periodic Discharges on Routine EEG | 72% |
| Requirement for Pharmacological Paralysis and Risk for Seizures | 98% |
| Clinical Paroxysmal Events Suspected to be Seizures | 98% |
| Pathways / Algorithms / Ordersets Include CEEG | |
| Cardiac Arrest | 44% |
| CAR T-Cell Therapy | 14% |
| Central Nervous System Infections | 12% |
| Congenital Heart Disease - Neonate with Surgery Requiring Bypass | 36% |
| Congenital Heart Disease - Neonate with Surgery Not Requiring Bypass | 20% |
| Extracorporeal Membrane Oxygenation | 54% |
| Neuro-Inflammatory Conditions | 16% |
| Paralyzed Patient | 22% |
| Sepsis | 10% |
| Status Epilepticus | 74% |
| Stroke - Ischemic | 24% |
| Stroke - Hemorrhagic | 24% |
| Traumatic Brain Injury | 38% |
CEEG Process – Initiation, Screening, and Discontinuation
Neurologist approval for CEEG initiation was always needed at only 38% of US institutions but 82% of Canadian institutions.
Technologists reviewed the initial portion of EEG quickly (defined as within one hour) to identify seizures or major abnormalities almost always at 69% and 27% of US and Canadian institutions, respectively. Similarly, physicians reviewed the initial portion of EEG quickly almost always at 65% and 45% of US and Canadian institutions, respectively. The initial EEG physician review was generally completed by EEG attendings (65% US; 36% Canada).
Physicians screened 100% of pages at 69% of institutions. Screening frequency was standardized for all patients at 54% of institutions or individualized based on patient variables at 46% of institutions. Physicians screened EEG 2–6 times per day (19–25%). Technologists engaged in screening EEG at 67% of institutions, and technologists screened all EEG pages at 62% of institutions. Screening frequency by technologists ranged from once per day (8%) to nearly continuously (39%) (Figure 2). During the day, technologists screened EEG for 3–4 patients (56%) or 5–8 patients (25%). During the night, technologists screened EEG for 3–4 patients (37%), 5–8 patients (37%), or 9–12 patients (19%).
Figure 2.

CEEG review frequency (by technologists and physicians) and approach.
CEEG was usually performed for 24 hours (70%) if screening for ES in patients without EEG risk factors for seizures and longer in patients with EEG risk factors for seizures (24 hours in 52%; 48 hours in 32%) (Figure 3a and 3b). Among patients with ES, CEEG was usually continued for 24 hours after the last seizure (76%) (Figure 3c). Under ideal circumstances (without resource limitations), 48 hours of CEEG was preferred when screening for ES among patients with EEG risk factors for seizures (42%) and after ES terminated (26%). The CEEG duration was guided by patient characteristics without specific criteria (44%), by pathways (27%), or by systematic tailored approaches (29%). Decisions to end CEEG were most often made by the Neuro-ICU Consult Team (100%) with rare guidance from pathways (25%) or other teams (21–33%).
Figure 3.



Continuous EEG monitoring (CEEG) durations. Black bars represent current practice and gray bars represent ideal practice.
3A. CEEG duration when screening for electrographic seizures in patients with no EEG risk factors.
3B. CEEG duration when screening for electrographic seizures in patients with EEG risk factors.
3C. CEEG duration after electrographic seizures terminate.
Remote access and time-locked video were almost always available at 92% and 96% of institutions, respectively. Reactivity assessment was performed by technologists using a specific procedure at only 50% of institutions. Some institutions standardized reactivity stimuli (42%), assessment frequency (23%), and EEG changes constituting reactivity (19%).
Communication and Reports
Reports were generated daily at 94% and 64% of institutions in the US and Canada, respectively. American Clinical Neurophysiology Society terminology24 was used in almost all (50%) or some (47%) reports, but only 46% of institutions almost always used templated reports.
There was a policy regarding urgent result communication at 57% of institutions. Technologists most often communicated urgent EEG findings to an EEG clinician (53%) who then communicated the findings to the Neuro-ICU Consult Team (79%).
Respondents at 43% of institutions indicated that they have meetings to discuss EEG findings 1–2 times per day with technologists, EEG attendings and fellows, and/or Neuro-ICU Consult clinicians.
Seizure Management
Anti-seizure medications (ASM) were selected and ordered when seizures occurred (50%), selected in advance but not ordered (21%), or both selected and ordered in advance (27%) (Figure 4). ASM selection was guided by an institutional pathway (52%) or an individualized seizure action plan (40%). The most common first-line ASM administered for ES were lorazepam (48%) and levetiracetam (42%). Second-line ASM selections were more variable, including levetiracetam (33%), fosphenytoin (29%), phenytoin (17%), or lacosamide (8%). Clinicians usually (65%) aimed to terminate all non-convulsive seizures.
Figure 4.
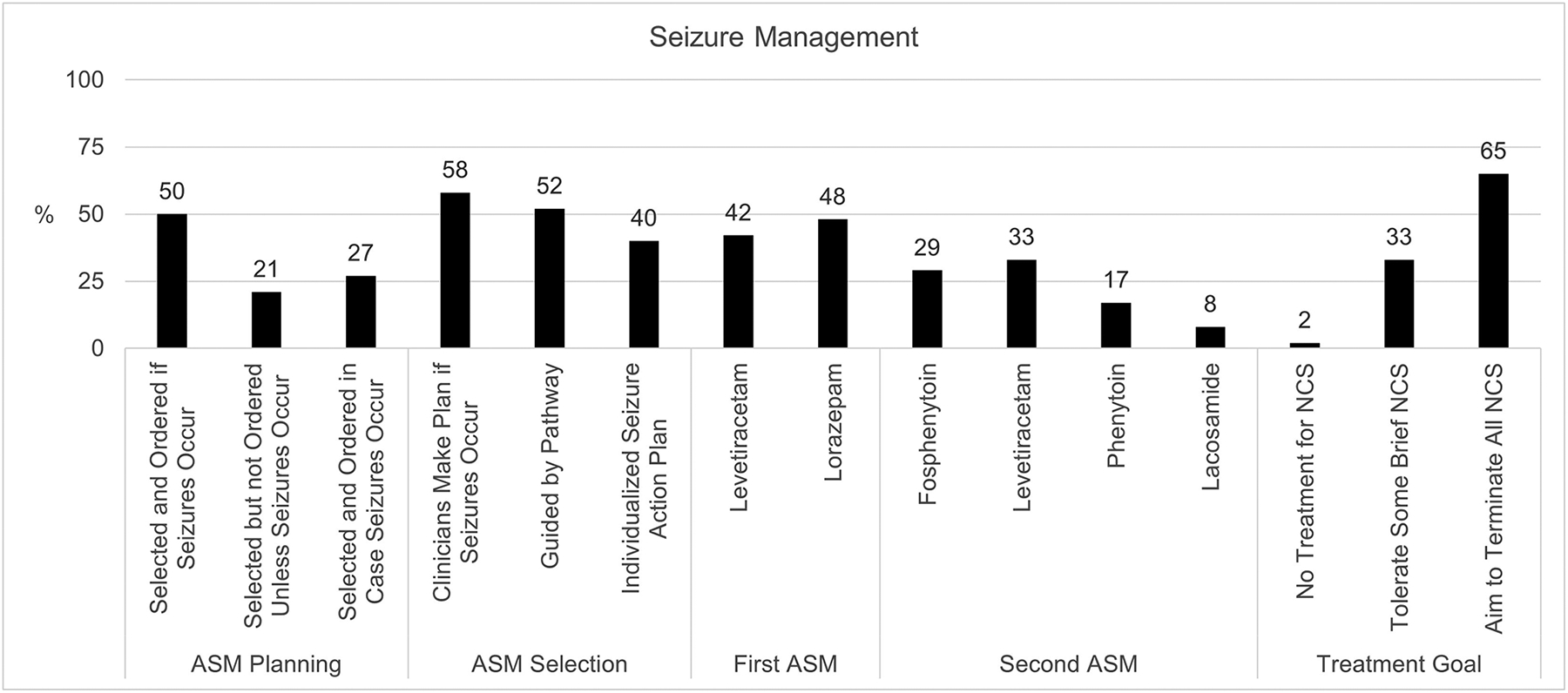
Anti-seizure medication (ASM) planning, selection, and treatment goals.
Quantitative EEG, Quality Improvement, and Affiliated Institutions
QEEG was used by electroencephalographers for some patients (40%) or most patients (29%), most commonly for seizure identification (63%). QEEG was used less by bedside clinicians (21%).
There were ongoing QI projects related to ICU CEEG processes at 31% of institutions. Fifty percent of institutions performed CEEG for affiliated institutions (57% US; 27% Canada), and 8% used external companies for ICU CEEG.
Changes from 2011 to 2022
Addendum Figure 1 summarizes key changes from 20111 to 2022, including an increase in the number of patients undergoing CEEG per month, an increase in the proportion of centers with ICU CEEG pathways, and variable changes in CEEG availability. There was increased involvement of technologists in EEG screening but variable changes in technologist review frequency.
Discussion
This survey regarding institutional ICU CEEG resources and practice was intended as an update to a survey performed in 20111 given substantial literature expansion and publication of guidelines and consensus statements during the intervening decade.18–20 While the 77% response rate was slightly lower than the 2011 survey, it is on the high end of the 5–95% range reported by recent similar surveys1, 25–32 and likely provides a reasonable representation of large academic institutions in the US and Canada.
There are several key findings. First, CEEG use has increased 3–4 fold over the last decade (US use increased from 10 to 40 patients per month; Canadian use increased from 3 to 10 patients per month).1 This increase likely reflects increasing data describing the high incidence of ES in critically ill children,2–17 data establishing an association between high ES exposure and outcome,6, 7, 10, 15, 16 publication of consensus statements and guidelines which call for the broad use of CEEG,18–20 and studies identifying many PICU patients meet criteria for CEEG initiation.33, 34 These findings are consistent with surveys addressing adult ICU CEEG monitoring26, 27, 30 and observational data of PICU patients undergoing CEEG.35
Second, the indications for CEEG endorsed by respondents generally aligned with recommendations from consensus statements and guidelines,18–20 although the use of CEEG to identify ischemia was uncommon. The current indications were similar to the 2011 survey data, except that there was increased monitoring of patients with unexplained altered mental status without a known acute brain injury. This may reflect increased recognition that ES may occur with systemic disorders in the absence of clinically evident convulsions. Educational initiatives regarding CEEG indications and implementation of neurocritical care programs may increase utilization.33, 36
Respondents endorsed less frequent inclusion of CEEG into care pathways for specific etiologic conditions, suggesting that they may consider the overall clinical scenario rather than solely the etiologic diagnosis when deciding whether CEEG is indicated. Targeted CEEG use based on clinical and EEG risk factors37–40 may reduce CEEG utilization but may also fail to identify ES in some patients. In contrast to our survey of neurologists, a 2021 survey of pediatric intensivists and neurologists focused on neurocritical care reported higher use of CEEG for specific etiologic conditions such as cardiac arrest and traumatic brain injury.31 This difference suggests that ICU-focused providers may use broader screening CEEG approaches.
Third, most US institutions always had technologist coverage available in-hospital or on-call. The decreased availability of technologists on-call may have shifted in favor of increased in-hospital availability. In contrast, most Canadian institutions did not always have technologists available, particularly on evenings and weekends. Similar limitations regarding CEEG access have been observed in surveys in many countries focused on both pediatric and adult care.26–30, 41
Fourth, screening of the initial EEG within one hour by both technologists and physicians was reported as less common now than in 2011.1 This change may relate to increasing ICU EEG demand with insufficient technologist and physician availability. Given ES are particularly common near CEEG initiation,2, 4, 5, 8, 9, 11, 39, 40 approaches to screen CEEG early after initiation may be beneficial. Both technologists and physicians screened the ongoing CEEG recording more frequently than in 2011, although screening remained periodic. Expansion of ICU CEEG capacity and timely interpretation may require workflow modifications, involvement of non-technologists, and development of QEEG strategies.
There is increasing evidence that QEEG may enable effective, albeit imperfect, seizure detection after brief training programs.42 Respondents indicated that QEEG was used for some or most patients by 69% of electroencephalographers and only 21% of bedside clinicians. These findings are consistent with prior surveys.25–27, 29, 31, 43 Physicians reported reading all or most pages of CEEG, suggesting QEEG implementation may not be substantially reducing the time required to screen CEEG in current practice. The use of QEEG by pediatric intensivists at the bedside is sensitive for ES detection, including for rapid detection when neurologists were unavailable.44
Fifth, the use of written pathways increased from 31% in 2011 to 68%. This expansion likely reflects the accumulation of sufficient data to develop comprehensive pathways, the publication of guidelines and consensus statements,18–20 critical care EEG educational initiatives, and an overall trend across institutions toward pathway-driven care. However, a recent survey of practice in 27 countries indicated CEEG protocols were less available in PICUs than adult Neuro-ICUs.30
Ten other findings deserve mention. First, there was a substantial increase in the proportion of institutions that could obtain CEEG without neurology approval in the US, indicating CEEG is becoming a standard diagnostic service. Second, remote access to CEEG remained widely available in the US and had increased substantially in Canada. Third, while all institutions had Neuro-ICU consultation available, consulting neurologists were not specifically focused on Neuro-ICU care. This may be evolving with development of the inpatient pediatric neurology fields.32, 45 Fourth, many institutions separated physician coverage of ICU EEG and EMU. This may reflect increasing ICU EEG volume and/or increasing sub-specialization. Fifth, attendings generally cover 5–7 days in a row and were often awoken at night. Increasing ICU CEEG volume along with efforts to rapidly detect and manage ES may underlie this increasing overnight workload, potentially necessitating modification of current care and practice models for ICU EEG. Sixth, while there was a slight increase in the number of institutions with policies guiding communication, dissemination of urgent EEG findings often involved multiple steps in which the technologist called the EEG service and then the EEG service called the Neuro-ICU consult service. Direct communication between the EEG service and the ICU clinicians was rare and had decreased since 2011 (18% to 8%).1 This is particularly striking given the increase in CEEG initiation without neurology approval. Seventh, only a small proportion of institutions had interdisciplinary reading sessions attended by technologists or ICU clinicians. Expansion of these interdisciplinary sessions could provide an opportunity for continued education which may enhance inter-rater agreement between technologists.46 Similarly, if ICU clinicians use QEEG at the bedside,44 interdisciplinary conferences could enhance the use of hybrid CEEG-QEEG approaches. Eighth, ASM are mostly planned and ordered only if ES occur, using ASM consistent with status epilepticus management guidelines18, 47 which are likely safe and effective for many PICU patients with ES.48 Ninth, half of the institutions reported performing CEEG at affiliated institutions, enabling hub and spoke networks for more widespread provision of ICU CEEG services.49 Tenth, one-third of institutions had ongoing QI projects regarding CEEG utilization and clinical management. Studies indicate that targeted strategies may improve management through streamlined workflow and communication approaches.33, 50
This study has several limitations. First, there may be selection bias in assessing primarily large academic institutions which may have more access to CEEG resources and subspecialty care and may not be generalizable to all institutions providing pediatric critical care. Second, the data were derived from respondent estimates and not observational studies of contemporary practice. Third, the multiple-choice responses may not capture the full complexity of ICU CEEG practice, introducing potential information bias. Strengths of the survey include alignment with recent survey recommendations,23 a high response rate, minimal missing data among responses, and a broad geographic distribution.
Over the last decade, there has been a substantial increase in volume of ICU CEEG, as well as increased agreement regarding the indications for ICU CEEG. Further there has been increased standardization, including greater use of formal written pathways. Increasing standardization can enable learning health system approaches to assess areas of practice variability, assist institutions in sharing best practices, and shift practice towards optimized and equitable care across institutions. However, there remains substantial international and inter-institutional variability in available resources and workflow, particularly regarding technologist availability and EEG screening approaches. The identification of these practice gaps should prompt deployment of strategies to target CEEG to patients most like to benefit, development of efficient and accurate seizure detection approaches, and expansion of the workforce necessary to provide comprehensive ICU EEG services.
Supplementary Material
Addendum Figure 1. Changes in key characteristics between 2011 and 2022 for (A) US and (B) Canada.
Financial Disclosures:
France W. Fung – Reports no disclosures.
Jessica L. Carpenter – Reports no disclosures.
Kevin E. Chapman – Reports no disclosures.
William Gallentine – Reports no disclosures.
Christopher C. Giza - Grants/Research Support: Hit-IQ (2022–2023); NIH NINDS (R01 NS110757 2019–2024); NINDS (U54 NS121688 2021–2026); UCLA Brain Injury Research Center, UCLA Steve Tisch BrainSPORT program, Easton Clinic for Brain Health. Clinical Consultant (provide clinical care to athletes): NBA, NFL-Neurological Care Program, NHL/NHLPA, Los Angeles Lakers. Advisory Board (Noncompensated): Major League Soccer, National Basketball Association, US Soccer Federation. Advisory Board (Compensated): Highmark Interactive. Stock Shareholder: Highmark Interactive stock options (2018). Other Financial or Material Support: Book royalties – Blackwell/Wiley Publishing: Prioritized Neurological Differential Diagnosis.
Joshua L. Goldstein – Reports no disclosures.
Cecil D. Hahn - Takeda Pharmaceuticals (consulting), UCB Pharma (consulting), Holberg EEG (consulting), Cambridge University Press (royalties).
Tobias Loddenkemper – ‘Tobias Loddenkemper is part of pending patent applications to detect and predict seizures and to diagnose epilepsy. He receives research support from the NIH, the Epilepsy Research Fund, and received research grants from Sage, Lundbeck, Eisai, Upsher-Smith, Mallinckrodt, and Pfizer. He served as a consultant for Engage and Upsher Smith, in the past.’
Joyce H. Matsumoto – Reports no disclosures.
Craig A. Press – Reports no disclosures.
James J. Riviello, Jr. – Reports no disclosures.
Nicholas S. Abend – Funding from NIH (NINDS) K02NS096058 and Wolfson Foundation for this study. Other funding from PCORI (to institution), UCB Pharma (to institution), Epilepsy Foundation (consulting), and Demos Publishing (royalties).
Footnotes
Conflicts of Interest: None
References
- 1.Sanchez SM, Carpenter J, Chapman KE, et al. Pediatric ICU EEG monitoring: current resources and practice in the United States and Canada. J Clin Neurophysiol. 2013. Apr;30:156–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shahwan A, Bailey C, Shekerdemian L, Harvey AS. The prevalence of seizures in comatose children in the pediatric intensive care unit: a prospective video-EEG study. Epilepsia. 2010. Jul;51:1198–1204. [DOI] [PubMed] [Google Scholar]
- 3.Abend NS, Topjian A, Ichord R, et al. Electroencephalographic monitoring during hypothermia after pediatric cardiac arrest. Neurology. 2009. Jun 2;72:1931–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williams K, Jarrar R, Buchhalter J. Continuous video-EEG monitoring in pediatric intensive care units. Epilepsia. 2011. Jun;52:1130–1136. [DOI] [PubMed] [Google Scholar]
- 5.Greiner HM, Holland K, Leach JL, Horn PS, Hershey AD, Rose DF. Nonconvulsive status epilepticus: the encephalopathic pediatric patient. Pediatrics. 2012. Mar;129:e748–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kirkham FJ, Wade AM, McElduff F, et al. Seizures in 204 comatose children: incidence and outcome. Intensive Care Med. 2012. May;38:853–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abend NS, Arndt DH, Carpenter JL, et al. Electrographic seizures in pediatric ICU patients: cohort study of risk factors and mortality. Neurology. 2013. Jul 23;81:383–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCoy B, Sharma R, Ochi A, et al. Predictors of nonconvulsive seizures among critically ill children. Epilepsia. 2011. Nov;52:1973–1978. [DOI] [PubMed] [Google Scholar]
- 9.Schreiber JM, Zelleke T, Gaillard WD, Kaulas H, Dean N, Carpenter JL. Continuous video EEG for patients with acute encephalopathy in a pediatric intensive care unit. Neurocrit Care. 2012. Aug;17:31–38. [DOI] [PubMed] [Google Scholar]
- 10.Payne ET, Zhao XY, Frndova H, et al. Seizure burden is independently associated with short term outcome in critically ill children. Brain. 2014. May;137:1429–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abend NS, Gutierrez-Colina AM, Topjian AA, et al. Nonconvulsive seizures are common in critically ill children. Neurology. 2011. Mar 22;76:1071–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vlachy J, Jo M, Li Q, et al. Risk Factors for Seizures Among Young Children Monitored With Continuous Electroencephalography in Intensive Care Unit: A Retrospective Study. Front Pediatr. 2018;6:303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sansevere AJ, Duncan ED, Libenson MH, Loddenkemper T, Pearl PL, Tasker RC. Continuous EEG in Pediatric Critical Care: Yield and Efficiency of Seizure Detection. J Clin Neurophysiol. 2017. Sep;34:421–426. [DOI] [PubMed] [Google Scholar]
- 14.Sanchez SM, Arndt DH, Carpenter JL, et al. Electroencephalography monitoring in critically ill children: current practice and implications for future study design. Epilepsia. 2013. Aug;54:1419–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Topjian AA, Gutierrez-Colina AM, Sanchez SM, et al. Electrographic Status Epilepticus is Associated with Mortality and Worse Short-Term Outcome in Critically Ill Children. Crit Care Med. 2013;41:215–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wagenman KL, Blake TP, Sanchez SM, et al. Electrographic status epilepticus and long-term outcome in critically ill children. Neurology. 2014. Feb 4;82:396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanchez Fernandez I, Sansevere AJ, Guerriero RM, et al. Time to electroencephalography is independently associated with outcome in critically ill neonates and children. Epilepsia. 2017. Mar;58:420–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brophy GM, Bell R, Claassen J, et al. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care. 2012. Aug;17:3–23. [DOI] [PubMed] [Google Scholar]
- 19.Herman ST, Abend NS, Bleck TP, et al. Consensus statement on continuous EEG in critically ill adults and children, part I: indications. J Clin Neurophysiol. 2015. Apr;32:87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herman ST, Abend NS, Bleck TP, et al. Consensus statement on continuous EEG in critically ill adults and children, part II: personnel, technical specifications, and clinical practice. J Clin Neurophysiol. 2015. Apr;32:96–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of biomedical informatics. 2009. Apr;42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.News U. Best Children’s Hospitals for Neurology and Neurosurgery. Available at: https://health.usnews.com/best-hospitals/pediatric-rankings/neurology-and-neurosurgery. Accessed July, 2022.
- 23.Sharma A, Minh Duc NT, Luu Lam Thang T, et al. A Consensus-Based Checklist for Reporting of Survey Studies (CROSS). J Gen Intern Med. 2021. Oct;36:3179–3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hirsch LJ, Fong MWK, Leitinger M, et al. American Clinical Neurophysiology Society’s Standardized Critical Care EEG Terminology: 2021 Version. J Clin Neurophysiol. 2021. Jan 1;38:1–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abend NS, Dlugos DJ, Hahn CD, Hirsch LJ, Herman ST. Use of EEG monitoring and management of non-convulsive seizures in critically ill patients: a survey of neurologists. Neurocrit Care. 2010. Jun;12:382–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gavvala J, Abend N, LaRoche S, et al. Continuous EEG monitoring: a survey of neurophysiologists and neurointensivists. Epilepsia. 2014. Nov;55:1864–1871. [DOI] [PubMed] [Google Scholar]
- 27.Swisher CB, Sinha SR. Utilization of Quantitative EEG Trends for Critical Care Continuous EEG Monitoring: A Survey of Neurophysiologists. J Clin Neurophysiol. 2016. Dec;33:538–544. [DOI] [PubMed] [Google Scholar]
- 28.Hilkman DMW, van Mook W, Mess WH, van Kranen-Mastenbroek V. The Use of Continuous EEG Monitoring in Intensive Care Units in The Netherlands: A National Survey. Neurocrit Care. 2018. Oct;29:195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bruns N, Felderhoff-Muser U, Dohna-Schwake C, Woelfle J, Muller H. aEEG Use in Pediatric Critical Care-An Online Survey. Front Pediatr. 2020;8:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koffman L, Rincon F, Gomes J, et al. Continuous Electroencephalographic Monitoring in the Intensive Care Unit: A Cross-Sectional Study. J Intensive Care Med. 2020. Nov;35:1235–1240. [DOI] [PubMed] [Google Scholar]
- 31.Kirschen MP, LaRovere K, Balakrishnan B, et al. A Survey of Neuromonitoring Practices in North American Pediatric Intensive Care Units. Pediatr Neurol. 2022. Jan;126:125–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nash KB, Palaganas J, Abend NS, et al. The State of Inpatient Child Neurology: A Survey of North American Academic Programs. Neurology. 2022. Sep 12. [DOI] [PubMed] [Google Scholar]
- 33.Ghossein J, Alnaji F, Webster RJ, Bulusu S, Pohl D. Continuous EEG in a Pediatric Intensive Care Unit: Adherence to Monitoring Criteria and Barriers to Adequate Implementation. Neurocrit Care. 2021. Apr;34:519–528. [DOI] [PubMed] [Google Scholar]
- 34.Gutierrez-Colina AM, Topjian AA, Dlugos DJ, Abend NS. EEG Monitoring in Critically Ill Children: Indications and Strategies. Pediatric Neurology. 2012;46:158–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chang N, Rasmussen L. Exploring Trends in Neuromonitoring Use in a General Pediatric ICU: The Need for Standardized Guidance. Children (Basel). 2022. Jun 22;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Herman I, Nguyen T, Chan SW, Erklauer J, Riviello JJ, Lai YC. Implementation of a Pediatric Neurocritical Care Program for Children With Status Epilepticus: Adherence to Continuous Electroencephalogram Monitoring. Pediatr Crit Care Med. 2022. Dec 1;23:1037–1046. [DOI] [PubMed] [Google Scholar]
- 37.Fung FW, Jacobwitz M, Parikh DS, et al. Development of a model to predict electroencephalographic seizures in critically ill children. Epilepsia. 2020. Mar;61:498–508. [DOI] [PubMed] [Google Scholar]
- 38.Fung FW, Parikh DS, Jacobwitz M, et al. Validation of a model to predict electroencephalographic seizures in critically ill children. Epilepsia. 2020. Dec;61:2754–2762. [DOI] [PubMed] [Google Scholar]
- 39.Fung FW, Fan J, Vala L, et al. EEG monitoring duration to identify electroencephalographic seizures in critically ill children. Neurology. 2020. Sep 15;95:e1599–e1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fung FW, Fan J, Parikh DS, et al. Validation of a Model for Targeted EEG Monitoring Duration in Critically Ill Children. J Clin Neurophysiol. 2022. Apr 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Butler E, Mills N, J JPA, Hart AR. Knowledge and attitudes of critical care providers towards neurophysiological monitoring, seizure diagnosis, and treatment. Dev Med Child Neurol. 2021. Aug;63:976–983. [DOI] [PubMed] [Google Scholar]
- 42.Lalgudi Ganesan S, Hahn CD. Spectrograms for Seizure Detection in Critically Ill Children. J Clin Neurophysiol. 2022. Mar 1;39:195–206. [DOI] [PubMed] [Google Scholar]
- 43.Ng MC, Gillis K. The state of everyday quantitative EEG use in Canada: A national technologist survey. Seizure. 2017. Jul;49:5–7. [DOI] [PubMed] [Google Scholar]
- 44.Rowberry T, Kanthimathinathan HK, George F, et al. Implementation and Early Evaluation of a Quantitative Electroencephalography Program for Seizure Detection in the PICU. Pediatr Crit Care Med. 2020. Jun;21:543–549. [DOI] [PubMed] [Google Scholar]
- 45.LaRovere KL, Murphy SA, Horak R, et al. Pediatric Neurocritical Care: Evolution of a New Clinical Service in PICUs Across the United States. Pediatr Crit Care Med. 2018. Nov;19:1039–1045. [DOI] [PubMed] [Google Scholar]
- 46.Ahrens S, Twanow JD, Vidaurre J, Gedela S, Moore-Clingenpeel M, Ostendorf AP. Electroencephalography Technologist Inter-rater Agreement and Interpretation of Pediatric Critical Care Electroencephalography. Pediatr Neurol. 2021. Feb;115:66–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Glauser T, Shinnar S, Gloss D, et al. Evidence-Based Guideline: Treatment of Convulsive Status Epilepticus in Children and Adults: Report of the Guideline Committee of the American Epilepsy Society. Epilepsy Curr. 2016. Jan-Feb;16:48–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fung FW, Jacobwitz M, Vala L, et al. Electroencephalographic seizures in critically ill children: Management and adverse events. Epilepsia. 2019. Oct;60:2095–2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fitzgerald MP, Massey SL, Fung FW, et al. Expanding Access to Continuous EEG Monitoring in Neonatal Intensive Care Units. J Clin Neurophysiol. 2021. Nov 1;38:525–529. [DOI] [PubMed] [Google Scholar]
- 50.Williams RP, Banwell B, Berg RA, et al. Impact of an ICU EEG monitoring pathway on timeliness of therapeutic intervention and electrographic seizure termination. Epilepsia. 2016. May;57:786–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Addendum Figure 1. Changes in key characteristics between 2011 and 2022 for (A) US and (B) Canada.


